Abstract
Objective
To summarize the clinical characteristics and immunological and genetic features of patients who developed autoimmune polyendocrine syndrome type II (APS-2) after treatment with immune checkpoint inhibitors (ICIs).
Design and methods
Several databases (MEDLINE/EMBASE/Cochrane) were searched for studies published between January 2000 and February 2020 involving patients with two or more endocrine disorders after ICI therapy.
Results
Our final review included 22 articles comprising 23 patients (median age 56 years; 65.2% male patients). Of these patients, 60.9% received anti-programmed cell death 1 (PD-1) therapy, 17.4% received anti-programmed cell death ligand 1 (PD-L1) therapy, and 4.3% received anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) monotherapy. Patients underwent a median of four treatment cycles before the onset of the primary adverse event; the median time of onset was 8.5 weeks. Endocrine organs affected by ICI administration included the thyroid gland (18/23, 78.3%), pancreatic islets (17/23, 73.9%), pituitary gland (11/23, 47.8%), and adrenal gland (2/23, 8.7%). Related autoantibodies were detected in 65.2% of patients. In patients with diabetes, glutamic acid decarboxylase antibody was closely related to the development of diabetes ketoacidosis. The human leukocyte antigen genotype was reported in 34.8% (8/23) of patients, 5 (62.5%) of which had risk genotypes.
Conclusions
As a serious adverse event of ICI treatment, APS-2 is presented with abrupt initiation time and rapid development. Physicians should be aware of potential endocrine disorders and continue monitoring hormone status when treating cancer patients with ICIs.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02699-1) contains supplementary material, which is available to authorized users.
Keywords: Autoimmune polyendocrine syndrome, Immune checkpoint inhibitors, Endocrinopathy, Immune-related adverse effect
Introduction
Immune checkpoints are negative modulators of the immune system to prevent an overactivated immune response. Monoclonal antibodies called immune checkpoint inhibitors (ICIs) have been developed to blockade these regulators, including cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1), as well as its ligand, programmed cell death ligand 1 (PD-L1). These agents have shown remarkable antitumor effects on diverse cancer types by overcoming immune tolerance and enhancing immunity of the tumor microenvironment. The anti-CTLA-4 antibody, ipilimumab, has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of melanoma. In addition, PD-1 inhibitors (pembrolizumab and nivolumab) and PD-L1 antibodies (atezolizumab, durvalumab, and avelumab) have been shown to improve overall survival in various cancers and have also been approved by the FDA [1].
While activating the immune system, ICIs are also expected to induce autoimmune diseases, often named immune-related adverse events (irAEs) [2]. These side effects could involve many organs including the gastrointestinal tract, skin, and endocrine glands. Among the endocrinopathies caused by PD-1 or PD-L1 inhibitors, hypothyroidism (6.1%) and hyperthyroidism (2.8%) are most commonly observed [3]. By contrast, Type 1 Diabetes Mellitus (T1DM, 0.2%) and primary adrenal insufficiency (0.7%) is rare in patients treated with ICIs [4]. Hypophysitis is a rare adverse event in patients on PD-1 inhibitors (0.4%) and PD-L1 antibodies (< 0.1%) while its incidence is relatively high in those treated with CTLA-4 inhibitors (3.2%) [4].
Sometimes, patients treated with ICIs develop autoimmune polyendocrine syndrome type II (APS-2), defined as having two or three of the following endocrinopathies: autoimmune thyroid disease, type 1 diabetes, and Addison’s disease (adrenal insufficiency) [5]. In 1926, the occurrence of adrenal insufficiency and hypothyroidism in a single patient was firstly described by Schmidt [6]. APS-2, a polygenetic disease, is characterized by increased autoantibodies in the blood and infiltrating lymphocytes in the impaired glands, leading to failure of multiple endocrine glands. It is known that patients at risk of APS-2 have mutations in CTLA-4 or human leukocyte antigen (HLA) DR3-DQ2 and DR4-DQ8 [5]. Several cases have reported two or more endocrine irAEs in an individual patient after the administration of ICIs [7]. However, whether APS-2 is associated with immune checkpoint inhibition has yet to be elucidated. Herein, we provide an overview of case studies on APS-2 related to PD-1/PD-L1 or CTLA-4 inhibitors to provide some evidence of the association.
Methods
Literature search
We conducted a search of several databases (MEDLINE/EMBASE/Cochrane) for studies on patients with two or more endocrine disorders after immune checkpoint inhibition from January 2000 to February 2020. Different terms of “autoimmune polyendocrine syndrome” and various current available ICIs were combined by Boolean operators AND/OR to form the search approach, which is provided in Supplementary Table 1. In addition, we enrolled five more case reports from two published reviews [7, 8].
Study selection
We intended to select cancer patients who developed two or three autoimmune endocrinopathies, including autoimmune thyroid disease, T1DM, and adrenal insufficiency, during the treatment of ICIs with adequate clinical data. After removing the duplicates, two independent investigators reviewed the title and abstract to initially exclude the articles that did not fit our inclusion criteria. Language was restricted to English, and only case reports were included. We excluded conference abstracts, reviews, systematic review or meta-analysis articles, corresponding letters, and off-topic articles. Then, further selection was conducted by reviewing full texts. Patients with only one endocrine disorder after the checkpoint inhibition or with any history of autoimmune or endocrine disease were excluded.
Data extraction
The following data were extracted from each report: year and author of publication, age, gender, country, primary cancer situation, checkpoint inhibitor treatment, number of therapy cycles, endocrine adverse event (hypophysitis, thyroiditis, hypothyroidism, hyperthyroidism, primary adrenal insufficiency, and diabetes mellitus), time intervals between the onset of disorders, corresponding laboratory test results, autoimmune antibodies, and HLA genotypes.
Statistical analysis
We conducted the statistical analysis using SPSS version 20.0 software. Descriptive statistics were utilized to evaluate data. For continuous data, median and range were used and for categorical variables, frequencies and percentages were used. For two-group comparison, the Mann–Whitney U test was utilized. P values under 0.05 were considered statistically significant.
Results
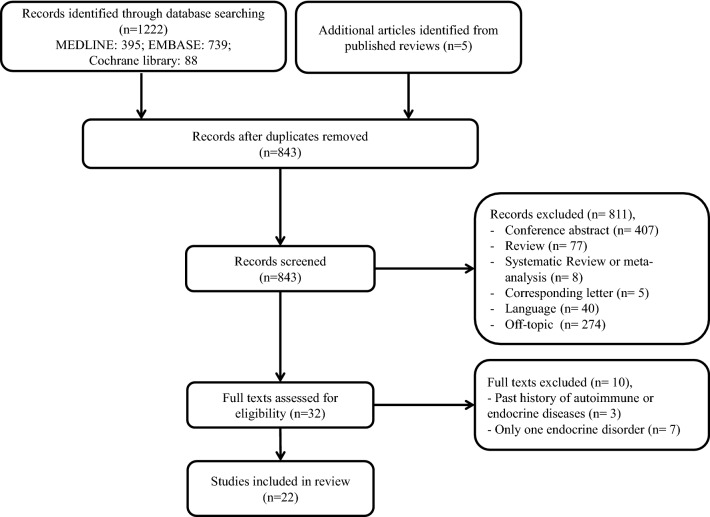
Our search yielded a total of 843 articles after the removal of duplicates. Of these articles, 811 articles were excluded by reviewing title and abstract. The remaining 32 publications were further assessed based on the full text. Seven reports were excluded because only one endocrine disorder was present in each patient. In addition, three other articles were excluded because they were diagnosed with type 2 diabetes before receiving immune checkpoint therapy. Finally, 22 articles with 23 patients were enrolled in our review [7–28]. The flow chart of publication selection is shown in Fig. 1.
