Abstract
Purpose
While immune checkpoint inhibitors (ICI) have had success with various malignancies, their efficacy in brain cancer is still unclear. Retrospective and prospective studies using PD-1 inhibitors for recurrent glioblastoma (GBM) have not established survival benefit. This study evaluated if ICI may be effective for select patients with recurrent GBM.
Methods
This was a single-center retrospective study of adult patients diagnosed with first recurrence GBM and received pembrolizumab or nivolumab with or without concurrent bevacizumab. Archival tissue was used for immunohistochemistry (IHC) and targeted DNA next-generation sequencing (NGS) analysis.
Results
Median overall survival (mOS) from initial diagnosis was 24.5 months (range 10–42). mOS from onset of ICI was 10 months (range 1–31) with 75% surviving > 6 months and 46% > 12 months. Additional IHC analysis on tumors from eight patients demonstrated a trend of longer survival after ICI for those with elevated PD-L1 expression. NGS of samples from 15 patients identified EGFR amplification at initial diagnosis and at any time point to be associated with worse survival after ICI (HR 12.2, 95% CI 1.37–108, p = 0.025 and HR 3.92, 95% CI 1.03–14.9, p = 0.045, respectively). This significance was corroborated with previously tested EGFR amplification via in situ hybridization.
Conclusion
ICI did not extend overall survival for recurrent GBM. However, molecular sequencing identified EGFR amplification as associated with worse survival. Prospective studies can validate if EGFR amplification is a biomarker of ICI resistance and determine if its use can stratify responders from non-responders.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03381-y.
Keywords: EGFR amplification, Next-generation sequencing, Nivolumab, Pembrolizumab, Recurrent glioblastoma
Introduction
Despite standard of care treatment, the survival of patients with glioblastoma (GBM) remains poor with limited therapeutic options in the recurrent setting. A current benchmark for recurrent GBM mOS is 9.2 months for patients treated with bevacizumab monotherapy [1]. ICI, such as PD-1 inhibitors, have seen clinical efficacy in various cancers including advanced melanoma [2]. Based on results with other malignancies, ICI might be a therapeutic option for GBM. Initial trials of ICI in GBM patients have shown good tolerability [3]. However, studies examining PD-1 inhibitor treatment of recurrent high-grade gliomas and GBMs have demonstrated no survival benefit or similar survival results to bevacizumab monotherapy [4–8]. A phase 3 clinical trial for recurrent GBM, Checkmate 143, treated patients with either nivolumab or bevacizumab, and found no significant difference in overall survival between the nivolumab group (9.8 mOS) and bevacizumab group (10.0 mOS) [7]. Similarly, a recent randomized phase 2 trial comparing pembrolizumab + / − bevacizumab for recurrent GBM concluded no survival benefit of pembrolizumab monotherapy or in combination with bevacizumab compared to historical monotherapy bevacizumab treatment [8]. Nonetheless, there is still thought that ICI may be an effective therapeutic option for select patients, as evidenced by a small study examining neoadjuvant ICI for recurrent GBM [9]. Due to the lack of efficacious treatment options and well-tolerated nature of ICI, we treated a group of patients with recurrent GBM with either pembrolizumab or nivolumab with or without bevacizumab. This retrospective review evaluates this subset of patients, determining survival and analyzing pathological and molecular markers that could influence survival and treatment outcomes.
Methods
Study population
This study is an Icahn School of Medicine at Mount Sinai Institutional Review Board approved single-center observation retrospective study conducted with a waiver of consent at Mount Sinai Hospital, New York. We retrospectively evaluated patients with recurrent GBM treated with ICI of pembrolizumab or nivolumab with the addition of bevacizumab. These were consecutive patients with no apparent risks of treating with ICI for whom the treating physicians thought ICI was a reasonable option. To comply with the WHO 2021 classification of GBM, we excluded two patients with IDH mutant GBM. We included in our analysis patients who had pathology of IDH wildtype GBM, were treated with initial standard of care therapy as per Stupp protocol, had the first recurrence with radiographic progression of their GBM, at least 18 years of age, and who received at least 2 cycles of either pembrolizumab or nivolumab between July 2014 and February 2019, after diagnosis of recurrent GBM. Patients were treated with nivolumab 240 mg every 2 weeks or pembrolizumab 200 mg every 3 weeks. Bevacizumab was dosed at either 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks. Toxicity was assessed retrospectively according to the Common Terminology Criteria for Adverse Events 4.03.
