Abstract
Chimeric antigen receptor (CAR)-T cell therapy has impressive efficacy in hematological malignancies, but its application in solid tumors remains a challenge. Multiple hurdles associated with the biological and immunological features of solid tumors currently limit the application of CAR-T cells in the treatment of solid tumors. Using syngeneic mouse models, we recently reported that CAR-T cells engineered to concomitantly produce interleukin (IL)-7 and chemokine (C–C motif) ligand 19 (CCL19)-induced potent anti-tumor efficacy against solid tumors through an improved ability of migration and proliferation even in an immunosuppressive tumor microenvironment. In this study, for a preclinical evaluation preceding clinical application, we further explored the potential of IL-7/CCL19-producing human CAR-T cells using models that mimic the clinical features of solid tumors. Human anti-mesothelin CAR-T cells producing human IL-7/CCL19 achieved complete eradication of orthotopic pre-established malignant mesothelioma and prevented a relapse of tumors with downregulated antigen expression. Moreover, mice with patient-derived xenograft of mesothelin-positive pancreatic cancers exhibited significant inhibition of tumor growth and prolonged survival following treatment with IL-7/CCL19-producing CAR-T cells, compared to treatment with conventional CAR-T cells. Transfer of IL-7/CCL19-producing CAR-T cells resulted in an increase in not only CAR-T cells but also non-CAR-T cells within the tumor tissues and downregulated the expression of exhaustion markers, including PD-1 and TIGIT, on the T cells. Taken together, our current study elucidated the exceptional anti-tumor efficacy of IL-7/CCL19-producing human CAR-T cells and their potential for clinical application in the treatment of patients with solid tumors.
Supplementary Information
The online version contains supplementary material available at (10.1007/s00262-021-02853-3)
Keywords: CAR-T, CCL19, IL-7, Mesothelin, Patient-derived xenograft (PDX), T cell exhaustion
Introduction
While anti-CD19 chimeric antigen receptor (CAR)-T cell therapy has been remarkably successful in the treatment of hematological malignancies [1–4], its clinical application in solid tumors is yet to be achieved [5, 6]. Cumulative evidence has revealed multiple challenges in the application of CAR-T cells in the eradication of solid tumors. These include, and are not limited to, the difficulty in getting CAR-T cells to efficiently migrate into the stroma-rich tumor tissues, dysfunction of CAR-T cells in the immunosuppressive tumor microenvironment, and relapse of antigen-loss variant tumors due to the heterogeneous nature of the tumor cells with target antigens [7–10]. These challenges must be overcome to apply CAR-T cells for effective therapy of solid cancers, and various novel technologies have been proposed toward this purpose. Some of the promising approaches developed thus far include modifications to endow CAR-T cells with the capacity to produce specific cytokines, chemokines, and/or other immune-regulatory factors and the incorporation of novel intracellular signaling domains to increase the activation status and/or exhaustion resistance of CAR-T cells [11–16].
Regarding novel technologies to improve CAR-T cells, our group recently reported the generation of CAR-T cells that concomitantly express interleukin (IL)-7 and chemokine (C–C motif) ligand 19 (CCL19) (hereafter referred to as 7 × 19 CAR-T cells) to improve their anti-tumor efficacy against solid cancers in syngeneic mouse models [17]. The concept of 7 × 19 CAR-T cells was driven by the immunological finding that the specific combination of IL-7 and CCL19 is crucial for the formation and maintenance of a T cell zone in the secondary lymphoid organs [18, 19]. We found that 7 × 19 CAR-T cells enhanced the mobilization of the administered CAR-T cells and endogenous immune cells, including host T cells and dendritic cells (DCs) into the tumor tissues, and resulted in superior therapeutic effect against solid tumors, with long-term memory responses [17].
