Abstract
Hepatocellular carcinoma (HCC) is a malignant tumor with high mortality, but lacks effective treatments. Carcinoembryonic antigen glypican-3 (GPC3) is a tumor-associated antigen overexpressed in HCC but rarely expressed in healthy individuals and thus is one of the most promising therapeutic targets. T cell epitope-based vaccines may bring light to HCC patients, especially to the patients at a late stage. However, few epitopes from GPC3 were identified to date, which limited the application of GPC3-derived epitopes in immunotherapy and T cell function detection. In this study, a total of 25 HLA-A0201 restricted GPC3 epitopes were in silico predicted and selected as candidate epitopes. Then, HLA-A0201+/GPC3+ HCC patients’ PBMCs were collected and co-stimulated with the candidate epitope peptides in ex vivo IFN-γ Elispot assay, by which five epitopes were identified as real-world epitopes. Their capacity to elicit specific CD8+ T cells activation and proliferation was further confirmed by in vitro co-cultures of patients’ PBMCs with peptide, in vitro co-cultures of healthy donors’ PBLs with DCs and peptide, T2 cell binding assay as well as HLA-A2 molecule stability assay. Moreover, the in vivo immunogenicity of the five validated epitopes was confirmed by peptides cocktail/poly(I:C) vaccination in HLA-A0201/DR1 transgenic mice. Robust epitope-specific CD8+ T cell responses and cytotoxicity targeting HepG2 cells were observed as detected by IFN-γ Elispot, intracellular IFN-γ staining and cytolysis assay. This study provided novel GPC3 CTL epitopes for the development of T cell epitope vaccines and evaluation of GPC3 specific T cell responses.
Keywords: Hepatocellular carcinoma, Glypican-3, HLA-A0201, T cell epitope, Cytotoxic T lymphocyte
Introduction
Liver cancer is the second and third most common cause of cancer death in China and worldwide to date, respectively [1]. Hepatocellular carcinoma (HCC) accounts for about 75–85% of primary liver cancers. When diagnosed, around 57% of HCC patients are of late or even terminal stage and few treatments are available. The high rate of relapse post-treatment and lack of standard adjuvant therapy inevitably result in a bad prognosis. Despite the therapeutic effects, systemic therapies such as Sorafenib generally prolong survival only by a few months. More effective therapies are urgently needed for HCC treatment.
CD8+ T cells which are capable of recognizing epitope peptides presented by human leukocyte antigens (HLA) class I molecules onto tumor cells are considered critical T cells in anti-tumor immune responses [2]. The epitope-specific CD8+ cytotoxic T lymphocyte (CTL) responses could be elicited by T cell epitope peptides in previous studies [2–4] and could be enhanced by collecting more well-characterized CD8+ T cell epitopes [5]. Therefore, identifying abundant T cell epitopes from target antigens is an essential task in the development of epitope-based cellular immunotherapy. Carcinoembryonic antigen glypican-3 (GPC3) is a tumor-associated antigen (TAA) specifically overexpressed in HCC but rarely expressed in healthy individuals and thus is used as an ideal target in immunotherapies of HCC [6]. The prognosis of GPC3-positive HCC patients was poor following initial hepatectomy [7], but GPC3 specific CD8+ T cell response may indicate a relatively longer tumor-free survival in HCC patients [8], and the epitope peptides derived from GPC3 could efficiently stimulate specific CTL responses in clinical trials [8]. Thus, GPC3-targeted immunotherapy may bring light to GPC3-positive HCC patients. However, only four CD8+ T cell epitopes have been identified from GPC3 thus far [9–11]. Finding CD8+ T cell epitopes from GPC3 as many as possible is urgently needed, which may contribute greatly to the design and development of T cell epitope-based immunotherapy and also facilitate detection of GPC3 specific cellular immunity.
HLA-A*02:01 is one of the most prevalent HLA class I alleles among various ethnic groups, including Asians. In this study, HLA-A0201 restricted CD8+ T cell epitopes from GPC3 were screened and identified using several approaches. Firstly, putative epitopes were in silico predicted using four widely used T cell epitope prediction algorithms and 25 epitopes were selected as candidate epitopes, then ex vivo IFN-γ Elispot assay was used to determine whether the epitope-specific memory T cells could be detected in the peripheral blood mononuclear cells (PBMCs) of HLA-A0201+/GPC3+ HCC patients, by which five epitopes were identified as real-world epitopes. Then, their capacity to stimulate CD8+ T cell responses in vitro was further confirmed by patients’ PBMCs co-cultures with peptides, healthy donor’s peripheral blood lymphocytes (PBLs) co-cultures with DC and peptides, and T2 cell binding as well as HLA-A0201 molecule stability assay. Finally, the peptides cocktail/poly(I:C) vaccinations elicited robust epitope-specific CTLs responses in HLA-A0201/DR1 transgenic mice, indicating their in vivo immunogenicity. These data provided novel targets for epitope-based and CTL-based immunotherapy for GPC3-positive HCC patients.
Materials and methods
Ethical approval, PBMCs preparation and HLA-A genotyping
HCC patients were recruited from the Department of Hepatic Oncology, Nanjing Second Hospital affiliated to Southeast University. According to the updated treatment recommendations for HCC from the ESMO Clinical Practice Guidelines [12], the patients had clinical, biochemical, imaging and pathological evidences of HCC, additionally carried HLA-A*02:01 allele, and expressed GPC3 as detected by immunohistochemical staining (IHC) in HCC tissue sections. The exclusion criteria for these participants were the infection with hepatitis C virus, hepatitis A virus or human immunodeficiency virus, and other malignant tumors. Partial HLA-A0201−/GPC3+ HCC patients were also recruited. The study was conducted according to the Declaration of Helsinki principles and approved by Clinical Ethics Committee of Nanjing Second Hospital (ref: 2018-LY-kt054, 2019-LY-ky011, 2021-LS-ky013). Heparinized peripheral blood samples were collected from HCC patients at hospital. All subjects gave written, informed consent. Meanwhile, healthy donors’ blood samples were collected from the Blood Component Preparation Section of Jiangsu Province Blood Center in the form of white blood cell filter trays following red blood cells filtering. In this instance, informed consent was waived because the white blood cell filter trays were the biological specimens obtained from past clinical diagnosis and treatment, but consent was obtained from Jiangsu Province Blood Center.
PBMCs were instantly isolated by Ficoll density gradient centrifugation and then were either used directly or cryopreserved at − 80 °C until further use. HLA-A alleles were identified using PCR sequencing-based tying. Primers as described [13] were synthesized by Sangon Biotech Co., Ltd (Shanghai) and are displayed in Table 1. The DNA from exon 1 to exon 3 of HLA-A was amplified in PCR using primer combination A1/A3 followed by sequencing using primer combination A2F/A2R for exon 2 and A3F/A3R for exon 3. The sequencing data were aligned and analyzed using Lasergene software.
Table 1.
Primers used in this study for HLA-A genotyping
| Primers | Sequence (5′-3′) | Anneal site | Product |
|---|---|---|---|
| A1 | GAAACSGCCTCTGYGGGGAGAAGCAA | Intron l: 21–26 | 985 bp |
| A3 | TGTTGGTCCCAATTGTCTCCCCTC | Intron 3: 66–89 | |
| A2F | AGCCGCGCCKGGASGAGGGTC | Exon 2- intron 2: 99–119 | 270 bp |
| A2R | GGCCCGTCCGTGGGGGATGAG | Exon 2- intron 2: 37–57 | |
| A3F | GTTTCATTTTGRTTKAGGCCA | Exon 3- intron 3: 150–171 | 276 bp |
| A3R | TGTGGGAGGCCAGCCCGGGAGA | Exon 3- intron 3: 41–66 |
Mice and cell lines
Male HLA-A0201/DR1 transgenic and H-2-β2m−/−/IAβ−/− C57BL/6 mice at six weeks were generous gifts from the Academy of Military Medical Sciences of China. All mice were maintained at the specific pathogen-free Animal Centre of Southeast University (Nanjing, China). Animal welfare and experimental procedures were performed by the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology of China, 2006), and were approved by the Animal Ethics Committee of Southeast University. HepG2, T2, K562 and Yac-1 cell lines were purchased from Fuheng Biotechnology Co., Ltd (Shanghai, China) and maintained in house.
