Abstract
Background
Pancreatic cancer is a highly lethal malignancy often presenting with advanced disease and characterized by resistance to standard chemotherapy. Immune-based therapies such checkpoint inhibition have been largely ineffective such that pancreatic cancer is categorized as an immunologically “cold tumor”. In the present study, we examine the therapeutic efficacy of a personalized cancer vaccine in which tumor cells are fused with dendritic cells (DC) resulting in the broad induction of antitumor immunity.
Results
In the KPC spontaneous pancreatic cancer murine model, we demonstrated that vaccination with DC/KPC fusions led to expansion of pancreatic cancer specific lymphocytes with an activated phenotype. Remarkably, vaccination led to a reduction in tumor bulk and near doubling of median survival in this highly aggressive model. In a second murine pancreatic model (Panc02), vaccination with DC/tumor fusions similarly led to expansion of tumor antigen specific lymphocytes and their infiltration to the tumor site. Having shown efficacy in immunocompetent murine models, we subsequently demonstrated that DC/tumor fusions generated from primary human pancreatic cancer and autologous DCs potently stimulate tumor specific cytotoxic lymphocyte responses.
Conclusions
DC/tumor fusions induce the activation and expansion of tumor reactive lymphocytes with the capacity to infiltrate into the pancreatic cancer tumor bed.
Keywords: Cancer vaccines, Pancreatic cancer, Dendritic cells
Introduction
Pancreatic cancer is a leading cause of cancer related death (1, 2). Patients are characteristically diagnosed with advanced stage disease such that surgical resection is often not possible (2). In patients with localized disease, neoadjuvant chemotherapy has demonstrated modest therapeutic efficacy, but most patients succumb to disease progression due to the intrinsic resistance of pancreatic cancer to cytotoxic therapy. Similarly, pancreatic cancer has been described as a “cold tumor” due to the lack of efficacy of immunomodulatory therapies, such as checkpoint blockade (3, 4). Pathologic evaluation of the tumor site demonstrates a lack of tumor specific lymphocytes potentially due to poor immunogenicity of the tumor, steric hindrance of T cell migration into the tumor bed and the potential tolerizing effect of the associated microenvironment (1, 5, 6).
A major area of investigation is the use of vaccine-based therapy to stimulate the expansion and activation of pancreatic cancer specific lymphocytes (7, 8). Vaccine platforms have targeted oncogenic proteins including K-ras, MUC1, MUC4 and WT1 (9–13). A clinical trial testing mutant Ras vaccine in 11 pancreatic cancer patients resulted in 92% of the patients exhibiting an immune response but only 2 out of 11 with prolonged survival (10). Single antigen approaches may demonstrate limited therapeutic efficacy due to the presence of tumor heterogeneity and the potential for immune escape due to the emergence of antigen negative variants. Targeting of mutationally derived neoantigens may be more efficacious than shared tumor targets as they are less susceptible to tolerance-based mechanisms. In addition, vaccine platforms that do not provide effective antigen presentation may not result in sustained T cell activation.
We have pioneered a strategy for personalized cellular immunotherapy, in which patient specific hybridomas are created by fusing primary tumor cells with autologous dendritic cells (DC) (14–16). In this way, a broad array of tumor antigens, including patient specific neoantigens, is presented in the context of DC mediated co-stimulation. In a phase II clinical trial, vaccination of patients with acute leukemia resulted in the dramatic and durable expansion of leukemia specific T cells in the peripheral blood and bone marrow and the targeting of multiple shared leukemia associated antigens. Of note, despite a median age of 63 in this cohort of non-transplant eligible patients, 71% of patients have remained in remission at 5 years of follow-up, and no patients relapsed after the first 6 months of therapy (16). Similarly, vaccination of patients with myeloma led to a near doubling of the complete response rate following high dose chemotherapy (14, 15).
In this study, we examined the capacity of the DC/tumor fusion vaccine to induce a therapeutically effective immune response targeting pancreatic cancer. In a spontaneous pancreatic cancer model that mimics human disease, we demonstrate the DC/tumor fusions potently induce the expansion of pancreatic cancer lymphocytes with an activated phenotype resulting in a dramatic enhancement of survival. Similarly, in the PANC02 syngeneic immunocompetent model, we demonstrate that vaccination elicits the expansion of antigen specific T cells and results in the infiltration of T cells into the tumor bed associated with reduction of disease bulk. Finally, we have demonstrated that patient derived DC/pancreatic cancer fusions induce CTL responses targeted the primary pancreatic cancer cells.
Results
Generation of DC/tumor fusions
We investigated the potency of the DC/tumor fusion vaccine in two pancreatic cancer murine models: a spontaneous pancreatic cancer model (KPC) and subcutaneous tumor model (Panc02). The KPC model is based on Cre-Lox mediated conditional activation of mutant endogenous alleles of the Kras and Trp53 genes in which an activating point mutation (G12D) in Kras and a dominant negative mutation inTrp53 (R172H) are conditionally activated in the mouse pancreas (17). The KPC model recapitulates many of the salient clinical and pathological features of disease evolution in human disease including evolution from pre-malignant ductal involvement, malignant transformation and metastatic spread, and the relative absence of effector T cells in the tumor bed.
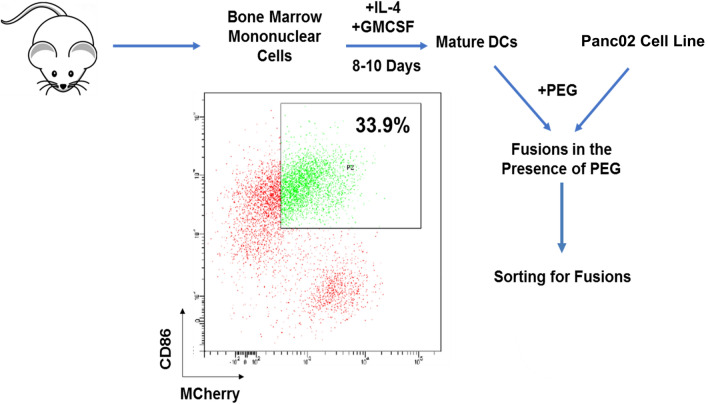
KPC cells were isolated from spontaneous murine tumors and cultured in the presence of Matrigel to form organoid like structures as described in methods. Tumor organoids were dissociated after 2–3 weeks to generate a single cell suspension. Syngeneic DCs were generated by culture of adherent bone marrow mononuclear cells with GM-CSF and IL-4 for 1 week. Prior to fusing, KPC cells were stained using red cell tracker live staining. DC/tumor fusions were generated by coculture of tumor cells and DCs at a ratio of (1:5) in the presence of PEG and fusion cells were isolated by flow cytometric sorting of the cell population that strongly expressed unique DC (CD86) and tumor (red cell tracker) markers. Similarly, syngeneic DC/Panc02 fusion cells were generated as outlined above using Panc02 cells transduced with mCherry as a tumor marker. Fusion cells were identified by dual expression of CD86 and mCherry and isolated by flow cytometric sorting of the population that strongly co-expressed the two surface markers (Fig. 1).
