Abstract
As the main immune checkpoint, PD-L1-PD-1 interaction plays a critical role in the dysregulation of effector T cells, which contributes to the failure of Chimeric Antigen Receptor T-cell (CAR-T) and other immunotherapies. Presently, most research focuses on the extracellular function of PD-L1. Membrane PD-L1 can interact with its receptor PD-1 and decrease T cell-induced cancer immunity. However, the function of PD-L1 in cancer cells is still unclear. Recent studies have shown the separated clinical significance of PD-L1 expression in various cancer types, showing the complexity of PD-L1 in cancer cell regulation. As a novel regulatory pathway, the nuclear translocation of PD-L1 in cancer cells receives more attention. Results of these preclinical studies demonstrated that nuclear PD-L1 has an essential role in cancer development and other immune checkpoint molecules transcription. Herein, we summarized the mechanisms involved in PD-L1 nuclear transportation and identify the key regulatory factors in this process. Furthermore, we also summarize the function of nuclear PD-L1 in cancer immunity. These findings suggested the novel PD-L1 regulation in cancer development, which showed that nuclear PD-L1 is a potential therapeutic target in future cancer therapy.
Keywords: PD-L1, Nuclear transportation, Immune escape, Transcriptional regulation, Cancer immunity and immunotherapy
Background
The development in immunologic research increases the attention in cancer immunotherapy. The therapeutic effect of immunotherapy relies on the activation of the T cell-based immune system [1]. The exposed antigen expressed on the surface of tumor cells promotes the functional activation of T cells by binding with T cell receptor (TCR). However, the tumor immunologic recognition and cytotoxicity induced by TCR is adjusted by the interaction of several co-expressed receptors and their ligands, such as immune checkpoints. They can synergistically stimulate or suppress the function and viability of T cells and alternate the magnitude and duration of T-cell immune response [2]. The immune checkpoints are a series of suppressant pathways through the receptor-ligand binding between T cell and target cells, including PD-L1/PD-1, CTLA-4/B7, CD47/SIRP-α, and LAG-3/MHC-II, which perform as an essential supervisor in physiologic immunity and prevent the occurrence of autoimmune diseases [3]. Unfortunately, the immune checkpoint-mediated immunologic inhibition also contributes to the T cell exhaustion in anti-tumor immunity and results in the immune escape of cancer cells [4, 5]. Among the immune checkpoint, programmed cell death protein ligand 1 (PD-L1) and its receptor become the center of attention by researchers. The antibodies against PD-1/PD-L1 therapy is Food and Drug Administration (FDA) approved and promise as an exciting therapeutic effect in cancer immunotherapy [6–8]. However, the dominating investigation of PD-L1 focused on the membrane PD-L1 (mPD-L1). Recent studies proved that besides membrane expression, PD-L1 also has nuclear expression in cancer and normal cells, and the translocation of nuclear PD-L1 (nPD-L1) is involved in the immunologic variation of cancer cells [9–11]. In this review, we mainly summarized the nPD-L1 and the mechanism of nPD-L1 nuclear transportation (Table 1). Meanwhile, the functional differences between mPD-L1 and nPD-L1 are also discussed to clarify the PD-L1 regulation in tumor development and immunity, which may contribute to the therapeutic difference in the PD-L1/PD-1 targeted therapy.
Table 1.
The overview of concerning nPD-L1 in various cancer researches
| Cancer type | Condition | Binding site | Transcription | Function | Ref. |
|---|---|---|---|---|---|
| TNBC | Loss of Sororin |
SMC1/SMC3/ SA2/SCC1/ PDS5B/WAPL |
– | Sister chromatid cohesion | [89] |
| NSCLC | – | KPNB1/SP1 | Gas6 | Cell proliferation | [54] |
| BRCA | Deacetylation |
HIP1R/KPNA2/ Vimentin |
BIRC3/TRAF1/ RELB/HLA-A/ HLA-B/PD-L2/ VISTA/PD-L1/ PD-L2 |
Immune response enhancement | [9] |
| TNBC | Hypoxia | p-STAT3 | GSDMC | Pyroptosis and necrosis | [10] |
| BRCA | Doxorubicin | p-AKT (prediction) | – | Anti-apoptosis | [44] |
| PRAD/BRCA/COAD/OS | Circulating tumor cells | Vimentin (prediction) | – | Overall-survival decreasion | [19] |
| LADC/LSCC/RCC/HCC | – | - | – | Nuclear immunoreactivity | [46] |
| HNSCC | – | AKT-1 | – | Radioresistant | [80] |
| ESCC | – | – | – | Invasion promotion | [45] |
| PRAD | Primary radiotherapy | – | – | Higher Gleason score and cT stage | [91] |
| CC | BRAF-mutated CRC cells | – | – | Promoting cell proliferation | [90] |
TNBC Triple-Negative Breast Cancer, NSCLC Non-Small Cell Lung Cancer, BRCA Breast Cancer, PRAD Prostate Adenocarcinoma, CC Colorectal cancer, COAD Colon Adenocarcinoma, OS Osteosarcoma, LADC Lung Adenocarcinoma, LSCC Lung Squamous Cell Carcinoma, RCC Renal Cell Carcinoma, HCC Hepatocellular Carcinoma, HNSCC Neck Squamous Cell Carcinoma, ESCC Esophageal Squamous Cell Carcinoma
The PD-1/PD-L1 Axis in cancer immunity
PD-L1, also called B7-H1 or CD274, has been regarded as a vital immune regulator in anti-cancer immunity, which can be recognized by PD-1 in T and NK cells and cause the exhaustion of these immune cells [12, 13]. Mediated by inflammation and different cytokines and growth factors, the expression of PD-L1 is regulated by two separated pathways, including transcription and post-transcription pathways. The ChIP-seq analysis indicated that the binding of NF-κB1/p65 in the PD-L1 enhancer region is increased by paclitaxel-induced reactive oxygen species and further upregulate the expression of PD-L1 in tumor-associated macrophages [14]. On the other hand, IL-27 can activate the JAK1 and JAK2 and promote the phosphorylation of STAT1 after binding with IL-27Rα and IL-6Rβ. The phosphorylation of STAT1 leads to the nucleus transportation and enhances the PD-L1 transcription [15]. Meanwhile, Lin’s researches suggested that the ERK-induced C/EBPβ phosphorylation causes Bcl-2-associated athanogene-1 transcription and PD-L1 expression, which promotes the development of non-small cell lung cancer (NSCLC) [16]. These results showed the importance of NF-κB, ERK, and STAT pathway in PD-L1 transcriptional expression.
The function of PD-L1 also requires post-transcription regulation, including Ubiquitination, N-Glycosylation, Phosphorylation, Palmitoylation, and so on. Post-translational modifications of PD-L1 can alternate the structure of PD-L1 and adjust the PD-L1 stability inside the cancer cells and prevent the proteasome-induced degradation [17]. Meanwhile, the intracellular localization of PD-L1 also requires post-translational modifications, such as in ER, Golgi, nucleus, and cytoplasm [18, 19]. However, the specific effect of its subcellular localization remains unknown.
