Abstract
Treatment with immune checkpoint inhibitors (ICIs) can be complicated by cardiovascular toxicity, including pericardial disease. To date, no prospective studies specifically investigated the optimal treatment of ICI-associated pericardial disease, and the available evidence is based on case reports and series only. We performed a systematic review of case reports and series including 20 publications for a total of 28 cases of ICI-associated pericardial disease. In this review, pericardial disease was reversible in the majority of cases (75%), although 2 deaths were reported. The majority of cases were life-threatening (G4, 53.6%) or severe (G3, 21.4%), requiring pericardiocentesis. Higher rates of improvement were associated with administration of corticosteroids (86.7% vs 61.5%), presence of other immune-related adverse events (90.9% vs. 64.7%), and non-malignant effusions (86.7% vs 42.8%). ICIs were discontinued in the majority of cases and then restarted in 7 patients with no recurrence of pericardial disease. Based on these results, ICI-associated G3–G4 pericardial disease as well as G2 pericardial disease with moderate–severe effusion should be treated with ICIs discontinuation and high-dose steroids, also performing pericardiocentesis, pericardial drainage or pericardial window in case of cardiac tamponade. For G2 with small effusion or G1 pericardial disease, ICIs might be continued and colchicine or NSAIDs could be considered. For patients requiring ICIs discontinuation, a rechallenge with ICIs seems to be feasible after resolution or meaningful improvement of pericardial disease.
Keywords: Pericarditis, Pericardial effusion, Cardiac tamponade, Cardiotoxicity, Immune checkpoint inhibitors, Rechallenge
Introduction
Over the last decade, immune checkpoint inhibitors (ICIs) have revolutionized the treatment of cancer and extended survival across several tumor types [1]. ICIs are monoclonal antibodies directed against immune checkpoint proteins such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1) or programmed death-ligand 1 (PD-L1), that are involved in key negative regulatory pathways of the immune system [2]. ICIs work by unleashing the brakes on the immune system, thus eliciting an anti-tumor immune response. On the other hand, they may also induce immune-related adverse events (irAEs) that potentially involve any organ or system, including the cardiovascular system [3, 4].
Pericardial disease in the form of pericarditis, pericardial effusion or cardiac tamponade represents one of the clinical manifestations of ICI-associated cardiovascular toxicity [5]. An observational retrospective pharmacovigilance study reported 95 cases of ICI-related pericardial disease. In this study, pericardial disease had a median time to onset of 30 days (IQR 9–90) and it was severe in 81% of cases with a fatality rate of 21% [6]. The exact incidence of ICI-associated pericardial disease is unknown. In a single-institution retrospective study on 2,830 patients treated with ICIs, pericardial toxicity was uncommon with an incidence rate of approximately 0.1% [7], but a retrospective Italian study reported a non-negligible 6.7% incidence of pericardial effusion among 60 patients treated with ICIs for non-small cell lung cancer (NSCLC) [8].
Although several well-established guidelines on irAEs are available, no recommendation is specifically provided for the management of pericardial disease [9, 10]. Since there are no prospective studies investigating the optimal management of ICI-associated pericardial disease, the available evidence is limited to case reports and series. We performed a systematic review of case reports with the aim to assess the available evidence on the treatment of ICI-associated pericardial disease.
Materials and methods
Published studies were identified by searching PubMed until May 2020 for the following combination of terms: (‘Neoplasms’ OR ‘cancer’ OR ‘tumor’) AND (‘checkpoint inhibitors’ OR ‘checkpoint blockade’ OR ‘checkpoint inhibition’ OR ‘anti-CTLA4′ OR ‘anti-PD1′ OR anti-PDL1′ OR ‘nivolumab’ OR ‘pembrolizumab’ OR ‘ipilimumab’ OR ‘atezolizumab’ OR ‘durvalumab’ OR avelumab’ OR ‘cemiplimab’) AND (‘pericarditis’ OR ‘pericardial toxicity’ OR ‘pericardial effusion’ OR ‘cardiac tamponade’ OR ‘cardiac toxicity’ OR ‘cardiac adverse event’ OR ‘cardiac irAE’ OR ‘cardiac complication’ OR cardiac side-effect’). Three reviewers (A.I., G.M., A.C.) independently performed the study selection and data extraction. Case reports or case series of pericardial disease in cancer patients receiving ICIs were included if they reported data on the treatment and outcome related to pericardial complication. Severity of pericardial disease was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0 [11], as reported in Table 1. When not specifically stated by the authors, NCI-CTCAE grading was inferred based on clinical information. Associations among categorical variables were evaluated by the Fisher’s exact test.
Table 1.
NCI-CTCAE v. 5.0 grading of pericardial disease
| CTCAE term | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Pericardial effusion | – | Asymptomatic effusion size small to moderate | Effusion with physiologic consequences | Life-threatening consequences; urgent intervention indicated | Death |
| Pericardial tamponade | – | – | – | Life-threatening consequences; urgent intervention indicated | Death |
| Pericarditis | Asymptomatic, ECG or physical findings (e.g., rub) consistent with pericarditis | Symptomatic pericarditis (e.g., chest pain) | Pericarditis with physiologic consequences (e.g., pericardial constriction) | Life-threatening consequences; urgent intervention indicated | Death |
Results
The search strategy identified 116 records. An additional publication was included from authors’ knowledge. All records were screened by abstract review, and 20 publications including 28 cases of pericardial disease were selected (Fig. 1) [7, 12–30]. A descriptive summary of the 28 cases is reported in Table 2.
