Abstract
Cancer immunotherapy, which blocks immune checkpoint molecules, is an effective therapeutic strategy for human cancer patients through restoration of tumor-infiltrating (TI) cell function. However, evaluating the efficacy of immune checkpoint inhibitors (ICIs) is difficult because no standard in vitro assay for ICI efficacy evaluation exists. Additionally, blocking a particular immune checkpoint receptor (ICR) is insufficient to restore T cell functionality, because other ICRs still transduce inhibitory signals. Therefore, limiting inhibitory signals transduced via other ICRs is needed to more accurately assess the efficacy of ICIs targeting a particular immune checkpoint. Here, we introduce a newly developed in vitro coculture assay using human peripheral blood mononuclear cells (hPBMCs) and engineered human cancer cell lines. We enriched CD8+ T cells from hPBMCs of healthy donors through low-dose T cell receptor stimulation and cytokine (human IL-2 and IL-7) addition. These enriched CD8+ T cells were functional and expressed multiple ICRs, especially TIM-3 and TIGIT. We also established immune checkpoint ligand (ICL) knockout (KO) cancer cell lines with the CRISPR-Cas9 system. Then, we optimized the in vitro coculture assay conditions to evaluate ICI efficacy. For example, we selected the most effective anti-TIM-3 antibody through coculture of TIM-3+CD8+ T cells with PD-L1-/-PVR-/- cancer cells. In summary, we developed a mechanism-based in vitro coculture assay with hPBMCs and ICL KO cancer cell lines, which could be a useful tool to identify promising ICIs by providing reliable ICI efficacy information.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03201-9.
Keywords: Human peripheral blood mononuclear cells, Immune checkpoint ligand knockout cell lines, In vitro coculture assay, Immune checkpoint receptor-blocking antibody
Introduction
Immune checkpoint receptors (ICRs) on tumor-infiltrating (TI) CD8+ T cells interact with their counterpart ligands on tumor cells, thereby suppressing the functionality of TI CD8+ T cells [1, 2]. Given the high extent of ICR expression on TI CD8+ T cells in various tumor types, blockade of ICRs on TI CD8+ T cells enhances antitumor immunity by restoring the functionality of CD8+ T cells [3–7]. PD-1 blockade has been shown to be more effective in enhancing antitumor immunity than other therapies [8]. Although PD-1 blockade is more effective than other therapies, the response rate to PD-1 blockade is still approximately 30% and needs to be improved [9, 10]. Combination therapy with blockade of PD-1 and other ICRs has recently shown a better response rate and prognosis than PD-1 blockade alone in various tumor types [11–17]. To further understand the mechanism of cancer immunotherapy, many studies have revealed the ICR signaling pathway and TI CD8+ T cell characteristics [18–25]. Because of the emphasized importance of cancer immunotherapy, many companies are participating in developing their own immune checkpoint inhibitors (ICIs). Evaluating the specific efficacy of ICIs is important for effective cancer immunotherapy. The efficacy of ICIs is evaluated by in vitro and in vivo assays. Because it is difficult to examine the efficacy of all ICIs by in vivo assays, most ICIs are usually evaluated by in vitro assays. However, existing in vitro assays used for the evaluation of ICI efficacy have several limitations.
One in vitro assay evaluates the efficacy of ICIs by using Jurkat cells with stable PD-1 and NFAT-luciferase reporter expression. Since Jurkat cells harbor several mutations in the T cell receptor (TCR) signaling pathway, their response cannot reflect the actual T cell response [26]. To overcome this limitation, some in vitro assays use human peripheral blood mononuclear cells (hPBMCs) isolated from healthy donors [27, 28]. In some in vitro assays, hPBMCs are usually cultured under TCR stimulation for 3–4 days, and culture supernatants are then collected [27]. The amount of IFNγ in the collected culture supernatant is determined by an IFNγ enzyme-linked immunosorbent assay (ELISA). However, various immune cells are present among hPBMCs cultured for 3–4 days. Therefore, it is unclear whether the amount of IFNγ measured by ELISA is derived solely from CD8+ T cells. Although using hPBMCs is a more accurate and physiological approach for measuring the efficacy of ICR-blocking antibodies than using lymphocyte cell lines, a disadvantage of this approach is that hPBMCs are heterogeneous among individuals. Additionally, obtaining a sufficient number of vials of hPBMCs from healthy donors is difficult. These limitations emphasize the importance of a consistent and reliable in vitro assay for evaluating the efficacy of ICR-blocking antibodies. However, since there is no standard and consistent in vitro assay for the measurement of ICI efficacy, it is difficult to compare and evaluate the efficacy of each ICI developed by different companies.
Here, we introduce a protocol for human CD8+ T cell enrichment and a newly developed in vitro coculture assay for evaluating the efficacy of ICR-blocking antibodies. We enriched CD8+ T cells from hPBMCs of healthy donors without magnetic bead-dependent isolation. We also established immune checkpoint ligand (ICL) knockout (KO) HCC4006 cell lines by using the CRISPR-Cas9 system. Then, with an in vitro coculture assay system, we evaluated the efficacy of ICIs and showed that ICIs increased the functionality of ICR+CD8+ T cells. Additionally, we demonstrated that the efficacy of ICIs was augmented by deletion of other ICLs expressed on cancer cell lines. In summary, we developed a standard and physiologically relevant in vitro coculture assay based on direct interactions between CD8+ T cells and tumor cells.
Materials and methods
Isolation of hPBMCs
Peripheral blood was obtained from healthy donors, a single patient with non-small cell lung cancer (NSCLC), and a single patient with head and neck squamous cell carcinoma (HNSCC). PBMCs were isolated from whole blood by Percoll density gradient centrifugation. Isolated hPBMCs were cryopreserved until use.
Reagents and antibodies
The following fluorochrome-conjugated monoclonal antibodies (clone, manufacturer, catalog number) were used for multicolor flow cytometry: anti-CD4-BV785 (OKT4, Biolegend, 317,442), anti-CD8-PerCP/Cy5.5 (SK1, Biolegend, 344,710), anti-PD-1-BV421 (EH12.2H7, Biolegend, 329), anti-IFNγ-PE (4S.B3, Biolegend, 502,509), anti-TNFα-BV605 (MAb11, Biolegend, 502,936), streptavidin-PerCP/Cy5.5 (Biolegend, 405,214), anti-PD-L1-BV421 (29E.2A3, Biolegend, 329,714), anti-PVR-PE (TX24, Biolegend, 337,507), anti-Galectin-9-PerCP/Cy5.5 (9M1-3, Biolegend, 348,910), mouse IgG2b κ-BV421 (MPC-11, Biolegend, 400,342), mouse IgG2a κ-PE (MOPC-173, Biolegend, 400,212), mouse IgG1 κ-PerCP/Cy5.5 (MOPC-21, Biolegend, 400,150), anti-CCR7-BV421 (G043H7, Biolegend, 353,207), anti-human IgG-PE/Dazzle594 (QA19A42, Biolegend, 366,920), anti-CD107a-BV785 (H4A3, Biolegend, 328,644), anti-TIM-3-Alexa488 (R&D Systems, FAB2365G), anti-TIGIT-APC (R&D Systems, FAB7898A), anti-Ceacam1-APC (CC1, Invitrogen, 17-0661-80), mouse IgG1 κ-APC (P3.6.2.8.1, Invitrogen, 17-4714-81), anti-CD45RA-PE/Cy7 (L48, BD Biosciences, 337,167) and anti-IgG4-biotin (G17-4, BD Biosciences, 555,882). For the in vitro coculture assay, anti-PD-1 (pembrolizumab), anti-TIM-3 (Novartis), anti-TIGIT (Merck), and human IgG4 isotype control (QA16A15, Biolegend, 403,702) antibodies were used.
