Introduction
Generalized eruptive keratoacanthomas (GEKA) is a distinctive and uncommon variant of keratoacanthoma, presenting with hundreds of small skin-colored to erythematous, umbilicated keratotic papules.1,2 While the etiology of GEKA is unknown, associations have been made with environmental factors such as exposure to ultraviolet radiation, immunological abnormalities, viral exposures, trauma, and immunomodulatory medications. Systemic treatment is often most practical given the widespread distribution of GEKA; however, results are variable and often associated with adverse effects.3
GEKA poses treatment challenges, primarily due to its rapid progression and lack of standardized treatment approach. Accurate diagnosis and timely intervention are crucial, as misdiagnosis or delayed treatment can lead to complications and unnecessary patient distress. Here, we present the management approach to a patient with GEKA as well as a brief literature review regarding trends in management of GEKA.
Case
A 75-year-old male presented with a 6-month history of persistent, pruritic bumps on the arms, shins, thighs, feet, and back. On physical exam, numerous 5 to 15 mm erythematous crateriform papules were present on the trunk and extremities, accompanied by pronounced erythema on the shins bilaterally (Fig 1, A). There was no mucosal involvement. Biopsies revealed multiple keratoacanthomas (GEKA; Fig 1, B-D). He was initially treated with higher dose acitretin (titrated to 50 mg daily), which lead to an elevation in liver enzymes.
Fig 1.
Generalized eruptive keratoacanthomas before treatment. A, Clinical presentation of generalized eruptive keratoacanthomas with secondary retention hyperkeratosis on the right distal extremity prior to treatment. Histopathology from a shave biopsy of generalized eruptive keratoacanthomas on the left distal pretibial region at (B) 4×, (C) 10×, and (D) 20× demonstrating a verrucous endophytic epithelial proliferation containing large keratinocytes with abundant pale eosinophilic cytoplasm and peripheral atypia.
The patient was transitioned to the epidermal growth factor receptor inhibitor erlotinib 150 mg daily by his oncology team but developed a pruritic acneiform dermatitis on the face and trunk within 1 week. He continued erlotinib for a 3-month trial, as recommended by his medical oncologist, but due to both rash and lack of clinical response, erlotinib was discontinued with resolution of the dermatitis.
The patient was next treated with the programmed cell death protein-1 inhibitor cemiplimab which resulted in a rapidly progressive, severe lichenoid eruption affecting large areas of the trunk and extremities. Biopsies of the left proximal pretibial region and left ventral proximal forearm were consistent with a lichenoid drug reaction. Due to the severity of his pruritus, cemiplimab was held, and the patient was prescribed a slow prednisone taper (beginning at 1 mg/kg) over 1.5 months as well as triamcinolone 0.1% ointment, with ultimate resolution of the lichenoid eruption.
Though the lichenoid drug reaction had resolved, his GEKA remained untreated. The patient was next treated with topical 5-fluorouracil weekly with unna boots; however, did not tolerate this secondary to pain. Intralesional triamcinolone (40 mg/mL) had no effect on GEKA lesions. Oral methotrexate (MTX) was not used given the patient’s prior history of transaminitis and the patient declined intralesional MTX. It was then decided to reinitiate treatment with oral acitretin at 10 mg daily and perform therapeutic shave removals of the most symptomatic lesions. Acitretin 10 mg daily was tolerated well without side effects. The patient returned to clinic 1 month later with decreased burden of keratotic papules and nodules on the lower extremities. Upon continuing this regimen of acitretin 10 mg daily for an additional 4 months, the patient experienced significantly improved disease burden, with only few remaining keratotic papules and nodules of the bilateral lower extremities noted at each visit (Fig 2). He is now on a maintenance dose of acitretin 10 mg every other day and very satisfied with his improvement.
Fig 2.
