Abstract
Chimeric antigen receptor (CAR) T cell therapy, a type of adoptive cell therapy, has been successfully used when treating lymphoma malignancies, but not nearly as successful in treating solid tumors. Trophoblast cell surface antigen 2 (Trop2) is expressed in various solid tumors and plays a role in tumor growth, invasion, and metastasis. In this study, a CAR targeting Trop2 (T2-CAR) was developed with different co-stimulatory intercellular domains. T2-CAR T cells demonstrated a powerful killing ability in the presence of Trop2-positive cells following an in vitro assay. Moreover, T2-CAR T cells produced multiple effector cytokines under antigen stimulation. In tumor-bearing mouse models, the CD27-based T2-CAR T cells showed a higher antitumor activity. Additionally, more CD27-based T2-CAR T cells survived in tumor-bearing mice spleens as well as in the tumor tissue. CD27-based T2-CAR T cells were also found to upregulate IL-7Rα expression, while downregulating PD-1 expression. In conclusion, the CD27 intercellular domain can enhance the T2-CAR T cell killing effect via multiple mechanisms, thus indicating that a CD27-based T2-CAR T cell approach is suitable for clinical applications.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-020-02838-8.
Keywords: Trop2, CAR, Solid tumor, CD27
Background
Adoptive cell therapy (ACT) utilizes a patient’s own immune cells to treat diseases, such as cancer, chronic infection, and autoimmunity [1], with emerging preclinical data suggesting that ACT approaches provide multiple advantages relative to other cancer immunotherapies [2]. One implementation of ACT utilizes genetically modified T cells that express chimeric antigen receptors (CARs) target CD19, which is associated with B cell lymphoid malignancies [3]. Therefore, this therapy is called CAR T cell therapy and has been approved by the US Food and Drug Administration (FDA) for treating leukemia and lymphoma (tisagenlecleucel, CTL019). Recently, the use of CAR T cell therapy in solid tumors is also being explored [4].
In any immunotherapy, identifying an abnormal protein that only expressed on the surface of tumor cells, which is a tumor-specific antigen (TSA), is ideal, but identifying one that can serve as a CAR T cell target is rare. Alternatively, tumor-associated antigens (TAAs) that tend to be highly expressed on tumor cells, but less common on normal cells are instead utilized. In leukemia, the TAA CD19 has been successfully utilized as a CAR T cell target [5], while other TAAs, including GD2, MUC1, and Her2, have been examined in solid tumors [6]. Owing to the high heterogeneity associated with tumors, current studies examining CAR T cell targets have only involved in a portion of tumors. So, it is still necessary to identify additional targets for CAR T cell applications.
Trophoblast cell surface antigen 2 (Trop2) is a cell surface glycoprotein that is highly expressed in a variety of late-stage epithelial carcinomas, such as triple-negative breast cancer (TNBC) [7, 8], and has only been identified in normal trophoblast cells, prostate stem cells, and liver oval cells [9, 10]. A Trop2 mutation has been associated with gelatinous drop-like corneal dystrophy [11], and Trop2 has been shown to play an important role in cancer cell proliferation, migration, invasion, and metastasis [12]. Increased Trop2 expression is associated with a poor prognosis, increased tumor aggressiveness and metastasis, and decreased overall survival rate [13–15]. In previous studies, the administration of Trop2 antibody in the treatment of lung cancer, breast cancer, and urothelial carcinoma was shown to be safe and efficacious [8, 16–18]. Overall, these findings suggest that Trop2 may be a promising target for CAR T cell cancer therapy.
CAR T cells could specifically recognize and eliminate tumor cells in a major histocompatibility complex (MHC)-independent manner. The essential components of the CAR structure include an extracellular antigen binding domain, spacer domain such as a CD8 hinge, a transmembrane domain, and an intracellular signaling domain containing CD3ζ chains linked with co-stimulatory molecules. In these elements, the antigen-binding domain, which is often a single-chain fragment variant (scFv) of a monoclonal antibody, recognizes and specifically binds to the tumor antigen. Furthermore, the receptor also contains a co-stimulatory molecule domain that could promote CAR T cell activation with the cooperation of the CD3 signaling [19]. Moreover, these co-stimulatory molecules, which include CD28, CD27, ICOS, 4-1BB, or OX40, could enhance T cell proliferation and cytotoxic function [20]. When constructing CARs, different co-stimulatory domains could induce different downstream signaling. Thus, selecting a co-stimulatory domain that would enhance the killing ability of CAR T cells is essential. After antigen stimulation, CAR T cells are activated and release multiple cytokines that stimulate various immune responses to promote tumor elimination [21, 22]. While CAR T cells have been successful in treating hematologic malignancies, their efficacy in treating solid tumors is still limited by the paucity of specific antigens and the immunosuppressive microenvironment in solid tumors [23, 24].
CD27, which belongs to the TNFR superfamily, is constitutively expressed on both CD8 and CD4 T cells, natural killer T cells, and other immune cells[25]. CD27 co-stimulatory signaling promotes T cell activation, clonal expansion, and effector T cell differentiation, survival and memory. During T cell priming, the absence of CD27 signaling results in abortive T cell clonal expansion, dysfunctional CD8 T cell memory and antitumor responses[25]. When activated T cells undergo clonal expansion, CD27 co-stimulation counteracts apoptosis by increasing expression of the antiapoptotic molecule Bcl-xL [26, 27], downregulating the expression of FasL and decreasing the sensitivity to FasL-induced apoptosis [28]. Moreover, CD27 signaling enhances CAR T cell expansion, effector functions, and survival in vitro, as well as augmenting CAR T cell persistence and antitumor activity in vivo [29]. However, it remains unclear if CD27 signaling could enhance CAR T cell activity in a solid tumor.
In this study, Trop2-specific CAR (T2-CAR) T cells were developed with different co-stimulatory signaling molecules. The killing ability of T2-CAR T cells was then determined in both in vitro and in vivo models. The results revealed that T2-CAR T cells could serve as a potential treatment for Trop2-positive tumors, and that CD27 signaling may have a potential application in treating solid tumors with CAR T cell therapy.
Materials and methods
CAR construction
The sequence encoding the anti-Trop2 scFv antibody in the VL-VH orientation (Supplementary Fig. 1a) was based on the sequence of hRS7 [30], while the anti-human CD19 scFv was derived from a FMC-63 clone as previously described [31]. The constructed CARs contained a scFv, a human CD8a hinge domain, a transmembrane domain, a co-stimulatory intracellular signaling domain (varied), and a CD3ζ intracellular signaling domain. All of the constructed CARs were then cloned into the lentiviral vector-CSII. To generate the murine CARs, the same structures were employed, with the exception of the intracellular signaling domains being murine, and these CARs were cloned into the MIGR1 retroviral vector.