Enrichment of CD8+ T cells from hPBMCs of healthy donors
Cryopreserved hPBMCs were thawed and rested in complete RPMI 1640 medium supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 mg/ml) for 16 h in 24-well non-tissue culture-treated plates at a concentration of 5 × 106 cells/ml. Rested hPBMCs were harvested and cultured in 24-well non-tissue culture-treated plates with the anti-CD3 antibody (BD Biosciences, 555,336, 1 μg/ml), human interleukin-2 (hIL-2) (PeproTech, 200-02, 10 ng/ml) and human interleukin-7 (hIL-7) (PeproTech, 200-07, 10 ng/ml) at a concentration of 1 × 106 cells/ml for 3 days. After 3 days, the above process was repeated for 4 weeks. For T cell culture by using αCD3/CD28 dynabeads, rested hPBMCs were cultured in 24-well non-tissue culture-treated plates with αCD3/CD28 dynabeads (Gibco™, 11131D, the ratio of 1:1), hIL-2 (PeproTech, 200-02, 10 ng/ml) at a concentration of 1 × 106 cells/ml for 3 days. After 3 days, the above process was repeated for 4 weeks.
Establishment of knock-out cancer cell lines
The HCC4006 lung adenocarcinoma cell line was obtained from ATCC in 2010 and was cultured in complete RPMI 1640 medium supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 mg/ml). HCC4006 cells tested negative for mycoplasma contamination. To generate cell lines with PD-L1 and/or PVR KO, HCC4006 cells were transiently transfected with a PX459 plasmid (Addgene) expressing Cas9 and an optimized single-guide RNA (sgRNA) targeting PD-L1 (PD-L1 sgRNA sequence, 5’-TCTTTATATTCATGACCTAC-3’) or PVR (PVR sgRNA sequence, 5’-CCTGTTCGTCACGTTCCCGC-3’) (GenScript), respectively. After transfection and transient selection with puromycin (Invitrogen), single cells were seeded into 96-well plates without puromycin. To select KO clones, the expression of PD-L1 and PVR was evaluated by flow cytometry.
In vitro coculture assay and intracellular staining
Ninety-six well U-bottom plates were coated with an anti-CD3 antibody (BD Biosciences, 555,329, 20 μg/ml) at 4 °C. Before coculture, CD8+ T cells enriched for 14 days were preincubated with isotype control or ICR-blocking antibodies for 20 min in a 37 °C incubator. Preincubated CD8+ T cells were cocultured with HCC4006 cell lines for 6 h at a ratio of 1:1 under restimulation with the anti-CD3 antibody (20 μg/ml, plate-coated), the anti-CD28 antibody (5 μg/ml, plate-coated), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml). To detect degranulation and intracellular cytokines in CD8+ T cells, GolgiPlug/GolgiStop, anti-CD107a antibodies and DNase I were added to the coculture plates prior to intracellular cytokine staining using anti-IFN-γ and anti-TNF-α antibodies. Intracellular staining was performed after surface staining using a BD Cytofix/Cytoperm fixation/permeabilization Kit (BD Biosciences) according to the manufacturer’s instructions. A LIVE/DEAD Fixable Dead Cell Stain Kit (Invitrogen, Carlsbad, CA, USA) was used in most staining procedures to remove the dead cell population. All stained samples were analyzed in a CytoFLEX or CytoFLEX LX instrument (Beckman Coulter) using FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analysis
Statistical analysis was performed using Prism software version 7.00 (GraphPad, San Diego, CA, USA). Significant differences between groups were analyzed by two-tailed unpaired Student’s t-test or one-way ANOVA with post hoc Tukey’s multiple comparison test.
Results
Enrichment of CD8+ T cells from hPBMCs of healthy donors
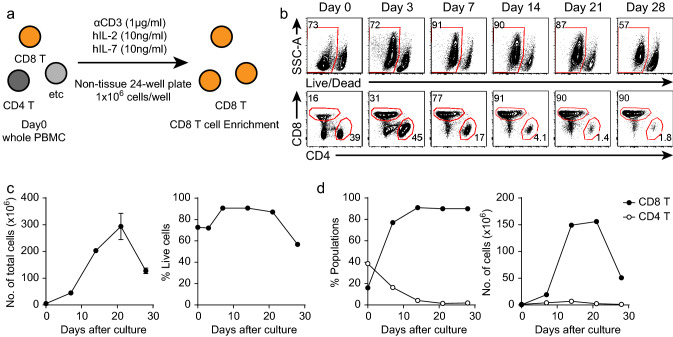
To establish a more accurate in vitro coculture assay for evaluating the efficacy of immune checkpoint-blocking antibodies, it is important to obtain sufficient numbers of ICR-expressing CD8+ T cells from hPBMCs of healthy donors. Thus, we first developed a CD8+ T cell enrichment protocol. For enrichment of CD8+ T cells from hPBMCs of healthy donors, we cultured 1 × 106 hPBMCs under low-dose TCR stimulation (with an anti-CD3 antibody). Additionally, TIM-3 expression on CD8+ T cells has been shown to be induced by common γ-chain cytokines [29, 30]. Therefore, we supplemented the cultures with common γ-chain cytokines (human IL-2 and IL-7) to induce TIM-3 expression (Fig. 1a). hPBMCs expanded to approximately 3 × 108 cells after culture with the anti-CD3 antibody, hIL-2 and hIL-7 (Fig. 1b, c). The viability of the cultured hPBMCs was reduced after 21 days (Fig. 1b, c). Because of this reduction in viability, the total number of cultures hPBMCs was decreased after 21 days (Fig. 1c). We next examined the frequencies of CD8+ and CD4+ T cells among the cultured hPBMCs. The frequency and number of CD8+ T cells were increased until 21 days after culture initiation and decreased thereafter because of reduced viability (Fig. 1b, d). From 14 to 28 days after culture initiation, CD8+ T cells accounted for the greatest proportion of the cultured hPBMCs (Fig. 1b, d). In contrast, the frequency and number of CD4+ T cells were decreased as culture progressed (Fig. 1b, d). These results showed that CD8+ T cells can easily be enriched from hPBMCs of healthy donors following this culture protocol without using T cell-isolating magnetic beads.
Fig. 1.
Enrichment of CD8+ T cells from hPBMCs of healthy donors. a hPBMCs were isolated from the peripheral blood of healthy donors. Isolated hPBMCs were cultured with anti-CD3 antibodies (1 μg/ml, soluble), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml) in 24-well non-tissue culture-treated plates. b Representative FACS plots of live cells and T cells among the cultured hPBMCs at the indicated time points. c Kinetics of the number of total cells and the frequency of live cells among the cultured hPBMCs. d Kinetics of the frequency and number of T cells among the cultured hPBMCs. The experiment was performed with hPBMCs from five donors. The data are representative of triplicate samples from a single donor. The error bars indicate the means ± SEMs
T cell culture protocol using αCD3/CD28 dynabeads has been usually used to expand T cells. To identify which protocol was suitable to expand and enrich CD8+ T cells from hPBMCs, we cultured hPBMCs according to each protocol. When cultured by αCD3/CD28 dynabeads, not only CD8+ T cells, CD4+ T cells were also expanded (Supplementary Fig. 1). This result demonstrated that CD8+ T cell enrichment protocol introduced here was appropriate for the enrichment of CD8+ T cells from hPBMCs. Additionally, using hPBMCs of cancer patients could be clinically relevant. However, there are several limitations of using hPBMCs from cancer patients. First, it was hard to obtain the sufficient hPBMCs of cancer patients. Second, according to the stage of cancer patients, hPBMCs might exhibit different characteristics (T cell frequency, ICR expression, cytotoxicity, etc.). These patient-dependent characteristics could lead to the inconsistency of CD8+ T cell enrichment and further inaccuracy of the ICI efficacy assessment. To verify that these limitations could affect to the CD8+ T cell enrichment, we compared the frequencies of CD8+ and CD4+ T cells among the cultured hPBMCs of healthy donors and cancer patients (a single patient with non-small cell lung cancer (NSCLC) and a single patient with head and neck squamous cell carcinoma (HNSCC)). In HNSCC cancer patient, although CD8+ T cells were enriched as same extent as in healthy donors, the cell number of enriched CD8+ T cells was fewer than that in healthy donors (Supplementary Fig. 2a–c). In NSCLC cancer patient, CD8+ T cells could not be enriched as same extent as those in healthy donors (Supplementary Fig. 2a–c). These result demonstrated that using hPBMCs of healthy donors was suitable to obtain the sufficient and highly enriched CD8+ T cells.
