Abstract
Background
Ectopic lymphoid formations are called tertiary lymphoid structures (TLSs). TLSs in cancer have been reported to be associated with good prognosis and immunotherapy response. However, the relationship between TLSs and lymph node (LN) metastasis is unclear.
Methods
We analyzed 218 patients with radically resected lung adenocarcinoma. TLSs were defined as the overlap of T cell zone and B cell zone. Granzyme B + cells were defined as cytotoxic lymphocytes. We evaluated phenotypes of lymphocytes in TLSs, tumor-infiltrating lymphocytes (TILs) and LNs by immunohistochemistry. We divided the patients into mature TLS (DC-Lamp high) and immature TLS (DC-Lamp low) groups. The relationship between TLS maturation and clinicopathological factors was analyzed.
Results
The mature TLS group was associated with significantly lower frequency of LN metastasis (P < 0.0001) and early cancer stage (P = 0.0049). The mature TLS group had significantly more CD8 + (P = 0.0203) and Foxp3 + (P = 0.0141) cells in TILs than the immature TLS group had. Mature TLSs were independently associated with a favorable overall survival (hazard ratio [HR] = 0.17, P = 0.0220) and disease-free survival (HR = 0.54, P = 0.0436). Multivariate analysis showed that mature TLS was an independent low-risk factor for LN metastasis (odds ratio = 0.06, P = 0.0003). The number of cytotoxic lymphocytes in LNs was higher in the mature TLS group than in the immature group (20.0 vs. 15.1, P = 0.017).
Conclusion
Mature TLSs were associated with an increased number of cytotoxic lymphocytes in draining LNs, a lower frequency of LN metastasis, and favorable outcomes. Mature TLSs may support antitumor immunity by lymphocyte activation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03353-8.
Keywords: Cytotoxic lymphocyte, Lung adenocarcinoma, Lymph node metastasis, Tertiary lymphoid structure
Introduction
Immunotherapy using immune checkpoint inhibitors is associated with improved therapeutic effects in patients with lung cancer [1, 2]; however, biomarkers for predicting responsiveness to immune checkpoint inhibitors remain unclear, aside from the expression of programmed cell death-ligand 1 (PD-L1). Recently, tertiary lymphoid structures (TLSs) have attracted increasing attention as important factors associated with clinical response [3].
TLSs are ectopic lymphoid formations present in inflammatory tissue, such as in autoimmune disease, infection, and malignant tumors [4]. TLSs comprise a T cell zone with mature dendritic cells (DCs), a germinal center with follicular dendritic cells and proliferating B cells, and high endothelial venules (HEVs) [5]. TLSs are associated with expression of chemokines, such as CCL19, CCL21 and CXCL13 [6–10]. It is considered that naïve T cells, B cells and DCs attracted by these chemokines modulate the antitumor immune response [11]. In relation to those findings, TLSs have also been reported to be a positive prognostic factor in various malignant tumors, such as lung, colorectal, urothelial and breast cancers [5, 11–14]. Other studies have shown that the presence of TLS relates to the response to immunotherapy [15–18]. Expression of TLS signature, including chemokine and DC genes such as CCL19, CXCL13, CCR7, CXCR5, SELL and LAMP3, has been reported to be significantly higher in immunotherapy responders than in non-responders [16]. Additionally, the TLS signature impacts outcome of immunotherapy more than do typical T cell signatures such as CD8+ cells [17]. Thus, TLSs are considered to be an important factor associated with the prognosis and efficacy of immunotherapy for patients with lung cancer.
Lymph nodes (LNs) have been considered an important site for activating antitumor immunity [19], and it has been reported that ablation of draining LNs in mice diminishes the effect of immunotherapy [20]. Recently, not only LNs but also TLSs are revealed to be important for antitumor immunity [19]. In terms of the relationship between TLSs and draining LNs, a review about TLSs mentioned a hypothesis that lymphocytes may circulate between TLSs, draining LNs and tumors [4]. However, the effect of intratumoral TLSs on the composition of draining LNs is still incompletely understood.
In this study, we evaluated the relationship between TLS maturation and clinicopathological factors, especially draining LNs. Our results imply that TLS maturation might be associated with an increased number of cytotoxic lymphocytes in the LNs and a decreased frequency of LN metastasis.
Materials and methods
Patients
This study included 240 patients with stage I–IVa lung adenocarcinoma who had undergone surgery between December 2007 and December 2012 at the Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University. A total of 218 formalin-fixed, paraffin-embedded specimens were available for immunohistochemical staining. Clinicopathological characteristics, disease-free survival (DFS) and overall survival (OS) were retrospectively analyzed. Clinicopathological characteristics assessed included age, sex, smoking history, pathological T (pT) status, pathological N (pN) status, pathological M status, histological differentiation grade, pleural invasion, vascular invasion, lymphatic invasion, operative method, adjuvant chemotherapy status, EGFR mutations and PD-L1 positivity. Pleural invasion and vascular invasion were evaluated using Elastica van Gieson (EVG) staining, and lymphatic invasion was evaluated using D2-40 staining; these evaluations were performed by pathologists at Kyushu University. This study was approved by our Institutional Review Board (Kyushu University, IRB No. 2019–232).
Immunohistochemical staining
Immunohistochemical staining was performed in 218 surgically resected lung adenocarcinomas and their draining LNs. Sections were cut at 4-μm thickness from formalin-fixed, paraffin-embedded tissue blocks, then dewaxed with xylene, and rehydrated through a graded concentration series of ethanol. After inhibition of endogenous peroxidase activity with 3% hydrogen peroxide in methanol for 5 min, the sections were pretreated with Target Retrieval Solution (Dako) in a decloaking chamber at 110 °C for 15 min and then incubated with primary antibodies at 4 °C overnight. The primary antibodies were: mouse monoclonal anti-human CD20cy antibody (clone #L26, dilution 1:200; Dako); rat monoclonal anti-mouse DC-Lamp antibody (clone #1010E1.01, dilution 1:100; eurobio SCIENTIFIC); mouse monoclonal anti-human CD3 antibody (clone #F7.2.38, dilution 1:50; DAKO); mouse monoclonal anti-human CD8 antibody (clone #C8/144B, dilution 1:100; Dako); rabbit monoclonal anti-human CD4 antibody (clone #sp35, dilution 1:100; abcam); mouse monoclonal anti-human Foxp3 antibody (clone #236A/E7, dilution 1:100; eBioscience); rabbit polyclonal anti-granzyme B antibody (dilution 1:200; abcam); mouse monoclonal anti-human NKp46/NCR1 antibody (clone #195,314, dilution 1:200; R&D systems); rabbit polyclonal anti-MIP-3 beta/CCL19 antibody (dilution 1:500; abcam); rabbit polyclonal anti-CCL21 antibody (dilution 1:200; abcam); and rabbit polyclonal anti-CCR7 antibody (dilution 1:200; abcam). The immune complex was detected with a Dako EnVision Detection System. The sections were finally reacted in 3,30-diaminobenzidine, counterstained with hematoxylin, and mounted under coverslips. Sections of tonsil were used as positive controls for CD20cy, DC-Lamp, CD3, CD8, CD4, Foxp3, granzyme B, CCL19, CCL21 and CCR7. Stained slides were scanned using the NanoZoomer (Hamamatsu Photonics KK). PD-L1 expression was detected by immunohistochemical staining with rabbit monoclonal anti-human PD-L1 antibody (clone #SP142, dilution 1:100; Spring Bioscience), as described previously [21]. In this study, positive staining of > 1% of tumor cells was considered to denote PD-L1 positivity.