Lentivirus and Retrovirus production
For lentivirus production, HEK293T cells were cultured with DMEM medium and 8 × 106 cells were seeded per 10-cm dish. On the next day, the cells were transfected with CAR-expressing plasmids (12 μg), an encoding vesicular stomatitis virus (VSV-G) envelope protein plasmid pMD.2G (5 μg) and a packaging plasmid psPAX2 (10 μg) using the calcium phosphate method. Retrovirus production was performed in the same way, with the exception of the MIGR1 plasmid and pCL-Eco retrovirus packaging being utilized instead. The culturing medium was changed at 6 h post-transfection, with the viral supernatants were harvested 48 h later. For T cells transduction, 1 × 106 activated T cells were plated onto a 12-well plate coated with retronectin (Takara Bio), followed by the addition of 5 ml of fresh supernatant with 8 μg /ml polybrene (Sigma) and 10 ng/ml IL-2. The plates were then centrifuged for 90 min at 600 g and incubated at 37 °C overnight, with the medium changed to RPMI 1640 medium for culturing.
Human T cells and mouse T cells isolation and modification
Peripheral blood mononuclear cells (PBMCs) were isolated from the whole blood of healthy volunteer donors (Guangzhou Blood Center, Guangzhou) by using the Ficoll-Hypaque density gradient centrifugation methods. Primary human CD8+ T cells were negatively purified from the PBMCs using a MACS magnetic column with a human CD8 T lymphocyte enrichment set DM (BD-IMag™) according to the manufacturer's protocol. T cells were activated using Dynabeads® Human T-Expander CD3/CD28 (11141D, Thermo Fisher Scientific) and recombinant IL-2 at 10 ng/ml (R&D) for 2 d. The activated T cells were then infected with fresh lentiviral supernatants. The transduced T cells were continually cultured in RPMI 1640 (Invitrogen) with 10% fetal bovine serum (Gibco) for an additional 5 d in the presence of 10 ng/ml IL-2, 10 ng/ml IL-7 and 10 ng/ml IL-15. The transduction efficacy was determined with flow cytometry analysis (Supplementary Fig. 1b, c). Similarly, mouse CD8+ T cells generated from wild-type C57BL/6 female mice were purified with a mouse CD8+ T cell negative enrichment kit (eBioscience, USA). T cells were activated with immobilized anti-CD3e antibody (5 μg/mL, eBioscience) and anti-CD28 (2 μg/mL, eBioscience) antibodies in the presence of murine IL-2 (ProSpec) for 24 h and then were infected with retroviral supernatants followed by additional incubation for 2 d with 10 ng/ml murine IL-2. The transduction efficacy was determined with flow cytometry analysis (Supplementary Fig. 4).
Cell lines and cell culture
All cells lines, including MDA-MB-231, MDA-MB-231-fLuc, SKOV3, EC109, A549, A549-Trop2+, MCF7, BGC823, and 293 T, were cultured in high-glucose DMEM (Gibco, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin/streptomycin (Invitrogen). LLC-Trop2+, MC38-Trop2+, BxPC-3 and H1975 cells were maintained in conditioned RPMI 1640 (Invitrogen) containing 10% FBS (Gibco) and 100 U/ml penicillin/streptomycin. MDA-MB-231, SKOV3, EC109, A549, MCF7, BGC823, BxPC-3, H1975, MC38, LLC, and 293 T cells were obtained from ATCC (Manassas, VA, USA). A549-Trop2+ cells were established by infecting A549 with lentivirus carrying Trop2-IRES-GFP, followed by sorting GFPhigh cells (BD FACSAria II). MDA-MB-231-fLuc cells were established by infecting MDA-MB-231 cells with lentiviruses carrying luciferase-IRES-RFP, followed by sorting RFPhigh cells. LLC-Trop2+ and MC38-Trop2+ were established by infecting LLC and MC38 cells with recombinant retroviruses carrying Trop2-IRES-GFP moiety, followed by GFPhigh sorting. All cell lines were maintained in a humidified atmosphere containing 37 °C and 5% CO2.
In vitro cytotoxicity assay
The cytotoxic activity of the CAR T cells was evaluated using the CytoTox 96® Non-Radioactive Cytotoxicity Assay (G1781, Promega) following manufacture guideline. The lactate dehydrogenase (LDH) release was evaluated after 8 h in the supernatant with effector-to-target (E: T) ratios of 1:1, 2:1, 5:1, and 10:1.
Intracellular cytokine staining and flow cytometry analysis
T cells were co-cultured with target cells at a 2:1 ratio, with 2 × 106 cell/ml seeded in 24-well flat bottom tissue-culture plates at 37 °C and 5% CO2 for 16 h with RPMI-1640 plus 10% FBS. Brefeldin A inhibitor was added at 8 h after co-culture. Cells were harvested, and surface staining for anti-human CD3 was performed. The cells were then fixed, permeabilized, and stained with cytokine antibodies according to the manufacture’s guidelines (88–8824-00, eBioscience Intracellular Fixation & Permeabilization Buffer Set). Human T2-CAR T cells were detected with Alexa-Fluor 488 conjugated goat anti-mouse IgG(H + L) antibodies (A-11001, Invitrogen). For the detection of murine T2-mCAR T cells, the GFP within the retroviral MIGR1 vector was employed. The following Abs were used in flow cytometry analysis: PE-conjugated anti-human Trop2 (clone MR54, eBioscience), Alexa-Fluor 488 conjugated goat anti-mouse IgG(H + L) antibodies (A-11001, Invitrogen), Pacific blue conjugated mouse anti-human CD3 (clone UCHT1, Invitrogen), APC conjugated mouse anti-human IFN-γ (clone 4S.B3, Invitrogen), PE-Cyanine7 conjugated mouse anti-human TNF-α (clone MAb11, Invitrogen), and PE-conjugated mouse anti-human Granzyme-B(clone GB11, Invitrogen). The following Abs were used in mouse T cell detection: PE-conjugated anti-mouse TCR (clone H57-597, Invitrogen), PE-Cyanine7 conjugated anti-mouse CD8 (clone 53–6.7, Invitrogen), APC conjugated anti-mouse PD-1 (clone J43, Invitrogen), and PerCP-Cyanine5.5 conjugated anti-mouse CD127 (clone A7R34, Invitrogen). FACS analysis was performed with flow cytometry (LSR Fortessa, BD) and FlowJo software (FlowJo_V10, BD). An Annexin V-PE Apoptosis Detection Kit (BD Pharmingen) was used to evaluate apoptosis levels according to the manufacture’s guidelines.