Pathology
Tumor characteristics, including basic histology and immunohistochemical (IHC) markers, were collected through chart review. Additional IHC testing was performed on archival tissue from both initial and recurrent samples assessing the percentage and distribution of CD3, CD8, CD20, CD163, PD-L1 expressing cells, and Ki-67 to determine cell proliferation. EGFR and chromosome 7 were routinely evaluated using the FDA-approved Ventana UltraView silver-enhanced in situ hybridization (SISH) and red ISH DNP Detection kits with EGFR and CEP7 ISH DNA probes. Molecular sequencing was performed internally at Icahn School of Medicine at Mount Sinai on the Ion Torrent platform using DNA from archival tissue from both initial and recurrent samples with a validated commercially available Oncomine™ Comprehensive Assay v3M panel. The cohort of Samstein et al. was utilized as a comparative data source for molecular sequencing on glioma patients treated with immunotherapy [10]. Briefly, this was a multi-cancer cohort of immunotherapy-treated patients, including 82 with GBM who also had next-generation sequencing of their tumors.
Statistical analysis
Kaplan–Meier survival curves accessible in the supplementary section were created using GraphPad Prism version 9.4.0. The date of death was obtained through either chart review of medical records, family notification to treating physician, or search of available public records. Genomic correlates of overall survival after PD-1 blockade were analyzed by univariable Cox proportional hazards models. Since there were some differences in genomic findings between initial biopsy and biopsy at recurrence, we conducted the regression analysis three ways: using genomic alterations found at any time, at initial biopsy (only among those with sequencing at initial biopsy), and at recurrence (only among those with sequencing at recurrence). Genomic correlates of overall survival after immunotherapy in the Samstein et al. cohort were analyzed using univariable and multivariable Cox proportional hazards models [10]. Multivariable models were adjusted for age, sex, tumor mutation burden, and immune checkpoint inhibitor class. A two-sided p value of less than 0.05 was considered statistically significant. All analyses were conducted in R 4.0.0.
Results
Twenty-four patients with a diagnosis of IDH wildtype GBM who received either pembrolizumab or nivolumab were reviewed through electronic medical records. Full patient characteristics are noted in Table 1.
Table 1.
Patients and tumor characteristics
| Median age | 62 (range 36–78) |
| Sex | |
| Male | 14 (58%) |
| Female | 10 (42%) |
| Ethnicity | |
| White | 13 (54%) |
| African-American | 3 (13%) |
| Hispanic | 3 (13%) |
| Asian | 1 (4%) |
| Other | 4 (17%) |
| Median KPS prior to ICI | 70 (range 60–100) |
| Initial Resection Type | |
| Gross Total | 10 (42%) |
| Subtotal | 10 (42%) |
| None | 2 (8%) |
| Unknown | 2 (8%) |
| MGMT Promotor Methylation | |
| Methylated | 8 (33%) |
| Unmethylated | 15 (63%) |
| Indeterminate | 1 (4%) |
| Initial Radiotherapy/TMZ regimen | |
| 6 weeks RT/TMZ | 22* (88%) |
| 3 weeks RT/TMZ | 2 (8%) |
| Treated with adjuvant TMZ | |
| Adjuvant TMZ | 19 (79%) |
| No adjuvant TMZ | 5 (21%) |
| Median adjuvant cycles TMZ | 3 (range 1–14) |
| Patients with second resection prior to ICI | 8 (33%) |
| Patients with re-RT | |
| Less than 2 months prior to ICI | 9 (38%) |
| During ICI | 5 (21%) |
| Type of ICI Treatment | |
| Nivolumab | 17 (71%) |
| Pembrolizumab | 7 (29%) |
| Median Cycles ICI | 10 (range 4–31) |
| Corticosteroid use | |
| At onset of ICI | 6 (25%) |
| Started during ICI | 10 (42%) |
| None prior to or during ICI | 8 (33%) |
| Patients receiving concurrent bevacizumab | 22 (92%) |
| Median concurrent bevacizumab cycles | 6 (1–26) |
ICI Immune checkpoint inhibitor, KPS Karnofsky Performance Status, RT radiation therapy, TMZ temozolomide
*One patient treatment was interrupted at 4 weeks due to severe thrombocytopenia and subsequently developed myelodysplastic syndrome
The median age was 62 years (range 36–78) with 14 men (58%) and 10 women (42%). The median initial Karnofsky Performance Status prior to initiation of ICI was 70 (range 60–100). For initial surgical treatment of the 24 patients, 10 (42%) had gross total resections and 10 (42%) had subtotal resections. Eight patients (33%) had tumors with MGMT promoter methylated, 15 (63%) unmethylated and one (4%) was indeterminate. The indeterminate tumor on subsequent resection after initiation of ICI was determined to be MGMT methylated. Five patients (21%) did not receive adjuvant temozolomide after concurrent radiation and chemotherapy due to either severe side effect of temozolomide or progression of disease. Eight patients (33%) had a second surgical resection due to recurrence prior to initiation of PD-1 inhibitor. Nine patients (38%) received a second radiation treatment within two months of starting ICI with six of those patients receiving re-irradiation within one month of ICI. Seventeen patients (71%) were treated with nivolumab and seven patients (29%) with pembrolizumab. The entire cohort received a median of 10 cycles (range 4–31) of ICI. Twenty-two patients (92%) were treated concurrently with a median number of six cycles (range 1–26) of bevacizumab. Six patients (25%) received corticosteroids prior to initiation of ICI and 10 (42%) during ICI treatment either for increased cerebral edema or ICI adverse effects.