To translate 7 × 19 CAR-T cell therapy into clinical application, its efficacy and immunological mechanisms need to be confirmed experimentally in human T cells. Therefore, in this study, we generated a human version of 7 × 19 CAR-T cells and examined whether the 7 × 19 CAR-T cells derived from human T cells also exhibit superior therapeutic efficacy over conventional CAR-T cells in models of human solid cancers engrafted in immunodeficient mice. We employed two experimental models of human solid cancers: an orthotopic cancer model using a mesothelin-positive human malignant mesothelioma cell line and a patient-derived xenograft (PDX) model by implanting mesothelin-positive pancreatic cancer tissues. Our current study revealed that human anti-mesothelin 7 × 19 CAR-T cells induced potent therapeutic effects in both these models. The underlying mechanisms were further investigated through the analysis of tumor-infiltrating lymphocytes (TILs) and tumor re-challenge experiments.
Materials and methods
Mice and cell lines
NOD.Cg-PrkdcscidIl2rgtm1Wjl/Szj (NSG) mice (female, 7–9 weeks old) purchased from Charles River Laboratories Japan Inc. (Kanagawa, Japan) were used in all the experiments. The mice were maintained under specific pathogen-free conditions in the animal facility at the Yamaguchi University. All the animal procedures were approved by the Institutional Animal Care and Use Committee of the Yamaguchi University. Human malignant mesothelioma cell line, ACC-MESO1, was established at the Aichi Cancer Research Center Institute (Aichi, Japan), and transduced to stably express GFP and firefly luciferase genes and (hereafter referred to as ACC-MESO1-GFP-Luc) at the Yamaguchi University. Another human malignant mesothelioma cell line, NCI-H28, was purchased from American Type Culture Collection (Manassas, VA, USA). ACC-MESO1, ACC-MESO1-GFP-Luc and NCI-H28 cells were cultured in RPMI-1640 (Gibco, Grand Island, NY, USA) medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gemini Bio Products, West Sacramento, CA, USA), 1% penicillin–streptomycin sulfate (Wako, Osaka, Japan), 25 mM HEPES (Sigma-Aldrich, St. Louis, MO, USA), and 50 mM 2-mercaptoethanol (Thermo Fisher Scientific, Waltham, MA, USA). Human colorectal cancer cell line, SW620, was purchased from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan) and cultured in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 10% FBS and 1% penicillin–streptomycin sulfate. Murine ovarian cancer cell line, OV2944-HM-1 (HM-1), was purchased from the RIKEN BioResource Research Center (Ibaraki, Japan) and genetically modified to stably express human mesothelin. HM-1 cells were cultured in minimum essential medium-alpha (MEM-alpha, Gibco) supplemented with 10% FBS and 1% penicillin–streptomycin sulfate.
Construction of CAR-expressing vectors and transduction of human T cells
Anti-human mesothelin single-chain variable fragment (scFv) was generated as described in previous reports [20, 21]. CAR construct was designed by ligating the scFv to the transmembrane domain of human CD8α chain and cytoplasmic regions of human CD28, 4-1BB, and CD3ζ molecules and then cloned into the retroviral vector, pMSGV1 [22, 23]. To express human IL-7, CCL19, and EGFP in addition to CAR, self-cleaving 2A peptide sequence was inserted among these genes. Transduction of human T cells was performed as described previously [23]. Briefly, retroviruses were produced by the transfection of the CAR-expressing plasmid into GP2-293 packaging cells together with pAmpho envelope vector plasmid (Retro-X Universal Packaging System, Clontech, Mountain View, CA, USA). The culture supernatant containing the retroviruses was harvested and used to infect healthy donor-derived activated PBMCs in the presence of RetroNectin (Takara Bio, Shiga, Japan). The cells were incubated with OpTmizer (Gibco) supplemented with OpTmizer CTS, CTS Immune Cell serum replacement, L-Glutamine (Gibco), 1% penicillin–streptomycin sulfate, and amphotericin B (Bristol Myers Squibb, New York, NY, USA) for 5 d in the presence of IL-2. The transduction efficiency of CAR was assessed using flow cytometry. To evaluate cytokine production, the concentrations of human IL-7 and CCL19 in the culture supernatants were measured using the corresponding ELISA kits (R&D, Minneapolis, MN, USA).