Establishment of a tumor-bearing model
All studies complied with the ethical regulations established by the Institutional Animal Care and Use Committee of Sun Yat-Sen University (IACUC, SYSU). NCG mice (NOD/ShiLtJGpt-Prkdcem26Cd52Il2rgem26Cd22/Gpt, Nanjing GemPharmatech) were used to establish the human carcinoma tumor-bearing models. Female NCG mice (6–8 weeks old) were inoculated subcutaneously (s.c.) with 1 × 106 MDA-MB-231 cells. At 3 d, 1 × 106 transduced CAR T cells were adoptively transferred into the tumor-bearing mice via the tail vein. Tumor size was measured, and the tumor volume was calculated as follows: (major axis of tumor) × (minor axis of tumor)2/2. To observe the inhibition of the T2-CAR T cells in tumor metastasis in vivo. NCG mice were inoculated intravenously (i.v.) in the tail with 5 × 105 MDA-MB-231-fLuc cells. On day 3, the mice were intraperitoneally (i.p.) infused with 150 mg/kg of VivoGlo Luciferin (Promega). The mice were anesthetized, and the tumor was imaged using an in vivo imaging system (IVIS, PerkinElmer). Next, 1 × 106 CAR T cells were administered intravenously (i.v.) into the tumor-bearing mice. The mice were re-analyzed at 7 and 10 d using the IVIS imaging system with Living Image software (PerkinElmer). To assess the antitumor effects of mCAR T cells in the established MC38-Trop2+ mice tumor models, 6–8 weeks old female C57BL/6 mice were inoculated subcutaneously (s.c.) with 5 × 105 tumor cells in the right flank (0 d). At 7 d, 2 × 106 CAR T cells were injected intravenously (i.v.). In the LLC-Trop2+ model, C57BL/6 mice were inoculated (s.c.) with 4 × 106 tumor cells in right flank (0 d), and then injected (i.v.) with 2 × 106 CAR T cells at 7 d. In some experiments, mice were inoculated (s.c.) with 5 × 105 MC38-Trop2+ cells (0 d), and then injected (i.v.) with 1 × 106 CAR T cells at 14 d. On day 21, spleen cells and tumor‐infiltrating lymphocytes were harvested to assess the ratio and kinetics of the CAR T cells.
Immunohistochemistry
Tumors were formalin fixed, embedded in paraffin, and sectioned into 5 μm sections. The sections were then stained with anti-CD3 (ab5690, Abcam) according to the manufacture’s guidelines (DGSP-H12, DINGGUO), and images were obtained using an optical microscope (DM6000B, Leica).
Statistical Analysis
Data were analyzed using Prism (version 7.0, GraphPad) software and the results were presented as mean ± SEM where indicated. Statistical significance was determined by using a one-way ANOVA with Dunnett’s test or a two-way ANOVA analysis. Survival curves were calculated by implementing a Log-rank (Mantel-Cox) test. *p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 were considered significant.
Results
T2-CAR T cells specifically recognized and killed Trop2-positive cells
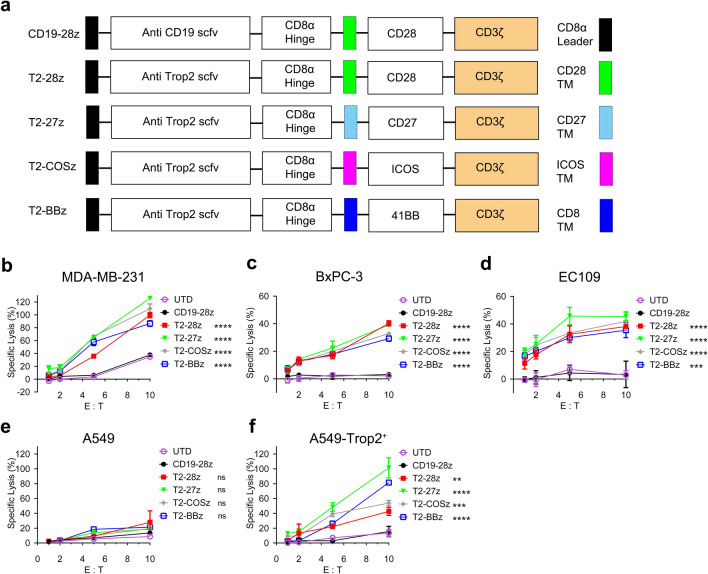
Four different T2-CARs including CD28 (named T2-28z CAR), CD27 (T2-27z CAR), ICOS (T2-COSz CAR), and 4-1BB (T2-BBz CAR), were constructed (Fig. 1a). Trop2 expression was evaluated using flow cytometry analysis on tumor cell lines (Supplementary Fig. 2). Trop2 highly expressed on MDA-MB-231, BxPC-3, and EC109, but not on A549 (Supplementary Fig. 2a-d). A549-Trop2+ cell was established with Trop2 over-expression into A549 cell (Supplementary Fig. 2e). LDH release assay was adopted to determine the tumor killing abilities of the T2-CAR T cells. All of the T2-CAR T cells, regardless of the co-stimulatory element, could efficiently kill MDA-MB-231, BxPC-3, and EC109 tumor cells (Fig. 1b-d). The enhanced T2-CAR T cell cytotoxicity against tumor cells was accompanied with an elevated E: T ratio. To test T2-CAR cell specificity, A549 and the A549-Trop2+ cells were compared in a lysis study. As expected, T2-CAR T cells killed the A549-Trop2+ cells, but not the A549 cells (Fig. 1e, f), which demonstrating the specificity and potent cytotoxicity of these T2-CAR T cells in targeting Trop2-positive cells.
Fig. 1.
T2-CAR T cells specifically recognize and kill Trop2-positive cells. a Schematic diagram representing different Trop2-specific CARs and CD19-28z CAR. b–f The cytotoxic activity of different second-generation T2-CAR T cells with different tumor cells. Statistical significance was determined relative to the CD19-28z group. All data are presented as mean ± SEM from experiments (one-way ANOVA with Dunnett’s test). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Cytokines produced by T2-CAR T cells
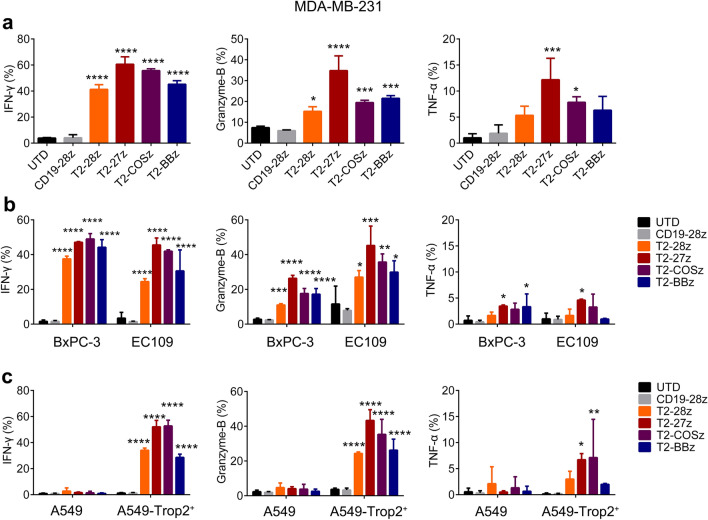
T2-CAR T cell effectors production was measured by flow cytometry with intracellular staining (Fig. 2). The production levels of IFN-γ, Granzyme-B and TNF-α were upregulated in all T2-CAR T cells co-cultured with MDA-MB-231, BxPC-3, or EC109 when compared to the negative control CD19-CAR T cells (Fig. 2a, b). Compared to A549-Trop2+ cells, lower level effectors production was observed when co-cultured with A549 cells (Fig. 2c), thus showing that T2-CAR T cells specifically modulate effectors production in the presence of Trop2 antigen stimulation. Comparing to T2-28z or T2-BB CAR T cell, T2-27z CAR T cell produced more effectors when co-cultured with any Trop2-positive cells (Fig. 2a-c). Furthermore, when compared to T2-COS CAR T cells, T2-27z CAR T cells showed better Granzyme-B production in all of the tumor cells, then with varied performance on IFN-γ and TNF-α amongst different tumor cells (Fig. 2a, b). Overall, T2-27z CAR T cells performed better on effectors production.