Fig. 1.
Process of DC/Panc02 fusions generation. Dendritic cells (DC) were isolated from the bone marrow mononuclear cells harvested from C57BL/6 J mice cultured with IL-4 and GM-CSF for 5–7 days and fused with M-Cherry/Luciferase + syngeneic Panc02 cells in the presence of polyethylene glycol (PEG). Fusion cells were identified as cells with strong coexpression of DC and tumor markers, CD86 and mCherry, respectively, and isolated by flow cytometric sorting. Gating for the quadrants defining the fusion population was based on integration of single staining for each marker and negative controls. To optimize the purity of the fusion cell population we sorted on the cells that strongly co-expressed CD86 and mCherry
Impact of vaccination on tumor bulk and survival
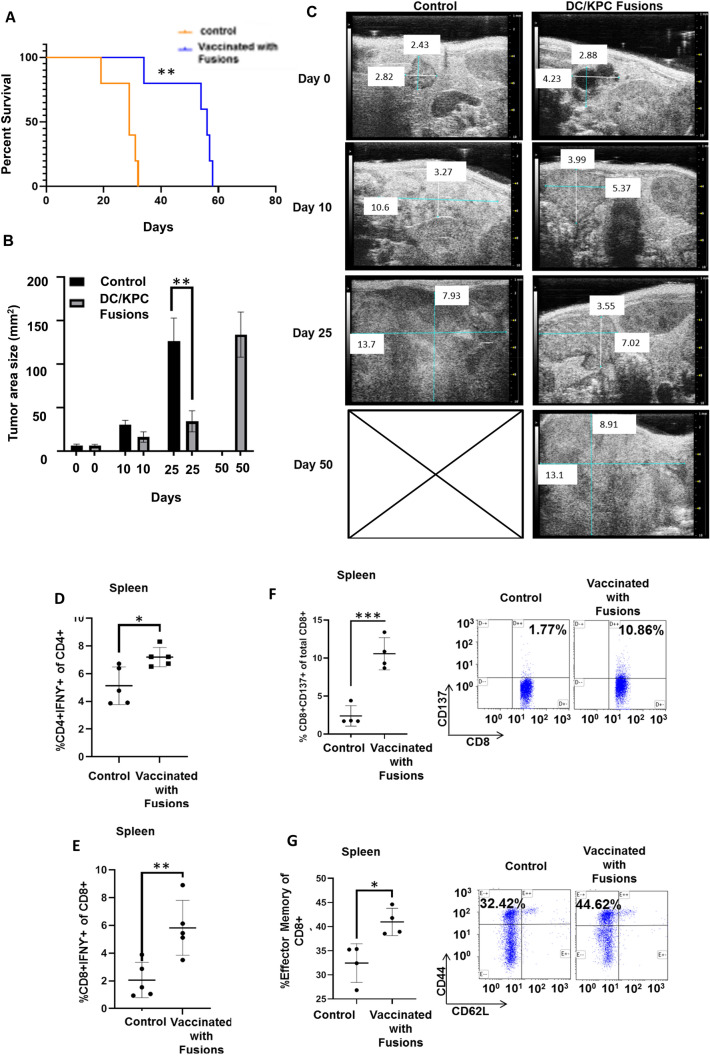
KPC animals characteristically develop malignant disease at 10–20 weeks after birth that is rapidly progressive resulting in death. KPC mice were assessed for the development of spontaneous tumors by serial ultrasound imaging and considered appropriate for vaccination once they had developed tumor size of at least 40 mm2. Based on this criteria, ten mice were enrolled and randomized to vaccination with 105 DC/tumor fusions subcutaneously or observation. Animals were assessed for tumor bulk by serial imaging by ultrasound (US) and monitored daily for survival based on standard criteria for euthanasia based on tumor bulk and symptoms. Remarkably vaccinated animals demonstrated markedly improved survival as compared to the control cohort (Fig. 2A). Vaccinated animals demonstrated significantly slower tumor growth at day 10 and 25 following tumor challenge as compared to untreated animals as determined by serial US imaging (Fig. 2B, C). However, eventually mice in both groups required euthanasia due to pancreatic cancer progression and large tumor volumes.
Fig. 2.
Vaccination with DC/KPC fusions results in improved survival, decreased tumor bulk, and expansion of tumor specific T cells in animals developing spontaneous pancreatic cancer cells. KPC mice developing spontaneous pancreatic tumors > 40 mm2 by ultrasound imaging were enrolled and randomized to undergo vaccination with DC/KPC tumor fusions (n = 5) as compared to observation (n = 5). The mice were followed for survival and euthanized as per criteria outlined above. A Survival curve of treated and control KPC mice showing significantly longer survival for treated mice compared to control mice. N = 5, p < 0.05. B Quantification of tumor area for vaccinated and unvaccinated animals at enrollment, 10 and 25 days post-vaccination demonstrating decreased tumor size for vaccinated animals as compared to unvaccinated animals reaching statistical significance at 25 days post vaccination (p < 0.01, n = 5). C Representative ultrasound scans demonstrating measurement of the tumor at enrollment, and 10, 25, and 50 days post enrollment. D–E At time of euthanasia, splenocytes isolated from vaccinated as compared to control animals demonstrated a significant increase in intracellular IFN-ƴ expressing in CD4 + and CD8 + T cells after ex vivo culture with KPC tumor lysate. F–G Splenocytes isolated from vaccinated as compared to control animals demonstrated a significant increase in CD137 expressing CD8 + T cells and CD8 + T cells expressing CD44 + /CD62L-. A representative flow cytometric analysis is provided
Impact of vaccination with DC/KPC fusions on tumor specific immunity
The induction of tumor specific immunity was interrogated at time of sacrifice by quantifying the percent of CD4 and CD8 splenocytes that express IFNγ following ex vivo exposure to syngeneic tumor lysate as determined by multichannel flow cytometry. Vaccinated animals demonstrated a marked increase in CD4 + and CD8 + T cells expressing IFNγ as compared to control animals (Fig. 2D, E). Consistent with these findings, we demonstrated that vaccination resulted in a significant increase in antigen specific T cell activated cells as manifested by the expansion of the CD8 + CD137 + population as compared to control animals (Fig. 2F). Further analysis of the CD8 + compartment in vaccinated animals demonstrated a relative increase in the CD44 + /CD62L- fraction in contrast to control animals consistent with the vaccine induced expansion of memory cells potentially better able to maintain long term immunity (Fig. 2G).