Epitopes prediction and peptides synthesis
CD8+ T cell epitopes spanning overall GPC3 protein (P51654) and presented by HLA-A0201 molecule were in silico predicted using four epitope prediction algorithms (SYFPEITHI, NetMHC, EPIJEN, IEDB). 9-mer or 10-mer peptides with high scores (high affinity) in at least two tools were selected as candidate epitopes for further identification. Epitope peptides were synthesized from China Peptides Co., Ltd (Suzhou) with a purity of > 95% as defined by HPLC purification and mass spectrometry. Lyophilized peptides were reconstituted at a stock concentration of 2 mg/mL in DMSO-PBS solution and stored in aliquots at -80 ℃.
IFN-γ Elispot assay
IFN-γ Elispot assays were performed to detect the epitope-specific T cells in patient’s PBMCs or splenocytes from immunized mice. Briefly, PBMCs from HLA-A0201+/GPC3+ HCC patients or splenocytes from immunized mice were seeded in the 96-well plates coated with anti-human IFN-γ or anti-mouse IFN-γ antibody at the cell density of 2 × 105 cells per well in 100 μL of serum-free cell culture medium (Dakewe Biotech, Shenzhen), and co-cultured, respectively, with peptide pools (8 or 9 epitopes/pool, 20 μg/mL/epitope), single peptide (20 μg/mL), phytohemagglutinin (PHA) (10 μg/mL, positive control), irrelevant epitope peptide (HLA-A24 restricted AFP424-432, 20 μg/mL, irrelevant control) and no peptide (negative control) for different purposes. After incubation for 20 h at 37 °C, 5% CO2 incubator, the plates were washed and incubated with biotinylated anti-human or anti-mouse IFN-γ detecting antibody (BD Bioscience) for 2 h at room temperature (RT). Then, the plates were washed and incubated with streptavidin-conjugated HRP (BD Bioscience) for 1 h at RT. After washing, AEC solution (BD Bioscience) was added to develop spots. The spot forming units (SFUs) were imaged and enumerated. Positive T cell response was defined according to the criterion as follows: (SFUs in peptide well—SFUs in negative control well) ≧ 5, while SFUs in negative control well was 0–5; or (SFUs in peptide well)/(SFUs in negative control well) ≧ 2, while SFUs in negative control well was > 5.
Peptide-PBMC co-culture experiment using patient’s PBMCs
Briefly, PBMCs from HLA-A0201+/GPC3+ HCC patients were prelabeled with CFSE at a final concentration of 1.5 μM for 20 min at 37 °C, 5% CO2 incubator. After washing, CFSE-labeled PBMCs were seeded in 96-well plates (2 × 105 cells/well) and incubated for 7 days with a single validated epitope peptide (20 μg/mL) or PHA (10 μg/mL) in RPMI1640 culture medium with 10% FBS. Then, cells were harvested, blocked with anti-CD16/CD32 for 20 min (eBioscience) and stained with PE-labeled anti-human CD3 and APC-labeled anti-human CD8a monoclonal antibodies (mAbs, Biolegend) for 30 min at 4 °C followed by flow cytometry analysis. The proliferation percentage of CD8+ T cells in CD3+/CD8+ population was calculated according to the reduction of CFSE-staining brightness.
DC-peptide-PBL co-culture experiment using healthy donor’s PBMCs
Mature DCs and peptide-specific CTLs were induced from HLA-A0201 matched healthy donors’ PBMCs as we have described in a recent submission [14]. Briefly, healthy donor’s PBMCs were incubated in serum-free RPMI 1640 for 2 h in 5% CO2 at 37 °C. Then, non-adherent cells (peripheral blood lymphocytes, PBLs) were removed and cryopreserved at − 80 °C until further use. The resulting adherent cells were cultured in complete RPMI 1640 medium containing 10% FBS, 1% penicillin/streptomycin, recombinant human GM-CSF (rhGM-CSF, 1000 IU/mL, PrepoTech) and rhIL-4 (500 IU/mL, PrepoTech). Half of the complete medium was changed every two or three days. On day 5, lipopolysaccharide (1 μg/mL, Sigma) was added to induce mature DCs (mDCs). On day 7, cells were harvested and the phenotype of mDCs was verified by detecting the expression of CD83, CD80, CD86, HLA-DR, HLA-ABC and CD1a, respectively, with flow cytometry. Then, mDCs (5 × 104 cells/well) were incubated with a single validated epitope peptide (20 μg/mL) or no peptide (negative control) in serum-free RPMI 1640 in 48-well plates for 4 h in 5% CO2, at 37 °C incubator. PBLs from the same donor were thawed in advance and recovered overnight and then were added in each well (1 × 106 cells/well) and co-cultured with peptide and mDCs for 14 days. Recombinant human IL-2 was added on day 11 (20 IU/mL) and day 17 (10 IU/mL). On day 14, the corresponding validated epitope peptide (20 μg/mL) was added one more time. On day 21, cells were harvested and followed by intracellular IFN-γ staining.
Intracellular IFN-γ staining
PBLs co-cultured with mDC and peptide for 14 days or splenocytes from immunized mice were harvested, seeded in 48-well plates, and re-stimulated with corresponding epitope peptide (20 μg/mL), PHA (positive control, 10 μg/mL) or no peptide (negative control) for 16 h at 37 °C, 5% CO2. After that, BFA/Monensin mixture (MultiSciences Biotech, China) was added for another 6-h co-culture. Then, cells were harvested, blocked with anti-CD16/CD32 for 20 min, and stained with fluoresce-labeled anti-CD3 and anti-CD8 mAbs (eBioscience) for 30 min at 4 ℃. After washing, cells were fixed and permeabilized according to the protocol and were further stained with fluoresce-labeled anti-IFN-γ antibody (eBioscience) for another 30 min at 4 °C followed by flow cytometry. The frequency of IFN-γ+ cells in CD3+/CD8+ population was calculated.
T2 cell binding assay and HLA-A2 molecule stability assay
The HLA-A0201 expressing and TAP-1 deficient human T cell line was used. The peptide-induced stabilization of HLA-A0201 molecules onto T2 cells was measured to evaluate the binding affinity of epitope peptide with HLA-A0201 molecules as described with minor modification [4]. Briefly, T2 cells were seeded in 96-well plates (1 × 105 cells/well) and incubated, respectively, with candidate peptides, CMV pp65495-503 peptide (NLVPMVATV, HLA-A0201 restricted, as positive control) and OVA257-264 peptide (SIINFKEL, H-2 Kb restricted, as negative control) at a gradient concentration; then, β2-m (Sigma) was added in each well (3 μg/mL). After co-culture for 16 h at 37 °C and 5% CO2 incubator, T2 cells were harvested, washed with PBS, and stained with PE-labeled anti-HLA-A2.1 mAb (BB7.2, BD Bioscience) for 30 min at 4 °C. Then, the expression of HLA-A0201 molecules on the surface of T2 cells was detected by flow cytometry using FACSCalibur (BD Bioscience). The fluorescence index (FI) was calculated using mean fluorescence intensity (MFI) at 200 μM of peptide in the wells with given peptide (MFIpeptide) and in the well without peptide (MFIno peptide) as follows: FI = (MFIpeptide—MFIno peptide)/MFIno peptide. FI ≥ 1.0 means high binding affinity, 0.5 < FI < 1.0 means intermediate binding affinity, and FI < 0.5 means low or no binding affinity. In another independent experiment (peptide disconnection assay), peptides were washed out and BFA/Monensin was added in each well after 16-h co-incubation of T2 cells with peptide and β2-m. Then, HLA-A0201 expression onto T2 cells was measured at different time points using the same way as described above. The horizon of the half-life of peptide dissociation from HLA-A0201 molecules (predicted t1/2) was confirmed according to the MFI-Time curve.