Fig. 2.
Cytokines produced by T2-CAR T cells. Cytokines released by a variety of T2-CAR T cells when co-cultured with MDA-MB-231 cells (a), BxPC-3 and EC109 cells (b), or A549/A549-Trop2+ cells (c) were analyzed. All data are displayed as a mean ± SEM (n = 3), with differences determined relative to the CD19-28z group (one-way ANOVA with Dunnett’s test). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
T2-27z CAR T cells exhibit antitumor activity in a NCG tumor-bearing model
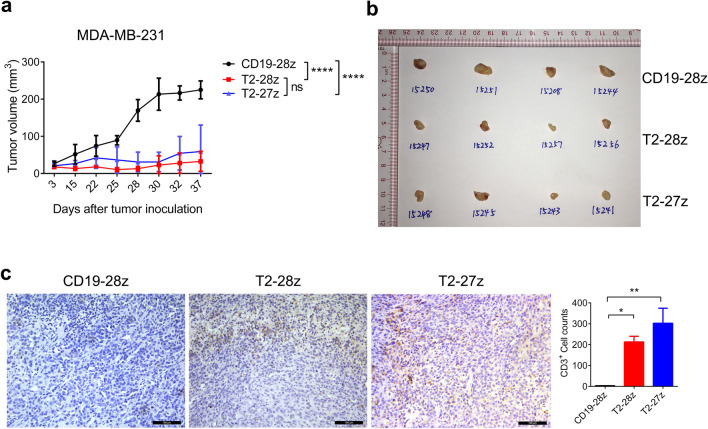
Previous studies have shown that CD28-based CAR T cells have been broadly used in leukemia therapy, and many solid tumors clinical studies [32]. 4-1BB has a better effect on the survival of CAR T cells than CD28, and also shows comparable therapeutic effects in the in vivo study and clinical practice [33, 34]. ICOS signaling domain enhances the persistence of CAR-expressing CD4 T cells and promotes TH17 cell function in tumor-bearing mice [35]. For T cells, CD27 co-stimulatory could promote survival of activated T cells and help establishment of the effector T cell pool [36]. T2-27z CAR T cells performed better on effectors production in the in vitro study (Fig. 2). However, whether CD27 signaling benefit for CAR T cells therapeutic effects still remain unknown. Therefore, T2-27z CAR T cells were selected to further investigate their therapeutic effect on solid tumors with in vivo models which compared to the effective of CD28-based CAR T cells. The results showed that the tumor growth was dramatically inhibited by both T2-28z and T2-27z CAR T cells with no significant difference in the NCG tumor-bearing model (Fig. 3a, b). This inhibition was maintained until day 37, two mice in CD19-28z CAR T cell treatment group displaying morbidity symptoms that met the experimental endpoint criterion. Findings showed that the T2-27z and T2-28z CAR T cells exhibited higher tumor infiltration compared to the CD19-28z CAR T cells (Fig. 3c). To determine whether T2-CAR T cells could inhibit tumor metastasis, MDA-MB-231 metastasis model was established via a tail vein injection. Bioluminescence imaging results showed that T2-27z and T2-28z CAR T cells could effectively suppress tumor growth and lung metastasis when comparing to the CD19-28z CAR T cells (Supplementary Fig. 3). Overall, both T2-27z and T2-28z CAR T cells showed potent antitumor efficacy in NCG tumor-bearing models.
Fig. 3.
Antitumor activities of T2-CAR T cells in a tumor-bearing NCG mouse model. a Tumor size was measured by digital calipers. b Tumor tissues were examined at 37 days postinoculation. c CAR T cells were detected by immunohistochemical staining with anti-human CD3 Ab (shown in brown), scale bar = 100 μm. All data are presented as mean ± SEM (N = 4 mice/group (two-way ANOVA)). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
T2-27z CAR T cells exhibit an enhanced antitumor activity in an immunocompetent tumor-bearing mice model
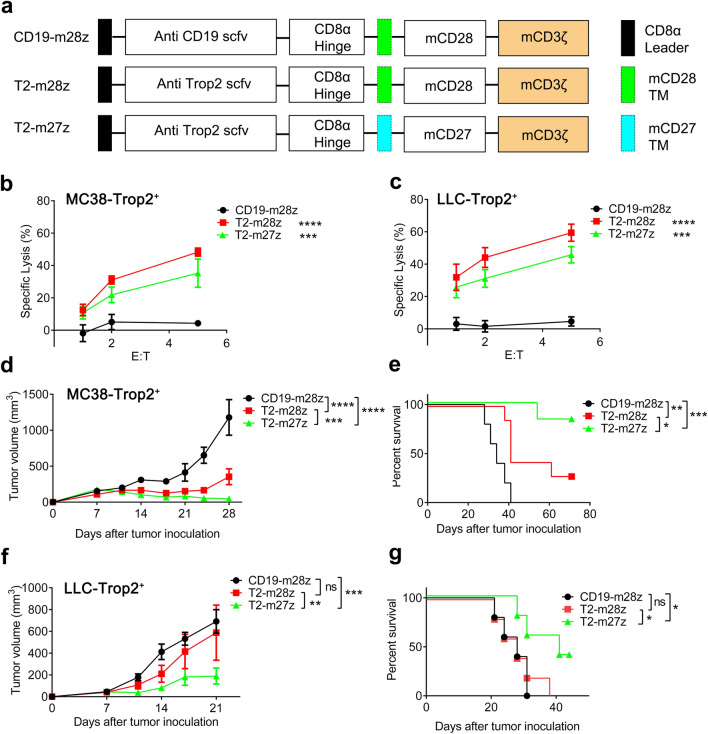
Given the limitation of immunodeficient mice tumor models, an immunocompetent mice tumor model was chosen to examine the antitumor effect of T2-CAR T cells. Second-generation murine T2-CAR (T2-mCAR) T cells targeting the human Trop2 antigen were established. The structure was the same as the human CARs, but the intracellular signaling domains were changed into murine signaling domains (Fig. 4a). The transduction efficacies of all mCAR T cells were similar (Supplementary Fig. 4). Both T2-m27z and T2-m28z CAR T cells dramatically induced MC38-Trop2+ and LLC-Trop2+ cytotoxicity, which demonstrating the potent killing effect in response to Trop2 antigen stimulation (Fig. 4b, c). In MC38-Trop2+ and LLC-Trop2+ tumor-bearing mice, T2-m27z CAR T cell treatment more significantly inhibited the tumor compared to T2-m28z CAR T cells (Fig. 4d, f). Moreover, T2-m27z CAR T cell treated mice showed an extended survival time when compared to the T2-m28z group (Fig. 4e, g). These results demonstrate that T2-m27z CAR T cells displayed better antitumor activity in vivo than T2-m28z CAR T cell in immunocompetent tumor models.
