Abstract
Dermatofibrosarcoma protuberans (DFSP) stands as a rare and locally aggressive soft tissue tumor, characterized by intricated molecular alterations. The imperative to unravel the complexities of intratumor heterogeneity underscores effective clinical management. Herein, we harnessed single-cell RNA sequencing (scRNA-seq) to conduct a comprehensive analysis encompassing samples from primary sites, satellite foci, and lymph node metastases. Rigorous preprocessing of raw scRNA-seq data ensued, and employing t-distributed stochastic neighbor embedding (tSNE) analysis, we unveiled seven major cell populations and fifteen distinct subpopulations. Malignant cell subpopulations were delineated using infercnv for copy number variation calculations. Functional and metabolic variations of diverse malignant cell populations across samples were deciphered utilizing GSVA and the scMetabolism R packages. Additionally, the exploration of differentiation trajectories within diverse fibroblast subpopulations was orchestrated through pseudotime trajectory analyses employing CytoTRACE and Monocle2, and further bolstered by GO analyses to elucidate the functional disparities across distinct differentiation states. In parallel, we segmented the cellular components of the immune microenvironment and verified the presence of SPP1+ macrophage, which constituted the major constituent in lymph node metastases. Remarkably, the CellChat facilitated a comprehensive intercellular communication analysis. This study culminates in an all-encompassing single-cell transcriptome atlas, propounding novel insights into the multifaceted nature of intratumor heterogeneity and fundamental molecular mechanisms propelling metastatic DFSP.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03577-2.
Keywords: Dermatofibrosarcoma protuberans, Metastasis, Single-cell RNA sequencing analysis, Intratumoral heterogeneity
Introduction
Dermatofibrosarcoma protuberans (DFSP), an uncommon soft tissue sarcoma, has long captivated the medical community due to its distinctive characteristics, including slow growth, local invasiveness, and a propensity for recurrence [1]. With an annual incidence of approximately 0.8 to 5 cases per million population [2–4], gaining a comprehensive understanding of its etiological triggers and risk factors has been challenging. DFSP predominantly affects adults in their prime, exhibiting a characteristic age distribution with a peak occurrence in the third and fourth decades of life [5]. The recurrent COL1A1-PDGFB fusion gene, resulting from a t(17;22) chromosomal translocation, acts as a driver mutation observed in approximately 90% of DFSP cases [6, 7]. This fusion gene plays a pivotal role in orchestrating oncogenic signaling pathways within the tumor microenvironment [8]. Moreover, the landscape of DFSP’s molecular intricacies is further enriched by the identification of other gene rearrangements, such as the SLC2A5-BTBD7 [t(1;14)] fusion [9]. Clinical diagnosis often presents difficulties due to its variable presentation and potential confusion with other benign or malignant entities. While histopathological analysis revealing storiform proliferation of spindle cells and CD34 expression remains central to definitive diagnosis [10, 11]. Recent advancements in immunohistochemistry and radiographic techniques have partially enhanced diagnostic precision [3]. The management of DFSP adopts a multidisciplinary approach, tailored to the tumor's unique behavior. Surgical excision serves as the primary modality, with the extent of resection and adjuvant therapies adjusted according to tumor size, location, and recurrence risk [12]. In advanced or unresectable cases, Imatinib, a tyrosine kinase inhibitor targeting PDGF receptor (PDGFR) pathways, has emerged as a promising therapeutic avenue [13, 14]. However, the evolving therapeutic landscape of DFSP, including the exploration of targeted agents and immunotherapeutic interventions, underscores the necessity for ongoing research.
In this study, we embark on an investigative odyssey into the cellular heterogeneity underpinning DFSP, harnessing the power of single-cell RNA sequencing (scRNA-seq) to dissect the intricate interplay of cell populations within neoplastic lesions. By unraveling diverse transcriptional profiles, molecular drivers, and critical intercommunications, we aim to unveil concealed facets of DFSP pathogenesis and offer insights that hold promise for precision treatment.
Results
Multifocal DFSP with sequential metastasis
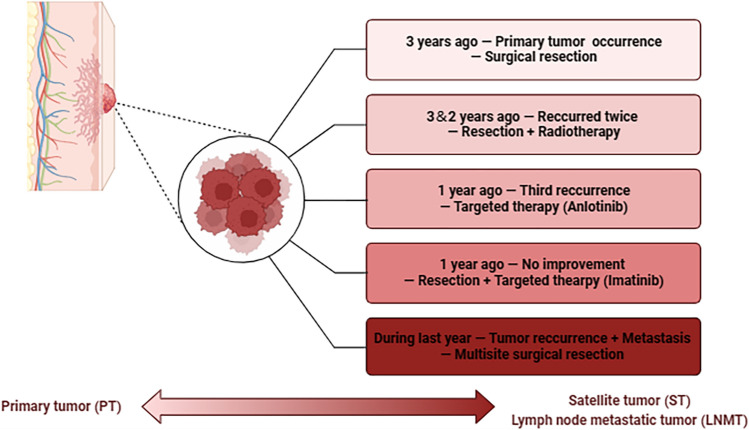
We present a compelling case of a 40-year-old female patient with multifocal DFSPs, demonstrating primary, satellite, and lymph node metastatic tumors (Fig. 1). The initial presentation occurred three years ago as a painless mass, measuring approximately 2 × 2 cm, situated in the right supraclavicular area. Following surgical resection at a local hospital, the disease recurred in situ, necessitating a second surgical intervention within the same year. Despite these interventions, the disease continued to resurface, resulting in a third surgical resection two years ago, followed by postoperative radiotherapy. However, another recurrence was observed a year ago, prompting treatment with anlotinib, which yielded no improvement. Subsequently, the patient went through a fourth surgical removal and underwent imatinib-targeted therapy. Referring to the pathology report, all previous surgeries achieved negative margins. During this visit, the clinical examination revealed multiple lumps, extending across the right anterior chest wall, right breast, and right axilla with deep tissue infiltration. Utilizing preoperative imaging along with intraoperative pathological observations, the conclusive diagnosis of DFSP with satellite and lymph node metastasis was established (Fig. 2). This case underscores the challenges of managing metastasis DFSP and highlights the importance of comprehensive analysis in unraveling the underlying mechanisms.