Fig. 1.
Flow chart of article selection
Case characteristics
The median age of patients selected was 56 years (range 43–83 years), with a male predominance (15/23, 65.2%). Most patients were from United States (7/23, 30.4%), followed by Italy (3/23, 13.0%) and Japan (3/23, 13.0%). Other cases were reported in diverse countries including Australia, France, United Kingdom, Ireland et al. The major cancer types of the identified cases were melanoma (11/23, 47.8%) and lung cancer (7/23, 30.4%). The rest of the cases were other cancer types, including breast cancer, bladder cancer, gastric cancer et al.
More than half of the patients (14/23, 60.9%) received anti-PD-1 therapies (nivolumab or pembrolizumab), and four patients (17.4%) received anti-PD-L1 therapies (avelumab, atezolizumab, or durvalumab). Among these patients, one 55-year-old man received a combination therapy of durvalumab and bacillus Calmette-Guérin [28]. Additionally, only one patient was treated with the anti-CTLA-4 therapy ipilimumab alone, and two patients (8.7%) received a combination therapy of ipilimumab with nivolumab. Furthermore, two patients received ICI treatments in a sequence: one was treated with ipilimumab followed by pembrolizumab, and the other received nivolumab followed by a combination of ipilimumab and nivolumab. These case characteristics are summarized in Table 1.
Table 1.
Summary of patients characteristics
| Characteristics | Value | Percentage (%) |
|---|---|---|
| Age (years) | ||
| Median (range) | 56 (43–83) | |
| Gender | ||
| Male | 15 | 65.2 |
| Female | 8 | 34.8 |
| Country | ||
| US | 7 | 30.4 |
| Italy | 3 | 13.0 |
| Japan | 3 | 13.0 |
| Australia | 2 | 8.7 |
| France | 2 | 8.7 |
| UK | 2 | 8.7 |
| Irland | 1 | 4.3 |
| Korea | 1 | 4.3 |
| Portugal | 1 | 4.3 |
| Spain | 1 | 4.3 |
| Cancer type | ||
| Melanoma | 11 | 47.8 |
| Lung cancer | 7 | 30.4 |
| Bladder cancer | 1 | 4.3 |
| Breast cancer | 1 | 4.3 |
| Gastric cancer | 1 | 4.3 |
| Renal cell carcinoma | 1 | 4.3 |
| Parotid gland adenocarcinoma | 1 | 4.3 |
| Type of checkpoint inhibitor | ||
| PD-1 | 14 | 60.9 |
| PD-L1 | 4 | 17.4 |
| CTLA-4 | 1 | 4.3 |
| CTLA-4 + PD-1 | 2 | 8.7 |
| Othersa | 2 | 8.7 |
CTLA-4 cytotoxic T-lymphocyte antigen 4, PD-1 programmed cell death 1, PD-L1 programmed cell death ligand 1, UK United Kingdom, US United States
aOne was treated with ipilimumab followed by pembrolizumab, and the other received nivolumab followed by a combination of ipilimumab and nivolumab
Clinical features
Detailed patient characteristics and critical laboratory test results are presented in Table 2. The median immune checkpoint therapy course received before onset of the first adverse event was 4 cycles (range 1–13 cycles), and the median time from therapy initiation to irAE onset was 8.5 weeks (range 2–30 weeks; Fig. 2a). Early-onset autoimmune disorders (within 2 cycles) were witnessed in 30.4% patients (8/23) who received nivolumab, pembrolizumab, atezolizumab, durvalumab, or a combination of ipilimumab and nivolumab.
Table 2.
Summary of case reports of APS-2 related to ICI therapies
| Author (year) | Age | Gender | Country | Cancer type | Therapy | Onset duration (week/cycle)a | Condition | Autoantibody | HLA genotype | Response |
|---|---|---|---|---|---|---|---|---|---|---|
| Hansen (2016) | 58 | Male | US | Melanoma | Pembrolizumab | NR | Hypothyroidism | GAD ( +) | NR | PR |
| 52/17 | T1DM | |||||||||
| Kong (2016) | 68 | Male | Korea | Lung cancer | Pembrolizumab | 21/7 | T1DM (DKA) | (−) | DRB1*0901-DQB1*0303 | PR |
| 0 | Hyperthyroidism | |||||||||
| Lowe (2016) | 54 | Male | US | Melanoma | Ipilimumab and nivolumab | 2/1 | Hyperthyroidism | TSHRAb (+), GAD (+) | A2, DQB1*0602 | CR |
| 6/1 | Hypothyroidism | |||||||||
| 11/1 | T1DM (DKA) | |||||||||
| 0 | Hypophysitis (adrenal insufficiency) | |||||||||
| Humayun (2016) | 55 | Male | UK | Melanoma | Ipilimumab | 8/4 | Hypophysitis (hypopituitarism) | (−) | NR | SD |
| Pembrolizumab | NR/9 | T1DM (DKA) | ||||||||
| Kuru (2017) | 83 | Female | US | Melanoma | Nivolumab | 30/15 | Thyroiditis (hypothyroidism) | TPO (+) | NR | PR |