After engagement with PD-1 on T cells, PD-L1 induces the conformation change of PD-1 and promotes the phosphorylation of SHP-2 in the cytoplasmic tail of PD-1 [20, 21]. The PD-1-induced SHP-2 decreases the TCR signal pathway by LCK-induced ZAP70 phosphorylation and cause T cell dysfunction [22]. On the other hand, the interaction between PD-L1/PD-1 decreases the activation of PI3K/AKT and causes a series of cellular biological alternations, promoting p21Cip/p27Kip-induced proliferation inhibition and FOXO1-induced apoptosis [23]. Meanwhile, PD-L1/PD-1 pathway also interferes with the therapeutic effect of active immunotherapy such as chimeric antigen receptor (CAR)-T therapy and enhances the anti-tumor activity and safety profile [24]. Clinical researches have emerged that PD-1 gene disrupted CAR-T cells therapies have an attractive result by monoclonal antibody or CRISPR/Cas9 strategy [25, 26]. Thus, the PD-L1/PD-1 targeted therapy received a great deal of attention from cancer immunologic therapies.
Although multiple kinds of research have suggested the unfavorable prognosis in cancer with high expression of PD-L1 [27, 28], a part of the studies showed the different results of PD-L1 in some cancer types. Sideras and his colleagues found that the expression of PD-L1 indicated the higher survival probability in hepatocellular carcinoma (HCC), which can be the superior predictors of HCC mortality together with Galectin-9 and low CD8 + TIL [29]. Similarly, in advanced gastric cancer, the patients with better prognostic had a high grade of PD-L1 and a high density of CD8 + T cells[30]. Also, some other clinical prognosis data showed that high expression of PD-L1 is related to the favorable prognosis in certain cancer types, including NSCLC, HCC, BRCA, OS et.al, which challenge the conventional opinion of PD-L1. Parts of relevant research have summarized in Table 2 [29–43]. These results suggested that PD-L1 has a disparate regulatory mechanism in cancer cell immunity and development.
Table 2.
The overview of the research indicating the favorable prognosis in PD-L1 high expression cancer
| Cancer type | Item | Subtype condition | Detection methods | Ref. |
|---|---|---|---|---|
| Colorectal cancer | OS | Mismatch repair proficient | IHC | [31] |
| Nasopharyngeal carcinoma | OS/PFS | – | IHC | [32] |
| Endometrial carcinoma | OS | – | IHC | [33] |
| Non-Metastatic Nasopharyngeal carcinoma | LRFFS |
Intensity-modulated radiation therapy |
IHC | [34] |
| Bladder squamous cell carcinoma | OS/DSS |
Radical cystectomy |
IHC | [35] |
| Non-Small Cell Lung Cancer | PFS | Immunotherapy | IHC | [36] |
| Advanced lung adenocarcinoma | PFS | Pemetrexed maintenance therapy | IHC | [37] |
| HER2‐positive invasive breast cancer | OS | – | IHC | [38] |
|
Skin cutaneous melanoma & Clear cell renal cell carcinoma |
OS/PFS | – | RNA-Seq | [39] |
| Hepatocellular carcinoma | DSS | – | IHC | [29] |
|
High-grade serous carcinoma |
PFS | – | IHC | [40] |
| Esophageal squamous cell carcinoma | OS/PFS | – | IHC | [41] |
| Advanced gastric cancer | OS | – | IHC | [30] |
| Osteosarcoma | OS | – | RT-qPCR | [42] |
| Non-Small Cell Lung Cancer | OS |
Adjuvant therapy Squamous cell pT2-4 (tumor size) Positive lymph node status |
IHC | [43] |
OS Overall survival, PFS Progression free survival; loco-regional failure-free survival (LRFFS), DSS Disease specific survival, IHC Immunohistochemistry, RNA-Seq RNA Sequencing, RT-qPCR Real-time quantitative PCR
The nPD-L1 in cancer cell
The nPD-L1 was first illustrated in 2010 in breast cancer cells. Ghebeh and his colleagues [44] found that the expression of nPD-L1 plays a role as the anti-apoptosis molecule. The depletion of PD-L1 significantly reduced the chemotherapy-induced cytotoxicity. In their research, the dose-dependent treatment of doxorubicin in breast cancer repressed the expression of mPD-L1 and increased the PD-L1 nuclear expression induced by phosphorylation of nuclear AKT, which suggested the chemotherapy-induced PD-L1 nuclear transportation. They also found that doxorubicin-induced nPD-L1 existed in heart tissues, indicating the non-tissue specific expression pattern of nPD-L1. As the poor prognostic biomarker, nPD-L1 also exists in invasive esophageal cancer [45]. Besides these findings, Parra and his colleagues [46] investigated the PD-L1 expression in malignant and nonmalignant cells and discovered that the nPD-L1 occurs in some other samples of lung adenocarcinoma (LADC), lung squamous cell carcinoma (LSCC), renal cell carcinoma (RCC), and hepatocellular carcinoma (HCC). Meanwhile, the immunofluorescence research also clarified the nPD-L1 expression in circulating tumor cells isolated from prostate cancer (PRAD), breast cancer (BRCA), colon cancer (COAD), and osteosarcoma (OS) patients [19]. These findings suggested that the nuclear transportation of PD-L1 is ubiquitous and has a critical regulatory function in cancer development.
Interestingly, the research from Polioudaki [47] found that the nuclear location of PD-L1 may result from the error of experimental operation. The immunostaining using standard conditions of fixation and permeabilization showed the nuclear-location of PD-L1 in MDA-MB-231 cell lines while the mild detergent and rigorous fixation conditions maintain the membrane localization of PD-L1, similar to the immunostaining pattern of CD24. Further research achieved no significant PD-L1 translocation with 24 h-treatment of doxorubicin, which is opposite to the data from Ghebeh. However, these results are still unverified in the following research. Nowadays, FDA has approved the application of three PD-L1 assessments, including PD-L1 IHC 22C3 PharmDx kit (Dako North America), the PD-L1 28–8 PharmDx kit (Dako North America), and the PD-L1 SP142 Ventana test (Ventana Medical Systems Inc). These PD-L1 test platforms have a high affinity with different PD-L1 domains [48]. So, cross-validation by diverse test platforms is necessary for the investigation of nPD-L1 expression in cancer cells. In this regard, we should consider the authenticity in the research of nPD-L1, and the experimental methods of PD-L1 detection require further improvement.
Initiation of nPD-L1
As the special regulation in cancer cells compared to the mPD-L1 function, the initiation of nPD-L1 requires a specific cancer microenvironment. Hou and his colleagues investigated different nPD-L1 initiation conditions including PD-L1 antibody, small molecule drugs, cytokines, autophagy, and cell stress, and found that hypoxia is the most effective initiation factor of nPD-L1 in a hypoxia-inducible factor-1α (HIF-1α)- independent manner [10]. Gao and his colleagues [9] also found that the HDAC2-dependent PD-L1 deacetylation initiates its internalization, which can be reversed by p300/CBP-dependent PD-L1 acetylation. However, the acetylation-dependent nPD-L1 transportation remains unclear. Acetyl-CoA, one of the key products in aerobic metabolism, is essential in the p300/CBP-dependent PD-L1 acetylation. Hypoxia can suppress the activation of Acetyl-CoA Synthetase and cause the decline of Acetyl-CoA [49], which may play a crucial role in the process of PD-L1 acetylation and nuclear transportation. Meanwhile, the research found that Epidermal Growth Factor (EGF) enhances the Lysine acetylation of PD-L1, which can be detected by Acetyl lysine binding beads [50]. In this process, EGF promotes the p300 phosphorylation at the Ser1834 site induced by PI3K/AKT signaling pathway after binding with EGFR[51]. These results indicate that the absence of EGF can promote the nuclear transportation of PD-L1.
