Abstract
CD8+CD103+ tissue-resident memory T cells (TRMs) are involved in tumor immune response and linked to favorable clinical outcome in human cancer. However, the distribution, phenotype, functional properties and clinical relevance of these cells in gastric cancer (GC) remain elusive. Here, our data show that, in comparison to non-tumor tissues, the percentages of CD8+CD103+ TRMs in tumors are significantly decreased. Most tumor-infiltrating CD8+CD103+ TRMs are CD45RA−CCR7− effector-memory cells with higher PD-1 and 4-1BB expression than those from non-tumor tissues. Further, tumor-infiltrating CD8+CD103+ TRMs show impaired cytolytic capacity due to decreased granzyme B and perforin expression. Moreover, ex vivo PD-1 blockade could restore the cytolytic capacity of tumor-infiltrating CD8+CD103+ TRMs, and such anti-PD-1-mediated reinvigoration of CD8+CD103+ TRMs could be further enhanced by 4-1BB co-stimulation. Finally, lower levels of Tumor-infiltrating CD8+CD103+ TRMs are positively correlated with GC progression and poor patients’ survival. Our data suggest that restoring CD8+CD103+ TRM function by combining PD-1 blockade and 4-1BB co-stimulation may be a promising strategy for treating GC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03105-0.
Keywords: Gastric cancer, CD8+CD103+ tissue-resident memory T cells, PD-1, 4-1BB
Introduction
Gastric cancer (GC) is the fifth most frequent malignancy and the fourth leading cause of cancer death worldwide [1]. Despite significant improvement in GC prevention and treatment, controlling advanced-stage disease remains challenging [2]. It is well established that tumor progression is influenced by the cross-talk between cancer cells and the host immune system and that, tumor-infiltrating CD8+ T cells are associated with improved clinical outcomes of GC patients [3–5]. Therefore, understanding the functional properties of these CD8+ T cells could contribute to developing more effective therapeutic strategies against GC.
Tumor-infiltrating CD8+ T cells are heterogeneous, comprising multi-subpopulations with special phenotypes and functions [6]. Among them, CD103 expressing CD8+ T cells are Tissue-resident memory T cells (TRMs) with stronger anti-tumor immune activity [7, 8]. Recent studies have linked increased CD8+CD103+ TRMs in tumors to improved patients’ survival and this is often a more accurate indicator for better prognosis than total CD8+ T cell number [9–11]. In GC, tumor-infiltrating CD8+CD103+ TRMs are also associated with favorable clinical outcome and a superior anti-tumor activity compared with their CD8+CD103− counterparts [12]. However, the distribution, characteristics, and functional properties of CD8+CD103+ TRMs within tumor and tumor-adjacent normal (non-tumor) tissues in GC remain unknown. Additionally, CD8+CD103+ TRMs in normal tissues have been also reported to produce higher levels of cytokines, such as IFN-γ than their CD8+CD103− counterparts [13], which further emphasizes a urgent need to investigate CD8+CD103+ TRMs in both tumor and non-tumor tissues to define their functional status in GC microenvironment.
Here, we show that the percentages of CD8+CD103+ TRMs in tumors are significantly lower than those in non-tumor tissues, and we also observe altered expression of molecules associated with T cell inhibition and activation such as PD-1 and 4-1BB. Furthermore, tumor-infiltrating CD8+CD103+ TRMs exhibit an impaired cytolytic capacity, which could be restored by anti-PD-1 antibodies, and anti-4-1BB agonists could further enhance anti-PD-1-mediated CD8+CD103+ TRM reinvigoration. These findings suggest that restoring CD8+CD103+ TRM function by combining PD-1 blockade and 4-1BB co-stimulation may have clinical implications for a tissue-localized immunotherapy against GC.
Materials and methods
Patients and specimens
Peripheral blood and tissue samples were obtained from 155 patients with pathologically confirmed GC. None of the patients had received anti-tumor therapy before sampling, and patients with autoimmune diseases, infectious diseases, or multi-primary cancers were excluded. Fresh peripheral blood, non-tumor (at least 5-cm distant from the tumor site) and tumor tissues from 50 patients (group 1) were obtained for preparing single cell suspensions during surgery between July 2019 and April 2021 at the First and second Affiliated Hospital of the Third Military Medical University. One hundred and five patients (group 2) who received curative resection between May 2016 and August 2017 at the Affiliated Hospital of the North Sichuan Medical College with follow-up data were enrolled for survival analysis. The clinical stages of tumors were determined according to the TNM classification system of International Union against Cancer (Edition 8). The Ethics Committee of the Third Military Medical University and North Sichuan Medical College approved the study, and written informed consent was obtained from all subjects. The clinical characteristics of GC patients were present in Supplemental Table 1.
Cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation. Non-tumor and tumor tissues were digested for obtaining single cell suspensions as previously described [14]. Briefly, tissues were cut into small pieces and collected in RPMI 1640 medium containing 1 mg/ml collagenase IV (Gibco, Carlsbad, CA) and 10 mg/ml DNase I (Invitrogen, Carlsbad, CA), then mechanically dissociated by using a gentle MACS Dissociator (Miltenyi Biotec, Auburn, CA). Dissociated cell suspensions were further incubated 1 h at 37 °C under continuous rotation and filtered through 40 μm cell strainers to obtain single cell suspensions. The cell suspensions were then used for flow cytometry analysis.
Flow cytometric analysis
Cell suspensions were stained with Fixable Viability Stain 700 (BD Biosciences) to exclude dead cells; cells were then washed and appropriate surface antibodies were added. For intracellularly staining granzyme A, granzyme B, granzyme K, perforin and granulysin, cells were washed, fixed, then permeabilized for 20 min using Cytofix/Cytoperm reagent (BD Biosciences) before being stained. For intracellular staining of ki-67, the Foxp3-Staining Buffer Set (Invitrogen) was used according to the manufacturer’s protocol. For intracellular cytokine staining of IFN-γ and TNF-α, cells were stimulated for 4 h with Leukocyte Activation Cocktail (BD Biosciences) before staining. The fluorochrome-labeled antibodies were listed in Supplemental Table 2.
Immunohistochemistry
Paraffin-embedded samples were cut into 5-μm sections. After being deparaffinized and hydrated, the sections in citrate buffer (pH 6.0) were subjected to heat-induced antigen retrieval in a microwave oven, and then treated with 3% hydrogen peroxide. The sections were washed and incubated with normal horse serum and tipped off excess serum from sections and incubated with rabbit anti-human CD103 antibody and mouse anti-human CD8 antibody (Abcam) overnight at 4 °C. Sections were stained and visualized by using ImmPRESS® Duet Double Staining Polymer Kit (Vector Laboratories) according to the manufacturer’s protocol. CD103 and CD8 were separately stained brown and red, and CD8+CD103+ cells were dark red (Fig. 1c). Three most representative high-power fields (× 400 magnifications) were captured for each tumor section by using a microscope (Nikon, Tokyo, Japan), and the images were reviewed by two experienced histopathologists to calculate the numbers of CD8+CD103+ cells in all specimens. Finally, the numbers of CD8+CD103+ cells from the three fields in each tumor sample were averaged.
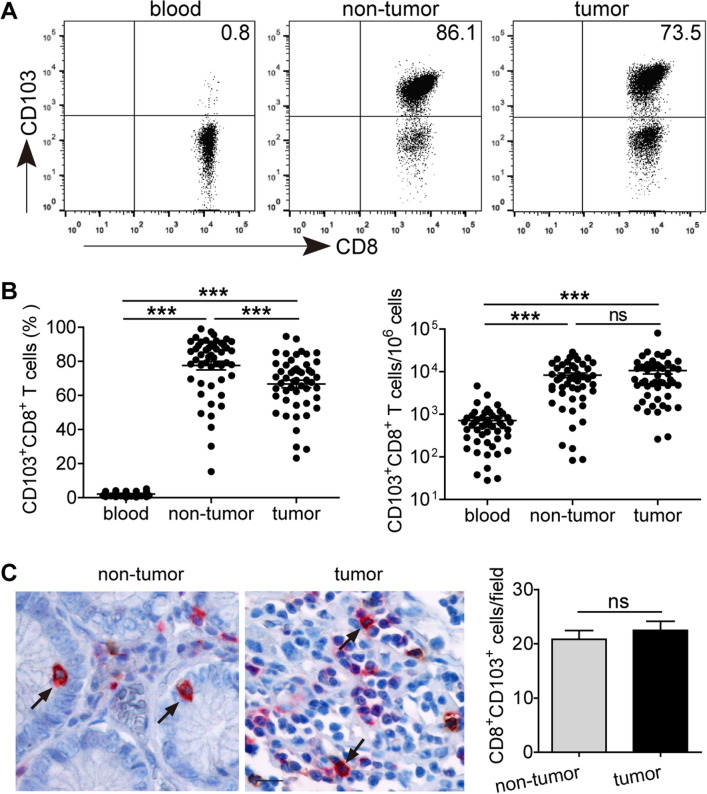
Fig. 1.