Enriched CD8+ T cells exhibit heterogeneity by expressing multiple ICRs and with naïve/memory phenotype
We next examined the expression patterns of ICRs on the enriched CD8+ T cells. Notably, PD-1 expression peaked 3 days after culture initiation and decreased thereafter as culture progressed (Fig. 2a, b). TIM-3 was expressed 7 days after culture initiation and decreased thereafter (Fig. 2a, b). TIGIT expression increased gradually as culture progressed (Fig. 2a, b). Additionally, we examined the coexpression patterns of ICRs on the enriched CD8+ T cells 14 days after culture initiation. The enriched CD8+ T cells were heterogeneous in terms of ICR expression patterns (Fig. 2c, d). Among the heterogeneous populations in the enriched CD8+ T cells, TIM-3 single-positive cells were the most abundant among the enriched CD8+ T cells (Fig. 2c, d). In CD4+ T cells, PD-1 and TIM-3 were highly expressed during culture progression (Fig. 2c, d). However, TIGIT expression in CD4+ T cells was maintained at a low level during culture progression (Fig. 2a, b). However, because compared to CD8+ T cells, CD4+ T cells accounted for only a small proportion of the cultured hPBMCs, ICR expression on CD4+ T cells might be irrelevant. We next identified which CD8+ T cells were preferentially expanded and enriched in terms of naïve/memory phenotype. Before CD8+ T cell enrichment, effector memory (EM) CD8+ T cells were most abundant in hPBMCs (Supplementary Fig. 3a, b). We found that a proportion of naïve CD8+ T cells in the cultured hPBMCs were increased compared to that in precultured CD8+ T cells (Supplementary Fig. 3a, b). However, EM CD8+ T cells were still major populations in the cultured hPBMCs (Supplementary Fig. 3a, b). This result indicated that naïve CD8+ T cells were preferentially expanded compared to other CD8+ T cells although EM CD8+ T cells were still most of the cultured hPBMCs. Taken together, we found that enriched CD8+ T cells were heterogeneous in terms of the expression of ICRs and naïve/memory phenotype.
Fig. 2.
Expression patterns of T cells among cultured hPBMCs. hPBMCs were isolated from the peripheral blood of healthy donors. Isolated hPBMCs were cultured with anti-CD3 antibodies (1 μg/ml, soluble), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml) in non-tissue 24-well culture plates. a Representative FACS plot of the expression patterns of ICRs on CD8+ and CD4+ T cells among the cultured hPBMCs at the indicated time points. b Kinetics of PD-1, TIM-3, and TIGIT expression on CD8+ and CD4+ T cells. c Representative FACS plot of the expression patterns of ICRs on CD8+ T cells at 14 days after culture initiation. d Quantification of the percentages of ICR+ populations among CD8+ T cells 14 days after culture initiation. The experiment was performed with hPBMCs from five donors. The data are representative of triplicate samples from a single donor. The error bars indicate the means ± SEMs
The functionality of CD8+ T cells is determined by the total ICR inhibitory signaling
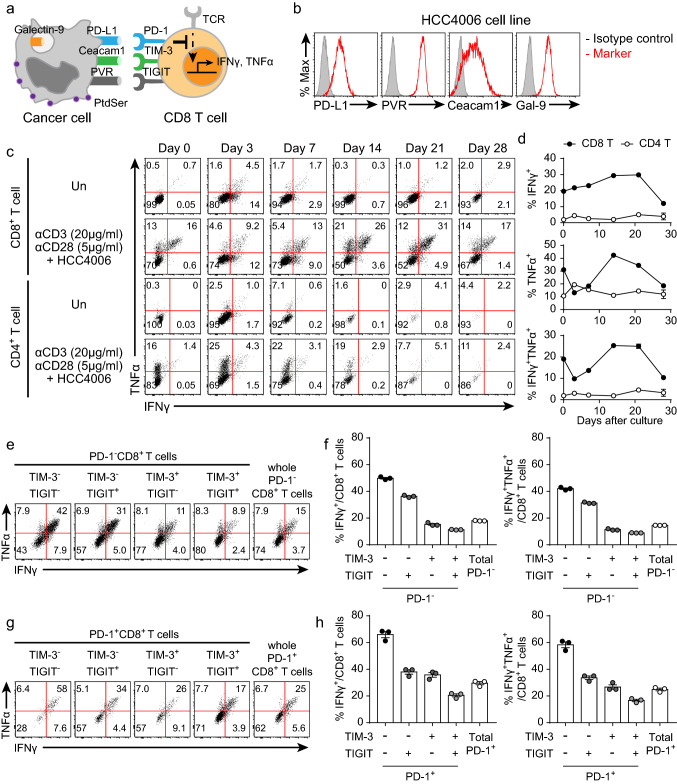
To examine the functional kinetics of the enriched CD8+ T cells, we cocultured these CD8+ T cells with cancer cell lines at a ratio of 1:1 under anti-CD3 and anti-CD28 antibody restimulation (Fig. 3a). HCC4006 human lung adenocarcinoma cell lines were used as the main source of ICLs because these cell lines express multiple ICLs (Fig. 3b). CD8+ T cells cultured for 14–21 days had the greatest functionality, although their functionality decreased after 21 days (Fig. 3c, d). Thus, considering the ICR expression patterns and functionality of the enriched CD8+ T cells, CD8+ T cells cultured for 14–21 days were used to optimize the in vitro coculture assay conditions. As the CD8+ T cells cultured for 14 days were heterogeneous (Fig. 2c, d), we examined their functionality based on ICR expression. TIM-3 and/or TIGIT expression on PD-1−CD8+ T cells resulted in impairment of cytokine secretion (Fig. 3e, f). Additional ICR expression on PD-1+CD8+ T cells also reduced their functionality (Fig. 3g, h). These results indicated that the functionality of CD8+ T cells was negatively correlated with the total ICR inhibitory signaling. Unexpectedly, PD-1+CD8+ T cells were more functional than PD-1−CD8+ T cells (Fig. 3e–h). To find out why PD-1+CD8+ T cells more secreted cytokines, we examined the distribution of naïve/memory T cells in the CD8+ T cells. EM CD8+ T cells were prevalent in PD-1+CD8+ T cells, while naïve CD8+ T cells were dominant in PD-1−CD8+ T cells (Supplementary Fig. 3c, d). Since EM CD8+ T cells were more functional than naïve CD8+ T cells [31], PD-1+CD8+ T cells more secreted cytokines than PD-1−CD8+ T cells (Fig. 3e–h).
Fig. 3.