Fig. 1.
PRISMA flow diagram
Table 2.
Cases of pericardial disease in cancer patients treated with ICIs
| Author [Ref] | Age, sex | Cancer | ICI | Pericardial event (grading)^ | Time to onset | Cardiac imaging | Best response to ICI | Cancer cells in pericardial fluid cytology/tissue biopsy | Additional data on pericardial fluid cytology/tissue biopsy | Associate myocarditis | ICI discontinuation | Treatment | Outcome | ICI rechallenge | Pericardial disease recurrence after rechallenge | Associated irAEs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altan [12] | 73, M | nSqNSCLC | Anti-CTLA4 + Anti-PD1, NOS | Cardiac tamponade (G4) | 78 days | NR | PR | No | Diffuse fibrinous pericarditis with inflammatory infiltrate | Small collections of lymphocytes within myocardium | Yes | Resuscitation | Death (cardiac arrest) | N/A | N/A | None |
| Altan [12] | 65, F | nSqNSCLC | Anti-PD1/PDL1, NOS | Cardiac tamponade (G4) | 131 days | NR | PR | No |
Fibrinous pericarditis with mild chronic lymphocytic infiltrate |
NR | Yes |
Pericardial window Pacemaker (due to arrhythmias) |
Death | N/A | N/A | Hypothyroidism |
| Altan [12] | 57, M | nSqNSCLC | Anti-PD1/PDL1, NOS | Cardiac tamponade (G4) | 98 days | NR | SD | No |
Fibrosis, hemorrhage, edema, moderate lymphoplasmacytic infiltrate, and fibrinous exudate |
NR | Yes | Pericardial window | Recovery | Yes | No | None |
| Asai [13] | 62, M | nSqNSCLC | Nivolumab | Cardiac tamponade (G4) | 1 cycle (7 days) | CT scan | PR | Yes | NR | NR | Yes | Pericardiocentesis | Recurrent effusion (6 weeks after 1st pericardiocentesis); treated with second pericardiocentesis and intrapericardial bleomycin | Yes (after recurrence) | No | None |
| Atallah-Yunes [14] | 66, F | SqNSCLC | Pembrolizumab | Pericardial effusion (G3) | 1 cycle (10 days) | TTE | Not assessed | No | exudative effusion with lymphocytic predominance | NR | Yes |
Pericardiocentesis Steroid (prednisone 60 mg/day) |
Recovery (within 5 days after starting steroids) | No | N/A | NR |
| Chahine [7] | 70, M | nSqNSCLC | Nivolumab |
Acute pericarditis/ Pericardial effusion (G2) |
13 weeks | TTE | SD | N/A | N/A | NR | Yes | Steroid (prednisone 75 mg/day) | Recovery | Yes | No | None |
| Chahine [7] | 60, F | nSqNSCLC | Nivolumab + THU-decitabine (clinical trial) |
Acute pericarditis /Pericardial effusion (G2) |
9 weeks | TTE | NR | N/A | N/A | NR | Yes | Colchicine/Ibuprofen | Recovery | No | N/A | None |
| Clahine [7] | 58,M | NSCLC | Nivolumab | Pericardial effusion (G2) | 10 weeks | TTE | NR | N/A | N/A | NR | Yes | Steroid (prednisone 80 mg/day) | Recovery | No | N/A | Pneumonitis |
| Chu [15]¶ | 59, M | nSqNSCLC | Nivolumab | Cardiac tamponade (G4) | 3 cycles (6 weeks) | CT scan | PR | No | Granulomatous inflammation and acid-fast bacilli (culture of pericardial effusion positive for M. tuberculosis) | NR | Yes |
Pericardiocentesis¶ Prednisone 1 mg/kg Anti-tubercular treatment† |
Recovery (within 1 month) | Yes (only 1 cycle skipped) | No | NR |
| Dasanu [16] | 65, F | Melanoma | Ipilimumab | Cardiac tamponade (G4) | 4 cycle (9 months, i.e., 6 months after ipilimumab completion) | Chest X-Ray, CT scan, TTE | CR | No | Lymphocytic pericarditis and reactive mesothelial cells | NR | N/A |
Pericardiocentesis Steroids (methylprednisolone 60 mg × 4/day) |
Recovery (within 2 weeks) | No | N/A |
Hyperthyroidism, transaminitis, skin rash, arthritis |
| de Almeida [17] | 69, M | nSqNSCLC | Nivolumab | Pericarditis/Pericardial effusion (G3) | 24 cycle (12 months) | CT scan, TTE | PR | No | Nonspecific chronic inflammation with extensive fibrosis and lymphocyte infiltration | NR | Yes |
Pericardiocentesis and pericardial window Steroids (prednisone 1 mg/kg/day to prevent constrictive pericarditis) |
Recovery with pericardial window (3 days after the onset) | No | N/A | Hypothyroidism |
| Dhenin [18] | 79, F | nSqNSCLC | Pembrolizumab | Pericardial effusion (G2) | 3 cycles (10 weeks) | CT scan, TTE | CR | Not assessed | NR | NR | No | Steroids (methylprednisolone 16 mg/day) | Recovery (within 7 days) | Yes | No (ICI discontinued after 22 weeks due to colitis) | Skin rash, Colitis, myasthenia gravis |
| Khan [19] | 62, M | SqNSCLC | Pembrolizumab | Cardiac tamponade (G4) | 5 cycles (15 weeks) | CT scan, TTE | PR | No | Reactive mesothelial and chronic inflammatory cells | NR | Yes |
