Abstract
Background
CD47 has been identified as an innate immune checkpoint and found to be associated with inferior survival in various types of cancer. However, the critical role of CD47 in gastric cancer and its association with tumor associated macrophages remain unclear.
Methods
Tumor tissues of gastric cancer from Zhongshan Hospital and data from GSE62254, GSE84437 and TCGA datasets were analyzed. Immunohistochemistry was performed to detect the expression of CD47, CD11c, CD163 and CD68 in gastric cancer tissues. Kaplan–Meier curves and Cox model were used for comparing the clinical outcomes of patients belonging to different subgroups.
Results
Gastric cancer patients with high CD47 expression exhibited poor prognosis and inferior therapeutic responsiveness to fluorouracil-based adjuvant chemotherapy (ACT). A positive correlation was found between M1-polarized macrophage infiltration and CD47 expression in gastric cancer; however, the prognostic value of M1-polarized macrophages was attenuated in CD47-high gastric cancer patients. Moreover, we found that CD47 mRNA level was enriched in microsatellite-instable (MSI) subtype of gastric cancer and associated with ARID1A mutation and FGFR2 signaling pathway activation.
Conclusions
Aberrant CD47 expression represented an independent predictor for adverse survival outcome and ACT resistance in gastric cancer. Targeting CD47 might be a promising strategy for gastric cancer patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-020-02806-2.
Keywords: CD47, Gastric cancer, Prognosis, Adjuvant chemotherapy, Tumor-associated macrophage
Introduction
Gastric cancer remains one of the most common and deadly cancers and presents a serious threat to global health. Most patients are diagnosed at an advanced stage due to a lack of early specific symptoms, which contributes to a poor prognosis [1]. For these advanced-stage patients, standard gastrectomy combined with a 5-fluorouracil (5-FU)-based adjuvant chemotherapy (ACT) regimen is commonly recommended as the first-line choice, especially in Asian countries [2–4]. However, drug resistance and poisonous side effect limit the clinical application of ACT. Therefore, it is in urgent need to better identify patients who will benefit from ACT and to explore new agents for those who are not responsive to chemotherapy.
It has been well elucidated that tumor-reactive T-cell responses play a critical role in eliminating tumor cells [5]. Cancer immunotherapies that interfere with immune-inhibitory receptors mainly on T cells (so-called checkpoint inhibitors), such as cytotoxic T lymphocyte-associated protein 4 (CTLA-4) [6] or programmed cell death 1 (PD-1) [7], have shown promising efficacy in many malignancies. However, despite the success, only a subset of patients respond to existing immunotherapy [8]. To extend the benefit of such T-cell-targeted immunotherapies to more patients, greater knowledge of the relationship between cancer and the immune system is required [9].
The innate immune response constitutes an essential weapon of the immune system that dictates the type of adaptive immune response. As the most abundant innate immune cells in tumor microenvironment, although macrophages were shown to have predominantly pro-tumor and immunosuppressive effects in many cancer types, they are also indispensable to establish effective anti-tumor immunity [10]. Based on the environmental cues, tumor-associated macrophages can display a spectrum of activation states that fulfill specific functions and are often catalogued as classically (M1; commonly activated by IFN-γ and TLR ligands, express higher levels of IL-12, IL-23, TNF-α, MHCII, IL-6, and inducible nitric oxide synthase or iNOS) or alternatively activated (M2; commonly activated by IL-4 and IL-13, express higher levels of IL-10 and TGF-β) for simplicity [11]. Through a process known as phagocytosis, macrophages, especially the M1-polarized subsets, can capture and eliminate transformed malignant cells, and present the tumor-derived antigens to prime T cells and activate downstream adaptive immune responses [12]. And our previous study suggested that M1-polarized macrophages, which exhibit stronger phagocytic function, were associated with improved survival of gastric cancer [13]. However, recent studies revealed that tumor cells could upregulate the anti-phagocytic markers and evade the phagocytosis by macrophages [14]. Therefore, it is critical to comprehensively explore the significance of anti-phagocytic marker expression in cancers.
CD47 is an immunoglobulin-like trans-membrane protein that is widely expressed on normal cells, but often overexpressed on cancer cells [15]. By binding with its receptor, Signal-Regulatory Protein alpha (SIRPα), CD47 expression can inhibit phagocytosis of macrophages, and attenuate the presentation of tumor antigens to T lymphocytes, thereby impairing both macrophage-mediated and cytotoxic CD8+ T-cell-mediated anti-tumor effects, which made CD47–SIRPα axis a promising innate immune checkpoint (ICK) in cancer [16]. Studies largely led by the Weissman group provided compelling indication that CD47 blockade markedly enhanced the ability of macrophages to engulf tumor cells in vitro [17–19]. Moreover, CD47-high expression has been found to be associated with poor prognosis in patients with non-small-cell lung cancer [20], melanoma [21], oral squamous cell carcinoma [22], etc. In gastric cancer, Yoshida K has identified CD47 as an adverse prognostic factor, but its relationship with chemotherapy responsiveness and tumor-associated macrophages remains ambiguous [23].