| 3/0 | Hypophysitis (adrenal insufficiency) | |||||||||
| Marchand (2017) | 55 | Male | France | Lung cancer | Nivolumab | 19/9 | T1DM (DKA) | (−) | NR | PR |
| 4/0 | Hypophysitis (adrenal insufficiency) | |||||||||
| Paepegaey (2017) | 55 | Female | France | Melanoma | Pembrolizumab | 17/6 | Thyroiditis (hyperthyroidism) | 21-OH Ab (+), ACA (+) | NR | PD |
| 2/0 | Hypothyroidism | |||||||||
| 12/4 | Adrenalitis (Acute adrenal crisis) | |||||||||
| Li (2017) | 63 | Male | US | Lung cancer | Nivolumab | 4/1 | T1DM (DKA) | GAD (+), TPO (+) | NR | PD |
| 9/NR | Hypothyroidism | |||||||||
| Aziz (2018) | 69 | Male | US | Gastric cancer | Avelumab | 13/7 | Thyroiditis (hyperthyroidism) | NR | NR | NR |
| 17/8 | Hypothyroidism | |||||||||
| 0 | Adrenalitis (adrenal insufficiency) | |||||||||
| Sakurai (2018) | 68 | Female | Japan | Renal cell carcinoma | Nivolumab | 2/1 | Thyroiditis (hyperthyroidism) | TgAb (+), TPO (+) | DRB1*09:01-DQB1*03:03 | NR |
| 4/2 | Hypothyroidism | |||||||||
| 8/4 | T1DM | |||||||||
| Sum (2018) | 75 | Male | US | Melanoma | Nivolumab and ipilimumab | 5/3 | T1DM | GAD (+) | NR | CR |
| 2/0 | Hypophysitis (adrenal insufficiency) | |||||||||
| Tzoulis (2018) | 56 | Female | UK | Lung cancer | Nivolumab | 6.5/3 | T1DM (DKA) | GAD (+) | NR | PR |
| 30/14 | Hypothyroidism | |||||||||
| Okahata (2019) | 52 | Female | Japan | Breast cancer | Nivolumab | 30/13 | Hypophysitis (adrenal insufficiency) | (−) | DRB1*14:05, 14:06 | NR |
| 4/0 | T1DM | DQB1*03:01, 03:03 | ||||||||
| Erra (2019) | 63 | Male | US | Melanoma | Ipilimumab | 9/4 | Hypophysitis (adrenal insufficiency) | (−) | NR | PR |
| 43/0 | Hypothyroidism | |||||||||
| Gunjur (2019) | 77 | Female | Australia | Melanoma | Pembrolizumab | 3/1 | T1DM (DKA) | GAD (+), IA2 (+) | DRB1*04.16; DQB1*02.05 | CR |
| 6/3 | Thyroiditis (hypothyroidism) | DQA1*01.03 | ||||||||
| Hakami (2019) | 52 | Male | Irland | Melanoma | pembrolizumab | 12/5 | Thyroiditis (hypothyroidism) | (−) | NR | PR |
| 8/2 | T1DM (DKA) | |||||||||
| Lanzolla (2019) | 60 | Male | Italy | Lung cancer | Atezolizumab | 6/2 | T1DM (DKA) | 21-OH Ab (+), APA (+) | DRB1*04; DQB1*03 | PD |
| 5/2 | Hypophysitis (adrenal insufficiency, hypogonadotropic hypogonadism) | |||||||||
| Lupi (2019) | 80 | Male | Italy | Melanoma | Pembrolizumab | 4/2 | Thyroiditis (hypothyroidism) | TgAb (+), TPO (+) | NR | SD |
| 26/7 | Hypophysitis (adrenal insufficiency) | |||||||||
| Lupi (2019) | 43 | Female | Italy | Melanoma | Nivolumab | 3/2 | Thyroiditis (hypothyroidism) | TPO (+), APA (+) | DQB1*02; DQB1*0602 | SD |
| 8/4 | T1DM (DKA) | DQA1*0102 | ||||||||
| Ipilimumab and nivolumab | 17/1 | Hypophysitis (adrenal insufficiency) | ||||||||
| Machado (2019) | 55 | Male | Portugal | Lung cancer | Nivolumab | 12/7 | Thyroiditis (hyperthyroidism) | (−) | NR | PR |
| 4/0 | Hypothyroidism | |||||||||
| 53/33 | Hypophysitis (adrenal insufficiency) | |||||||||
| Mengíbar (2019) | 55 | Male | Spain | Bladder cancer | Durvalumabb | 3/1 | T1DM (DKA) | GAD (+), IA2 (+), TPO (+), TSHRAb (+), TgAb (+) | NR | SD |
| 2/0 | Thyroiditis (hypothyroidism) | |||||||||
| Patel (2019) | 49 | Female | Australia | Lung cancer | Durvalumab | 13/6 | T1DM (DKA) | GAD (+) | NR | PR |
| 4/0 | Hypothyroidism | |||||||||
| Kurihara (2020) | 48 | Male | Japan | Parotid gland adenocarcinoma | Nivolumab | 18/5 | T1DM | TSHRAb(+) | DRB1*04:05 | SD |
| 0 | Thyroiditis (hyperthyroidism) |
21-OH Ab 21-hydroxylase antibody, ACA adrenal cortex antibody, APA anti-pituitary antibody, CR complete response, DKA diabetes ketoacidosis, GAD Glutamic Acid Decarboxylase, HLA human leukocyte antigen, IA2 islet cell autoantibody, NR not reported, PD progressive disease, PR partial response, SD stable disease, T1DM Type 1 Diabetes Mellitus, TgAb thyroglobulin antibody, TPO thyroid peroxidase antibody, TSHRAb thyrotropin receptor antibody, UK United Kingdom, US United States
aFor the primary onset disorder, it is time from initiation of therapy; for the secondary disorder, it is time from the last disorder
bCombined with BCG (Bacillus Calmette–Guérin)
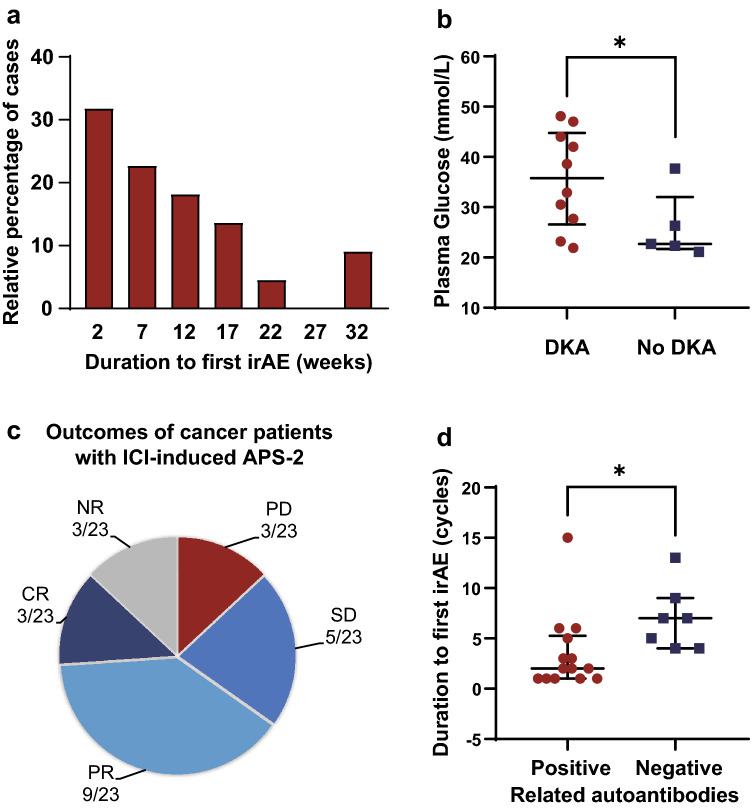
Fig. 2.