Pericardiocentesis Steroids (prednisone 1 mg/kg/day) |
Improvement (within 6 days) | No | N/A | NR |
| Kolla [20] | 46, M | SCLC | Nivolumab | Cardiac tamponade (G4) | 9 weeks | NR | PR | Yes | 5% lymphocytes in pericardial fluid analysis | NR | No | Pericardiocentesis | Recovery with pericardiocentesis | N/A | N/A | NR |
| Kolla [20] | 54, F | nSqNSCLC | Nivolumab | Cardiac tamponade (G4) | 7 weeks | NR | PR | Yes |
30% lymphocytes in pericardial fluid Analysis |
NR | Yes |
Pericardiocentesis Steroids (prednisone 20–60 mg/day) |
Recurrent effusions | No | N/A | NR |
| Kushnir [21] | 67, M | SqNSCLC | Nivolumab | Cardiac tamponade (G4) | 5 cycles (10 weeks) | CT scan, TTE | PR | No | leukocytes | NR | Yes |
Pericardiocentesis Steroids (prednisone 30 mg/day) |
Improvement | No | N/A | NR |
| Nesfeder [22] | 64, M | nSqNSCLC | Nivolumab | Cardiac tamponade (G4) | 3 months | TTE | NR | No |
Focal mild acute inflammation, mild fibrosis, and scattered atypical cells |
NR | NR |
Pericardiocentesis Pericardial window |
Improvement (10 days) | NR | NR | Hypothyroidism, pneumonia |
| Oristrell [23] | 55, F | Breast cancer | Pembrolizumab (neoadjuvant) | Pericardial effusion (G3) | 12 months (6 months after end of ICI treatment) | TTE | N/A | No |
Exudate with abundant cellularity and a predominant acute inflammatory component (93% neutrophils) |
NR | N/A |
Pericardiocentesis Pericardiectomy Noradrenaline (for refractory hypotension) Steroids (drug not stated, 2 mg/kg/day) |
Recovery (rapid improvement with steroids) | N/A | N/A | Adrenal insufficiency, hypophysitis, hypothyroidism |
| Saade [24] | 58, F | nSqNSCLC | Nivolumab | Pericardial effusion (G3) | 4 cycles (8 weeks) | Chest X-Ray, CT scan, TTE | PD | No |
Hemorrhagic and discretely inflammatory cytology; small reactive T-lymphocytes predominantly CD4 + at pericardial biopsy |
NR | Yes |
Pericardiocentesis Steroids (dose not reported)* |
Improvement (rapidly after pericardiocentesis and steroids) | No | - | NR |
| Saade [24] | 65, M | nSqNSCLC | Nivolumab | Pericardial effusion (G4) | 35 cycles (71 weeks) | Chest X-Ray, TTE | PR | No |
hemorrhagic and inflammatory cytology; pericardial hyperplasia with T-lymphocyte infiltrate, mostly CD4 + |
NR | Yes |
Surgical drainage Steroids (dose not reported)* |
Improvement | Yes (16 months after onset) | No | Colitis |
| Saade [24] | 55, F | nSqNSCLC | Nivolumab | Pericardial effusion (G2) | 3 cycles (6 weeks) | CT scan, TTE | PD | Not assessed | Not assessed | NR | Yes | None* | Improvement (after ICI discontinuation) | No | N/A | Colitis |
| Shaheen [25] | 70, F | nSqNSCLC | Nivolumab | Pericardial effusion (G3) | 1 cycle (4 days) | TTE | NR | Not assessed | Not assessed | NR | No |
Colchicine Steroids (prednisone 1 mg/kg/day) |
Resolution (in 5 weeks). Recurrence (1 week after steroids were stopped): nivolumab was discontinued, and steroids restarted (prednisone 1 mg/kg/day), with resolution of recurrent pericardial effusion (in 3 weeks) | Yes (with low-dose steroids) | No | NR |
| Tachihara [26] | 70, M | nSqNSCLC | Pembrolizumab | Cardiac tamponade (G4) | 3 cycles (9 weeks) | Chest X-ray, CT scan, TTE | PR | Yes | NR | NR | No | Pericardiocentesis | Recovery with pericardiocentesis | N/A | N/A | NR |
| Vittorio [27] | 71, M | nSqNSCLC | Nivolumab | Pericardial effusion (G2) | 3 cycles (5 weeks) | TTE | PR | Yes | NR | NR | No | Pericardiocentesis, pericardial window | Recurrent effusion after 6 weeks (nivolumab permanently interrupted after recurrence) | N/A | N/A | NR |
| Yamasaki [28] | 65, M | nSqNSCLC | Nivolumab | Cardiac tamponade (G4) | 4 cycles (8 weeks) | Chest X-ray, TTE | PR | Yes | WBC: 3025/μL, 84% lymphocytes | No | No (discontinued after 5 more cycles due do PD) | Pericardiocentesis | Improvement (with pericardiocentesis) | N/A | N/A | None |
| Yamasaki [28] | 71, M | nSqNSCLC | Nivolumab | Cardiac tamponade (G4) | 2 cycles (4 weeks) | Chest X-ray, CT scan, TTE | PD | Yes | WBC: 756/μL, 3% lymphocytes | No | Yes | Pericardiocentesis | Recurrent effusion after 1 month | No | N/A | None |
| Yun [29] | 59, M | Melanoma | Ipilimumab | Pericardial effusion (G3) | 4 cycles (6 months, i.e., 3 months after ipilimumab completion) | CT scan, TTE | NR | No | fibrinous pericarditis | NR | N/A |
Pericardiocentesis Indomethacin Steroids (methylprednisolone 125 mg/day) |
Recovery | N/A | N/A | Skin rash, hypothyroidism, adrenal insufficiency, diarrhea |