Toxicity
Overall, treatment was well tolerated. A total of nine patients (38%) had ten documented immune-related adverse events (IRAE) ranging from Grade I to Grade III as listed in supplementary section table: three grade I, four grade II and three grade III. A patient with pneumonitis required cessation of ICI.
Survival analysis
The mOS for the total cohort from initial diagnosis was 24.5 months (range 10–42). The eight patients with MGMT methylated tumors had a mOS of 26.5 months (range 19–42) compared to a mOS of 22 months (range 10–34) for the 15 patients with MGMT unmethylated. The patient that had undetermined MGMT methylation status at initial resection with MGMT methylated at subsequent resection had an overall survival of 34 months. The mOS for all patients from onset of ICI was 10 months (range 1–31), with 18 patients (75%) surviving > 6 months and 11 patients (46%) surviving > 12 months.
Molecular analysis
IHC testing to assess proliferation index and the percentage and distribution of immunological markers from initial and recurrent tumor tissue was done on samples from eight of the 24 patients (Table 2).
Table 2.
Immunohistochemical analysis of tumors from patients treated with immune checkpoint inhibitor
| Survival Post PD1 (months) | CD3% | Dist | CD8% | Dist | CD20% | Dist | CD163% | Dist | PDL1% | Dist | MIB-1% | Dist | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pt20 Initial Recurrent |
5 |
1 10–20 |
PV TB |
1 5–10 |
PV NA |
0 2–3 |
NA NA |
40 20 |
F MAC |
4–5 30–40 |
F F |
50 NA |
NA NA |
|
Pt10 Initial Recurrent |
5 |
2–3 2–3 |
TB TB |
1 2–3 |
TB TB |
0 0 |
NA NA |
60 40–50 |
F NA |
1 1 |
TB TB |
40–50 15–20 |
F NA |
|
Pt23 Initial |
9 | 2–3 | PV | 2–3 | PV | 0 | NA | 40 | MAC | 1 | PV | ||
|
Pt2 Recurrent |
16 | 3–5 | TB | 4 | TB | 3–4 | PV | 60–70 | F, P | < 1 | F | ||
|
Pt8 Initial Recurrent |
17 | 4–5 | PV | 2 | PV | 6–7 | PV | 10–15 | MAC | 2–3 | S |
40–50 40 |
NA NA |
|
Pt17 Initial Recurrent |
18 | 5 | PV | 5 | PV | 0 | NA | 20 | MAC | 5 | F, P |
10 30–40 |
F F |
|
Pt1 Initial Recurrent |
19 |
2–3 2–3 |
PV S |
2 2–3 |
PV S |
< 1 0 |
PV NA |
20 10–20 |
P NA |
20 5–10 |
NA F |
40 10 |
NA NA |
|
Pt15 Initial Recurrent |
31 |
5–10 3–5 |
PV PV |
3 3 |
PV PV |
1–2 0 |
PV NA |
50–60 50–60 |
TB NA |
20 40 |
D NA |
60–70 10–20 |
F F |
Dist. distribution, PV perivascular, MAC macrophage, F focal, P periphery, TB tumor bed, D diffuse, S scattered, NA not applicable
Each column represents a specific immunological marker and the adjacent column represents the specific distribution (if any) of that particular marker. Patients are listed in ascending order of survival from start of PD-1 inhibitor
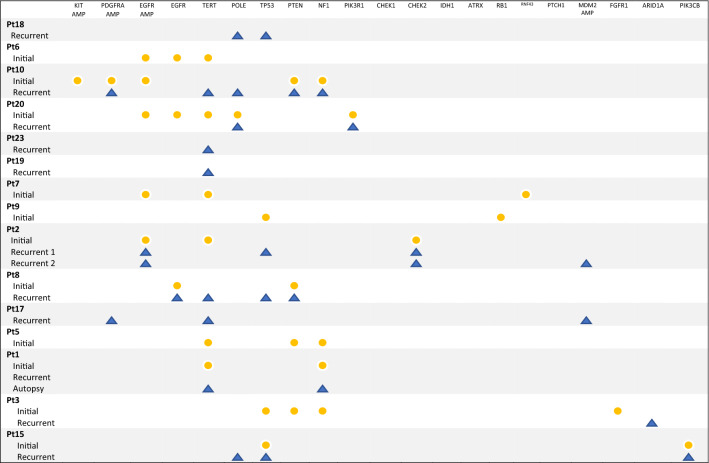
NGS assessment using targeted DNA sequencing of the initial diagnostic and recurrent tumor tissue was performed on archival samples of 15 patients (Table 3).