The prevalence of CD8+CD103+ TRMs in the peripheral blood, non-tumor and tumor tissues of GC patients. (a) Peripheral blood, non-tumor and tumor tissue-derived cell suspensions were stained with Fixable Viability Stain 700, anti-CD45, CD3, CD8 and CD103 antibodies, then CD8+CD103+ TRM percentages were analyzed after gating on CD3+CD8+ T lymphocytes. (b) The percentages of CD8+CD103+ TRMs and their absolute numbers per million cells in the peripheral blood, non-tumor and tumor tissues of 50 GC patients. (c) A representative immunohistochemical staining of CD8+ cells (red) and CD103+ cells (brown) in non-tumor and tumor tissue of the same section of one GC patient (Scale bars = 50 μM). CD8+CD103+ cells are in dark brown color (pointed by black arrows) and their numbers were analyzed from 105 GC patients. ***P < 0.001; ns, not significant
Ex vivo functional restoration assay
Cell suspensions obtained from tumor digests were cultured in RPMI 1640 containing 10% fetal calf serum (Gibco) with anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) antibodies (Biolegend). An anti-PD-1 blocking antibody (pembrolizumab, 5 μg/ml, MedChem Express) and/or an anti-4-1BB agonistic antibody (10 μg/ml, R&D systems) were also added in the culture system. After 24 h, cells were collected for flow cytometry analysis.
CD8+CD103+ TRM co-culture with GC cell line and apoptosis assays
The co-culture system as previously described [15] was used to analyze the killing capacity of CD8+ T cell subset. Briefly, tumor-infiltrating CD8+CD103+ TRMs were sorted using a FACSAria III (BD Biosciences), and the purity was more than 95%. Such CD8+CD103+ TRMs were added into U-bottom 96-well plate with pre-coated anti-CD3 antibody (10 μg/ml) and soluble anti-CD28 antibody (1 μg/ml, Biolegend). Before co-culture, GC cell line SGC-7901 was labeled with 10 μM CFSE (Invitrogen) according to the manufacturer’s instruction. Next, CFSE-labeled SGC-7901 was co-cultured with the CD8+CD103+ TRMs at a 1:1 ratio, and an anti-PD-1 blocking antibody (5 μg/ml) and/or an anti-4-1BB agonistic antibody (10 μg/ml) were also added in the co-culture system. After 18 h, CFSE labeled cells were analyzed for their survival and apoptosis by Annexin V-PE and 7-AAD (BD Biosciences) according to the manufacturer’s instructions.
Statistical analysis
All results were summarized as mean ± standard error of the mean (SEM), and statistical analysis was performed with the Prism 7.0 Software. Differences between groups were evaluated by two-tailed Student’s t test. When variance was detected, Mann–Whitney U test was used to analyze the difference between groups. The correlation analysis between groups was performed using the Spearman’s correlation test. Cumulative survival time was measured in months and calculated by the Kaplan–Meier method, and the log-rank test was applied to compare between groups. Multivariate analysis of prognostic factors for patient survival was performed using the Cox proportional hazards model. P < 0.05 was considered as statistically significant.
Results
Enumerating GC-infiltrating CD8+CD103+ TRMs
Using flow cytometry, we first analyzed the proportion of CD8+CD103+ TRMs in the peripheral blood, non-tumor and tumor tissues of 50 GC patients (Fig. 1a, b). Compared with peripheral blood, the percentages of tissue-infiltrating CD8+CD103+ TRMs were significantly increased, and similar observations were made when analyzing the absolute number of CD8+CD103+ TRMs per million cells in each sample. However, within patients’ tissues, the percentages of CD8+CD103+ TRMs in tumor tissues were significantly lower than those in non-tumor tissues, whereas the absolute number of CD8+CD103+ TRMs per million cells was equivalent between non-tumor and tumor tissues. Immunohistochmical staining further showed that similar numbers of infiltrating CD8+CD103+ TRMs were observed in tumor and non-tumor tissues (Fig. 1c).