DC/PANC02 fusions elicit tumor specific immunity In vitro
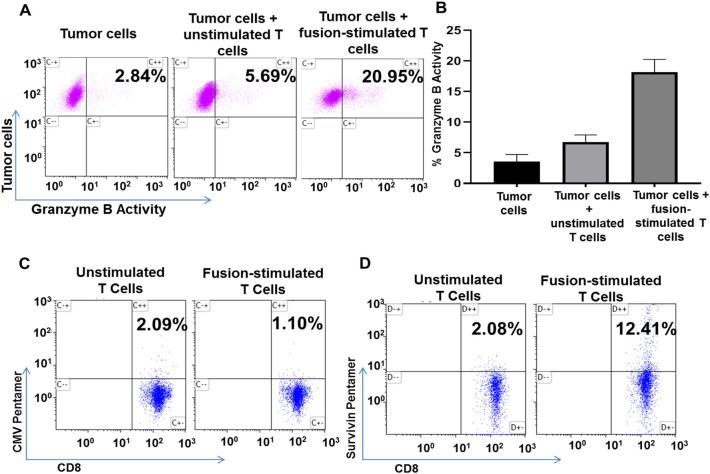
We subsequently studied vaccine efficacy in a second immunocompetent murine pancreatic cancer model involving the Panc02 cell line. Coculture of DC/Panc02 fusion cells with syngeneic T cells elicited the expansion of tumor specific cytotoxic T cells as determined by the enhanced lysis of Panc02 tumor targets (20.95%) as compared to unstimulated T cells (5.69%) in a standard CTL assay (Fig. 3A, B) To further define tumor specificity of the response we assessed the capacity of DC/Panc02 to induce the expansion of T cell recognizing the tumor antigen, survivin, by tetramer analysis (18, 19). Coculture of syngeneic T cells with DC/Panc02 fusions resulted in increased levels of survivin specific T cells while a similar expansion of CMV reactive T cells was not observed (Fig. 3C, D).
Fig. 3.
DC/Panc02 fusions elicit cytotoxic T lymphocyte (CTL) responses and stimulate expansion of tumor antigen specific T cells. Syngeneic T cells harvested from C57BL/6 J mice were cocultured with DC/Panc02 at a ratio of 10:1. After 3–5 days T cells were collected and further analyzed for CTL activity as compared to unstimulated T cells. Fusion stimulated T cells as compared to unstimulated T cells were also assessed regarding the percent of CD8 cells specific to the survivin tumor antigen. A–B T cells cytolytic capacity was quantified using the cytotoxic T lymphocyte (CTL) assay, GranToxiLux. Target Panc02 cells were labeled and cultured with fusion stimulated or control T cells in the presence of a fluorogenic granzyme B substrate which was quantified by flow cytometric analysis. A representative flow plot and summary data (n = 3) showing increased killing of Panc02 cells by fusions stimulated T cells as compared to unstimulated T cells is presented. C To assess expansion of antigen specific T cells, the fusion stimulated and control T cells underwent flow cytometric analysis using H-2 Db pentamer targeting the tumor antigen, survivin, expressed on Panc02 cells. Cells were stained with anti-CD8APC- Cy7 and murine survivin specific APC-conjugated pentamers ATFKNWPFL or CMV specific PE—conjugated pentamers HGIRNASFI as a control. C–D Fusion stimulated T cells demonstrate expansion of survivin specific T cells as compared to unstimulated T cells while CMV specific T cells are not selectively expanded (n = 3, p < 0.05)
Vaccination with DC/Panc02 fusions elicits tumor specific immunity in vivo
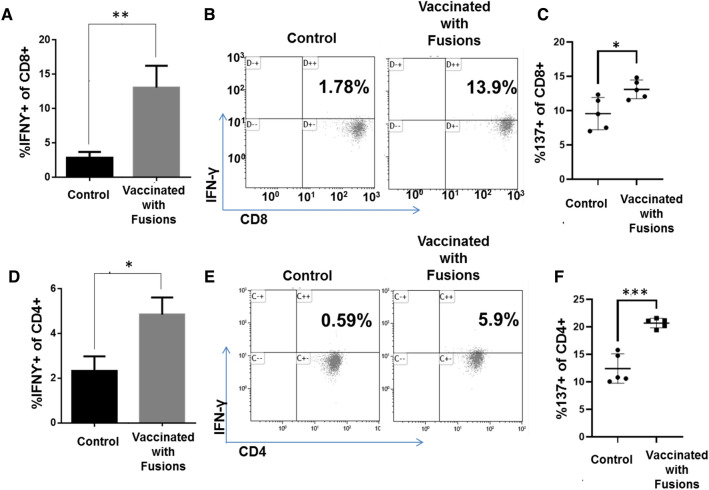
C57BL/6 J mice were inoculated with 5 × 105 luciferase/mCherry Panc02 cells subcutaneously and a cohort of ten animals subsequently underwent vaccination with 1 × 105 DC/Panc02 fusions 24 h after tumor challenge. Control animals (N = 10) underwent tumor challenge alone. Vaccination of fusion cells isolated by flow cytometric sorting did not result in the formation of subcutaneous tumors. At 14 days after vaccination, five animals in each cohort were sacrificed, splenic tissue was harvested, and the immune response of the splenocyte derived CD4 and CD8 T cell population was isolated assessed via multichannel flow cytometric analysis. Vaccination resulted in the significant expansion of tumor reactive CD8 + (Fig. 4A, B; p = 0.035) and CD4 + (Fig. 4D, E; p = 0.025) T cell populations as defined by the percent of cells expressing IFNγ following ex-vivo exposure to syngeneic tumor lysate. Furthermore, vaccination led to significant increase in antigen specific T cells, as detected by CD137 expression in both CD8 (Fig. 4C) and CD4 (Fig. 4F) T cells.
Fig. 4.