Kinetic analysis of the cytokine secretion capacity of CD8+ T cells cocultured with HCC4006 cell lines. Enriched CD8 + T cells were cocultured with HCC4006 cell lines for 6 h at a ratio of 1:1 under restimulation with the anti-CD3 antibody (20 μg/ml, plate-coated), the anti-CD28 antibody (5 μg/ml, plate-coated), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml). a Graphical explanation of the mechanism by which CD8+ T cell functionality is inhibited by cancer cells. b Representative histograms of ICLs expressed on HCC4006 cell lines. c Representative FACS plot of cytokine secretion from CD8+ T cells at the indicated time points. d Kinetics of the frequencies of CD8+ and CD4+ T cells secreting cytokines. e Representative FACS plot of PD-1−CD8+ T cells secreting cytokines 14 days after culture initiation. f Quantification of the percentage of PD-1−CD8+ T cells secreting cytokines 14 days after culture initiation. g Representative FACS plot of PD-1+CD8+ T cells secreting cytokines 14 days after culture initiation. h Quantification of the percentage of PD-1+CD8+ T cells secreting cytokines 14 days after culture initiation. The experiment was performed with hPBMCs from three donors. The data are representative of triplicate samples from a single donor. The error bars indicate the means ± SEMs
Accurate identification of the ICR-positive CD8+ T cell population is a prerequisite for evaluating the efficacy of ICR-blocking antibodies
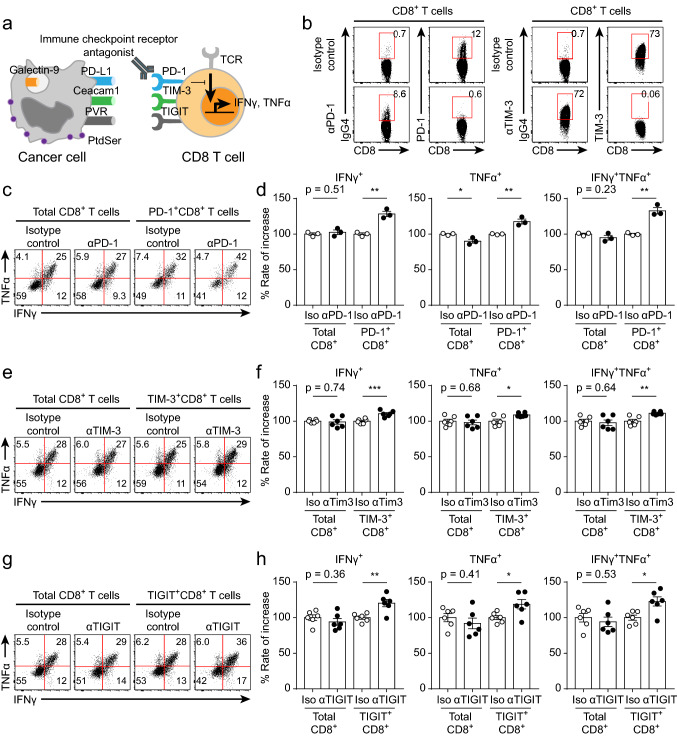
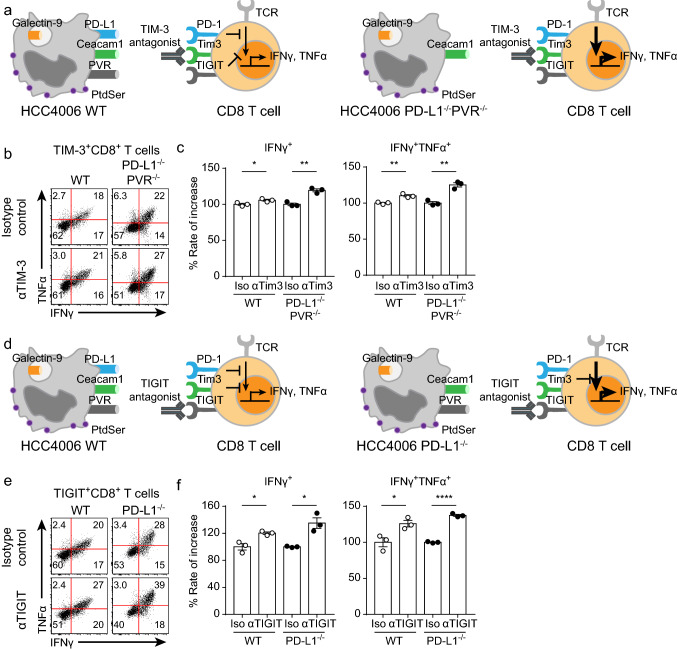
We next evaluated the efficacy of ICR-blocking antibodies by using an in vitro coculture assay (Fig. 4a). Because of the heterogeneous expression of ICRs on the enriched CD8+ T cells (Fig. 2c, d), evaluating the efficacy of ICR-blocking antibodies in the total CD8+ T cell population would lead to inaccurate results in this in vitro coculture assay. Therefore, it was important to accurately identify CD8+ T cells directly bound to the ICR-blocking antibody. Using an anti-IgG4 secondary antibody, we identified CD8+ T cells directly bound to the ICR-blocking antibody (Fig. 4b). We first evaluated the efficacy of an anti-PD-1 antibody (pembrolizumab) by using the in vitro coculture assay. As expected, the efficacy of the anti-PD-1 antibody was not accurately measured in the total CD8+ T cell population (Fig. 4c, d). Moreover, PD-1+CD8+ T cell function (cytokine secretion (IFNγ, TNFα) and degranulation) was enhanced by anti-PD-1 antibody treatment (Fig. 4c, d, Supplementary Fig. 4a, b). This result suggested that identifying specific ICR+ populations among CD8+ T cells is important for accurately evaluating the efficacy of ICR-blocking antibodies. We next evaluated the efficacies of the anti-TIM-3 and anti-TIGIT antibodies. Consistent with the above result, the functionality (cytokine secretion (IFNγ, TNFα) and degranulation) of the total CD8+ T cell population was not enhanced by treatment with the anti-TIM-3 or anti-TIGIT antibody, while the function of TIM-3+CD8+ and TIGIT+CD8+ T cells was induced by treatment with these antibodies (Fig. 4e–h, Supplementary Fig. 4c, d). Thus, we established the identification of ICR+CD8+ T cells as a prerequisite for accurate evaluation of ICR antibody efficacy.
Fig. 4.
Evaluation of ICR-blocking antibodies by using the in vitro coculture assay. Before coculture, CD8+ T cells enriched for 14 days were preincubated with isotype control or ICR-blocking antibodies for 20 min in a 37 °C incubator. Preincubated CD8+ T cells were cocultured with HCC4006 cell lines for 6 h at a ratio of 1:1 under restimulation with the anti-CD3 antibody (20 μg/ml, plate-coated), the anti-CD28 antibody (5 μg/ml, plate-coated), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml). a Graphical explanation of the mechanism by which ICR-blocking antibodies restored the functionality of CD8+ T cells. b Representative FACS plot of ICR-expressing CD8+ T cells detected by the appropriate secondary antibodies. c Representative FACS plot of cytokine-secreting CD8+ and PD-1+CD8+ T cells cultured with and without anti-PD-1 antibodies. d Rate of increase in CD8+ and PD-1+CD8+ T cell function induced by anti-PD-1 antibody treatment. e Representative FACS plot of cytokine-secreting CD8+ and TIM-3+CD8+ T cells cultured with and without anti-TIM-3 antibodies. f Rate of increase in CD8+ and TIM-3+CD8+ T cell function induced by anti-TIM-3 antibody treatment. g Representative FACS plot of cytokine-secreting CD8+ and TIGIT+CD8+ T cells cultured with and without anti-TIGIT antibodies. h Rate of increase in CD8+ and TIGIT+CD8+ T cell function induced by anti-TIGIT antibody treatment. The experiment was performed with hPBMCs from three donors. The data are representative of triplicate samples from a single donor. The data were analyzed by two-tailed unpaired Student’s t-test (d, f, h). The error bars indicate the means ± SEMs. *p < 0.05; **p < 0.01; ***p < 0.001
To compare between the efficacy of ICIs at the coculture ratio 1:1 and 1:0.1, we assessed the functionality of TIGIT+CD8+ T cells at the ratio of 1:0.1. At the coculture ratio of 1:0.1, the functionality (cytokine secretion (IFNγ, TNFα) and degranulation) of the TIGIT+CD8+ T cells was also augmented by anti-TIGIT antibody treatment (Supplementary Fig. 5a–d). The enhancement of TIGIT+CD8+ T cell function at the coculture ratio of 1:0.1 was comparable with that at the coculture ratio of 1:1 (Supplementary Fig. 5a–d). This result indicated that the coculture ratio of 1:0.1 was also available to evaluate the efficacy of ICIs. Although it was evident that both coculture ratio could be used for evaluating the ICI efficacy, we choose the coculture ratio of 1:1 to provide the sufficient ICLs.