Fig. 4.
CD27 signal enhances murine T2-CAR T cells antitumor activity. a Schematic representation of murine CARs constructs. Cytotoxic activity of mCAR T cells co-cultured MC38-Trop2+ (b) or LLC-Trop2+ (c) cells. Tumor growth curve of MC38-Trop2+ (d) (N = 5, CD19-m28z group; N = 7, T2-m28z group; N = 6, T2-m27z group (two-way ANOVA)). e The survival of the mice was shown. p values; were calculated by Log-rank (Mantel-Cox) test. p = 0.0089 (T2-m28z vs. CD19-m28z.); p = 0.0003 (T2-m27z vs CD19-m28z); p = 0.0297(T2-m28z vs. T2-m27z). Tumor growth curve (f) and survival (g) of LLC-Trop2+ (N = 5 mice/group; p values were calculated by Log-rank (Mantel-Cox) test. p = 0.6854 (T2-m28z vs. CD19-m28z.); p = 0.0431 (T2-m27z vs CD19-m28z); p = 0.0466(T2-m28z vs. T2-m27z). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
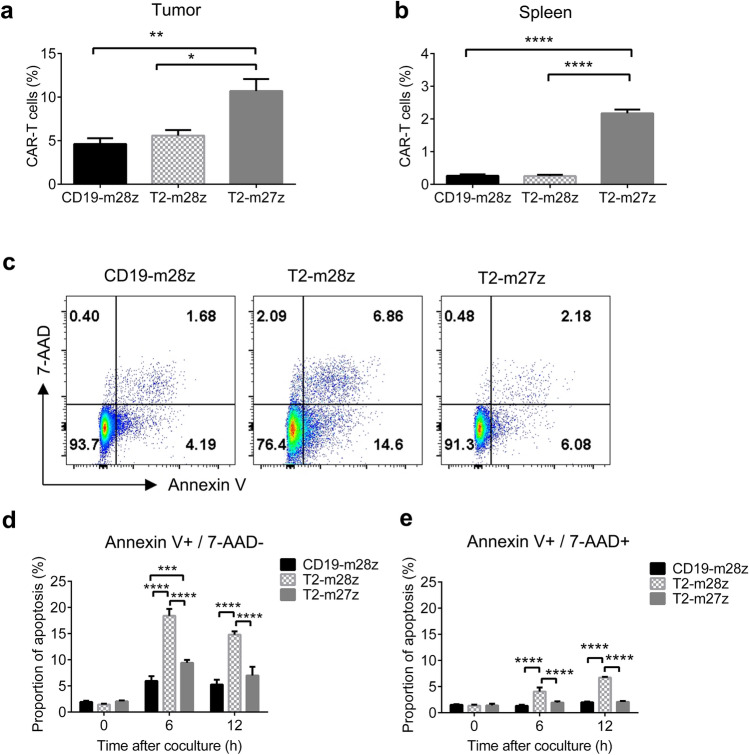
CD27 signaling increases T2-m27z CAR T cell survival
To reveal the reason that T2-m27z CAR T cells display a better antitumor activity in vivo, CAR T cells were examined in tumor-bearing mouse models. The results showed that the proportion of CAR T cells were significantly higher in tumor tissues treated with T2-m27z than with T2-m28z (Fig. 5a), as well as in splenocytes (Fig. 5b), indicating the better survival of T2-m27z than T2-m28z in the in vivo model. To reveal the role of CD27 signaling in regulating cell survival and apoptosis, the apoptosis status was examined in different mCAR T cells co-cultured with MC38-Trop2+ cells (Fig. 5c). The T2-m27z CAR T cells showed less apoptosis, both in early and late stages, relative to the T2-m28z CAR T cells (Fig. 5d, e), suggested that CD27 enhances T2-m27z CAR T cell survival, consistent to previous studies [36].
Fig. 5.