The mechanisms of nPD-L1 transportation
The PD-L1 nuclear transportation is a complicated process and requires accurate adjustment. Until now, a few factors elucidated to participate in nPD-L1 regulation, including Karyopherin protein family (nuclear transportation recognizer), Vimentin (nPD-L1 carrier), and STAT3 and AKT pathway (co-transcription factor). We will discuss them in the following sections.
Karyopherin protein family
Karyopherin (KPNs) family protein, also called Importin, is a series of nuclear import proteins, which controls the nucleocytoplasmic transport progress by forming the heterodimer of importin α and β subunits [52]. During the nuclear-transportation process, the protein-containing nuclear localization signals (NLSs) are identified by importin α and then orientated to the nuclear pore complex with the assistance of importin β [53]. The studies have suggested that KPNs engage in the regulation of the nuclear translocation of PD-L1. The mass spectrometry assay in Du’s research demonstrated that PD-L1 has the highest binding capacity with the β1 subunits of Karyopherin (KPNB1). The abrogation of KPNB1 causes the downregulation of nPD-L1 in NSCLC. The KPNB1-induced PD-L1 nuclear transportation enhances the activation of Sp1 and promotes the transcription of Gas6 as well as Mertk pathway [54]. Gao and his colleagues [9] also found that the deacetylation of PD-L1 leads to the bond with importin-α1 (KPNA1), which carries the PD-L1 through nuclear pore complexes into the nucleus. These two studies showed that the nuclear transportation of PD-L1 requires the synergetic interaction of importin α and β.
However, the research in human head and neck squamous cancer cells (HNSCC) revealed that the suppression of KPNB1 inhibited the expression of PD-L1 on the surface of cancer cells induced by radiotherapy [55]. This result is opposite to the phenomenon of mPD-L1 decrease in condition of KPNs-induced mPD-L1 internalization. The reduction of PD-L1 induced by KPNB1 knock-down may result from the interaction between KPNB1 and interferons (IFNs)-related pathway. The Interferon Regulatory Factor 1 (IRF1) contains the NLS structure, which can be recognized and carried into nuclear by KPNA2 after being activated by IFNs [56, 57]. The depletion of KPNB1 reduced the nuclear location and transcription activation of IRF1, consequently diminishing the IRF1-related PD-L1 expression [58]. Furthermore, the employment of KPNB1 inhibitor, importazole, diffuses the nuclear localization of IRF1 and causes the downregulation of PD-L1 after irradiation therapy, confirming the pivotal role of IRF1 in KPNB1-induced IRF1 and PD-L1 nuclear transportation [59]. Second, the KPNs are also responsible for the activation of stress-responsive transcription factors (SRTFs), including Nuclear Factor Kappa B (NF-κB), Activator Protein 1 (AP-1), Nuclear Factor of Activated T-cells (NFAT), and Signal Transducer and Activator of Transcription 1 alpha (STAT-1α), and dominate the cytokines and chemokines-mediated proinflammatory response [60]. Lim and his colleagues have illustrated that the expression of PD-L1 is related to the macrophage-secreted inflammatory cytokines induced by Tumor Necrosis Factor alpha (TNF-α) [61]. These findings reveal that the KPNs-induced SRTFs pathway promotes the expression of PD-L1. The role of KPNs in nPD-L1 and mPD-L1 will be also discussed in the next section.
STAT3 pathway
In response to the stimulation of cytokines including IFNs, EGF, Interleukin (IL)-6, Transducer and Activator of Transcription 3 (STAT3) facilitates the formation of heterodimers by kinases-induced phosphorylation, which moves into the nuclear and transduces the signal from receptor to nuclear [62–64]. Hou and his colleagues found that the Y705 phosphorylation of STAT3 enhances the nuclear aggregation of PD-L1 and regulates the transcription of gasdermin C (GSDMC) [10]. Coincidentally, it is reported that KPNs participate in the nuclear transportation of STAT3. The knock-down of KPNA2 impaired the expression of STAT3 in nuclear in pancreatic ductal adenocarcinoma cells [65]. The Ma’s data elucidated that KPNA5 and KPNA7 have a similar function in binding with STAT3, rather than KPNA1 or KPNA3. These results suggested the complicacy in PD-L1 and STAT3 transportation [66]. On the other hand, the nuclear relocation of STAT3 induced by EGFR also requires the interaction between KPNA5 and phosphorylated Tripartite Motif-containing 59 (TRIM59), a ubiquitin ligase, in glioblastoma, suggested the TRIM59 in PD-L1 transportation [67]. In this regard, we hypothesize that the complex of PD-L1 translocator consists of KPNs, STAT3, and TRIM59 in the PD-L1 regulation in nuclear.
Interestingly, the activation of STAT3 is also involved in the IFNs-induced transcription diversity of PD-L1 and reduces of immunotherapy effect in multiple cancer types [68–70]. Xiang and his colleagues also elucidated that the dexamethasone inhibits the nuclear translocation of GR/STAT3 complex and suppresses the expression PD-L1, decreasing the cancer cell immune evasion [71]. Meanwhile, together with p65, the activation of STAT3 promotes the transcription of COP9 signalosome complex subunit 5 (CSN5), which functions as the deubiquitin enzyme and reduces ubiquitination-induced PD-L1 degradation [70]. So, it is easy for us to get the hypothesis that the nPD-L1-induced STAT3 activation can in turn feedback the transcription of PD-L1 and increase the mPD-L1-induced immune escape. The hypothesis indicates the close relationship between nPD-L1 and mPD-L1, which has been elucidated in Gao’s research [9]. The expression of KPNs and STAT3 engage in the acceleration of the membrane-nuclear-membrane expression loop of PD-L1, which plays an essential role in the rotation of cell death and tumor immunity in cancer cells.
AKT pathway
The activation of AKT is related to cell viability, apoptosis, and proliferation, which manipulates the development of various cancer types and can be the therapeutic target in cancer treatment [72, 73]. The research has emerged that AKT plays a crucial role in PD-L1 regulation. The investigation showed that phosphorylation of AKT promotes immune escape by driving the expression of PD-L1 [74, 75]. Meanwhile, the stimulation from c-MET amplification, the HGF, and EGFR-T790M mutation caused the activation of PI3K and sequentially improved the phosphorylation of AKT, increasing the transcription of PD-L1 [76]. These results revealed the crucial role of an inside-out regulator in PD-L1 expression.
On the other side, AKT also controls the outside-in transportation of PD-L1. The phosphorylation of AKT increased after the treatment of doxorubicin, along with the enhancement of nPD-L1 expression in breast cancer cells [44]. 112-480aa fragment of AKT is the fundamental structure that can recognize 128-237aa fragment of PD-L1 with the stimulation of starvation and facilitates glioma cell invasion [77]. However, the specific mechanism of AKT in PD-L1 nuclear transportation is still unclear. Jeong’s research provided an explanation that phosphorylation of AKT promotes nuclear translocation by enhancing the binding and transportation ability of importin α, which facilitates the PD-L1 nuclear translocation [78]. On the other hand, The AKT and its phosphorylation contribute to the phosphorylation of STAT3, suggesting the sequential activation from AKT to STAT3 in nPD-L1 transportation [79]. Moreove, Schulz and his colleagues [80] proved that PD-L1 can directly bind with AKT-1, especially in the radio-resistant neck squamous cancer cells. The irradiation enhanced the binding capacity between PD-L1 and AKT-1 with the time extension. Different from the previous result, the higher binding affinity between AKT and PD-L1 reveals the lower nuclear enrichment of nPD-L1 in radio-resistant neck squamous cancer cells. Their results indicated that the AKT has various function in nPD-L1 expression in different cells, which requires further research.