Immunophenotype of GC-infiltrating CD8+CD103+ TRMs
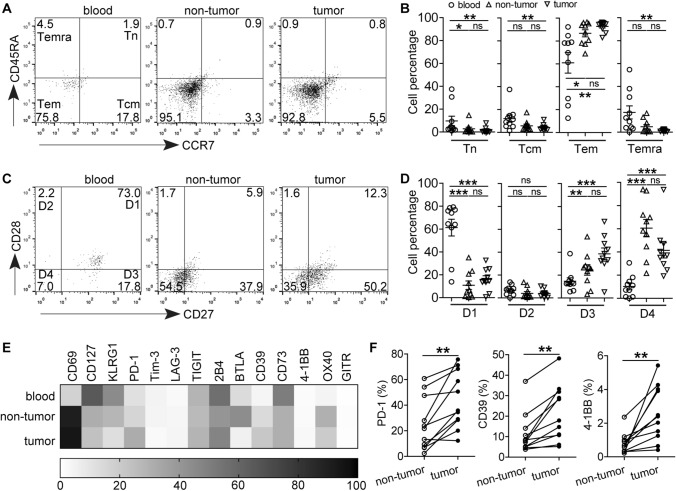
Next, we investigated the phenotypic feature of CD8+CD103+ TRMs at tumor site. TRM cells were distinguished into four subpopulations based on CD45RA and CCR7 expression: naive (Tn, CD45RA+CCR7+), central memory (Tcm, CD45RA−CCR7+), effector memory (Tem, CD45RA−CCR7−), and terminally differentiated effector memory (Temra, CD45RA+CCR7−). CD8+CD103+ TRMs in the peripheral blood were mainly composed of Tcm and Tem subsets; however, most of the tissue-infiltrating CD8+CD103+ TRMs belonged to Tem (95% and 92%) subset, and CD8+CD103+ TRMs in non-tumor and tumor tissues contained similar proportions of Tn, Tcm, Tem and Temra subsets (Fig. 2a, b). CD27 and CD28 co-expression was used to analyze the differentiation status of TRM cells: early differentiated (D1, CD28+CD27+), early-like differentiated (D2, CD28+CD27−), intermediate differentiated (D3, CD28−CD27+), and late differentiated (D4, CD28−CD27−). Again, no significant differences in the percentages of D1, D2, D3 and D4 subpopulations between CD8+CD103+ TRMs isolated from non-tumor and tumor tissues. However, there were more D3 and D4 subpopulations and fewer D1 subpopulation in tissues than those in the peripheral blood (Fig. 2c, d), suggesting that the majority of GC-infiltrating CD8+CD103+ TRMs are effector memory cells with intermediate or late differentiated status.
Fig. 2.
Phenotypic features of GC-infiltrating CD8+CD103+ TRMs. a Peripheral blood, non-tumor and tumor tissue-derived cell suspensions were stained with Fixable Viability Stain 700, anti-CD45, CD3, CD8, CD103, CD45RA and CCR7 antibodies, A representative flow cytometry analysis of GC patients shows the percentages of different subsets of CD8+CD103+ TRMs indicated by CD45RA and CCR7 expression: Tn (CD45RA+CCR7+), Tcm (CD45RA−CCR7+), Tem (CD45RA−CCR7−), and Temra (CD45RA+CCR7−). b Statistical analysis of the percentages of different subsets of CD8+CD103+ TRMs in 10 GC patients. c A representative flow cytometry analysis for the expression of CD28 and CD27 on CD8+CD103+ TRMs: early differentiated (CD28+CD27+), early-like differentiated (CD28+CD27−), intermediate differentiated (CD28−CD27+), and late differentiated (CD28−CD27−). d The differentiation status of CD8+CD103+ TRMs from 10 GC patients. e Converted flow cytometry phenotyping results of CD8+CD103+ TRMs isolated from paired blood, non-tumor, and tumor tissues; data represent mean results from at least four GC patients (n = 4–10). f PD-1, CD39 and 4-1BB expression on CD8+CD103+ TRMs from tumor or non-tumor tissues of GC patients (n = 11). *P < 0.05, **P < 0.01; ***P < 0.001; ns, not significant
We further characterized the expression of other surface molecules on these CD8+CD103+ TRMs. Compared with peripheral blood, an increased level of CD69 expression but decreased CD127, KLRG1 and CD73 expression on tissue-infiltrating CD8+CD103+ TRMs were observed, and there was no significant difference for the expression of Tim-3, LAG-3, TIGIT, 2B4 and GITR (Fig. 2e). Additionally, the expression of co-inhibitory molecule PD-1 and ecto-enzyme CD39 as well as costimulatory molecule 4-1BB on tumor-infiltrating CD8+CD103+ TRMs was significantly higher than those on their counterparts of non-tumor tissues (Fig. 2f), suggesting that GC microenvironment may endow CD8+CD103+ TRMs with distinct immuno-phenotypes from non-tumor tissues.