| Zarogoulidis [30] | 60, M | nSqNSCLC | Nivolumab | Pericarditis (unknown) | 4 months | NR | No | NR | NR | Yes |
Pericardiocentesis Steroids (methylprednisolone 16 mg × 2/day) |
Recovery | No | - | Bowel perforation |
CR, complete response; N/A, not applicable; NR, not reported; nSqNSCLC, non-squamous non-small cell lung cancer; PR, partial response; PD, progressive disease; SCLC, small cell lung cancer; SqNSCLC, squamous non-small cell lung cancer; TTE, trans-thoracic echocardiogram; WBC, white blood cells count
^Grading was defined according to NCI-CTCAE v 5.0; when the authors did not explicitly state the grade of severity, it was inferred on the basis of case description [11]
¶Pericardiocentesis was not explicitly reported by the authors but since pericardial effusion culture and pericardial biopsy were performed, in all probability pericardiocentesis or other forms of pericardial drainage/aspiration were done;
†Mycobacterium tuberculosis was found in the pericardial effusion, the case was interpreted as tubercular reactivation during therapy with immune checkpoint inhibitors, and the patient received antitubercular therapy in addition to steroids
*One of these 3 patients also received colchicine (but authors did not state who)
Main characteristics of patients are summarized in Table 3. Median time to onset of pericardial disease was 70 days (IQR 44–116) after the start of ICIs, although some cases of early toxicity occurring within the first week of treatment, or late toxicity occurring after ≥ 1 year of ICIs treatment [23, 24] or even after treatment completion [16, 23, 29], were described.
Table 3.
Characteristics of patients with pericardial disease under ICI treatment
| N = 28 | |
|---|---|
| Gender | n (%) |
| Male | 18 (64.3) |
| Female | 10 (35.7) |
| Primary cancer | n (%) |
| NSCLC | 24 (85.7) |
| SCLC | 1 (3.6) |
| Melanoma | 2 (7.1) |
| Breast cancer | 1 (3.6) |
| ICI | n (%) |
| Nivolumab | 18 (64.3) |
| Pembrolizumab | 5 (17.9) |
| Ipilimumab | 2 (7.1) |
| Anti-PD1/PDL1, NOS | 2 (7.1) |
| Anti-PD1 + Anti-CTLA4, NOS | 1 (3.6) |
| Median time of onset, day (min–max) [IQR} | 70 (4–497) [44–116] |
| Best response to ICI | n (%) |
| CR | 2 (7.1) |
| PR | 13 (46.4) |
| SD | 2 (7.1) |
| PD | 3 (10.7) |
| Not reported/not applicable | 8 (28.6) |
| Other irAEs | n (%) |
| No | 17 (60.7) |
| Yes | 11 (39.3) |
| Type of irAEs | |
| Dysthyroidism (hyper/hypo) | 6 (21.4) |
| Colitis/diarrhea/bowel perforation | 5 (17.9) |
| Skin rash | 3 (10.7) |
| Adrenal insufficiency | 2 (7.1) |
| Pneumonitis | 2 (7.1) |
| Arthritis | 1 (3.6) |
| Hypophysitis | 1 (3.6) |
| Myasthenia gravis | 1 (3.6) |
| Transaminitis | 1 (3.6) |
| Grading of pericardial disease at presentation | n (%) |
| G4 | 15 (53.6) |
| G3 | 6 (21.4) |
| G2 | 6 (21.4) |
| G1 | – |
| Unknown | 1 (3.6) |
| Malignant cells in pericardial effusion/tissue | n (%) |
| Yes | 7 (25.0) |
| No | 15 (53.6) |
| Unknown | 6 (21.4) |
| Treatment | n (%) |
| Pericardiocentesis/drainage | 19 (67.8) |
| Pericardial window | 5 (17.9) |
| Steroids | 16 (57.1) |
| Other* | 6 (21.4) |
| No treatment | 1 (3.6) |
| Outcome | n (%) |
| Death | 2 (7.1) |
| Recovery/improvement | 21 (75.0) |
| Recurrence | 5 (17.9) |
| Management of ICI | n (%) |
| Treatment already completed at the onset | 3 (10.7) |
| Continued | 6 (21.4) |
| Temporarily discontinued, then restarted | 7 (25.0) |
| Permanently discontinued | 11 (39.3) |
| Not reported | 1 (3.6) |
CR, complete response; NOS, not otherwise specified; PD, progressive disease; PR, partial response; SD, stable disease
*Other treatments included: colchicine in 2 patients; colchicine plus ibuprofen in 1 patient; indomethacin in 1 patient; intrapericardial bleomycin in 1 patient; anti-tubercular therapy in 1 patient
Among the 28 included cases, patients were mostly male (64.3%), with lung cancer (89.3%), and treated with anti-PD-1/PD-L1 as single agent (89.3%). Pericardial effusion cytology and/or pericardial biopsy was positive for malignant cells in 7 (25%) patients. Pericardial disease was severe (G3) or life-threatening (G4) in the majority of the cases (75%). No patients with G1 pericardial disease were included. Although pericardial disease was largely reversible with a recovery or improvement rate of 75%, 2 deaths (7.1%) related to this condition were reported.
