Abstract
Vδ2+ γδ T cell, one of promising strategies for tumor immunotherapy, recognizes and kills cancer cells in a non-MHC dependent manner. Previously, we pioneeringly proved the clinical safety and efficacy of allogeneic Vδ2+ γδ T cells, in vitro expanded from healthy donors, in the treatment of late-stage cancer patients. Nevertheless, how to profoundly potentiate cytotoxic function of expanded Vδ2+ γδ T cells remains to be further explored. Here, we proposed that 40 °C-Shock could be a simple and reliable approach to in vitro boost the effector function. We found that 40 °C-shock could phosphorylate two MAPK proteins ERK and p38 through HSP70, which facilitated actyl-α-tubulin and actin augments and reorganization, elevated Ki-67 expression and cell surface adhesion, and promoted releases of cytokines IFN-γ, perforin and granzyme B, as well as downregulated LAG3 expression. We also observed 40 °C-shock induced elevations of mitochondrial metabolism. These altogether led to potentiated cytotoxic responses against cancer cells. This proof-of-concept work demonstrated that 40 °C-shock would be probably developed into an effective method to in vitro boost the cytotoxicity of Vδ2+ γδ T cell before applying it in immunotherapy, and provided scientific evidences for the view that fever can activate immune responses of innate immune cells.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03164-x.
Keywords: Vδ2+ γδ T cell, 40 °C-shock, HSP70, Cytotoxicity
Introduction
Since the successful application of CAR-T cells in tumor therapy, research and development based on immune cells has become one of the most important frontiers for tumor immunotherapy [1–3]. Comparing with routine immune cell candidates that can be engineered into CAR-T, for example, CD8+ T cell and natural killer cells, γδ T cell is a new player but is receiving increasing attentions in the field of anti-tumor immunity. γδ T cell is the second population of T lymphocytes other than αβ T cell (e.g., CD4+, CD8+ T cells), mainly consists of Vδ1, Vδ2, and Vδ3 subsets. γδ T cell expresses numerous surface receptors including NKG2D, Fas/FasL, Trail, DNAM1, CD16, CD107a, etc., cytokines including IFN-γ, TNF-α, perforin and granzyme B, and other chemokines. Notably, we previously revealed that γδ T cell is the earliest producer of IFN-γ in the tumor microenvironment and acute spinal cord injury [4, 5]. Importantly, γδ T cell was discovered as one of the best prognostic markers of pan-cancer [6], and demonstrated to be able to regulate both innate and adaptive immunity [3]. Among three γδ T subsets, Vδ2+ γδ T cell predominantly circulates in peripheral blood and can directly kill tumor cells in non-MHC dependent manner. In our previous published works [2, 7–9], we pioneeringly proved the clinical safety of allogeneic Vδ2+ γδ T cell originated from healthy donors in treating cancer patients, then clinically evidenced that Vδ2+ γδ T cell could prolong survival of late-stage cancer patients, and discovered that cytoskeleton play a key role in regulating cytotoxicity of Vδ2+ γδ T cell against tumor cells. Our research goal is to produce off-the-shelf and low-cost Vδ2+ γδ T cell product with optimal cytotoxicity using the simple and reliable approaches, which can indeed benefit cancer patients. Therefore, in the present work, we proposed a hypothesis that thermal stress, specifically 40 °C-shock, can potentiate effector functions of Vδ2+ γδ T cells. This will provide scientific evidences for applying 40 °C-shock in immunotherapy if it is proved to be reliable.
Another circumstance that immune cells can encounter 40 °C-shock is fever. For human beings, evolution restrains the body temperature at ~ 37 °C (ranges 36.1–37.2 °C). Fever (38–41 °C) is result of inflammatory response usually triggered by pathogen infection, injury, and transformation [10–12]. Clinical observation showed fever could lead to tumor remission [13, 14], indicating fever-induced enhancement of immune responses. Therefore, investigations of the underlying mechanism on how fever regulates host immunity have attracted increasing attentions. Previously reported that fever can activate innate immunity [15–17], including neutrophil accumulating, cytokine releasing, NO producing, leukocyte trafficking, and APC potentiating, and can activate adaptive immunity, including heat shock protein up-regulating, T cell migration enhancing, T cell homing [18–20], enhanced differentiation from naïve to effector cells [21], IFN-γ up-regulating, and others [22]. Nevertheless, as one important population of T lymphocytes that bridges innate and adaptive immunity, how γδ T cell alters in the context of fever stress remains to be addressed yet. Our hypothesis is that fever stress, specifically 40 °C-shock, can also elevate immune responses of Vδ2+ γδ T cell.
To address above two hypotheses, we compared effects of different thermal stresses (4 °C, 25 °C, and 40 °C-shock) on Vδ2+ γδ T cells. mRNA-Seq results revealed that 4 °C only slightly alter Vδ2+ γδ T cells, while 25 °C adversely suppresses cell function. 40 °C-shock, however, could significantly enhance cell function and cell proliferation, augment cytoskeleton, and promote metabolism. Investigations of molecular mechanism demonstrated that 40 °C-shock up-regulates expressions of cytotoxicity-related molecules, such as IFN-γ, perforin, and granzyme B, and meanwhile downregulated inhibitory molecules, such as LAG3 and TIM3 instead of PD-1. It further determined that enhanced cytotoxic responses of Vδ2+ γδ T cells were regulated by HSP70 through activation of MAPK key proteins, ERK and p38. Such biological processes ultimately lead to potentiated cytotoxic responses against cancer cells. Altogether, this work implicated that fever can indeed regulate, appeared to be positive, immune responses of peripheral Vδ2+ γδ T cells, which ultimately strengthens host’s immune defenses, and that 40 °C-shock in vitro could be potentially developed into a simple and reliable approach to further boost cytotoxicity of Vδ2+ γδ T cells before adoptive transfer for tumor immunotherapy.
Methods and materials
Selectively expansion of human Vδ2+ γδ T cells in vitro
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood of healthy donors by following standard Ficoll-Paque protocol. Then, PBMCs were cultured in RPMI1640 medium supplemented with recombination human IL-2 (10 ng/mL, Si Huan Sheng Wu, Beijing, People’s Republic of China), and 50 μM zoledronic acid monohydrate (Sigma-Aldrich, Shanghai, People’s Republic of China). Then the culture medium was replaced with new complete medium supplemented with only FBS and IL-2 every 2 or 3 days. Vδ2+ γδ T cells were identified using flow cytometry, and only those with > 90% purity was used in experiments.
Vδ2+ γδ T cell challenged with different temperatures
To investigate how different temperature condition would affect Vδ2+ γδ T cell, 4 temperature conditions were used, including 4 °C (temperature currently widely used during transportation), 25 °C (room temperature), 37 °C (normal cell culture condition), and 40 °C (extreme temperature condition). Notably, 40 °C can somehow simulate body temperature in the context of a few extreme conditions, such as high fever.