Preparation of peptides cocktail/poly(I:C) vaccine and mice immunization
The validated epitopes P8, P10, P13, P23 and P25 were grouped into one peptide pool and mixed with poly(I:C) for vaccinations. The HLA-A0201+/+/DR1+/+/mβ2m−/−/IAβ−/− C57BL/6 mice were randomly divided into two groups (5 mice/group) and immunized with peptides cocktail/poly(I:C) vaccine and normal saline (NS), respectively. On day 0, day 7 and day 21, mice were injected subcutaneously. In vaccine group, each mouse was injected with 300 μL of vaccine containing 50 μg peptides (10 μg/epitope) and 100 μg poly(I:C) at one time point, and at tail root, back of the neck and around the groin (100 μL/injection site). Seven days after the final immunization, mice were executed and splenocytes were collected for Elispot assay, intracellular IFN-γ staining and cytolysis assay.
Cytotoxicity assay
PBMCs from HLA-A0201+/GPC3+ HCC patients or splenocytes from the immunized mice were stimulated with a mixture of P8, P10, P13, P23 and P25 or a mixture of P8 and P23 (20 μg/mL for each epitope) in complete RPMI 1640 medium containing 10% FBS, 1% penicillin/streptomycin and IL-2 (20 IU/mL) in 48-well plates for 7 days. A negative control without peptide was also carried out. Then, cells were harvested and seeded into round-bottom 96-well plates as effector cells. HepG2 cells (HLA-A0201+/GPC3+) [15] or T2 cells (no peptide-loaded, as nonspecific target, HLA-A0201+/GPC3−) were prelabeled with CFSE and added in each well (1 × 104 cells/well) as target cells at an E: T ratio of 30: 1. K562 cells (for PBMCs) or Yac-1 cells (for splenocytes) were also added in each well (5 × 104 cells/well) to prevent nonspecific killing of NK cells to target cells. Each assay was performed in triplicate wells. After co-incubating for 4 h at 37 ℃, 5% CO2 incubator, cells were stained with 7-AAD (eBioscience) followed by flow cytometry analysis. The frequency of 7-AAD+ cells in CFSE+ cell population was calculated.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9 (GraphPad, La Jolla, CA, USA). Two-way ANOVA and multiple comparisons were used to compare the data across groups unless otherwise stated. p < 0.05 was considered as significant.
Results
Twenty-five GPC3 epitopes restricted by HLA-A0201 molecules were in silico predicted and selected as candidate epitopes
Using suited algorithms from four T cell epitope prediction tools, the amino acid sequence of GPC3 was scanned for 9-mer and 10-mer peptides predicted to bind to HLA-A0201 molecule, a prevalent HLA-A allotype around the world. Epitopes from the top 20 binders as predicted by at least two algorithms were selected as putative epitopes. Finally, 25 epitopes with the highest virtual affinity according to the scoring criteria of each prediction algorithms were selected as candidate epitopes and synthesized for further identification (Table 2).
Table 2.
25 GPC3 epitopes restricted by HLA-A0201 as predicted in silico were synthesized for further validation
| Epitope Serial number |
Position | Epitope Sequence |
Predicted binding scores | ||||
|---|---|---|---|---|---|---|---|
| SYFPEITHI | NETMHC | EPIJEN | IEDB < 500 nM | ||||
| ≥ 20 | < 2.0 | < 500 nM | ANN | SMM | |||
| P1 | 9–18 | CLVVAMLLSL | 27 | 2.5 | ns | 314.58 | 156.13 |
| P2 | 44–52 | RLQPGLKWV | 27 | 1.3 | 0.53 | 144.78 | 114.84 |
| P3 | 48–57 | GLKWVPETPV | 20 | 1.2 | ns | 127.92 | 210.13 |
| P4 | 92–100 | LLQSASMEL | 24 | 0.5 | 1.1 | 36.38 | 93.35 |
| P5 | 102–110 | FLIIQNAAV | 26 | 0.15 | 0.11 | 11.7 | 10.82 |
| P6 | 128–137 | AMFKNNYPSL | 24 | 0.8 | ns | 69.26 | 277 |
| P7 | 136–145 | SLTPQAFEFV | 23 | 0.6 | ns | 45.64 | 136.3 |
| P8 | 144–152 | FVGEFFTDV | 18 | 0.5 | 1.93 | 34.88 | 76.76 |
| P9 | 155–163 | YILGSDINV | 24 | 0.2 | 0.84 | 16.01 | 54.97 |
| P10 | 169–177 | ELFDSLFPV | 23 | 0.01 | 0.09 | 3.29 | 8.36 |
| P11 | 173–182 | SLFPVIYTQL | 25 | 0.4 | ns | 26.48 | 159.76 |
| P12 | 222–230 | SLQVTRIFL | 22 | 3.5 | 6.49 | 730.92 | 425.71 |
| P13 | 229–237 | FLQALNLGI | 22 | 0.12 | 0.35 | 10.33 | 32.67 |
| P14 | 232–240 | ALNLGIEVI | 27 | 3 | 0.36 | 585.42 | 359.01 |
| P15 | 281–289 | VMQGCMAGV | 24 | 0.2 | 0.65 | 15.45 | 28.39 |
| P16 | 299–307 | YILSLEELV | 23 | 0.2 | 0.04 | 16.01 | 54.34 |
| P17 | 319–327 | VLLGLFSTI | 26 | 0.5 | 0.26 | 31.96 | 59.86 |
| P18 | 322–331 | GLFSTIHDSI | 22 | 1 | ns | 91.96 | 225.67 |
| P19 | 325–334 | STIHDSIQYV | 22 | 1.6 | ns | 190.85 | 132.58 |
| P20 | 340–348 | KLTTTIGKL | 26 | 4.5 | 0.74 | 1113.97 | 604.1 |
| P21 | 367–375 | FIDKKVLKV | 27 | 0.5 | 0.32 | 37.64 | 69.68 |
| P22 | 420–429 | TLCWNGQELV | 20 | 1.1 | ns | 103.15 | 268.83 |
| P23 | 522–530 | FLAELAYDL | 27 | 0.03 | 0.66 | 4.94 | 7.85 |
| P24 | 563–572 | KLLTSMAISV | 25 | 0.17 | ns | 13.59 | 14.6 |
| P25 | 571–579 | SVVCFFFLV | 17 | 0.05 | 15.78 | 32.85 | 37.16 |
Position: The start and end positions of indicated epitope in the amino acid sequence of GPC3 (P51654); ns: no score available for this epitope in the prediction algorithm; ≥ 20, < 2.0 and < 500 nM: the judging criterion of T cell epitope for indicated prediction algorithm
Five candidate GPC3 epitopes were identified as real-world epitopes using HCC patients’ PBMCs
Twenty-four HLA-A0201+/GPC3+ patients with HCC were enrolled in this study, and their baseline features are shown in Table 3. Two rounds of IFN-γ Elispot assays using the patients’ PBMCs were used to screen the real-world GPC3 epitopes from the synthesized peptides. Firstly, the 24 PBMCs samples were ex vivo stimulated with the peptide pools of 25 candidate epitopes in the first-round Elispot assay, and of which 9 PBMCs samples (38%) displayed positive T cell responses against the peptide pools. Then, PBMCs from the nine patients were re-collected and co-cultured with each type of epitope peptides from the peptide pools which stimulated positive T cell responses in the first-round Elispot. After two rounds of Elispot assays, five epitope peptides (P8-GPC3144-152, P10-GPC3169-177, P13-GPC3229-237, P23-GPC3522-530 and P25-GPC3571-579) were identified as naturally processed GPC3 epitopes in at least two HCC patients, and P23 showed the highest positive rate (29.16%) in the 24 patients (Fig. 1), of which 3 epitopes (P10, P13 and P25) have not been reported previously. In parallel, almost no T cell response against these validated epitope peptides was observed in HLA-A0201+/GPC3− healthy donors’ PBMCs or HLA-A0201−/GPC3+ patients’ PBMCs (data not shown). These results imply that the five GPC3 peptides can be naturally processed and presented by malignant hepatocytes and initiate epitope-specific CD8+ T cells activation and proliferation followed by circulating in the patient’s peripheral blood as memory or activated T cell clones. Thus, in the 20-h ex vivo stimulation with epitope peptides, memory or activate T cells can produce IFN-γ and were detected in Elispot assay.
