Abstract
Purpose
Targeting of anti-programmed cell death protein-1 (PD-1) and anti-programmed death-ligand 1 (PD-L1) is a standard therapeutic strategy for various cancers. The aim of the present study was to investigate the prognostic effect of pretreatment PD-L1 expression levels in peripheral blood mononuclear cell (PBMC) subsets for patients with several cancer types receiving anti-PD-1 blockade therapies.
Patients and methods
Thirty-two patients undergoing anti-PD-L1 blockade therapy, including 15 with non-small cell lung cancer, 14 with gastric cancer, 1 with melanoma, 1 with parotid cancer, and 1 with bladder cancer, were recruited for the present study. PD-L1 expression levels in CD3+, CD4+, CD8+, CD45RA+ and CCR7+ T cells; CD20+ B cells; CD14+ and CD16+ monocytes were measured via flow cytometry before treatment. The percentages of PD-L1+ cells in respective PBMC subsets were compared with respect to different clinicopathological conditions and the association with overall survival (OS) was assessed.
Results
The percentages of PD-L1+ with CD3+, CD4+ and CD8+ T cells including naïve and memory T cell subsets, or CD20+ B cells during pretreatment were not markedly correlated with the OS of patients (p > 0.05); however, the percentage of the PD-L1+ CD14+ monocyte subset was significantly correlated with OS (p = 0.0426).
Conclusion
Increase in pretreatment expression levels of PD-L1 on CD14+ monocytes is associated with the OS of patients treated with immune checkpoint inhibitors. Further evaluation of large sample size and each specific cancer type might clarify the predictive role of PBMC in patients.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02686-6) contains supplementary material, which is available to authorized users.
Keywords: Programmed death-ligand 1, Programmed death-1, CD14, Prognosis, Nivolumab, Pembrolizumab
Introduction
Programmed death-1 (PD-1) and its ligand programmed death-ligand1 (PD-L1) play a pivotal role in immunosuppression [1–3]. The PD1/PD-L1 pathway is closely associated with resistance to antitumor immunity in the tumor microenvironment (TME) [4]. Therefore, targeting PD-1/PD-L1 signaling using immune checkpoint inhibitors (ICIs) such as anti-PD-1 antibodies (e.g., nivolumab and pembrolizumab) has provided remarkable therapeutic benefits in the treatment of various cancers [5–7]. PD-L1 expressed on cancer cells inhibits the activation of tumor-infiltrating lymphocytes, resulting in tumor progression, indicating that PD-L1 expression levels can potentially predict tumor dynamics [8–10]. Furthermore, accumulating evidence indicates that not only PD-L1-expressing tumor cells, as quantified by the tumor proportion score (TPS), but also PD-L1-expressing immune cells, referred to as the combined positive score (CPS), play an important role in predicting the response to ICIs [11–13]. The KEYNOTE-059 cohort 1 trial revealed that the CPS might improve the prediction of gastric cancer patients potentially benefiting from pembrolizumab, demonstrating its diagnostic utility [14]. Concurrently, the potential role of tumor-infiltrating PD-L1-expressing immune cells, especially lymphocytes and macrophages, has received increased research attention for improving ICI-based therapies. The recent most plausible explanation for this association may be that PD-L1-expressing immune cells maintain immunological self-tolerance by suppressing self-reactive lymphocytes such as regulatory T cells (Tregs) and M2 macrophages in the TME, suggesting potential therapeutic implications in various cancers [15, 16]. However, the prognostic significance and predictive role of circulating PD-L1-expressing immune cells in the peripheral blood for responses to ICIs remain unclear. Moreover, we previously reported that a reduction in sPD-L1 levels after four cycles of ICI treatment was significantly correlated with tumor regression in patients with non-small cell lung cancer (NSCLC) and gastric cancer, but not with overall survival (OS) [17].
To clarify these associations and suggest candidate biomarkers to improve patient selection and treatment outcome with ICIs, the aim of the present study was to identify prognostic factors in peripheral blood mononuclear cell (PBMC) subsets, including CD3+, CD4+, and CD8+ T cells; CD20+ B cells; and CD14+ monocytes, and assessed the PD-L1-expressing subsets of each of these cells including naïve and memory T cell subsets as well as classical, intermediate and non-classical monocyte subsets in patients with various cancer types, along with their clinical implications in anti-PD-1 immunotherapy.
Patients and methods
Study subject recruitment
A total of 32 patients were recruited for this study. Fifteen patients had NSCLC, 14 had gastric cancer, 1 had melanoma, 1 had parotid cancer, and 1 had bladder cancer. All patients had received treatment with an ICI (240 mg nivolumab intravenously every 2 weeks, or 200 mg pembrolizumab intravenously every 3 weeks) at Showa University Hospital from January 2017 to August 2019. Patient characteristics, immunohistochemical analysis of PD-L1 in the tumor tissue during pathological diagnosis, and the number of PD-L1+ cells in PBMC subsets during pretreatment were evaluated. OS was determined as the time from diagnosis to the final follow-up or death. Target lesions were assessed via computed tomographic imaging and the responses to ICIs were assessed in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 [18]. The percentage of PD-L1+ cells in PBMC subsets was also determined in blood samples collected from two healthy volunteers (control samples) for comparison. Written informed consent was obtained from all participants prior to specimen collection, and the study was approved by the Ethics Committee of Showa University Hospital and adhered to the tenets of the 1975 Declaration of Helsinki.
Assessment of tumor PD-L1 levels
Immunohistochemical staining of tumor PD-L1 was performed as previously described [17]. In brief, formalin-fixed paraffin-embedded tissue samples were prepared from biopsy specimens of the patients for pathological diagnosis. As a companion diagnostic method, PD-L1 immunohistochemistry 28-8 PharmaDX and PD-L1 IHC 22C3 PharmaDX kits were used in accordance with the manufacturer instructions (Dako, Glostrup, Denmark). PD-L1 expression was quantitatively evaluated as the TPS.