Cell viability assay
To examine the effect of thermal stress on Vδ2+ γδ T cell viability, the Annexin V-FITC apoptosis detection kit (KeyGEN) was used here. Briefly, after Vδ2+ γδ T cells incubated at different temperatures for different time period, cells were collected and then stained with Annexin V-FITC and propidium iodide (PI) for 10 min. Then, cells were assayed by flow cytometry and the results were analyzed using FlowJo software version 10.
Atomic force microscope visualization of cell topography
Cell topography of Vδ2+ γδ T cells before and after thermal stress was visualized using atomic force microscope (AFM) measuring and imaging. As for AFM methodology, it was described in our previous publications [23–25]. In brief, the tapping mode AFM (Bioscope Catalyst, Bruker) was used to image cells at room temperature. The spring constant of cantilever was calibrated at ~ 0.6 N/m. The values of cell stiffness (Young’s Modulus) were calculated from force curves via the Scanning Probe Image Processor (SPIP) software (Image Metrology, Denmark). And the average roughness (Ra) that describes biophysical properties of membrane surface
N represents the total number of data points of selected area, zn is the height of nth point and is the mean height.
Confocal microscopy imaging
The cytoskeleton components including β-actin and α-tubulin of Vδ2+ γδ T cells were analyzed using Confocal Microscopy (Leica SP8), and Flow Cytometry (BD, Verse) as well. After different temperature challenged, Vδ2+ γδ T cells were collected and stained with Alexa Fluor 647 anti-tubulin-α (Ex: 650 nm, Em: 668 nm, Biolegend, SanDiego, USA), Oregon Green 488 Phalloidin (Ex: 495 nm, Em: 518 nm, Invitrogen, USA), and Hoechst 33,342 (Ex: 346 nm, Em: 460 nm, Macklin). For mitochondria detection, the collected cells were stained with Mitotracker Green FM (Ex: 495 nm, Em: 516 nm, Invitrogen, USA). All the staining procedures were performed by referring to reagent provider’s protocols.
Metabolism phenotyping via Seahorse analysis
The Seahorse XF technology can provide two important metabolic phenotypes of living cells, mitochondrial respiration, and glycolysis. Oxygen consumption ratio (OCR) represents the efficiency of mitochondrial respiration, and the extracellular acidification rate (ECAR) shows the glycolysis capability of cells. The OCR and ECAR were measured using Seahorse XF Cell Mito Stress Kit and Glycolysis Stress Test Kit through Seahorse XFe 96 Extracellular Flux Analyzer (Agilent, Technologies, Inc.). Firstly, 2 × 105 Vδ2+ γδ T cells per well were seeded into Seahorse XF 96 cell culture micro-plates and incubated overnight. For OCR measurements, oligomycin, FCCP, and antenone/antimycin A were sequentially injected and generated the OCR curve. As for ECAR, glucose, oligomycin, and 2-DG were injected and generated the ECAR curve. Then, all the data were analyzed using the Seahorse XF 96 Wave software.
Cancer cell killing ability by Vδ2+ γδ T cells
Cancer cell lines including A549, MCF-7, K562, and Jurkat were used as model cell lines to text the killing ability of Vδ2+ γδ T cells. For sample preparation, cancer cells (target cell, T) were firstly stained with CFSE, and then incubated with Vδ2+ γδ T cells (effector cell, E) for 6 h. Vδ2+ γδ T cells were from normal culture condition or thermal stressed conditions. The effector cell versus target cell (E:T) ratio was 10:1. Afterward, the percentage of dead cancer cells was determined using flow cytometry after propidium iodide (PI) staining.
Western blotting
To determine alterations of certain proteins, thermal stressed Vδ2+ γδ T cells were collected and lysed in RIPA lysis buffer (Biosharp). Subsequently, total proteins were separated by SDS-PAGE, and primary antibodies including Acetyl-α-tubulin (CST, #5335), p-Cdc42 (CST, #2461), p-ERK (CST, #4370), p-JNK (CST, #9251), and p-p38 (CST, #9211) were used here. The WB images were captured by FluorChem8000 imaging system and gray values were analyzed by AlphaEaseFC 4.0.
Cytokine detection
To detect the effect of thermal stress on Vδ2+ γδ T cell function, the expression of surface receptors and intracellular cytokines was analyzed as well. For surface molecular expression, the thermal stressed cells were collected, washed with 4 °C PBS and stained using fluorochrome-conjugated antibody for 30 min at 4 °C. For intracellular cytokine detection, the thermal stressed cells were pre-stimulated with PMA, ionomycin and blocked with GolgiStop for 6 h, then fixed and permeabilized, followed by staining with corresponding cytokine antibodies. The samples were analyzed using flow cytometry.
mRNA sequencing
To perform transcriptome sequencing, the temperature-stressed Vδ2+ γδ T cell were collected and washed with cold PBS, and then total RNA was extracted using 1 mL Trizol. RNA purity and quantification were evaluated using the NanoDrop 2000 spectrophotometer. The libraries were constructed followed by RNA integrity. Then, RNA sequencing and analysis were conducted by the Longsee Co., Ltd. Afterward, we performed heatmap analysis of differential genes using R, and Go annotation and KEGG pathway enrichment by using David database (https://david.ncifcrf.gov/conversion.jsp). P < 0.05 were considered as the significance threshold. GSEA was carried out by using GSEA-Win-4.0.3 software, and gene association networks were performed by String (https://string-db.org/). Short Time-series Expression Miner (STEM) version1.3.9 was used to cluster expression patterns of all genes. Expression profiles of genes were clustered based on their FPKM values and correlation coefficients.
Statistical analysis
All data were presented as mean ± standard error of mean (SEM). The differences were determined by one-way ANOVA, and P-value of the results was performed using unpaired t-tests when data was consistent with normal distribution or nonparametric tests. Detailed statistical significance is described as *P < 0.05, **P < 0.01, ***P < 0.001.