GC-infiltrating CD8+CD103+ TRMs have an impaired cytolytic capacity
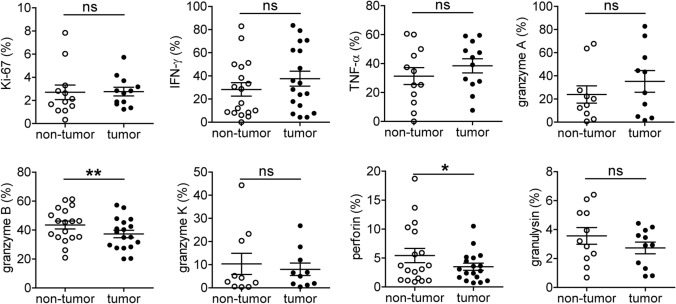
Based on the immunophenotype of CD8+CD103+ TRMs from tumor and non-tumor tissues, we investigated their proliferation and function (Fig. 3). We did not observe significant difference for the expression of proliferation-associated marker Ki-67 between CD8+CD103+ TRMs from non-tumor and tumor tissues; the percentages of IFN-γ and TNF-α-producing CD8+CD103+ TRMs from both tissues were also similar, suggesting the proliferation and cytokine production of these CD8+CD103+ TRMs were not altered. We further analyzed the levels of intracellular cytolytic molecules and detected similar levels of granzyme A, granzyme K and granulysin expression in CD8+CD103+ TRMs isolated from non-tumor and tumor tissues. Importantly, granzyme B and perforin were significantly decreased in tumor-infiltrating CD8+CD103+ TRMs compared to those from non-tumor tissues, suggesting a selective impairment of cytolytic capacity in GC-infiltrating CD8+CD103+ TRMs.
Fig. 3.
GC-infiltrating CD8+CD103+ TRMs show impaired function. Non-tumor and tumor tissue-derived cell suspensions were stained with Fixable Viability Stain 700, anti-CD45, CD3, CD8 and CD103 antibodies, then intracellular staining for Ki-67, IFN-γ, TNF-α, granzyme A, granzyme B, granzyme K, perforin and granulysin. The percentages of Ki-67+, IFN-γ+, TNF-α+, granzyme A+, granzyme B+, granzyme K+, perforin+ and granulysin+ cells among the CD8+CD103+ TRMs were analyzed. Symbols represent individual values from 10 to 18 GC patients analyzed. *P < 0.05; **P < 0.01; ns, not significant
The function of GC-infiltrating CD8+CD103+ TRMs could be restored by PD-1 blockade and enhanced by agonistic anti-4-1BB
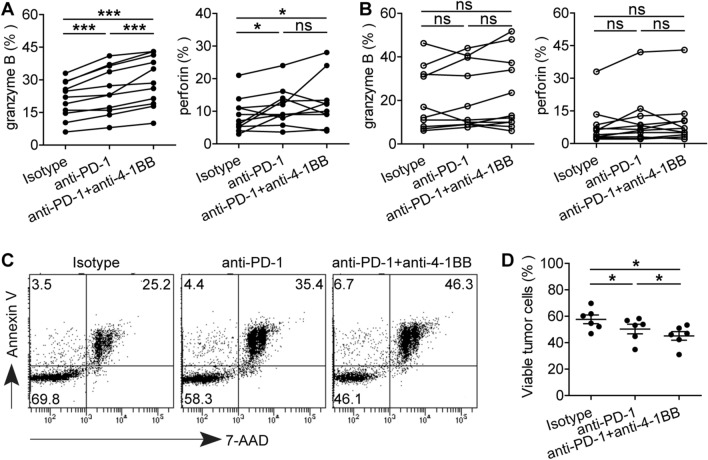
As GC-infiltrating CD8+CD103+ TRMs showed increased PD-1 and 4-1BB expression with impaired cytolytic capacity, we wondered whether their function might be restored by blocking PD-1. Indeed, PD-1 blockade increased tumor-derived CD8+CD103+ TRMs to express granzyme B, which was further enhanced by anti-4-1BB agonistic antibody (Fig. 4a). However, there was no increase in granzyme B and perforin expression in tumor-derived CD8+CD103− T cells following PD-1 blockade alone or combined with 4-1BB co-stimulation (Fig. 4b). In order to further investigate the killing ability of tumor-derived CD8+CD103+ TRMs by blocking PD-1 with or without 4-1BB co-stimulation, CD8+CD103+ TRMs were sorted and co-cultured with CFSE-labeled GC cell line SGC-7901. We demonstrated that significantly more SGC-7901 cells underwent apoptosis in the PD-1 blockade group, and even more so in the PD-1 blockade combined with 4-1BB co-stimulation group (Fig. 4c, d). These data not only confirm the role of PD-1 blockade on these TRMs but also suggest that 4-1BB co-stimulation may provide further beneficial effects in immunotherapies targeting PD-1.
Fig. 4.