Vimentin
Encoding a type III intermediate filament protein, Vimentin (VIM) intermediate filaments (VIFs) network is widely distributed from the nucleus surface to the plasma membrane [81]. VIM acts as one of the crucial components of the cytoskeleton to retain its stability, which monitors cell migration, adhesion, and differentiation [82, 83]. High expression of VIM has a positive relationship with PD-L1 expression and promotes the epithelial-mesenchymal transition (EMT) [83, 84]. Meanwhile, as the marker of circulating tumor cells, the cells with positive VIM expression on the cell surface showed a higher nPD-L1 expression in multiple cancer types, which indicates the regulatory function of VIM in PD-L1 nuclear location [19]. In addition, the mass spectrometry data has also illustrated that PD-L1 connected several cytoskeleton proteins by its C-terminal, including VIM and Keratins (KRTs). The depletion of VIM caused the down-regulation of PD-L1 accumulation in nuclear and cytoskeletal [9]. The VIM-induced PD-L1 binding to the cytoskeleton is associated with the functional PD-L1 interaction with Huntingtin-interacting-protein-1-related (HIP1R) protein, which is an essential factor in PD-L1 lysosomal degradation [9, 85]. These results implied that the nuclear import and export of PD-L1 depends on the route along with the cytoskeleton.
The function of nPD-L1 in cancer cell
Although the canonical modification of PD-L1 has been widely investigated in a series of research, which is considered as the criminal in anti-tumor immunity, the reverse signaling of PD-L1 in cancer cell regulation is still unknown [86]. The activation of PD-L1 induced by PD-1 reduced the susceptibility to apoptosis induced by Fas ligation or staurosporine [87]. Meanwhile, the antibody-induced PD-L1 blocking inhibited mTOR and reprogramed the metabolic process in tumor cells in a T cell-independent manner [88]. However, the current PD-L1 concerned research focuses on the effect in the tumor microenvironment, especially in the cancer cell-T cell interaction. The regulatory function of PD-L1 in cytoplasm and nuclear cancer cells remains unclear.
The function of PD-L1 in various cancers partly results from the nPD-L1-induced cell remodeling. Yu and his colleagues found that nPD-L1 replaces the role of Sororin and engaged in the formation of sister chromatid cohesion by binding to PDS5B. The abolishment of PD-L1 downregulated the proliferation, colony formation of cancer cells in vitro, and tumor growth in vivo [89]. On the other hand, nPD-L1-induced Gas6 expression is recognized by MerTK and enhanced cell proliferation with the activation of ERK and AKT signaling pathways [54]. The nPD-L1-induced proliferation promotion results from the increasing expression of cell cycle regulator BUB1 via thyroid hormone receptor-associated protein 3 (THRAP3), thereby accelerating cell cycle progression and enhancing cell proliferation [90]. Moreover, the nuclear transportation of PD-L1 increases the transcription of immunosuppressive molecules such as PD-L2, VISTA, and B7H3 and decrease the T cell-induced anti-tumor immunity [9]. Shim and his colleagues found that the high-level expression of nPD-L1 predicted the higher Gleason score and cT stage in the prostate cancer patients with the treatment of primary radiation therapy, which suggested the higher malignancy and poor prognosis [91]. Similarly, the research in HNSCC also showed upregulated nuclear distribution of PD-L1 in radiotherapy-resistant cancer cells as well as the cells post-radiation treatment [80]. In this regard, cancer cells enhance cell proliferation and the resistance of radiotherapy and immunotherapy via PD-L1 nuclear relocation.
However, the expression of nPD-L1 enhanced the STAT3-induced transcription of the GSDMC gene. GSDMC is a member of the conserved proteins family that includes gasdermin A, B, C, D, E, and DFNB59 [92]. Cleaved by Caspase family, GSDM family generates an N-terminal pore-forming fragment and gathers in the plasma membrane, forms the gasdermin pore and causes cell pyroptosis [93]. Hou’s research found that nPD-L1-induced GSDMC expression accelerates the TNFα-induced tumor necrosis and promotes the chemotherapy-mediated pyroptosis in breast cancer in hypoxia condition [10, 94]. The knockdown of PD-L1 reduces the function of PD-L1/STAT1 binding complex and suppresses the hypoxia-induced pyroptosis [95]. These results indicated that nPD-L1 increases the response to the cell death signal and causes the better prognosis after chemotherapy. This research explained the results from Schmidt and his colleagues, which elucidated that the PD-L1 positive cancer patients who received adjuvant therapy have a higher survival rate while it has no prognostic significance in whole patient analysis [43]. Meanwhile, two studies from BRAFV600E-induced human colon cancer cells showed that tumor intrinsic PD-L1 enhances chemotherapy-induced apoptosis [96, 97]. Knockout of tumor intrinsic PD-L1 reduces pro-apoptotic BIM and BIK, which cause the resistance to drug-induced apoptosis and show the better prognosis. These results suggested that the PD-L1 nuclear transportation has a different function in cancer development, which improves the sensibility of chemotherapeutic and prolong the survival time.
Conclusion
Separated from PD-L1/PD-1 interaction in the canonical immune checkpoints pathway, the expression of PD-L1 in nuclear reveals the novel transcription regulation in cancer development and immune escape. The nPD-L1 promotes cell proliferation by chromatin remodeling, while the nuclear translocation of PD-L1 induced by hypoxia shows a higher sensibility to pyroptosis and enhances the therapeutic effect. Moreover, the nuclear-translocation of PD-L1 also promotes the expression of immune checkpoint ligands such as PD-L1, PD-L2, VISTA and B7-H3. These immune checkpoint ligands interact with their receptors and cause the exhaustion of immune cells, further promoting the cancer immune escape (Fig. 1). These finding suggested that cancer cell with nPD-L1 expression has higher proliferation by cell cycle promoting. nPD-L1 also increase immunosuppression ability of cancer cells by further expression of immune check-point markers. However, these cells also facilitate the pyroptosis and show a higher sensibility to chemotherapy. This bidirectional regulation could contribute to the difference of prognosis in PD-L1 expression cancer patients (Fig. 2). The patients with nPD-L1+ cancer should consider the reinforce of chemotherapy and radiotherapy and have the higher frequency of follow-up. Thus, nPD-L1 is expected to be the biomarker of cancer development and therapeutic effect in future cancer treatment.
Fig. 1.