Evaluation of TLSs and TILs
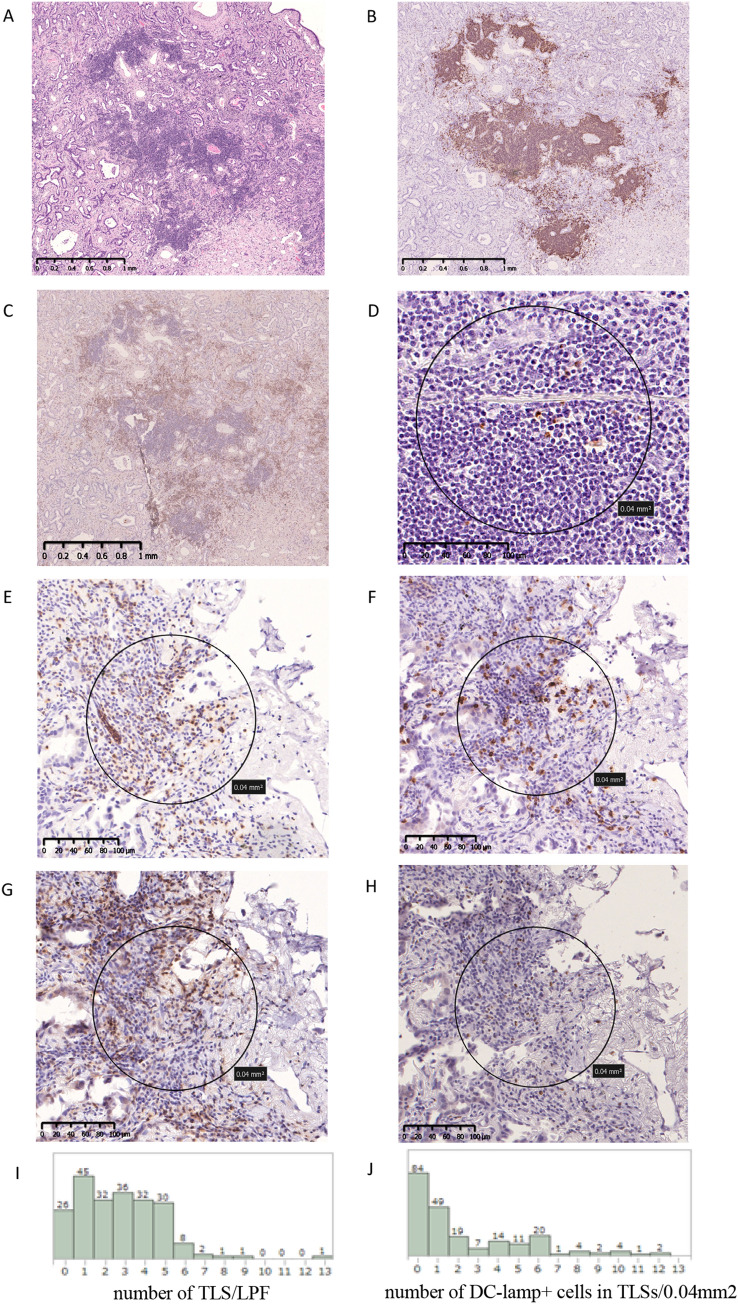
We defined TLSs as the overlap of the T cell zone and the B cell zone based on hematoxylin and eosin, CD20 and CD3 staining (Fig. 1 A, B, C). The densities of DC-Lamp+, granzyme B+, CCL19+, CCL21+, CCR7+ cells in TLSs were evaluated by counting the numbers of DC-Lamp+, granzyme B+, CCL19+, CCL21+ and CCR7+ cells per 0.04 mm2 over 3 TLSs and then averaging the cell counts. The median numbers of DC-Lamp+, granzyme B+, CCL19+, CCL21+, CCR7+ cells were 1.3 (0–12.2)/0.04 mm2,8.2 (0–23.6)/0.04 mm2, 1.0 (0–8.6)/0.04 mm2, 0 (0–4.2)/0.04 mm2 and 3.1 (0–11.2)/0.04 mm2, respectively. The densities of CD3+, CD8+, CD4+, Foxp3+, granzyme B+ and NKp46+ TILs were evaluated by counting the number of CD3+, CD8+, CD4+, Foxp3+, granzyme B+ and NKp46+ TILs per 0.04 mm2 over 5 fields in hotspots of the tumor stroma, then averaging the cell counts. The median numbers of CD3+, CD8+, CD4+, Foxp3+, granzyme B+ and NKp46+ TILs were 63.4 (10.2–121.2)/0.04 mm2, 52.4 (6–127)/0.04 mm2, 47.2 (0.2–104.6)/0.04 mm2, 13.6 (0–49.4)/0.04 mm2, 14.6 (0.6–42.6)/0.04 mm2 and 2.4 (0.2–10.6)/0.04 mm2, respectively. The densities of DC-Lamp+, granzyme B+, NKp46+, CCL19+, CCL21+, CCR7+ cells in draining LNs were evaluated by counting the number of DC-Lamp+, granzyme B+, NKp46+, CCL19+, CCL21+, CCR7+ cells per 0.04 mm2 over five fields in the LNs, then averaging the cell counts. The median numbers of DC-Lamp+, granzyme B+, NKp46+, CCL19+, CCL21+, CCR7+ cells in draining LNs were 35.8 (18.6–48.4)/0.04 mm2, 18.4 (9.4–36)/0.04 mm2, 0.8 (0–5.2)/0.04 mm2, 9.4 (0.4–22.6)/0.04 mm2, 1.7 (0–5.2)/0.04 mm2 and 2.2 (0.2–9.4)/0.04 mm2, respectively. The median numbers of peripheral and central granzyme B+ cells were 15.5 (0–42.8)/0.04 mm2 and 7.7 (0.2–48.8)/0.04 mm2, respectively. In this study, all hematoxylin–eosin staining and immunohistochemical staining was independently evaluated by at least two observers who were blinded to the clinical outcome. Cases with disagreement were reviewed by all observers to reach a consensus. The cut-off values for CD3+, CD8+, CD4+, Foxp3+ cells in TILs and DC-Lamp + cells in draining LNs were defined by the median numbers. The cut-off value for DC-Lamp+ cells in TLSs was set at 1.5 cells/0.04 mm2 by receiver operating characteristic curve. TLS subset was classified using the cut-off value of DC-Lamp+ cells as follows: mature TLS, ≥ 1.5 cells/0.04 mm2 and immature TLS, < 1.5 cells/0.04 mm2.