PBMC preparation
Blood samples were obtained prior to ICI treatment and stored in BD Vacutainer CPT Cell Preparation Tubes containing sodium heparin (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). The supernatant was separated via centrifugation at 1600 × g for 20 min at 20 °C, and the pellet was resuspended in phosphate-buffered saline (PBS) and washed once with PBS. The separated PBMCs were stored in BAMBANKER (GC LYMPHOTEC, Tokyo, Japan) at − 80 °C and then in liquid nitrogen until further analysis.
Staining and flow cytometry analysis
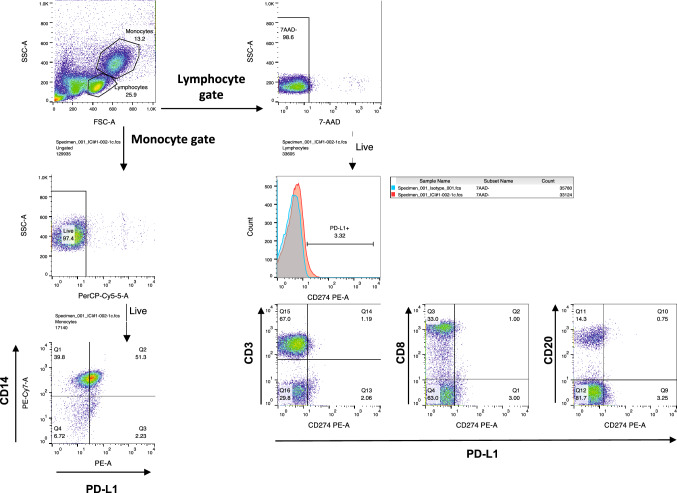
PD-L1+ cells in PBMC subsets were enumerated using a BD LSRFortessa X-20 Cell Flow Cytometer (Becton, Dickinson and Company). For individual assessments, 1 × 107 PBMCs were resuspended in PBS containing 2% fetal bovine serum (FBS), incubated with Human BD FC Block (Becton, Dickinson and Company) for 10 min at 25 °C, and stained with 7-AAD, PD-L1 phycoerythrin (PE)-conjugated antibody (PE Mouse Anti-Human CD274), and with the following antibodies on ice for 30 min: anti-CD3 BD Horizon BV480-conjugated antibody (Mouse Anti-Human CD3), anti-CD8 allophycocyanin (APC)-conjugated antibody (Mouse Anti-Human CD8), anti-CD4 PE-Cy7-conjugated antibody (Mouse Anti-Human CD4), anti-CD45RA APC-conjugated antibody (Mouse Anti-Human CD45RA), anti-CCR7 BV421-conjugated antibody (Rat Anti-Human CCR7), anti-CD20 fluorescein isothiocyanate (FITC)-conjugated antibody (Mouse Anti-Human CD20), anti-CD14 PE-Cy7-conjugated antibody (Mouse Anti-Human CD14), and anti-CD16 FITC-conjugated antibody (Mouse Anti-Human CD16), which targeted CD3+, CD8+, CD4+, CD45RA+, CCR7+ T cells, CD20+ B cells, or CD14+ and CD16+ monocytes, respectively (all used as supplied and obtained from Becton, Dickinson and Company). Thereafter, the cell suspension was washed twice in PBS with 2% FBS and detected at the respective wavelengths. A minimum of 50,000 events was acquired. The gating strategies used for flow cytometry were based on single-stained samples and isotype controls, and outlined in Fig. 2, Supplementary Fig. 1a, as well as Supplementary Fig. 2a, b. The data were analyzed using FlowJo version 10.5.3 (Tree Star, Inc., Ashland, OR, USA) software.
Fig. 2.
A schematic representation of gating strategy for PD-L1 expressing lymphocyte and monocyte subpopulations. Gating strategies for peripheral PD-L1-expressing CD14+ monocytes, CD3+ T cells, CD8+ T cells and CD20+ B cells
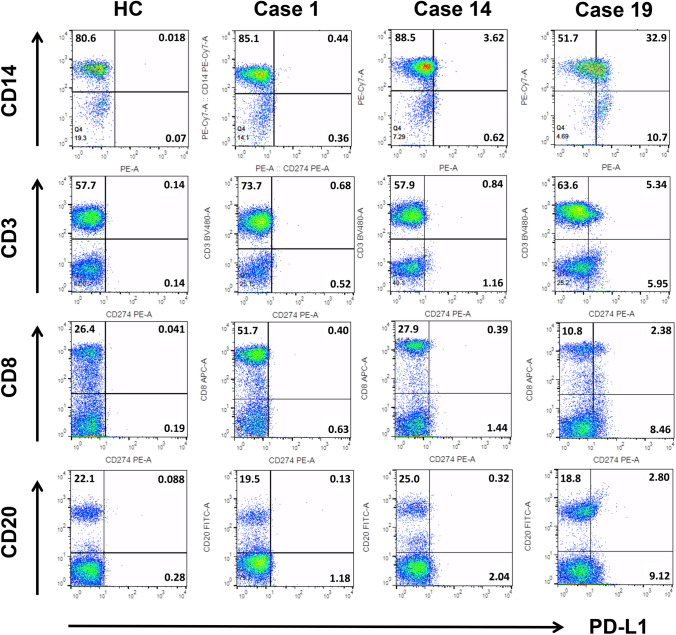
Fig. 1.