Vaccination with DC/Panc02 fusions results in increased T cells expressing IFNγ in response to syngeneic tumor lysate and T cells expressing CD137. C57BL/6 mice were inoculated subcutaneously with 5 × 105 luciferase/mCherry Panc02 cells and divided into cohorts assigned to vaccination with 105 DC/Panc02 fusion cells or observation 24 h after tumor challenge. Five animals in each cohort were sacrificed at day 14 following vaccination. Splenocytes were isolated by surgical resection and mechanical digestion, and then cultured with 1 µl/ml Panc02 tumor lysate for 3 days. The percent of CD4 and CD8 T cells within this population expressing IFN-γ in response to ex vivo exposure to Panc02 lysate was determined by multichannel flow cytometric analysis. Similarly, expression of CD137 by CD4 and CD8 cells was determined by multichannel flow cytometry. Vaccination resulted in a statistically significant increase (n = 5, p < 0.05) in the percentage of cells expressing intracellular IFN-ƴ and CD137 compared to unvaccinated animals by CD8 A–C and CD4 D–F as depicted as summary bar graphs and in representative flow cytometry blots
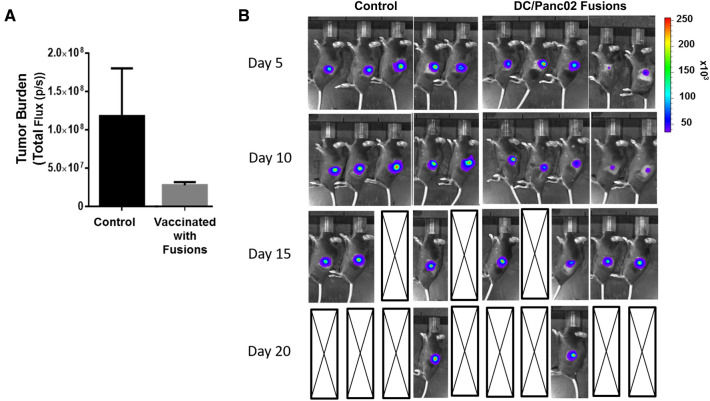
The impact of vaccination on disease bulk was assessed over time using serial bioluminescent imaging for five animals in the treatment and control cohorts, respectively. Vaccination resulted in a trend for reduced tumor burden in the vaccinated animals group compared to control group as defined by total flux recorded 10 days following tumor challenge (Fig. 5A; p = 0.12) Fig. 5B shows serial bioluminescence imaging of the tumors. Of note, after initial marked attenuation of tumor growth following vaccination, accelerated growth of Panc02 cells was observed and no significant survival advantage was demonstrated after vaccination in this highly aggressive pancreatic cancer model.
Fig. 5.
Tumor burden is decreased in mice DC/Panc02 fusions treated with DC/Panc02 fusions as imaged using BLI. C57BL/6 J mice were injected with 106 M-Cherry/luciferase transduced Panc02 cells subcutaneously. 24 h post inoculation cohorts of mice were assigned to vaccination with 105 DC/Panc02 fusions subcutaneously or control cohorts. Mice were followed by BLI imaging for tumor burden (n = 5 per cohort). A BLI imaging was analyzed to indicate total flux at day 10 post-vaccination demonstrating a trend supporting decreased tumor size in treated animals compared to control mice. B Serial BLI images over time showing decreased tumor size in treated mice compared to control mice with subsequent disease growth in both cohorts
DC/Panc02 vaccine treatment results in tumor infiltrating lymphocytes
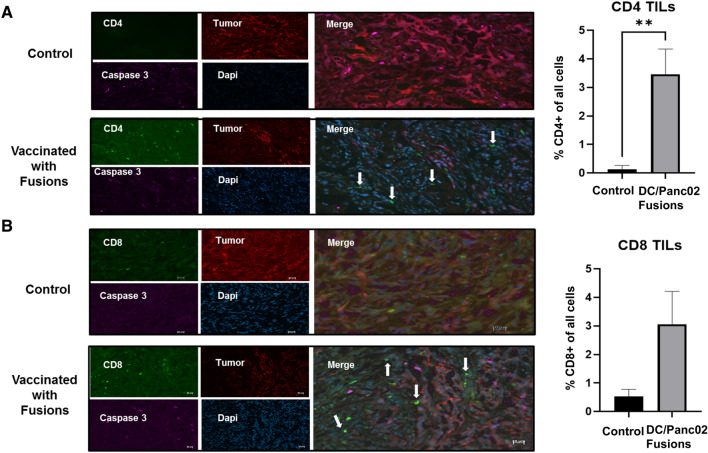
An important factor of immune escape in pancreatic cancer is the relative absence of tumor specific lymphocytes in the tumor bed. The Panco02 subcutaneous tumor model offers a unique platform to assess the impact of vaccination on the infiltration of T cells into the cancer microenvironment. In the present study, animals underwent tumor challenge, vaccination and subsequent resection and pathologic examination of the site of subcutaneous tumor inoculation 3–4 weeks later. The results were compared to that seen in the tumor bed of control animals. Of note, tissue harvested from vaccinated animals demonstrated the infiltration of CD4 + and CD8 + T cells and the near absence of mCherry tumor cells as determined by immunohistochemical staining and quantification of mean numbers of CD4 and CD8 tumor infiltrating lymphocytes and scored in five regions of the tumor bed. In contrast, control mice did not show evidence of CD4 + T cells and only minimal levels of CD8 lymphocyte infiltration within the population of M Cherry positive tumor cells (Fig. 6, B).
Fig. 6.
Cryosections indicating increased tumor infiltrating lymphocytes (TILs) in DC/Panc02 fusions treated mice compared to control mice. Subcutaneous tumors were removed from a vaccinated and control animal and tissue was fixed in 4% PFA. Cryosections were prepared and stained for CD4 and CD8 positive cells (green), caspase 3 (pink), and DAPI (blue). Tumor cells were identified by staining for M-cherry (red). Cryosection of tumor removed from control mouse indicating a large percentage of tumor cells and relative absence of tumor infiltrating CD4 A and CD8 B T cells. In contrast, vaccinated animals demonstrated relative paucity of tumor with presence of infiltrating CD4 A and CD8 B T cells. Bar graphs depict the percentage of CD4 TILs as quantified by the average value from enumeration of cells in five independent fields
Patient derived autologous DC/tumor fusions induce tumor specific immunity
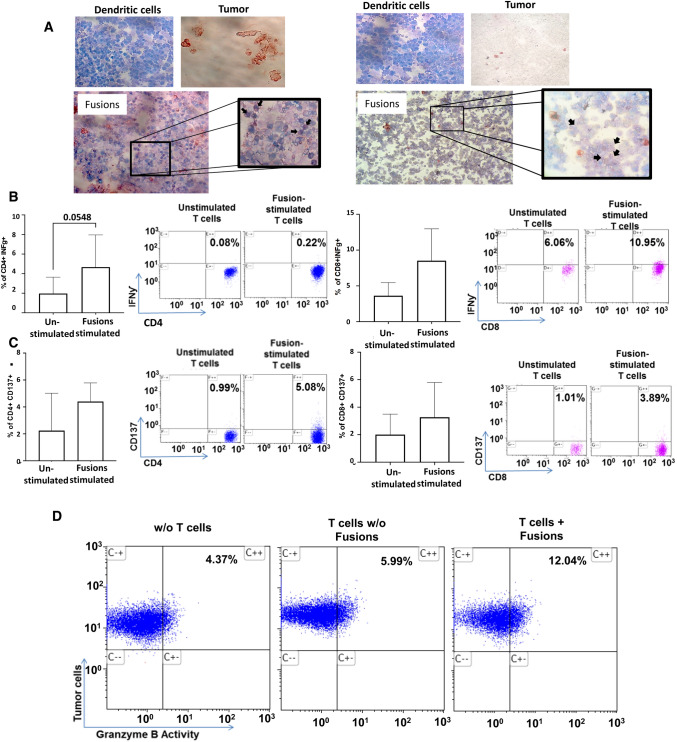
To demonstrate the feasibility of vaccine generation in the clinical setting, pancreatic tumor cells were obtained from four patients that underwent surgery as a part of standard of care therapy as provided by an IRB approved protocol. Tissue specimen average volume was 0.3cm3. Tumor tissue underwent digestion by incubation with 10 µg/ml proteinase K for 3 h at 37 °C and converted to a single cell suspension. Tumor cells were identified based on immunocytochemical staining for cytokeratin and EPCAM. Of four samples obtained, we demonstrated a median yield of 4 × 106 cells. Autologous DCs were generated from patient derived adherent mononuclear cells cultured for 1 week with GM-CSF and IL-4 and then matured in the presence of TNFα. DC and tumor cells were cocultured in the presence of PEG and DC/tumor cells were identified by coexpression of DC (CD86) and tumor (EPCAM, cytokeratin) markers (Fig. 7A).