Herein, our results indicated that CD47 could be used as an independent prognosticator for patients with gastric cancer and could identify patients who might benefit more from ACT. A positive correlation between M1-polarized macrophages and CD47 expression was found in gastric cancer; however, the prognostic value of M1-polarized macrophages was attenuated in CD47-high tumors. Moreover, we found that CD47 expression was up-regulated in microsatellite-instable (MSI) tumors and associated with ARID1A mutation status and FGFR2 signaling activation. These results may shed light on the clinical importance of CD47 in gastric cancer and provide a possible predictive system to evaluate outcomes for patients with gastric cancer.
Materials and methods
Study population
Initially, we recruited a cohort of 496 gastric cancer patients treated with radical gastrectomy at Zhongshan Hospital of Fudan University during 2007 and 2008 after approval of Clinical Research Ethics Committee of Zhongshan Hospital. After excluding patients with distant metastasis, incomplete data or dot loss, 453 patients were included in the subsequent analyses. All tissue samples were formalin fixed and paraffin embedded. Clinicopathological characteristics, including age, sex, tumor location, tumor size, tumor grade, Lauren classification, T classification, N classification, TNM stage and application of fluorouracil-based ACT, were retrospectively collected. The T, N classification and TNM stage were determined referring to the 7th edition of the American Joint Committee on Cancer (AJCC) TNM staging system. The cohort was randomly divided into 2 independent data sets: the discovery data set (n = 253) and the validation data set (n = 200). Postoperative ACT was administered to patients according to NCCN guidelines and patients’ will. No radiotherapy was given to any one of the patients enrolled. Overall survival (OS) was computed from the date of gastrectomy to the date of death or the last follow-up. Patients who died from other cause or were alive at last follow-up were censored.
Immunohistochemistry and evaluation of CD47 expression
Tissue microarray (TMA) was constructed and immunohistochemistry staining was performed according to the protocols as detailed previously [24]. Anti-CD47 monoclonal antibody (ab218810, diluted 1:2000; Abcam) was applied to identify CD47 expression in tissue. CD47 was stained yellowish to brown in cancer cells. Digitally Scanned with Nikon eclipse Ti-s microscope (Nikon, Tokyo, Japan), TMA sections were independently reviewed by Dr Zhang and Dr Chen who were blinded to clinical information. The representative images were illustrated in Supplementary Fig. 1. The level of CD47 expression was evaluated as the mean of “H-Scores” in 6 representative fields (each pathologist with 3 fields). The “H-score” was calculated using “∑pi (i + 1)” for all slides, in which “pi” represented the percentage of positive cells among all cells in the various intensity categories, and “i” represented the staining intensity (i = 0, weak positive; i = 1, median positive; i = 2, strong positive). The mean value of their evaluation was adopted for further analyses. The intraclass correlation between the two pathologists’ evaluation for CD47 expression level from the same slide was 0.944 (95% CI: 0.914–0.963, P = 0.001). Additionally, anti-CD68 monoclonal antibody (ab955, diluted1:300; Abcam), anti-CD11c monoclonal antibody (ab52632, diluted1:500; Abcam) and anti-CD163 monoclonal antibody (ab182422, diluted1:500; Abcam) were applied to identify CD68, CD11c and CD163 expression in tissue, which represented the infiltration of overall infiltrated, M1-polarized, and M2-polarized tumor-associated macrophages (TAMs), respectively.
Public dataset analysis
Three independent public data sets, GSE62254, GSE84437 and TCGA, including 300, 433, and 384 gastric cancer patients, respectively, were included in our analysis. Data of GSE84437 and GSE62254 were obtained from the respective depository in the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/). TCGA data were downloaded from the UCSC Xena. M0, M1 and M2 cell infiltration was estimated on microarray data by CIBERSPOT algorithm using LM22 as the expression signature [25]. The cut-off value of CD47 mRNA in three data sets was determined as median. Pre-defined gene signatures associated with gastric cancer molecular classification were applied for our analysis, which included EMT UP and EMT DOWN (up- and down-regulated genes in EMT program) [26], MSI UP (up-regulated genes in MSI tumors) [27] and TP53 (up-regulated in TP53 mutated tumors) [28]. We have also analyzed several most common mutated genes [29] and aberrantly activated signaling [30] in gastric cancer based on the Molecular Signatures Database (MSigDB). Gene set variation analysis (GSVA) R package and its ssGSEA method (http://www.bioconductor.org) were implemented to calculate the gene expression signature scores for each sample in GSE62254 dataset [31].