Results
mRNA-Seq analysis of Vδ2+ γδ T cell under 40 °C
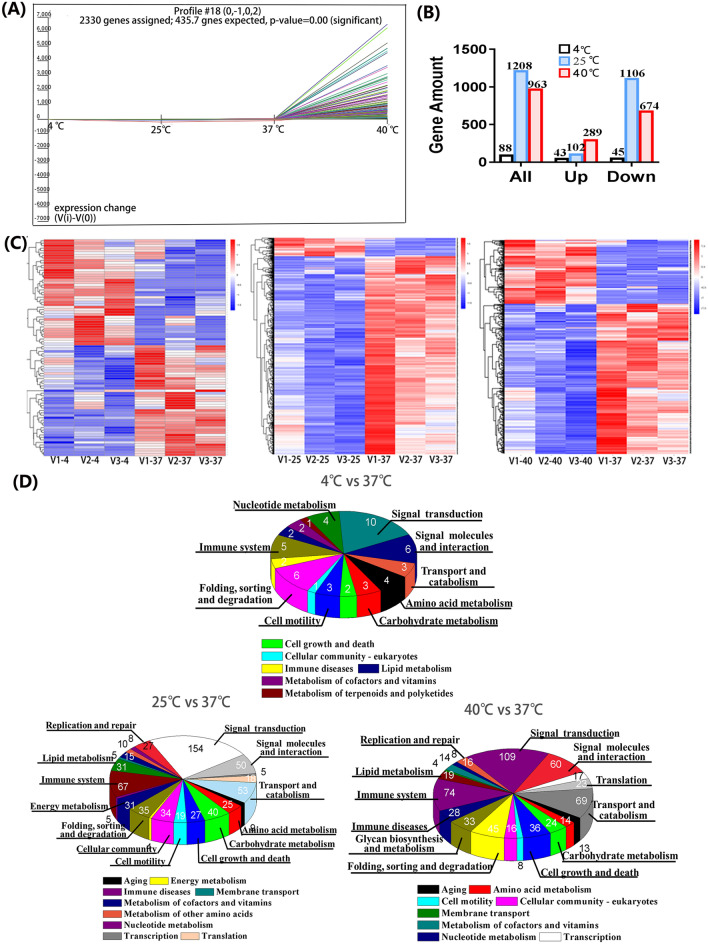
To examine how temperature stress affects biological function of Vδ2+ γδ T cell, we firstly pre-incubated cells at 40 °C for 6 h. 4 °C and 25 °C were used as controls since they are often used in the laboratory. Then the harvested cells were used to conduct mRNA sequencing (Fig. 1; Fig. S1). According to the PCA analysis, it intuitively shows that 40 °C challenged cells have significant difference in gene expression pattern comparing with cells of the rest conditions. Moreover, comparing with 37 °C, 4 °C but not 25 °C affects gene expressions very mildly. Then, clustering of sequence expression profiles was performed to identify the intersection of all gene expression patterns at four different temperature conditions. Further analysis showed that all genes can be clustered into 49 expression profiles (Fig. S1C), and the profile#18 was the most significantly enriched one (P < 0.001), with 2330 genes clustered (Fig. S1A). Figure 1A shows that the gene expression patterns have no obvious difference between 4 and 37 °C, and 25 °C can slightly down-regulate gene expression, as a contrast, 40 °C significantly up-regulates gene expression. This is endorsed by the statistical results shown in Fig. 1B and the heatmap in Fig. 1C. Furthermore, among 20,304 detected genes, only 88 differential genes (43 upregulated and 45 downregulated genes) were measured for 4 °C stressed cells comparing with 37 °C cultured cells. After cells pre-incubated at 25 °C, 1208 genes were altered, with 102 genes upregulated (8.4%) and 1106 genes downregulated (91.6%). As for 40 °C-shock, 963 differential genes were detected, with 289 genes upregulated (30%) and 674 downregulated (70%) (Fig. 1B). Further analysis revealed that six genes were co-downregulated after different temperature stressing (Fig. S1D), and the expression level of these six genes was showed in Fig. S1E. Among these six genes, TRIM32, ARPC5, and CPEB1 have been extensively studied. TRIM32 encodes a tripartite motif-containing protein with ubiquitin ligase activity and plays an important role in cell signal transduction, growth and apoptosis, innate immunity and autophagy. ARPC5 encode actin-related protein 2/3 complex subunit 5 which is involved in the assembly of actin, control of actin polymerization and affects the cell motor ability. The cytoplasmic polyadenylation element binding protein 1 (CPEB1) is a highly conserved RNA-binding protein, and is involved in various biological processes, such as cell cycle, cellular senescence, and inflammation. The rest three genes, F2RL1, LCTL, and TRAV4, remain to be fully studied yet.
Fig. 1.
The mRNA sequence (mRNA-seq) analysis of Vδ2+ γδ T cell at normal (37 °C) and temperature-stressed conditions (4 °C, 25 °C, 40 °C). A The profile#18 is significant enriched gene pattern that was produced by Short Time-Series Expression Miner (STEM) analysis. B The number of differential genes as well as up- and down- regulated genes at different thermal conditions. C The heatmap results. D The KEGG enrichment
To decipher functional annotations of differential genes, we performed gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses (Fig. 1D, F). Enriched GO terms revealed that biological process (BP) category was related to the cellular process, metabolic process, cell aggregation, signal, response to stimulus and immune system process, molecular function (MF) category was highly enriched for binding, activity of catalytic, signal transducer and transcription regulator, and the cell composition (CC) category was enriched for cell, organelle, membrane, and membrane-enclosed lumen. KEGG pathway analysis (Fig. 1D) also showed that the genes were dramatically enriched in signal transduction, immune system, cell growth and death, and metabolism (energy metabolism, carbohydrate metabolism, and lipid metabolism). Such results suggested that different temperature challenged cells have similar functional enrichment.
Then, Gene Set Enrichment Analysis (GSEA) was applied to analyze thermal stress on cell function (Fig. S1G). Since 4 °C stressing only mildly affects gene expression, we focused on analyses of differential genes at 40 °C and 25 °C. It turned out that, comparing with 37 °C condition, 25 °C stressing upregulated lipid metabolic process and locomotion related genes. As for 40°C stressing, 12 gene sets (cell junction, cell growth, reproduction, cell migration, cell motility, locomotion, immune response, immune system process, and signal transduction) were upregulated (Fig. S1G, H). Particularly, three gene sets including cell adhesion, protein folding, and response to temperature stimulus were significantly upregulated, as shown in heatmaps of Fig. S1I. This graph panel exhibits that the core enrichment genes of cell adhesion contain 17 genes such as DUSP1, HSPB1, CD34, THBS1, and SERPINE1, the protein folding gene set consists of 13 genes such as HSPA6, DNAJB1, and BAG3, and response to temperature stimulus gene set include 15 genes such as HSPA6, DNAJB1, CD34, AHSA1, and TCP1. Furthermore, we build a PPI network using String database (Fig. S1J), it shows that THBS1 bridges CD34 and SERPINE1 in cell adhesion related core enrichment genes, whereas, for core gene sets of protein folding and response to temperature stimulus, the HSP70 family genes, such as HSPA1A, HSPA1B, HSPA2, and HSPA4L, were found to play a central role in the network and bridge all other related genes. This finding implicates that HSP70 family genes play important roles in regulating immune response of Vδ2+ γδ T cell in the context of high temperature stressing.