Lymphocyte and monocyte subsets, and their PD-L1 expression levels in peripheral blood. Representative flow cytometry dot plots for peripheral CD3+, CD8+ T cells, CD20+ B cells, and CD14+ monocytes (vertical axis) and respective PD-L1+ cells (horizontal axis) from healthy controls (HC) and patients (Case 1, 14, and 19). Lymphocyte and monocyte subpopulations were gated on the basis of forward and side scattering, and their subsets were determined through cell surface markers. The percentage of double-positive cells is shown in the upper right area. PD-L1 programmed death-ligand 1; CD cluster of differentiation
Statistical analysis
An unpaired Student's t test was performed for between-group comparisons. Spearman’s rank correlation analysis was performed for linear correlation analysis. Statistical analysis was performed using Microsoft Excel (Microsoft Co., Redmond, WA, USA) and JMP version 14.0 (SAS Institute, Cary, NC, USA). The results are presented as mean ± standard deviation values. All tests were two sided, and a p value of less than 0.05 was considered statistically significant.
Results
Association between PBMC subsets and patient outcomes
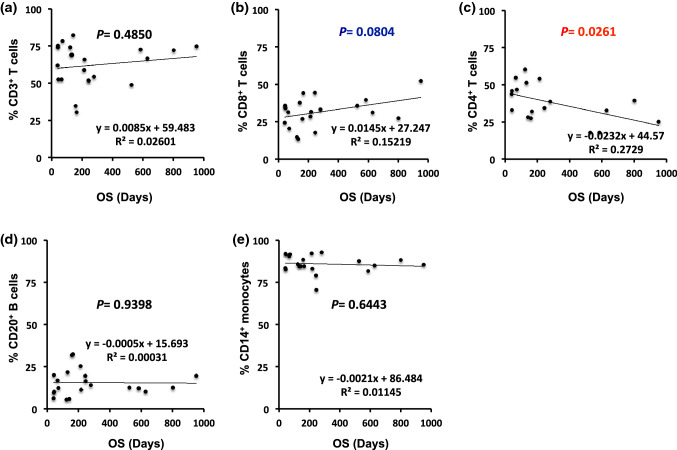
We assessed the potential prognostic predictors in different PBMC subsets. We enumerated CD3+, CD4+ and CD8+ T cells; CD20+ B cells; and CD14+ monocytes or the percentage of PD-L1+ cells of the respective subsets in 21 patients, including 11 with NSCLC, 9 with gastric cancer, and 1 with bladder cancer before ICI treatment, along with 2 healthy controls via flow cytometry (Fig. 1). Particularly, the configurations for initial gating were different and were set independently for lymphocytes and monocytes subsets. Therefore, the percentages of lymphocyte and monocyte subsets are represented as the proportion of cells within the independent lymphocyte and monocyte gates, respectively (Fig. 2). Four patients with NSCLC and all nine with gastric cancer were treated with nivolumab, and seven patients with NSCLC and the one patient with bladder cancer were treated with pembrolizumab, as previously described [17]. The clinicopathological characteristics of patients, including patient responses, and OS, the percentages of total or PD-L1+ cells in each subset are summarized in Tables 1 and 2, respectively. As mentioned above, we set different gates independently for lymphocyte and monocyte subsets initially. The percentage of PD-L1+ CD14+ monocytes indicated the proportion of PD-L1+ cells in total CD14+ monocytes (Table 2). We initially analyzed the correlation between the number of respective PBMC subsets and OS. No association was observed between the number of CD3+ T cells, CD20+ B cells, and CD14+ monocytes and OS (r = 0.1613, p = 0.4850; r = − 0.0175, p = 0.9398; r = − 0.1070, p = 0.6443; Fig. 3a, d, and e, respectively) among 21 patients before anti-PD-1 blockade therapy. An increased proportion of CD8+ T cells tended to be positively associated with OS, although the correlation was not statistically significant (r = 0.3901, p = 0.0804, Fig. 3b). These results suggest that changes in the distribution of CD8+ T cells may result from secondary factors influenced by unknown transitions in some PBMC subsets. Therefore, this finding prompted us to further investigate the percentages of CD4+ T cells in 18 patients whose PBMC samples were available for additional experiments. Predictably, we found an inverse correlation between the percentage of CD4+ T cells and OS (r = − 0.5224, p = 0.0261, Fig. 3c).
Table 1.