Fig. 7.
Patient derived DC/tumor fusions induce the activation of tumor specific T cell immunity. Autologous DC/tumor fusions were generated from patient derived tumor cells obtained at time of surgical resection and DCs generated from peripheral blood mononuclear cells. A Pictured are DC and tumor cells demonstrating expression of CD86 (blue) and cytokeratin (red), respectively, and fusion cells from two patients showing dual expression of CD86/cytokeratin. B IFNƴ expression in CD4 + and CD8 + T cells cultured with fusions compared to unstimulated T cells as depicted in a representative flow cytometry blot and as bar graphs showing summary data from four patients. C CD137 expression in CD4 and CD8 + T cells as depicted in a representative flow cytometry blot and as bar graphs showing summary data from four patients. D CTL assay depicting increase cytotoxicity of primary pancreatic cancer cells following culture with fusion educated T cells as compared to unstimulated T cells
The capacity of the patient derived DC/tumor fusions to elicit tumor specific immunity was then interrogated. Consistent with immune activation, fusion cells elicited the expansion of CD8 + cells expressing IFNγ (Fig. 7B, C). Furthermore, DC/tumor cells fusions induced the expansion of antigen specific T cells as quantified by the increase in CD4 + and CD8 + cells expressing CD137 as compared to unstimulated patient derived T cells (Fig. 7D, E). Most significantly, DC/Tumor fusions potently induced tumor specific cytotoxicity as measured by tumor release of Granzyme B in a standard CTL assay. Coculture of fusion cells and autologous T cells resulted in an increase in tumor cell lysis as compared to that observed with uneducated autologous T cells (Fig. 7F).
Discussion
Pancreatic cancer remains a leading cause of cancer-related death in the USA (17). A majority of patients present with advanced disease, surgical options are limited, and efficacy of systemic cytotoxic therapy is modest. In addition, there is a growing appreciation of the role of the immunosuppressive tumor microenvironment in promoting disease proliferation and protection from immune surveillance (20).
While immune checkpoint therapy has been highly efficacious in tumors with high mutational burden and intrinsic T cell response to neoantigens, pancreatic cancer has been characterized as a “cold” malignancy given the lack of productive immunity generated with these agents. Early clinical studies investigating safety and antitumor activity of PD-1/PDL1 antagonists showed minimal activity (21). Similar findings have been reported from clinical trials testing a combination of PD-L1 and CTLA-4 antagonists (21–25) and with a trial combining PD1 blockade with a small molecule inhibitor of indoleamine 2, 3-deoxygenase (IDO) (26). Recent studies examining combination strategies of immune checkpoint blockade and chemotherapy did not demonstrate clinical activity beyond what would be expected with chemotherapy alone (27–29). One explanation is that the majority of tumors show T cell populations skewed toward regulatory CD4 + T cells with minimal amount of effector CD8 + T cells within the tumor site that are further suppressed by the regulatory cells (4). The lack of effector CD8 T cells infiltrating into the tumor site is thought to arise from the absence of priming and activation of tumor specific lymphocytes and steric hindrance to their penetration into the tumor bed due to the dense tumor supporting stroma.
In the present study, we postulated that a potent tumor vaccine capable of presentation of multiple tumor antigens including neoantigens would effectively induce tumor specific immunity directed against pancreatic cancer resulting in the infiltration of effector cells into the tumor bed and blunting of disease, converting pancreatic cancer from a “cold” to immune responsive malignancy. We interrogated this hypothesis in a spontaneous pancreatic cancer model that is thought to recapitulate many of the critical aspects of evolution of disease in humans. The KPC mice are engineered to have mutant Kras, and changes in CDKN2A, P53, and DPC4/SMAD4 that are mutant in close to 90% of patients (30–33). Vaccination with DC/KPC fusions resulted in the expansion of tumor reactive lymphocytes demonstrating antigen specificity and an activated phenotype including expansion of the memory compartment. Most significantly, vaccination resulted in attenuation of tumor growth and in improvement in survival in this model which is characterized by rapid disease progression and death.
Similarly, vaccination of animals with subcutaneous tumor generated by the syngeneic Panc02 cell line resulted in the induction of productive tumor specific immunity. Furthermore, this model allowed for the evaluation of the subcutaneous tumor bed which demonstrated increased infiltration of CD4 and CD8 T cells after vaccination. While substantial reduction in tumor bulk was observed in the Panc02 model after vaccination, no statistically significant survival advantage was observed in contrast to the spontaneous pancreatic cancer tumors developed in the KPC model. This difference in the effect of vaccination on survival is potentially attributed to the subcutaneous site of the PANC02 tumor which resulted in ulceration and requirement for euthanasia at lower tumor burden. In addition, the biologic behavior of the immortalized Panc02 tumor cell line differs compared to more physiological behavior of the pancreatic tumors developed in the KPC model which recapitulates the behavior of spontaneously arising disease in humans.
While vaccinated mice initially demonstrated significant reduction in tumor bulk, all mice demonstrated disease progression and required euthanasia. Tumor resistance to vaccine mediated tumor specific immunity is likely multifactorial. While expansion of pancreatic cancer specific T cells following vaccination was observed, loss of T cell functional competency may be associated with T cell exhaustion mediated by the tumor microenvironment. Mediators of immune suppression include upregulation of negative co-stimulatory signaling. Consistent with this hypothesis, we have recently demonstrated in an immunocompetent murine leukemia model that vaccination in conjunction with checkpoint inhibition efficiently enhanced the potency of the syngeneic DC/Tumor fusion vaccine resulting in long-term survival in all of the animals receiving combination therapy.