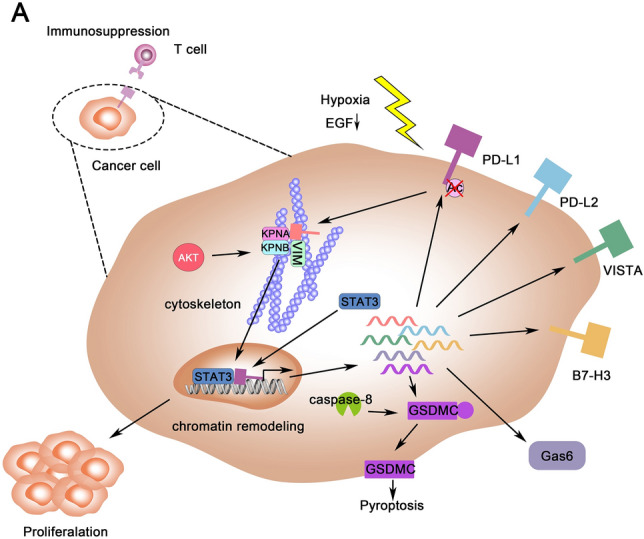
The overview of nPD-L1 in cancer cells. A The PD-L1 was deacetylated in hypoxia condition and start the process of nuclear transportation regulated by a series of factors including KPNs, STAT3, AKT and VIM. nPD-L1 regulates the cancer cells in these four apsects: (1) promote the proliferation of cancer cells; (2) enhance the transcription of GMDSC and facilitate pyroptosis; (3) increase the secretion of Gas6 and (4) upregulation the expression of immune check-point molecules such as PD-L1, PD-L2, VISTA and B7-H3 and cause the immune escape
Fig. 2.
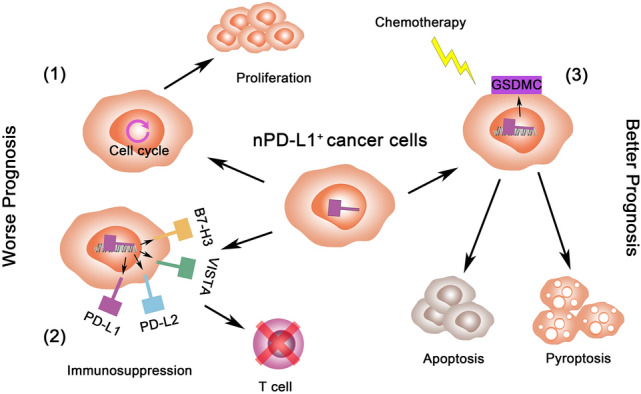
The nPD-L1-induced cancer prognosis regulation. High expression of nPD-L1 (1) causes the higher proliferation by cell cycle promoting; (2) enhances immunosuppression by immune check-point marker expression; (3) increase the sensibility to chemotherapy by cell pyroptosis
Acknowledgements
None.
Abbreviations
- AP-1
Activator protein 1
- BRCA
Breast cancer
- CAR-T
Chimeric antigen receptor T cell
- COAD
Colon cancer
- CSN5
COP9 signalosome complex subunit 5
- EGF
Epidermal growth factor
- FDA
Food and drug administration
- GSDMC
Gasdermin C
- HCC
Hepatocellular carcinoma
- HIP1R.
Huntingtin-interacting-protein-1-related
- IRF1
Interferon regulatory factor 1
- IFN
Interferons
- IL
Interleukin
- KPNs
Karyopherin
- KRTs
Keratins
- LADC
Lung adenocarcinoma
- LSCC
Lung squamous cell carcinoma
- HNSCC
Neck squamous cancer cells
- NSCLC
Non-small-cell lung cancer
- NF-κB
Nuclear factor kappa B
- NFAT
Nuclear factor of activated T-cells
- NLSs
Nuclear localization signals
- OS
Osteosarcoma
- PD-L1
Programmed cell death protein ligand 1
- PRAD
Prostate cancer
- RCC
Renal cell carcinoma
- STAT
Signal transducer and activator of transcription
- SRTFs
Stress-responsive transcription factors
- TCR
T cell receptor
- TRIM59
Tripartite motif-containing 59
- TNF-α
Tumor necrosis factor alpha
- VIM
Vimentin
Author contributions
LQ, JJ and JL drafted most of the manuscript. CQ, JL and AX participated in the modification of the manuscript. BL and MZ mainly drawing the schematic diagram. HT and WY participated in the review design. All authors read and approved the final manuscript.
Funding
This study was supported by grant from the National Natural Science Foundation of China (NO. 81872181), the program of Zhejiang medical and health technology (2019KY079), Natural Science Foundation of Zhejiang Province (NO. LY20H160025, LY21H160034 and LY21H060003) and Zhejiang Xinmiao Talents Program (2020R401234).
Data availability
Not applicable.
Declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huimin Tao, Email: 2187040@zju.edu.cn.
Wei Yu, Email: 11818244@zju.edu.cn.
References
- 1.Kishton RJ, Sukumar M, Restifo NP. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 2017;26(1):94–109. doi: 10.1016/j.cmet.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidman J, Principe N, Watson M, Lassmann T, Holt RA, Nowak AK, Lesterhuis WJ, Lake RA, Chee J: Characteristics of TCR repertoire associated with successful immune checkpoint therapy responses. Front Immunol 2020, 11:587014. [DOI] [PMC free article] [PubMed]
- 3.Huang C, Zhu HX, Yao Y, Bian ZH, Zheng YJ, Li L, Moutsopoulos HM, Gershwin ME, Lian ZX: Immune checkpoint molecules. Possible future therapeutic implications in autoimmune diseases. J Autoimmun 2019, 104:102333. [DOI] [PubMed]
- 4.Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276(1):145–164. doi: 10.1111/imr.12527. [DOI] [PubMed] [Google Scholar]
- 5.Bewersdorf JP, Shallis RM, Zeidan AM: Immune checkpoint inhibition in myeloid malignancies: Moving beyond the PD-1/PD-L1 and CTLA-4 pathways. Blood Rev 2021, 45:100709. [DOI] [PubMed]
- 6.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB: Review of Indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel) 2020, 12(3). [DOI] [PMC free article] [PubMed]
- 8.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Nihira NT, Bu X, Chu C, Zhang J, Kolodziejczyk A, Fan Y, Chan NT, Ma L, Liu J, et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat Cell Biol. 2020;22(9):1064–1075. doi: 10.1038/s41556-020-0562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu JM, Nie L, Chen Y, Wang YC, Liu C, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22(10):1264–1275. doi: 10.1038/s41556-020-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong W, Gao Y, Wei W, Zhang J. Extracellular and nuclear PD-L1 in modulating cancer immunotherapy. Trends Cancer. 2021;7(9):837–846. doi: 10.1016/j.trecan.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez M, Simonetta F, Baker J, Morrison AR, Wenokur AS, Pierini A, Berraondo P, Negrin RS. Indirect impact of PD-1/PD-L1 blockade on a murine model of NK cell exhaustion. Front Immunol. 2020;11:7. doi: 10.3389/fimmu.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roux C, Jafari SM, Shinde R, Duncan G, Cescon DW, Silvester J, Chu MF, Hodgson K, Berger T, Wakeham A, et al. Reactive oxygen species modulate macrophage immunosuppressive phenotype through the up-regulation of PD-L1. Proc Natl Acad Sci U S A. 2019;116(10):4326–4335. doi: 10.1073/pnas.1819473116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolvering C, Zimmer AD, Ginolhac A, Margue C, Kirchmeyer M, Servais F, Hermanns HM, Hergovits S, Nazarov PV, Nicot N, et al. The PD-L1- and IL6-mediated dampening of the IL27/STAT1 anticancer responses are prevented by alpha-PD-L1 or alpha-IL6 antibodies. J Leukoc Biol. 2018;104(5):969–985. doi: 10.1002/JLB.MA1217-495R. [DOI] [PubMed] [Google Scholar]
- 16.Lin PL, Wu TC, Wu DW, Wang L, Chen CY, Lee H. An increase in BAG-1 by PD-L1 confers resistance to tyrosine kinase inhibitor in non-small cell lung cancer via persistent activation of ERK signalling. Eur J Cancer. 2017;85:95–105. doi: 10.1016/j.ejca.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Hsu JM, Li CW, Lai YJ, Hung MC. Posttranslational modifications of PD-L1 and their applications in cancer therapy. Cancer Res. 2018;78(22):6349–6353. doi: 10.1158/0008-5472.CAN-18-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Arrigo P, Russo M, Rea A, Tufano M, Guadagno E, Del Basso De Caro ML, Pacelli R, Hausch F, Staibano S, Ilardi G et al A regulatory role for the co-chaperone FKBP51s in PD-L1 expression in glioma. Oncotarget 2017, 8(40):68291–68304. [DOI] [PMC free article] [PubMed]