Table 3.
Genomic profile of tumors from diagnosis and recurrence
Yellow circles—a mutation or amplification found in tissue from initial diagnosis. Blue triangles—a mutation or amplification found in tissue from recurrent disease
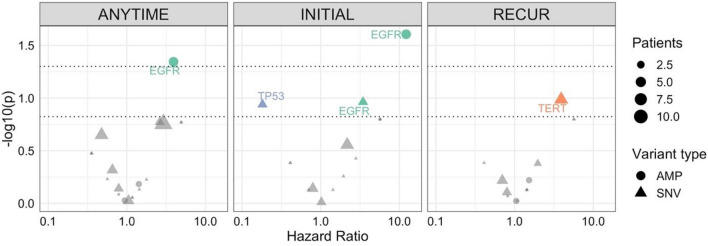
In eleven patients with NGS on an initial biopsy, EGFR amplification was observed in five samples and associated with worse overall survival (HR 12.2, 95% CI 1.37–108, p = 0.025) (Fig. 1).
Fig. 1.
Cox regression analysis of molecular mutations or amplifications found in tumor samples from 15 patients in the cohort. Three analyses were performed for mutations or amplifications found at any time point, at initial diagnosis only, or at recurrence only. Dotted lines represent p < 0.05 and p < 0.15 significance thresholds. Shapes correspond to amplifications (circles) or single nucleotide variants (triangles), with size corresponding to number of patients with the alteration. Hazard ratios greater than 1 represent an increased risk of death. AMP amplification, SNV single nucleotide variant
Among patients with sequencing at any time (N = 15), EGFR amplification was also associated with worse outcomes (N = 5, HR 3.92, 95% CI 1.03–14.9, p = 0.045). There were no other statistically significant genomic correlates of immunotherapy outcomes. However, some other notable but nonsignificant findings included TERT mutations at recurrence (N = 6, 3.91, 95% CI 0.76–20.1, p = 0.1) and TP53 mutations at initial biopsy/resection (N = 3, HR 0.182, 95% CI 0.0219–1.52, p = 0.12). We compared our data with the publicly available 82 immunotherapy-treated GBM patients in the dataset of Samstein et al. [10]. In univariable analyses, EGFRvIII mutations were significantly associated with worse OS (HR 1.81, 95% CI 1.13–2.89, p = 0.01), though this was no longer significant after adjusting for age, sex, tumor mutation burden, and immunotherapy class (HR 1.47, 95% CI 0.92–2.35, p = 0.11). In additional multivariable analyses, IDH1 (N = 6) and FAT1 (N = 4) mutations were associated with better (HR 0.15, 05% CI 0.03–0.80, p = 0.03) and worse outcomes (HR 12.0, 95% CI 1.66–85.8, p = 0.013), respectively.