CD27 signal enhances T2-CAR T cells survival. a The proportions of CAR T cells in CD8+ T cells in tumor tissue and spleen (b) at 7 d after CAR T cell infusion in MC38-Trop2+ tumor-bearing mice (mean ± SEM; n = 4 for each group). c T2-mCAR T cells were co-cultured with MC38-Trop2+ cells. Apoptosis levels in mCAR T cells were analyzed using Annexin V/7-AAD dye‐staining at 12 h post-stimulation. d, e Early and late apoptosis levels of co-cultured CAR-T cells were detected at different time periods (mean ± SEM; n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
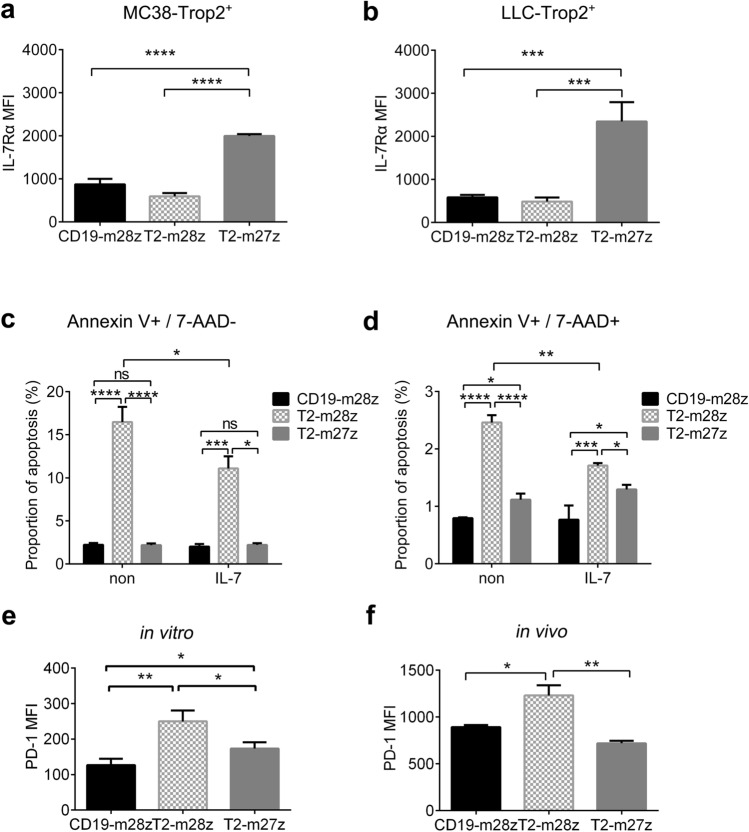
CD27 signaling regulated IL-7Rα and PD-1 expression in T2-m27z CAR T cell
Previous reports have suggested that CD27 stimulation could promote the expression of IL-7Rα, which is necessary for T cell survival. To delineate the role of IL-7Rα in enhancing T2-m27z CAR T cell survival, the expression of cell surface IL-7Rα was examined by flow cytometry at 12 h post-co-culturing with Trop2-positive cells. The results showed that IL-7Rα expression was significantly increased on T2-m27z CAR T cell, but no change was observed on T2-m28z CAR T cells (Fig. 6a, b). To examine whether this increase could enhance T2-m27z CAR T survival, IL-7 was added as a functional compensation to observe apoptosis changes in different CAR T cells. The addition of IL-7 dramatically inhibited T2-m28z CAR T cell apoptosis and narrowed the difference between it and T2-m27z (Fig. 6c, d), which is consistent with previous studies [37]. These results demonstrate that IL-7Rα plays an important role in T2-27z mCAR T cell survival and could be partially functionally compensated by IL-7 cytokine supplementation. PD-1, the T cell exhaustion marker, is also transcriptionally regulated by CD27 [38]. T2-m27z CAR T cells have a lower PD-1 expression, both stimulation co-culture in vitro and in tumor microenvironment, compared to T2-m28z CAR T cells (Fig. 6e, f). These findings indicate that CD27 could prevent T cell exhaustion in addition to the observed potent CAR T cell antitumor activity.
Fig. 6.
CD27 signaling regulated IL-7Rα and PD-1 expression. IL-7Rα expression in mCAR T cells was analyzed by flow cytometry at 12 h post-co-culturing with MC38-Trop2+ (a) or LLC-Trop2+ cells (b). Early (c) and late apoptosis (d) of the co-cultured CAR T cells was detected with/without IL-7 additive. PD-1 expression was analyzed by flow cytometry in mCAR T cells at 12 h post-co-culturing with MC38-Trop2+ (e) or TIL (f). *p < 0.05, ** p < 0.01, ***p < 0.001, ****p < 0.0001
Discussion
A major concern in cellular immunotherapy is the potential of “on-target, off-tumor” toxicity. Selecting a wide spectrum TAA is also important for a successful CAR T therapy. The Trop2 protein, which plays an important role in tumorigenesis and is associated with tumor aggressiveness and metastasis, is highly expressed in various solid tumor cells. Trop2 is overexpressed in colon cancer tissues and is associated with colon cancer pathogenesis. Furthermore, Trop2 expression is correlated with cancer patient’s survival rates. Clinical trials have revealed that the drug conjugated anti-Trop2 antibodies provide a good therapeutic efficiency in TNBC patients [39]. Therefore, Trop2 may provide an attractive and potential target for CAR T cell immunotherapy. Herein, CAR T cells targeting Trop2 could inhibit tumor growth in a tumor-bearing model (Fig. 3). Moreover, Trop2 targeted CAR T cells have also displayed a killing effect on gastric cancer [40]. Thus, these findings suggest that T2-CAR T cells could be used to treat Trop2-positive cancers.
In CAR T cells, the CAR intracellular domain determines the ultimate fate and function of CAR T cells. In the second generation of CAR T cell designs, co-stimulatory signaling was integrated into the intracellular domain and combined with CD3 signaling to generate fully functioning CAR T cells that effectively secrete cytokines, as well as having an effective tumor killing ability [41]. CD28 signaling enhances the T cell metabolic signature [34], and the incorporation of the CD28 domain into CARs improves T cell proliferation and IFN-γ production [32]. Meanwhile, other co-stimulatory domains have been assessed including 4-1BB, CD27, ICOS, and OX40 [42], different signals will have different impacts on CAR T cell antitumor activity and fate. Compared to CD28, 4-1BB signaling enhances T cell survival and antitumor activity by inducing mitochondria enlargement [43]. Incorporating an ICOS domain in the CAR leads to Th17/Th1 T cell polarization, and ICOS based Th17 CAR T mediates an efficient antitumor response [35]. However, CD27 has a greater effect on T cell proliferation [42] and enhances T cell survival to increase its tumor-infiltrating ability of T effector cells [29, 44]. OX40 is essential for T cell survival and enhances the function of CAR T cells by reducing IL-10 production [45]. In this study, CD27 signaling showed a potential role in antitumor activity (Fig. 4), with T2-27z CAR T cells showing a more powerful killing effect in immunodeficient NCG model (Fig. 3), which is consistent with previous studies [29]. Furthermore, T2-m27z CAR T cells displayed a better antitumor activity relative to T2-m28z CAR T cells in the immunocompetent model (Fig. 4). These results demonstrate that CD27 intracellular signaling can enhance T2- CAR T cell antitumor effects.
In the immunodeficient tumor-bearing model, the mice lack of intact immune environment and mouse cytokines cannot take effect on human CAR T cells, mouse MHC molecular cannot present antigen to human CAR T cells either, which will distort the CAR T cell immune response to tumor. As well, tumor growth and its microenvironment can be taken effect by lack of human cytokine or other signal stimulation. All of those may lead to eliminate the therapeutic differences by different CAR T cells. Syngeneic models of immunocompetent mice can provide integrated immune system and normal tumor microenvironment. In immunocompetent tumor-bearing model, CAR T cell would not only stimulated by CAR, but also through the endogenous T cell receptor (TCR) activation and other signaling. Previous study showed that TCR stimulation of CD8, rather than CD4, CAR T cell with CD28 co-stimulatory was associated with T cell exhaustion and apoptosis in immunocompetent mice [46]. In this study, we found that T2-27z CAR T cell therapy performed better than T2-28z CAR T cell in immunocompetent mice, rather immunodeficient mice (Fig. 4). These results demonstrated that immune system integration is critical for evaluating CAR T cell therapy efficiency.