Statistical analysis
Statistical analyses were carried out using GraphPad Prism (Version 6.00), R software (Version 3.5.3), MedCalc (Version 12.7.0) and IBM SPSS Statistics (Version 21) software. The cut-off value for classifying CD47-high/low subgroup was determined by the median value of “H-Score”. Ultimately, 70 was determined as the cut-off value of CD47 in both discovery set and validation set. Correlation with survival was evaluated by means of Kaplan–Meier curves, log-rank test, and univariate and multivariate analyses based on the Cox proportional hazards method. Student’s t test was applied for categorical variables. The correlation between two variables was assessed using Spearman’s correlation test. All grouped data in the figures are represented by the means ± SDs. For all tests in this study, a P value of < 0.05 was considered statistically significant.
Results
CD47 expression indicates poor clinical outcomes in gastric cancer patients
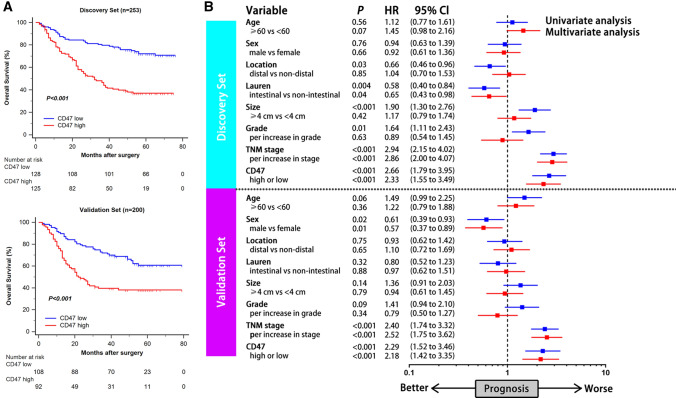
CD47 expression was evaluated by immunohistochemistry on tissue microarrays (representative images were illustrated in Supplementary Fig. 1). The relationship between tumor CD47 expression and clinicopathologic variables in gastric cancer was subsequently investigated (Table 1). In both Discovery set and Validation set, CD47 expression was correlated with increased tumor stage, which indicated that CD47 might be involved in tumor progression in gastric cancer. To further investigate the prognostic value of CD47 expression in gastric cancer, we applied Kaplan–Meier curves and log-rank test to compare overall survival (OS) between CD47-low and CD47-high patients. In both Discovery set and Validation set, CD47-high patients had significantly poorer OS (P < 0.001 and P < 0.001; Fig. 1a). Consistent with the above results, univariate and multivariate Cox analyses were performed to find out whether CD47 could serve as a potential independent prognostic factor for the survival outcomes. The analysis was conducted including age, sex, tumor location, Lauren classification, tumor size, tumor grade, TNM stage and CD47 expression. It revealed that CD47 expression was an independent prognosticator for worse OS according to multivariate analysis in both Discovery set and Validation set (Hazard Ratio (HR): 2.33, 95% Confidence Interval (CI): 1.55–3.49, P < 0.001 and HR: 2.18, 95% CI: 1.42–3.35, P < 0.001; Fig. 1b). Taken together, these results suggested that CD47 expression was associated with poor OS in patients with gastric cancer.
Table 1.
Relation between CD47 expression and clinical characteristics of patients with gastric cancer
| Group | Discovery set (n = 253) | Validation set (n = 200) | ||||
|---|---|---|---|---|---|---|
| CD47 | CD47 | |||||
| Low | High | P value | Low | High | P value | |
| Age(years) | 0.236 | 0.527 | ||||
| < 60 | 60 | 71 | 53 | 41 | ||
| ≥ 60 | 65 | 57 | 55 | 51 | ||
| Gender | 0.898 | 0.959 | ||||
| Male | 85 | 88 | 79 | 67 | ||
| Female | 40 | 40 | 29 | 25 | ||
| Location | 0.589 | 0.753 | ||||
| Proximal | 29 | 27 | 30 | 24 | ||
| Middle | 18 | 31 | 10 | 8 | ||
| Distal | 78 | 70 | 68 | 60 | ||
| Tumor size(cm) | 0.481 | 0.150 | ||||
| ≥ 4 cm | 55 | 62 | 43 | 46 | ||
| < 4 cm | 70 | 66 | 65 | 46 | ||
| Lauren’s classification | 0.426 | 0.682 | ||||
| Intestinal type | 77 | 67 | 78 | 64 | ||
| Non-intestinal type | 48 | 61 | 30 | 28 | ||
| Grade | 0.670 | 0.089 | ||||
| 1 | 7 | 8 | 5 | 3 | ||
| 2 | 25 | 20 | 30 | 16 | ||
| 3 | 93 | 100 | 73 | 73 | ||
| Depth of tumor invasion | 0.188 | 0.006 | ||||
| T1 | 29 | 22 | 27 | 11 | ||
| T2 | 19 | 15 | 20 | 8 | ||
| T3 | 18 | 25 | 17 | 27 | ||
| T4 | 59 | 66 | 44 | 46 | ||
| Lymph node metastasis | 0.002 | 0.310 | ||||
| N0 | 65 | 42 | 40 | 30 | ||
| N1 | 9 | 18 | 12 | 12 | ||
| N2 | 28 | 21 | 24 | 12 | ||
| N3 | 23 | 47 | 32 | 38 | ||
| TNM stage | 0.003 | 0.023 | ||||
| I | 43 | 23 | 34 | 15 | ||
| II | 30 | 33 | 21 | 21 | ||
| III | 52 | 72 | 53 | 56 | ||
P value < 0.05 marked in bold font shows statistical significance
Fig. 1.