With our NGS data indicating EGFR amplification was associated with worse survival for patients treated with ICI, we further evaluated the EGFR status in our cohort by reviewing previous pathology results from in situ hybridization of EGFR and chromosome 7. Using in situ hybridization, 19 tumors were tested for EGFR amplification at initial diagnosis: 11 (58%) were EGFR amplified compared to 8 (42%) that were not. Median overall survival from initial diagnosis for patients with tumors with EGFR amplified was 24 months (range 11–42) and for those with tumors not amplified was 23.5 months (range 10–34). However, mOS after initiation of PD-1 therapy was 8 months (range 1–26) for patients with tumors with EGFR amplification compared to 14.5 months (range 6–31) for those without. Nine recurrent tumors were tested for EGFR amplification via in situ hybridization: 4 tumors (44%) were EGFR amplified, and 5 (56%) were not. After initiation of PD-1 inhibitor for recurrent disease tissue with EGFR amplification, the mOS was 7 months (range 5–16) compared to 18 months (range 9–25) for those without EGFR amplification.
Discussion
Here, we report a single-center retrospective study evaluating the clinical course, survival, and correlative tissue biomarkers of patients with recurrent GBM treated with ICI. Pembrolizumab and nivolumab were generally well tolerated and there was no ICI treatment-related fatality in any of our patients. Previous retrospective studies focused on ICI in GBM have included either a mixture of high-grade gliomas or a heavily pre-treated GBM population with multiple recurrences for a range of mOS from 4 to 6.5 months [4, 6, 11]. With a mOS of 10 months for all patients from onset of ICI, our cohort had similar survival to patients with recurrent GBM treated with bevacizumab monotherapy [1], prospective studies using ICI with or without bevacizumab [8, 12] and nivolumab combined with bevacizumab [7].
IHC analysis showed no correlation of survival from ICI onset with T or B cell infiltration, macrophage presence, or cell proliferation index. However, there was an observational trend with longer survival from start of PD-1 inhibitor and increased expression of PD-L1 in tumor samples; the three longest survivors post-ICI each had PD-L1 expression ≥ 5% (Table 2). Nonetheless, these observations are from a small collection of patients and should not be interpreted as significant. Previous research showed no correlation between PD-L1 expression and survival from ICI in GBM patients [8, 13]. Studies in other cancers such as melanoma have suggested that a more comprehensive immune profiling may be required to differentiate responders from non-responders [14]. In addition, a few case reports of GBM tumors responding to ICI harbor mismatch repair deficiency [15, 16].
Utilizing a commercially available targeted NGS panel, we found that EGFR amplification at initial diagnosis and at any time was significantly associated with worse survival for patients treated with ICI. As a comparative data set for GBMs treated with ICI, Samstein et al.'s cohort did not share any molecular alterations with our data set associated with significantly worse survival [10]. However, their study focused on mutational load and did not include EGFR amplification status. For significant associations found in regression analysis in our cohort and Samstein et al.'s, high hazard ratios are most likely due to small sample sizes. Supporting our NGS findings, our in situ hybridization of EGFR and chromosome 7 also identified EGFR amplification at either initial diagnosis or recurrent disease associated with worse survival after ICI treatment. EGFR alterations such as amplifications and mutations are common in GBM and potentially associated with a worse prognosis [17–19]. Therefore, our findings could result from tumors with EGFR amplification having worse survival regardless of specific treatment, including ICI. The role of EGFR mutations in resistance to ICI has been studied in other malignancies, particularly non-small cell lung cancer (NSCLC) [20]. Subgroup analysis of clinical trials investigating the utility of ICI in NSCLC demonstrated that tumors harboring EGFR mutations had significantly worse outcomes to ICI than tumors with EGFR wildtype genotypes [21–23]. Multiple studies have identified that EGFR mutations in NSCLC are associated with tumoral T-lymphocyte depletion and an immunosuppressive tumor microenvironment (TME) that may hinder ICI's therapeutic benefit [21, 24, 25]. While the precise mechanism of how EGFR overexpression results in ICI resistance is unknown, it has been proposed that EGFR upregulation leads to TGFβ activation and subsequent local immune suppression [24]. TGFβ is a well-known cytokine contributing to immunosuppression of the TME in GBM [26]. Therefore, we can hypothesize that in GBM, EGFR upregulation may lead to TGFβ activation. For lung adenocarcinoma, different KRAS mutations have been identified to confer ICI resistance by affecting the expression of tumor-infiltrating lymphocytes and promoting tumor evasion with induction of Tregs, secretion of TGFβ and IL-10, and upregulation of PD-L1 potentially via MEK/ERK signaling [27–29]. MYC-KRAS axis was identified in a group of GBM patients via mass spectrometry, and those tumors exhibited a more invasive, proliferative phenotype and increased resistance of cell lines with KRAS signature to