Clinicopathological features, percentages of peripheral blood mononuclear cell subsets, and overall survival (OS) for all patients
| Case no | Sex | Age, | Cancer type | Stage | ICI | PD-L1 IHC (%) |
CD3+ T cells (%) |
CD4+ T cells (%) |
CD8+ T cells (%) |
CD20+ B cells (%) |
CD14+ monocytes (%) |
BOR | OS (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 78 | NSCLC | IV | NIVO | 20–30 | 74.38 | 24.90 | 52.10 | 19.63 | 85.54 | PR | 952 |
| 2 | F | 70 | NSCLC | IVB | NIVO | 50–60 | 30.32 | N/A | 43.82 | 32.28 | 84.61 | SD | 167 |
| 3 | M | 67 | NSCLC | IIIAR | NIVO | 70–80 | 72.37 | 31.80 | 39.39 | 12.12 | 81.65 | PR | 586 |
| 4 | M | 63 | GC | IIIAR | NIVO | N/A | 73.89 | 17.70 | 14.57 | 5.55 | 85.63 | PD | 125 |
| 5 | M | 74 | GC | IV | NIVO | N/A | 82.02 | 60.10 | 37.51 | 5.88 | 84.61 | PD | 144 |
| 6 | M | 68 | GC | IV | NIVO | N/A | 51.61 | N/A | 17.48 | 16.56 | 70.50 | SD | 248 |
| 7 | M | 67 | NSCLC | IIIA | NIVO | 50–60 | 51.81 | 28.00 | 44.23 | 19.59 | 79.08 | PD | 246 |
| 8 | F | 68 | GC | IV | NIVO | N/A | 52.38 | 34.20 | 31.30 | 16.98 | 90.30 | PD | 68 |
| 9 | M | 66 | GC | IIIB | NIVO | N/A | 65.62 | N/A | 31.41 | 11.42 | 82.90 | PD | 220 |
| 10 | M | 60 | GC | IIIBR | NIVO | N/A | 61.75 | 54.50 | 24.24 | 6.25 | 83.30 | PD | 43 |
| 11 | F | 49 | GC | IIIB | NIVO | N/A | 78.21 | 43.60 | 20.27 | 12.26 | 91.50 | PD | 74 |
| 12 | F | 75 | GC | IV | NIVO | N/A | 54.03 | 46.60 | 33.02 | 13.97 | 92.65 | SD | 281 |
| 13 | F | 57 | GC | IV | NIVO | N/A | 74.80 | 38.60 | 35.13 | 9.59 | 82.60 | PD | 45 |
| 14 | M | 72 | NSCLC | IV | PEMBRO | 70–80 | 58.74 | 32.80 | 28.29 | 25.32 | 92.12 | PD | 216 |
| 15 | M | 71 | NSCLC | IV | PEMBRO | 60–70 | 71.84 | 53.90 | 27.07 | 12.45 | 88.05 | PR | 803 |
| 16 | M | 59 | NSCLC | IV | PEMBRO | 60–70 | 73.75 | 39.20 | 35.44 | 20.04 | 91.90 | PD | 45 |
| 17 | M | 64 | NSCLC | IV | PEMBRO | 60–70 | 66.43 | 45.70 | 30.94 | 10.31 | 84.99 | PR | 630 |
| 18 | M | 70 | NSCLC | IV | PEMBRO | 70–80 | 52.33 | 32.50 | 33.76 | 10.26 | 91.60 | SD | 48 |
| 19 | M | 71 | NSCLC | IV | PEMBRO | 90 | 68.94 | 51.20 | 13.18 | 21.60 | 84.60 | PD | 133 |
| 20 | F | 70 | BLDC | IV | PEMBRO | N/A | 48.68 | 17.70 | 35.60 | 12.73 | 87.52 | PR | 527 |
| 21 | M | 68 | NSCLC | IVB | PEMBRO | 10–20 | 34.46 | 27.30 | 26.76 | 31.85 | 88.32 | PR | 161 |
BLDC bladder cancer; BOR best overall response; F female; GC gastric cancer; ICI immune checkpoint inhibitor; IHC immunohistochemistry; M male; NIVO Nivolumab; NSCLC non-small cell lung cancer; PEMBRO pembrolizumab; R recurrence
Table 2.
Percentage of PD-L1-expressing peripheral blood mononuclear cell (PBMC) subsets and overall survival (OS) among all patients
| Case no | PD-L1+CD3+ T cells (%) |
PD-L1+CD4+ T cells (%) |
PD-L1+CD8+ T cells (%) |
PD-L1+CD20+ B cells (%) |
PD-L1+CD14+ monocytes (%) | PD-L1+CD14high CD16− monocytes (%) |
PD-L1+CD14low CD16+ monocytes (%) |
PD-L1+CD14high CD16+ monocytes (%) |
OS (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.68 | 2.76 | 0.40 | 0.13 | 0.44 | 32.80 | 18.10 | 25.0 | 952 |
| 2 | 0.82 | N/A | 0.42 | 0.48 | 4.01 | N/A | N/A | N/A | 167 |
| 3 | 0.87 | 14.70 | 0.39 | 0.12 | 1.45 | 46.80 | 14.70 | 35.70 | 586 |
| 4 | 0.69 | 2.33 | 0.069 | 0.075 | 0.43 | 17.30 | 7.79 | 36.00 | 125 |
| 5 | 0.82 | 2.07 | 0.21 | 0.046 | 3.11 | 30.30 | 8.40 | 42.90 | 144 |
| 6 | 0.71 | N/A | 0.28 | 0.16 | 21.3 | N/A | N/A | N/A | 248 |
| 7 | 1.01 | 9.33 | 0.63 | 0.19 | 4.18 | 21.80 | 1.85 | 28.30 | 246 |
| 8 | 1.48 | 2.67 | 0.80 | 0.48 | 31.2 | 34.00 | 14.50 | 49.80 | 68 |
| 9 | 2.32 | N/A | 1.21 | 0.62 | 34.3 | N/A | N/A | N/A | 220 |
| 10 | 2.25 | 1.10 | 1.44 | 0.26 | 44.6 | 66.30 | 18.40 | 79.30 | 43 |
| 11 | 3.21 | 0.80 | 0.87 | 0.56 | 26.4 | 13.40 | 5.22 | 34.10 | 74 |
| 12 | 0.73 | 0.71 | 0.12 | 0.37 | 1.25 | 3.97 | 8.03 | 7.72 | 281 |
| 13 | 0.60 | 0.69 | 0.13 | 0.31 | 1.60 | 7.80 | 1.69 | 11.10 | 45 |
| 14 | 0.84 | 4.13 | 0.39 | 0.32 | 3.62 | 60.90 | 9.68 | 69.90 | 216 |
| 15 | 0.74 | 1.38 | 0.27 | 0.15 | 1.25 | 26.60 | 10.20 | 46.50 | 803 |
| 16 | 1.25 | 1.87 | 0.44 | 0.24 | 3.30 | 32.40 | 1.95 | 38.30 | 45 |
| 17 | 1.43 | 2.07 | 0.24 | 0.31 | 3.99 | 18.90 | 12.10 | 29.60 | 630 |
| 18 | 2.73 | 3.28 | 1.96 | 0.16 | 20.1 | 42.40 | 20.20 | 70.40 | 48 |
| 19 | 5.34 | 1.94 | 2.38 | 2.80 | 32.9 | 40.90 | 20.90 | 59.90 | 133 |
| 20 | 0.68 | 2.25 | 0.20 | 0.43 | 3.12 | 10.80 | 1.34 | 6.28 | 527 |
| 21 | 1.06 | 2.31 | 0.26 | 0.85 | 8.02 | 17.10 | 1.87 | 25.90 | 161 |
Fig. 3.