A significant challenge for designing effective immune therapy for pancreatic cancer is overcoming steric hindrance generated by the dense stromal layer surrounding the developing tumor bed. Of note, pancreatic tumors arising in the KPC model demonstrate a layer of desmoplastic stroma at the site of disease although not fully recapitulating levels seen in patients.31 Therapeutic efficacy of agents targeting the tumor stroma in combination with chemotherapy is being investigated including IPI-926 that inhibiting the Hh signaling pathway, calcipotriol targeting the tumor-associated fibroblasts, and PEGPH20 ablating hyaluronic acid (34–38). Targeting the tumor-stroma interaction as a component of immune-based therapy represents a promising therapeutic strategy against pancreatic cancer.
We have previously demonstrated efficacy of the DC/tumor fusion vaccine in hematological malignancies including acute myeloid leukemia and multiple myeloma (14, 15). Patients undergoing vaccination demonstrate the durable expansion of tumor reactive lymphocytes. In phase II study of patients with AML at high risk of relapse, 71% of patients remained in durable remission with a median follow up of nearly 5 years (16). In a study of myeloma patients following autologous transplant, vaccination with DC/MM fusions resulted in a near doubling of complete response between day 100 and 1 year post-transplant (14). These studies have served as the basis for larger randomized trials that are currently being conducted including a multicenter trial in multiple myeloma involving 18 centers under the auspices of the NHLBI/CTN cooperative oncology group. Vaccine production has been shown to be highly feasible.
Translation of personalized cancer vaccine for pancreatic cancer into the clinical setting presents several challenges. The isolation of pancreatic cancer from resected tumor tissue or biopsy specimens is complex given the heterogenous nature of the samples and the presence of dense stroma. In the present study, we demonstrated the successful generation of DC/tumor fusions from patient resected tumor samples and autologous DCs. DC/tumor fusions were characterized by co-expression of unique DC and tumor antigens and potently stimulated tumor specific CTL responses. The current results serve as a basis for a planned clinical trial for vaccination of patients with locally advanced pancreatic cancer undergoing standard therapy.
Methods
Generation of DC/KPC fusions
KPC tumors were harvested from animals developing spontaneous pancreatic tumors generated through the crossing of the LSL-KrasG12D (Jackson Laboratory, #008,179), Trp53fl (Jackson Laboratory, #008,462), and Pdx1-Cre (Jackson Laboratory, #014,647) to generate KrasLSL-G12D/ + ;Trp53fl/ + ;Pdx1-Cre (KPC). The DC/KPC fusions were generated as previously described (14–16). Tumors from KPC mice were minced with No.22 blades into 1–2 mm fragments then digested with 1 mg/ml collagenase/dispase (Roche) for 30–40 min. The digestion was stopped by adding equal volume of 1%BSA in DMEM, then centrifuged at 1500 rpm × 5 min. Pellets were further digested with Accutase (Sigma) for 30 min then collected by centrifugation at 1500 rpm × 5 min.
KPC cells were grown as organoids in an effort to preserve the pattern of antigen expression. KPC cells were resuspended in organoid growth medium containing Y-27632, 5% matrigel, and supplements: 0.5 µg/ml hydrocortisone, 10 µg/ml insulin, 100 ng/ml FGF2, 5 ng/ml EGF and 1% B27. The suspension was seeded onto 6-well plates pre-coated with matrigel. Culture media was replaced every 4 days. Organoids were passaged ever 12–14 days. At time of vaccine production, single cell suspension of tumor cells was generated by treatment with collagenase diluted in RPMI (1 mg/ml) for 1.5 h and digestion with trypsin for 10–20 min. Tumor cells were labeled with live cell red tracker to serve as a tumor marker.
Syngeneic DCs were generated from bone marrow mononuclear cells harvested from the femurs of KPC mice and cultured in media (RPMI, 10%FBS, 1% P/S) supplemented with IL-4 (500 IU/ml) and GM-CSF (1000 IU/ml) for 5–7 days. DCs were cocultured with KPC cells in the presence of PEG for 1–3 min which was then progressively diluted with RPMI. DC/Tumor fusion cells were identified as cells co-expressing of DC (anti-CD86-Alexa-647) and tumor (red cell tracker) markers and isolated via flow cytometric sorting. DC/Tumor fusion cells were identified as cells co-expressing of DC (anti-CD86-Alexa-647) and tumor (red cell tracker) markers and isolated via flow cytometric sorting on BD FACSAria II multicolor cell sorter at the BIDMC Flow cytometry core facility. Gating for the quadrants defining the fusion population was based on the integration of single staining for each marker and negative controls. To optimize the purity of the fusion cell population we sorted the cells that highly co-expressed unique DC and tumor markers.
Vaccination of KPC mice with DC/KPC fusions impact on tumor bulk and survival
KPC Mice were monitored by ultrasound using a Vevo 2100 Imaging System (VisualSonics) from 5 weeks of age and were enrolled on study once 40mm2 tumor were detectable by ultrasound. At time of meeting criteria for study enrollment, animals (n = 5) were assigned to undergo vaccination with 105 DC/KPC fusions injected subcutaneously or observation alone (n = 5). Tumor bulk was quantified serially by ultrasound performed every 5 days. Animals were monitored for survival with euthanasia performed based on defined criteria as per the institutionally approved protocol including: (a) ulceration of skin-based tumors; (b) interference with mobility and/or ability to acquire food or water; (c) debilitation, e.g., paralysis or general weakness; (d) labored respiration; (e) tumor burden exceeding 10% of normal body weight; or (f) weight loss exceeding 20% of baseline body weight.
Assessment of tumor specific immunity following vaccination with DC/KPC fusions
Splenocytes were isolated following euthanasia by surgical resection and mechanical digestion and cultured with 1 µl/ml KPC tumor lysate (prepared with 106 cells/1 ml) for 3 days. T cells were pulsed with GolgiStop (1 μg/ml; BD Pharmingen) for 4–6 h at 37 °C then labeled with CD4-BV and CD8-APC-Cy7. Permeabilization with Cytofix/Cytoperm (BD Pharmingen) containing formaldehyde and saponin was performed for 30 min at 4 °C. Cells were washed twice in Perm/Wash solution and incubated with PE-conjugated IFN-γ (Invitrogen, Camarillo, CA) or a matched isotype control for 30 min. Cells were washed in 1 × Perm/Wash solution prior to analysis.