therapy [30]. More common, however, in GBM are PI3K/AKT/mTOR pathway alterations occurring in approximately 89% of GBMs as reported in the Cancer Genome Atlas (TCGA) project and hyperactivity of the Ras/Raf/MAPK pathway from upregulation of upstream signals such as EGFR [31, 32]. Although a relative increase in MAPK pathway mutations (PTPN11 and BRAF) has been found in responders, and PTEN mutations have also been associated with immunosuppression in non-responders [33]. Recently in patients with colon adenocarcinoma and multiple other cancers treated with ICI, mutations in the PI3K/AKT/mTOR pathway, another downstream signaling cascade triggered by EGFR activation, were associated with better survival and the concurrent presence of immune effector cells in the TME [34, 35]. Less is known regarding the crosstalk of upregulation of the PI3K/AKT/mTOR pathway and the immune cell composition of the TME in GBM. Nevertheless, preclinical work showed that GBM initiating-cells activate mTOR pathways in microglia, the tissue-resident macrophages of the brain, generating an immunosuppressive microglial phenotype that leads to negative regulation of T cells and subsequent immune escape and proliferation of tumor cells [17, 36]. It is important to note that EGFR mutations and amplifications have different biological characteristics, as our study highlights amplification events. Interestingly, our report of a recurrent GBM ICI-responder with regional immunological heterogeneity uniformly lost focal amplifications and developed a new subclonal EGFR mutation at recurrence, accounting for the complexity and impact of tumor evolution on immunological and molecular heterogeneity [15]. EGFR variations, including focal amplification of EGFR and EGFRvIII mutation, have been identified in 57% of GBMs, and expression level of receptor tyrosine kinases such as EGFR can fluctuate over their disease course [37]. ICI trials in GBM may not have been successful partly due to the high frequency of EGFR alterations that may play a critical role in immune dysregulation and suppression. Future trials may benefit from a further subgroup analysis of molecular markers such as EGFR, especially with the 2021 WHO classification of CNS tumors integrating molecular markers to a greater extent into tumor characterization and grading [38]. Our study underscores the potential importance of molecularly profiling GBMs, as this heterogenous tumor may respond differently to therapies based on genetic makeup.
Our study has multiple limitations. First, it is a retrospective analysis, which resulted in omitting some standard metrics in treating patients with GBM and lacks a control group of patients not treated with ICI + / − bevacizumab. We did not include progression-free survival (PFS) analysis because standard radiographic criteria, such as Response Assessment in Neuro-Oncology, were not readily available for all patients. Of note, in a separate radiology retrospective study that evaluated some of these same patients, we demonstrated that an increase in the relative apparent diffusion coefficient may correlate with early treatment response of GBM patients to ICI and may represent a future biomarker [39]. Five patients did not start adjuvant temozolomide during the initial Stupp regimen, creating variability in the upfront treatment of the cohort; GBM is a very aggressive tumor, and it is not uncommon to find early progression shortly after first-line therapy requiring a change of the regimen. However, 4/5 (80%) underwent surgical biopsies confirming viable tumors to corroborate the clinical and radiographic progression. The remaining patient developed temozolomide-related myelodysplastic syndrome, a rare complication of temozolomide treatment, and could not undergo further tissue biopsy. However, she had a clear progression of disease based on clinical and radiographic features and disease course. We used bevacizumab to limit steroid use, with 75% of the patients not receiving steroids at the onset of ICI and 33% receiving them only when ICI was discontinued. There have been conflicting reports on the activity of bevacizumab in GBM [1, 40], and it is conceivable that it may affect outcomes in combination with ICI [41]. NGS and tissue biomarker analysis were only available for testing in a subset of the cohort due to patient’s lack of available archival tissue. Moreover, we utilized a targeted NGS panel, limiting the scope of alterations able to be identified. Whole exome sequencing or whole genome sequencing would allow a more comprehensive analysis of potential significant amplifications and variants. In addition, this study is underpowered, and multivariate analysis cannot be performed with our small sample size.
The finding of EGFR amplification’s potential impact on GBM survival with ICI is hypothesis-generating. A prospective analysis with stratification of EGFR with larger cohorts in multi-institutional trial settings should help clarify the subsets of tumors that do not profit from ICI and direct the therapy to the subset that will benefit. In conclusion, GBM immunotherapy responses may need systematic molecular analysis, which may uncover the tail of patients that appear to benefit from ICI.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Yayoi Kinoshita, DMD, for the DNA extraction and Ying-Chih Wang, MS, for depositing the data to SRA in the NCBI BioProject database.