Linear correlation between peripheral blood mononuclear cell subsets and overall survival (OS). Association between the percentage of peripheral a CD3+ T cells, b CD8+ T cells, c CD4+ T cells, d CD20+ B cells or e CD14+ monocytes and OS. Each dot represents one specimen from a total of 21 or 18 patients
PD-L1-expressing cells in PBMC subsets associated with different clinicopathological characteristics and clinical responses
As shown in Table 3, no significant correlation was observed between the median percentage of PD-L1+ cells in respective PBMC subsets and the patients’ sex, age, cancer type, pathological stage, and response to anti-PD-1 blockade therapy. Interestingly, an increased proportion of PD-L1+ CD14+ monocytes tended to be positively associated with progressive disease (PD) of the best overall response, although the association was not statistically significant (p = 0.083, Table 3). Therefore, a considerable improvement of guidelines for the assessment of response to immunotherapies, for instance, a modified RECIST 1.1 for immune-based therapeutics (iRECIST) [19], will be needed to open a window into the investigation of predictive markers for ICI response.
Table 3.
Association between clinical factors and the number of PD-L1-expressing peripheral blood mononuclear cell (PBMC) subsets
| Variable | All, n (%) | PD-L1+CD3+ Tcells (%) |
p value | PD-L1+CD8+ Tcells (%) |
p value | PD-L1+CD20+ Bcells (%) |
p value | PD-L1+CD14+ monocytes (%) |
p value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | 15 (71.4) | 1.01 | 0.549 | 0.39 | 0.213 | 0.19 | 0.847 | 3.99 | 0.863 |
| Female | 6 (28.6) | 0.78 | 0.31 | 0.46 | 3.57 | |||||
| Age | < 70 Years | 12 (57.1) | 1.16 | 0.751 | 0.42 | 0.541 | 0.29 | 0.556 | 6.10 | 0.259 |
| ≥ 70 Years | 9 (42.9) | 0.82 | 0.39 | 0.32 | 3.11 | |||||
| Cancer type | NSCLC | 11 (52.4) | 1.01 | 0.63 | 0.40 | 0.449 | 0.24 | 0.471 | 3.99 | 0.179 |
| Other | 10 (47.6) | 0.78 | 0.25 | 0.34 | 12.21 | |||||
| Clinical stage | III | 6 (28.6) | 0.82 | 0.603 | 0.28 | 0.649 | 0.31 | 0.394 | 3.62 | 0.319 |
| IV | 15 (71.4) | 1.63 | 0.75 | 0.23 | 15.29 | |||||
| Observed Response | PD | 10 (47.6) | 1.25 | 0.150 | 0.63 | 0.226 | 0.31 | 0.396 | 4.18 | 0.083 |
| PR and SD | 11 (52.4) | 0.78 | 0.275 | 0.24 | 3.56 |
Patients were divided on the basis of the median percentage of indicated subsets
NSCLC non-small cell lung cancer; PD progressive disease; SD stable Disease
Association between PD-L1+ PBMC subsets and patient outcomes
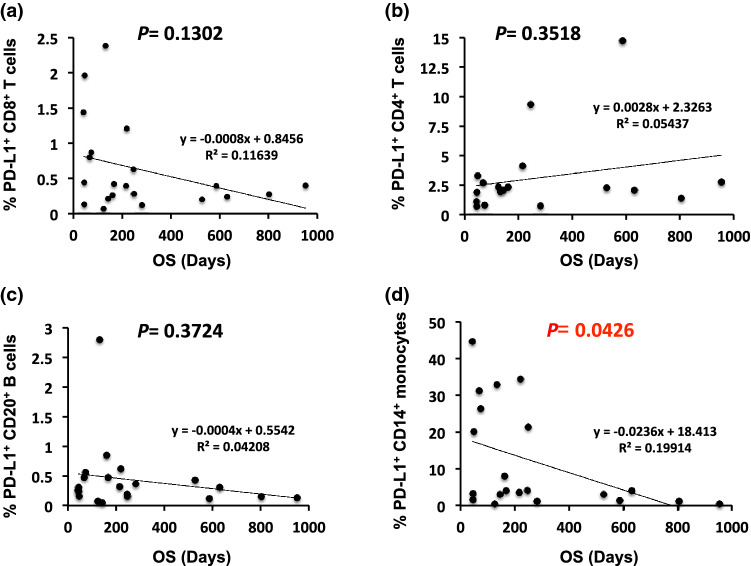
Among the various subsets examined, an increase in the percentage of PD-L1+ CD14+ monocytes was significantly correlated with a shorter OS (r = − 0.4463, p = 0.0426, Fig. 4d), whereas no significant association between OS and the number of PD-L1+ CD8+ T cells, PD-L1+ CD4+ T cells, or PD-L1+ CD20+ B cells (r = − 0.3412, p = 0.1302; r = 0.2332, p = 0.3518; r = − 0.2051, p = 0.3724, respectively) was observed among these 18 or 21 patients before anti-PD-1 blockade therapy (Fig. 4a, b, c).
Fig. 4.