Fig. 1.
Characterization of tertiary lymphoid structures and tumor-infiltrating lymphocytes. Characterization of tertiary lymphoid structures (TLSs) in lung adenocarcinoma by staining of formalin-fixed, paraffin-embedded tissues (A–D). TLS in lung adenocarcinoma stained by hematoxylin and eosin (A), CD20 (B), CD3 (C) and DC-Lamp (D). Tumor-infiltrating lymphocytes stained by CD3 (E), CD8 (F), CD4 (G) and Foxp3 (H). Histogram of the number of TLSs within low-power field (LPF) (I). Histogram of the number of DC-Lamp+ mature dendritic cells in TLSs (/0.04 mm2) (J)
Statistical analyses
Fisher’s exact test was used to analyze patients’ characteristics. DFS was defined as the period between surgery and the date of last follow-up, recurrence or death, and OS as the period between surgery and the date of last follow-up or death. Survival curves were estimated using the Kaplan–Meier method with the log-rank test. Cox proportional hazards regression analysis and logistic regression analysis were performed to estimate the hazard ratio (HR) and odds ratio (OR) for the risk factors with the backward elimination method. Wilcoxon’s rank sum test was used to compare the number of granzyme B+, CCL19+, CCL21+ and CCR7+ cells. All results were considered as statistically significant at P < 0.05. JMP pro 14.0 software (SAS Institute Japan Ltd.) was used for all statistical analyses.
Results
Clinicopathological characteristics of patients and their association with TILs
This study cohort included 218 patients with lung adenocarcinoma who had undergone surgical resection. The mean age was 68 years (range: 42–85 years), and 108 patients (49.5%) were female. Based on the histological examination of resected tumors, the number of patients with stage I, II, III and IV cancer were 177 (81.2%), 23 (10.6%), 14 (6.4%) and 4 (1.8%), respectively. The detailed clinical characteristics are listed in Supplementary Table 1. TLSs were present in 188 patients (86.2%, Supplementary Table 1). The median number of TLSs per low-power field was 3 (0–13) (Fig. 1I). There were 98 (45.0%) patients in the mature TLS group. Fifty-eight (26.6%) patients were positive for the expression of PD-L1, and 96 patients (44.0%) showed EGFR mutations (Supplementary Table 1). As shown in Table 1, Fisher’s exact test showed that mature TLSs were significantly associated with pN0 (P < 0.0001), early stage (I; P = 0.0049), and increased numbers of CD3+, CD8+, CD4+ and Foxp3+ TILs (P = 0.0001, P = 0.0203, P = 0.0017 and P = 0.0141, respectively). As shown in Supplementary Table 2, Fisher’s exact test showed that patients with TLS were significantly associated with pT status (> 2, P = 0.0260), histopathological differentiation grade (G2,3; P = 0.0098), Pleural invasion (P = 0.0125), vascular invasion (P = 0.0158) and increased the number of CD3+, CD8+, CD4+ and Foxp3+ TILs (P = 0.0101, P = 0.0290, P = 0.0172 and P = 0.0028, respectively).
Table 1.
Clinicopathological characteristics of the patients according to mature/immature TLS
| Characteristics | Immature TLS (n = 120) | Mature TLS (n = 98) | P value | |
|---|---|---|---|---|
| Age | < 70 | 63 (52.5%) | 57 (58.2%) | 0.4152 |
| ≥ 70 | 57 (47.5%) | 41 (41.8%) | ||
| Sex | Female | 59 (49.2%) | 49 (50.0%) | 1.0000 |
| Male | 61(50.8%) | 49 (50.0%) | ||
| Smoking | Never smoker | 60 (50.0%) | 44 (44.9%) | 0.4967 |
| Smoker | 60 (50.0%) | 54 (55.1%) | ||
| T | 1a,1b,1c | 84 (70.0%) | 75 (76.5%) | 0.2888 |
| 2a,2b,3,4 | 36 (30.0%) | 23 (23.5%) | ||
| N | 0 | 93 (77.5%) | 96 (98.0%) | < 0.0001* |
| 1,2,3 | 27 (22.5%) | 2 (2.0%) | ||
| M | 0 | 116 (96.7%) | 98 (100%) | 0.1292 |
| 1a | 4 (3.3%) | 0 (0%) | ||
| Stage | IA1,IA2,IA3,IB | 89 (74.2%) | 88 (89.8%) | 0.0049* |
| IIA,IIB,IIIA,IIIB,IVa | 31 (25.8%) | 10 (10.2%) | ||
| G | 1 | 60 (50.0%) | 50 (51.0%) | 0.8925 |
| 2,3 | 60 (50.0%) | 48 (49.0%) | ||
| Pleural invasion | Negative | 93 (77.5%) | 82 (83.7%) | 0.3057 |
| Positive | 27 (22.5%) | 16 (16.3%) | ||
| Vascular invasion | Negative | 82 (68.3%) | 73 (74.5%) | 0.3684 |
| Positive | 38 (31.7%) | 25 (25.5%) | ||
| Lymphatic invasion | Negative | 102 (85.0%) | 89 (90.8%) | 0.2201 |
| Positive | 18 (15.0%) | 9 (9.2%) | ||
| EGFR** | wild | 61 (54.5%) | 45 (50.0%) | 0.5720 |
| mutation | 51 (45.5%) | 45 (50.0%) | ||
| PD-L1 expression | Negative (< 1%) | 92 (76.7%) | 68 (69.4%) | 0.2809 |
| Positive (> 1%) | 28 (23.3%) | 30 (30.6%) | ||
| TILs CD3 | Low | 68 (56.7%) | 29 (29.6%) | 0.0001* |
| High | 52 (43.3%) | 69 (70.4%) | ||
| TILs CD8 | Low | 65 (54.2%) | 37 (37.8%) | 0.0203* |
| High | 55 (45.8%) | 61 (62.2%) | ||
| TILs CD4 | Low | 70 (58.3%) | 36 (36.7%) | 0.0017* |
| High | 50 (41.7%) | 62 (63.3%) | ||
| TILs Foxp3 | Low | 66 (55.0%) | 37 (37.8%) | 0.0141* |
| High | 54 (45.0%) | 61 (62.2%) |
*P < 0.05 **EGFR unexamined (n = 16) were excluded
TLS: tertiary lymphoid structures, EGFR: epidermal growth factor receptor, PD-L1: programmed cell death ligand-1, TILs: tumor-infiltrating lymphocytes, CD3: Cluster of differentiation 3, CD8: Cluster of differentiation 8, CD4: Cluster of differentiation 4, FoxP3: Forkhead boxprotein P3
Prognosis of patients with lung adenocarcinoma according to mature/immature TLSs
The presence of TLSs was not associated with the OS or DFS in a survival analysis by the Kaplan–Meier method (log-rank test P = 0.0672 and P = 0.1356; respectively; Supplementary Fig. 1A, B).