Pericardiocentesis, surgical drainage or pericardial window was performed in 19 out of 21 patients (90%) presenting with G3–G4 pericardial disease, and in 1 out of 6 patients (17%) presenting with G2 pericardial disease. Overall, corticosteroids were administered in 56% of cases. The most common corticosteroid schedule was prednisone 1 mg/kg/day. Colchicine and/or non-steroidal anti-inflammatory drugs were used in a minority of cases (4 patients), obtaining recovering or improvement of pericardial disease in 3 of them (75%).
The presence of other irAEs, the absence of malignant cells in pleural effusion or pericardial biopsy, and the administration of corticosteroids were associated with higher rates of recovery or improvement, although these associations were not statistically significant (Table 4).
Table 4.
Association between characteristics of patients and pericardial disease outcome
| Characteristics | Outcome | P |
|---|---|---|
| Grade at the onset | Improvement rate*, % (n/N) | |
| G4 (n = 16) | 68.8% ( 11/16) | 1.000 |
| ≤ G3 (n = 11) | 72.2% ( 8/ 11) | |
| Best response to ICI | Improvement rate, % (n/N) | |
| CR/PR (n = 15) | 66.7% (10/15) | 1.000 |
| SD/PD (n = 5) | 80.0% ( 4/ 5) | |
| Other irAEs | Improvement rate, % (n/N) | |
| Yes (n = 11) | 90.9% ( 10/11) | 0.191 |
| No (n = 17) | 64.7% ( 11/17) | |
| Malignant effusion | Improvement rate, % (n/N) | |
| Yes (n = 7) | 42.8% ( 3/ 7) | 0.053 |
| No (n = 15) | 86.7% (13/15) | |
| Pericardiocentesis/drainage/window | Improvement rate, % (n/N) | |
| Yes (n = 21) | 76.2% (16/21) | 1.000 |
| No (n = 7) | 71.4% ( 5/ 7) | |
| Steroid therapy | Improvement rate, % (n/N) | |
| Yes (n = 15) | 86.7% (13/15) | 0.197 |
| No (n = 13) | 61.5% ( 8/13) | |
| Discontinuation of ICI | Improvement rate, % (n/N) | |
| Yes (n = 18) | 72.2% ( 13/18) | 1.000 |
| No (n = 6) | 66.7% ( 4/ 6) | |
| Rechallenge of ICI | Recurrence rate, % (n/N) | |
| Yes (n = 7) | 0% ( 0/ 7) | 0.521 |
| No (n = 11) | 18% ( 2/ 11) |
*Improvement rate defined as rate of patients with full or partial recovery of pericardial disease, with no evidence of recurrence; recurrence rate defined as rate of patients with initial resolution/improvement of pericardial disease, followed by recurrent/worsening pericardial disease
At the onset of pericardial disease, only 6 patients (21.4%) continued ICIs, whereas 18 patients (64.3%) discontinued ICIs, temporarily (7 patients) or permanently (11 patients). Interestingly, none of the 7 patients who restarted ICIs experienced recurrent pericardial disease.
Discussion
The present systematic review included the largest number of published case reports of pericardial disease in cancer patients treated with ICIs.
The exact incidence of pericardial disease in cancer patients treated with ICIs is still unknown, with reported incidence rates ranging from 0.1% to approximately 7% in different series [7, 8, 31]. This broad range is possibly due to the heterogeneity, both in terms of different grades of severity of the cases included in the studies and in terms of different distribution of potential risk factors for ICI-associated pericardial disease across the studies. Regarding severity of pericardial disease, studies that included only symptomatic patients or pericardial effusions requiring pericardiocentesis reported lower incidence rates [7, 31], whereas higher incidence rates were reported in studies that included asymptomatic patients with incidental findings of pericardial disease [8]. Regarding potential risk factors, male sex, lung cancer and treatment with anti-PD-1/PD-L1 seem to increase the risk of ICI-associated pericardial disease. In fact, in a large retrospective pharmacovigilance study, patients with pericardial disease were more often males (60%), with lung cancer (56%), and treated with anti-PD-1/PD-L1 as single agent (78%) [6]. Consistently with these reports, also the patients included in the present review were mostly male patients receiving anti-PD-1/PD-L1 for NSCLC.