Linear correlation between PD-L1-expressing peripheral blood mononuclear cell subsets and overall survival (OS). Association between the percentage of peripheral PD-L1-expressing a CD8+ T cells, b CD4+ T cells, c CD20+ B cells, and d CD14+ monocytes and OS. Each dot represents one specimen from a total of 18 or 21 patients
To extend these findings to PD-L1+ T cell subsets, we investigated the possible association between patient outcomes and the percentages of PD-L1+ naïve or memory T cells. As shown in Supplementary Table 1, we analyzed the percentages of PD-L1+ CD4+ and PD-L1+ CD8+ T cell subsets in 18 patients based on expression of CCR7 in combination with CD45RA expression, which is a marker to classify naïve (CD45RA+CCR7+), central memory (CD45RA−CCR7+), effector memory (CD45RA−CCR7−), and terminal differentiated effector memory (CD45RA+CCR7−) T cell subsets [20, 21]. No significant correlations were observed between patients’ OS and PD-L1+ CD4+ naïve, central memory, effector memory, or terminally differentiated effector memory T cells (r = 0.0005, p = 0.9984, r = 0.2793, p = 0.2617; r = 0.2774, p = 0.2651; r = 0.0001, p = 0.9997, respectively; Supplementary Table 1 and Supplementary Fig. 2C a–d). Likewise, no significant correlations were detected between OS and PD-L1+ CD8+ T cell subsets regarding naïve, central memory, effector memory, or terminally differentiated effector memory T cells (r = − 0.3168, p = 0.2002; r = − 0.2329, p = 0.3524; r = − 0.2647, p = 0.2885; r = − 0.1811, p = 0.4721, respectively, Supplementary Table 1 and Supplementary Fig. 2C e–h).
In the past decade, CD16+ monocytes have been subdivided into two subsets, intermediate (CD14high) and non-classical (CD14low), whereas CD16− monocytes are considered the classical subset [22]. The intermediate and non-classical monocytes are considered to differentiate into M2 macrophages, which have a tumor-promoting function [23]. Therefore, we next evaluated whether these monocyte subsets with PD-L1+ expression could be correlated with patient outcomes. Although the PD-L1+ intermediate monocytes showed a tendency to be associated with patient outcomes among these three subsets, there was no significant correlation observed between OS and the percentages of PD-L1+ classical monocytes, PD-L1+ intermediate monocytes, or non-classical monocytes (r = − 0.0873, p = 0.7303; r = − 0.3148, p = 0.2032; and r = − 0.1618, p = 0.5213, respectively, Table 2 and Supplementary Fig. 2d).
To compensate for the relatively low sample size, which may have prevented detecting a significant effect, we recruited 11 additional patients to confirm whether the significant positive correlation between the percentages of PD-L1+ CD14+ monocytes and OS could be sustained in this setting. Including all 32 patients, we still found a significant association between the percentage of PD-L1+ CD14+ monocytes and the patients’ OS (higher percentage leads to shorter OS; r = − 0.3622, p = 0.04164; Supplementary Table 2 and Supplementary Fig. 1B). However, this analysis per cancer type revealed no significant correlation between the percentage of CD14+ PD-L1+ monocytes and the overall survival of patients: 15 NSCLC, p = 0.10459; 14 GC, p = 0.38005 (Supplementary Fig. 1b). Therefore, we could not exclude the possibility of a selection bias. For instance, our data set revealed that GC patients showed a shorter OS than that of NSCLC patients (p = 0.0307). For validating the results, this study should be conducted on large number of patients.
Collectively, these findings suggest that an increase in the percentage of PD-L1+ CD14+ monocytes might help to predict a poor prognosis before anti-PD-1 blockade therapy in patients with various cancers.
Inverse correlation between the percentage of PD-L1+CD14+monocytes and CD8+ T cells among PBMC subsets
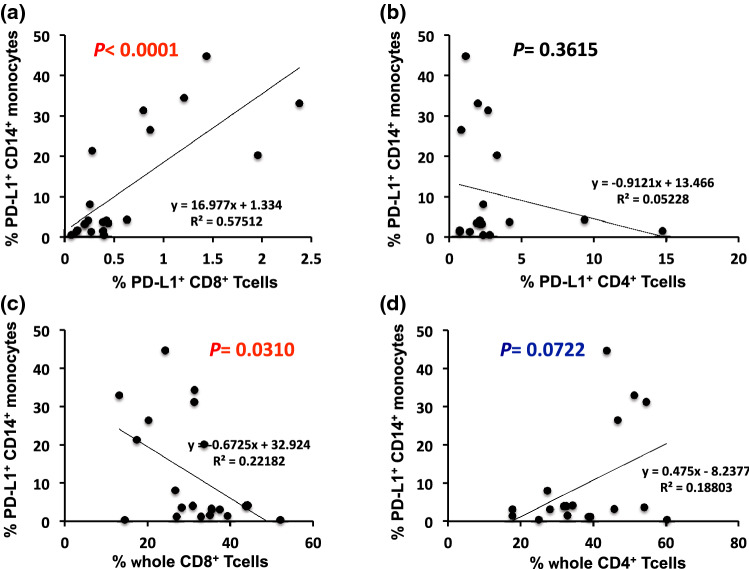
Given accumulating evidence that the number of immune cells can effectively predict the response to anti-PD-1 blockade therapy, PD-L1-positive tumor-infiltrating immune cells have been implicated for ICI therapeutics [11]. This prompted us to investigate the potential correlation between the percentage of PD-L1+ CD14+ monocytes and PD-L1+ CD3+ or PD-L1+ CD8+ T cells among PBMC subsets of our patients. A significant positive correlation was observed between the percentages of PD-L1+ CD14+ monocytes and PD-L1+ CD8+ T cells (r = 0.7584, p < 0.0001, Fig. 5a) or PD-L1+ CD3+ T cells (r = 0.7126, p = 0.0003, data not shown). However, there was no correlation between PD-L1+ CD14+ monocytes and PD-L1+ CD4+ T cells (r = − 0.2286, p = 0.3615, Fig. 5b). In contrast with PD-L1+ CD8+ T cells, the percentage of whole CD8+ T cells among all PBMC subsets was negatively correlated with the percentage of PD-L1+CD14+ monocytes (r = − 0.4710, p = 0.0312, Fig. 5c), supporting the tendency found for the reduction in the percentages of CD8+ T cells with a poor OS (Fig. 3b). Notably, a weak but nonsignificant correlation was observed between the percentages of whole CD4+ T cells and PD-L1+CD14+ monocytes (r = 0.4336, p = 0.0722, Fig. 5d). These results suggest that anti-PD-1 blockade therapy-related patient outcomes may be determined, at least in part, to changes in the relative proportions of CD4+ T cell subsets rather than CD8+ T cells.