Table 3.
Baseline features of 24 HCC patients enrolled in this study
| Number | Age | Gender | Stage | Diagnosis | HLA-A genotype | Positive T cell response to peptide |
|---|---|---|---|---|---|---|
| #1 | 59 | Male | II | HCC(CHB) | 02:01/11:01 | None |
| #2 | 47 | Male | III | HCC(CHB) | 02:01/30:01 | None |
| #3 | 61 | Female | III | HCC(CHB) | 02:01/02:03 | P8/P10/P13/P23/P25 |
| #4 | 82 | Male | IV | HCC(CHB) | 02:01/11:02 | None |
| #5 | 53 | Male | IV | HCC(CHB) | 02:01/02:06 | None |
| #6 | 51 | Male | III | HCC(CHB) | 02:01/24:02 | None |
| #7 | 43 | Female | IV | HCC(CHB) | 02:01/24:02 | P8/P23 |
| #8 | 71 | Female | III | HCC(CHB) | 02:01/11:01 | P23 |
| #9 | 65 | Male | IV | HCC(CHB) | 02:01/31:01 | None |
| #10 | 51 | Female | III | HCC(CHB) | 02:01/33:03 | None |
| #11 | 57 | Male | IV | HCC(CHB) | 02:01/11:01 | P23 |
| #12 | 67 | Male | III | HCC(CHB) | 02:01/02:06 | None |
| #13 | 50 | Male | IV | HCC(CHB) | 02:01/24:02 | None |
| #14 | 37 | Male | IV | HCC(CHB) | 02:01/02:03 | P10/P13/P23 |
| #15 | 62 | Male | II | HCC(CHB) | 02:01/01:01 | None |
| #16 | 56 | Male | III | HCC(CHB) | 02:01/11:01 | None |
| #17 | 68 | Female | IV | HCC(CHB) | 02:01/24:02 | P23 |
| #18 | 53 | Female | III | HCC(CHB) | 02:01/24:02 | None |
| #19 | 46 | Male | IV | HCC(CHB) | 02:01/11:01 | None |
| #20 | 62 | Male | III | HCC(CHB) | 02:01/33:03 | P13/P23 |
| #21 | 51 | Female | III | HCC(CHB) | 02:01/24:02 | P25 |
| #22 | 56 | Male | IV | HCC(CHB) | 02:01/03:01 | None |
| #23 | 59 | Female | III | HCC(CHB) | 02:01/02:07 | P25 |
| #24 | 64 | Female | IV | HCC(CHB) | 02:01/24:02 | None |
Positive T cell response to peptide: the putative epitope peptides induced positive T cell responses in the IFN-γ Elispot assays in at least two patients’ PBMCs; None: no epitope peptide-induced positive T cell response in the patient’s PBMCs
Fig. 1.
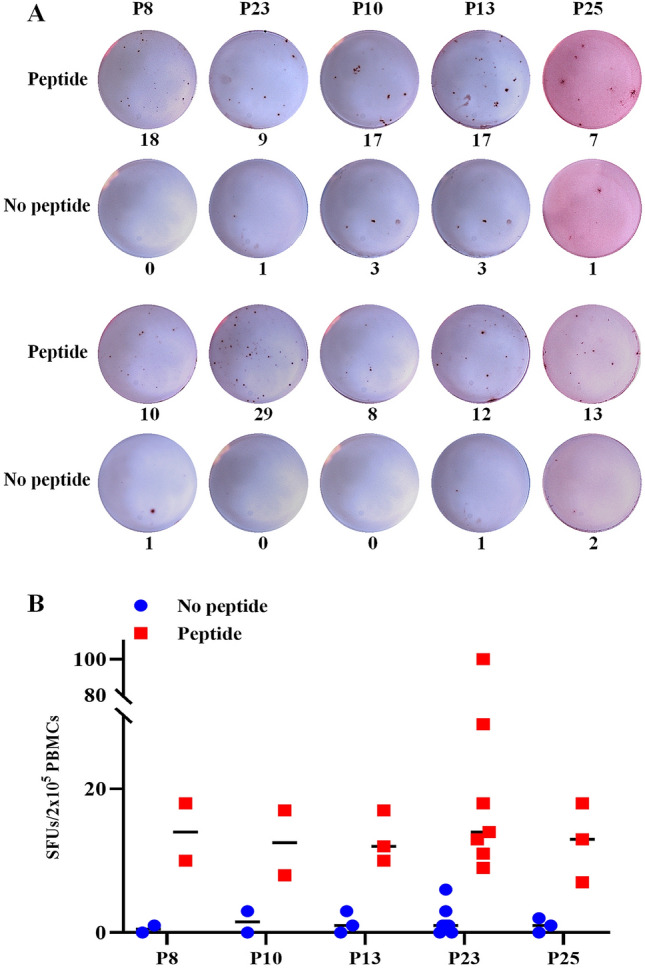
Five candidate GPC3 epitopes were validated as real-world T cell epitopes by IFN-γ Elispot assay using patients’ PBMCs. PBMCs from HLA-A0201+/GPC3+ HCC patients were co-cultured for 20 h with the peptide pools of 25 candidate epitopes in the first-round Elispot assays and with single candidate epitope peptide in the second-round Elispot assays. a Representative SFUs plots of 5 validated epitope peptides in the second-round Elispot assays. b The scatter grams of SFUs numbers for each validated epitope peptide and its negative control well (no peptide) in the patients’ PBMCs which displayed positive T cell responses in the second-round Elispot assays
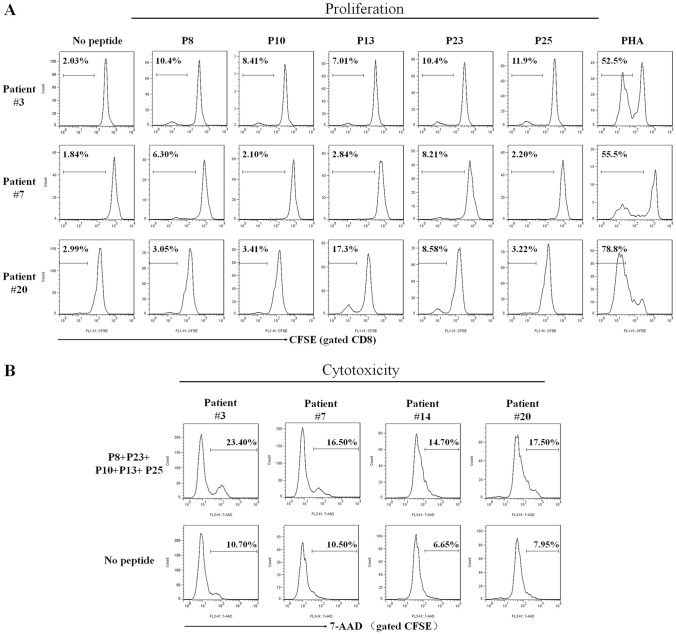
Furthermore, the 5 validated epitopes were assessed for their in vitro capacity to induce memory CD8+ T cell proliferation. PBMCs were collected from three HLA-A0201+/GPC3+ HCC patients, prelabeled with CFSE, and stimulated with indicated epitope peptides for seven days in vitro; then, epitope-specific CD8+ T cells expansions were observed as detected by flow cytometry. As shown in Fig. 2a, each epitope peptide induce obvious expansion of CD8+ T cells in the PBMCs from at least one patient. The frequencies of CD8 T cells were 2.86–6.37 times higher in the peptide stimulation groups than in the no peptide control groups. PBMCs from patient #3 displayed CD8+ T cell proliferation responded to all five validated epitope peptides, while the PBMCs from the other two patients showed responses only to 1–3 of the five epitope peptides, implying the diverse T cell immune responses in different individuals against same antigen or epitope.
Fig. 2.