40 °C-shock regulates proliferation and facilitates cytoskeletal reorganization
Since mRNA sequencing revealed that 40 °C-shocking could upregulate genes relating with cell proliferation, cell metabolism, cytoskeleton, and cell adhesion, therefore, functional alterations of Vδ2+ γδ T cells under 40 °C were intensively evaluated afterward. Firstly, we examined the effects of 40 °C-shock on cell viability using flow cytometry, and found that, comparing with the control group (37 °C), 40 °C did not significantly alter cell proportion of necrotic cells, early apoptosis, and late apoptosis cells at different incubation time (2 h, 6 h and 12 h) (Fig. S2A-B). Then, how 40 °C would affect cell proliferation was also studied by assaying expression of cell proliferation marker molecule Ki67. It found that 40 °C rather than 25 °C upregulated Ki67 expression in a time-dependent manner, and 6 h of incubation could significantly upregulate Ki67 expression and then this augment tends to be stabilized afterward (Fig. 2A; Fig. S2C). Further analysis based on γδ T cells derived different donors supported such observation as well (Fig. 2B).
Fig. 2.
Effects of 40 °C-shock on the proliferation, cytoskeleton rearrangement and topography of Vδ2 + γδ T cells. A The Ki67 (cell proliferation marker) expression of different time at 40 °C. Cells were from the same batch, and three duplicates tested. B Comparisons of Ki67 expression between 37 and 40 °C-shock. Cells originated from eight donors, showing similar augments in all individuals. C Representative images of cellular surface topography acquired by AFM. D Membrane surface adhesion force and Young’s modulus (quantitively describing cell stiffness) of the cells were calculated by performing cellular mechanical force curve analyses (n > 200 per group). Roughness (Ra, describing membrane surface smoothness) was obtained by analyzing membrane surface microstructures. E Cytoskeleton components (actin and tubulin) visualized using laser scanning confocal microscopy. F Quantified analyses of tubulin and actin expression by flow cytometry, showing increase tendency but no statistical significance. G The acetylated α-tubulin protein expression was assessed using the Western blot; H Rho family related protein expression was assessed using the Western blot (‘P’ represents phosphorylated). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
Because cell proliferation, mobility, morphology, recognition, and cell–cell interaction closely link with cytoskeleton infrastructures, we thus visually evaluated how 40 °C would alter cytoskeleton using atomic force microscope (AFM). We can see that, after thermal stress, the cell pseudopodia became indiscernible (Fig. 2C), the cell membrane average roughness (Ra) significantly decreased, and adhesion force of membrane surface gently increased (Fig. 2D). Notably, cell stiffness (Young’s modulus), a mechanical indicator of cytoskeletal amount, reassembling, and integrity, significantly increased by ~ 4.7-fold after 40 °C challenge (Fig. 2D).
In terms of cell stiffness alteration, we further visualized cytoskeleton using confocal microscopy and flow cytometry. We found that two major cytoskeleton components, F-actin and α-tubulin, obviously augmented after 40 °C-incubation, evident by enhanced fluorescence intensity of F-actin and α-tubulin (Fig. 2E). This was further supported by quantified assessments of flow cytometry (Fig. 2F). Notably, confocal visualization indicated that both F-actin and α-tubulin polarized in the context of 40 °C-shock, which will facilitate immune recognition and formation of immune synapse [26, 27]. Post-translational modifications regulate organization and dynamics of microtubules, for example acetylation of α-tubulin [9, 28]. Therefore, we further validated the acetylation of α-tubulin of Vδ2+ γδ T cells after challenged at 40 °C for 6 h. As shown in Fig. 2G, 40 °C greatly upregulated expression level of acetyl-α-tubulin. This indicated that 40°C stress can potentiate immune responses of Vδ2+ γδ T cell by regulating cytoskeleton organization.
Additionally, cell division cycle 42 (cdc42) is a small GTPase of Rho family, and plays an important role in regulating cytoskeleton related biological processes, including actin rearrangement, migration, and motility. Therefore, we also analyzed how the Rho family related proteins were altered by the thermal stress (Fig. 2H). The results showed that total cdc42 expression was not changed; however, the activated cdc42 (p-cdc42) was greatly upregulated by ~ 3.8-fold. This implicated that thermal (40 °C) stress can activate cdc42 which can be regulated by heat shock protein [29] to facilitate cytoskeletal rearrangement, and finally to potentiate effector function of Vδ2+ γδ T cells.
40 °C-shock promotes cellular energy metabolism
Since all biological processes are regulated by cellular energy metabolism such as glycolysis, mitochondrial oxygen respiration, and others, therefore, cellular energy metabolism analyzer (Seahorse) was used to detect how mitochondrial respiration (Oxygen Consumption Rate, OCR) and glycolysis (Extracellular Acidification Rate, ECAR) (Fig. 3A) of Vδ2+ γδ T cells were altered in the context of 40 °C-shock. Firstly, we found that, after stressed at 40 °C for 6 h, the metabolic potential (= (Maximal Respiration)/(Basal Respiration)) of OCR but not ECAR significantly elevated (Fig. 3B). Further analysis on OCR indicated that the basal respiration, ATP production, proton leak, maximal respiration, spare respiratory capacity, and non-mitochondrial respiration were all significantly elevated after thermal stress (Fig. 3C), clearly exhibited 40 °C-shock induced enhancement of mitochondrial function. This could be further supported by examinations of both confocal microscope and flow cytometry, which either visualized or quantified augments of mitochondrial mass in the context of 40 °C-shock (Fig. 3D). As for ECAR, glycolysis of Vδ2+ γδ T cells was significantly elevated by 40 °C-shock as well, even though glycolytic capacity and glycolytic reserve were surprisingly reduced (Fig. S3). Together, these results suggested that 40 °C-shock promotes metabolic function of Vδ2+ γδ T cells.
Fig. 3.
40 °C-shock promotes the mitochondrial respiration of Vδ2 + γδ T cells. A Illustration of how key parameters of mitochondria respiration (OCR) were detected by the Seahorse XF Cell Mito Stress Test Kit, and a representative graph of OCR assay. B Cellular metabolic potential calculated from the OCR curve. C Quantitative comparison of basal respiration, APT production, H + proton leak, spare respiration capacity, maximal respiration, and non-mitochondrial oxygen consumption between 37 °C group and 40 °C-shock group. D Cellular mitochondrion labeled with Mitotracker Green was visualized by confocal imaging (fluorescence) and flow cytometry (MFI). *P < 0.05; **P < 0.01; ***P < 0.001
40 °C-shock potentiates Vδ2+ γδ T cell cytotoxicity
To assess the effects of 40 °C-shock on cytotoxic activity of Vδ2+ γδ T cells, we firstly examined cytotoxicity-related cytokines releasing, including IFN-γ, perforin, and granzyme B. It showed that 40 °C-shock could upregulate expressions of IFN-γ and perforin after cells were incubated for different time (Fig. 4A). Moreover, upregulations of IFN-γ, perforin and granzyme B were confirmed in different individuals (Fig. 4B). Notably, we also examined expressions of inhibitory molecules, which negatively regulate activation and effector functions of immune cells, and found that the expressions of LAG3 and TIM3, but not PD-1, were significantly reduced in the context of 40 °C-shock (Fig. 4C; Fig. S4A). This also endorsed the conclusion that 40 °C-shock did upregulate cytotoxic function of Vδ2+ γδ T cells. Moreover, in order to detect phagocytic antigen function and ability to recognize the tumor cells of heat-treated Vδ2+ γδ T cells, expression level of CD36 and NKG2D were checked using flow cytometry (Fig. S4B, C). The results showed that 40 °C did not affect CD36 and NKG2D expression in Vδ2+ γδ T cells.