Fig. 1.
Diagram illustrating the course of disease advancement and the treatment journey in the context of a metastatic case of dermatofibrosarcoma protuberans (DFSPs)
Fig. 2.
Description of the detailed clinical information. a Demonstration of the patient’s right supraclavicular primary tumor, chest wall satellite metastases, and lymph node metastases (internal) lesion sites. *: the approximate location of 3 tumor samples; b CT imaging for diagnosis of multisite tumor lesions; c Pathologic findings including Hematoxylin–Eosin (HE) staining and immunohistochemistry for diagnosis of DFSP at different sites
Comprehensive single-cell transcriptome atlas of progressive DFSP lesions
Our investigation commenced with the execution of scRNA-seq on distinct tumor samples encompassing the primary lesion, chest wall satellite metastases, and lymph node metastases. After conducting the initial quality control assessment (Supplementary Fig. S1), a total of 9151 cells were selected for subsequent analysis, from which single-cell expression profiles were extracted (Fig. 3a). We then partitioned these single cells into seven distinct subclusters, characterized by unique expression patterns using t-distributed stochastic neighbor embedding (t-SNE) analysis (Fig. 3b). To showcase their unique identities, we identified all differentially expressed genes (DEGs) (Supplementary Table S1) and presented the top five in a dot plot format (Fig. 3c). Leveraging insights from SingleR annotation and established markers (Supplementary Fig. S2), we categorized these clusters as follows: (1) cancer stem cell cluster enriched in cell cycle-associated mRNAs (MKI67, CDK1, TOP2A, CDC20); (2) fibroblast cluster exhibiting expression of DCN and COL1A1; (3) endothelial cell cluster characterized by PECAM and VWF signature molecules; (4) pericyte cluster demonstrating elevated ACTA2 and RGS5 expression; (5) macrophage cluster marked by CD68 and CD163; (6) T cell cluster expressing CD2 and CD8A; (8) mast cell cluster with heightened TPSAB1 and CPA3 expression(Fig. 3d). Further, the proportions of each cell type across the three samples are presented in Fig. 3e, unraveling the intertumoral heterogeneity driving progression.
Fig. 3.
Single-cell transcriptional atlas of DFSP. a Flowchart of single-cell transcriptome analysis of 3 DFSP samples; b Visualization of the 7 predominant cell types in metastatic DFSP lesions using a t-distributed stochastic neighbor embedding (t-SNE) plot; c Dot plot illustrating the top 5 differentially expressed genes (DEGs) for 7 major cell clusters; d Feature plot revealing cell-specific characteristics across 7 distinct subgroups; e Stacked bar graph depicting cell proportions across 3 tumor samples
Copy number variations revealing distinct patterns in DFSP tumor cells
In the tripartite context of the tumor samples, fibroblasts emerge as the prevailing cellular cluster, attaining a numerical count of up to 5891 cells. This fibroblast abundance is distinctly categorized into five subpopulations, delineated by top DEGs as illustrated in Fig. 4a, b. To discern malignant entities within DFSP, we conducted a comprehensive analysis of chromosomal copy number variations (CNVs), grounded in transcriptomic data. Individual and composite heatmaps were presented, offering insights into different genomic landscapes. We designated immune cell clusters as benchmarks. Gain regions, depicted in red, and loss regions, in blue, populated the derived CNV heatmaps. Notably, in primary tumors and lymph node metastases, all fibroblast subpopulations and cancer stem cells exhibited malignancy. However, in satellite foci, a higher CNV variability was observed in cancer stem cells and the Fib02, Fib03, and Fib04 subpopulations (Fig. 4c–f). The discerned tumor clonal heterogeneity across progression stages may partially contribute to the heightened recurrence rate of DFSP.
Fig. 4.
Distinct subpopulations of tumor cells in metastatic DFSPs. a t-SNE plot illustrating the presence of 6 subclusters within malignant cells. Fan charts representing the relative proportions of distinct malignant cell groups within 3 tumor samples; b Dot plot for the visualization of the top 5 DEGs across various subpopulations of tumor cells; (c-f) The hierarchical heatmaps show significant copy number variations (CNVs) within different tumor lesions (primary tumor, satellite tumor, lymph node metastasis tumor, all samples merged)
Functional and metabolic heterogeneity among malignant cells in DFSP
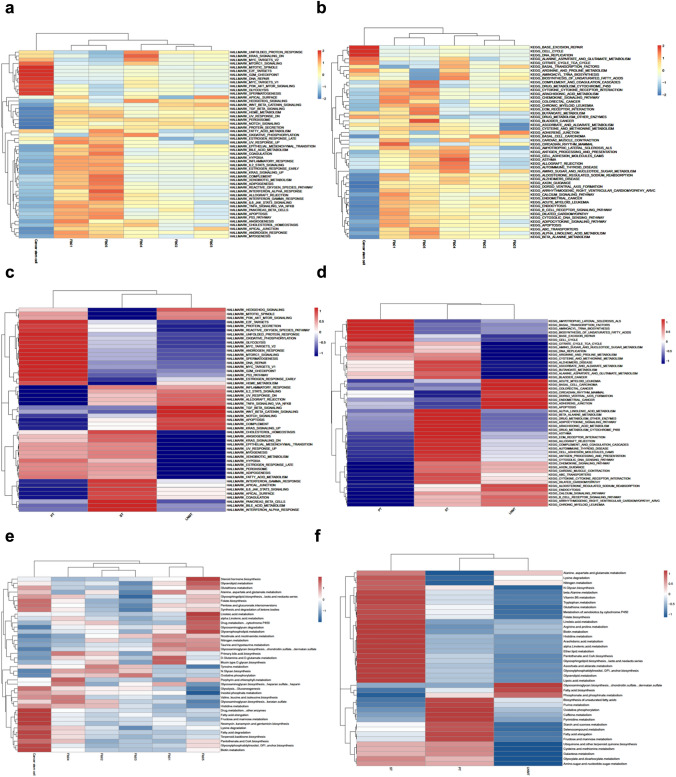
To unravel the contributions of diverse malignant cells to the emergence and advancement of DFSP, we conducted a comprehensive Gene Set Variation Analysis (GSVA) analysis, shedding light on both Hallmark and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. Remarkably, the cancer stem cell subset displayed significant enrichment in cell cycle and DNA repair pathways intricately linked to tumor proliferation. Fib01 and Fib05 displayed heightened involvement in IL-6/JAK/STAT3, TGF-β, and epithelial-mesenchymal transition (EMT)-associated pathways, suggestive of their potential roles in facilitating tumor metastasis. In contrast, Fib04 exhibited prominent enrichment in inflammation-associated pathways. Additionally, Fib02 and Fib03 showcased upregulation in Wnt-β and NOTCH signaling pathways, indicating their heightened malignancy potential (Fig. 5a, b). This comprehensive evaluation underscores the plausible roles of these distinct populations in driving the intricate pattern of DFSP evolution.