CD47 expression predicts poor prognosis in gastric cancer patients. a Kaplan–Meier curves of OS according to CD47 expression in Discovery set (top, n = 253) and Validation set (bottom, n = 200). Data were analyzed by log-rank test. b Univariate and multivariate analyses based on clinicopathological characteristics in Discovery set (top, n = 253) and Validation set (bottom, n = 200). HR refers to hazard ratio; CI refers to confidence interval
CD47 expression yields inferior ACT response in stage II/III gastric cancer patients
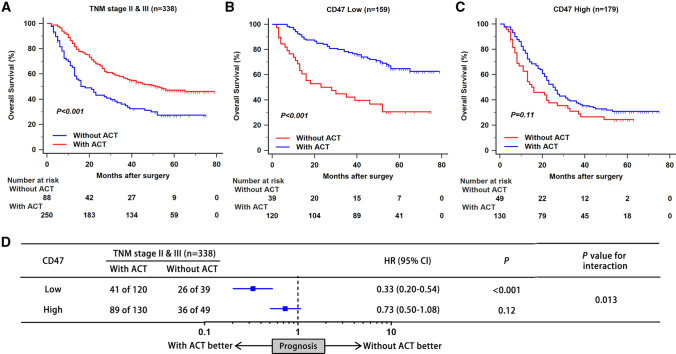
As depicted in Fig. 2a, treatment by fluorouracil-based ACT was associated with better OS in patients with stage II/III gastric cancer (P < 0.001). To evaluate the predictive value of CD47 expression for ACT, we compared prognosis of patients stratified by ACT application in different CD47 expression subgroup. In CD47-low subgroup, ACT could significantly lead to better OS (P < 0.001; Fig. 2b). However, no statistically significant difference was found in CD47-high subgroup (P = 0.11; Fig. 2c). Furthermore, a test for interaction also indicated that the benefit of ACT was superior among CD47-low patients (HR: 0.33, 95% CI: 0.20–0.54, P value for interaction: 0.013; Fig. 2d) than CD47-high patients. Consequently, these results provided an indication that CD47 expression could possibly identify patients with stage II/III gastric cancer who were suitable candidates for adjuvant chemotherapy.
Fig. 2.