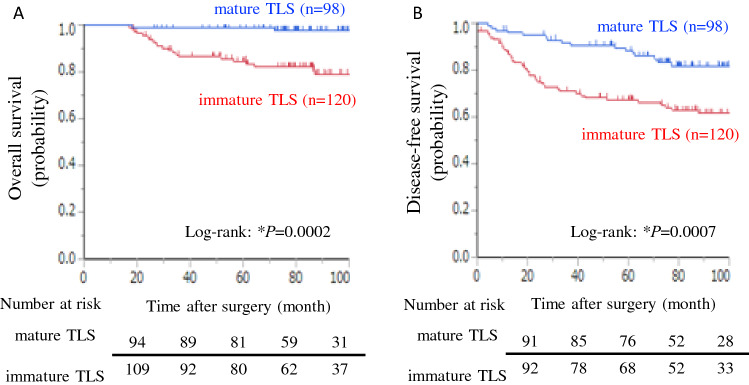
A survival analysis using the Kaplan–Meier method showed that the OS and DFS of patients with mature TLSs were significantly better than those of patients with immature TLSs (log-rank test P = 0.0002 and P = 0.0007; respectively; Fig. 2A, B). Univariate analysis by Cox proportional hazards regression model showed that patients with mature TLSs, age ≥ 70 years, higher tumor stage, adjuvant chemotherapy and positivity for PD-L1 expression were associated with OS (mature vs. immature TLS: HR = 0.10, P = 0.0022; Table 2). Patients with mature TLSs, higher tumor stage and adjuvant chemotherapy were associated with DFS (mature vs. immature TLS: HR, 0.38, P = 0.0011; Supplementary Table 3). In the multivariate analysis, inclusion in the mature TLS group remained a good predictor of the OS (mature vs. immature TLS: HR = 0.17, P = 0.0220; Table 2) and DFS (mature vs. immature TLS: HR = 0.54, P = 0.0436; Supplementary Table 3). Age, tumor stage, and PD-L1 positivity remained worse predictors of OS (Table 2). Age, tumor stage and adjuvant chemotherapy remained worse predictors of DFS (Supplementary Table 3).
Fig. 2.
The maturation of tertiary lymphoid structure relates to prognosis. Kaplan–Meier curves of overall survival (A) and disease-free survival (B) for patients with lung adenocarcinoma depending on mature/immature tertiary lymphoid structure. *P < 0.05
Table 2.
Univariate and multivariate analysis of overall survival
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | ≥ 70 | 2.74 | 1.161–6.472 | 0.0215* | 3.65 | 1.503–8.843 | 0.0042* |
| Sex | Male | 0.96 | 0.425–2.186 | 0.9310 | |||
| Smoking | Smoker | 0.72 | 0.314–1.631 | 0.4257 | |||
| Stage | ≥ II | 10.7 | 4.539–25.33 | < 0.0001* | 6.60 | 2.579–16.90 | < 0.0001* |
| Operative method | Lobectomy | 0.95 | 0.373–2.404 | 0.9094 | |||
| Adjuvant chemotherapy | + | 2.87 | 1.264–6.497 | 0.0117* | |||
| PD-L1 expression | Positive (≥ 1%) | 3.29 | 1.449–7.447 | 0.0044* | 3.12 | 1.294–7.506 | 0.0112* |
| EGFR | mutation | 0.56 | 0.235–1.339 | 0.1935 | |||
| TLS | mature | 0.10 | 0.024–0.442 | 0.0022* | 0.17 | 0.037–0.775 | 0.0220* |
*P < 0.05
HR: hazard ratio, CI: confidence interval, PD-L1: programmed cell death ligand 1, EGFR: epidermal growth factor receptor, TLS: tertiary lymphoid structures
Relationship between mature/immature TLS and LN metastasis
Univariate analyses demonstrated that inclusion in the mature TLS group was associated with a low risk of LN metastasis (mature vs. immature TLS: OR = 0.07, P = 0.0004; Table 3). Pathological T status was associated with a high risk of LN metastasis (Table 3). In the multivariate analysis, the mature TLS group was also independently associated with a low risk of LN metastasis (mature vs. immature TLS: OR = 0.06, P = 0.0003; Table 3). Sex and pT status independently predicted LN metastasis (Table 3).
Table 3.