Fig. 5.
Disparities in enrichment pathways and metabolic profiles between 3 samples and 6 clusters of malignant cells. a Heatmap depicting the characteristic pathway terms of enriched Hallmark pathways within each cellular subgroup; b Heatmap depicting the characteristic pathway terms of enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways within each cellular subgroup; c Heatmap illustrating the distinctive pathway terms enriched within each tumor sample according to Hallmark pathways; d Heatmap illustrating the distinctive pathway terms enriched within each tumor sample according to KEGG pathways; e Heatmap demonstrating variations in metabolic profiles across diverse tumor cell clusters; f Heatmap demonstrating variations in metabolic profiles across primary and metastatic tumor samples
Our study further unveiled substantial diversity in the functional enrichment of tumor cells across these samples. In primary foci, a significant enrichment of signaling pathways associated with malignancy, notably PI3K-AKT-MTOR and P53, was evident. Notably, satellite foci exhibited a prominent upregulation of angiogenic and EMT-related pathways, suggesting their potential involvement in pathways conducive to metastasis. Additionally, our analysis of lymph node metastatic foci highlighted enriched signaling pathways, including KRAS and apoptosis, which could serve as drivers of their distant metastasis (Fig. 5c, d). At the tissue level, we observed a higher presence of cleaved Caspase-3-positive cells in lymph node metastases (Supplementary Fig. S3).
At the metabolic level, we revealed noteworthy metabolic distinctions among various malignant cell clusters (Fig. 5e). Notably, tumor cells within primary foci exhibited a prevalent reliance on oxidative phosphorylation metabolism, whereas satellite and lymph node metastatic lesions displayed a marked upregulation in fatty acid and amino acid metabolism (Fig. 5f).
Dynamic transcriptomic profiles of fibroblast populations throughout DFSP progression
The rarity of DFSP as a tumor entity has resulted in a dearth of comprehensive investigations into its origins. In this study, we identified a compact yet vigorously proliferating cluster of stem-like cancer cells exhibiting pronounced CNVs across all tumor lesions. While fibroblasts constitute a substantial portion of the fibrous tumor matrix, the precise functions attributed to their distinct subpopulations within the tumor microenvironment warrant further elucidation. The utilization of CytoTRACE R package provided insights into the temporal order of cell state transitions within the context of DFSP (Fig. 6a). Furthermore, we meticulously categorized individual cells to construct a comprehensive tree-like framework that delineates differentiation trajectory (Fig. 6b). This trajectory unfolded sequentially with three distinct states: the initial pre-branch point, as well as two subsequent branches known as Fate1 and Fate2 (Fig. 6c). Notably, the Fib02, Fib03, and Fib04 subpopulations predominantly occupied the terminal portion of trajectory 1, while Fib01 and Fib05 primarily populated the terminus of trajectory 2 (Fig. 6d, e). During differentiation, we witnessed the aggregation of molecules intricately associated with EMT, notably TWIST1, and MMP2, as cells advanced within trajectory 2. In contrast, the dispersion pattern of ACTA2, a molecule linked to fibroblast activation, concurred with the trajectory 1 (Fig. 6f). Furthermore, we conducted a comprehensive analysis of gene expression patterns during varied cell state transitions. The dynamic genes undergoing evolution were systematically classified into two distinct clusters corresponding to cell fates (Supplementary Table S2). Further gene ontology (GO) difference analysis provides a comprehensive perspective on altered gene expression patterns across distinct trajectories (Supplementary Fig. S4).
Fig. 6.