Flow cytometry
PE-conjugated anti-human mesothelin mAb (clone 420,411, R&D) was used to detect the surface mesothelin. The CAR-transduced T cells were stained with APC-conjugated anti-CD8 (clone RPA-T8, BioLegend, San Diego, CA, USA), 6-His-tagged recombinant human mesothelin protein (BioLegend), and secondary PE-conjugated anti-6-His mAb (clone RM146, Abcam, Cambridge, UK). Zombie Yellow viability dye (BioLegend) and PE-conjugated anti-CD45 mAb (clone HI30, BioLegend) were used for in vitro co-culture assay. TILs were analyzed using 7-AAD staining solution (BD Biosciences, San Jose, CA, USA), APC-Cy7-conjugated anti-CD3 mAb (clone SK7, BD Biosciences), BV480-conjugated anti-CD4 mAb (clone SK3, BD Biosciences), PE-Cy7-conjugated anti-CD8 mAb (clone RPA-T8, BD Biosciences), BV421-conjugated anti-PD-1 mAb (clone EH12.1, BD Biosciences), and APC-conjugated anti-TIGIT mAb (clone MBSA43, eBioscience). Flow cytometric data were acquired using the BD LSRFortessa™ X-20 cell analyzer (BD Biosciences), EC800 (SONY, Tokyo, Japan), or CytoFLEX (Beckman Coulter, Brea, CA, USA). Data were analyzed using the FlowJo software (FlowJo LLC, Ashland, OR, USA).
In vitro cytotoxicity assay
For in vitro cytotoxicity assay, CAR-T or un-transduced T cells (1 × 105 cells/well) were co-cultured with tumor cells at an effector to target (E:T) ratio of 1:1, 1:3, or 1:5 for 48 h. The cultured cells were then harvested and stained with Zombie Yellow viability dye and anti-CD45 mAb, followed by flow cytometric analysis to detect the residual tumor cells and T cells. The level of interferon (IFN)-γ secreted by the stimulated T cells into the culture supernatants was assessed using an ELISA kit (BioLegend).
In vivo orthotopic mouse model of pleural mesothelioma
Anesthetized NSG mice were intrapleurally inoculated with 2 × 106 ACC-MESO1-GFP-Luc tumor cells on day 0. On days 1 or 10, 1 × 105 CAR-T or un-transduced T cells were injected intravenously (i.v.) through the tail vein. CAR transduction efficiency, which varied among the CAR constructs, was adjusted to the same percentage by adding un-transduced T cells prior to the injection. Tumor burden was periodically measured using the IVIS Spectrum In Vivo Imaging System (Perkin Elmer, Waltham, MA, USA) and analyzed using the Living Image Software (Perkin Elmer).
In vivo PDX tumor mouse model
Pancreatic cancer patient-derived tumor tissues were purchased from the Central Institute for Experimental Animals (Kanagawa, Japan). The PDX tumors were maintained by successive implantation into NSG mice. For use in the experiments, the PDX tumors were resected, carved into 3 × 3 mm blocks, and implanted in an incision in the subcutaneous cavity on the right flank of the NSG mice. Nine days later, the mice were treated with an i.v. injection of 3 × 106 CAR-T cells. Tumor size was measured twice a week using a digital caliper and the tumor volume was calculated using the following formula: (major axis of tumor) × (minor axis of tumor)2/2. The mice were euthanized when the tumor volume reached 1000 mm3. To analyze T cells infiltrating into the PDX tumors, the tumors were resected 5 d after the administration of CAR-T cells, minced with scissors, and digested with medium containing liberase TL (Roche Diagnostics, Basel, Switzerland) and DNase I (Roche Diagnostics) for 30 min at 37 ℃. The digested tumor samples were homogenized by repetitive pipetting and passed through a cell strainer to generate single-cell suspension, followed by staining with antibodies for flow cytometric analyses. For the tumor re-challenge experiments, the murine ovarian cancer cell line, HM-1, was genetically modified to stably express human mesothelin. NSG mice that had previously rejected PDX tumor following treatment with 7 × 19 CAR-T cells were inoculated subcutaneously (s.c.) with 1 × 106 mesothelin-positive HM-1 or parental mesothelin-negative HM-1 cells on the right and left flanks of the mice, respectively. The tumor size was measured twice a week with a digital caliper.