4-1BB co-stimulation enhances anti-PD-1-mediated CD8+CD103+ TRM reinvigoration. The effect of PD-1 blockade with or without combining 4-1BB co-stimulation on cytolytic marker granzyme B and perforin expression in CD8+CD103+ TRMs (a) and CD8+CD103− T cells (b) from tumor tissues of GC patients (n = 11). (c) A representative apoptosis profile of GC cell line SGC-7901 following co-culture with purified GC-infiltrating CD8+CD103+ TRMs in vitro. (d) The viable GC cell line SGC-7901 in all co-cultures are shown for different groups (n = 6). *P < 0.05; ns, not significant
GC-infiltrating CD8+CD103+ TRMs correlated with disease progression
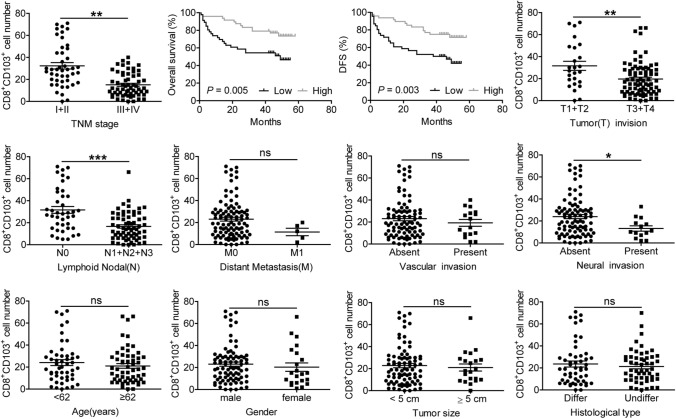
We next studied the relation of GC-infiltrating CD8+CD103+ TRM numbers in 105 GC patients with regards to disease progression (Fig. 5). We found that GC-infiltrating CD8+CD103+ TRM numbers were significantly lower in advanced disease (TNM stage III + IV) than those in early disease (TNM stage I + II), and lower CD8+CD103+ TRM numbers were positively correlated with poor overall survival (OS) and disease-free survival (DFS) of GC patients when using the medium value of all tumor-infiltrating CD8+CD103+ TRM numbers as a comparison point. We also found that tumor-infiltrating CD8+CD103+ TRM numbers could independently predict patient survival, which was verified by multivariate analyses using a Cox proportional hazard model (Supplementary Table 3). Moreover, tumor-infiltrating CD8+CD103+ TRM numbers were negatively correlated with tumor invasion, lymph node metastasis and neural invasion status. However, no correlation was observed between tumor-infiltrating CD8+CD103+ TRM numbers and distant metastasis, vascular invasion status, age, gender, tumor size as well as histological types. These results suggest that decreased tumor-infiltrating CD8+CD103+ TRMs are associated with GC progression and poor patients’ survival.
Fig. 5.
The number of tumor-infiltrating CD8+CD103+ TRMs correlates with multiple clinical parameters of GC patients. The number of tumor-infiltrating CD8+CD103+ TRMs was analyzed for putative correlations with multiple clinical parameters, such as tumor stage, patient overall survival time. For the cumulative survival curves, patients were separated into two groups by the median value of tumor-infiltrating CD8+CD103+ TRM numbers, and Kaplan–Meier plots were used to show cumulative survival differences. Each dot represents one patient. Ab, antibody; Diff, differentiated; Undiff, undifferentiated; ns, not significant. *P < 0.05; **P < 0.01; ***P < 0.001
Discussion
In this study, we systematically investigated the distribution, phenotype and functional profile of CD8+CD103+ TRMs from tumor and non-tumor tissues of GC patients. We observed, in comparison to non-tumor tissues, the percentages of GC-infiltrating CD8+CD103+ TRMs were significantly decreased accompanied with increased PD-1 expression and impaired cytolytic function. We further demonstrated that blocking PD-1 could restore the cytolytic capacity of GC-infiltrating CD8+CD103+ TRMs and that 4-1BB co-stimulation with its agonistic antibody further enhanced anti-PD-1-mediated CD8+CD103+ TRM function. Finally, we show that the number of GC-infiltrating CD8+CD103+ TRMs had strong correlation with tumor progression and patients’ survival. These findings imply that strategies boosting CD8+CD103+ TRMs’ number and function should be considered for immunotherapy against GC.
CD8+CD103+ TRM infiltration in human cancer has been extensively examined, including GC [12, 16–19]. However, these studies largely focused on the prognostic values of CD8+CD103+ TRMs and their functional comparison with that of their CD8+CD103− counterparts from the same tumors, and no investigation has compared the distribution and characteristics of CD8+CD103+ TRMs from tumor and non-tumor tissues of GC patients. In this study, we show a significantly lower percentage of CD8+CD103+ TRMs in tumor tissues than those in non-tumor tissues. However, the absolute number of CD8+CD103+ TRMs in tumor and non-tumor tissues are similar even though CD8+ and CD8+CD103− T cell numbers were both higher in tumor tissues than those in non-tumor tissues (Supplemental Fig. 1). We speculated that both the infiltrating CD8+CD103+ TRMs and their CD8+CD103− counterparts in tumor tissues might be initially increased, but due to an enhanced apoptosis of CD8+CD103+ TRMs compared to their CD8+CD103− counterparts [19], the percentages of tumor-infiltrating CD8+CD103+ TRMs were ultimately decreased, while their numbers were still equal to those in non-tumor tissues. However, along with disease progression, the numbers of CD8+CD103+ TRMs in tumors were gradually decreased, and Low numbers of tumor-infiltrating CD8+CD103+ TRMs were positively associated with poor patient’ survival, suggesting that GC-infiltrating CD8+CD103+ TRM number may be a useful clinical marker of tumor progression.