CD47 expression indicates inferior responsiveness to fluorouracil-based adjuvant chemotherapy in gastric cancer patients. a–c Kaplan–Meier curves of OS according to ACT application in all stage II/III patients (a), patients with low CD47 expression (b), and patients with high CD47 expression (c). d The interaction between CD47 expression and therapeutic responsiveness to ACT. HR refers to hazard ratio; CI refers to confidence interval; ACT refers to adjuvant chemotherapy
CD47 expression correlates with macrophage infiltration in gastric cancer patients
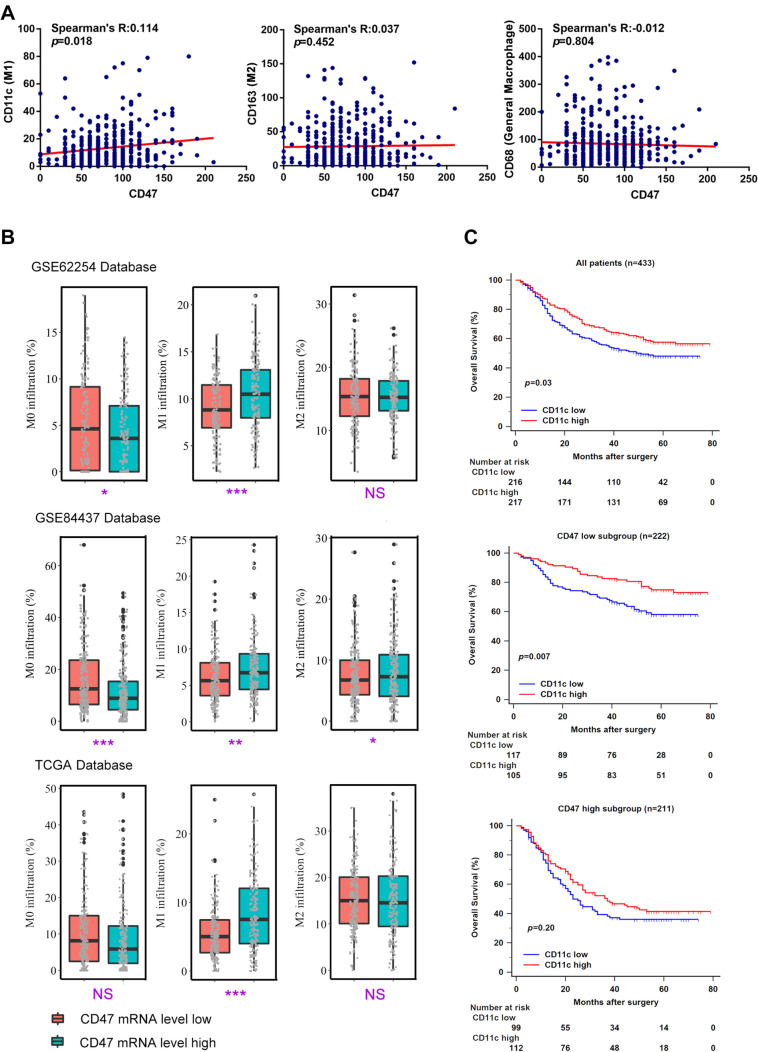
We next investigate the association between CD47 expression and macrophage infiltration. Interestingly, it was found that CD47 expression was highly associated with CD11c expression, which represented the level of M1-polarized macrophage infiltration (Spearman’s R: 0.114, P = 0.018; Fig. 3a). To further illustrate our results, we used CIBERSORT to calculate the relative proportion of M0, M1 and M2-polarized macrophage infiltration in different CD47 mRNA level subgroup. As shown in Fig. 3b, high proportion of M1-polarized macrophages infiltration was found in CD47 mRNA level high subgroup in GSE62254, GSE84437 and TCGA (P < 0.001, P = 0.002, and P < 0.001; Fig. 3b). Our previous study has revealed that increased M1-polarized macrophages indicated better prognosis in gastric cancer [13]. To explore the impact of CD47 expression on the prognostic value of M1-polarized macrophages in gastric cancer, we applied Kaplan–Meier curves and log-rank test to compare OS of patients stratified by CD11c expression in different CD47 expression subgroup. Consistent with previous studies [32], high CD11c expression was a prognosticator for better OS generally (P = 0.03; Fig. 3c). Intriguingly, CD47-low patients gained more significant survival benefit from CD11c abundance (P = 0.007; Fig. 3c). However, high expression of CD11c failed to improve survival of CD47-high patients, which suggested that CD47 attenuates the prognostic value of M1-polarized macrophages in gastric cancer patients (P = 0.20; Fig. 3c).
Fig. 3.
Characterization of tumor-associated macrophages based on CD47 expression. a Correlation of CD47 expression with CD11c expression (M1), CD163 expression (M2), and CD68 expression (general macrophages). Data were analyzed by Spearman’s correlation test. b Relative proportion of M0, M1 and M2-polarized macrophage infiltration between low/high CD47 mRNA expression subgroup in GSE62254, GSE84437 and TCGA database. Data were analyzed by Student’s t test, and presented as median and interquartile range (IQR). c Kaplan–Meier curves according to CD11c expression (M1) in all patients (top, n = 433), CD47-low subgroup (middle, n = 222), and CD47-high subgroup (bottom, n = 211). Data were analyzed by log-rank test. *P < 0.05, **P < 0.01, ***P < 0.001, and NS indicates not significant
CD47 expression associates with genomic features in gastric cancer patients
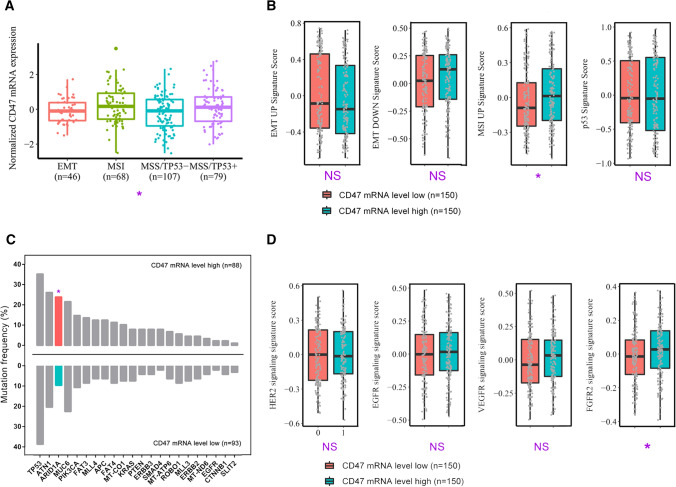
Previous studies have illustrated that genomic features of gastric cancer provided a possible avenue for patients’ stratification and personalized anti-tumor therapies [33]. Remarkably, we found that CD47 mRNA expression in GSE62254 showed difference across 4 molecular subtypes, and was highly accumulated in MSI subtype (P = 0.016; Fig. 4a). Next, we applied molecular subtype signatures to further illustrate our results. Tumors with high CD47 expression showed an increased MSI UP signature score (P = 0.024; Fig. 4b), which validated the finding that CD47 mRNA was overexpressed in MSI subtype of gastric cancer. However, no statistically significant difference of EMT UP, EMT DOWN or p53 signature score was