Author contributions
First author J.F. perform data acquisition, analysis, drafting of the paper. Lead author A.H., provide conception, design, analysis, interpretation and drafting, O.R. and N.T. immunohistochemical and molecular data acquisition and analysis; E.E., and R.S. next-generation sequencing acquisition, analysis and drafting of results; K.L.H. and T.J. genomic and statistical analysis and drafting of results; P.K., K.N. and P.B. radiological data acquisition and analysis.
Funding
This work was supported by the Neuro-Oncology Fin Fogg Fund.
Data availability
The data have been deposited to SRA under the accession PRJNA922203 in the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/).
Declarations
Conflict of interest
JSF, OR, HKL, EE, PK, PB, KN, NT have nothing to disclose. TJ is employed by Sema4. RS is VP of Technology Development at Sema4. AH is on the advisory board of TargTex and is the recipient of grants from Novocure, EMD Serono (Merck KGaA), National Brain Tumor Society and Cancer Research Institute.
Ethical approval
This study was approved by the Mount Sinai Hospital Institutional Review Board as a retrospective observational study that confirmed no ethical approval is required.
Consent for publication
This study is not publishing any identifiable individual details such as images or videos which would require consent to publish.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 2.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 3.Omuro A, Vlahovic G, Lim M, Sahebjam S, Baehring J, Cloughesy T, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674–686. doi: 10.1093/neuonc/nox208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurz SC, Cabrera LP, Hastie D, Huang R, Unadkat P, Rinne M, et al. PD-1 inhibition has only limited clinical benefit in patients with recurrent high-grade glioma. Neurology. 2018;91(14):e1355–e1359. doi: 10.1212/WNL.0000000000006283. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain MC, Kim BT. Nivolumab for patients with recurrent glioblastoma progressing on bevacizumab: a retrospective case series. J Neurooncol. 2017;133(3):561–569. doi: 10.1007/s11060-017-2466-0. [DOI] [PubMed] [Google Scholar]
- 6.Reiss SN, Yerram P, Modelevsky L, Grommes C. Retrospective review of safety and efficacy of programmed cell death-1 inhibitors in refractory high grade gliomas. J Immunother Cancer. 2017;5(1):99. doi: 10.1186/s40425-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the Checkmate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayak L, Molinaro AM, Peters K, Clarke JL, Jordan JT, de Groot J, et al. Randomized phase II and biomarker study of pembrolizumab plus bevacizumab versus pembrolizumab alone for patients with recurrent glioblastoma. Clin Cancer Res. 2021;27(4):1048–1057. doi: 10.1158/1078-0432.CCR-20-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. doi: 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantica M, Pritchard A, Lieberman F, Drappatz J. Retrospective study of nivolumab for patients with recurrent high grade gliomas. J Neurooncol. 2018;139(3):625–631. doi: 10.1007/s11060-018-2907-4. [DOI] [PubMed] [Google Scholar]
- 12.Ahluwalia MS, Rauf Y, Li H, Wen PY, Peereboom DM, Reardon DA. Randomized phase 2 study of nivolumab (nivo) plus either standard or reduced dose bevacizumab (bev) in recurrent glioblastoma (rGBM) J Clin Oncol. 2021;39(15 suppl):2015–2015. doi: 10.1200/JCO.2021.39.15_suppl.2015. [DOI] [Google Scholar]
- 13.Reardon DA, Kim TM, Frenel JS, Simonelli M, Lopez J, Subramaniam DS, et al. Treatment with pembrolizumab in programmed death ligand 1-positive recurrent glioblastoma: results from the multicohort phase 1 KEYNOTE-028 trial. Cancer. 2021;127(10):1620–1629. doi: 10.1002/cncr.33378. [DOI] [PubMed] [Google Scholar]
- 14.Morrison C, Pabla S, Conroy JM, Nesline MK, Glenn ST, Dressman D, et al. Predicting response to checkpoint inhibitors in melanoma beyond PD-L1 and mutational burden. J Immunother Cancer. 2018;6(1):32. doi: 10.1186/s40425-018-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Restrepo P, Yong R, Laface I, Tsankova N, Nael K, Akturk G, et al. Tumoral and immune heterogeneity in an anti-PD-1-responsive glioblastoma: a case study. Cold Spring Harb Mol Case Stud. 2020;6(2):a004762. doi: 10.1101/mcs.a004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–2211. doi: 10.1200/JCO.2016.66.6552. [DOI] [PubMed] [Google Scholar]