Clinical features of APS-2 cases induced by ICI treatments. a Histogram of duration to the first adverse event (weeks). n = 22 reporting time to first onset. b Plasma glucose levels between T1DM patients with or without DKA. n = 10 T1DM cases with DKA, n = 5 T1DM cases with no DKA. c Outcomes of cancer in APS-2 patients triggered by ICIs. d Duration to the onset of first adverse event (cycles) by autoantibody positivity. In this figure, the duration to the onset of first adverse event is presented as the number of cycles that patients received. n = 14 patients reporting time to first onset with positive autoantibodies, n = 7 patients reporting time to first onset with negative autoantibodies. Values are presented as the median ± interquartile range; *P < 0.05. APS-2 autoimmune polyendocrine syndrome type II, CR complete response, DKA diabetes ketoacidosis, ICI immune checkpoint inhibitor, irAE immune-related adverse event, NR not reported, PD progressive disease, PR partial response, SD stable disease
Endocrine organs affected by ICI administration included the thyroid gland (18/23, 78.3%), pancreatic islets (17/23, 73.9%), pituitary gland (11/23, 47.8%), and adrenal gland (2/23, 8.7%). In most patients (21/23, 91.3%), two sites were involved in immune checkpoint therapy. Nearly half of them presented with thyroiditis and T1DM, followed by five patients who developed T1DM with hypophysitis. In addition, two other patients showed disorders of three endocrine glands, displaying thyroiditis, T1DM and hypophysitis.
Among the 18 patients who presented with a thyroid disorder, 5 (27.8%) transitioned from hyperthyroidism to hypothyroidism, 11 (61.1%) had a single hypothyroidism phase, and 2 (11.1%) had hyperthyroidism. Patients with hypothyroidism exhibited increased thyroid stimulating hormone (TSH) levels with low free thyroxine (FT4) or free triiodothyronine levels, whereas those with hyperthyroidism presented with the opposite laboratory values. Another most frequently affected organ was the pancreatic islet, precipitating the onset of T1DM. Most patients with T1DM (12/17, 70.6%) presented with diabetes ketoacidosis (DKA), with a median plasma glucose of 35.8 mmol/L (range 21.9–48.1 mmol/L). T1DM patients without DKA (5/17) had a lower median plasma glucose of 22.7 mmol/L (range 21.1–37.7 mmol/L) (P < 0.05, Fig. 2b). Specific laboratory tests results are shown in Table 2. Eleven patients with the pituitary gland affected developed hypophysitis. In nine of these patients (81.8%), only the adrenal axis was affected, with decreased cortisol and adrenocorticotropic hormone. The other two patients with hypophysitis had more axes involved. Besides adrenal insufficiency, one patient presented with lower luteinizing hormone (LH) and follicle-stimulating hormone (FSH) or testosterone, suggesting the diagnosis of hypogonadotropic hypogonadism induced by autoimmune hypophysitis. In another patient, the thyroid axis was also involved, with decreased TSH and FT4 [13]. In addition, two patients had adrenal gland impairment with decreased cortisol [12, 25]. Thorough endocrine function evaluations and imaging results suggested these two patients were diagnosed as having adrenalitis.
Of the 20 patients with reported outcomes, only three of them (15.0%) exhibited progressive disease. Three patients showed complete response, nine showed partial response, and five presented with stable disease (Fig. 2c).
Immunological and HLA features
At least one of the relative autoantibodies was detected in 65.2% (15/23) patients. It is notable that one patient presented with two positive islet-related antibodies (glutamic acid decarboxylase [GAD] autoantibody; islet cell autoantibody) and three elevated thyroid antibodies (thyroglobulin antibody; thyroid peroxidase [TPO] antibody; thyrotropin receptor antibody). In the remaining 14 patients, only one or two positive autoantibodies were detected. The median onset of first irAE was 2 cycles (range 1–15 cycles) of ICI treatments for autoantibody-positive patients and 7 cycles (range 4–13 cycles) for autoantibody-negative patients (P < 0.05, Fig. 2d). Meanwhile, autoantibody-positive patients showed earlier onset time than negative patients (positive, 4.5 weeks; negative, 12 weeks; P < 0.05).
The GAD antibody is the most frequently detected positive autoantibody. Of 17 patients with T1DM, 8 (47.1%) exhibited positive GAD antibody and 75.0% of these GAD-positive patients (6/8) developed T1DM with DKA. Thyroid-related autoantibodies were detected as positive in 38.9% patients (7/18) who later developed immune-related thyroid disorders. Moreover, test results for other autoantibodies, including 21-hydroxylase antibody (21-OH Ab), adrenal cortex antibody (ACA), and anti-pituitary antibody, were also positive in patients with relative endocrine events.
The HLA genotype was analyzed and reported in 34.8% (8/23) of patients. Among these reported results, five patients (62.5%) presented with risk genotypes. A well-known susceptible allele, HLA-DR4, was detected in three patients, whereas the protective haplotype HLA-DQ6 was present in two patients.
Discussion
Immune checkpoint inhibition therapies have shown strong effects against various types of malignant cancer, and the number of patients receiving these treatments has been increasing rapidly. Meanwhile, more and more immune-related or inflammatory side effects have been reported. Although rare, APS-2 is an endocrine irAE that can cause severe outcomes. To summarize the clinical characteristics and immunological and genetic features of patients with APS-2 after immune checkpoint inhibition, we conducted the current systematic review of cases reports on this rare adverse event induced by ICI treatments.
Immune checkpoint blockade therapy has significantly changed strategies for treating cancer as a supplement to comprehensive treatment including surgery, radiotherapy, chemotherapy, and target therapy. Under physiological conditions, CTLA-4 and PD-1/PD-L1 pathways are critical in self-tolerance and restricting autoimmune response. However, in tumor tissues, these immune checkpoint pathways are triggered to help cancer cells escape from immunity [29]. Therefore, ICIs which can block the overactivation of these pathways are used to enhance the antitumor immune response, providing impressive survival improvement for cancer patients. Meanwhile, the immune checkpoint blockade can promote the generation of autoantibodies by stimulating humoral immune response [30] as well as activate autoimmune T-cell reaction, thus causing immune-related adverse events [31] (Fig. 3). Increasing evidence from meta-analyses, systematic reviews, clinical trials, and case reports support that a wide range of organs are involved in inflammatory or immune-related side effects after ICI treatments [2, 32–39]. The severity of these side effects varies from mild to life threatening. Nevertheless, the co-occurrence of two or more endocrine disorders (e.g., APS) during immunotherapy has been far from well summarized due to its low incidence.