Fig. 5.
Linear correlation between PD-L1-expressing CD14+ monocytes and T cell subsets. Association between the percentage of peripheral PD-L1-expressing CD14+ monocytes and PD-L1-expressing a CD8+ T cells or b CD4+ T cells, and peripheral PD-L1-expressing CD14+ monocytes and whole c CD8+ T cells or d CD4+ T cells. Each dot represents one specimen from a total of 21 or 18 patients
Discussion
Promotion of T cell-specific immune responses is a central feature of cancer immunotherapy. Effector cytotoxic T cells (CTLs) express the CD8 antigen and play an essential role in the direct elimination of cancer cells. Accumulating evidence has clarified the mechanism underlying cancer cell immune resistance through which the functions of CTLs are suppressed by tumor stromal cells, including cancer-associated fibroblasts, Tregs, and tumor-associated macrophages (TAMs) in the TME, in a phenomenon known as “immune exhaustion” [24, 25]. PD-1/PD-L1 blockade therapy is performed primarily to prevent CTL suppression, and the number of infiltrating CD8+ T cells in the tumor, and PDL-1 expression in the TME have been proposed as candidate biomarkers of immunotherapy responses [12, 26]. Moreover, considering the recent success in using the CPS as a companion diagnostic tool for predicting responses to pembrolizumab, PD-L1 expression in either the tumor membrane or tumor-infiltrating lymphocytes and monocytes has emerged as a potential predictor of the treatment response [11]. However, the clinical implication of each mononuclear cell subset in the peripheral blood, including lymphocytes and monocytes, with respect to their predictive potential has remained unclear. This study shows the potential prognostic significance of patient PBMC subsets before ICI treatment by assessing the correlation between OS and different PBMC subsets or the PD-L1+ PBMC subset in samples from patients with various cancer types. The most prominent feature of our results is that high rates of PD-L1-expressing CD14+ monocytes detected in the peripheral blood before treatment of nivolumab/pembrolizumab displayed prognostic effects based on an association with poor patient survival.
Most peripheral blood CD14+ monocytes differentiate into macrophages in the TME [27]. Macrophages are classified into at least two subsets as tumoricidal macrophages and tumor-promoting macrophages or TAMs, also referred to as M1 and M2 macrophages, respectively. There is a strong association between poor patient survival and high TAM infiltration in various cancers, including lung, gastric, and bladder cancers [28, 29]. Although it remains controversial whether TAM heterogeneity originates from independent lineages and/or environmental cues [30], the correlation between PD-L1-expressing CD14+ monocytes in PBMCs and patient outcomes is of particular interest. Concurrent with the present results, a previous study reported that the number of PD-L1-expressing CD14+ monocytes was increased in patients with cervical cancer and intraepithelial neoplasia in comparison with that of healthy controls, suggesting the potential involvement of these factors in tumorigenesis [31]. One plausible explanation for our findings is that a large number of PD-L1+ TAMs might be mobilized at tumor sites when PD-L1+ monocytes emerge in PBMC subsets.
Another remarkable finding of this study is that the percentage of PD-L1-expressing CD14+ monocytes was inversely correlated with the percentage of CD8+ T cells. In addition, a weak positive correlation was observed between percentages of PD-L1-expressing CD14+ monocytes and CD4+ T cells in PBMC subsets. A reduction in the percentage of CD8+ T cells displayed a limited tendency to be positively associated with patient survival, whereas the percentage of CD4+ T cells showed a significant negative correlation with patient survival. Thus, we presume that the relative increase in some CD4+ T cell subsets, including Tregs, might influence patient survival. Tregs, as an immunosuppressive subset of CD4+ T cells, are central players in cancer immunity; therefore, targeting Tregs has been an attractive method to potentiate immune therapy [32, 33]. Regarding infiltrating lymphocytes in the tumor tissue, an increasing number of FoxP3+ Tregs has been significantly correlated with a poor patient prognosis in various tumors, whereas a high CD8+/FoxP3+ Tregs ratio was significantly associated with improved OS in certain cancers [34]. Similarly to the tumor tissue, an increase in the number of CD4+ Tregs in PBMC subsets might result in a relative reduction in the number of CD8+ subsets. However, in our experimental conditions, we observed no correlation between the percentage of CD4+/FOXP3+ Tregs and OS (r = 0.0430, p = 0.9526, Supplementary Fig. 3, Supplementary Table 3 and Supplementary Materials and Methods). Therefore, the predictive importance of the CD4+/FOXP3+ Tregs before ICI treatment might not lie on their presence in PBMC. Additionally, recent evidence suggests that the biological interaction between T cells and monocytes results in a significant proportion of cell doublets in flow cytometry [35]. Further improvements in evaluation focusing on CD4+ and/or PD-L1+ CD14+ PBMC subsets will clarify their predictive importance for poor patient outcomes before ICI treatment.