The 5 validated epitopes induced CD8+ T cell proliferation and cytolysis of patients’ PBMCs in vitro. The PBMCs from three HLA-A0201+/GPC3+ HCC patients were prelabeled with CFSE and co-cultured with each validated epitope peptide for 7 days followed by flow cytometry to detect the proliferation frequency of CD8+ T cells. a The proliferation profiles of CD8+ T cells in response to each epitope peptide, PHA (positive control) or no peptide (negative control) for each patient’s PBMCs. In parallel, the PBMCs from four HLA-A0201+/GPC3+ HCC patients were co-cultured with the cocktail of 5 validated epitope peptides for 7 days, then cells were harvested and co-cultured as effector cells with K562 cells and CFSE-prelabeled HepG2 cells for 4 h. After 7-AAD staining, cytotoxicity was measured by flow cytometry. b Cytolysis profiles and the frequencies of 7-AAD+ cells in the CFSE+ cell populations at an E: T ratio of 30:1
In parallel, another co-culture of the patient’s PBMCs with validated epitope peptides was also carried out for cytolysis experiments. PBMCs from HLA-A0201+/GPC3+ HCC patients were stimulated with a mixture of P8, P10, P13, P23 and P25 for 7 days. Then, cells were harvested and seeded in 96-well plates as effector cells, CFSE-prelabeled HepG2 cells (HLA-A0201+/GPC3+) were seeded as target cells at an E: T ratio of 30: 1. K562 cells were also added in each well to prevent nonspecific killing of NK cells to target cells. After co-incubating for 4 h at 37 °C, 5% CO2 incubator, cells were stained with 7-AAD and the frequency of 7-AAD+ cells in CFSE+ cell population was calculated. As shown in Fig. 2b, the PBMCs following 7-day stimulation with the five validated epitope peptides displayed much stronger cytotoxicity than the PBMCs without peptide stimulation, implying the contribution of expanded epitope-specific CD8+ T cells in the cytolysis against target cells. The increase in cytotoxicity, to some extent, was consistent with the expansion of epitope-specific CD8+ T cells in 7-day in vitro stimulation of patient’s PBMCs with epitope peptides.
The five validated epitopes induced activation of naive T cells in vitro
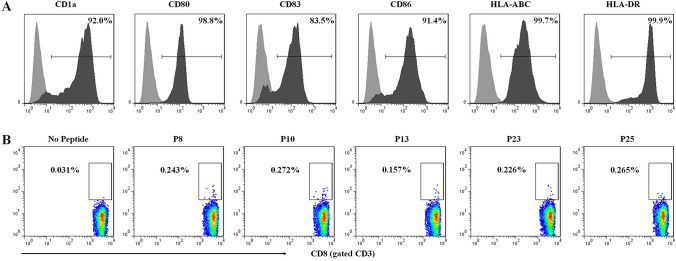
To further investigate in vitro immunogenicity of the five validated epitopes, DC-peptide-PBL co-culture experiments were carried out using healthy donor’s PBMCs. Firstly, mature DCs were successfully induced from adherent PBMCs of HLA-A0201+ healthy donors and were confirmed with the high expression of CD1a, CD80, CD83, CD86, HLA class I and HLA-DR molecules (Fig. 3a), as our previous study [14]. Then, the mDCs were co-cultured with epitope peptides and autologous PBLs for 14 days. IFN-γ-producing CD8+ T cells were markedly elicited by each epitope peptides with the frequencies of 5.06–8.77 times higher than the no peptide control group, as detected by ICS and flow cytometry (Fig. 3b).
Fig. 3.
The five validated epitopes induced activation of naive T cells in vitro. Monocyte-derived DCs were induced from PBMCs of three HLA-A0201+ healthy donors and were matured by LPS. Then, autologous PBLs were co-incubated with mature DCs and each epitope peptide for 14 days, and followed by IFN-γ intracellular cytokine staining. a Phenotypes of mature DCs induced from PBMCs. b Representative flow cytometric dot plots of IFN-γ+/CD8+ T cells in the DC-peptide-PBL co-cultures
The five validated epitopes displayed high binding affinity with HLA-A0201 molecules
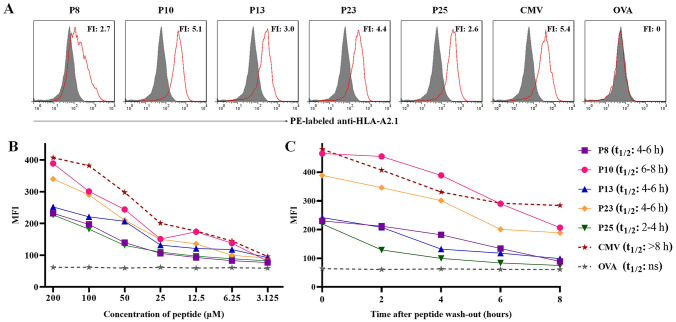
In order to evaluate the binding efficiency of validated epitope peptides to HLA-A0201 molecules, the increase in HLA-A0201 molecules on the surface of T2 cells following 16 h of co-culture with each peptide and β2-m was detected by flow cytometry. As shown in Fig. 4a, all five validated epitope peptides induced an obvious upregulation of HLA-A0201 molecules onto T2 cells and were considered high-affinity epitopes (FI > 1.0). As expected, the positive control peptide (CMVpp65495-503 peptide presented by HLA-A0201) exhibited strong binding to HLA-A0201, while the negative control peptide (OVA257-264 peptide presented by H-2 Kb) exhibited no binding. When T2 cells incubated for 16 h with each epitope peptide at a gradient concentration, a concentration-dependent mean fluorescence intensity (MFI) was also observed, implying the peptide concentration-dependent stabilization of HLA-A0201/peptide complexes onto T2 cells (Fig. 4b). After incubating for 16 h with each epitope peptide at a given concentration, T2 cells were washed and BFA/Monensin was added for further incubation, and followed by the detection of HLA-A0201 molecules at different time points. As shown in Fig. 4c, a time-dependent MFI and stabilization of HLA-A0201 molecules were observed onto T2 cells, and the half-life of peptide dissociation from HLA-A0201 molecules was also depicted. The positive control group displayed similar MFI dynamic curves while negative control with no change.
Fig. 4.
The five validated epitopes displayed high binding affinity with HLA-A0201 molecules onto T2 cells. a T2 cells were incubated with β2-m and each validate epitope peptide (200 μM) for 16 h, and followed by PE-labeled anti-HLA-A2.1 mAb staining as well as flow cytometry. Gray filled line represented the fluorescence strength of T2 cells under incubation with only β2-m, while the red solid line represented the fluorescence strength of T2 cells after co-incubation with β2-m and indicated peptides. CMV (CMVpp65495-503 epitope presented by HLA-A0201) and OVA (OVA257-264 epitope presented by H-2 Kb) were positive control and negative control peptides, respectively. The fluorescence index (FI) for each peptide was calculated and displayed. b T2 cells were incubated with each epitope peptide at 7 gradient concentrations and β2-m for 16 h followed by flow cytometry as described. The mean fluorescence intensities (MFI) of T2 cells at different concentrations of peptides were exhibited as dynamic curves. c T2 cells were incubated with β2-m and each epitope peptide (200 μM) for 16 h, then peptides were washed out and BFA/Monensin was added in each well for another incubation followed by flow cytometry as described. The MFI of T2 cells at different time points were exhibited as dynamic curves. The horizon of the half-life of each peptide dissociation from HLA-A0201 molecules (predicted t1/2) was calculated according to the MFI-Time curve and displayed also
The five validated epitope peptides elicited robust specific CTL responses in HLA-A0201/DR1 transgenic mice
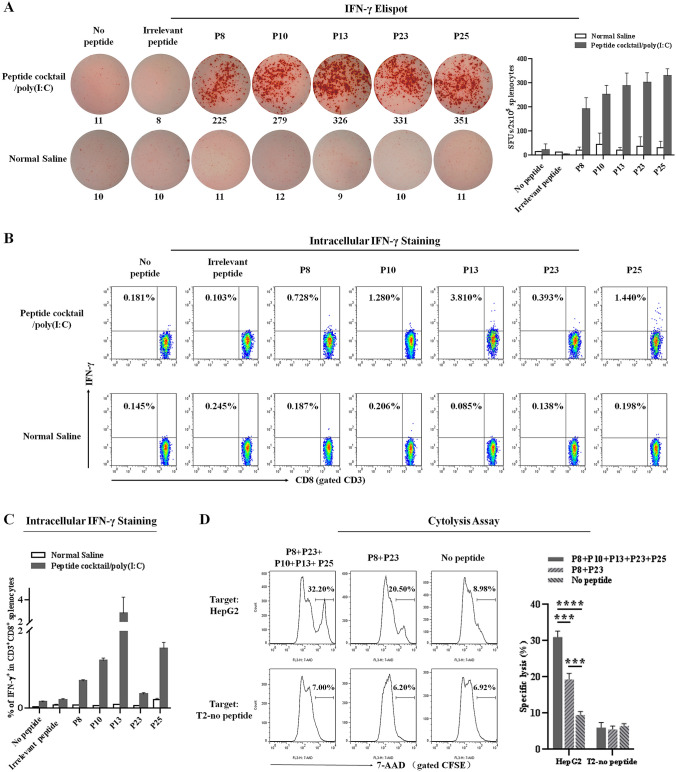
To determine whether the five validated epitope peptides can induce T cell responses in vivo, the humanized C57BL/6 mice (HLA-A0201+/+/DR1+/+/mβ2m−/−/IAβ−/−) were immunized with the peptides cocktail vaccine for three times. Seven days after the final immunization, spleen cells were isolated and stimulated with each validated epitope peptide ex vivo. As detected by IFN-γ Elispot assays, the splenocytes from each mouse in vaccine group displayed 6–13 times more SFUs than the splenocytes from normal saline group (Fig. 5a). The irrelevant peptide (HLA-A24 restricted AFP424-432 epitope) was also co-incubated with immunized splenocytes and obtained negative results similar to the no peptide group (negative control). Furthermore, ICS confirmed that the percentages of IFN-γ+ /CD8+ T cells in splenocytes from vaccine group were 5–30 times higher than that from normal saline group, after ex vivo stimulation with each epitope peptide (Fig. 5b and c). The irrelevant peptide and no peptide stimulation groups remained baseline percentages.