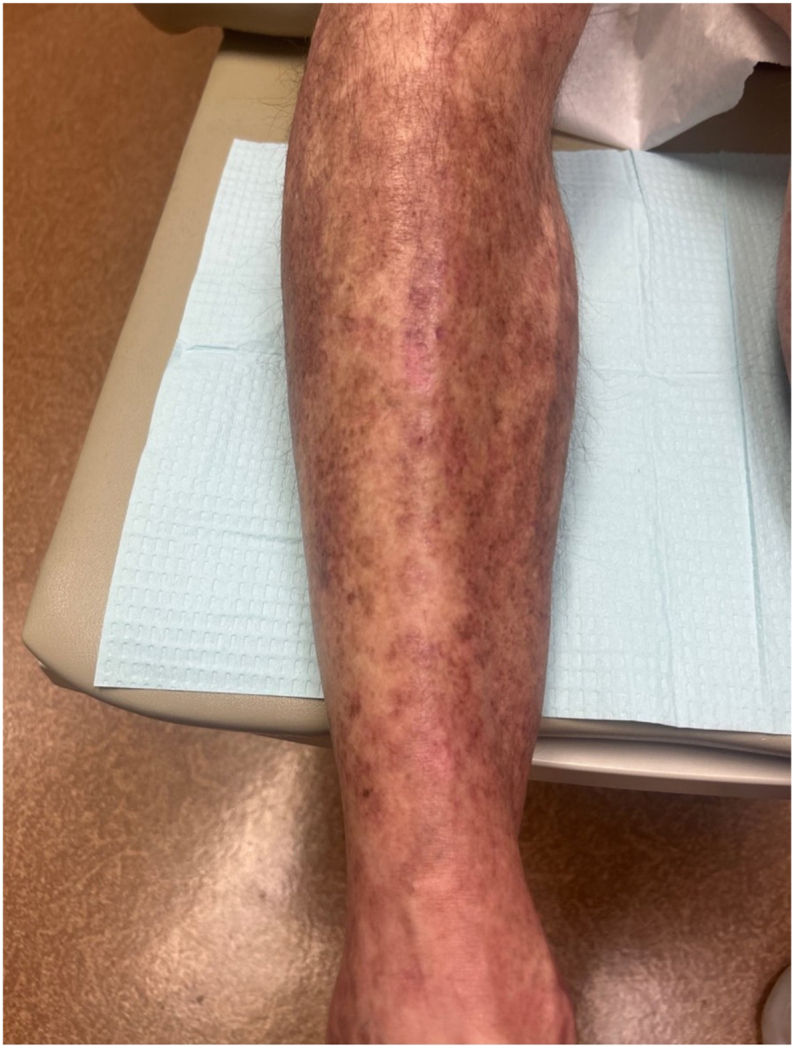
Generalized eruptive keratoacanthomas after treatment and resolution of lichenoid drug reaction. Right distal extremity showing resolution of generalized eruptive keratoacanthomas with associated postinflammatory hyperpigmentation changes after treatment.
Discussion
Forty-one cases of GEKA have been published worldwide from 2018 to 2023, each with unique provoking factors (Table I). Common offenders are checkpoint inhibitor therapeutics—namely nivolumab and pembrolizumab. Other etiologies have included trauma, tattoos, 5-fluorouracil, and idiopathic causes. Due to the variety of etiologies and stubborn course of disease, multiple treatments have been utilized, yielding inconsistent results, and varied adverse events.4
Table I.