found between CD47-low and CD47-high tumors (Fig. 4b). Furthermore, we focused on profiles of key gastric cancer gene mutations and therapeutic targets-associated signatures. It was shown that ARID1A gene mutations were enriched in CD47-high tumors, which agreed with features of MSI subtype (P = 0.010; Fig. 4c) [34]. Additionally, CD47-high tumors presented elevated FGFR2 signature score, which indicated FGFR2 signaling activation in these tumors (P = 0.044; Fig. 4d). Collectively, our results indicated that CD47 mRNA expression was elevated in MSI subtype of gastric cancer and associated with ARID1A mutation and FGFR2 signaling activation.
Fig. 4.
Association between CD47 mRNA expression and tumor-intrinsic genomic features. a Quantification analyses of normalized CD47 mRNA expression in GSE62254 database across molecular classification systems (n = 300). Data were analyzed by one-way ANOVA, and presented as median and interquartile range (IQR). b Quantification of molecular subtype signatures between low/high CD47 mRNA expression subgroup (n = 300). Data were analyzed by Student’s t test, and presented as median and IQR. c Association of genes mutation frequency with CD47 mRNA expression level (n = 181). Data were analyzed by Chi-square test. d Quantification analyses of therapy-associated gene signatures between low/high CD47 mRNA expression subgroup (n = 300). Data were analyzed by Student’s t test, and presented as median and IQR. *P < 0.05, **P < 0.01, ***P < 0.001, and NS indicates not significant
Discussion
Prognostic assessment is crucial for choosing appropriate treatment for gastric cancer patients. Recently, the association of ICK expression with patients’ prognosis has attracted more and more attention in different cancer due to its impact on immune contexture [35–37]. In this study, we found that CD47 was an independent prognosticator and indicated inferior overall survival in gastric cancer in both Discovery set and Validation set. Expanding researches have pointed that overexpressed CD47 functioned as a “don’t eat me” signal on cancer cells, inhibiting phagocytosis and clearance by macrophages and enhancing tumor cell in vivo, which supports our observation [38].
Fluorouracil-based ACT is recommended as a first-line adjuvant therapy regimen for stage II/III gastric cancer patients [2, 3]. To prevent excessive toxic effects, we believed it was important to identify patients who might benefit more from postoperative ACT. Our previous studies have demonstrated the contribution of gammadelta T cells [39], IL17A+ cells [40] and O6-methylguanine-DNA methyltransferase (MGMT) [24] to the beneficial of ACT in gastric cancer. In this study, we found that CD47-high patients yielded poorer ACT response compared to CD47-low patients. As a result, our findings suggested that CD47 might be a significant biomarker to stratify stage II/III patients who might be suitable for ACT. Additionally, ACT in combination with CD47-targeting therapy could be investigated in further researches of gastric cancer.
Our analyses on Zhongshan cohort and GSE62254, GSE84437, TCGA database both demonstrated the positive correlation between CD47 expression and M1-polarized macrophage infiltration. Previous studies have revealed that M1-polarized macrophages had a cytotoxic effect and increased apoptosis in cancer cells [41]. Thus, M1 abundance in cancer is considered as a sign for better prognosis for patients, which is consistent to our finding. However, we found that M1 infiltration failed to predict patients’ prognosis in CD47-high subgroup, which suggested that CD47 might promote gastric cancer progression by attenuating the anti-tumor effects of M1-polarized macrophages in gastric cancer.