CytoTRACE and monocle2 analysis reveal the differentiation trajectories and distinctive gene expression patterns within 5 separate subpopulations of tumor fibroblasts. a Cell differentiation level; b Pseudotime trajectory of fibroblasts; c Cell type distribution; d Cell state transition; e Individual trajectories of each fibroblast subcluster; f Expression pattern of representative genes (TWIST1, MMP2, ACTA2)
Immune microenvironment diversity across different stages of DFSP advancement
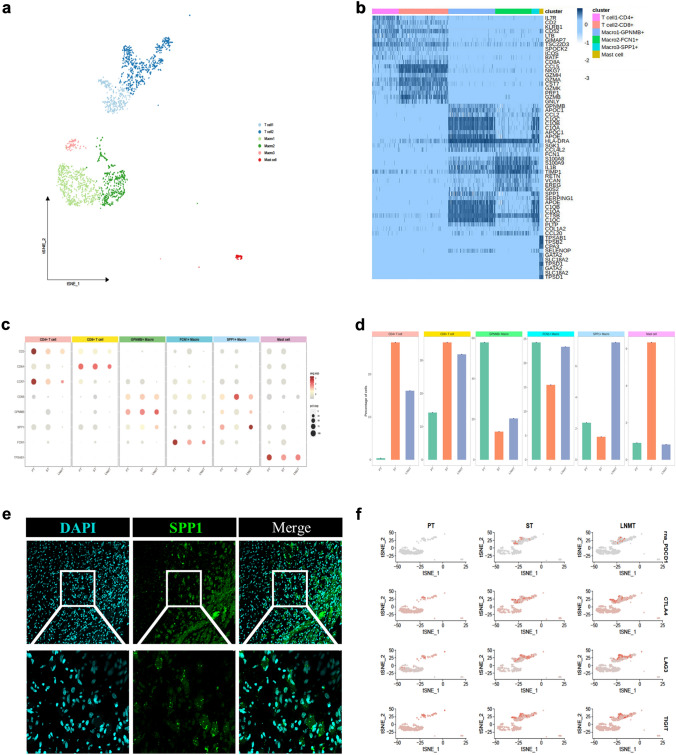
In DFSP samples, the immune microenvironment exhibited a prevailing presence of three discrete macrophage subpopulations, coupled with two T cell subpopulations and one mast cell subpopulation (Fig. 7a). To delve deeper into the intricate dynamics, we visualized this data with the heatmap highlighting the top DEGs. As a result, we designated the diverse T and macrophage subpopulations as CD4+ T cells, CD8+ T cells, GPNMB+ macrophages, FCN1+ macrophages, and SPP1+ macrophages, rooted in their distinctive expression profiles (Fig. 7b). The unique expression profiles of molecules within each of these six immune cell populations across the three samples are detailed in Fig. 7c. Additionally, the proportions of these distinct immune cell subpopulations across the three samples are depicted in Fig. 7d. The findings demonstrated heightened T-cell infiltration within metastatic tumor foci. Additionally, in correlation with our extrapolation of macrophage developmental trajectories (Supplementary Fig. S5), we observed that FCN1+ macrophages, displaying a lesser degree of monocyte-like differentiation, constituted a larger proportion within primary foci. Conversely, SPP1+ macrophages, characterized as M2-type macrophages, exhibited a notably higher frequency in lymph node metastasis (Fig. 7e). Furthermore, our investigation revealed notable upregulation of immune checkpoint-associated molecules, including PD1 (PDCD1), CTLA2, LAG3, and TIGHT, in satellite and lymph node metastases compared to the primary tumor (Fig. 7f).
Fig. 7.
Heterogeneity of immune cell profiles within metastatic DFSP tumors. a t-SNE diagram visualizing different immune cell subpopulations; b Heatmap displaying the top 5 DEGs for the annotations of various immune cell clusters; c Dot plots representing gene expression levels that define unique subclusters of immune cells across 3 tumor samples; d Bar graph depicting the proportions of various immune cell clusters in 3 tumor samples; e Representative immunofluorescence staining of lymph node metastatic tumor tissue at 20 × and 60 × magnifications. DAPI (cyan), SPP1 (green), in individual and merged channels are shown; f Feature plot showing the distribution of exhausted T cell-related genes in different samples
Cell–cell communication networks within the tumor microenvironment
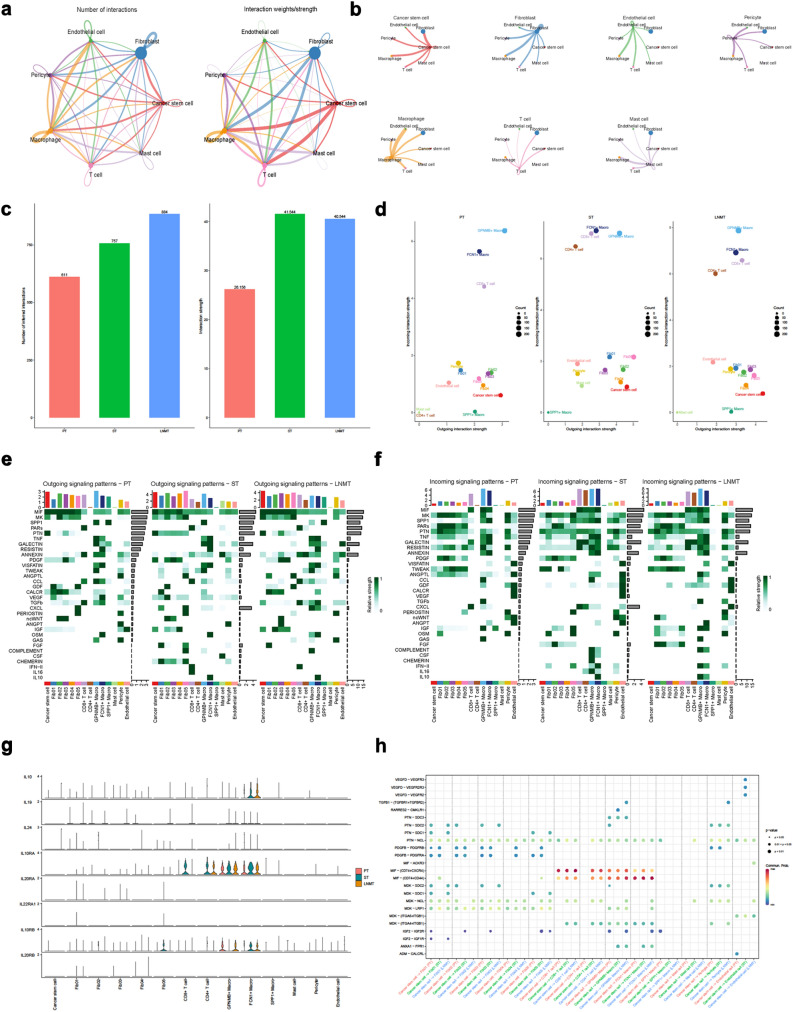
The identified sets of ligand-receptor pairs using CellChat showed extensive molecular crosstalk among the major cell types in DFSP (Fig. 8a, b). We conducted a quantitative comparison of intercellular communication across the three samples, revealing a significant enhancement of cell–cell interactions in metastatic foci in terms of both quantity and strength (Fig. 8c). Regarding the contributions of distinct cell populations to intercellular communication, our findings indicate the crucial involvement of immune cells in signaling communication across all three samples. Additionally, malignant cell populations emerged as significant participants in the efferent signaling pathway (Fig. 8d). Moreover, we utilized heatmaps to graphically illustrate the fluctuations observed in specific signaling pathways across the three tumor samples, while also highlighting the unique roles played by various cell types in shaping these signaling cascades (Fig. 8e, f). In contrast to the primary focus, we observed distinct signaling pathways involving Fibroblast Growth Factor (FGF), Complement, Colony-stimulating Factor (CSF), Chemerin, IL-10, and IL-16 in adjacent and lymph node metastases (Fig. 8e–g, Supplementary Fig. S6). Furthermore, our exploration of the complex intercellular communication network involving cancer stem cells and their diverse cellular counterparts has brought to light a strategic role played by the Macrophage Migration Inhibitory Factor (MIF)-CD74 pathway, particularly in its interactions with various immune cells (Fig. 8h).