Univariate and multivariate analysis of lymph node metastasis
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Age | ≥ 70 | 1.01 | 0.548–2.207 | 0.9883 | |||
| Sex | Male | 0.47 | 0.206–1.060 | 0.0689 | 0.25 | 0.091–0.672 | 0.0062* |
| Smoking | Smoker | 0.51 | 0.228–1.138 | 0.1005 | |||
| T | ≥ 2a | 4.96 | 2.195–11.20 | 0.0001* | 7.61 | 2.856–20.27 | < 0.0001* |
| PD-L1 expression | Positive (≥ 1%) | 1.85 | 0.813–4.189 | 0.1424 | |||
| EGFR | mutation | 0.64 | 0.285–1.429 | 0.2748 | |||
| TLS | mature | 0.07 | 0.017–0.310 | 0.0004* | 0.06 | 0.013–0.284 | 0.0003* |
*P < 0.05
CI: confidence interval, PD-L1: programmed cell death ligand 1, EGFR: epidermal growth factor receptor, TLS: tertiary lymphoid structures
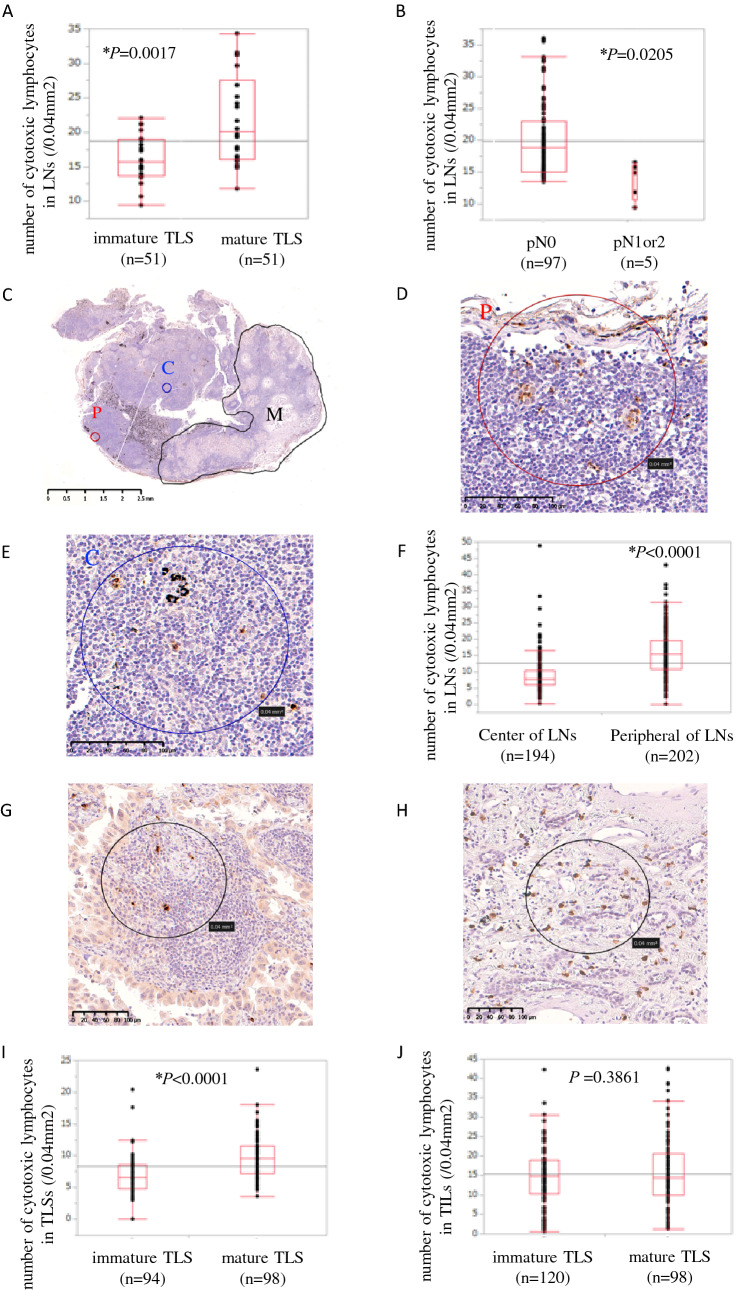
The lower frequency of LN metastasis in the mature TLS group implied a protective effect of mature TLS against LN metastasis. To support this hypothesis, we examined the difference in granzyme B+ cells as activated cytotoxic lymphocytes in draining LNs between the mature and immature TLS groups. The LNs of the mature TLS group were infiltrated by significantly more cytotoxic lymphocytes than the LNs of the immature TLS group (20.0 vs. 15.1; P = 0.017; Fig. 3A). Additionally, comparison between the LN metastasis group (pN1 or 2) and non-LN metastasis group (pN0) showed that the latter had significantly more cytotoxic lymphocytes in the LNs (18.8 vs. 14.9; P = 0.0205; Fig. 3B).
Fig. 3.
The relationship between the maturation of tertiary lymphoid structure and cytotoxic lymphocytes. The mean number of peripheral cytotoxic lymphocytes in non-metastatic lymph nodes (LNs) depending on mature/immature tertiary lymphoid structures (TLSs) (A) and LN metastasis (+ / −) (B). Characterization of cytotoxic lymphocytes stained by granzyme B in LNs (C–E). The number of cytotoxic lymphocytes in LNs at the center and periphery (F). Characterization of cytotoxic lymphocytes stained by granzyme B in TLS (G) and tumor-infiltrating lymphocytes (TILs) (H). The mean number of cytotoxic lymphocytes in TLSs (I) and TILs (J) depending on mature/immature TLS. *P < 0.05. **P, C, M in Fig. 3C-E mean periphery of LN, center of LN and metastasized lesion, respectively
The number of cytotoxic lymphocytes was higher in the LNs of the non-LN metastasis group than the LN metastasis group. This may suggest that cytotoxic lymphocytes have an important role in preventing LN metastasis. Although we demonstrated a relationship between TLS maturation and the number of cytotoxic lymphocytes in LNs, the mechanism by which TLS maturation affects draining LNs is unclear. To determine the effect of mature TLSs on draining LNs, we examined the location of cytotoxic lymphocytes in LNs, and compared the numbers of cytotoxic lymphocytes in the primary tumor of the mature and immature TLS groups. With regard to the number of cytotoxic lymphocytes in LNs (Fig. 3C–E), there were significantly more cytotoxic lymphocytes in the periphery than center of LNs (15.5 vs. 7.7, P < 0.0001; Fig. 3F). This might imply that cytotoxic lymphocytes in the LNs infiltrated from the tumor via the afferent lymphatic vessels in the periphery of the LNs. The median numbers of cytotoxic lymphocytes in TLSs and TILs were evaluated according to the TLS maturation (Fig. 3G, H). There was a significantly higher number of cytotoxic lymphocytes in mature than in immature TLSs (9.8 vs. 6.7, P < 0.0001; Fig. 3I). Patients without TLS were excluded from immature TLS group for this analysis, because the number of cytotoxic lymphocytes in TLSs could not be evaluated. In contrast, no significant difference in the numbers of cytotoxic lymphocytes in TILs was found between the mature and immature TLS groups (14.8 vs. 14.3, P = 0.3861; Fig. 3J). Cytotoxic lymphocytes (granzyme B+ cells) included cytotoxic T lymphocytes and NK cells. For further evaluation, the numbers of NK cells in TILs and LNs were also investigated. There was a significantly higher number of NK cells, but not likely cytotoxic T cells, in the TILs of the mature TLS group than the immature TLS group (3.2 vs. 2.0; P = 0.0016; Supplementary Fig. 2B). On the other hand, the number of NK cells in the LNs of the mature TLS and immature TLS groups did not differ to a statistically significant extent (1.2 vs. 0.8, P = 0.5536; Supplementary Fig. 2D). This suggested that the cytotoxic lymphocytes increasing in LNs were most likely cytotoxic T lymphocytes.