Pericardial disease can be also associated with a number or anticancer agents including anthracyclines, platinum agents, alkylating agents, antimetabolites, microtubule-targeting drugs and tyrosine kinase inhibitors [32], but some retrospective evidence suggests that patients treated with ICIs have higher risk of pericardial disease when compared with patients receiving other therapies [6, 7, 31]. Particularly, in their pharmacovigilance study, Salem et al. showed a 3.8-fold increased risk of reporting pericardial disease for patients treated with ICI as compared with the full database (0.30% vs 0.08%) [6]. In a small retrospective study on NSCLC patients, the incidence of pericardial effusion among patients treated with ICIs (n = 60) was higher (6.7%) when compared with a control group of patients (n = 60) with NSCLC receiving other anticancer agents (3.3%), even when patients with contemporary pleural effusion were excluded (adjusted incidence: 3.3% vs 1.6%) [7]. Similarly, a retrospective study on 3,966 patients treated with ICIs and 82,517 patients treated with anticancer agents other than ICIs at the MD Anderson from 2015 to 2017, showed that the prevalence of hemodynamically significant pericardial effusion among patients treated with ICIs was higher than that observed among patients not receiving ICIs (0.38% vs 0.11%) [31]. Therefore, although relatively rare, pericardial disease is more frequently associated with ICIs than with other anticancer agents, and this finding may represent a relevant aspect due to the expanding role of ICIs across a number of different tumor types and, consequently, the ever-growing number of cancer patients treated with these drugs.
ICI-associated pericardial disease represents a clinically relevant problem also because it may be potentially fatal, with mortality rates ranging from approximately 7%, as reported in the present analysis, up to more than 20%, as reported in the pharmacovigilance study [6]. In our review, however, pericardial disease was reversible in the majority of cases, with an improvement rate of 75%.
ICI-associated pericardial disease is often a diagnosis of exclusion. Differential diagnosis mainly includes progression or pseudo-progression of the underlying cancer, and infectious disease [13, 15, 19]. Although the differential diagnosis between immune-related toxicity and progression/pseudoprogression of cancer is often challenging, some clinical and pathological elements may be helpful for a proper assessment. Particularly, the presence of other irAEs, the absence of malignant cells in pericardial effusion or pericardial biopsy, and/or objective response or stable disease of the other tumor sites are more suggestive for an immune-related toxicity rather than cancer progression. Interestingly, we observed that patients with other irAEs and/or without malignant effusion (i.e., those with highly suspected immune-related pericardial disease) had higher improvement rates as compared to patients without other irAEs and those with malignant effusion, respectively. When progressive cancer in patients with malignant pericardial effusion is suspected, intrapericardial injection of chemotherapy such as bleomycin could represent an option, although it was used only in 1 out of the 28 case reports included in this review [13]. Also, other possible etiologies such as infectious disease should be ruled out. Particularly, Chu et al. reported a case of pericardial tamponade caused by a hypersensitivity response to tuberculosis reactivation after ICIs. In such case, antitubercular treatment was given in addition to corticosteroids, achieving pericardial disease recovery [15].
In the present review, clinical presentation of pericardial disease was severe or life-threatening in most patients, consistently with data reported in the pharmacovigilance study, where 81% of pericardial disease were severe events [6]. However, given the retrospective nature of these evidence, it could not be excluded that asymptomatic, mild cases were under-detected or under-reported, thus leading to an overestimation of severe cases.
In most patients, pericardial disease was characterized by effusion with tamponade physiology, often requiring invasive interventions such as pericardiocentesis, surgical drainage or pericardial window. As reported above, the MD Anderson study on hemodynamically significant pericardial effusion among patients treated with ICIs revealed a low incidence rate (0.38%), but the relative risk of pericardiocentesis was significantly increased (3.1) for patients receiving ICIs when compared with those not receiving ICIs [31]. These data suggest that patients on ICIs are more likely to need pericardiocentesis, but the reason remains unclear. In our review, 21 out of 28 patients (75%) with any grade pericardial disease and 19 out of 21 patients (90%) with G3–G4 pericardial disease needed an invasive management (i.e., pericardiocentesis, surgical drainage or pericardial window) of pericardial effusion. Only 5 patients were successfully treated with a conservative approach, and all of them had a low-grade (G2) pericardial disease [7, 18, 24].
Regarding medical treatment, corticosteroids represent the cornerstone of management for most irAEs [3]. Although in the present review only slightly more than half patients (57.1%) received corticosteroids, they achieved a better improvement rate as compared to those who did not receive steroids, thus suggesting that corticosteroids have a role for the treatment of ICI-associated pericardial disease.