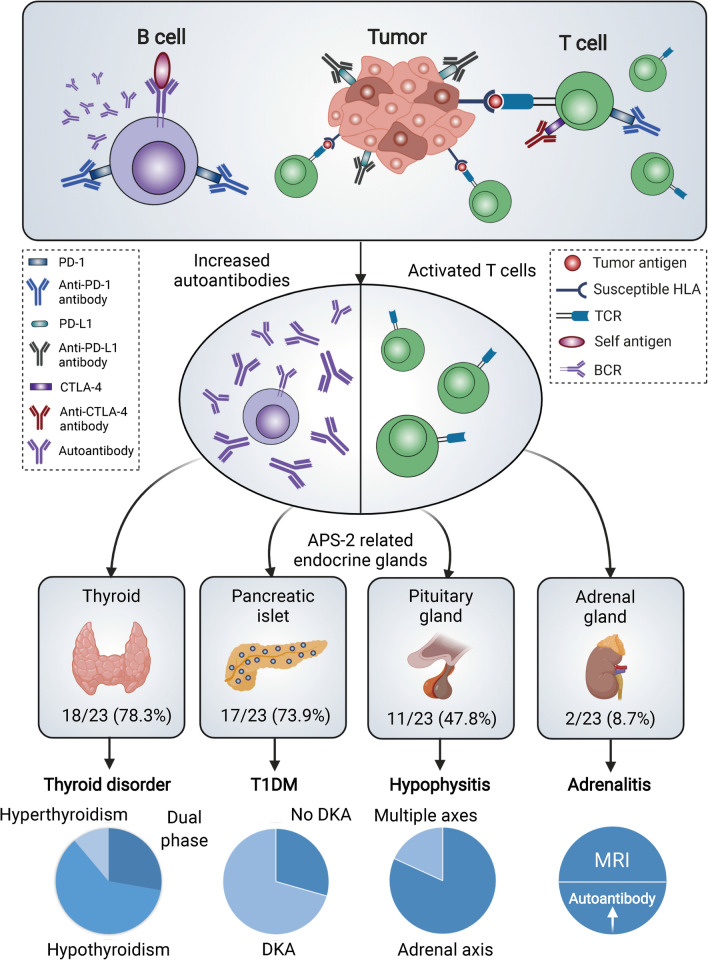
Fig. 3.
Schematic diagram of immune-related APS-2 induced by ICIs. Immune checkpoint blockade activates humoral immune response as well as cytotoxic T cells. Both increased level of autoantibodies and activated T cells contribute to the destruction of targeted endocrine glands. The glands involved in immune-related APS-2 include thyroid, pancreatic islets, pituitary gland, and adrenal gland. Dual phase means that patients with thyroid disorder experienced a transition from hyperthyroidism to hypothyroidism. More than 70% patients with T1DM had DKA. Most cases with hypophysitis presented with adrenal axis affected while two had multiple axes affected. Two patients manifested with adrenalitis were diagnosed by elevated autoantibodies or MRI results. APS-2 autoimmune polyendocrine syndrome type II, BCR B cell receptor, CTLA-4 cytotoxic T-lymphocyte antigen 4, DKA diabetes ketoacidosis, HLA human leukocyte antigen, MRI Magnetic Resonance Imaging, PD-1 programmed cell death 1, PD-L1 programmed cell death ligand 1, T1DM Type 1 Diabetes Mellitus, TCR T-cell receptor
To better examine APS-2 during immune checkpoint blockade, we searched three databases of medical publications. We identified five additional case reports which were not included in the searching results from two published literature reviews [7, 8]. After careful review, only 23 cancer patients who presented with two or more endocrine glands affected by ICIs and had not been diagnosed with any autoimmune or endocrine diseases before treatment were included in our research. A review of case reports can provide detailed manifestations of rare but significant side effects that are seldom described in clinical trials. However, it cannot display the general profile of a disorder. It is well known that women are at a higher risk of autoimmune diseases because of sex-related differences in both innate and adaptive immune responses to self-antigens. Existing studies have suggested that these differences are caused by genes and hormones [40]. We hypothesized that as a special autoimmune adverse event, APS-2 also affects a greater proportion of women. Interestingly, approximately twice the number of male patients with ICI-induced APS-2 than the number of female patients were enrolled in our study. We speculate that the main reason why a higher proportion of male patients was observed in our systematic review is that more men receive ICI treatments than women, a common feature of many immunotherapy clinical trials [41–43]. For example, in a meta-analysis of 20 eligible randomized controlled trials of patients with advanced cancer, 67% of 11,351 patients were male, which was very similar to the male percentage of our results [41]. However, it has not yet been determined whether men are more susceptible to APS-2 induced by ICIs than women; hence, this requires further investigation. Meanwhile, because metastatic melanoma and lung cancer are earlier approved indications for immune checkpoint antibodies, they were more frequently treated with ICIs and were the predominant cancer types among all the included patients. Additionally, all the available antibodies targeting CTLA-4, PD-1, or PD-L1, as well as combination therapies could trigger multiple endocrine side effects. Notably, more APS-2 patients induced by PD-1/PD-L1 blockade than CTLA-4 inhibition may be due to the wider usage of PD-1/PD-L1 antibodies. To date, five PD-1/PD-L1 blockade drugs have been approved for a wide range of indications whereas only ipilimumab has been approved for melanoma treatment as an anti-CTLA-4 therapy [31]. Thus, it is not surprising that more patients with cancer receive PD-1/PD-L1 therapies than CTLA-4 blockade. Moreover, other studies suggested that patients receiving PD-1/PD-L1 inhibitor treatment had a higher risk of immune-related thyroid disorder than those receiving anti-CTLA-4 treatment [44, 45]. Consistent with this, a systematic review concluded that some other irAEs including pneumonitis, hypothyroidism, arthralgia, and vitiligo were more common with the use of PD-1 antibodies [46]. Therefore, we inferred that APS-2 induced by ICIs may also be associated with the PD-1 pathway. However, this requires validation in further studies.
Induced by various types of immunotherapies, most patients developed two disorders, with the co-occurrence of autoimmune thyroiditis and type 1 diabetes most common. Given that both hypothyroidism and hyperthyroidism are observed with high incidence in patients undergoing anti-PD-1/PD-L1 treatments [3], it is not surprising that the thyroid gland was most frequently affected gland in this study. However, the relatively rare autoimmune disease, T1DM, also occurred in most of the included cases, indicating APS-2 and autoimmune diabetes share some similarities of pathogenesis. Most T1DM patients included experience rapidly developing DKA in our research, suggesting that APS-2 triggered by immunotherapy has life-threatening outcomes. Therefore, T1DM patients induced by ICI treatment with other endocrine organs affected should receive immediate insulin therapy to prevent the occurrence of DKA.
Another important manifestation of APS-2, adrenal insufficiency, is related to an autoimmune reaction in the pituitary or adrenal glands. Most patients with hypophysitis only showed an impaired adrenal axis, whereas two patients exhibited reduced production of FSH, LH (gonadal axis), and TSH (thyroid axis) in addition to adrenal insufficiency. Three enrolled patients who received therapies containing ipilimumab all developed hypophysitis, which is consistent with the association between ipilimumab and high hypophysitis incidence [4]. Besides autoimmune hypophysitis, two patients were diagnosed with autoimmune adrenalitis. Paepegaey et al. [25] reported a patient who presented with acute adrenal crisis. Further laboratory tests confirmed the diagnosis of autoimmune adrenalitis by positive autoantibodies (21-OH Ab, ACA). Aziz et al. [12] diagnosed adrenal cortical atrophy using abdomen Magnetic Resonance Imaging (MRI). Abrupt onset of adrenal insufficiency can cause life-threatening acute adrenal crisis, which requires immediate medical attention. The key clinical features of APS-2 cases enrolled in our study are illustrated in Fig. 3.