Fig. 4.
In vitro cytotoxicity evaluation of Vδ2 + γδ T cells before and after 40 °C-shock. A Representative flow graphs and statistical histograms of IFN-γ, perforin, and granzyme B expressions of cells at either 37 °C or incubated at 40 °C for 2 h, 6 h, or 12 h. B Comparisons of IFN-γ, perforin, and granzyme B expressions of cells incubated either at 37 °C or 40 °C (6 h). Each paired data point originated from an individual. C The immune checkpoint molecules LAG3, Tim3, and PD1 expression before and after 40 °C-shock. D Statistical results of Vδ2 + γδ T cell cytotoxicity (killing assay) against different tumor cells (lung cancer cell A549, breast cancer cell MCF7, chronic leukemia cell K562, and acute leukemia cell Jurkat) obtained by flow cytometry. ns, no statistical significance; *P < 0.05; **P < 0.01; ***P < 0.001
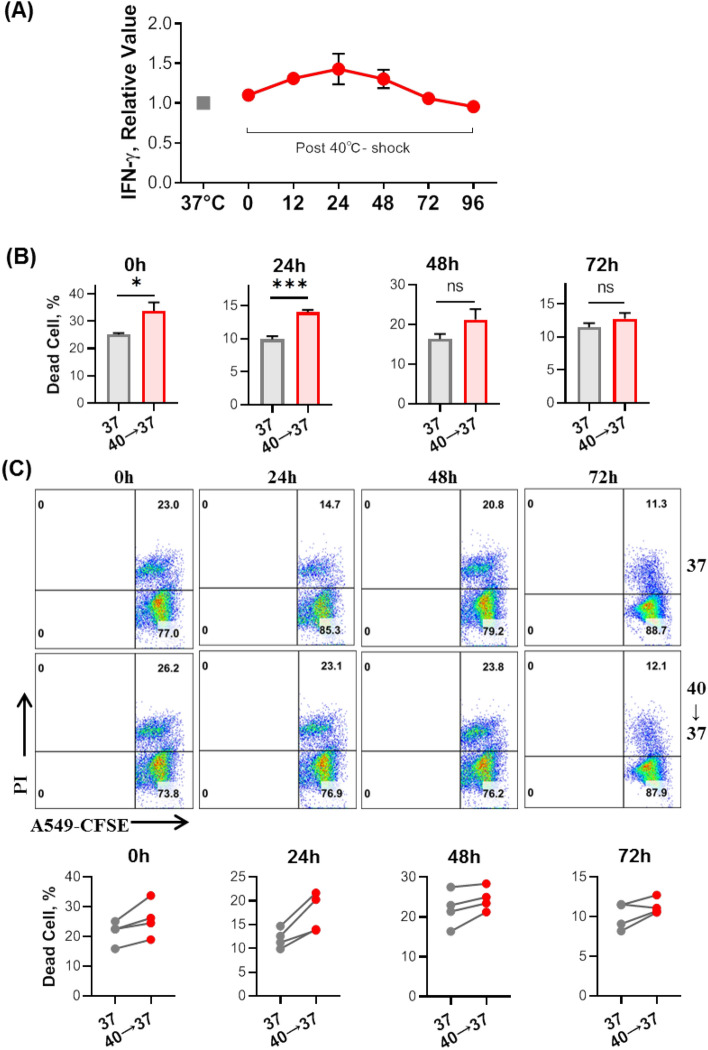
To further examine the cytotoxic response of 40 °C-shocked Vδ2+ γδ T cells, in vitro killing assay was conducted based on model cancer cell lines, A549, MCF-7, K562, and Jurkat cells. Cancer cells were pre-stained with dye CFSE, following by coincubation with stressed Vδ2+ γδ T cells for 6 h at the E:T ratio of 10:1, then percentage of dead cancer cells was determined by flow cytometry. It turned out that thermal stressed Vδ2+ γδ T cells have obvious higher killing ability against cancer cells (Fig. 4D). Additionally, it is interesting to explore how long 40 °C-shock enhanced cytotoxicity of Vδ2+ γδ T cells can be sustained in vitro, therefore, 40 °C-shocked cells were re-incubated at 37 °C for different time (24, 48, 72 h). Then, IFN-γ expression and killing ability were assayed (Fig. 5). We can see that IFN-γ releasing peaks at 24 h, and then down to the original level after 72 h (Fig. 5A). This can be supported by subsequent killing assay using A549 cancer cells, showing that cytotoxicity of Vδ2+ γδ T cells was still sustained at relative high level after 24 h of thermal stress comparing with the control condition (Fig. 5B). This was also confirmed in γδ T cells expanded from different donors (Fig. 5C). Together, such data indicated that 40 °C-shock could strengthen the cytotoxicity of Vδ2+ γδ T cells, scientifically supporting the hypothesis that high fever, far infrared physical hyperthermia, or steaming therapy could enhance the cytotoxic response of Vδ2+ γδ T cells, and that 40 °C-shocked Vδ2+ γδ T cells possess better anti-tumor activity.
Fig. 5.
Evaluation on how long 40 °C-shock induced functional enhancements of Vδ2+ γδ T cell could be sustained in vitro (37 °C). A After cells were firstly shocked at 40 °C for 6 h, they were subsequently put back to 37 °C. Then IFN-γ expression over time was detected. It shows IFN-γ releasing peaks at 24 h. B Tumor killing ability assay based on lung cancer cell line A549. C Killing assay of Vδ2+ γδ T cell that originated from different donors, showing similar enhanced cytotoxicity (against A549 cell) in all individuals. ‘40 → 37’ means cells were firstly shocked at 40 °C for 6 h, and then re-incubated at 37 °C for 24, 48, or 72 h. ns, no statistical significance; *P < 0.05; ***P < 0.001
In order to more fully simulate the fever environment, we detected the cell mortality after 6 h of co-heat treatment of tumor cells (A549) and Vδ2+ γδ T cells. The results were showed in Fig. S5, there was no significant change in A549 cell mortality after 40 °C co-heat treatment as compared with heat-treated Vδ2+ γδ T cells alone. This may be attributed to the cytotoxicity to tumor cells at temperatures above 42 °C [30]. Therefore, the co-heating at 40 °C cannot effectively cause the enhancement of cytotoxicity.