We further studied CD8+CD103+ TRMs’ surface molecule expression and function. Although these CD8+CD103+ TRMs exhibited a similar differentiation status, they clearly showed distinct immuno-phenotypic and functional properties compared to those from non-tumor tissues. For instance, tumor-infiltrating CD8+CD103+ TRMs expressed higher co-inhibitory molecule PD-1, a surrogate marker of tumor-exhausted CD8+ T cells [20, 21]. Additionally, these CD8+CD103+ TRMs were functionally impaired, as indicated by their decreased granzyme B and perforin expression, which was further confirmed restored functionality via PD-1 blockade. Interestingly, tumor-infiltrating CD8+CD103+ TRMs expressed a higher level of 4-1BB than their counterparts from non-tumor tissues, and their activation by 4-1BB agonist antibody combined with PD-1 blockade led to better function restoration of these CD8+CD103+ TRMs. In fact, it has been reported that 4-1BB is expressed on exhausted tumor-infiltrating PD-1+CD8+ T cells, and combining 4-1BB co-stimulation and PD-1 blockade act synergistically to promote ex vivo functions of tumor-infiltrating CD8+ T cells isolated from ovarian cancer and hepatocellular carcinoma [22, 23]. Thus, our results demonstrate that, in combination with PD-1 blockade, 4-1BB costimulatory signal overcomes CD8+CD103+ TRM exhaustion.
In summary, our study characterized the phenotypic and functional properties of CD8+CD103+ TRMs in the tumor and non-tumor tissues of GC patients and revealed an impaired cytolytic capacity of the CD8+CD103+ TRMs in tumor tissues compared with those in non-tumor tissues. Importantly, such impaired function of GC-derived CD8+CD103+ TRMs was restored by PD-1 blockade, which was further enhanced by 4-1BB co-stimulation. Our study suggests that restoring CD8+CD103+ TRMs’ function by combining PD-1 blockade with 4-1BB co-stimulation could be a promising therapeutic strategy for GC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants of the National Natural Science Foundation of China (NSFC, No. 81872331 and No. 81773042). We thank all the patients for their trust, understanding, and willingness to provide their samples for the research.
Abbreviations
- BTLA
B and T lymphocyte attenuator
- CFSE
5, 6-Carboxyfluorescein diacetate succinimidyl ester
- GC
Gastric cancer
- GITR
Glucocorticoid-induced tumor necrosis factor receptor
- IFN-γ
Interferon γ
- KLRG1
Killer cell lectin-like receptor G1
- LAG-3
Lymphocyte activation gene 3
- PD-1
Programmed cell death protein 1
- PBMC
Peripheral blood mononuclear cells
- TIGIT
T cell immunoglobulin and ITIM domain
- Tim-3
T cell immunoglobulin domain and mucin domain-3
- TNF-α
Tumor necrosis factor α
- TNM
Tumor-lymph node-metastasis
- TRMs
Tissue-resident memory T cells
Author contributions
Conception and design: L.-s. Peng, Y. Shen, Y. Qiu, Q.-m. Zou. Collection and assembly of data: L.-s. Peng, Y. Shen, X.-l. Li,Y.-x. Li, Z.-g. Shan, Y.-l. Zhao, P. Chen and Z. Zhao. Data analysis and interpretation: L.-s. Peng, Y. Shen, J.-y. Zhang, Y. Zhuang, D.-y. Ma, Y. Qiu and Q.-m. Zou. Manuscript writing: L.-s. Peng. Revision of the manuscript: L.-s. Peng, Y. Qiu and W. Chen. Final approval of manuscript: all authors.
Availability of data and material
Data are available upon reasonable request. All data relevant to the study are either included in the article or uploaded as supplementary information.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the Ethics Committee of the Third Military Medical University. Full written informed consent was obtained from all patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Shen and Xiao-long Li are contributed equally to this work.
Contributor Information
Yuan Qiu, Email: xiaoq2037@qq.com.