The genomic features of gastric cancer were gradually unveiled and attracting more and more attention, which provided more and more suggestions for targeted therapies [33, 42, 43]. We discovered that CD47 expression was enriched in MSI subtype, which was validated by the positive correlation of CD47 expression with MSI UP signature score. Meanwhile, the mutation of ARID1A gene was up-regulated in CD47 mRNA-high tumors, which could promote DNA mutability and increase tumor sensitivity to immune checkpoint blockade (ICB) therapies [44]. Moreover, FGFR2 signaling showed higher activation level in CD47-high tumors. This correlation suggested possible combination of CD47 blockade and FGFR2-targeted therapy. However, verification of above findings at cellular level and CD47 blockade combined with other targeted therapies need further investigation.
In conclusion, our study clarified that CD47-high expression was an independent prognosticator for inferior prognosis and ACT resistance in gastric cancer. Further analysis showed that CD47 could possibly attenuate the prognostic value of M1-polarized macrophages. Moreover, we found that CD47 mRNA level was the highest in MSI subtype of gastric cancer and associated with ARID1A mutation and FGFR2 signaling pathway activation. Thus, CD47 could be a predictor for prognosis and adjuvant chemotherapy, as well as a clinical target for monotherapy and combined therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Peipei Zhang (Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) for their contribution to the evaluation of immunohistochemistry results and excellent pathological technology help.
Author contributions
M. Shi, Y. Gu, K. Jin and H. Fang for acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; Y. Chen, Y. Cao, X. Liu, K. Lv, X. He, C. Lin, H. Liu, H. Li, H. He and J. Qin for technical and material support; R. Li, H. Zhang and W. Zhang for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Funding
This study was funded by grants from National Natural Science Foundation of China (81671628, 81871306, 81871926, 81871930, 81902402, 81902901, 81972219), Shanghai Municipal Natural Science Foundation (18ZR1432900), and Shanghai Sailing Program (17YF1402200, 18YF1404600, 19YF1407500). All these study sponsors have no roles in the study design, in the collection, analysis and interpretation of data.
Data availability
All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr. Zhang upon reasonable request.
Code availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The protocol of all study was approved by the institutional review board and ethics committee of Zhongshan Hospital, Fudan University.
Informed consent
Written informed consent was obtained from each patient included.
Consent for publication
All authors provide their consent for publication of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mingsu Shi, Yun Gu, Kaifeng Jin and Hanji Fang contributed equally to this work.
Contributor Information
Ruochen Li, Email: rcli12@fudan.edu.cn.
Heng Zhang, Email: zhang.heng@zs-hospital.sh.cn.
Weijuan Zhang, Email: weijuanzhang@fudan.edu.cn.
References
- 1.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 2.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 3.Group G, Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303(17):1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 4.Petrillo A, Smyth EC. Multimodality treatment for localized gastric cancer: state of the art and new insights. Curr Opin Oncol. 2020;32(4):347–355. doi: 10.1097/CCO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 5.O'Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16(3):151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 6.Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131(1):58–67. doi: 10.1182/blood-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21(1):24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitt JM, Vetizou M, Daillere R, Roberti MP, Yamazaki T, Routy B, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44(6):1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Rothlin CV, Ghosh S. Lifting the innate immune barriers to antitumor immunity. J Immunother Cancer. 2020;8(1):e000695. doi: 10.1136/jitc-2020-000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demaria O, Cornen S, Daeron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574(7776):45–56. doi: 10.1038/s41586-019-1593-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Wang X, Shen Z, Xu J, Qin J, Sun Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer. 2015;18(4):740–750. doi: 10.1007/s10120-014-0422-7. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Zheng DX, Yu XJ, Sun HW, Xu YT, Zhang YJ, et al. Macrophages induce CD47 upregulation via IL-6 and correlate with poor survival in hepatocellular carcinoma patients. Oncoimmunology. 2019;8(11):e1652540. doi: 10.1080/2162402X.2019.1652540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veillette A, Chen J. SIRPalpha-CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol. 2018;39(3):173–184. doi: 10.1016/j.it.