CD8+ T cells cocultured with ICL KO HCC4006 cell lines are more functional than those cocultured with the WT HCC4006 cell lines
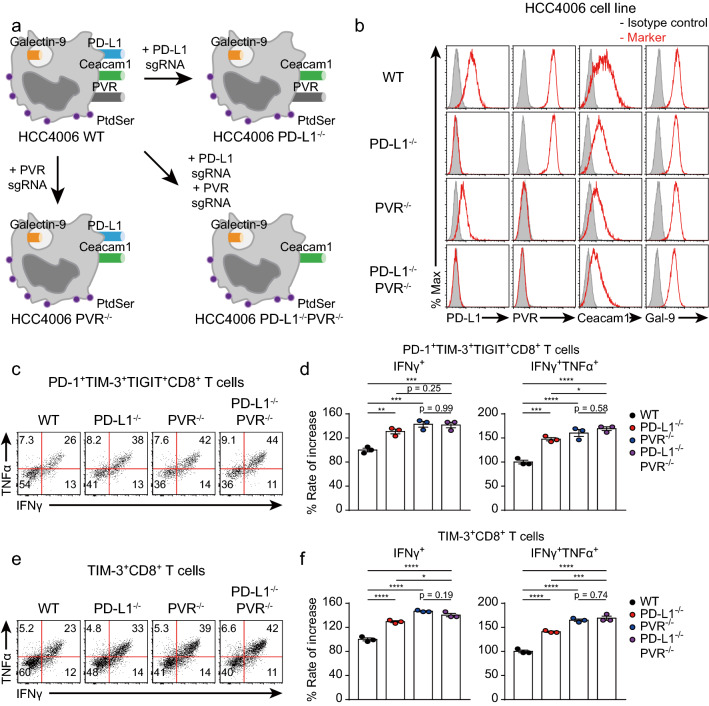
We found that each ICR independently suppressed CD8+ T cell function (Fig. 3e–h). Given that inhibitory signals induced by other ICRs prevent restoration of CD8+ T cell function, blockade of other ICRs is needed to evaluate the actual efficacy of specific ICR-blocking antibodies. However, in this in vitro coculture assay, blockade of multiple ICRs makes it difficult to accurately identify ICR+CD8+ T cells because secondary antibodies cannot distinguish each ICR-blocking antibody. Therefore, we decided to manipulate ICLs on tumor cells to exclude inhibitory signals induced by other ICRs. Using the CRISPR-Cas9 system, we genetically manipulated the expression of ICLs (Fig. 5a). Additionally, we transiently transfected HCC4006 cells with a vector containing the sequences encoding the Cas9 protein and a sgRNA targeting a specific ICL to prevent off-target effects caused by insertion of the vector into chromosomal DNA. Then, we established HCC4006 cell lines with PD-L1 and/or PVR KO (Fig. 5b). To identify whether CD8+ T cells cocultured with ICL KO HCC4006 cell lines were more functional than those cocultured with the WT HCC4006 cell line, we cocultured enriched CD8+ T cells with ICL KO and ICL WT HCC4006 cell lines. We next examined the functionality of TIM-3+ and PD-1+TIM-3+TIGIT+CD8+ T cells (Fig. 5c). PD-1+TIM-3+TIGIT+CD8+ T cells cocultured with the PVR−/− or PD-L1−/−PVR−/− HCC4006 cell line were more functional than those cocultured with the WT or PD-L1−/− HCC4006 cell line (Fig. 5d, e). The same results were observed for TIM-3+CD8+ T cells (Fig. 5f, g). This finding indicated that the PD-L1−/−PVR−/− HCC4006 cell line could be optimal for evaluating the efficacy of the anti-TIM-3 antibody.
Fig. 5.
The functionality of CD8+ T cells is enhanced by deleting ICL in human cancer cell lines. CD8+ T cells enriched for 16 days were cocultured with ICL WT or KO HCC4006 cell lines for 6 h at a ratio of 1:1 under restimulation with the anti-CD3 antibody (20 μg/ml, plate-coated), the anti-CD28 antibody (5 μg/ml, plate-coated), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml). a Graphical explanation of the establishment of the ICL KO HCC4006 cell lines with the CRISPR-Cas9 system. b Representative histograms of ICL expression on the indicated HCC4006 cell lines. c Representative FACS plot of cytokine-secreting PD-1+TIM-3+TIGIT+CD8+ T cells cocultured with the indicated HCC4006 cell lines. d Rate of increase in PD-1+TIM-3+TIGIT+CD8+ T cell function after coculture with the indicated HCC4006 cell lines. e Representative FACS plot of cytokine-secreting TIM-3+CD8+ T cells cocultured with the indicated HCC4006 cell lines. f Rate of increase in TIM-3+CD8+ T cell function after coculture with the indicated HCC4006 cell lines. The experiment was performed with hPBMCs from three donors. The data are representative of triplicate samples from a single donor. The data were analyzed by one-way ANOVA with Tukey’s post hoc multiple comparisons test (d, f). The error bars indicate the means ± SEMs. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
Evaluation of ICR-blocking antibodies using the appropriate in vitro coculture assay
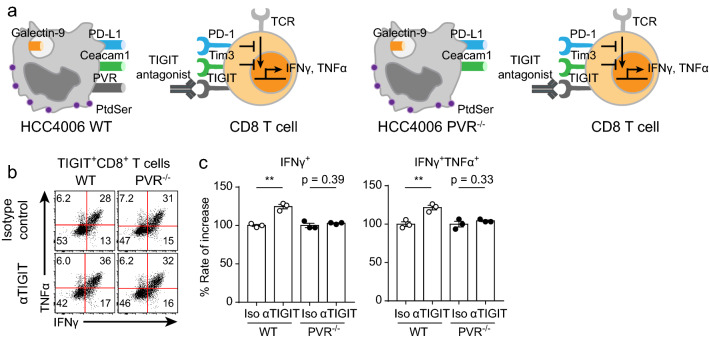
To identify that using cell lines with KO of other ICLs was indeed optimal for specific ICR-blocking antibodies, we evaluated the efficacy of anti-TIM-3 antibodies using the PD-L1−/−PVR−/− HCC4006 cell line (Fig. 6a). The efficacy of the anti-TIM-3 antibody evaluated by using the WT HCC4006 cell line was statistically significant, but the percentage increase was only 110% (Fig. 6b, c). Notably, the efficacy of the anti-TIM-3 antibody evaluated by using the PD-L1−/−PVR−/− HCC4006 cell line was higher than that evaluated by using the WT HCC4006 cell line (Fig. 6b, c). We next evaluated the efficacy of the anti-TIGIT antibody. Interestingly, there are several ICLs (Ceacam1, galectin-9, phosphatidylserine (PS), and HMGB1) of TIM-3. Because it was difficult to manipulate all ICLs of TIM-3, we alternatively used the PD-L1−/− HCC4006 cell line to evaluate the efficacy of the anti-TIGIT antibody (Fig. 6d). TIGIT+CD8+ T cells cocultured with PD-L1−/− HCC4006 cells were more strongly reinvigorated by anti-TIGIT antibody treatment than those cocultured with the WT HCC4006 cell line (Fig. 6e, f). Taken together, these results indicated that regulating other ICLs facilitates the efficacy evaluation of ICR-blocking antibodies. Finally, to identify whether this in vitro coculture assay is dependent on direct interactions between ICRs and ICLs, we evaluated the efficacy of the anti-TIGIT antibody by using the PVR−/− HCC4006 cell line (Fig. 7a). As expected, the functionality of TIGIT+CD8+ T cells cocultured with PVR−/− HCC4006 cells was not enhanced by anti-TIGIT antibody treatment, while TIGIT+CD8+ T cells cocultured with WT HCC4006 cells were reinvigorated by anti-TIGIT antibody treatment (Fig. 7b, c). These results indicated that the mechanism of the in vitro coculture assay was dependent on direct interactions between ICRs and ICLs. In summary, we developed an in vitro coculture assay to optimally evaluate the efficacy of ICR-blocking antibodies by using enriched human CD8+ T cells and human cell lines with KO of other ICLs.