GEKA cases that have been published worldwide from 2018 to 2023
| Anatomic locations | Etiology (Weeks to onset) |
Age range | Attempted treatments | Successful treatments | References |
|---|---|---|---|---|---|
| Face | PM8001 [PDL1 & TGF-β Inhibitor] (6) | 67 | d/c PM8001 | d/c PM8001 | Qi et al6 |
| Trunk | s/p excision | 84 | Intralesional MTX | Intralesional MTX | Gualdi et al12 |
| Extremities | Dupilumab (6) | 33-92 | Excision Topical 5-FU |
Excision Topical 5-FU |
Patchinsky et al7 |
| Pembrolizumab (15, 22, 40) & prednisolone | Acitretin Nicotinamide |
Acitretin Nicotinamide |
Star et al10 | ||
| Pembrolizumab (28, 61) & prednisolone | Excision Nicotinamide Topical steroids Decreased pembrolizumab frequency |
Excision Nicotinamide Topical steroids Decreased pembrolizumab frequency |
|||
| Pembrolizumab & ipilimumab (8, 14, 20, 22, 50) | Excision Acitretin Nicotinamide |
Excision Acitretin Nicotinamide |
|||
| Pembrolizumab with either epacadostat or placebo (10, 42) | Excision Oral & topical steroids |
Excision Oral & topical steroids |
|||
| Pembrolizumab (15) | Topical steroids Oral steroids Acitretin |
Acitretin | Schwartz et al11 | ||
| Pembrolizumab & ipilimumab (8) | |||||
| Pembrolizumab & ipilimumab (16) | |||||
| Nivolumab (18) | |||||
| Nivolumab (19) | |||||
| Pembrolizumab (12) | |||||
| Nivolumab (51) | |||||
| Dupilumab (4) | Excision | Excision Spontaneous regression |
Gleeson et al13 | ||
| Moderna mRNA-1273 Covid-19 vaccination | Mohs Intralesional 5-FU |
Mohs Intralesional 5-FU |
Ahmed et al14 | ||
| Topical 5-FU (2) | Acitretin Excision Intralesional 5-FU |
Acitretin Excision Intralesional 5-FU |
Cruzval-O’Reilly et al16 | ||
| Spironolactone (3) | Oral MTX Excision |
Oral MTX Excision |
Kunadia et al18 | ||
| Idiopathic | Excision Topical imiquimod Lapacho tea dressings Topical 5-FU |
Excision Topical imiquimod Lapacho tea dressings Topical 5-FU |
Havenith et al19 | ||
| Tattoo | Excision Topical tazarotene Cryotherapy |
Excision Topical tazarotene Cryotherapy |
Giberson et al20 | ||
| Idiopathic | Acitretin | Acitretin | Relvas et al21 | ||
| Tattoo (2) | Intralesional MTX | Spontaneous regression Intralesional MTX |
Linares-Gonzales et al22 | ||
| Laser tattoo removal (1) | Intralesional triamcinolone | Spontaneous regression Intralesional triamcinolone |
Hoss et al23 | ||
| Idiopathic | Excision Intralesional 5-FU Acitretin Oral nicotinamide Intralesional MTX Topical 5-FU PDT Intralesional triamcinolone |
Excision Intralesional 5-FU Acitretin Oral nicotinamide Intralesional MTX Topical 5-FU PDT Intralesional triamcinolone |
Marka et al25 | ||
| Nivolumab | Weekly wound care with antibacterial wound dressing, conforming stretch bandages, and thromboembolism deterrent hose Weekly occlusion and compression with zinc oxide gauze bandage (unna boot) |
Weekly wound care with antibacterial wound dressing, conforming stretch bandages, and thromboembolism deterrent hose Weekly occlusion and compression with zinc oxide gauze bandage (unna boot) |
Crow et al26 | ||
| Idiopathic | Excision Intralesional 5-FU |
Intralesional 5-FU | Seger et al27 | ||
| Idiopathic | Mohs Acitretin |
Mohs Acitretin |
Dyson et al28 | ||
| Nivolumab (4) | None | Spontaneous regression | Fujimura et al31 | ||
| Nivolumab | ED&C Spontaneous regression after d/c nivolumab |
ED&C Spontaneous regression after d/c nivolumab |
Antonov et al33 | ||
| Nivolumab | Topical clobetasol Intralesional triamcinolone |
Topical clobetasol Intralesional triamcinolone |
Bednarek et al34 | ||
| Trunk & extremities | Idiopathic | 52-80 | Acitretin | Acitretin | Current |
| Idiopathic | Excision Acitretin Subcutaneous MTX |
Subcutaneous MTX | Filippi et al9 | ||