Fig. 5.
The five validated epitope peptides elicited robust specific CTL responses in HLA-A0201/DR1 transgenic mice. The five validated epitope peptides cocktail was mixed with poly(I:C) and administered into HLA-A0201/DR1 transgenic mice on day 0, 7 and 21. Splenocytes were collected 7 days after the last booster, and ex vivo stimulated with each epitope peptide, irrelevant peptide (HLA-A24 restricted AFP424-432 epitope), or no peptide (negative control) overnight, and followed by IFN-γ Elispot and ICS. a Representative SFUs spot plots and SFUs numbers of splenocytes from each group in the IFN-γ Elispot assays under stimulation with each epitope peptide. Data in the histograms were presented as mean ± SEM, n = 4–5 per group. b Representative flow cytometric dot plots of IFN-γ+/CD8+ T cells in CD3+/CD8+ T cell populations of splenocytes from each group in IFN-γ ICS under stimulation with each epitope peptide. c Frequencies of IFN-γ+/CD8+ T cells in CD3+/CD8+ T cell populations of splenocytes from each group in IFN-γ ICS. Data in the histograms were presented as mean ± SEM, n = 4–5 per group. In parallel, splenocytes from each group were re-stimulated for 7 days in vitro with the cocktail of 5 validated epitope peptides or mixture of P8 and P23. Then, cells were co-cultured as effector cells with Yac-1 cells and CFSE-prelabeled HepG2 cells or T2 cells (no peptide loading) for 4-h cytolysis assay. d Representative cytolysis profiles and the frequencies of 7-AAD+ cells in the CFSE+ cell populations for each group. Data in the histograms were mean ± SEM, n = 4–5 per group. E: T ratio = 30:1. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001
In order to ascertain target killing activity of the CD8+ T cells elicited by peptides cocktail vaccine, spleen cells were isolated 7 days after the final immunization, and stimulated with a mixture of P8, P10, P13, P23 and P25 or a mixture of P8 and P23 for 7 days in vitro, then followed by 4-h cytolysis experiments. As shown in Fig. 5d, splenocytes from vaccine group exhibited strong cytotoxicity to HepG2 cells (HLA-A0201+/GPC3+), but not to T2 cells (HLA-A0201+/GPC3−, no peptide-loaded), as compared with the negative control groups without peptide in vitro stimulation. In addition, splenocytes re-stimulated in vitro with five peptides showed much stronger cytotoxicity than the splenocytes re-stimulated with only P8 and P23, implying that the epitope-specific T cells contributed greatly to the cytolysis of target cells. These data from cellular functional experiments indicate that the 5 validated GPC3 epitope peptides can induce robust and target-specific CTL responses in vivo.
Discussion
GPC3 is overexpressed in most HCCs but almost no expressed on healthy adult tissues [6] and has been demonstrated to enhance proliferation of malignant cells and accelerate disease progression [16–18]. Therefore, GPC3 is widely investigated in diagnosis, clinical management and molecular targeting therapy for HCC [6, 11, 19–22]. The safety and clinical effects of GPC3-derived peptide vaccines have been confirmed in previous clinical trials [8]. As well known, multiple antigenic epitopes are required for the control of cancers [5, 11, 23, 24]. Thus, identifying more T cell epitopes of GPC3 will contribute to the design and development of epitope-based vaccines and the tools monitoring GPC3-specific cellular immune responses for GPC3-positive HCC patients.
However, only four T cell epitopes of GPC3 have been reported thus far, to our knowledge. Komori in silico predicted nine HLA-A0201 restricted epitopes from GPC3 followed by immunization of HLA-A2.1 (HHD) transgenic mice with the peptides-loaded DCs. Only GPC3144-152 could trigger specific CTL responses in HHD transgenic mice and ex vivo co-cultures with patients’ PBMCs. The resulting CTLs could lyse HLA-A2+/GPC3+ HepG2 cells or GPC3144-152 loaded T2 cells in vitro and reduce the growth of HLA-A2+/GPC3+ SK-Hep-1/GPC3 cells in NOD/SCID mice. Meanwhile, GPC3298-306 cross-presented by H-2Kd and HLA-A2402 could induce GPC3-reactive CTLs response in the PBMCs of HLA-A2402+ HCC patients [9]. In the study from James O’Beirne [10], GPC3-specific CTLs were generated in the co-cultures of HLA-A2+ healthy donors’ PBMCs with GPC3 mRNA transfected autologous monocyte-derived DCs. Of six putative peptides, GPC3522-530 was identified as a naturally processed, HLA-A2-restricted T cell epitope. However, GPC3522-530 was not immunogenic epitope in the study from Komori [9], which may be explained by the difference in T cell repertoires between humans and HLA-A transgenic mice. Later, the mass spectroscopy analysis was performed to screen epitopes presented on the targeted tumor tissue. Among 45 putative epitopes, GPC3367-375 was identified to be naturally presented by HLA-A2 molecules. Then, the engineered TCR-T cell clone with the expression of GPC3367-375-specific T cell receptor could recognize and kill HLA-A2+/GPC3+ tumor in vitro and in mice [11]. It is a successful pre-clinical application of epitope-based adoptive T cell therapy.