Fig. 6.
The efficacy of ICR-blocking antibodies was more effectively evaluated by using appropriate ICL KO cancer cell lines. Before coculture, CD8+ T cells enriched for 20 days were preincubated with isotype control, anti-TIM-3, or anti-TIGIT antibodies for 20 min in a 37 °C incubator. Preincubated CD8+ T cells were cocultured with the PD-L1−/−PVR−/− or PD-L1−/− HCC4006 cell line for 6 h at a ratio of 1:1 under restimulation with the anti-CD3 antibody (20 μg/ml, plate-coated), the anti-CD28 antibody (5 μg/ml, plate-coated), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml). a Graphical explanation of the optimal coculture conditions for evaluating anti-TIM-3 antibody efficacy. b Representative FACS plot of cytokine-secreting TIM-3+CD8+ T cells cocultured with the WT and PD-L1−/−PVR−/− HCC4006 cell lines. c Rate of increase in TIM-3+CD8+ T cell function induced by anti-TIM-3 antibody treatment after coculture with the WT and PD-L1−/−PVR−/− HCC4006 cell lines. d Graphical explanation of optimal coculture conditions for evaluating anti-TIGIT antibody efficacy. e Representative FACS plot of cytokine-secreting TIGIT+CD8+ T cells cocultured with the WT and PD-L1−/− HCC4006 cell lines. f Rate of increase in TIGIT+CD8+ T cell function induced by anti-TIGIT antibody treatment after coculture with the WT and PD-L1−/− HCC4006 cell lines. The experiment was performed with hPBMCs from three donors. The data are representative of triplicate samples from a single donor. The data were analyzed by two-tailed unpaired Student’s t-test (c, f). The error bars indicate the means ± SEMs. *p < 0.05; **p < 0.01; ****p < 0.0001
Fig. 7.
The mechanism of the in vitro coculture assay is dependent on direct interactions between ICRs and ICLs. Before coculture, CD8+ T cells enriched for 20 days were preincubated with isotype control or anti-TIGIT antibodies for 20 min in a 37 °C incubator. Preincubated CD8+ T cells were cocultured with the PVR−/− HCC4006 cell line for 6 h at a ratio of 1:1 under restimulation with the anti-CD3 antibody (20 μg/ml, plate-coated), the anti-CD28 antibody (5 μg/ml, plate-coated), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml). a Graphical explanation of the validation of the in vitro coculture assay results. b Representative FACS plot of cytokine-secreting TIGIT+CD8+ T cells cocultured with the WT and PVR−/− HCC4006 cell lines. c Rate of increase in TIGIT+CD8+ T cell function induced by anti-TIGIT antibody treatment after coculture with the WT and PVR−/− HCC4006 cell lines. The experiment was performed with hPBMCs from three donors. The data are representative of triplicate samples from a single donor. The data were analyzed by two-tailed unpaired Student’s t-test (c). The error bars indicate the means ± SEMs. **p < 0.01
Discussion
ICIs are effective in restoring the functionality of TI CD8+ T cells, thereby enhancing antitumor immunity in various tumor types [3–5, 9, 11–14]. Many companies develop their own ICIs, but currently available in vitro assays for evaluating the efficacy of ICIs have several limitations [26, 27]. Because of the limitations of current in vitro assays, the development of a standard and consistent in vitro assay is needed. We developed an in vitro coculture assay with CD8+ T cells enriched from hPBMCs of healthy donors and engineered human cancer cell lines. Without using magnetic beads, we enriched CD8+ T cells from hPBMCs of healthy donors under low-dose TCR stimulation (Fig. 1). The proportion of CD8+ T cells was increased and that of CD4+ T cells was decreased after culture progression (Fig. 1b and d). Fourteen days after culture initiation, CD8+ T cells heterogeneously expressed ICRs (Fig. 2c and d). These enriched CD8+ T cells were also mainly composed of EM and naïve CD8+ T cells (Supplementary Fig. 3). These result indicated that naïve CD8+ T cells were preferentially expanded although EM CD8 + T cells were most abundant among cultured hPBMCs (Supplementary Fig. 3). Additionally, the enriched CD8+ T cells were functional, and their functionality was dependent on the expression of ICRs (Fig. 3). We evaluated the efficacy of ICIs through coculture of these CD8+ T cells with HCC4006 cell lines (Fig. 4). As we demonstrated that the functionality of the enriched CD8+ T cells was regulated by the expression of ICRs (Fig. 3), we manipulated ICL expression in HCC4006 cells. CD8+ T cells cocultured with ICL KO HCC4006 cell lines were more functional than those cocultured with the WT HCC4006 cell line (Fig. 5). We next evaluated the efficacy of ICIs by using the appropriate ICL KO HCC4006 cell lines and found that the efficacy of ICIs can be evaluated more accurately by using ICL KO HCC4006 cell lines and that the mechanism of this in vitro coculture assay was based on direct interactions between ICRs and ICLs (Fig. 6 and Fig. 7). In this study, we examined the efficacy of two representative antibodies—an anti-TIM-3 antibody and an anti-TIGIT antibody—by using PD-L1−/−PVR−/− and PD-L1−/− HCC4006 cell lines, respectively (Fig. 6).
In terms of CD8+ T cell enrichment, this method was more suitable than existing protocols. When we cultured hPBMCs with anti-CD3/CD28 dynabeads and hIL-2, CD4+ T cells were more preferentially expanded than CD8+ T cells (Supplementary Fig. 1). Additionally, using hPBMCs of human cancer patients could be more clinically relevant than using those of healthy donors. However, it was difficult to expand and enrich CD8+ T cells by using hPBMCs from cancer patients (Supplementary Fig. 2). Therefore, using the CD8+ T cell enrichment protocol introduced here was superior to the existing protocols for CD8+ T cell enrichment.