| Nilotinib (1) | ED&C | ED&C | Crain et al24 | ||
| Pembrolizumab | Hydroxychloroquine | Hydroxychloroquine | Crow et al26 | ||
| UVB phototherapy & crude coal tar Topical clobetasol & intralesional triamcinolone |
UVB phototherapy & crude coal tar Topical clobetasol & intralesional triamcinolone |
||||
| Idiopathic | Antihistamines Topical corticosteroids Phototherapy Excision Acitretin |
Excision Acitretin |
Mascitti et al30 | ||
| Face, trunk, & extremities | Nivolumab (4) | 52-79 | Topical clobetasol & oral acyclovir Topical halobetasol propionate Prednisone |
Prednisone | Sullivan et al8 |
| Idiopathic | Isotretinoin Antihistamines Oral MTX Cyclophosphamide |
None | Liu et al15 | ||
| Sorafenib (14) | Excision d/c sorafenib Acitretin |
Excision Acitretin |
Abbas et al17 | ||
| Idiopathic | Acitretin & topical tretinoin | Acitretin & topical tretinoin | Rotola et al29 | ||
| Ruxolitinib (20) | Excision | Excision | March-Rodriguez et al32 |
Table References in Supplementary Material, available via Mendeley at https://doi.org/10.17632/9kvg3s5j49.1.
5-FU, 5-Fluorouricil; ED&C, electrodessication and curettage; MTX, methotrexate; PDL1, program death ligand 1; PDT, photodynamic therapy; TGF-β, transforming growth factor beta.
Treating GEKA is clinically challenging, with no uniform approach established in past literature. Therapeutic modalities such as surgical excision or physical destruction with cautery, cryotherapy, curettage, and laser ablation have been trialed for large keratoacanthomas in GEKA.2 Additionally, utilization of topical therapies, including corticosteroids, 5-fluorouracil, MTX, and triamcinolone have yielded success in some cases. Other systemic therapies have included aminopterin, MTX, cyclophosphamide, γ-interferon, and α-interferon, as generalized keratoacanthoma may be resistant to initial treatment with first-line agents.1,2,4,5 Systemic acitretin or other oral retinoids remain first-line options for GEKA. In the last 5 years, acitretin was utilized in 12 out of 41 cases (Table I).
In this case, our patient had tried topical and non-pharmacologic treatment modalities before finally responding to low-dose acitretin. This case further supports the complicated course of GEKA, the difficulties of choosing the most beneficial treatment regimen, and the utility of acitretin in promoting disease improvement and remission.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
Patient consent: The authors obtained written consent from patients for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available. Patient consent forms were not provided to the journal but are retained by the authors.
IRB approval status: Not applicable.
References
- 1.Anzalone C.L., Cohen P.R. Generalized eruptive keratoacanthomas of Grzybowski. Int J Dermatol. 2014;53(2):131–136. doi: 10.1111/ijd.12318. [DOI] [PubMed] [Google Scholar]
- 2.Nofal A., Assaf M., Ghonemy S., Nofal E., Yosef A. Generalized eruptive keratoacanthoma: a diagnostic and therapeutic challenge. Int J Dermatol. 2015;54(2):160–167. doi: 10.1111/ijd.12308. [DOI] [PubMed] [Google Scholar]
- 3.Mascitti H., De Masson A., Brunet-Possenti F., et al. Successful treatment of generalized eruptive keratoacanthoma of Grzybowski with acitretin. Dermatol Ther (Heidelb) 2019;9(2):383–388. doi: 10.1007/s13555-019-0287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwiek B., Schwartz R.A. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74(6):1220–1233. doi: 10.1016/j.jaad.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 5.Chu D.H., Hale E.K., Robins P. Generalized eruptive keratoacanthomas. Dermatol Online J. 2003;9(4):36. [PubMed] [Google Scholar]