Guidelines of the European Society of Cardiology for the management of pericardial disease recommend the use of colchicine and aspirin or non-steroidal antiinflammatory drugs (NSAIDs) for the treatment of acute pericarditis and recurrent pericarditis, and also for the treatment of pericardial effusion when associated with systemic inflammation [33]. In the present review, however, only 5 patients received colchicine and/or NSAIDs with or without corticosteroids, achieving complete recovery in 4 cases, and initial improvement followed by effusion recurrence in 1 case. Due to the paucity of data, definitive conclusion on the role of colchicine and NSAIDs for ICIs-associated pericardial disease cannot be drawn, but they may possibly have a role for the management of mild–moderate cases.
Among the 28 case reports included in our review, the majority of patients discontinued ICIs. After recovery, treatment was then restarted in 7 patients with no recurrence or worsening of the pericardial disease. This finding suggests that a rechallenge of ICIs after recovery of pericardial disease may be a feasible strategy.
The present study has several limitations, mainly due to its nature of retrospective review of case reports: (1) some details on risk factors, diagnostic work-up or management of cardiac symptoms could be missing; (2) a potential publication bias cannot be excluded, and mild cases or cases with fatal outcome could have been under-reported; particularly, no cases of G1 pericardial disease have been reported; (3) sample size is limited, and the observed associations between patients’ characteristics and outcome were not statistically significant, and thus, our conclusions are merely speculative; (4) patients selected for rechallenge were probably those in better clinical conditions, and in the daily clinical practice the choice of the rechallenge should be considered carefully on an individual basis.
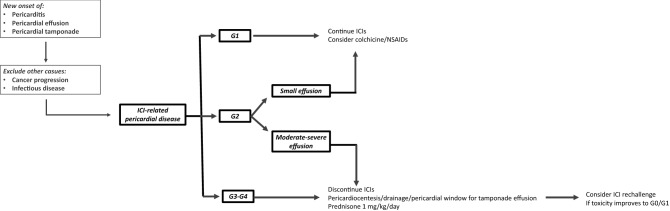
Our systematic review shows that, although potentially fatal, this condition may be reversible in the majority of cases. Based on this review, a reasonable approach to manage ICI-associated pericardial disease could be as it follows: for severe cases (G3–G4), perform pericardiocentesis or other invasive interventions to treat pericardial effusions with tamponade physiology, discontinue ICIs, administer high-dose corticosteroids (prednisone 1 mg/kg/day); for moderate cases (G2 pericardial disease), in selected patients with small effusions consider to continue ICIs and administer colchicine ± NSAIDs, whereas in patients with moderate–severe effusions discontinue ICIs and administer high-dose corticosteroids (prednisone 1 mg/kg/day); for mild cases (G1 pericardial disease), continue ICIs and consider colchicine ± NSAIDs. Once full recovery or meaningful clinical improvement has been achieved and steroids tapered to low-dose (prednisone < 10 mg/day) or stopped, a rechallenge of ICIs seems to be feasible (Fig. 2).
Fig. 2.
Proposed approach for the management of ICI-associated pericardial disease
Funding
The authors did not receive funding for this work.
Availability of data and materials
All relevant data and materials are included in the present publication.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–2519. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 3.Inno A, Metro G, Bironzo P, et al. Pathogenesis, clinical manifestations and management of immune checkpoint inhibitors toxicity. Tumori. 2017;103(5):405–421. doi: 10.5301/tj.5000625. [DOI] [PubMed] [Google Scholar]
- 4.Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19(9):e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 5.Hu JR, Florido R, Lipson EJ, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors [published correction appears in Cardiovasc Res. 2019 Apr 15;115(5):868]. Cardiovasc Res 2019;115(5):854–868. 10.1093/cvr/cvz026 [DOI] [PMC free article] [PubMed]
- 6.Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chahine J, Collier P, Maroo A, Tang WW, Klein AL. Myocardial and pericardial toxicity associated with immune checkpoint inhibitors in cancer patients. J Am Coll Cardiol Case Rep. 2020;2(2):191–199. doi: 10.1016/j.jaccas.2019.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canale ML, Camerini A, Casolo G, et al. Incidence of pericardial effusion in patients with advanced non-small cell lung cancer receiving immunotherapy. Adv Ther. 2020;37(7):3178–3184. doi: 10.1007/s12325-020-01386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haanen JBAG, Carbonnel F, Robert C et al (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(suppl_4):iv119–iv142. 10.1093/annonc/mdx225. Erratum in: Ann Oncol 2018; 29(Suppl 4):iv264–iv266. Erratum in: Ann Oncol 2018;29 Suppl 4:iv264–iv266 [DOI] [PubMed]
- 11.National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 12.Altan M, Toki MI, Gettinger SN, et al. Immune checkpoint inhibitor-associated pericarditis. J Thorac Oncol. 2019;14(6):1102–1108. doi: 10.1016/j.jtho.2019.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asai M, Kato Y, Kawai S, et al. Management of cardiac tamponade during nivolumab of lung cancer with intrapericardial bleomycin: case report. Immunotherapy. 2019;11(6):467–472. doi: 10.2217/imt-2019-0003. [DOI] [PubMed] [Google Scholar]