Co-stimulatory receptors amplified the T cell response in concert with TCR signaling, and subsequently affected the strength and lifespan of the response. CD28 and CD27, which are the two most important co-stimulatory elements in T cells, belong to different families. CD28 belongs to the immunoglobulin family, which serves largely as a TCR signal amplifier [47], while CD27 belongs to the TNF receptor family, which mediates a different signaling pathway. Furthermore, CD27 co-stimulation counteracts apoptosis by downregulating the expression of FasL [28] and increasing Bcl-xL [26, 27], an antiapoptotic factor that promotes T cell clonal expansion. In this study, T2-m27z CAR T cells demonstrated a higher resistance to apoptosis when co-cultured with tumor cells (Fig. 5c, d), with more tumor-infiltrating lymphocytes (TIL) and splenocytes accumulated in T2-m27z CAR T cell tumor-bearing mice than in mice treated with T2-m28z CAR T cells (Fig. 5a, b). These results show that CD27 signaling can increase T2-CAR T cells antiapoptotic abilities and overall cell survival in immunocompetent tumor models.
CD27 signaling promotes IL-7Rα transcriptional re-expression upon TCR signaling stimulation [48]. IL-7Rα is broadly expressed throughout the lymphoid system and contains a common gamma chain (γc) that is ubiquitously expressed on lymphocytes, with IL-7 responsiveness largely controlled by the presence or absence of IL-7Rα [49]. Furthermore, IL-7/IL-7R signaling could potentially promote cell viability, cell cycle progression, and growth [50]. T cells continue to express IL-7R in both naive and memory states, and IL-7 signaling is critical for the long-term maintenance of all T cell populations. Furthermore, IL-7 promotes cell survival by modulating the intrinsic pathway, which enhances CAR T cell survival and provides apparent anticancer benefits [37]. Herein, the findings suggest that IL-7Rα expression is significantly increased in T2-m27z CAR T cells (Fig. 6a, b), and IL-7 supplement could benefit T2-m28z cell survival and narrow the difference seen between it and T2-m27z (Fig. 6c, d). As the apoptosis level of T2-m27z CAR T after tumor cell activation was slightly increased, it may be the reason to observe no effect on IL-7 addition to T2-m27z CAR cell apoptosis that have significantly decreased on T2-m28z CAR cell. Thus, increasing IL-7Rα expression could help to enhance T2-m27z survival.
In exhausted CD8 T cells, high PD-1 expression is the hallmark and contributes to the exhaustion. This exhaustion is characterized by a high expression of co-inhibitory receptors, including PD-1, Tim3, and LAG3 [51]. In a previous study, anti-CD27 treatment correlates with lower PD-1 levels in CD8+ TILs in a lung metastases tumor model [52]. In this study, PD-1 expression was increased in activated T2-m28z CAR T cells and TILs relative to the T2-m27z CAR T cells (Fig. 6e, f). The lower PD-1 levels in the T2-m27z CAR T cells may prevent T cell exhaustion, which may be another mechanism by which CD27 signaling enhances the T2-mCAR T cell killing effect.
Overall, these findings have revealed that CD27 signaling performs more effectively than CD28 when utilizing Trop2 CAR T cells in solid tumor treatment. Furthermore, CD27 signal enhances the killing effect of T2-CAR T cells by increasing CAR T cell survival and antiapoptotic abilities. This enhancement was also partially due to the alleviation of IL-7Rα expression, as well as preventing T cell exhaustion via reduced PD-1 expression.
Précis: Trop2 may provide an attractive and potential target for CAR T cell immunotherapy. Furthermore, the addition of a CD27 intercellular domain can enhance the killing effect of CAR T cells via multiple mechanisms, thus indicating that T2-27z CAR is more suitable for clinical application.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Abbreviations
- ACT
Adoptive cell transfer
- Trop2
Trophoblast cell surface antigen 2
- CAR
Chimeric antigen receptor
- T2-CAR
Trop2-specific CAR
- TAA
Tumor-associated antigen
- FDA
Food and Drug Administration
- scFv
Single-chain fragment variant
- MHC
Major histocompatibility complex
- IL-2
Interleukin-2
- IL-7
Interleukin-7
- IL-15
Interleukin-15
- TNF-α
Tumor necrosis factor-α
- UTD
Untransduced
- IFN-γ
Interferon-γ
- PBMCs
Peripheral blood mononuclear cells
- IVIS
In Vivo Imaging System
- LDH
Lactate dehydrogenase
- TNBC
Triple-negative breast cancer
- TIL
Tumor infiltrated lymphocyte
Author contributions
H.Z and C.H designed the study. C.H, Y.M, W.F, Z.Q, Y.B, and H.Z performed experiments, collected and analyzed data. L.Z provided technical support on the mouse model. C.H and H.Z drafted the manuscript. All authors revised and approved the manuscript.
Funding
This research was supported by the Science and Technology Department of Guangdong Province (2017A050501010 and 2016A050503023) and Guangzhou Science, Technology and Innovation Commission (201807010042).
Availability of data and materials
All data generated during this study have included in this article.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The research was approved by the Zhongshan School of Medicine, Sun Yat-sen University (Guangzhou, China). This research was performed in accordance with the Declaration of Helsinki and according to national and international guidelines. Whole blood from anonymous healthy volunteer donors was obtained from Guangzhou Blood Center, Guangzhou, in which all volunteers had known and confirmed that the blood was going to be used for scientific research. All mouse experiments were approved by and followed to the ethical regulations of IACUC, SYSU.
Consent for publication
All authors agree to publish this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/8/2024
Missing supplementary file and the supplementary figure links which were wrongly linked to main text figures have been updated.