Fig. 8.
Intricate cellular communication between tumor cells and microenvironmental components in DFSP samples. a Overall view of the ability of different cell–cell communication. The lines link to cell types expressing corresponding receptors. The thickness of the lines is proportional to the number of ligands expressed by the recipient cell types; b Detailed perspective of each cell type engaging in ligand-receptor interactions with other cells; c Assessment of the overall intensity of cellular interaction across 3 distinct samples; d Dot plot displaying the intensities of efferent and afferent signals from different cell clusters in 3 tumor samples; e Enriched outgoing signaling pathways involved in the communication within 3 distinct tumor samples and their corresponding predominant cell clusters; f Enriched incoming signaling pathways involved in the communication within 3 distinct tumor samples and their corresponding predominant cell clusters; g Expression patterns of key ligands and receptors within the IL-10 signaling pathway among different cell types across 3 samples; h Dot plot showing the interaction pathways of cancer stem cells with those of other cells across 3 tumor samples
Discussion
DFSP, characterized by its rare incidence, distinctive clinical behavior, and intricate molecular underpinnings, demands a nuanced exploration across various facets [15]. To date, the cellular heterogeneity and clonal diversity that contribute to DFSP’s complex biology remain inadequately explored [9]. By elucidating the diverse genomic landscapes at the single-cell level, this study seeks insights into the cellular dynamics, heterogeneity, and therapeutic targets underlying metastatic DFSP.
The identification and characterization of distinct cellular populations, including cancer stem cells and malignant fibroblasts within DFSP represents a significant breakthrough. The presence of a cancer stem cell population is of paramount importance, as these cells are recognized for their pivotal role in tumor initiation, propagation, and therapeutic resistance [16, 17]. These findings provide additional support for the previously posited hypothesis regarding the origin of mesenchymal stem cells as a contributing factor in DFSP origin [18, 19]. Furthermore, the recognition of specific subclusters of malignant fibroblasts highlights their potential involvement in intratumoral diversity.
Recently, metabolic reprogramming has emerged as a hallmark feature of cancer cells, enabling adaption and thriving in new microenvironments. Metastatic tumors exhibit distinct metabolic profiles compared to their primary counterparts, marked by altered mitochondrial function and augmented nutrient uptake. These changes fuel energy demands, and confer advantages in immune evasion, angiogenesis, and therapy resistance [20, 21]. Additionally, recent published studies have indicated that metastatic tumors frequently exhibit a reliance on alternative nutrient sources such as fatty acids and amino acids to sustain their growth and survival [22–25]. The evolving understanding of metabolic reprogramming in metastatic tumors has shed light on the intriguing phenomenon observed in DFSP metastases, where tumor cell metabolism also undergoes a distinctive shift towards fatty acid and amino acid utilization. This metabolic preference may be linked to the need for increased energy production and biosynthetic building blocks required for rapid proliferation in the new microenvironment. Furthermore, this metabolic alteration could potentially influence the modulation of immune cells and promote an immunosuppressive phenotype [24, 26].
The heightened expression of checkpoint molecules, such as PD1, CTLA2, LAG3, and TIGHT, could serve to dampen anti-tumor immune responses, thereby creating an immunosuppressive milieu that allows tumor cells to evade immune surveillance [27, 28]. Furthermore, the distinct activation of signaling pathways, including FGF, Complement, CSF, Chemerin, IL-10, and IL-16, in satellite and lymph node metastases compared to the primary tumor suggests a dynamic interplay between tumor cells and the surrounding microenvironment. The upregulation of these signaling pathways in metastatic foci could be indicative of an altered immune response and an adaptive mechanism by which tumor cells evade immune surveillance. For instance, the involvement of FGF signaling has been linked to tumor cell proliferation, angiogenesis, and metastasis, potentially facilitating the establishment of secondary tumor sites [29, 30]. The activation of Complement pathways could contribute to immune evasion, as complement proteins can inhibit immune cell responses and promote tumor cell survival. CSF, Chemerin, IL-10, and IL-16 recognized regulators of immune responses and inflammation, are likely instrumental in sculpting the immune microenvironment in metastatic tumors [30–32]. IL-10, for example, has been implicated in exerting a wide array of immunosuppressive functions by interacting with its corresponding receptor (IL-10R) and subsequently activating the STAT3 pathway [33]. Traditionally, IL-10 hampers the activity of antigen-presenting cells, thereby impeding T-cell function [34]. Elevated circulating levels of IL-10 have been correlated with advanced disease stages, compromised survival outcomes, or an increased propensity for recurrence in various other tumor types [35]. Consequently, the unique expression profile of IL-10 within metastatic DFSP tumors has the potential to facilitate immunotherapy research.
Moreover, the MIF-CD74 signaling axis has garnered recognition for its multifaceted involvement in immune modulation and tumor dynamics [36, 37]. The implications of our findings suggest a substantial role for the MIF-CD74 pathway in orchestrating an immunosuppressive milieu, enabling cancer stem cells to elude immune surveillance and exploit immune tolerance.
The utilization of three samples from a single patient for scRNA-seq analysis represents a robust approach to unraveling intratumoral complexity in DFSP. While this strategy offers significant advantages in terms of data depth and reliability, it is imperative to recognize and address the potential limitations. Further studies involving larger patient cohorts are warranted to validate and extend the findings presented here, ultimately contributing to a more comprehensive and nuanced understanding of DFSP heterogeneity and its implications for disease progression and management.
Materials and methods
Sample collection
Surgical specimens were obtained from a female patient, aged 40, who had received a diagnosis of DFSP. These samples comprised primary tumor tissue, satellite foci, and lymph node metastases. This study was approved by the institutional review board of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (NO. YBKA201901). Ethical guidelines and institutional protocols were strictly followed.