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21(10):1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrera L, Montes-Servin E, Hernandez-Martinez JM, Garcia-Vicente MLA, Montes-Servin E, Herrera-Martinez M, et al. CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer. 2017;117(3):385–397. doi: 10.1038/bjc.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu W, Li J, Zhang W, Li P. High expression of CD47 predicts adverse prognosis in Chinese patients and suppresses immune response in melanoma. Biomed Pharmacother. 2017;93:1190–1196. doi: 10.1016/j.biopha.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Pai S, Bamodu OA, Lin YK, Lin CS, Chu PY, Chien MH, et al. CD47-SIRPalpha signaling induces epithelial-mesenchymal transition and cancer stemness and links to a poor prognosis in patients with oral squamous cell carcinoma. Cells. 2019;8(12):1658. doi: 10.3390/cells8121658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida K, Tsujimoto H, Matsumura K, Kinoshita M, Takahata R, Matsumoto Y, et al. CD47 is an adverse prognostic factor and a therapeutic target in gastric cancer. Cancer Med. 2015;4(9):1322–1333. doi: 10.1002/cam4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y, Liu H, Li H, Lin C, Li R, Wu S, et al. Association of O6-methylguanine-DNA methyltransferase protein expression with postoperative prognosis and adjuvant chemotherapeutic benefits among patients with stage II or III gastric cancer. JAMA Surg. 2017;152(11):e173120. doi: 10.1001/jamasurg.2017.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loboda A, Nebozhyn MV, Watters JW, Buser CA, Shaw PM, Huang PS, et al. EMT is the dominant program in human colon cancer. BMC Med Genomics. 2011 doi: 10.1186/1755-8794-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cancer Genome Atlas N Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21(5):449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 30.Elimova EWR, Shiozaki H, Sudo K, Estrella JS, Badgwell BD, Das P, et al. Molecular biomarkers in gastric cancer. J Natl Compr Canc Netw. 2015;13(4):e19–29. doi: 10.6004/jnccn.2015.0064. [DOI] [PubMed] [Google Scholar]
- 31.Hänzelmann SCR, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013 doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Xu B, Hu WW, Chen LJ, Wu CP, Lu BF, et al. High expression of CD11c indicates favorable prognosis in patients with gastric cancer. World J Gastroenterol. 2015;21(31):9403–9412. doi: 10.3748/wjg.v21.i31.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J, Yu W, Su H, Pang X. Genomic landscape of gastric cancer: molecular classification and potential targets. Sci China Life Sci. 2017;60(2):126–137. doi: 10.1007/s11427-016-0034-1. [DOI] [PubMed] [Google Scholar]
- 34.Kim YS, Jeong H, Choi JW, Oh HE, Lee JH. Unique characteristics of ARID1A mutation and protein level in gastric and colorectal cancer: a meta-analysis. Saudi J Gastroenterol. 2017;23(5):268–274. doi: 10.4103/sjg.SJG_184_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Shi D, Miao J, Wu H, Chen J, Zhou X, et al. PD-L1 predicts poor prognosis for nasopharyngeal carcinoma irrespective of PD-1 and EBV-DNA load. Sci Rep. 2017;7:43627. doi: 10.1038/srep43627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider S, Kadletz L, Wiebringhaus R, Kenner L, Selzer E, Fureder T, et al. PD-1 and PD-L1 expression in HNSCC primary cancer and related lymph node metastasis - impact on clinical outcome. Histopathology. 2018;73(4):573–584. doi: 10.1111/his.13646. [DOI] [PubMed] [Google Scholar]
- 37.Marisa L, Svrcek M, Collura A, Becht E, Cervera P, Wanherdrick K, et al. The balance between cytotoxic T-cell lymphocytes and immune checkpoint expression in the prognosis of colon tumors. J Natl Cancer Inst. 2018 doi: 10.1093/jnci/djx136. [DOI] [PubMed] [Google Scholar]
- 38.Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276(1):145–164. doi: 10.1111/imr.12527. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Lin C, Li H, Li R, Wu Y, Liu H, et al. Tumor-infiltrating gammadeltaT cells predict prognosis and adjuvant chemotherapeutic benefit in patients with gastric cancer. Oncoimmunology. 2017;6(11):e1353858. doi: 10.1080/2162402X.2017.1353858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang JT, Li H, Zhang H, Chen YF, Cao YF, Li RC, et al. Intratumoral IL17-producing cells infiltration correlate with antitumor immune contexture and improved response to adjuvant chemotherapy in gastric cancer. Ann Oncol. 2019;30(2):266–273. doi: 10.1093/annonc/mdy505. [DOI] [PubMed] [Google Scholar]
- 41.Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 2015;15:577. doi: 10.1186/s12885-015-1546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizuguchi A, Takai A, Shimizu T, Matsumoto T, Kumagai K, Miyamoto S, et al. Genetic features of multicentric/multifocal intramucosal gastric carcinoma. Int J Cancer. 2018;143(8):1923–1934. doi: 10.1002/ijc.31578. [DOI] [PubMed] [Google Scholar]
- 43.Pectasides E. Genomic alterations and targeted therapy in gastric and esophageal adenocarcinoma. Clin Ther. 2016;38(7):1589–1599. doi: 10.1016/j.clinthera.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Shen J, Ju Z, Zhao W, Wang L, Peng Y, Ge Z, et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med. 2018;24(5):556–562. doi: 10.1038/s41591-018-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr. Zhang upon reasonable request.
Not applicable.