References
- 1.June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. SciTranslMed. 2015;7(280):2807–7. doi: 10.1126/scitranslmed.aaa3643. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tchou J, Zhao Y, Levine BL, et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol Res. 2017;5(12):1152–1161. doi: 10.1158/2326-6066.CIR-17-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang LN, Song Y, Liu D. CD19 CAR-T cell therapy for relapsed/refractory acute lymphoblastic leukemia: factors affecting toxicities and long-term efficacies. J Hematol Oncol. 2018;11(1):41. doi: 10.1186/s13045-018-0593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Li W, Huang K, et al. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol. 2018;11(1):22. doi: 10.1186/s13045-018-0568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cubas R, Li M, Chen C, et al. Trop2: a possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta. 2009;1796(2):309–314. doi: 10.1016/j.bbcan.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Bardia A, Mayer IA, Diamond JR, et al. Efficacy and safety of Anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol. 2017;35(19):2141–2148. doi: 10.1200/jco.2016.70.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukahara Y, Tanaka M, Miyajima A. TROP2 expressed in the trunk of the ureteric duct regulates branching morphogenesis during kidney development. PLoS ONE. 2011;6(12):e28607. doi: 10.1371/journal.pone.0028607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okabe M, Tsukahara Y, Tanaka M, et al. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136(11):1951–1960. doi: 10.1242/dev.031369. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Yao Y-F. Gelatinous drop-like corneal dystrophy with a novel mutation of TACSTD2 manifested in combination with spheroidal degeneration in a Chinese patient. Mol Vis. 2010;16:1570–1575. [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Day R, Dong Y, et al. Identification of Trop-2 as an oncogene and an attractive therapeutic target in colon cancers. Mol Cancer Ther. 2008;7(2):280–285. doi: 10.1158/1535-7163.MCT-07-2003. [DOI] [PubMed] [Google Scholar]
- 13.Tang G, Tang Q, Jia L, et al. High expression of TROP2 is correlated with poor prognosis of oral squamous cell carcinoma. Pathol Res Pract. 2018;214(10):1606–1612. doi: 10.1016/j.prp.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Trerotola M, Jernigan DL, Liu Q, et al. Trop-2 promotes prostate cancer metastasis by modulating beta(1) integrin functions. Cancer Res. 2013;73(10):3155–3167. doi: 10.1158/0008-5472.CAN-12-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bignotti E, Todeschini P, Calza S, et al. Trop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. Eur J Cancer. 2010;46(5):944–953. doi: 10.1016/j.ejca.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Heist RS, Guarino MJ, Masters G, et al. Therapy of advanced non-small-cell lung cancer with an sn-38-anti-trop-2 drug conjugate, sacituzumab Govitecan. J Clin Oncol. 2017;35(24):2790–2797. doi: 10.1200/jco.2016.72.1894. [DOI] [PubMed] [Google Scholar]
- 17.Faltas B, Goldenberg DM, Ocean AJ, et al. Sacituzumab govitecan, a novel antibody-drug conjugate, in patients with metastatic platinum-resistant urothelial carcinoma. Clin Genitourin Cancer. 2016;14(1):e75–e79. doi: 10.1016/j.clgc.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Starodub AN, Ocean AJ, Shah MA, et al. First-in-human trial of a novel anti-trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res. 2015;21(17):3870–3878. doi: 10.1158/1078-0432.CCR-14-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16(9):566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boleto G, Allanore Y, Avouac J. Targeting costimulatory pathways in systemic sclerosis. Front Immunol. 2018;9:2998. doi: 10.3389/fimmu.2018.02998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13(8):525–541. doi: 10.1038/nrc3565. [DOI] [PubMed] [Google Scholar]
- 22.Labanieh L, Majzner RG, Mackall CL. Programming CAR-T cells to kill cancer. Nat Biomed Eng. 2018;2(6):377–391. doi: 10.1038/s41551-018-0235-9. [DOI] [PubMed] [Google Scholar]
- 23.Yu S, Li A, Liu Q, et al. Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol. 2017;10(1):78. doi: 10.1186/s13045-017-0444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacchetti B, Botticelli A, Pierelli L, et al. CAR-T with License to Kill Solid Tumors in Search of a Winning Strategy. Int J Mol Sci. 2019; https://doi.org/10.3390/ijms20081903 [DOI] [PMC free article] [PubMed]
- 25.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9(4):271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Oosterwijk MF, Juwana H, Arens R, et al. CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int Immunol. 2007;19(6):713–718. doi: 10.1093/intimm/dxm033. [DOI] [PubMed] [Google Scholar]
- 27.Peperzak V, Veraar EA, Keller AM, et al. The Pim kinase pathway contributes to survival signaling in primed CD8+ T cells upon CD27 costimulation. Journal of immunology. 2010;185(11):6670–6678. doi: 10.4049/jimmunol.1000159. [DOI] [PubMed] [Google Scholar]
- 28.Dolfi DV, Boesteanu AC, Petrovas C, et al. Late signals from CD27 prevent Fas-dependent apoptosis of primary CD8+ T cells. Journal of immunology. 2008;180(5):2912–2921. doi: 10.4049/jimmunol.180.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song DG, Ye Q, Poussin M, et al. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119(3):696–706. doi: 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- 30.Goldenberg DM, Cardillo TM, Govindan SV, et al. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget. 2015;6(26):22496–512. https://doi.org/10.18632/oncotarget.4318 [DOI] [PMC free article] [PubMed]
- 31.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pegram HJ, Park JH, Brentjens RJ. CD28z CARs and armored CARs. Cancer J. 2014;20(2):127–133. doi: 10.1097/PPO.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ying Z, He T, Wang X, et al. Parallel Comparison of 4–1BB or CD28 Co-stimulated CD19-Targeted CAR-T Cells for B Cell Non-Hodgkin's Lymphoma. Mol Ther Oncolytics. 2019;15:60–68. doi: 10.1016/j.omto.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawalekar OU, O'Connor RS, Fraietta JA, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44(2):380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Guedan S, Chen X, Madar A, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124(7):1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198(9):1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adachi K, Kano Y, Nagai T, et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018;36(4):346–351. doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 38.Ramakrishna V, Sundarapandiyan K, Zhao B, et al. Characterization of the human T cell response to in vitro CD27 costimulation with varlilumab. Journal for immunotherapy of cancer. 2015;3:37. doi: 10.1186/s40425-015-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2019;380(8):741–751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 40.Zhao W, Jia L, Zhang M, et al. The killing effect of novel bi-specific Trop2/PD-L1 CAR-T cell targeted gastric cancer. Am J Cancer Res. 2019;9(8):1846–1856. [PMC free article] [PubMed] [Google Scholar]
- 41.Finney HM, Akbar AN, Lawson ADG. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR chain. J Immunol. 2003;172(1):104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 42.Croft M. Costimulation of T cells by OX40, 4–1BB, and CD27. Cytokine Growth Factor Rev. 2003;14(3–4):265–273. doi: 10.1016/s1359-6101(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 43.Teijeira A, Labiano S, Garasa S, et al. Mitochondrial Morphological and Functional Reprogramming Following CD137 (4–1BB) Costimulation. Cancer Immunol Res. 2018;6(7):798–811. doi: 10.1158/2326-6066.CIR-17-0767. [DOI] [PubMed] [Google Scholar]
- 44.Bullock TN. Stimulating CD27 to quantitatively and qualitatively shape adaptive immunity to cancer. Curr Opin Immunol. 2017;45:82–88. doi: 10.1016/j.coi.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hombach AA, Heiders J, Foppe M, et al. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology. 2012;1(4):458–466. doi: 10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, Kohler ME, Chien CD, et al. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aag1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3(12):939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 48.Dong H, Buckner A, Prince J, et al. Frontline Science: Late CD27 stimulation promotes IL-7Rα transcriptional re-expression and memory T cell qualities in effector CD8(+) T cells. J Leukoc Biol. 2019;106(5):1007–1019. doi: 10.1002/jlb.1hi0219-064r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174(11):6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira ML, Akkapeddi P, Ribeiro D, et al. IL-7R-mediated signaling in T-cell acute lymphoblastic leukemia: An update. Adv Biol Regul. 2019;71:88–96. doi: 10.1016/j.jbior.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 52.Roberts DJ, Franklin NA, Kingeter LM, et al. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J Immunother. 2010;33(8):769–79. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study have included in this article.