This method can be used to evaluate the efficacy of newly developed ICIs and select the best candidate. Additionally, this method can be applied to examine the efficacy of ICIs targeting newly discovered ICRs. In evaluating the efficacy of ICIs targeting newly identified ICRs, several factors must be considered. An ICL of a specific ICR should be well documented. However, the counterpart ICLs of some ICRs have not yet been identified (e.g., B7-H3) [32]. Because this method is dependent on direct interactions between ICRs and ICLs, identification of ICLs of newly discovered ICRs is crucial for evaluating the efficacy of ICIs. Additionally, the cancer cell lines used should express the counterpart ICL of the ICR. Moreover, optimal conditions for ICR expression on enriched CD8+ T cells should be developed. If these prerequisites are satisfied, this method is helpful for evaluating the efficacy of ICIs and selecting the best candidate.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figure 1 Comparison between the CD8+ T cell enrichments by the existing protocol and the newly developed protocol. hPBMCs were isolated from the peripheral blood of healthy donors. For the existing protocol using αCD3/CD28 dynabeads, isolated hPBMCs were cultured with αCD3/CD28 dynabeads (the ratio of 1:1) and hIL-2 (10 ng/ml). For the newly developed protocol using soluble anti-CD3 antibodies, isolated hPBMCs were cultured with anti-CD3 antibodies (1 μg/ml, soluble), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml) in non-tissue 24-well culture plates. (a) Representative FACS plot of singlets, lymphocytes, live cells and T cells of the cultured hPBMCs for 15 days according to the indicated protocol. The experiment was performed with hPBMCs from three donors. The data are representative of a single donor. (PDF 436 KB)
Supplementary Figure 2 Comparison between the CD8+ T cell enrichments by using hPBMCs of healthy donors and cancer patients. hPBMCs were isolated from the peripheral blood of healthy donors, a single patient with NSCLC, and a single patient with HNSCC. Isolated hPBMCs were cultured with anti-CD3 antibodies (1 μg/ml, soluble), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml) in non-tissue 24-well culture plates. (a) Representative FACS plot of the T cell frequency at the indicated time point after culture initiation. (b) Kinetics of the number of total cells among the cultured hPBMCs. (c) Kinetics of the frequency of T cells, and the number of T cells among the cultured hPBMCs. The experiment was performed with hPBMCs from healthy donors, a single patient with NSCLC, and a single patient with HNSCC. (PDF 332 KB)
Supplementary Figure 3 Naïve/memory phenotype of enriched CD8+ T cells. hPBMCs were isolated from the peripheral blood of healthy donors. Isolated hPBMCs were cultured with anti-CD3 antibodies (1 μg/ml, soluble), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml) in non-tissue 24-well culture plates. (a) Representative FACS plot of naïve/memory phenotype of cultured CD8+ T cells at the indicated time point after culture initiation. (b) Quantification of the percentage of effector memory (EM), effector memory expressing CD45RA (EMRA), central memory (CM), and naïve CD8+ T cells at the indicated time point after culture initiation. (c) Representative FACS plot of the ICR expression patterns of CD8+ T cells enriched 15 days according to naïve/memory phenotype. (d) Representative FACS plot of naïve/memory CD8+ T cell frequency in PD-1+ and PD-1-CD8+ T cells at 15 days after culture initiation (left). Quantification of the percentage of naïve/memory CD8+ T cell frequency in PD-1+ and PD-1-CD8+ T cells at 15 days after culture initiation (right). The experiment was performed with hPBMCs from two donors. The data are representative of triplicate samples from a single donor. The data were analyzed by two-tailed unpaired Student’s t-test (b, d). The error bars indicate the means ± SEMs. **p < 0.01; ****p < 0.0001. (PDF 938 KB)
Supplementary Figure 4 Degranulation in ICR-positive CD8+ T cells was augmented by ICIs. Before coculture, CD8+ T cells enriched for 21 days were preincubated with isotype control or ICR-blocking antibodies for 20 min in a 37°C incubator. Preincubated CD8+ T cells were cocultured with HCC4006 cell lines for 6 hr at a ratio of 1:1 under restimulation with the anti-CD3 antibody (20 μg/ml, plate-coated), the anti-CD28 antibody (5 μg/ml, plate-coated), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml). (a) Representative FACS plots of cytokine secretion and degranulation in PD-1+CD8+ T cells cultured with and without anti-PD-1 antibodies. (b) Rate of increase in PD-1+CD8+ T cell degranulation induced by anti-PD-1 antibody treatment. (c) Representative FACS plots of cytokine secretion and degranulation in TIGIT+CD8+ T cells cultured with and without anti-TIGIT antibodies. (d) Rate of increase in TIGIT+CD8+ T cell degranulation induced by anti-TIGIT antibody treatment. The data were concatenated in each group (a, c). The data were analyzed by two-tailed unpaired Student’s t-test (b,d). The error bars indicate the means ± SEMs. **p < 0.01; ****p < 0.0001. (PDF 496 KB)
Supplementary Figure 5 Comparison between the increased cytokine secretion in TIGIT+CD8+ T cells in the ratio of 1:1 and 0.1:1. Before coculture, CD8+ T cells enriched for 21 days were preincubated with isotype control or TIGIT-blocking antibodies for 20 min in a 37°C incubator. Preincubated CD8+ T cells were cocultured with HCC4006 cell lines for 6 hr at a ratio of 1:1 and 0.1:1 under restimulation with the anti-CD3 antibody (20 μg/ml, plate-coated), the anti-CD28 antibody (5 μg/ml, plate-coated), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml). (a) Representative FACS plot of cytokine secreting-TIGIT+CD8+ T cells cultured with and without anti-TIGIT antibodies in the ratio of 1:1 and 0.1:1. (b) Rate of increase in TIGIT+CD8+ T cell function induced by anti-TIGIT antibodies in the ratio of 1:1 and 0.1:1. (c) Representative FACS plot of degranulation in TIGIT+CD8+ T cells cultured with and without anti-TIGIT antibodies in the ratio of 1:1 and 0.1:1. (d) Rate of increase in TIGIT+CD8+ T cell degranulation induced by anti-TIGIT antibodies in the ratio of 1:1 and 0.1:1. The data were concatenated in each group (a, c). The data were analyzed by two-tailed unpaired Student’s t-test (b,d). The error bars indicate the means ± SEMs. ***p < 0.001; ****p < 0.0001. (PDF 490 KB)
Abbreviations
- CRISPR
Clustered regularly interspaced short palindromic repeats
- Cas9
CRISPR-associated protein 9
- hPBMC
Human peripheral blood mononuclear cell
- ICI
Immune checkpoint inhibitor
- ICL
Immune checkpoint ligand
- ICR
Immune checkpoint receptor
- TI
Tumor-infiltrating
Author contributions
MJK and S-JH designed and interpreted the study, wrote the manuscript and edited the manuscript. MJK performed the experiments and analyzed the data. KHH and BRL assisted with experiments. S-JH supervised the study. All authors approved the final version of the article, including the authorship list.
Funding
The authors declare no competing financial interests. This study was supported by grants funded by the Ministry of Food and Drug Safety (18182MFDS408) and the Ministry of Science and ICT (MSIT) (2017R1A5A1014560, 2019M3A9B6065221). This study was also supported by Korean Health Technology R&D Project through the Korean Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare (HV20C0144) and Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (HN21C1410). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
All data generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Ethical approval
The studies were approved by the Institutional Review Board of Yonsei University Severance Hospital with IRB no. 4-2016-0788 for a single patient with NSCLC and a single patient with HNSCC. All patients who participated in these studies provided written informed consent prior to enrollment and sample collection at Yonsei University Severance Hospital. The research conformed to the principles of the Helsinki Declaration.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauvin JM, Pagliano O, Fourcade J, et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci USA. 2017;114:4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thommen DS, Koelzer VH, Herzig P, et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24:994–1004. doi: 10.1038/s41591-018-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emens LA, Ascierto PA, Darcy PK, Demaria S, Eggermont AMM, Redmond WL, Seliger B, Marincola FM. Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer. 2017;81:116–129. doi: 10.1016/j.ejca.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JE, Patel MA, Mangraviti A, et al. Combination therapy with anti-PD-1, Anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res. 2017;23:124–136. doi: 10.1158/1078-0432.CCR-15-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung AL, Maxwell R, Theodros D, et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology. 2018;7:e1466769. doi: 10.1080/2162402X.2018.1466769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris-Bookman S, Mathios D, Martin AM, et al. Expression of LAG-3 and efficacy of combination treatment with anti-LAG-3 and anti-PD-1 monoclonal antibodies in glioblastoma. Int J Cancer. 2018;143:3201–3208. doi: 10.1002/ijc.31661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yonesaka K, Haratani K, Takamura S, et al. B7–H3 negatively modulates CTL-mediated cancer immunity. Clin Cancer Res. 2018;24:2653–2664. doi: 10.1158/1078-0432.CCR-17-2852. [DOI] [PubMed] [Google Scholar]