40 °C-shock enhances cytotoxicity through HSP70
Attempt to reveal the underlying molecular mechanism on how 40 °C-shock enhances cytotoxic responses of Vδ2+ γδ T cells, we further mined the data of mRNA sequencing. Via KEGG analysis, we found that thermal stress upregulated genes were significantly enriched in MAPK signal pathway (Fig. 6A), which is further confirmed by the GSEA analysis (Fig. 6B). MAPK signal pathway has been identified to include three major parts, ERK, JNK, and P38, majorly involving in cell proliferation, differentiation, migration, cytokine secretion, and others [31, 32]. Moreover, the most significant upregulated genes of the MAPK signal pathway are listed in heatmap, including HSPA6, HSPA1A, GADD45R, JUN, and DUSP1 (Fig. 6C). Importantly, the PPI network of core enrichment genes further indicates that the HSP70 family genes (HSPA1A, HSPA1B, HSPA2, HSPA6, HSPA8), which modulates various physiological processes including stress responses, proliferation, and apoptosis [33], play a central role in the regulation network (Fig. 6D). This phenotype proposed a hypothesis that HSP70 is a key regulator for 40 °C-shock strengthened cytotoxic responses of Vδ2+ γδ T cell.
Fig. 6.
mRNA-Seq analysis of upregulated genes of Vδ2+ γδ T cell stressed at 40 °C for 6 h. A The KEGG enrichment of 40 °C-shock upregulated genes. B The GSEA plot of MAPK signal pathway gene set. C The differential gene heatmap of MAPK pathway gene set. D The string PPI analysis of core gene enrichment of MAPK pathway
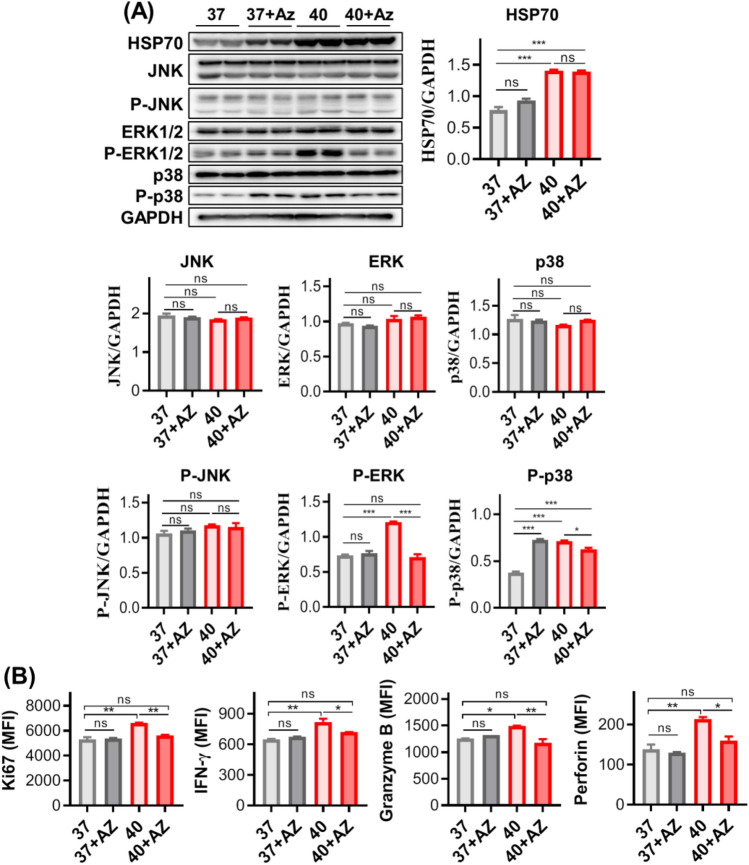
Therefore, we examined the expression of HSP70 and MAPK signaling pathway related proteins before and after 40 °C-shock using Western blot. It shows that total expressions of JNK, phosphate JNK (P-JNK), ERK, and p38 were not statistically altered. As a contrast, expressions of HSP70, activated ERK (P-ERK) and p38 (P-p38) were all significantly upregulated (Fig. 7A). In order to further determine the correlation between HSP70 and MAPK, T cells were pre-treated with Apoptozole (AZ), a specific inhibitor for HSP70 by binding to its ATPase domain [34], for 30 min before 40 °C-shock. The results (Fig. 7A) turned out that both P-ERK and P-p38 were downregulated by inhibitor AZ, which suggested that HSP70 may be an upstream regulator for activation of ERK and p38.
Fig. 7.
40 °C-shock regulates functional alterations of Vδ2 + γδ T cell through heat shock protein 70 (HSP70). A The expression of HSP70 protein and MAPK signal pathway related proteins before and after 40 °C-shock as well as treatment of HSP70 inhibitor (Apoptozole, Az). It shows that total protein levels of JNK, ERK, and p38 were not affected, and phosphorylated ERK and p38 but not JNK were elevated by 40 °C-shock. Inhibitor Az treatment could inhibit such phosphorylation. For HSP70, 40 °C-shock significantly upregulated its expression, and Az did not suppress on its expression level. B Cell proliferation and cytokine release could be regulated by HSP70 as well. Upregulated Ki67 expression by 40 °C-shock was lowered by Az treatment, so do cytokine release Vδ2 + γδ T cell (IFN-γ, perforin, and granzyme B)
Because the activation of ERK and p38 involves in regulating cellular proliferation and cytokine releasing, we also detected Ki67 expression and cytokine production in the context of HSP70 inhibitor and 40 °C-shock. We found that, comparing with 40 °C-shock alone, the expressions of IFN-γ, Ki67, perforin, and granzyme B were all significantly reduced after the inhibitor introduced (40 °C + AZ) (Fig. 7B; Fig. S6A). It should be mentioned here that the inhibitor AZ has no effects on inhibitory molecule expression (LAG3, Tim3) (Fig. S6B). Altogether, such findings indicate that 40 °C-shock potentiated cytotoxic responses of Vδ2+ γδ T cell were regulated by HSP70.
Discussion
Under certain circumstances, the body temperature of human could be greatly higher than average 37 °C and reach as high as 40 °C, such as very high fever. Currently, it recognized that fever is closely related with activation of both innate and adaptive immunity [16, 17]. Nevertheless, fever-related increase of body temperature belongs to intrinsic thermal stress. As a contrast, extrinsic thermal stress normally includes both low and high temperature challenge, which can greatly affect physiological function because both low and high temperature exceeded normal body temperature range became lethal. However, down to the cell level, how thermal stress would regulate immune responses of immune cells remains to be fully addressed. For years, we have focused on anti-tumor immunity of γδ T cell. Therefore, in the present work, we focused on Vδ2+ γδ T cells to examine how thermal stress, specifically 40 °C-shock, can regulate cellular effector functions.