Immunohistochemistry and immunofluorescence staining
The sections underwent a deparaffinization and rehydration process, followed by antigen retrieval in 10 mM sodium citrate buffer (pH 6.0). For immunohistochemistry, endogenous peroxidase activity was inhibited using 3% H2O2, and nonspecific protein binding sites were blocked with 3% bovine serum albumin (BSA). Subsequently, the sections were incubated overnight at 4 °C with anti-CD34 (1:2500, Abcam, ab81289) and anti-SOX-10 (1:200, Abcam, ab180862) antibodies. Afterward, the sections were washed with phosphate-buffered saline (PBS) and incubated with a biotinylated secondary antibody for 20 min at 37 °C. The staining was visualized using 3,3'-diaminobenzidine (DAB) substrate. For immunofluorescence, the sections were incubated overnight at 4 °C with anti-SPP1 (1:100, Abcam, ab214050) and anti-cleaved Caspase-3 (1:300, Cell Signaling Technology, #9661) antibodies. Following PBS washes, the sections were incubated with fluorescently labeled secondary antibodies for 2 h at room temperature and observed using Leica microscopy.
Single-cell data preprocessing
The Seurat object containing gene expression data was imported into the Seurat (version 4.0.2) R package [38]. Only high-quality cells satisfying the following criteria were retained for further analysis: (1) each cell should exhibit expression of over 200 genes; (2) every gene should be detected in a minimum of 3 cells; (3) the proportion of mitochondrial RNA content per cell should not exceed 10%. Following data filtration, we proceeded to normalize the expression values via the “NormalizeData” function. Subsequently, the “FindVariableFeatures” function was employed to identify 2000 genes exhibiting significant variability. These genes were subsequently standardized using the “ScaleData” function. Notably, data integration was accomplished with 2000 anchors, facilitating harmonious incorporation of three datasets.
Dimensionality reduction, single-cell clustering and annotation
To streamline the dataset and extract features, we used the “RunPCA” function. This step involved selecting a subset of high-variant genes to undergo dimensionality reduction. Subsequently, we employed the “FindNeighbors” and “FindClusters” functions to effectively group cells with similar expression patterns. Following the clustering analysis, we harnessed the power of the “RunTSNE” function and gained a visually informative representation of the cellular subgroups. For comprehensive cell type annotation, we employed the Single R package, which utilizes gene expression profiles to infer cell types based on the comparison with reference datasets (HSPA and BP) [39]. Additionally, to ensure precise identification, we conducted manual annotation referring to the CellMarker database [40].
Copy number variation analysis with infercnv
We employed the infercnv R package (Version 1.17.0) to investigate CNVs within our scRNA-seq data. By leveraging the expression profiles of individual cells, infercnv allows us to infer the copy number states of specific genomic regions, revealing potential chromosomal gains and losses. In our analysis, we utilized distinct groups of immune cells as reference. This approach enabled us to identify potentially malignant cells within DFSP, enhancing our understanding of the intricate genetic landscape and heterogeneity in the tumor microenvironment.
Trajectory analysis with CytoTRACE and Monocle2
To unravel the dynamic developmental trajectories of cell populations, we conducted trajectory analysis using the CytoTRACE and Monocle2 R package [14, 41]. CytoTRACE (version 0.3.3) enabled us to infer cellular differentiation orders by quantifying the similarity of gene expression profiles between individual cells. Additionally, we employed Monocle2 (version 2.26.0) to further dissect the trajectory characteristics of distinct fibroblast subpopulations. Through these trajectory analyses, we gained a comprehensive understanding of the evolving cell states and lineage relationships at single-cell resolution.
Gene set variation analysis
To elucidate functional variations across different cell populations and samples, we employed GSVA (version 1.46.0) [42]. Specifically, we employed GSVA to assess the pathway activity profiles of cancer stem cells and various subpopulations of fibroblasts. Moreover, we extended this analysis to evaluate the relative pathway activity within the malignant cell populations across three tumor samples. By quantifying the pathway activity levels, we gained deeper insights into the functional implications of these distinct cell populations.
Metabolic analysis with scMetabolism
To unravel the intricate metabolic landscape within tumors, we employed the scMetabolism R package (version 0.2.1) [43], which integrates scRNA-seq data with metabolic pathway information. This approach allowed us to discern the dynamic modulation of metabolic pathways within distinct malignant cell clusters. Notably, our investigation extended beyond intratumoral variation, as we elucidated the metabolic dynamics characterizing the progression of metastatic DFSP. This was achieved through an incisive analysis of metabolic disparities across three diverse samples, underscoring the relevance of metabolism in the context of disease advancement.
Cell–cell communication analysis with CellChat
The network of cell–cell communication within the tumor microenvironment was systematically investigated using the CellChat R package (version 1.6.1) [44]. We deciphered the intricate interactions among fibroblasts, cancer stem cells, immune cells, and other cellular constituents within the tumor. Through the identification of ligand-receptor pairs and their corresponding molecular interactions, we gained deeper insights into the orchestration of intercellular crosstalk that contributes to the regulatory dynamics and functional adaptations within the metastatic DFSP microenvironment.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
LL G, ZC W, and QF L contributed to conceptualization; LL G, ZC W, and QF L contributed to methodology; LL G, ZC W and CJ W contributed to investigation; LL G, ZC W and CJ W contributed to resources; LL G, ZC W and CJ W contributed to data curation; LL G contributed to writing—original draft preparation; ZC W and CJ W contributed to writing—review and editing; LL G contributed to visualization; ZC W, CJ W, and QF L contributed to supervision; ZC W and QF L contributed to project administration; ZC W and QF L contributed to funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from National Natural Science Foundation of China (82102344; 82172228); Science and Technology Commission of Shanghai Municipality (22MC1940300); Natural Science Foundation of Shanghai (22ZR1422300); Innovative research team of high-level local universities in Shanghai (SSMU-ZDCX20180700); Clinical Research Plan of SHDC (SHDC2020CR1019B).
Data availability
The authors state that the primary data endorsing the results of this study are accessible in the main text and the Supplementary materials. The raw dataset of this study can be requested from the corresponding authors for research purposes.