- 19.Satelli A, Batth IS, Brownlee Z, Rojas C, Meng QH, Kopetz S, Li S. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci Rep. 2016;6:28910. doi: 10.1038/srep28910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zak KM, Kitel R, Przetocka S, Golik P, Guzik K, Musielak B, Domling A, Dubin G, Holak TA. Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1. Structure. 2015;23(12):2341–2348. doi: 10.1016/j.str.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209(6):1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ai L, Xu A, Xu J. Roles of PD-1/PD-L1 pathway: signaling, cancer, and beyond. Adv Exp Med Biol. 2020;1248:33–59. doi: 10.1007/978-981-15-3266-5_3. [DOI] [PubMed] [Google Scholar]
- 23.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGowan E, Lin Q, Ma G, Yin H, Chen S, Lin Y: PD-1 disrupted CAR-T cells in the treatment of solid tumors: Promises and challenges. Biomed Pharmacother 2020, 121:109625. [DOI] [PubMed]
- 25.Chen N, Morello A, Tano Z, Adusumilli PS: CAR T-cell intrinsic PD-1 checkpoint blockade: a two-in-one approach for solid tumor immunotherapy. Oncoimmunology 2017, 6(2):e1273302. [DOI] [PMC free article] [PubMed]
- 26.Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, Marson A. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7(1):737. doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nduom EK, Wei J, Yaghi NK, Huang N, Kong LY, Gabrusiewicz K, Ling X, Zhou S, Ivan C, Chen JQ, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18(2):195–205. doi: 10.1093/neuonc/nov172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vital D, Ikenberg K, Moch H, Rossle M, Huber GF. The expression of PD-L1 in salivary gland carcinomas. Sci Rep. 2019;9(1):12724. doi: 10.1038/s41598-019-49215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sideras K, Biermann K, Verheij J, Takkenberg BR, Mancham S, Hansen BE, Schutz HM, de Man RA, Sprengers D, Buschow SI et al (2017) PD-L1, Galectin-9 and CD8(+) tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. Oncoimmunology 6(2):e1273309. [DOI] [PMC free article] [PubMed]
- 30.Wang Y, Zhu C, Song W, Li J, Zhao G, Cao H. PD-L1 Expression and CD8(+) T cell infiltration predict a favorable prognosis in advanced gastric cancer. J Immunol Res. 2018;2018:4180517. doi: 10.1155/2018/4180517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A, Rosso R, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49(9):2233–2242. doi: 10.1016/j.ejca.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Q, Cai MY, Chen CL, Hu H, Lin HX, Li M, Weng DS, Zhao JJ, Guo L, Xia JC: Tumor cells PD-L1 expression as a favorable prognosis factor in nasopharyngeal carcinoma patients with pre-existing intratumor-infiltrating lymphocytes. Oncoimmunology 2017, 6(5):e1312240. [DOI] [PMC free article] [PubMed]
- 33.Zhang S, Minaguchi T, Xu C, Qi N, Itagaki H, Shikama A, Tasaka N, Akiyama A, Sakurai M, Ochi H, et al. PD-L1 and CD4 are independent prognostic factors for overall survival in endometrial carcinomas. BMC Cancer. 2020;20(1):127. doi: 10.1186/s12885-020-6545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee VH, Lo AW, Leung CY, Shek WH, Kwong DL, Lam KO, Tong CC, Sze CK, Leung TW: Correlation of PD-L1 expression of tumor cells with survival outcomes after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinoma. PLoS One 2016, 11(6):e0157969. [DOI] [PMC free article] [PubMed]
- 35.Owyong M, Lotan Y, Kapur P, Panwar V, McKenzie T, Lee TK, Zi X, Martin JW, Mosbah A, Abol-Enein H, et al. Expression and prognostic utility of PD-L1 in patients with squamous cell carcinoma of the bladder. Urol Oncol. 2019;37(7):478–484. doi: 10.1016/j.urolonc.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 36.Tseng JS, Yang TY, Wu CY, Ku WH, Chen KC, Hsu KH, Huang YH, Su KY, Yu SL, Chang GC. Characteristics and predictive value of PD-L1 status in real-world non-small cell lung cancer patients. J Immunother. 2018;41(6):292–299. doi: 10.1097/CJI.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 37.Qin Y, Jiang L, Yu M, Li Y, Zhou X, Wang Y, Gong Y, Peng F, Zhu J, Liu Y, et al. PD-L1 expression is a promising predictor of survival in patients with advanced lung adenocarcinoma undergoing pemetrexed maintenance therapy. Sci Rep. 2020;10(1):16150. doi: 10.1038/s41598-020-73013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou Y, Nitta H, Wei L, Banks PM, Lustberg M, Wesolowski R, Ramaswamy B, Parwani AV, Li Z. PD-L1 expression and CD8-positive T cells are associated with favorable survival in HER2-positive invasive breast cancer. Breast J. 2018;24(6):911–919. doi: 10.1111/tbj.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanze J, Wegner M, Noessner E, Hofmann R, Hegele A. Co-regulation of immune checkpoint PD-L1 with interferon-gamma signaling is associated with a survival benefit in renal cell cancer. Target Oncol. 2020;15(3):377–390. doi: 10.1007/s11523-020-00728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Molberg K, Strickland AL, Castrillon DH, Carrick K, Jiang Q, Niu S, Rivera-Colon G, Gwin K, Hinson S, et al. PD-L1 Expression and CD8+ tumor-infiltrating lymphocytes in different types of tubo-ovarian carcinoma and their prognostic value in high-grade serous carcinoma. Am J Surg Pathol. 2020;44(8):1050–1060. doi: 10.1097/PAS.0000000000001503. [DOI] [PubMed] [Google Scholar]
- 41.Guo W, Zhang F, Shao F, Wang P, Li Z, Yang X, He Z, Shi S, Gao Y, He J. PD-L1 expression on tumor cells associated with favorable prognosis in surgically resected esophageal squamous cell carcinoma. Hum Pathol. 2019;84:291–298. doi: 10.1016/j.humpath.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Wunder JS, Lee MJ, Nam J, Lau BY, Dickson BC, Pinnaduwage D, Bull SB, Ferguson PC, Seto A, Gokgoz N, et al. Osteosarcoma and soft-tissue sarcomas with an immune infiltrate express PD-L1: relation to clinical outcome and Th1 pathway activation. Oncoimmunology. 2020;9(1):1737385. doi: 10.1080/2162402X.2020.1737385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt LH, Kummel A, Gorlich D, Mohr M, Brockling S, Mikesch JH, Grunewald I, Marra A, Schultheis AM, Wardelmann E et al. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS One 2015, 10(8):e0136023. [DOI] [PMC free article] [PubMed]
- 44.Ghebeh H, Lehe C, Barhoush E, Al-Romaih K, Tulbah A, Al-Alwan M, Hendrayani SF, Manogaran P, Alaiya A, Al-Tweigeri T, et al. Doxorubicin downregulates cell surface B7–H1 expression and upregulates its nuclear expression in breast cancer cells: role of B7–H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010;12(4):R48. doi: 10.1186/bcr2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang J, Wu C. B7–H1 expression associates with tumor invasion and predicts patient's survival in human esophageal cancer. Int J Clin Exp Pathol. 2014;7(9):6015–6023. [PMC free article] [PubMed] [Google Scholar]