Firstly, for the purpose of comparison, 4 °C and 25 °C were tested. 4 °C is experientially used as cell transportation temperature in short-term, and the experimental condition in laboratory. 25 °C (room temperature) was only a control condition because it is rarely used to manipulate cells. Transcriptome mRNA sequencing clearly revealed that, comparing with 37 °C (in vitro cell culture condition), 4 °C affects cell gene expression very mildly, evident by only detected 88 differential genes. Whereas, 1208 genes were altered under 25 °C-stress. These findings scientifically support that 4 °C is the optimal temperature condition for cellular short-distance or short-term transportation, and probably the best experimental condition in laboratory as well. As a contrast, room temperature stress is an adverse condition for maintaining cell function.
Our focus was to illustrate how 40 °C-shock would benefit cellular responses. Bioinformatic analyses revealed that 40 °C-shock could indeed promote cell function related gene expression, such as proliferation, cell adhesion, cytoskeleton, effector function, and cell metabolism. Firstly, gradual increases of Ki67 expression over incubation time indicated that 40 °C-shock can elevate cell proliferation, and 6-h incubation appeared to be an optimal condition. While longer than 12-h incubation has no obvious increasing trend for the Ki67 expression. This suggested that short-term stimulation at 40 °C could be probably developed into a simple and reliable approach to enhance cell function before adoptive transfer of Vδ2+ γδ T cell in tumor immunotherapy. Meanwhile, 40 °C-shock could increase cell adhesion as well. This will facilitate Vδ2+ γδ T cell to interact with target cells, since cell–cell adhesion is an initiation step for immune recognition.
It reported that cell proliferation, differentiation and mobility are closely regulated by cytoskeleton [35]. It observed that 40 °C-shock could indeed not only increase expression of cytoskeleton proteins (F-actin and tubulin) but also induce spatial reorganization and polarization of cytoskeleton. Furthermore, the acetylation of α-tubulin expression was significantly upregulated as well, which contributes to stabilization of cytoskeletal structures and facilitates the formation of immune synapse, resulting in increased immune recognition and cytotoxicity. The phenotype that elevated cell stiffness caused by 40 °C-shock also supports such explanations. More importantly, our further investigation revealed that the phosphorylation of cdc42, a GTPase of Rho family that regulates the dynamic organization of cytoskeleton [36–38], was significantly upregulated by around 3.8-fold. This suggests that 40 °C-shock regulates cytoskeletal alterations through cdc42 phosphorylation which can be regulated by heat shock protein. [29, 39] Notably, 40 °C-shock evaluated both mitochondrial respiration and glycolysis, which contributes to cytoskeletal alterations and enhanced cytotoxic responses (upregulated cytokines, downregulated inhibitory molecules, strengthened killing ability, etc.). It should be marked here that, 40 °C-shock induced upregulation of cytotoxic responses of Vδ2+ γδ T cells could sustain around 72 h. This would be used to boost effector function of Vδ2+ γδ T cells before adoptive transfer for tumor immunotherapy.
Further molecular mechanism investigation showed that heat shock protein (HSP) family genes played a key role in regulating cell function in the context of 40 °C-shock. Both mRNA-Seq and WB analyses identified that HSP70 regulated activations of MAPKs signal pathway ERK and p38, as well as the production of cytokines. Previous reports showed that ERK regulates cell proliferation [39], and p38 MAP kinase regulates cytokine expression [40]. Therefore, we can conclude that 40 °C-shock activated both ERK and p38 through upregulation of HSP70, leading to enhanced proliferation and anti-tumor cytotoxicity of Vδ2+ γδ T cell. It should be marked here that P-p38 of normal cultured cells (37 °C) was significantly upregulated in the context of HSP70 inhibition (AZ treated), which may be because HSP70 is a cytoprotective protein which activity is inhibited and stimulates cells to express more HSP70 and regulates the activation of P38 for cell protection. And the specific reason is not yet clear and needs to be further studied.
In conclusion, our results demonstrated that different temperature stresses alter transcriptome of Vδ2+ γδ T cell diversely. 4 °C affect cells very mild and is the optimal condition for short-term or distance transportation and experimental condition in the laboratory. 40 °C can activate ERK and p38 through up-regulating HSP70, subsequently triggers cytoskeletal rearrangement and metabolic elevation, finally leads to enhancements of cytotoxic responses. This is summarized as the sketch graph in Fig. 8. The findings based on 40 °C-shock provide scientific evidences for the view that fever can positively maneuver immune responses, and more importantly, proposed a simple and reliable strategy for in vitro boosting cytotoxic function of immune cells before adoptive transfer for tumor immunotherapy.
Fig. 8.
The sketch graph of transient 40 °C-shock potentiates cytotoxic response of Vδ2+ γδ T cell
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Prof Jianlei Hao and Guangchao Cao from the Biomedical Translational Research Institute of Jinan University for their suggestions on data analyzing, and thank technician Zonghua Liu for help on experimental preparations. Funding was provided by Young Scientists Fund (Grant Nos. 32000534, 82002787), Startup Foundation of the Zhuhai People’s Hospital, Natural Science Foundation of Guangdong Province (Grant No. 2020A1515010132), Key Program of the National Natural Science Foundation of China (Grant Nos. 32030036, 31830021).
Author contributions
YZW, LL and ZNY Work supervising and protocol design. LL, YC DC Experiments. LL, YZW, ZNY, YC, DC, JS and YH Data analysis and discussion. LL and YZW Manuscript drafting. YZW, LL and YH Manuscript proof-reading and revision. All authors approved the final version of manuscript.
Funding
This work was partially supported by the Key Program of the National Natural Science Foundation of China (32030036, 31830021) (ZNY), and by the National Natural Science Foundation of China (32000534) (LL) and (82002787) (YH). YZW is supported by the Startup Foundation of the Zhuhai People’s Hospital (YNXM20210305) and the Natural Science Foundation of Guangdong Province, China (2020A1515010132).
Declarations
Conflict of interest
All authors declare no conflict of interest.
Footnotes
Li Lin and Yan Chen authors share the first authorship.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhinan Yin, Email: tzhinan@jnu.edu.cn.
Yangzhe Wu, Email: tyzwu@jnu.edu.cn.