The occurrence of irAEs, including APS-2 in our research, always suggests effective activation of the immune system via immune checkpoint inhibition. In a retrospective study of patients with advanced non-small cell lung cancer (NSCLC) treated with PD-1 blockade, thyroid dysfunction was found to be associated with longer progression-free and overall survival [47]. Moreover, a recent prospective study of NSCLC revealed improved survival in patients with skin-related adverse events induced by PD-1 treatment [48]. Other reports also suggested that the development of autoimmune adverse events was associated with a higher response rate to ICI treatments [49–51], although not all investigations have supported the conclusion [52, 53]. In our study, as one of the rarest irAEs, APS-2 triggered by ICIs indicated better efficacy of the immune checkpoint blockade. Notably, only 15.0% of the cancer patients with reported outcomes presented with progressive disease. Most patients with cancer showed at least stable disease in response to ICI therapies, suggesting that the presentation of APS-2 may be related to a good response to ICI therapies. Therefore, more efforts should be made to overcome the severe adverse event, APS-2, to ensure better survival.
APS-2 is a relatively more common variety of autoimmune polyendocrine syndrome (prevalence, 1:1,000) than APS type 1 [5]. Although the pathogenesis of this disease is still not clear, it is thought to be a polygenetic disease associated with HLA genotypes and other immune-related genes. Among these genes of interest, CTLA-4 with heterozygous mutations that reduce its expression and with missense mutations that disturb ligand binding were reported to interfere with T and B cell homeostasis, leading to a complex autoimmune disease [54]. Based on this study, we suggested that CTLA-4 blockade promotes both T-cell activation and humoral immunity to induce APS-2 in cancer patients, similar to the mechanisms to induce other irAEs. In addition, the study that analyzed skin toxicity adverse events in patients with NSCLC receiving PD-1 therapy identified several T-cell antigens shared between lung tumors and skin tissues. The T cells responding to these antigens present in blood biopsy samples were found to infiltrate skin lesions and tumor tissues, providing evidence that inhibition of the PD-1 pathway may trigger APS-2 by enhancing T-cell activation [48]. Although several relevant studies have indicated the possible mechanisms of APS-2 induced by ICIs, more specific and direct studies are still required.
Clinical laboratory tests related to mechanisms, including HLA risk allele analysis and autoantibody test, were carried out in our enrolled cases. One patient in our review had genotype HLA-DR4-DQ3, which shows genetic susceptibility to T1DM and adrenal insufficiency [7, 16]. Two more patients had the DR4 genotype, which confers the risk of T1DM and thyroiditis [8, 27, 55]. Furthermore, HLA DR9-DQ3 was detected in two Asian patients [10, 24], and this haplotype is closely related to T1DM in Asia [56, 57]. However, a protective haplotype, DQ6 [58], was identified in two patients [16, 19]. These HLA results support that APS-2 is closely associated with HLA subtypes and suggest that all autoimmune endocrinopathies, including APS-2, T1DM, thyroiditis, and adrenal insufficiency, may share some similar pathogenesis and mechanisms. In addition, autoantibody results have suggested a significant role of autoantibodies in the development of immune-related APS-2. In one article, a close association between ICI-induced thyroid disorders and anti-thyroid antibodies was reported [30]. Consistent with this, patients with APS-2 or related autoimmune endocrinopathies have been found to generate positive autoantibodies, such as GAD, TPO, and 21-OH Ab [5, 59–61]. Notably, nearly two-thirds of the patients enrolled in our study were positive for related autoantibodies. Moreover, the onset of the primary adverse event varies from weeks to nearly 1 year, and autoantibody-negative patients showed a significantly later onset of APS-2 than autoantibody-positive patients. Collectively, these results suggest that immune checkpoint blockade also enhances humoral immunity, consequently generating higher levels of autoantibodies during the pathogenesis of APS-2 triggered by ICIs. Similar results of HLA genotypes and autoantibodies reported in both spontaneous and ICI-induced endocrinopathies indicate that immune checkpoint inhibition would be a potential approach to studying the mechanisms of these complex autoimmune disorders.
To the best of our knowledge, this is the largest and most comprehensive systematic review of case reports on APS-2 after ICI therapies. Lanzolla et al. [7] summarized 16 cases of immune-related APS after anti-PD-1 or anti-CTLA-4 therapy with limited clinical features concluded. Additionally, Gunjur et al. [8] conducted a review of cases of anti-PD-1/PD-L1–induced APS-2. However, both studies only included partial reports of ICIs. Moreover, some patients had previous history of autoimmune or endocrine diseases while some didn’t have adequate clinical data. However, our study has some limitations. Case reports tend to describe side effects with rare, unique, and severe features, whereas unremarkable cases remain unreported. Therefore, the included cases may not represent all APS-2 cases induced by ICIs. These cases were published by different authors, with some incomplete clinical information and laboratory test results. Moreover, we could not know which population these patients were selected from, so we could not estimate an incidence of this disorder and identify risk factors.
We conducted this comprehensive review primarily to provide a larger scale view of APS-2 induced by ICI therapy, including clinical characteristics, immunological features, and genetic findings. We also aimed to increase the awareness of this severe adverse event during the increasing administration of these novel immune therapies. Our results show that APS-2 triggered by ICIs can suddenly cause lethal events including adrenal crisis and DKA. Thus, we suggest detecting autoantibodies and monitoring the related hormone levels during treatments for early discovery of endocrine disorders. Moreover, our study provides some evidence for elucidating the mechanisms of irAEs and APS-2. However, because the data of these reports are far from enough to research the underlying mechanisms and discover novel predictive markers, we suggest oncologists collect related genetic and clinical information of more APS-2 patients induced by ICIs.