- 17.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Wang X, Xu L, Zhang J, Cao H. Analysis of the EGFR amplification and CDKN2A deletion regulated transcriptomic signatures reveals the prognostic significance of SPATS2L in patients with glioma. Front Oncol. 2021;11:551160. doi: 10.3389/fonc.2021.551160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Liang R, Song C, Xiang Y, Liu Y. Prognostic significance of epidermal growth factor receptor expression in glioma patients. Onco Targets Ther. 2018;11:731–742. doi: 10.2147/OTT.S155160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santaniello A, Napolitano F, Servetto A, De Placido P, Silvestris N, Bianco C, et al. Tumour microenvironment and immune evasion in EGFR addicted NSCLC: hurdles and possibilities. Cancers Basel. 2019;11(10):1419. doi: 10.3390/cancers11101419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer—a meta-analysis. J Thorac Oncol. 2017;12(2):403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapoor SS, Zaiss DMW. Emerging role of EGFR mutations in creating an immune suppressive tumour microenvironment. Biomedicines. 2021;10(1):52. doi: 10.3390/biomedicines10010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6(11):e1356145. doi: 10.1080/2162402X.2017.1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaim H, Shanley M, Basar R, Daher M, Gumin J, Zamler DB, et al. Targeting the alphav integrin/TGF-beta axis improves natural killer cell function against glioblastoma stem cells. J Clin Invest. 2021;131(14):9–21. doi: 10.1172/JCI142116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C, Zheng S, Wang Z, Wang S, Wang X, Yang L, et al. KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer. Cancer Commun Lond. 2022;42(9):828–847. doi: 10.1002/cac2.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen N, Fang W, Lin Z, Peng P, Wang J, Zhan J, et al. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother. 2017;66(9):1175–1187. doi: 10.1007/s00262-017-2005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamarsheh S, Gross O, Brummer T, Zeiser R. Immune modulatory effects of oncogenic KRAS in cancer. Nat Commun. 2020;11(1):5439. doi: 10.1038/s41467-020-19288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam KHB, Leon AJ, Hui W, Lee SC, Batruch I, Faust K, et al. Topographic mapping of the glioblastoma proteome reveals a triple-axis model of intra-tumoral heterogeneity. Nat Commun. 2022;13(1):116. doi: 10.1038/s41467-021-27667-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cancer Genome Atlas Research N Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo HW. Targeting Ras-RAF-ERK and its interactive pathways as a novel therapy for malignant gliomas. Curr Cancer Drug Targets. 2010;10(8):840–848. doi: 10.2174/156800910793357970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J, Chen AX, Gartrell RD, Silverman AM, Aparicio L, Chu T, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25(3):462–469. doi: 10.1038/s41591-019-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng L, Wang Y, Qiu L, Chang Y, Lu H, Liu C, et al. mTOR pathway gene mutations predict response to immune checkpoint inhibitors in multiple cancers. J Transl Med. 2022;20(1):247. doi: 10.1186/s12967-022-03436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin A, Gu T, Hu X, Zhang J, Luo P. Comprehensive analysis identifies PI3K/Akt pathway alternations as an immune-related prognostic biomarker in colon adenocarcinoma patients receiving immune checkpoint inhibitor treatment. J Immunol Res. 2022;2022:8179799. doi: 10.1155/2022/8179799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumas AA, Pomella N, Rosser G, Guglielmi L, Vinel C, Millner TO, et al. Microglia promote glioblastoma via mTOR-mediated immunosuppression of the tumour microenvironment. EMBO J. 2020;39(15):e103790. doi: 10.15252/embj.2019103790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furnari FB, Cloughesy TF, Cavenee WK, Mischel PS. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat Rev Cancer. 2015;15(5):302–310. doi: 10.1038/nrc3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song J, Kadaba P, Kravitz A, Hormigo A, Friedman J, Belani P, et al. Multiparametric MRI for early identification of therapeutic response in recurrent glioblastoma treated with immune checkpoint inhibitors. Neuro Oncol. 2020;22(11):1658–1666. doi: 10.1093/neuonc/noaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. doi: 10.1056/NEJMoa1707358. [DOI] [PubMed] [Google Scholar]
- 41.Chiu D, Qi J, Thin TH, Garcia-Barros M, Lee B, Hahn M. A phase I trial of VEGF-A inhibition combined with PD-L1 blockade for recurrent glioblastoma. Cancer Res Commun. 2023 doi: 10.1158/2767-9764.CRC-22-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data have been deposited to SRA under the accession PRJNA922203 in the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/).