References
- 1.Xiang Z, Tu W. Dual face of Vγ9Vδ2-T cells in tumor immunology: anti- versus pro-tumoral activities. Front Immunol. 2017;8:1041–1041. doi: 10.3389/fimmu.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alnaggar M, et al. Allogenic Vgamma9Vdelta2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J Immunother Cancer. 2019;7(1):36. doi: 10.1186/s40425-019-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva-Santos, Mensurado S, Coffelt SB (2019) gammadelta T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer 19(7):392–404 [DOI] [PMC free article] [PubMed]
- 4.Gao Y, et al. gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198(3):433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun G, et al. gammadelta T cells provide the early source of IFN-gamma to aggravate lesions in spinal cord injury. J Exp Med. 2018;215(2):521–535. doi: 10.1084/jem.20170686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentles AJ, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin L, et al. Chitosan nanoparticles strengthen Vgamma9Vdelta2 T-cell cytotoxicity through upregulation of killing molecules and cytoskeleton polarization. Int J Nanomed. 2019;14:9325–9336. doi: 10.2147/IJN.S212898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Y, et al. Allogeneic Vgamma9Vdelta2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell Mol Immunol. 2021;18(2):427–439. doi: 10.1038/s41423-020-0515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Y et al (2019) Selenium nanoparticles as new strategy to potentiate γδ T cell anti-tumor cytotoxicity through upregulation of tubulin-α acetylation. Biomaterials 222:119397 [DOI] [PubMed]
- 10.Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect. 2000;2(15):1891–1904. doi: 10.1016/S1286-4579(00)01337-X. [DOI] [PubMed] [Google Scholar]
- 11.Zhang HG, et al. Hyperthermia on immune regulation: a temperature’s story. Cancer Lett. 2008;271(2):191–204. doi: 10.1016/j.canlet.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Pasikhova Y, Ludlow S, Baluch A. Fever in patients with cancer. Cancer Control. 2017;24(2):193–197. doi: 10.1177/107327481702400212. [DOI] [PubMed] [Google Scholar]
- 13.Hobohm U. Fever therapy revisited. Br J Cancer. 2005;92(3):421–425. doi: 10.1038/sj.bjc.6602386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cann SAH, Van Netten JP, Van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad Med J. 2003;79(938):672–680. [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, et al. Febrile temperature critically controls the differentiation and pathogenicity of T helper 17 cells. Immunity. 2020;52(2):328. doi: 10.1016/j.immuni.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Evans SS, Repasky EA, Fisher DT (2015) Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol 15(6):335–349 [DOI] [PMC free article] [PubMed]
- 17.Hasday JD, Thompson C, Singh IS. Fever, immunity, and molecular adaptations. Compr Physiol. 2014;4(1):109–148. doi: 10.1002/cphy.c130019. [DOI] [PubMed] [Google Scholar]
- 18.Kumada K, et al. HSP70/DNAJA3 chaperone/cochaperone regulates NF-κB activity in immune responses. Biochem Bioph Res Co. 2019;513(4):947–951. doi: 10.1016/j.bbrc.2019.04.077. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol. 2006;7(12):1299–1308. doi: 10.1038/ni1406. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi Y et al (2014) Fever-range whole-body heat treatment stimulates antigen-specific T-cell responses in humans. Immunol Lett 162(1 Pt A):256–261 [DOI] [PubMed]
- 21.Mace TA, et al. Differentiation of CD8+ T cells into effector cells is enhanced by physiological range hyperthermia. J Leukoc Biol. 2011;90(5):951–962. doi: 10.1189/jlb.0511229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mace TA, et al. Effector CD8 + T cell IFN-γ production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperther. 2012;28(1):9–18. doi: 10.3109/02656736.2011.616182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Sims RC, Zhou A. AFM resolves effects of ethambutol on nanomechanics and nanostructures of single dividing mycobacteria in real-time. Phys Chem Chem Phys. 2014;16(36):19156–19164. doi: 10.1039/C4CP01317D. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, et al. BRMS1 expression alters the ultrastructural, biomechanical and biochemical properties of MDA-MB-435 human breast carcinoma cells: an AFM and Raman microspectroscopy study. Cancer Lett. 2010;293(1):82–91. doi: 10.1016/j.canlet.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Mcewen GD, et al. Subcellular spectroscopic markers, topography and nanomechanics of human lung cancer and breast cancer cells examined by combined confocal Raman microspectroscopy and atomic force microscopy. Analyst. 2013;138(3):787–797. doi: 10.1039/C2AN36359C. [DOI] [PubMed] [Google Scholar]
- 26.Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol. 2007;7(2):131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn JR, Poenie M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity. 2002;16(1):111–121. doi: 10.1016/S1074-7613(02)00262-5. [DOI] [PubMed] [Google Scholar]
- 28.Wei D, et al. α-Tubulin acetylation restricts axon overbranching by dampening microtubule plus-end dynamics in neurons. Cereb Cortex. 2018;28(9):3332–3346. doi: 10.1093/cercor/bhx225. [DOI] [PubMed] [Google Scholar]
- 29.Selva E, et al. Inhibition of apoptosis induced by heat shock preconditioning is associated with decreased phagocytosis in human polymorphonuclear leukocytes through inhibition of Rac and Cdc42. Immunol Cell Biol. 2007;85(3):257–264. doi: 10.1038/sj.icb.7100029. [DOI] [PubMed] [Google Scholar]
- 30.Stewart JR et al (1984) Hyperthermia in the treatment of cancer. Perspectives on its promise and its problems. Cancer 54 (11 Suppl):2823–2830 [DOI] [PubMed]
- 31.Sun Y, et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 32.Jakhar R, et al. Immunosuppressive potential of astemizole against LPS activated T cell proliferation and cytokine secretion in RAW macrophages, zebrafish larvae and mouse splenocytes by modulating MAPK signaling pathway. Int Immunopharmacol. 2018;65:268–278. doi: 10.1016/j.intimp.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Hao Y, et al. Role of MAPKs in HSP70’s protection against heat stress-induced injury in rat small intestine. BioMed Res Int. 2018;2018:1–10. doi: 10.1155/2018/1571406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko S-K, et al. A small molecule inhibitor of ATPase activity of HSP70 induces apoptosis and has antitumor activities. Chem Biol. 2015;22(3):391–403. doi: 10.1016/j.chembiol.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Cui Y, et al. Airborne particulate matter impairs corneal epithelial cells migration via disturbing FAK/RhoA signaling pathway and cytoskeleton organization. Nanotoxicology. 2018;12(4):312–324. doi: 10.1080/17435390.2018.1440651. [DOI] [PubMed] [Google Scholar]
- 36.Etienne-Manneville S. Cdc42–the centre of polarity. J Cell Sci. 2004;117(Pt 8):1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- 37.Cerione RA. Cdc42: new roads to travel. Trends Cell Biol. 2004;14(3):127–132. doi: 10.1016/j.tcb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16(13):1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 39.Ostberg JR, et al. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukocyte Bio. 2007;82(5):1322–1331. doi: 10.1189/jlb.1106699. [DOI] [PubMed] [Google Scholar]
- 40.Rincón M, Pedraza-Alva G. JNK and p38 MAP kinases in CD4+ and CD8+ T cells. Immunol Rev. 2003;192:131–142. doi: 10.1034/j.1600-065X.2003.00019.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.