Declarations
Conflict of interest
The authors state no conflict of interest.
Ethics approval
This study was approved by the institutional review board of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (NO. YBKA201901).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ling-Ling Ge, Zhi-Chao Wang and Cheng-Jiang Wei have equally contributed to this work.
References
- 1.Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503–2508. doi: 10.1002/cncr.20678. [DOI] [PubMed] [Google Scholar]
- 2.Criscione VD, Weinstock MA. Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. J Am Acad Dermatol. 2007;56:968–973. doi: 10.1016/j.jaad.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Mujtaba B, Wang F, Taher A, Aslam R, Madewell JE, Spear R, Nassar S. Dermatofibrosarcoma protuberans: pathological and imaging review. Curr Probl Diagn Radiol. 2021;50:236–240. doi: 10.1067/j.cpradiol.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Kreicher KL, Kurlander DE, Gittleman HR, Barnholtz-Sloan JS, Bordeaux JS. Incidence and survival of primary dermatofibrosarcoma protuberans in the United States. Dermatol Surg. 2016;42(Suppl 1):S24–31. doi: 10.1097/dss.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 5.Larbcharoensub N, Kayankarnnavee J, Sanpaphant S, Kiranantawat K, Wirojtananugoon C, Sirikulchayanonta V. Clinicopathological features of dermatofibrosarcoma protuberans. Oncol Lett. 2016;11:661–667. doi: 10.3892/ol.2015.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon MP, Pedeutour F, Sirvent N, Grosgeorge J, Minoletti F, Coindre JM, Terrier-Lacombe MJ, Mandahl N, Craver RD, Blin N, Sozzi G, Turc-Carel C, O'Brien KP, Kedra D, Fransson I, Guilbaud C, Dumanski JP. Deregulation of the platelet-derived growth factor B-chain gene via fusion with collagen gene COL1A1 in dermatofibrosarcoma protuberans and giant-cell fibroblastoma. Nat Genet. 1997;15:95–98. doi: 10.1038/ng0197-95. [DOI] [PubMed] [Google Scholar]
- 7.Sirvent N, Maire G, Pedeutour F. Genetics of dermatofibrosarcoma protuberans family of tumors: from ring chromosomes to tyrosine kinase inhibitor treatment. Genes Chromosomes Cancer. 2003;37:1–19. doi: 10.1002/gcc.10202. [DOI] [PubMed] [Google Scholar]
- 8.Heldin CH, Lennartsson J, Westermark B. Involvement of platelet-derived growth factor ligands and receptors in tumorigenesis. J Intern Med. 2018;283:16–44. doi: 10.1111/joim.12690. [DOI] [PubMed] [Google Scholar]
- 9.Peng C, Jian X, Xie Y, Li L, Ouyang J, Tang L, Zhang X, Su J, Zhao S, Liu H, Yin M, Wu D, Wan M, Xie L, Chen X. Genomic alterations of dermatofibrosarcoma protuberans revealed by whole-genome sequencing. Br J Dermatol. 2022;186:997–1009. doi: 10.1111/bjd.20976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao X, Billings SD, Wu F, Stultz TW, Procop GW, Mirkin G, Vidimos AT. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020 doi: 10.3390/jcm9061752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thway K, Noujaim J, Jones RL, Fisher C. Dermatofibrosarcoma protuberans: pathology, genetics, and potential therapeutic strategies. Ann Diagn Pathol. 2016;25:64–71. doi: 10.1016/j.anndiagpath.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Baig IT, Lauck K, Nguyen QD. Tumor size is the most significant risk factor for local recurrence in dermatofibrosarcoma protuberans: a large-scale retrospective cohort analysis. J Am Acad Dermatol: 2023 doi: 10.1016/j.jaad.2023.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Maki RG, Awan RA, Dixon RH, Jhanwar S, Antonescu CR. Differential sensitivity to imatinib of 2 patients with metastatic sarcoma arising from dermatofibrosarcoma protuberans. Int J Cancer. 2002;100:623–626. doi: 10.1002/ijc.10535. [DOI] [PubMed] [Google Scholar]
- 14.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods. 2017;14:979–982. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483–488. doi: 10.1016/j.det.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Prasetyanti PR, Medema JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16:41. doi: 10.1186/s12943-017-0600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781. doi: 10.1016/j.lfs.2019.116781. [DOI] [PubMed] [Google Scholar]
- 18.Sellheyer K, Nelson P, Krahl D. Dermatofibrosarcoma protuberans: A tumour of nestin-positive cutaneous mesenchymal stem cells? Br J Dermatol. 2009;161:1317–1322. doi: 10.1111/j.1365-2133.2009.09363.x. [DOI] [PubMed] [Google Scholar]
- 19.Sellheyer K, Nelson P, Patel RM. Expression of embryonic stem cell markers SOX2 and nestin in dermatofibrosarcoma protuberans and dermatofibroma. J Cutan Pathol. 2011;38:415–419. doi: 10.1111/j.1600-0560.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 20.Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer. 2021;21:162–180. doi: 10.1038/s41568-020-00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottfried E, Kreutz M, Mackensen A. Tumor metabolism as modulator of immune response and tumor progression. Semin Cancer Biol. 2012;22:335–341. doi: 10.1016/j.semcancer.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer. 2020;122:4–22. doi: 10.1038/s41416-019-0650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferraro GB, Ali A, Luengo A, Kodack DP, Deik A, Abbott KL, Bezwada D, Blanc L, Prideaux B, Jin X, Posada JM, Chen J, Chin CR, Amoozgar Z, Ferreira R, Chen IX, Naxerova K, Ng C, Westermark AM, Duquette M, Roberge S, Lindeman NI, Lyssiotis CA, Nielsen J, Housman DE, Duda DG, Brachtel E, Golub TR, Cantley LC, Asara JM, Davidson SM, Fukumura D, Dartois VA, Clish CB, Jain RK, Vander Heiden MG. Fatty acid synthesis is required for breast cancer brain metastasis. Nat Cancer. 2021;2:414–428. doi: 10.1038/s43018-021-00183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Med. 2020;52:15–30. doi: 10.1038/s12276-020-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porporato PE, Payen VL, Baselet B, Sonveaux P. Metabolic changes associated with tumor metastasis, part 2: Mitochondria, lipid and amino acid metabolism. Cell Mol Life Sci. 2016;73:1349–1363. doi: 10.1007/s00018-015-2100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CK, Jeong SH, Jang C, Bae H, Kim YH, Park I, Kim SK, Koh GY. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 2019;363:644–649. doi: 10.1126/science.aav0173. [DOI] [PubMed] [Google Scholar]