- 46.Parra ER, Villalobos P, Rodriguez-Canales J. The multiple faces of programmed cell death ligand 1 expression in malignant and nonmalignant cells. Appl Immunohistochem Mol Morphol. 2019;27(4):287–294. doi: 10.1097/PAI.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 47.Polioudaki H, Chantziou A, Kalyvianaki K, Malamos P, Notas G, Mavroudis D, Kampa M, Castanas E, Theodoropoulos PA. Nuclear localization of PD-L1: artifact or reality? Cell Oncol (Dordr) 2019;42(2):237–242. doi: 10.1007/s13402-018-00419-7. [DOI] [PubMed] [Google Scholar]
- 48.Ma J, Li J, Qian M, Han W, Tian M, Li Z, Wang Z, He S, Wu K. PD-L1 expression and the prognostic significance in gastric cancer: a retrospective comparison of three PD-L1 antibody clones (SP142, 28–8 and E1L3N) Diagn Pathol. 2018;13(1):91. doi: 10.1186/s13000-018-0766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS, Goodwin LM, Smethurst E, Mason S, Blyth K, et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell. 2015;27(1):57–71. doi: 10.1016/j.ccell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horita H, Law A, Hong S, Middleton K. Identifying regulatory posttranslational modifications of PD-L1: a focus on monoubiquitinaton. Neoplasia. 2017;19(4):346–353. doi: 10.1016/j.neo.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang C, Niu J, Wang X, Zhang ZS, Yang RH, Yao X, Liu FY, Li WQ, Pei SH, Sun H, et al. P300-dependent acetylation of histone H3 is required for epidermal growth factor receptor-mediated high-mobility group protein A2 transcription in hepatocellular carcinoma. Cancer Sci. 2021;112(2):679–690. doi: 10.1111/cas.14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han Y, Wang X (2020) The emerging roles of KPNA2 in cancer. Life Sci 241:117140. [DOI] [PubMed]
- 53.Soniat M, Chook YM. Nuclear localization signals for four distinct karyopherin-beta nuclear import systems. Biochem J. 2015;468(3):353–362. doi: 10.1042/BJ20150368. [DOI] [PubMed] [Google Scholar]
- 54.Du W, Zhu J, Zeng Y, Liu T, Zhang Y, Cai T, Fu Y, Zhang W, Zhang R, Liu Z et al KPNB1-mediated nuclear translocation of PD-L1 promotes non-small cell lung cancer cell proliferation via the Gas6/MerTK signaling pathway. Cell Death Differ 2020. [DOI] [PMC free article] [PubMed]
- 55.Hazawa M, Yoshino H, Nakagawa Y, Shimizume R, Nitta K, Sato Y, Sato M, Wong RW, Kashiwakura I: Karyopherin-beta1 regulates radioresistance and radiation-increased programmed death-ligand 1 expression in human head and neck squamous cell carcinoma cell lines. Cancers (Basel) 2020, 12(4). [DOI] [PMC free article] [PubMed]
- 56.Feng H, Zhang YB, Gui JF, Lemon SM, Yamane D: Interferon regulatory factor 1 (IRF1) and anti-pathogen innate immune responses. PLoS Pathog 2021, 17(1):e1009220. [DOI] [PMC free article] [PubMed]
- 57.Umegaki N, Tamai K, Nakano H, Moritsugu R, Yamazaki T, Hanada K, Katayama I, Kaneda Y. Differential regulation of karyopherin alpha 2 expression by TGF-beta1 and IFN-gamma in normal human epidermal keratinocytes: evident contribution of KPNA2 for nuclear translocation of IRF-1. J Invest Dermatol. 2007;127(6):1456–1464. doi: 10.1038/sj.jid.5700716. [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19(6):1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshino H, Sato Y, Nakano M. KPNB1 inhibitor importazole reduces ionizing radiation-increased cell surface PD-L1 expression by modulating expression and nuclear import of IRF1. Curr Issues Mol Biol. 2021;43(1):153–162. doi: 10.3390/cimb43010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veach RA, Liu Y, Zienkiewicz J, Wylezinski LS, Boyd KL, Wynn JL, Hawiger J: Survival, bacterial clearance and thrombocytopenia are improved in polymicrobial sepsis by targeting nuclear transport shuttles. PLoS One 2017, 12(6):e0179468. [DOI] [PMC free article] [PubMed]
- 61.Lim SO, Li CW, Xia W, Cha JH, Chan LC, Wu Y, Chang SS, Lin WC, Hsu JM, Hsu YH, et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30(6):925–939. doi: 10.1016/j.ccell.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao FL, Qin CF. EGF promotes HIF-1alpha expression in colorectal cancer cells and tumor metastasis by regulating phosphorylation of STAT3. Eur Rev Med Pharmacol Sci. 2019;23(3):1055–1062. doi: 10.26355/eurrev_201902_16993. [DOI] [PubMed] [Google Scholar]
- 63.Siersbaek R, Scabia V, Nagarajan S, Chernukhin I, Papachristou EK, Broome R, Johnston SJ, Joosten SEP, Green AR, Kumar S et al: IL6/STAT3 signaling hijacks estrogen receptor alpha enhancers to drive breast cancer metastasis. Cancer Cell 2020, 38(3):412–423 e419. [DOI] [PMC free article] [PubMed]
- 64.Liu Y, Lv J, Liu J, Liang X, Jin X, Xie J, Zhang L, Chen D, Fiskesund R, Tang K, et al. STAT3/p53 pathway activation disrupts IFN-beta-induced dormancy in tumor-repopulating cells. J Clin Invest. 2018;128(3):1057–1073. doi: 10.1172/JCI96329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou KX, Huang S, Hu LP, Zhang XL, Qin WT, Zhang YL, Yao LL, Yu Y, Zhou YQ, Zhu L, et al. Increased nuclear transporter KPNA2 contributes to tumor immune evasion by enhancing PD-L1 expression in PDAC. J Immunol Res. 2021;2021:6694392. doi: 10.1155/2021/6694392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma J, Cao X. Regulation of Stat3 nuclear import by importin alpha5 and importin alpha7 via two different functional sequence elements. Cell Signal. 2006;18(8):1117–1126. doi: 10.1016/j.cellsig.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Sang Y, Li Y, Zhang Y, Alvarez AA, Yu B, Zhang W, Hu B, Cheng SY, Feng H. CDK5-dependent phosphorylation and nuclear translocation of TRIM59 promotes macroH2A1 ubiquitination and tumorigenicity. Nat Commun. 2019;10(1):4013. doi: 10.1038/s41467-019-12001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cao D, Qi Z, Pang Y, Li H, Xie H, Wu J, Huang Y, Zhu Y, Shen Y, Zhu Y, et al. Retinoic acid-related orphan receptor c regulates proliferation, glycolysis, and chemoresistance via the PD-L1/ITGB6/STAT3 signaling axis in bladder cancer. Cancer Res. 2019;79(10):2604–2618. doi: 10.1158/0008-5472.CAN-18-3842. [DOI] [PubMed] [Google Scholar]