In this study, we mainly focused on the clinical significance of PD-L1-expressing PBMC subsets for patients with several cancer types receiving treatment with anti-PD-1 antibodies. The following were the limitations of this study: the sample size was relatively small; the study relied on the evaluation of mixed samples from patients with several cancer types who were undergoing ICI treatment, implicating the focus was not on a single cancer. Nonetheless, to our knowledge, this is the first study to report the prognostic significance of the distribution of PD-L1-expressing subsets in circulating PBMCs, particularly CD14+ monocytes. The main advantage in identifying circulating levels of these subsets is that the blood is more accessible than tumor samples in a clinical setting, thereby offering a non-invasive biomarker to facilitate repeated testing and monitoring of the treatment response. Further studies on improving cell surface markers distinguishing PBMC subsets, including CD4+ lymphocyte and CD14+ monocyte subsets, are expected to reveal their prognostic impact and predictive factors of responses to ICI treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
KH and SW conceived and designed this study. KH, RO, YK, AH, HM, TI, YH, HA, TA, and SW collected samples and recorded the general data and patient indications. MS, MW, and RO prepared the samples. MS and RO performed all flow cytometry analyses. KA analyzed the data and wrote the first draft of the manuscript. JT, KY, TT, SK, and SW critically reviewed and revised the manuscript. All Authors reviewed and approved the final version of the manuscript.
Funding
No sponsor was involved.
Compliance with ethical standards
Conflict of interest
None of the Authors declares any conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The “Showa University Ethics Committee” approved the study with number 2165 and 2253.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 3.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 4.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 5.Zou W, Wolchok JD, Chen L. PD-L1 (B7–H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21(3):462–473. doi: 10.1007/s10147-016-0959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7–H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Can Res. 2006;66(7):3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Liu F, Liu L. Prognostic significance of PD-L1 in solid tumor: An updated meta-analysis. Medicine. 2017;96(18):e6369. doi: 10.1097/MD.0000000000006369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulangara K, Zhang N, Corigliano E, Guerrero L, Waldroup S, Jaiswal D, Ms MJ, Shah S, Hanks D, Wang J, Lunceford J, Savage MJ, Juco J, Emancipator K. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143(3):330–337. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 12.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell Infiltration and PD-L1. Can Res. 2015;75(11):2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41(10):868–876. doi: 10.1016/j.ctrv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 Trial. JAMA oncology. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown JM, Recht L, Strober S. The promise of targeting macrophages in cancer therapy. Clin Cancer Res. 2017;23(13):3241–3250. doi: 10.1158/1078-0432.CCR-16-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka A, Sakaguchi S. Targeting Treg cells in cancer immunotherapy. Eur J Immunol. 2019;49(8):1140–1146. doi: 10.1002/eji.201847659. [DOI] [PubMed] [Google Scholar]
- 17.Ando K, Hamada K, Watanabe M, Ohkuma R, Shida M, Onoue R, Kubota Y, Matsui H, Ishiguro T, Hirasawa Y, Ariizumi H, Tsurutani J, Yoshimura K, Tsunoda T, Kobayashi S, Wada S. Plasma levels of soluble PD-L1 correlate with tumor regression in patients with lung and gastric cancer treated with immune checkpoint inhibitors. Anticancer Res. 2019;39(9):5195–5201. doi: 10.21873/anticanres.13716. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 11) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litiere S, Dancey J, Chen A, Hodi FS, Therasse P, Hoekstra OS, Shankar LK, Wolchok JD, Ballinger M, Caramella C, de Vries EGE, group Rw iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 21.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 22.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 23.Lee HW, Choi HJ, Ha SJ, Lee KT. Kwon YG (2013) Recruitment of monocytes/macrophages in different tumor microenvironments. Biochem Biophys Acta. 1835;2:170–179. doi: 10.1016/j.bbcan.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol. 2019;234(6):8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 25.Kato T, Noma K, Ohara T, Kashima H, Katsura Y, Sato H, Komoto S, Katsube R, Ninomiya T, Tazawa H, Shirakawa Y, Fujiwara T. Cancer-associated fibroblasts affect intratumoral CD8(+) and FoxP3(+) T cells Via IL6 in the tumor microenvironment. Clin Cancer Res. 2018;24(19):4820–4833. doi: 10.1158/1078-0432.CCR-18-0205. [DOI] [PubMed] [Google Scholar]
- 26.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarif JC, Hernandez JR, Verdone JE, Campbell SP, Drake CG, Pienta KJ. A phased strategy to differentiate human CD14+monocytes into classically and alternatively activated macrophages and dendritic cells. Biotechniques. 2016;61(1):33–41. doi: 10.2144/000114435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE. 2012;7(12):e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laviron M, Boissonnas A. Ontogeny of tumor-associated macrophages. Front Immunol. 2019;10:1799. doi: 10.3389/fimmu.2019.01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Zhu W, Zhang X, Qu Q, Zhang L. Expression and clinical significance of programmed death-1 on lymphocytes and programmed death ligand-1 on monocytes in the peripheral blood of patients with cervical cancer. Oncol Lett. 2017;14(6):7225–7231. doi: 10.3892/ol.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Quiros J, Mahuron K, Pai CC, Ranzani V, Young A, Silveria S, Harwin T, Abnousian A, Pagani M, Rosenblum MD, Van Gool F, Fong L, Bluestone JA, DuPage M. Targeting EZH2 reprograms intratumoral regulatory T cells to enhance cancer immunity. Cell Rep. 2018;23(11):3262–3274. doi: 10.1016/j.celrep.2018.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7(11):880–887. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 34.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burel JG, Pomaznoy M, Lindestam Arlehamn CS, Weiskopf D, da Silva AR, Jung Y, Babor M, Schulten V, Seumois G, Greenbaum JA, Premawansa S, Premawansa G, Wijewickrama A, Vidanagama D, Gunasena B, Tippalagama R, deSilva AD, Gilman RH, Saito M, Taplitz R, Ley K, Vijayanand P, Sette A, Peters B. Circulating T cell-monocyte complexes are markers of immune perturbations. eLife. 2019 doi: 10.7554/eLife.46045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.