- 27.Serratì S, Guida M, Di Fonte R, De Summa S, Strippoli S, Iacobazzi RM, Quarta A, De Risi I, Guida G, Paradiso A, Porcelli L, Azzariti A. Circulating extracellular vesicles expressing PD1 and PD-L1 predict response and mediate resistance to checkpoint inhibitors immunotherapy in metastatic melanoma. Mol Cancer. 2022;21:20. doi: 10.1186/s12943-021-01490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franzin R, Netti GS, Spadaccino F, Porta C, Gesualdo L, Stallone G, Castellano G, Ranieri E. The use of immune checkpoint inhibitors in oncology and the occurrence of AKI: Where do we stand? Front Immunol. 2020;11:574271. doi: 10.3389/fimmu.2020.574271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao P, Cohen O, Kowalski KJ, Kusiel JG, Buendia-Buendia JE, Cuoco MS, Exman P, Wander SA, Waks AG, Nayar U, Chung J, Freeman S, Rozenblatt-Rosen O, Miller VA, Piccioni F, Root DE, Regev A, Winer EP, Lin NU, Wagle N. Acquired FGFR and FGF alterations confer resistance to estrogen receptor (ER) targeted therapy in ER(+) metastatic breast cancer. Clin Cancer Res. 2020;26:5974–5989. doi: 10.1158/1078-0432.Ccr-19-3958. [DOI] [PubMed] [Google Scholar]
- 30.Giacomini A, Grillo E, Rezzola S, Ribatti D, Rusnati M, Ronca R, Presta M. The FGF/FGFR system in the physiopathology of the prostate gland. Physiol Rev. 2021;101:569–610. doi: 10.1152/physrev.00005.2020. [DOI] [PubMed] [Google Scholar]
- 31.Richmond J, Tuzova M, Cruikshank W, Center D. Regulation of cellular processes by interleukin-16 in homeostasis and cancer. J Cell Physiol. 2014;229:139–147. doi: 10.1002/jcp.24441. [DOI] [PubMed] [Google Scholar]
- 32.Goralski KB, Jackson AE, McKeown BT, Sinal CJ. More than an adipokine: The complex roles of chemerin signaling in cancer. Int J Mol Sci. 2019 doi: 10.3390/ijms20194778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan KM, Jiang X, Guha P, Lausted C, Carter JA, Hsu C, Labadie KP, Kohli K, Kenerson HL, Daniel SK, Yan X, Meng C, Abbasi A, Chan M, Seo YD, Park JO, Crispe IN, Yeung RS, Kim TS, Gujral TS, Tian Q, Katz SC, Pillarisetty VG. Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases. Gut. 2023;72:325–337. doi: 10.1136/gutjnl-2021-325808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saraiva M, Vieira P, O'Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020 doi: 10.1084/jem.20190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 36.Bozzi F, Mogavero A, Varinelli L, Belfiore A, Manenti G, Caccia C, Volpi CC, Beznoussenko GV, Milione M, Leoni V, Gloghini A, Mironov AA, Leo E, Pilotti S, Pierotti MA, Bongarzone I, Gariboldi M. MIF/CD74 axis is a target for novel therapies in colon carcinomatosis. J Exp Clin Cancer Res. 2017;36:16. doi: 10.1186/s13046-016-0475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Azevedo RA, Shoshan E, Whang S, Markel G, Jaiswal AR, Liu A, Curran MA, Travassos LR, Bar-Eli M. MIF inhibition as a strategy for overcoming resistance to immune checkpoint blockade therapy in melanoma. Oncoimmunology. 2020;9:1846915. doi: 10.1080/2162402x.2020.1846915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33:495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao G, Xuan X, Li Y, Hu J, Zhang R, Jin H, Dong H. Single-cell RNA sequencing reveals the vascular smooth muscle cell phenotypic landscape in aortic aneurysm. Cell Commun Signal. 2023;21:113. doi: 10.1186/s12964-023-01120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Lan Y, Xu J, Quan F, Zhao E, Deng C, Luo T, Xu L, Liao G, Yan M, Ping Y, Li F, Shi A, Bai J, Zhao T, Li X, Xiao Y. Cell Marker: a manually curated resource of cell markers in human and mouse. Nucleic Acids Res. 2019;47:D721–d728. doi: 10.1093/nar/gky900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulati GS, Sikandar SS, Wesche DJ, Manjunath A, Bharadwaj A, Berger MJ, Ilagan F, Kuo AH, Hsieh RW, Cai S, Zabala M, Scheeren FA, Lobo NA, Qian D, Yu FB, Dirbas FM, Clarke MF, Newman AM. Single-cell transcriptional diversity is a hallmark of developmental potential. Science. 2020;367:405–411. doi: 10.1126/science.aax0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D, Cheng Y, Huang S, Liu Y, Jiang S, Liu J, Huang X, Wang X, Qiu S, Xu J, Xi R, Bai F, Zhou J, Fan J, Zhang X, Gao Q. Spatiotemporal immune landscape of colorectal cancer liver metastasis at single-cell level. Cancer Discov. 2022;12:134–153. doi: 10.1158/2159-8290.Cd-21-0316. [DOI] [PubMed] [Google Scholar]
- 44.Jin S, Guerrero-Juarez CF, Zhang L, Chang I, Ramos R, Kuan CH, Myung P, Plikus MV, Nie Q. Inference and analysis of cell-cell communication using cell chat. Nat Commun. 2021;12:1088. doi: 10.1038/s41467-021-21246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that the primary data endorsing the results of this study are accessible in the main text and the Supplementary materials. The raw dataset of this study can be requested from the corresponding authors for research purposes.