CD8 T cells exhibiting. an activated phenotype via antigen recognition or effector memory profile were quantified by determining the percentage of CD8 cells that expressed CD137 and CD44 + /CD62L-, respectively, by multichannel flow cytometry. Cells were incubated with FcR blocking reagent (Miltenyi, Bergisch Gladbach, Germany) for 10 min at room temperature followed by anti-CD62L APC (BD Pharmingen), anti-44-PE (BD Pharmingen), anti-137- PE (BioLegend, San Diego, CA), anti-CD8-APC-cy7 (BioLegend, San Diego, CA) or appropriate isotype control. The data were obtained using FACS Gallius (BD Biosciences, San Jose, CA, USA). Kaluza software (Beckman Coulter, Brea, CA) was used to analyze the obtained data.
Assessment of cytotoxic T lymphocyte killing and expansion of tumor antigen specific T cells following in vitro stimulation of syngeneic T cells with DC/Panc02 fusions
DC/Panc02 fusions were generated as outlined above. Briefly, the murine pancreatic cancer cell line Panc02 was transduced with luciferase/mCherry using a lentiviral vector (pCDH-EF-eFFLy-T2A-mCherry) (kindly provided by Prof. Irmela Jeremias) to serve as a tumor marker. DCs were generated from bone marrow mononuclear cells harvested from the femurs of C57BL/6 J mice as described. DCs were cocultured with Panc02 cells in the presence of PEG. DC/Tumor fusion cells were identified as cells co-expressing of DC (anti-CD86-Alexa-647) and tumor (mCherry) markers and isolated via flow cytometric sorting as outlined above. Syngeneic T cells harvested from C57BL/6 J mice were cocultured with DC/Panc02 at a ratio of 10:1 for 3–5 days.
T cells cytolytic capacity was quantified using the cytotoxic T lymphocyte (CTL) assay, GranToxiLux (OncoImmunin, Inc), according to manufacturer’s instructions. Target Panc02 cells were incubated in APC-labeled phosphate-buffered saline (PBS) (1 µl of reconstituted TFL4 in PBS at 1:3000 ratio) at 106 cells/ml for 30 min at 37 °C. Labeled cells were washed twice in PBS. Fusion stimulated or control T cells were co-incubated with labeled target cells in the presence of a fluorogenic granzyme B substrate for 1 h at 37 °C. Prior to analysis cells were washed in washing buffer. Dead target cells were identified through dual staining for granzyme B and APC label. As a negative control, granzyme B positive tumor cells not co-incubated with T cells were quantified. The data were analyzed using FACS Gallius (BD Biosciences, San Jose, CA, USA). Kaluza software (Beckman Coulter, Brea, CA).
To assess expansion of antigen specific T cells, the fusion stimulated and control T cells underwent flow cytometric analysis using H-2 Db pentamer targeting the tumor antigen, survivin, expressed on Panc02 cells. Cells were stained with anti-CD8APC- Cy7 and murine survivin specific APC-conjugated pentamers ATFKNWPFL (ProImmune, Inc; Sarasota, FL, USA). CMV specific PE—conjugated pentamers HGIRNASFI (ProImmune, Inc; Sarasota, FL, USA) were used as control. Percent of pentamer positive CD8 T cells was assessed using multichannel flow cytometry.
Assessment of tumor specific immunity, tumor bulk and survival following in vivo vaccination with DC/Panc02 fusions
Wild type C57BL/6 mice (Jackson Laboratory) were inoculated subcutaneously with 5 × 105 luciferase/mCherry Panc02 cells. Cohorts of mice (n = 10 per cohort) were assigned to treatment with 105 DC/Panc02 fusion cells via subcutaneous injection or observation 24 h after tumor challenge. Five animals in each cohort were sacrificed at day 14 following vaccination. Splenocytes were isolated by surgical resection and mechanical digestion, and then cultured with 1 µl/ml Panc02 tumor lysate (prepared with 106 cells/1 ml) for 3 days. The percent of CD4 and CD8 T cells within this population expressing IFN-γ in response to ex vivo exposure to Panc02 lysate was determined by multichannel flow cytometric analysis as described above. Similarly, expression of CD137 by CD4 and CD8 cells was determined by multichannel flow cytometry. The remaining mice (n = 5 per cohort) were followed for disease development and tumor burden using serial bioluminescence imaging (BLI) and survival with indications for euthanasia outlined above.
Assessment of tumor infiltrating lymphocytes in the following vaccination with DC/Panc02 fusions
Infiltration of CD4 and CD8 T cells into the subcutaneous Panc02 tumor bed was assessed after vaccination and in the control animal population. Tumor tissue was resected from euthanized animals and fixed in 4% PFA for 24 h and sucrose solution for 24 h to maintain tumor florescence. Tumor tissue was embedded in OCT before freezing and cut in sections to enable staining. Staining for CD4 + and CD8 + cells penetrating the tumor bed was done for treated and control samples. Cryosections were prepared in which staining for CD4 or CD8 positive cells (green), caspase 3 (pink), and DAPI (blue). Tumor cells were transduced to express M-cherry and stained accordingly (red). T cell infiltration was quantified and scored in five independent fields and compared between the vaccinated and control animals.
Generation of DC/pancreatic fusions from patient derived samples
Patient tumor samples were obtained from four patients undergoing primary tumor resection as part of standard or care therapy according to an approved IRB protocol. The average volume of the tumor samples was 0.3cm2. The sample was mechanically digested using a scalpel and was incubated at 37 °C in PBS supplemented with 10 µg/ml proteinase K (Sigma). After 3 h the incubated samples were pipetted with a 10 ml pipette for at least five times. Cells were collected and washed in complete media (RPMI, 10%FBS, 1%P/S). Cells viability was quantified using Trypan Blue. Tumor cells were identified by immunocytochemical staining for cytokeratin and EPCAM.
Autologous DCs were generated from patient derived peripheral blood adherent mononuclear cells cultured for 1 week in complete media supplemented with GM-CSF (1000 IU/ml) and IL-4 (500 IU/ml) followed by 2 days of culture in complete media supplemented with GM-CSF (1000 IU/ml), IL-4 (500 IU/ml), and TNFα (2750 IU/ml) to achieve DCs maturation. Mature DCs and autologous tumor cells were fused in the presence of PEG as described earlier. Percent of fusion was assessed by identifying dual labelling of DCs (CD86) and tumor (EPCAM, cytokeratin) markers using immunocytochemical staining.
Primary DC/pancreatic cancer fusions were cocultured with autologous T cells at a 1:10 ratio for 3–5 days in complete media supplemented with IL-2 (10UI/ml) and functional assessments were performed. T cells activation was quantified by measuring intracellular IFN-γ expression by CD4 and CD8 T cells by intracellular flow cytometric analysis as outlined above. Similarly, antigen specific responses were interrogated by expansion of CD4 and CD8 T cells expressing CD137. Finally, CTL response targeting primary autologous pancreatic cancer cells was determined using the standard Granzyme release assay in which patient derived tumor cells were incubated in APC-labeled phosphate-buffered saline (PBS) and then cocultured with the vaccine stimulated or control T cells as outlined above.