- 16.Lee YH, Martin-Orozco N, Zheng P, et al. Inhibition of the B7–H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27:1034–1045. doi: 10.1038/cr.2017.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38:255. doi: 10.1186/s13046-019-1259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamphorst AO, Wieland A, Nasti T, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui E, Cheung J, Zhu J, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan O, Giles JR, McDonald S, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han HS, Jeong S, Kim H, et al. TOX-expressing terminally exhausted tumor-infiltrating CD8(+) T cells are reinvigorated by co-blockade of PD-1 and TIGIT in bladder cancer. Cancer Lett. 2021;499:137–147. doi: 10.1016/j.canlet.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 22.Kim K, Park S, Park SY, et al. Single-cell transcriptome analysis reveals TOX as a promoting factor for T cell exhaustion and a predictor for anti-PD-1 responses in human cancer. Genome Med. 2020;12:22. doi: 10.1186/s13073-020-00722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 24.Kim KH, Cho J, Ku BM, et al. The first-week proliferative response of peripheral blood PD-1(+)CD8(+) T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin Cancer Res. 2019;25:2144–2154. doi: 10.1158/1078-0432.CCR-18-1449. [DOI] [PubMed] [Google Scholar]
- 25.Kim KH, Hur JY, Koh J, et al. Immunological characteristics of hyperprogressive disease in patients with non-small cell lung cancer treated with anti-PD-1/PD-L1 abs. Immune Netw. 2020;20:e48. doi: 10.4110/in.2020.20.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–856. doi: 10.1158/2326-6066.CIR-14-0040. [DOI] [PubMed] [Google Scholar]
- 28.Kwon M, Choi YJ, Sa M, Park SH, Shin EC. Two-round mixed lymphocyte reaction for evaluation of the functional activities of Anti-PD-1 and Immunomodulators. Immune Netw. 2018;18:e45. doi: 10.4110/in.2018.18.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mujib S, Jones RB, Lo C, Aidarus N, Clayton K, Sakhdari A, Benko E, Kovacs C, Ostrowski MA. Antigen-independent induction of Tim-3 expression on human T cells by the common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway. J Immunol. 2012;188:3745–3756. doi: 10.4049/jimmunol.1102609. [DOI] [PubMed] [Google Scholar]
- 30.Zhang ZN, Zhu ML, Chen YH, Fu YJ, Zhang TW, Jiang YJ, Chu ZX, Shang H. Elevation of Tim-3 and PD-1 expression on T cells appears early in HIV infection, and differential Tim-3 and PD-1 expression patterns can be induced by common gamma -chain cytokines. Biomed Res Int. 2015;2015:916936. doi: 10.1155/2015/916936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin MD, Badovinac VP. Defining memory CD8 T cell. Front Immunol. 2018;9:2692. doi: 10.3389/fimmu.2018.02692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picarda E, Ohaegbulam KC, Zang X. Molecular pathways: targeting B7–H3 (CD276) for human cancer immunotherapy. Clin Cancer Res. 2016;22:3425–3431. doi: 10.1158/1078-0432.CCR-15-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Comparison between the CD8+ T cell enrichments by the existing protocol and the newly developed protocol. hPBMCs were isolated from the peripheral blood of healthy donors. For the existing protocol using αCD3/CD28 dynabeads, isolated hPBMCs were cultured with αCD3/CD28 dynabeads (the ratio of 1:1) and hIL-2 (10 ng/ml). For the newly developed protocol using soluble anti-CD3 antibodies, isolated hPBMCs were cultured with anti-CD3 antibodies (1 μg/ml, soluble), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml) in non-tissue 24-well culture plates. (a) Representative FACS plot of singlets, lymphocytes, live cells and T cells of the cultured hPBMCs for 15 days according to the indicated protocol. The experiment was performed with hPBMCs from three donors. The data are representative of a single donor. (PDF 436 KB)
Supplementary Figure 2 Comparison between the CD8+ T cell enrichments by using hPBMCs of healthy donors and cancer patients. hPBMCs were isolated from the peripheral blood of healthy donors, a single patient with NSCLC, and a single patient with HNSCC. Isolated hPBMCs were cultured with anti-CD3 antibodies (1 μg/ml, soluble), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml) in non-tissue 24-well culture plates. (a) Representative FACS plot of the T cell frequency at the indicated time point after culture initiation. (b) Kinetics of the number of total cells among the cultured hPBMCs. (c) Kinetics of the frequency of T cells, and the number of T cells among the cultured hPBMCs. The experiment was performed with hPBMCs from healthy donors, a single patient with NSCLC, and a single patient with HNSCC. (PDF 332 KB)
Supplementary Figure 3 Naïve/memory phenotype of enriched CD8+ T cells. hPBMCs were isolated from the peripheral blood of healthy donors. Isolated hPBMCs were cultured with anti-CD3 antibodies (1 μg/ml, soluble), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml) in non-tissue 24-well culture plates. (a) Representative FACS plot of naïve/memory phenotype of cultured CD8+ T cells at the indicated time point after culture initiation. (b) Quantification of the percentage of effector memory (EM), effector memory expressing CD45RA (EMRA), central memory (CM), and naïve CD8+ T cells at the indicated time point after culture initiation. (c) Representative FACS plot of the ICR expression patterns of CD8+ T cells enriched 15 days according to naïve/memory phenotype. (d) Representative FACS plot of naïve/memory CD8+ T cell frequency in PD-1+ and PD-1-CD8+ T cells at 15 days after culture initiation (left). Quantification of the percentage of naïve/memory CD8+ T cell frequency in PD-1+ and PD-1-CD8+ T cells at 15 days after culture initiation (right). The experiment was performed with hPBMCs from two donors. The data are representative of triplicate samples from a single donor. The data were analyzed by two-tailed unpaired Student’s t-test (b, d). The error bars indicate the means ± SEMs. **p < 0.01; ****p < 0.0001. (PDF 938 KB)
Supplementary Figure 4 Degranulation in ICR-positive CD8+ T cells was augmented by ICIs. Before coculture, CD8+ T cells enriched for 21 days were preincubated with isotype control or ICR-blocking antibodies for 20 min in a 37°C incubator. Preincubated CD8+ T cells were cocultured with HCC4006 cell lines for 6 hr at a ratio of 1:1 under restimulation with the anti-CD3 antibody (20 μg/ml, plate-coated), the anti-CD28 antibody (5 μg/ml, plate-coated), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml). (a) Representative FACS plots of cytokine secretion and degranulation in PD-1+CD8+ T cells cultured with and without anti-PD-1 antibodies. (b) Rate of increase in PD-1+CD8+ T cell degranulation induced by anti-PD-1 antibody treatment. (c) Representative FACS plots of cytokine secretion and degranulation in TIGIT+CD8+ T cells cultured with and without anti-TIGIT antibodies. (d) Rate of increase in TIGIT+CD8+ T cell degranulation induced by anti-TIGIT antibody treatment. The data were concatenated in each group (a, c). The data were analyzed by two-tailed unpaired Student’s t-test (b,d). The error bars indicate the means ± SEMs. **p < 0.01; ****p < 0.0001. (PDF 496 KB)
Supplementary Figure 5 Comparison between the increased cytokine secretion in TIGIT+CD8+ T cells in the ratio of 1:1 and 0.1:1. Before coculture, CD8+ T cells enriched for 21 days were preincubated with isotype control or TIGIT-blocking antibodies for 20 min in a 37°C incubator. Preincubated CD8+ T cells were cocultured with HCC4006 cell lines for 6 hr at a ratio of 1:1 and 0.1:1 under restimulation with the anti-CD3 antibody (20 μg/ml, plate-coated), the anti-CD28 antibody (5 μg/ml, plate-coated), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml). (a) Representative FACS plot of cytokine secreting-TIGIT+CD8+ T cells cultured with and without anti-TIGIT antibodies in the ratio of 1:1 and 0.1:1. (b) Rate of increase in TIGIT+CD8+ T cell function induced by anti-TIGIT antibodies in the ratio of 1:1 and 0.1:1. (c) Representative FACS plot of degranulation in TIGIT+CD8+ T cells cultured with and without anti-TIGIT antibodies in the ratio of 1:1 and 0.1:1. (d) Rate of increase in TIGIT+CD8+ T cell degranulation induced by anti-TIGIT antibodies in the ratio of 1:1 and 0.1:1. The data were concatenated in each group (a, c). The data were analyzed by two-tailed unpaired Student’s t-test (b,d). The error bars indicate the means ± SEMs. ***p < 0.001; ****p < 0.0001. (PDF 490 KB)
Data Availability Statement
All data generated and analyzed during the current study are available from the corresponding author on reasonable request.