In conclusion, this systematic review examined APS-2, a special endocrine adverse event, after ICI therapy. The time from therapy initiation to APS-2 is abrupt, and this disorder develops rapidly. Life-threatening events, including adrenal crisis and severe DKA, may occur. Therefore, physicians should be aware of potential endocrine disorders during ICI treatments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ACA
Adrenal cortex antibody
- APS-2
Autoimmune polyendocrine syndrome type II
- CTLA-4
Cytotoxic T-lymphocyte antigen 4
- DKA
Diabetes ketoacidosis; FSH, follicle-stimulating hormone
- FT4
Free thyroxine
- GAD
Glutamic Acid Decarboxylase
- HLA
Human leukocyte antigen
- ICI
Immune checkpoint inhibitor
- irAE
Immune-related adverse event
- LH
Luteinizing hormone
- NSCLC
Non-small cell lung cancer
- 21-OH Ab
21-Hydroxylase antibody
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death ligand 1
- T1DM
Type 1 Diabetes Mellitus
- TPO
Thyroid peroxidase antibody
- TSH
Thyroid stimulating hormone
Author contributions
We declare that all authors made fundamental contributions to the manuscript. All authors contributed to the study conception and design. Database search and data analysis was conducted by WX. Study selection and data extraction were performed by NJ and SM. The manuscript was written by ZZ. BX and ZD reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Sciences Foundation of China (81630097 and 81773718), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016-I2M-3–011), the Chinese Academy of Medical Sciences Fundamental Research Funds for the Central Universities (2018RC350002), and the Drug Innovation Major Project (2018ZX09711001-003–005, 2018ZX09711001-008–005, and 2018ZX09711001-003–020).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–e551. doi: 10.1016/s1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, Lanoy E, Texier M, Libenciuc C, Eggermont AMM, Soria J-C, Mateus C, Robert C. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, Shen C, Duma N, Vera Aguilera J, Chintakuntlawar A, Price KA, Molina JR, Pagliaro LC, Halfdanarson TR, Grothey A, Markovic SN, Nowakowski GS, Ansell SM, Wang ML. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5:1008–1019. doi: 10.1001/jamaoncol.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4:173–182. doi: 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husebye ES, Anderson MS, Kampe O. Autoimmune polyendocrine syndromes. N Engl J Med. 2018;378:1132–1141. doi: 10.1056/NEJMra1713301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt MB. Eine biglandulare Erkrankung (Nebennieren und Schilddruse bei Morbus Addisonni) Dtsch Pathol Ges. 1926;21:212–221. [Google Scholar]
- 7.Lanzolla G, Coppelli A, Cosottini M, Del Prato S, Marcocci C, Lupi I. Immune checkpoint blockade anti-PD-L1 as a trigger for autoimmune polyendocrine syndrome. J Endocr Soc. 2019;3:496–503. doi: 10.1210/js.2018-00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunjur A, Klein O, Kee D, Cebon J. Anti-programmed cell death protein 1 (anti-PD1) immunotherapy induced autoimmune polyendocrine syndrome type II (APS-2): a case report and review of the literature. J Immunother Cancer. 2019 doi: 10.1186/s40425-019-0713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuru S, Khan N, Shaaban H. Acute hypophysitis secondary to nivolumab immunotherapy in a patient with metastatic melanoma. Int J Crit Illn Inj Sci. 2017;7:177–180. doi: 10.4103/ijciis.Ijciis_15_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong SH, Lee SY, Yang YS, Kim TM, Kwak SH. Anti-programmed cell death 1 therapy triggering diabetic ketoacidosis and fulminant type 1 diabetes. Acta Diabetol. 2016;53:853–856. doi: 10.1007/s00592-016-0872-y. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Masood A, Bari S, Yavuz S, Grosbach AB. Autoimmune diabetes and thyroiditis complicating treatment with nivolumab. Case Rep Oncol. 2017;10:230–234. doi: 10.1159/000456540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aziz K, Shahbaz A, Umair M, Sachmechi I. Avelumab inducing hypothyroidism and hypoadrenalism: a case report and review of literature. Excli J. 2018 doi: 10.17179/excli2018-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humayun MA, Poole R. A case of multiple immune toxicities from Ipilimumab and pembrolizumab treatment. Hormones (Athens) 2016 doi: 10.14310/horm.2002.1656. [DOI] [PubMed] [Google Scholar]
- 14.Hakami OA, Ioana J, Ahmad S, Tun TK, Sreenan S, McDermott JH. A case of pembrolizumab-induced severe DKA and hypothyroidism in a patient with metastatic melanoma. Endocrinol Diabetes Metab Case Rep. 2019 doi: 10.1530/edm-18-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen E, Sahasrabudhe D, Sievert L. A case report of insulin-dependent diabetes as immune-related toxicity of pembrolizumab: presentation, management and outcome. Cancer Immunol Immunother. 2016;65:765–767. doi: 10.1007/s00262-016-1835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupi I, Brancatella A, Cosottini M, Viola N, Lanzolla G, Sgrò D, Di Dalmazi G, Latrofa F, Caturegli P, Marcocci C. Clinical heterogeneity of hypophysitis secondary to PD-1/PD-l1 blockade: insights from four cases. Endocrinol Diabetes Metab Case Rep. 2019 doi: 10.1530/EDM-19-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel S, Chin V, Greenfield JR. Durvalumab-induced diabetic ketoacidosis followed by hypothyroidism. Endocrinol Diabetes Metab Case Rep. 2019 doi: 10.1530/edm-19-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okahata S, Sakamoto K, Mitsumatsu T, Kondo Y, Noso S, Ikegami H, Shiba T. Fulminant type 1 diabetes associated with Isolated ACTH deficiency induced by anti-programmed cell death 1 antibody-insight into the pathogenesis of autoimmune endocrinopathy. Endocr J. 2019;66:295–300. doi: 10.1507/endocrj.EJ18-0328. [DOI] [PubMed] [Google Scholar]
- 19.Lowe JR, Perry DJ, Salama AK, Mathews CE, Moss LG, Hanks BA. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J Immunother Cancer. 2016 doi: 10.1186/s40425-016-0196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sum M, Garcia FV. Immunotherapy-induced autoimmune diabetes and concomitant hypophysitis. Pituitary. 2018;21:556–557. doi: 10.1007/s11102-018-0880-8. [DOI] [PubMed] [Google Scholar]
- 21.Marchand L, Paulus V, Fabien N, Pérol M, Thivolet C, Vouillarmet J, Saintigny P. Nivolumab-induced acute diabetes mellitus and hypophysitis in a patient with advanced pulmonary pleomorphic carcinoma with a prolonged tumor response. J Thorac Oncol. 2017;12:e182–e184. doi: 10.1016/j.jtho.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Tzoulis P, Corbett RW, Ponnampalam S, Baker E, Heaton D, Doulgeraki T, Stebbing J. Nivolumab-induced fulminant diabetic ketoacidosis followed by thyroiditis. Endocrinol Diabetes Metab Case Rep. 2018 doi: 10.1530/edm-18-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martins Machado C, Almeida Santos L, Barroso A, Oliveira MJ. Nivolumab-induced hypothyroidism followed by isolated ACTH deficiency. BMJ Case Rep. 2019 doi: 10.1136/bcr-2019-231236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakurai K, Niitsuma S, Sato R, Takahashi K, Arihara Z. Painless thyroiditis and fulminant type 1 diabetes mellitus in a patient treated with an immune checkpoint inhibitor, nivolumab. Tohoku J Exp Med. 2018;244:33–40. doi: 10.1620/tjem.244.33. [DOI] [PubMed] [Google Scholar]