Statistics
All statistical analysis between two groups was preformed using two tailed T test when p < 0.05 was considered significant. For all bar graphs data represents mean ± SEM. Survival statistics was performed using the Wilcoxon test (p < 0.05 was considered significant. All statistical analysis was performed using Prisma software.
Author contribution
S.O contributed by writing the manuscript, designing and conducting experiments, and acquiring and analyzing data. L.H. and O.G. provided cells and helped writing the manuscript. J.M. and C.D. provided patient samples. D.S. helped in designing experiments, analyzing data, and writing of the manuscript. A.E. helped in conducting experiments. J.R. helped in designing experiments and analyzing the data. D.A. contributed to designing experiments, analyzing data and writing of the manuscript. J.L. and G.C. helped in experiment designing and data analysis while D.T. and C.T. helped in conducting experiments. DK, MH, and SM participated in experimental design and review of the manuscript.
Declarations
Competing interests
Funded in part by V Foundation.
Study approval
All animal studies were approved by BIDMC IACUC.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Banerjee K, et al. Emerging trends in the immunotherapy of pancreatic cancer. Cancer Lett. 2018;417:35–46. doi: 10.1016/j.canlet.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGuigan A, et al. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment, and outcomes. World J Gastroenterol. 2018;24:4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Bosch N, Vinaixa J, Navarro P. Immune evasion in pancreatic cancer: from mechanisms to therapy. Cancers (Basel) 2018;10:1–16. doi: 10.3390/cancers10010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balachandran VP, Beatty GL, Dougan SK, Sloan M, Cancer K. Broadening the impact of immunotherapy to pancreatic cancer: Challenges and opportunities. Gastroenterology. 2020;156:2056–2072. doi: 10.1053/j.gastro.2018.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin IH, Askan G, Hu ZI, Reilly EMO. Immunotherapy in pancreatic ductal adenocarcinoma : An emerging entity ? Ann Oncol. 2017 doi: 10.1093/annonc/mdx503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng M, et al. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57–65. doi: 10.1016/j.canlet.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;80(359):1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 8.Luo W, et al. Novel therapeutic strategies and perspectives for metastatic pancreatic cancer: vaccine therapy is more than just a theory. Cancer Cell Int. 2020;20:1–10. doi: 10.1186/s12935-020-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou-Alfa GK, et al. Targeting mutated K-ras in pancreatic adenocarcinoma using an adjuvant vaccine. Am J Clin Oncol. 2011;34(3):321–325. doi: 10.1097/COC.0b013e3181e84b1f. [DOI] [PubMed] [Google Scholar]
- 10.Wedén S, et al. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer. 2011;128(5):1120–1128. doi: 10.1002/ijc.25449. [DOI] [PubMed] [Google Scholar]
- 11.Torres MP, Chakraborty S, Batra SK. Mucin-based targeted pancreatic cancer therapy. Curr Pharm Des. 2012;18(17):2472–2481. doi: 10.2174/13816128112092472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishida S, et al. Combination gemcitabine and WT1 peptide vaccination improves progression-free survival in advanced pancreatic ductal adenocarcinoma: a phase II randomized studywt1 vaccine Plus GEM in pancreatic cancer. Cancer Immunol Res. 2018;6(3):320–331. doi: 10.1158/2326-6066.CIR-17-0386. [DOI] [PubMed] [Google Scholar]
- 13.Koido S, Okamoto M, Shimodaira S, Sugiyama H. Wilms’ tumor 1 (WT1)-targeted cancer vaccines to extend survival for patients with pancreatic cancer. Immunotherapy. 2016;8(11):1309–1320. doi: 10.2217/imt-2016-0031. [DOI] [PubMed] [Google Scholar]
- 14.Rosenblatt J, et al. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood. 2011;117:393–402. doi: 10.1182/blood-2010-04-277137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenblatt J, et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res. 2013;19:3640–3648. doi: 10.1158/1078-0432.CCR-13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenblatt J, et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci Transl Med. 2016;8(368):368–368. doi: 10.1126/scitranslmed.aag1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2004;5(1):103. doi: 10.1016/S1535-6108(03)00335-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhu K, et al. Survivin DNA vaccine generated specific antitumor effects in pancreatic carcinoma and lymphoma mouse models. Vaccine. 2007;25:7955–7961. doi: 10.1016/j.vaccine.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 19.Ishizaki H, et al. Modified vaccinia Ankara expressing survivin combined with gemcitabine generates specific antitumor effects in a murine pancreatic carcinoma model. Cancer Immunol Immunother. 2011;60:99–109. doi: 10.1007/s00262-010-0923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansari D, et al. Pancreatic cancer: Yesterday, Today and Tomorrow. Futur Oncol. 2016;12:1929–1946. doi: 10.2217/fon-2016-0010. [DOI] [PubMed] [Google Scholar]
- 21.Brahmer JR, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. New Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly F, Victoria K, Elizabeth J, Lei Z. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016;381:244–251. doi: 10.1016/j.canlet.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patnaik A, et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res. 2015;21:4286–4293. doi: 10.1158/1078-0432.CCR-14-2607. [DOI] [PubMed] [Google Scholar]
- 25.Royal RE, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naing A, et al. Epacadostat plus durvalumab in patients with advanced solid tumors preliminary results of the ongoing open-label phase I/II ECHO-203 study. Cancer Res. 2018;78:13. doi: 10.1158/1538-7445.AM2018-CT177. [DOI] [Google Scholar]
- 27.Aglietta M, et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25(9):1750–1755. doi: 10.1093/annonc/mdu205. [DOI] [PubMed] [Google Scholar]
- 28.Von Hoff DD, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conroy T, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 30.Gabriel ANA, et al. Differences between KC and KPC pancreatic ductal adenocarcinoma mice models, in terms of their modeling biology and their clinical relevance. Pancreatology. 2020;20(1):79–88. doi: 10.1016/j.pan.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto D, et al. Heterogeneity of KRAS mutations in pancreatic ductal adenocarcinoma. Pancreas. 2016;45(8):1111–1114. doi: 10.1097/MPA.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 32.Waddell N, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hingorani SR, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 34.Provenzano PP, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ijichi H, et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121:4106–4117. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massó-Vallés D, et al. Ibrutinib exerts potent antifibrotic and antitumor activities in mouse models of pancreatic adenocarcinoma. Cancer Res. 2015;75:1675–1681. doi: 10.1158/0008-5472.CAN-14-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Tuveson DA. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherman MH, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159(1):80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]