It is reasonable that if memory or active T cells specific for certain epitopes exist in peripheral blood of patients, these epitopes should be real-world T cell epitopes naturally processed by target cells and thus could be considered the first-line candidate peptides for vaccine design. This theory has already been applied to identify epitopes for many TAAs [4, 25–27] and foreign antigens [28, 29]. However, none of GPC3 epitopes was identified by this rationale in previous studies. Herein, HCC patients’ PBMCs and IFN-γ Elispot assay were used to identify the immunogenicity of 25 putative GPC3 epitopes. During the 20-h ex vivo co-culture of PBMCs with putative epitope peptides, only pre-existed memory or active T cells specific for indicated peptide could be stimulated to produce IFN-γ and display positive spots, while naive T cells could not be elicited to activate. Of note is that the PBMCs used in this assay were collected from HCC patients and not pre-stimulated with indicated peptides. Different from acute infectious diseases, HCC usually happens in the patients with chronic HBV infection. Their memory T cells are partially exhausted or weakly responsive [30, 31]. Meanwhile, the 20-h ex vivo IFN-γ Elispot assay can activate but not expand specific T cells efficiently. Therefore, only a relatively low frequency of responsive T cells was observed, but this approach revealed, to the largest extent, the native functional states of antigen-specific T cells in HCC patients. IFN-γ Elispot assay is one of the widely used methods functionally evaluating antigen-specific T cell and has been used to screen and identify T cell epitopes of tumor, bacteria and virus antigens using patient’s PBMCs [26, 32, 33]. In clinical diagnosis of tuberculosis infection, IFN-γ Elispot assay has been routinely used to date, in which the general criterion to judging positive T cell response is that SFUs/2 × 105 PBMCs > 6 in experimental well while SFUs in negative control well ≤ 5. In this study, much stricter criterion to judging positive T cell response was used as described. Actually, we cannot exclude this possibility that some positive responses were neglected. Among 25 putative epitopes, only five candidate epitopes were finally identified as real-world T cell epitopes. Of which P8 (GPC3144-152) and P23 (GPC3522-530) have been reported by Komori, Yoshikawa [9, 15] and O’ Beirne [10], respectively, and also induced a median SFUs of 10–20 in this assay. More importantly, other cellular functional experiments with distinct designs and principles were further used here to confirm the 5 epitopes validated by IFN-γ Elispot assay. The CD8+ T cell proliferation after patients’ PBMCs in vitro co-stimulation and IFN-γ+/CD8+ T cell increase after healthy donors’ PBLs in vitro co-stimulation confirmed the in vitro immunogenicity of the 5 validated epitopes in inducing memory T cells proliferation or eliciting naive T cells activation. HLA-A2 transgenic mice immunization and the resulting cytolysis against HepG2 cells demonstrated the in vivo immunogenicity of the 5 validated epitopes in eliciting cytotoxic T cell responses. As compared with the weak positive T cell responses in ex vivo IFN-γ Elispot assays, the results from the later cellular functional experiments were more convincing, because the positive T cell responses induced by the five epitope peptides were much stronger in these experiments than in the ex vivo IFN-γ Elispot assays. Furthermore, the binding affinity of the 5 epitopes with HLA-A0201 molecule was evaluated by T2 cell binding assays.
As found, individual HLA-A0201+/GPC3+ HCC patients had T cell responses to different epitopes [9, 10], which underscore the need of identifying the epitopes as many as possible for vaccine design. To evaluate the potential of the five validated epitopes for inducing GPC3-specific CTL responses in vivo, the peptides cocktail was prepared as vaccine to immunize humanized mice in this study. As compared with the HLA-A0201 transgenic mice (HHD mice) generally used in identifying HLA-A0201-restricted epitopes and in evaluating peptide vaccines [34, 35], the HLA-A0201+/+/DR1+/+/H-2-β2m−/−/IAβ−/− C57BL/6 mice should be more suitable to simulate the antigen procession and presentation in human, since the interference caused by mouse H-2 molecules presentation was weakened. As expected, the peptides cocktail vaccine induced strong CD8+ T cell responses specific for the five T cell epitopes as detected by IFN-γ Elispot and ICS. More importantly, the resulting CD8+ T cells could specifically recognize and kill HepG2 target cells, and displayed obviously stronger cytotoxicity than the CD8+ T cells expanded in the 7-day in vitro co-cultures of HCC patients’ PBMCs with the peptides cocktail. These data provide evidence that CTLs in vivo induced by these GPC3-derived peptides are functional and cytotoxic, which consistent with the clinical trial of GPC3-derived peptide vaccine [36]. In addition, splenocytes re-stimulated in vitro with five peptides cocktail showed much stronger cytotoxicity than the splenocytes re-stimulated with mixture of P8 and P23. The combinatorial use of more epitope peptides should be more effective in controlling cancer, which was also verified in other studies [5]. Considering these results, it is no doubt that the five epitope peptides validated here are good candidates for vaccine design and specific CD8+ T cells detection.
It is worth noting that although GPC3-derived peptides displayed encouraging results when used as vaccines in pre-clinical studies, the less satisfactory outcomes in clinical trials have impeded the clinical application of these vaccines as well as similar vaccines for other cancers. The less-than-ideal clinical effects may result from the concerted action of various factors: (i) Vaccination may induce the upregulation of inhibitory receptors on CTLs such as CTLA-4, PD-1 and Tim-3 [37, 38]; (ii) downregulation of GPC3 expression may occur after vaccination [37, 39]; (iii) loss of HLA alleles in patients aggravates the immune escapes of tumor cells [40]; (iv) HCC usually happens in patients with chronic HBV infection, whose CTLs are partially exhausted [30, 31]. Thus, it is reasonable that except for the identification of new T cell epitopes, overcoming T cell exhaustion and reducing immune escape are also key challenges. Combined use with immune-modulating antibodies, effective adjuvants, novel vaccine formulation may be the following topics to make the best use of the validated T cell epitopes.
Acknowledgements
This work was supported in part by the National Nature Science Foundation of China (82041006), Nanjing Municipal Hygiene and Health Fund of Jiangsu Province (zkx18043), and Jiangsu Provincial Hygiene and Health Fund (M2020088). The sponsors had no role in study design, data collection and analysis, preparation of the manuscript, or decision to submit the article for publication.
Abbreviations
- CTL
Cytotoxic T lymphocyte
- GPC3
Carcinoembryonic antigen glypican-3
- HCC
Hepatocellular carcinoma
- HLA
Human leukocyte antigens
- ICS
Intracellular cytokine staining
- MFI
Mean fluorescence intensity
- PBLs
Peripheral blood lymphocytes
- PBMCs
Peripheral blood mononuclear cells
- PHA
Phytohemagglutinin
- SFUs
Spot forming units
- TAA
Tumor-associated antigen
Author contributions
CS and JQ designed and supervised the research. XJ performed the main experiments of this study. XL and ZZ assisted in the in silico prediction of T cell epitopes and set up the functional validation experiments of candidate epitopes using patient’s PBMCs and IFN-γ Elispot assay. YD assisted in the preparation of mature DCs and DC-peptide-PBL co-culture experiments. YW collected blood samples, prepared PBMCs and performed HLA-A genotyping. JQ recruited the HCC patients. XJ and CS organized the whole data and wrote the manuscript with discussions from all authors.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jie Qiu, Email: 13851507229@163.com.