- 14.Atallah-Yunes SA, Kadado AJ, Soe MH. Pericardial effusion due to pembrolizumab-induced immunotoxicity: a case report and literature review. Curr Probl Cancer. 2019;43(5):504–510. doi: 10.1016/j.currproblcancer.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Chu YC, Fang KC, Chen HC, et al. Pericardial tamponade caused by a hypersensitivity response to tuberculosis reactivation after anti-PD-1 treatment in a patient with advanced pulmonary adenocarcinoma. J Thorac Oncol. 2017;12(8):e111–e114. doi: 10.1016/j.jtho.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Dasanu CA, Jen T, Skulski R. Late-onset pericardial tamponade, bilateral pleural effusions and recurrent immune monoarthritis induced by ipilimumab use for metastatic melanoma. J Oncol Pharm Pract. 2017;23(3):231–234. doi: 10.1177/1078155216635853. [DOI] [PubMed] [Google Scholar]
- 17.de Almeida DVP, Gomes JR, Haddad FJ, Buzaid AC. Immune-mediated pericarditis with pericardial tamponade during nivolumab therapy. J Immunother. 2018;41(7):329–331. doi: 10.1097/CJI.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 18.Dhenin A, Samartzi V, Lejeune S, Seront E. Cascade of immunologic adverse events related to pembrolizumab treatment. BMJ Case Rep. 2019;12(6):e229149. doi: 10.1136/bcr-2018-229149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan AM, Munir A, Thalody V, Munshi MK, Mehdi S. Cardiac tamponade in a patient with stage IV lung adenocarcinoma treated with pembrolizumab. Immunotherapy. 2019;11(18):1533–1540. doi: 10.2217/imt-2019-0067. [DOI] [PubMed] [Google Scholar]
- 20.Kolla BC, Patel MR. Recurrent pleural effusions and cardiac tamponade as possible manifestations of pseudoprogression associated with nivolumab therapy—a report of two cases. J Immunother Cancer. 2016;4:80. doi: 10.1186/s40425-016-0185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kushnir I, Wolf I. Nivolumab-induced pericardial tamponade: a case report and discussion. Cardiology. 2017;136(1):49–51. doi: 10.1159/000447053. [DOI] [PubMed] [Google Scholar]
- 22.Nesfeder J, Elsensohn AN, Thind M, Lennon J, Domsky S. Pericardial effusion with tamponade physiology induced by nivolumab. Int J Cardiol. 2016;222:613–614. doi: 10.1016/j.ijcard.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Oristrell G, Bañeras J, Ros J, Muñoz E. Cardiac tamponade and adrenal insufficiency due to pembrolizumab: a case report. Eur Heart J Case Rep. 2018;2(2):tyt038. doi: 10.1093/ehjcr/yty038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saade A, Mansuet-Lupo A, Arrondeau J et al (2019) Pericardial effusion under nivolumab: case-reports and review of the literature [published correction appears in J Immunother Cancer 7(1):335 (2019)]. J Immunother Cancer 7(1):266. 10.1186/s40425-019-0760-4 [DOI] [PMC free article] [PubMed]
- 25.Shaheen S, Mirshahidi H, Nagaraj G, Hsueh CT. Conservative management of nivolumab-induced pericardial effusion: a case report and review of literature. Exp Hematol Oncol. 2018;7:11. doi: 10.1186/s40164-018-0104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tachihara M, Yamamoto M, Yumura M, Yoshizaki A, Kobayashi K, Nishimura Y. Non-parallel anti-tumour effects of pembrolizumab: a case of cardial tamponade. Respirol Case Rep. 2019;7(3):e00404. doi: 10.1002/rcr2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vittorio A, Sharma R, Siejka D, Bhattarai K, Hardikar A. Recurrent pericardial effusion while receiving nivolumab for metastatic lung adenocarcinoma: case report and review of the literature. Clin Lung Cancer. 2018;19(5):e717–e720. doi: 10.1016/j.cllc.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Yamasaki M, Daido W, Saito N, et al. Pericardial effusion with tamponade in lung cancer patients during treatment with nivolumab: a report of two cases. Front Oncol. 2019;9:4. doi: 10.3389/fonc.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun S, Vincelette ND, Mansour I, Hariri D, Motamed S. Late onset ipilimumab-induced pericarditis and pericardial effusion: a rare but life threatening complication. Case Rep Oncol Med. 2015;2015:794842. doi: 10.1155/2015/794842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarogoulidis P, Chinelis P, Athanasiadou A, et al. Possible adverse effects of immunotherapy in non-small cell lung cancer; treatment and follow-up of three cases. Respir Med Case Rep. 2017;22:101–105. doi: 10.1016/j.rmcr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palaskas N, Morgan J, Daigle T, et al. Targeted cancer therapies with pericardial effusions requiring pericardiocentesis focusing on immune checkpoint inhibitors. Am J Cardiol. 2019;123(8):1351–1357. doi: 10.1016/j.amjcard.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Ala CK, Klein AL, Moslehi JJ. Cancer treatment-associated pericardial disease: epidemiology, clinical presentation, diagnosis, and management. Curr Cardiol Rep. 2019;21(12):156. doi: 10.1007/s11886-019-1225-6. [DOI] [PubMed] [Google Scholar]
- 33.Adler Y, Charron P. The 2015 ESC Guidelines on the diagnosis and management of pericardial diseases. Eur Heart J. 2015;36(42):2873–2874. doi: 10.1093/eurheartj/ehv479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data and materials are included in the present publication.