- 25.Paepegaey AC, Lheure C, Ratour C, Lethielleux G, Clerc J, Bertherat J, Kramkimel N, Groussin L. Polyendocrinopathy resulting from pembrolizumab in a patient with a Malignant Melanoma. J Endocr Soc. 2017;1:646–649. doi: 10.1210/js.2017-00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erra A, Pannu BS, Patel S, Qureshi F, Soliman M. A rare case of ipilimumab-induced reversible hypophysitis and permanent primary hypothyroidism. Cureus. 2019 doi: 10.7759/cureus.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurihara S, Oikawa Y, Nakajima R, Satomura A, Tanaka R, Kagamu H, Shimada A. Simultaneous development of graves' disease and type 1 diabetes during anti-programmed cell death-1 therapy: a case report. J Diabetes Investig. 2020 doi: 10.1111/jdi.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mengíbar JL, Capel I, Bonfill T, Mazarico I, Espuña LC, Caixàs A, Rigla M. Simultaneous onset of type 1 diabetes mellitus and silent thyroiditis under durvalumab treatment. Endocrinol Diabetes Metab Case Rep. 2019 doi: 10.1530/EDM-19-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok JD, Merghoub T, Rudin CM, Fish S, Hellmann MD. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28:583–589. doi: 10.1093/annonc/mdw640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 32.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA, National Comprehensive Cancer N. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F, Thompson JA, Obeid M. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 34.Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33:2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu C, Chen Y-P, Du X-J, Liu J-Q, Huang C-L, Chen L, Zhou G-Q, Li W-F, Mao Y-P, Hsu C, Liu Q, Lin A-H, Tang L-L, Sun Y, Ma J. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018 doi: 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol. 2018;14:569–579. doi: 10.1038/s41584-018-0074-9. [DOI] [PubMed] [Google Scholar]
- 38.Chang L-S, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40:17–65. doi: 10.1210/er.2018-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, Kelly RJ, Hann CL, Levy B, Feliciano JL, Brahmer JR, Feller-Kopman D, Lerner AD, Lee H, Yarmus L, D'Alessio F, Hales RK, Lin CT, Psoter KJ, Danoff SK, Naidoo J. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13:1930–1939. doi: 10.1016/j.jtho.2018.08.2035. [DOI] [PubMed] [Google Scholar]
- 40.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 41.Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, Gelber RD, Goldhirsch A. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19:737–746. doi: 10.1016/s1470-2045(18)30261-4. [DOI] [PubMed] [Google Scholar]
- 42.Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, Pennell NA, Velcheti V. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152:271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 43.Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, Zhao Z, Zhao J, Chen S, Song J, Qi C, Wang Q, Huang M, Zhang Y, Huang D, Bai Y, Sun F, Lee JJ, Wang Z, Wang J. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol. 2019;6:375–384. doi: 10.1001/jamaoncol.2019.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Min L. Immune-related endocrine disorders in novel immune checkpoint inhibition therapy. Genes Dis. 2016;3:252–256. doi: 10.1016/j.gendis.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51:145–156. doi: 10.1055/a-0843-3366. [DOI] [PubMed] [Google Scholar]
- 46.Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377–2385. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 47.Kim HI, Kim M, Lee SH, Park SY, Kim YN, Kim H, Jeon MJ, Kim TY, Kim SW, Kim WB, Kim SW, Lee DH, Park K, Ahn MJ, Chung JH, Shong YK, Kim WG, Kim TH. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology. 2017;7:e1375642. doi: 10.1080/2162402x.2017.1375642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berner F, Bomze D, Diem S, Ali OH, Fässler M, Ring S, Niederer R, Ackermann CJ, Baumgaertner P, Pikor N, Cruz CG, van de Veen W, Akdis M, Nikolaev S, Läubli H, Zippelius A, Hartmann F, Cheng HW, Hönger G, Recher M, Goldman J, Cozzio A, Früh M, Neefjes J, Driessen C, Ludewig B, Hegazy AN, Jochum W, Speiser DE, Flatz L. Association of Checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 2019;5:1043–1047. doi: 10.1001/jamaoncol.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xing P, Zhang F, Wang G, Xu Y, Li C, Wang S, Guo Y, Cai S, Wang Y, Li J. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer. 2019 doi: 10.1186/s40425-019-0779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, White DE, Steinberg SM, Rosenberg SA. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.Ccr-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, Jiang J, Robert C. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/jco.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 52.Ascierto PA, Simeone E, Sileni VC, Pigozzo J, Maio M, Altomonte M, Del Vecchio M, Di Guardo L, Marchetti P, Ridolfi R, Cognetti F, Testori A, Bernengo MG, Guida M, Marconcini R, Mandalà M, Cimminiello C, Rinaldi G, Aglietta M, Queirolo P. Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort. J Transl Med. 2014 doi: 10.1186/1479-5876-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Restifo NP, Haworth LR, Levy C, Mavroukakis SA, Nichol G, Yellin MJ, Rosenberg SA. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/jco.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, Bulashevska A, Petersen BS, Schäffer AA, Grüning BA, Unger S, Frede N, Baumann U, Witte T, Schmidt RE, Dueckers G, Niehues T, Seneviratne S, Kanariou M, Speckmann C, Ehl S, Rensing-Ehl A, Warnatz K, Rakhmanov M, Thimme R, Hasselblatt P, Emmerich F, Cathomen T, Backofen R, Fisch P, Seidl M, May A, Schmitt-Graeff A, Ikemizu S, Salzer U, Franke A, Sakaguchi S, Walker LSK, Sansom DM, Grimbacher B. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20:1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hashimoto K, Maruyama H, Nishiyama M, Asaba K, Ikeda Y, Takao T, Iwasaki Y, Kumon Y, Suehiro T, Tanimoto N, Mizobuchi M, Nakamura T. Susceptibility alleles and haplotypes of human leukocyte antigen DRB1, DQA1, and DQB1 in autoimmune polyglandular syndrome type III in Japanese population. Horm Res. 2005;64:253–260. doi: 10.1159/000089293. [DOI] [PubMed] [Google Scholar]
- 56.Awata T, Kuzuya T, Matsuda A, Iwamoto Y, Kanazawa Y. Genetic analysis of HLA class II alleles and susceptibility to type 1 (insulin-dependent) diabetes mellitus in Japanese subjects. Diabetologia. 1992;35:419–424. doi: 10.1007/bf02342437. [DOI] [PubMed] [Google Scholar]
- 57.Yasunaga S, Kimura A, Hamaguchi K, Ronningen KS, Sasazuki T. Different contribution of HLA-DR and -DQ genes in susceptibility and resistance to insulin-dependent diabetes mellitus (IDDM) Tissue Antigens. 1996;47:37–48. doi: 10.1111/j.1399-0039.1996.tb02512.x. [DOI] [PubMed] [Google Scholar]
- 58.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, Lavant E, Louey A, Moonsamy P, Type 1 Diabetes Genetics C HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57:1084–1092. doi: 10.2337/db07-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 60.Czarnocka B, Ruf J, Ferrand M, Carayon P, Lissitzky S. Purification of the human thyroid peroxidase and its identification as the microsomal antigen involved in autoimmune thyroid diseases. FEBS Lett. 1985;190:147–152. doi: 10.1016/0014-5793(85)80446-4. [DOI] [PubMed] [Google Scholar]
- 61.Winqvist O, Karlsson FA, Kämpe O. 21-Hydroxylase, a major autoantigen in idiopathic Addison's disease. Lancet. 1992;339:1559–1562. doi: 10.1016/0140-6736(92)91829-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.