Chuanlai Shen, Email: chuanlaishen@seu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Hiroishi K, Eguchi J, Baba T, et al. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:451–458. doi: 10.1007/s00535-009-0155-2. [DOI] [PubMed] [Google Scholar]
- 3.Ueda Y, Shimizu K, Itoh T, et al. Induction of peptide-specific immune response in patients with primary malignant melanoma of the esophagus after immunotherapy using dendritic cells pulsed with MAGE peptides. Jpn J Clin Oncol. 2007;37:140–145. doi: 10.1093/jjco/hyl136. [DOI] [PubMed] [Google Scholar]
- 4.Sun W, Shi J, Wu J, et al. A modified HLA-A*0201-restricted CTL epitope from human oncoprotein (hPEBP4) induces more efficient antitumor responses. Cell Mol Immunol. 2018;15:768–781. doi: 10.1038/cmi.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang XD, Guo SL, Wang GZ, Li N, Wu YY, Fang DC, Fan YH, Yang SM. In vitro and ex vivo evaluation of a multi-epitope heparinase vaccine for various malignancies. Cancer Sci. 2014;105:9–17. doi: 10.1111/cas.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shih TC, Wang L, Wang HC, Wan YY. Glypican-3: A molecular marker for the detection and treatment of hepatocellular carcinoma() Liver Res. 2020;4:168–172. doi: 10.1016/j.livres.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirakawa H, Suzuki H, Shimomura M, et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100:1403–1407. doi: 10.1111/j.1349-7006.2009.01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawada Y, Yoshikawa T, Nobuoka D, et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18:3686–3696. doi: 10.1158/1078-0432.CCR-11-3044. [DOI] [PubMed] [Google Scholar]
- 9.Komori H, Nakatsura T, Senju S, et al. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin Cancer Res. 2006;12:2689–2697. doi: 10.1158/1078-0432.CCR-05-2267. [DOI] [PubMed] [Google Scholar]
- 10.O'Beirne J, Farzaneh F, Harrison PM. Generation of functional CD8+ T cells by human dendritic cells expressing glypican-3 epitopes. J Exp Clin Cancer Res. 2010;29:48. doi: 10.1186/1756-9966-29-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dargel C, Bassani-Sternberg M, Hasreiter J, et al. T cells engineered to express a T-Cell receptor specific for glypican-3 to recognize and kill hepatoma cells in vitro and in mice. Gastroenterology. 2015;149:1042–1052. doi: 10.1053/j.gastro.2015.05.055. [DOI] [PubMed] [Google Scholar]
- 12.Vogel A, Martinelli E, clinicalguidelines@esmo.org EGCEa, Committee EG Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Ann Oncol. 2021;32:801–805. doi: 10.1016/j.annonc.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Voorter CEM, Palusci F, Tilanus MGJ (2014) Sequence-based typing of HLA: an improved group-specific full-length gene sequencing approach. In: Beksaç M (ed) Bone marrow and stem cell transplantation. Springer New York, New York, NY. pp 101–14 [DOI] [PubMed]
- 14.Jin X, Ding Y, Sun S et al. (2021) Screening of HLA-A restricted T cell epitopes of SARS-CoV-2 and induction of CD8+ T cell responses in HLA-A transgenic mice. bioRxiv preprint 10.1101/2021.04.01.438020. [DOI] [PMC free article] [PubMed]
- 15.Yoshikawa T, Nakatsugawa M, Suzuki S, et al. HLA-A2-restricted glypican-3 peptide-specific CTL clones induced by peptide vaccine show high avidity and antigen-specific killing activity against tumor cells. Cancer Sci. 2011;102:918–925. doi: 10.1111/j.1349-7006.2011.01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan Z, Chen C, Long H, Lei C, Tang G, Li L, Feng J, Chen F. Overexpression of GPC3 inhibits hepatocellular carcinoma cell proliferation and invasion through induction of apoptosis. Mol Med Rep. 2013;7:969–974. doi: 10.3892/mmr.2013.1279. [DOI] [PubMed] [Google Scholar]
- 17.Roncalli M, Borzio M, Di Tommaso L. Hepatocellular dysplastic nodules. Hepatol Res. 2007;37:S125–S134. doi: 10.1111/j.1872-034X.2007.00175.x. [DOI] [PubMed] [Google Scholar]
- 18.Gao W, Kim H, Feng M, Phung Y, Xavier CP, Rubin JS, Ho M. Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology (Baltimore, MD) 2014;60:576–587. doi: 10.1002/hep.26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Yang C, Lu W, Zeng Y. Prognostic significance of glypican-3 expression in hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2018;97:e9702. doi: 10.1097/MD.0000000000009702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu Y, Suzuki T, Yoshikawa T, Endo I, Nakatsura T. Next-generation cancer immunotherapy targeting glypican-3. Front Oncol. 2019;9:248. doi: 10.3389/fonc.2019.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishida T, Kataoka H. Glypican 3-targeted therapy in Hepatocellular carcinoma. Cancers (Basel) 2019 doi: 10.3390/cancers11091339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Gao F, Jiang L, et al. 32A9, a novel human antibody for designing an immunotoxin and CAR-T cells against glypican-3 in hepatocellular carcinoma. J Transl Med. 2020;18:295. doi: 10.1186/s12967-020-02462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chieochansin T, Thepmalee C, Grainok J, Junking M, Yenchitsomanus PT. Cytolytic activity of effector T-lymphocytes against hepatocellular carcinoma is improved by dendritic cells pulsed with pooled tumor antigens. Sci Rep. 2019;9:17668. doi: 10.1038/s41598-019-54087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Yang D, Li S, Gao Y, Jiang R, Deng L, Frankel FR, Sun B. Development of a Listeria monocytogenes-based vaccine against hepatocellular carcinoma. Oncogene. 2012;31:2140–2152. doi: 10.1038/onc.2011.395. [DOI] [PubMed] [Google Scholar]
- 25.Karyampudi L, Krco CJ, Kalli KR, et al. Identification of a broad coverage HLA-DR degenerate epitope pool derived from carcinoembryonic antigen. Cancer Immunol Immunother. 2010;59:161–171. doi: 10.1007/s00262-009-0738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson BM, Frye TP, Johnson LE, Fong L, Knutson KL, Disis ML, McNeel DG. HLA-A2-restricted T-cell epitopes specific for prostatic acid phosphatase. Cancer Immunol Immunother. 2010;59:943–953. doi: 10.1007/s00262-010-0820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiessling A, Stevanovic S, Fussel S, Weigle B, Rieger MA, Temme A, Rieber EP, Schmitz M. Identification of an HLA-A*0201-restricted T-cell epitope derived from the prostate cancer-associated protein prostein. Br J Cancer. 2004;90:1034–1040. doi: 10.1038/sj.bjc.6601642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou M, Xu D, Li X, et al. Screening and identification of severe acute respiratory syndrome-associated coronavirus-specific CTL epitopes. J Immunol. 2006;177:2138–2145. doi: 10.4049/jimmunol.177.4.2138. [DOI] [PubMed] [Google Scholar]
- 29.Tang ST, van Meijgaarden KE, Caccamo N, et al. Genome-based in silico identification of new Mycobacterium tuberculosis antigens activating polyfunctional CD8+ T cells in human tuberculosis. J Immunol. 2011;186:1068–1080. doi: 10.4049/jimmunol.1002212. [DOI] [PubMed] [Google Scholar]
- 30.Kakazu E, Ueno Y, Kondo Y, Fukushima K, Shiina M, Inoue J, Tamai K, Ninomiya M, Shimosegawa T. Branched chain amino acids enhance the maturation and function of myeloid dendritic cells ex vivo in patients with advanced cirrhosis. Hepatology. 2009;50:1936–1945. doi: 10.1002/hep.23248. [DOI] [PubMed] [Google Scholar]
- 31.Moudi B, Heidari Z, Mahmoudzadeh-Sagheb H. Meta-analysis and systematic review of prognostic significance of Glypican-3 in patients with hepatitis B-related hepatocellular carcinoma. Virusdisease. 2019;30:193–200. doi: 10.1007/s13337-019-00517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu SD, Su J, Zhang SM, et al. Identification of HLA-A*11:01-restricted Mycobacterium tuberculosis CD8(+) T cell epitopes. J Cell Mol Med. 2016;20:1718–1728. doi: 10.1111/jcmm.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacey SF, La Rosa C, Kaltcheva T, et al. Characterization of immunologic properties of a second HLA-A2 epitope from a granule protease in CML patients and HLA-A2 transgenic mice. Blood. 2011;118:2159–2169. doi: 10.1182/blood-2011-04-349951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takagi A, Matsui M. Identification of HLA-A*02:01-restricted candidate epitopes derived from the non-structural polyprotein 1a of SARS-CoV-2 that may be natural targets of CD8(+) T cell recognition in vivo. J Virol. 2020 doi: 10.1128/JVI.01837-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tada Y, Yoshikawa T, Shimomura M, Sawada Y, Sakai M, Shirakawa H, Nobuoka D, Nakatsura T. Analysis of cytotoxic T lymphocytes from a patient with hepatocellular carcinoma who showed a clinical response to vaccination with a glypican3derived peptide. Int J Oncol. 2013;43:1019–1026. doi: 10.3892/ijo.2013.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawada Y, Yoshikawa T, Ofuji K, et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5:e1129483. doi: 10.1080/2162402X.2015.1129483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hailemichael Y, Dai Z, Jaffarzad N, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki S, Shibata K, Kikkawa F, Nakatsura T. Significant clinical response of progressive recurrent ovarian clear cell carcinoma to glypican-3-derived peptide vaccine therapy: two case reports. Hum Vaccin Immunother. 2014;10:338–343. doi: 10.4161/hv.27217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paterson AC, Sciot R, Kew MC, Callea F, Dusheiko GM, Desmet VJ. HLA expression in human hepatocellular carcinoma. Br J Cancer. 1988;57:369–373. doi: 10.1038/bjc.1988.84. [DOI] [PMC free article] [PubMed] [Google Scholar]