- 69.Li T, Zhang C, Zhao G, Zhang X, Hao M, Hassan S, Zhang M, Zheng H, Yang D, Liu L, et al. IGFBP2 regulates PD-L1 expression by activating the EGFR-STAT3 signaling pathway in malignant melanoma. Cancer Lett. 2020;477:19–30. doi: 10.1016/j.canlet.2020.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu C, Yao Z, Wang J, Zhang W, Yang Y, Zhang Y, Qu X, Zhu Y, Zou J, Peng S, et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. 2020;27(6):1765–1781. doi: 10.1038/s41418-019-0460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiang Z, Zhou Z, Song S, Li J, Ji J, Yan R, Wang J, Cai W, Hu W, Zang L, et al. Dexamethasone suppresses immune evasion by inducing GR/STAT3 mediated downregulation of PD-L1 and IDO1 pathways. Oncogene. 2021;40(31):5002–5012. doi: 10.1038/s41388-021-01897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79(6):1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Q, Cao Y, Luo Q, Wang P, Shi P, Song C, E M, Ren J, Fu B, Sun H (2018) The transient receptor potential vanilloid-3 regulates hypoxia-mediated pulmonary artery smooth muscle cells proliferation via PI3K/AKT signaling pathway. Cell Prolif 51(3):e12436. [DOI] [PMC free article] [PubMed]
- 74.Lastwika KJ, Wilson W, 3rd, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76(2):227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 75.Zhao R, Song Y, Wang Y, Huang Y, Li Z, Cui Y, Yi M, Xia L, Zhuang W, Wu X et al PD-1/PD-L1 blockade rescue exhausted CD8+ T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway. Cell Prolif 2019, 52(3):e12571. [DOI] [PMC free article] [PubMed]
- 76.Peng S, Wang R, Zhang X, Ma Y, Zhong L, Li K, Nishiyama A, Arai S, Yano S, Wang W. EGFR-TKI resistance promotes immune escape in lung cancer via increased PD-L1 expression. Mol Cancer. 2019;18(1):165. doi: 10.1186/s12943-019-1073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen RQ, Xu XH, Liu F, Li CY, Li YJ, Li XR, Jiang GY, Hu F, Liu D, Pan F, et al. The binding of PD-L1 and Akt facilitates glioma cell invasion upon starvation via Akt/autophagy/F-actin signaling. Front Oncol. 2019;9:1347. doi: 10.3389/fonc.2019.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeong SA, Kim K, Lee JH, Cha JS, Khadka P, Cho HS, Chung IK. Akt-mediated phosphorylation increases the binding affinity of hTERT for importin alpha to promote nuclear translocation. J Cell Sci. 2015;128(12):2287–2301. doi: 10.1242/jcs.166132. [DOI] [PubMed] [Google Scholar]
- 79.Ryu KY, Lee HJ, Woo H, Kang RJ, Han KM, Park H, Lee SM, Lee JY, Jeong YJ, Nam HW, et al. Dasatinib regulates LPS-induced microglial and astrocytic neuroinflammatory responses by inhibiting AKT/STAT3 signaling. J Neuroinflammation. 2019;16(1):190. doi: 10.1186/s12974-019-1561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schulz D, Streller M, Piendl G, Brockhoff G, Reichert TE, Menevse AN, Beckhove P, Hautmann MG, Bauer RJ, Ettl T. Differential localization of PD-L1 and Akt-1 involvement in radioresistant and radiosensitive cell lines of head and neck squamous cell carcinoma. Carcinogenesis. 2020;41(7):984–992. doi: 10.1093/carcin/bgz177. [DOI] [PubMed] [Google Scholar]
- 81.Patteson AE, Vahabikashi A, Pogoda K, Adam SA, Mandal K, Kittisopikul M, Sivagurunathan S, Goldman A, Goldman RD, Janmey PA. Vimentin protects cells against nuclear rupture and DNA damage during migration. J Cell Biol. 2019;218(12):4079–4092. doi: 10.1083/jcb.201902046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Z, Fang Z, Ma J (2021) Regulatory mechanisms and clinical significance of vimentin in breast cancer. Biomed Pharmacother 133:111068. [DOI] [PubMed]
- 83.Cheng F, Shen Y, Mohanasundaram P, Lindstrom M, Ivaska J, Ny T, Eriksson JE. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-beta-Slug signaling. Proc Natl Acad Sci U S A. 2016;113(30):E4320–4327. doi: 10.1073/pnas.1519197113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim S, Koh J, Kim MY, Kwon D, Go H, Kim YA, Jeon YK, Chung DH. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum Pathol. 2016;58:7–14. doi: 10.1016/j.humpath.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Wang H, Yao H, Li C, Shi H, Lan J, Li Z, Zhang Y, Liang L, Fang JY, Xu J. HIP1R targets PD-L1 to lysosomal degradation to alter T cell-mediated cytotoxicity. Nat Chem Biol. 2019;15(1):42–50. doi: 10.1038/s41589-018-0161-x. [DOI] [PubMed] [Google Scholar]
- 86.Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48(3):434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7–H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111(7):3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu J, Qin B, Moyer AM, Nowsheen S, Tu X, Dong H, Boughey JC, Goetz MP, Weinshilboum R, Lou Z, et al. Regulation of sister chromatid cohesion by nuclear PD-L1. Cell Res. 2020;30(7):590–601. doi: 10.1038/s41422-020-0315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma R, Liu Y, Che X, Li C, Wen T, Hou K, Qu X. Nuclear PD-L1 promotes cell cycle progression of BRAF-mutated colorectal cancer by inhibiting THRAP3. Cancer Lett. 2021;527:127–139. doi: 10.1016/j.canlet.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 91.Shim KH, Kwon JE, Park SG, Choo SH, Kim SJ, Kim SI (2021) Cell membrane and nuclear expression of programmed death ligand-1 in prostate needle biopsy tissue in prostate cancer patients undergoing primary radiation therapy. Urol Oncol [DOI] [PubMed]
- 92.Kovacs SB, Miao EA. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27(9):673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen KW, Demarco B, Heilig R, Shkarina K, Boettcher A, Farady CJ, Pelczar P, Broz P (2019) Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J 38(10). [DOI] [PMC free article] [PubMed]
- 94.Feng S, Fox D, Man SM: Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J Mol Biol 2018, 430(18 Pt B):3068–3080. [DOI] [PubMed]
- 95.Zhang M, Xin W, Yu Y, Yang X, Ma C, Zhang H, Liu Y, Zhao X, Guan X, Wang X, et al. Programmed death-ligand 1 triggers PASMCs pyroptosis and pulmonary vascular fibrosis in pulmonary hypertension. J Mol Cell Cardiol. 2020;138:23–33. doi: 10.1016/j.yjmcc.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 96.Feng D, Qin B, Pal K, Sun L, Dutta S, Dong H, Liu X, Mukhopadhyay D, Huang S, Sinicrope FA. BRAF(V600E)-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts. Oncogene. 2019;38(41):6752–6766. doi: 10.1038/s41388-019-0919-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng D, Chen Z, He X, Huang S, Zhang Z. Loss of tumor intrinsic PD-L1 confers resistance to drug-induced apoptosis in human colon cancer. Neoplasma. 2021;68(1):144–153. doi: 10.4149/neo_2020_200531N589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