To investigate the effect of LNs on lymphocyte activation, the relationship between DC-Lamp+ cells and cytotoxic lymphocytes in draining LNs was analyzed. The numbers of DC-Lamp + cells in draining LNs were not associated with the numbers of cytotoxic lymphocytes in draining LNs (DC-Lamp high in LNs vs. DC-Lamp low in LNs: 18.4 vs. 18.2; P = 0.9786; Supplementary Fig. 2F). Furthermore, no significant differences in the numbers of cytotoxic lymphocytes in TLSs ((DC-Lamp high in LNs vs. DC-Lamp low in LNs: 7.4 vs. 7.2, P = 0.5161; Supplementary Fig. 2G) or TILs ((DC-Lamp high in LNs vs. DC-Lamp low in LNs: 15.4 vs. 12.3, P = 0.2427; Supplementary Fig. 2H) were found between the DC-Lamp+ cell high and low groups.
The effects of the maturation of TLS on chemokines
The mature TLS group was associated with higher numbers of cytotoxic lymphocytes in the LNs and TLSs. These findings implied the impact of the TLS maturation on lymphocyte activation. To elucidate the effect of the TLS maturation on chemokines, we examined the expression of some chemokines and chemokine receptors including CCL19, CCL21 and CCR7 in TLSs and draining LNs, and compared their expression status between the mature and the immature TLS groups. CCL19, CCL21 and CCR7 were mainly related to the migration of cytotoxic lymphocytes and DCs. As a result, a higher number of CCL19+, CCR7+ cells in TLS and a higher number CCL19+ cells in LNs were detected in the mature TLS group in comparison to the immature TLS group (P = 0.0431, P = 0.0069, P = 0.0002, respectively; Supplementary Fig. 3B). In contrast, the amount of DC-Lamp+ cells in LNs was not associated with these chemokines (Supplementary Fig. 3C).
Discussion
In this study, we evaluated the significance of TLSs in terms of LN metastasis in patients with lung adenocarcinoma. Mature TLSs were significantly associated with less LN metastasis and a good prognosis. The mature TLS group had significantly more cytotoxic lymphocytes and CCL19+ cells in draining LNs and TLSs in comparison to the immature TLS group. On the other hand, the amount of DC-Lamp+ cells in the LNs was not significantly associated with the infiltration of cytotoxic lymphocytes and chemokines. Moreover, the patients without LN metastasis had significantly more cytotoxic lymphocytes in their draining LNs in comparison to patients with LN metastasis. These results suggested that mature TLSs contributed to the increase in cytotoxic lymphocytes in the tumor microenvironment and therefore might prevent LN metastasis.
TLSs have been reported to be a good prognostic factor in several malignant tumors, including lung cancer [5, 11–14], and to be associated with a response to immunotherapy [15–18]. Among colorectal and breast cancer patients, it has been reported that mature TLSs are associated with a lower frequency of LN metastasis [22, 23], which is in line with our results. A review about TLSs mentioned a hypothesis that cytotoxic T lymphocytes circulated between TLSs, draining LNs, and tumors, and that this circulation is an important factor for the control of tumor metastasis [4]. In a study of tumor-bearing mice, which supported the hypothesis about cytotoxic T lymphocyte circulation, draining LNs were infiltrated by more tumor-specific granzyme B+ T cells than nondraining LNs were, and the T cells infiltrated the tumor through the bloodstream [24]. It has been reported that lymphatic vessels are abundant in TLSs [25], which was also found in the current study (data not shown). Generally, afferent lymphatic vessels exist at the periphery of LNs. In the present study, cytotoxic lymphocytes (granzyme B+ cells), including cytotoxic T lymphocytes, NK cells and gamma delta T cells, were more conspicuous at the periphery than at the center of the draining LNs. It has been suggested that the cytotoxic lymphocytes from mature TLSs in the tumor might infiltrate into the periphery of draining LNs through afferent lymphatic vessels, and these cytotoxic lymphocytes may contribute to the prevention of LN metastasis.
In terms of chemokines, since CCL19 is a secreted factor, immunohistochemical staining might be insufficient to evaluate this molecule. However, the mature TLS group showed the significant upregulation of CCL19 in draining LNs, although the number of DC-Lamp+ cells in LNs was not related to the chemokines in TLSs and draining LNs in this study. CCL19 attracts immune cells, such as T cells, NK cells and DCs [26]. CCL19 has been suggested to be secreted by DCs [27]. It has also been reported that CCL19 is upregulated by cytokines, such as tumor necrosis factor (TNF)-α and lymphotoxin (LT)-α[28]. These cytokines are induced by activated lymphocytes and macrophages. The upregulation of CCL19 in mature TLSs may affect the migration of cytotoxic lymphocytes in draining LNs.
Mature TLSs, which we defined by the number of DC-Lamp+ cells in the TLSs (≥ 1.5 cells/0.04 mm2), were independently associated with a good prognosis (Table 2). However, TLSs themselves, which were found in 188 patients (86.2%; Supplementary Table 1), were not associated with the prognosis (Supplementary Fig. 1A, B). We also divided patients into two groups based on the median number of TLSs/LPFs and compared the prognosis of the two groups but found no marked difference (Supplementary Fig. 1C, D). However, the TLS-density-high group had significantly more DCs in TLSs than the TLS-density-low group (Supplementary Fig. 1E). There was a significant prognostic difference between the mature and immature TLS groups but not between the TLS-density-high and TLS-density-low groups. Therefore, the maturity of TLSs might have more prognostic impact than their density. In some previous studies, the maturation of TLSs—as defined by the type of B cells present—was associated with a better prognosis and efficacy of immunotherapy in comparison to the presence of TLSs [14, 16, 17, 29]. Thus, TLS maturation is more important than the mere presence of TLSs for the evaluation of antitumor immunity.