Liu-sheng Peng, Email: pengliusheng06@163.com.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 3.Lee HE, Chae SW, Lee YJ, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704–1711. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pernot S, Terme M, Radosevic-Robin N, et al. Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric Cancer. 2020;23:73–81. doi: 10.1007/s10120-019-00983-3. [DOI] [PubMed] [Google Scholar]
- 5.Apetoh L, Smyth MJ, Drake CG, et al. Consensus nomenclature for CD8(+) T cell phenotypes in cancer. Oncoimmunology. 2015;4:e998538. doi: 10.1080/2162402X.2014.998538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng C, Zheng L, Yoo JK, et al. Landscape of Infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169:1342–1356.e16. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Mami-Chouaib F, Blanc C, Corgnac S, et al. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer. 2018;6:87. doi: 10.1186/s40425-018-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang T, Shen Y, Luyten S, et al. Tissue-resident memory CD8(+) T cells in cancer immunology and immunotherapy. Pharmacol Res. 2020;159:104876. doi: 10.1016/j.phrs.2020.104876. [DOI] [PubMed] [Google Scholar]
- 9.Hewavisenti R, Ferguson A, Wang K, et al. CD103+ tumor-resident CD8+ T cell numbers underlie improved patient survival in oropharyngeal squamous cell carcinoma. J Immunother Cancer. 2020 doi: 10.1136/jitc-2019-000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganesan AP, Clarke J, Wood O, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. 2017;18:940–950. doi: 10.1038/ni.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han L, Gao QL, Zhou XM, et al. Characterization of CD103(+) CD8(+) tissue-resident T cells in esophageal squamous cell carcinoma: may be tumor reactive and resurrected by anti-PD-1 blockade. Cancer Immunol Immunother. 2020;69:1493–1504. doi: 10.1007/s00262-020-02562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li R, Liu H, Cao Y, et al. Identification and validation of an immunogenic subtype of gastric cancer with abundant intratumoural CD103(+)CD8(+) T cells conferring favourable prognosis. Br J Cancer. 2020;122:1525–1534. doi: 10.1038/s41416-020-0813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piet B, de Bree GJ, Smids-Dierdorp BS, et al. CD8(+) T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest. 2011;121:2254–2263. doi: 10.1172/JCI44675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Y, Teng Y, Lv Y, et al. PD-1 does not mark tumor-infiltrating CD8+ T cell dysfunction in human gastric cancer. J Immunother Cancer. 2020 doi: 10.1136/jitc-2019-000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Zheng B, Goswami S, et al. PD1(Hi) CD8(+) T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J Immunother Cancer. 2019;7:331. doi: 10.1186/s40425-019-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb JR, Milne K, Watson P, et al. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. 2014;20:434–444. doi: 10.1158/1078-0432.CCR-13-1877. [DOI] [PubMed] [Google Scholar]
- 17.Djenidi F, Adam J, Goubar A, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 18.Egelston CA, Avalos C, Tu TY, et al. Resident memory CD8+ T cells within cancer islands mediate survival in breast cancer patients. JCI Insight. 2019 doi: 10.1172/jci.insight.130000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin R, Zhang H, Yuan Y, et al. Fatty Acid Oxidation Controls CD8(+) Tissue-Resident Memory T-cell Survival in Gastric Adenocarcinoma. Cancer Immunol Res. 2020;8:479–492. doi: 10.1158/2326-6066.CIR-19-0702. [DOI] [PubMed] [Google Scholar]
- 20.Thommen DS, Schreiner J, Muller P, et al. Progression of Lung Cancer Is Associated with Increased Dysfunction of T Cells Defined by Coexpression of Multiple Inhibitory Receptors. Cancer Immunol Res. 2015;3:1344–1355. doi: 10.1158/2326-6066.CIR-15-0097. [DOI] [PubMed] [Google Scholar]
- 21.Davidson TB, Lee A, Hsu M, et al. Expression of PD-1 by T Cells in Malignant Glioma Patients Reflects Exhaustion and Activation. Clin Cancer Res. 2019;25:1913–1922. doi: 10.1158/1078-0432.CCR-18-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leem G, Park J, Jeon M, et al. 4–1BB Co-stimulation further enhances anti-PD-1-mediated reinvigoration of exhausted CD39(+) CD8 T cells from primary and metastatic sites of epithelial ovarian cancers. J Immunother Cancer. 2020 doi: 10.1136/jitc-2020-001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HD, Park S, Jeong S, et al. 4–1BB Delineates distinct activation status of exhausted tumor-infiltrating CD8(+) T cells in hepatocellular carcinoma. Hepatology. 2020;71:955–971. doi: 10.1002/hep.30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are either included in the article or uploaded as supplementary information.