Concerning the tumor microenvironment, more CD8+ cells were found in the mature TLS group than in the immature TLS group in our study (Table 1), and this relationship between TLSs and CD8+ cells has also been reported previously [30]. A larger number of CD8+ cells has been reported to be associated with a good prognosis [31]; thus, mature TLS seems to contribute to antitumor immunity by increasing the number of CD8+ cells in the tumor. Additionally, we found that the PD-L1 expression rates in the mature TLS group were higher than in the immature TLS group (30.6% vs. 23.3%), even though the difference was not statistically significant. Several studies have revealed that TLS positivity is associated with high PD-L1 expression rates [4, 32, 33]. The PD-L1 expression is upregulated by interferon-γ, which is produced by CD8+ cells [34, 35]. Therefore, high PD-L1 positivity of mature TLSs may be induced by large numbers of CD8+ cells.
The present study was associated with some limitations. First, the design was retrospective, and the study cohort did not have many events. Second, only resectable lung adenocarcinoma cases were analyzed in this study. Therefore, whether or not the same results apply to all stages of lung adenocarcinoma is unclear. Third, we did not evaluate B cell subsets or other types of immune cells, such as macrophages and myeloid cells. The association between the presence of TLSs and the B cell subsets has been reported in previous studies. In particular, germ cell markers, a B cell subset, are considered to be associated with the maturation of TLSs [14, 32]. Further analyses including these cell markers are needed to assess the classification of TLS maturity in more detail. Fourth, inflammatory cell markers (CD3, CD4, CD8, Foxp3, Granzyme B, NKp46, CCR7, CCL19, CCL21) were quantified only in a limited number of tumor fields. The results should be carefully interpreted because the analysis may not reflect the whole tumor. Finally, our findings have not yet been confirmed in vivo, although a mouse model of the induction of TLSs has been reported [36, 37]. Hence, in the near future, we plan to investigate the dynamics of cytotoxic lymphocytes and the relationship between TLS maturation and the B cell subsets, macrophages or myeloid cells in vivo.
Conclusions
In the current study, we demonstrated that mature TLSs were associated with increased cytotoxic lymphocytes in draining LNs, a reduced frequency of LN metastasis, and favorable outcomes in patients with lung adenocarcinoma. Mature TLSs may contribute to antitumor immunity as a “relay station” facilitating cytotoxic lymphocytes between tumor and draining LNs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank MN and YK for her invaluable help with tissue processing. We also thank CK, BSc, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Abbreviations
- DC
Dendritic cell
- DFS
Disease-free survival
- HEV
High endothelial venule
- HR
Hazard ratio
- LN
Lymph node
- LT
Lymphotoxin.
- OR
Odds ratio
- OS
Overall survival
- PD-L1
Programmed cell death-ligand 1
- pN
Pathological N status
- pT
Pathological T status
- TIL
Tumor-infiltrating lymphocyte
- TLS
Tertiary lymphoid structure
- TNF
Tumor necrosis factor
Author contributions
Conception and design: SW, TT, MM. Development of methodology: SW, FK, TT, YO. Acquisition of data: SW, YO, YO, FK. Analysis and interpretation of data: SW, FK, NH, TT, TT, YO, MS, MM. Writing, review, and/or revision of manuscript: SW, FK, NH, TT, YO, MM. Study supervision: TT, MS, YO, MM.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
None.
Ethics approval
This study was approved by our Institutional Review Board (Kyushu University, IRB No. 2019–232).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, Investigators K. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC, Investigators K. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 3.Bruno TC. New predictors for immunotherapy responses sharpen our view of the tumour microenvironment. Nature. 2020;577(7791):474–476. doi: 10.1038/d41586-019-03943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35(11):571–580. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, Fridman WH, Cadranel J. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 6.Colbeck EJ, Ager A, Gallimore A, Jones GW. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in Disease? Front Immunol. 2017;8:1830. doi: 10.3389/fimmu.2017.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohee S, Garaud S, Noel G, Dang Chi VL, Lodewyckx JN, Naveaux C, Duvillier H, Goriely S, Larsimont D, Willard-Gallo K. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017 doi: 10.1172/jci.insight.91487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayar S, Campos J, Smith CG, Iannizzotto V, Gardner DH, Mourcin F, Roulois D, Turner J, Sylvestre M, Asam S, Glaysher B, Bowman SJ, Fearon DT, Filer A, Tarte K, Luther SA, Fisher BA, Buckley CD, Coles MC, Barone F. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc Natl Acad Sci U S A. 2019;116(27):13490–13497. doi: 10.1073/pnas.1905301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin L, Hu X, Zhang H, Hu H. Tertiary lymphoid organs in cancer immunology: mechanisms and the new strategy for immunotherapy. Front Immunol. 2019;10:1398. doi: 10.3389/fimmu.2019.01398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nerviani A, Pitzalis C. Role of chemokines in ectopic lymphoid structures formation in autoimmunity and cancer. J Leukoc Biol. 2018;104(2):333–341. doi: 10.1002/JLB.3MR0218-062R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, Validire P, Damotte D, Alifano M, Magdeleinat P, Cremer I, Teillaud JL, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189(7):832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 12.Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112(11):1782–1790. doi: 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koti M, Xu AS, Ren KYM, Visram K, Ren R, Berman DM, Siemens DR. Tertiary lymphoid structures associate with tumour stage in urothelial bladder cancer. Bladder Cancer. 2017;3(4):259–267. doi: 10.3233/BLC-170120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Posch F, Silina K, Leibl S, Mundlein A, Moch H, Siebenhuner A, Samaras P, Riedl J, Stotz M, Szkandera J, Stoger H, Pichler M, Stupp R, van den Broek M, Schraml P, Gerger A, Petrausch U, Winder T. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018;7(2):e1378844. doi: 10.1080/2162402X.2017.1378844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautes-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L, Wargo JA. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, van Schoiack A, Lovgren K, Warren S, Jirstrom K, Olsson H, Pietras K, Ingvar C, Isaksson K, Schadendorf D, Schmidt H, Bastholt L, Carneiro A, Wargo JA, Svane IM, Jonsson G. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 17.Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougouin A, Moreira M, Lacroix G, Natario I, Adam J, Lucchesi C, Laizet YH, Toulmonde M, Burgess MA, Bolejack V, Reinke D, Wani KM, Wang WL, Lazar AJ, Roland CL, Wargo JA, Italiano A, Sautes-Fridman C, Tawbi HA, Fridman WH. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 18.Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, Rekhtman N, Anders RA, Cuda JD, Illei PB, Gabrielson E, Askin FB, Niknafs N, Smith KN, Velez MJ, Sauter JL, Isbell JM, Jones DR, Battafarano RJ, Yang SC, Danilova L, Wolchok JD, Topalian SL, Velculescu VE, Pardoll DM, Brahmer JR, Hellmann MD, Chaft JE, Cimino-Mathews A, Taube JM. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC) Ann Oncol. 2018;29(8):1853–1860. doi: 10.1093/annonc/mdy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 20.Chamoto K, Chowdhury PS, Kumar A, Sonomura K, Matsuda F, Fagarasan S, Honjo T. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc Natl Acad Sci U S A. 2017;114(5):E761–E770. doi: 10.1073/pnas.1620433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takada K, Okamoto T, Shoji F, Shimokawa M, Akamine T, Takamori S, Katsura M, Suzuki Y, Fujishita T, Toyokawa G, Morodomi Y, Okano S, Oda Y, Maehara Y. Clinical significance of PD-L1 protein expression in surgically resected primary lung adenocarcinoma. J Thorac Oncol. 2016;11(11):1879–1890. doi: 10.1016/j.jtho.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Figenschau SL, Fismen S, Fenton KA, Fenton C, Mortensen ES. Tertiary lymphoid structures are associated with higher tumor grade in primary operable breast cancer patients. BMC Cancer. 2015;15:101. doi: 10.1186/s12885-015-1116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trajkovski G, Ognjenovic L, Karadzov Z, Jota G, Hadzi-Manchev D, Kostovski O, Volcevski G, Trajkovska V, Nikolova D, Spasevska L, Janevska V, Janevski V. Tertiary lymphoid structures in colorectal cancers and their prognostic value. Open Access Maced J Med Sci. 2018;6(10):1824–1828. doi: 10.3889/oamjms.2018.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dammeijer F, van Gulijk M, Mulder EE, Lukkes M, Klaase L, van den Bosch T, van Nimwegen M, Lau SP, Latupeirissa K, Schetters S, van Kooyk Y, Boon L, Moyaart A, Mueller YM, Katsikis PD, Eggermont AM, Vroman H, Stadhouders R, Hendriks RW, Thusen JV, Grunhagen DJ, Verhoef C, van Hall T, Aerts JG. The PD-1/PD-L1-checkpoint restrains T cell Immunity in tumor-draining lymph nodes. Cancer Cell. 2020 doi: 10.1016/j.ccell.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Engelhard VH, Rodriguez AB, Mauldin IS, Woods AN, Peske JD, Slingluff CL., Jr Immune cell infiltration and Tertiary lymphoid structures as determinants of antitumor immunity. J Immunol. 2018;200(2):432–442. doi: 10.4049/jimmunol.1701269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pesce S, Moretta L, Moretta A, Marcenaro E. Human NK cell subsets redistribution in pathological conditions: a role for CCR7 receptor. Front Immunol. 2016;7:414. doi: 10.3389/fimmu.2016.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71(20):6391–6399. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 28.Guedj K, Khallou-Laschet J, Clement M, Morvan M, Gaston AT, Fornasa G, Dai J, Gervais-Taurel M, Eberl G, Michel JB, Caligiuri G, Nicoletti A. M1 macrophages act as LTbetaR-independent lymphoid tissue inducer cells during atherosclerosis-related lymphoid neogenesis. Cardiovasc Res. 2014;101(3):434–443. doi: 10.1093/cvr/cvt263. [DOI] [PubMed] [Google Scholar]
- 29.Germain C, Gnjatic S, Dieu-Nosjean MC. Tertiary lymphoid structure-associated B cells are key players in anti-tumor immunity. Front Immunol. 2015;6:67. doi: 10.3389/fimmu.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M, Validire P, Remark R, Hammond SA, Cremer I, Damotte D, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74(3):705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 31.Zeng DQ, Yu YF, Ou QY, Li XY, Zhong RZ, Xie CM, Hu QG. Prognostic and predictive value of tumor-infiltrating lymphocytes for clinical therapeutic research in patients with non-small cell lung cancer. Oncotarget. 2016;7(12):13765–13781. doi: 10.18632/oncotarget.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silina K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, Wandres M, Isajevs S, Cheng P, Curioni-Fontecedro A, Foukas P, Levesque MP, Moch H, Line A, van den Broek M. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. 2018;78(5):1308–1320. doi: 10.1158/0008-5472.CAN-17-1987. [DOI] [PubMed] [Google Scholar]
- 33.Solinas C, Garaud S, De Silva P, Boisson A, Van den Eynden G, de Wind A, Risso P, Rodrigues Vitoria J, Richard F, Migliori E, Noel G, Duvillier H, Craciun L, Veys I, Awada A, Detours V, Larsimont D, Piccart-Gebhart M, Willard-Gallo K. Immune checkpoint molecules on tumor-infiltrating lymphocytes and their association with tertiary lymphoid structures in human breast cancer. Front Immunol. 2017;8:1412. doi: 10.3389/fimmu.2017.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6(6):223. doi: 10.1186/gb-2005-6-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodge G, Barnawi J, Jurisevic C, Moffat D, Holmes M, Reynolds PN, Jersmann H, Hodge S. Lung cancer is associated with decreased expression of perforin, granzyme B and interferon (IFN)-gamma by infiltrating lung tissue T cells, natural killer (NK) T-like and NK cells. Clin Exp Immunol. 2014;178(1):79–85. doi: 10.1111/cei.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson-Percival A, He B, Li ZJ, Kjellen A, Russell K, Li J, Larma I, Ganss R. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat Immunol. 2017;18(11):1207–1217. doi: 10.1038/ni.3836. [DOI] [PubMed] [Google Scholar]
- 37.Martin C, Thevenot G, Danel S, Chapron J, Tazi A, Macey J, Dusser DJ, Fajac I, Burgel PR. Pseudomonas aeruginosa induces vascular endothelial growth factor synthesis in airway epithelium in vitro and in vivo. Eur Respir J. 2011;38(4):939–946. doi: 10.1183/09031936.00134910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.