Immunohistochemical staining
To confirm the expression of mesothelin on the pancreatic cancer PDX tumor, formalin-fixed and paraffin-embedded tumor tissues were stained immunohistochemically using anti-human mesothelin mAb (clone 5B2, Leica Biosystems, Buffalo Grove, IL, USA). Anti-mouse IgG1 mAb (clone MG1-45, BioLegend) was used as an isotype control.
Statistical analysis
One-way analysis of variance was used to compare two groups. For the mouse survival data, Kaplan–Meier survival curves was generated, and statistical differences were analyzed using the log-rank test. JMP Pro 14 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. P < 0.05 was considered as statistically significant.
Results
Generation of anti-human mesothelin CAR-T cells producing IL-7 and CCL19
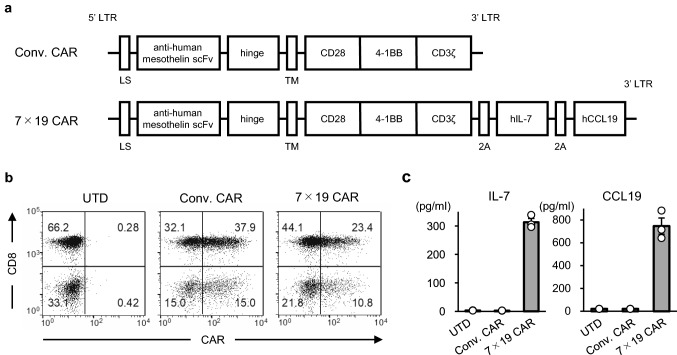
To investigate the anti-tumor efficacy of human CAR-T cells that simultaneously produce IL-7 and CCL19, we used anti-mesothelin CAR cells [20, 21]. We constructed anti-human mesothelin CAR containing signaling motifs consisting of CD28, 4-1BB and CD3ζ sequences, which was further connected with IL-7 and CCL19 sequences through a 2A self-cleavable linker sequence (hereafter referred to as 7 × 19 CAR) (Fig. 1a). As a control, conventional anti-human mesothelin CAR without IL-7 and CCL19 cassette was also constructed (hereafter referred to as Conv. CAR). When human PBMC were transduced with retroviral vectors encoding either Conv. CAR or 7 × 19 CAR, the transduction efficiencies were approximately 53 and 34%, respectively (Fig. 1b). Significant secretion of human IL-7 and CCL19 was detected in the culture supernatants of 7 × 19 CAR-T cells but not in that of Conv. CAR-T or un-transduced (hereafter referred to as UTD) T cells (Fig. 1c).
Fig. 1.
Generation of anti-human mesothelin CAR-T cells producing human IL-7 and CCL19. a Schematic representation of Conv. CAR and 7 × 19 CAR against human mesothelin. LS; leader sequence, TM; transmembrane domain. b Human PBMCs transduced with Conv. CAR or 7 × 19 CAR were stained with recombinant mesothelin protein to detect CAR expression, along with anti-CD8 Ab. UTD T cells were examined, as a control. The percentage of cells in each quadrant are indicated. c The culture supernatants from Conv. CAR-T and 7 × 19 CAR-T cells were harvested 4 days after gene transduction, and the concentrations of IL-7 and CCL19 were measured using ELISA. As a control, the culture supernatant from UTD T cells at the same time point were examined. Open circle represents the value of an individual sample. Data are shown as mean ± standard deviation (SD) of triplicate samples
In vitro anti-tumor response of anti-human mesothelin 7 × 19 CAR-T cells
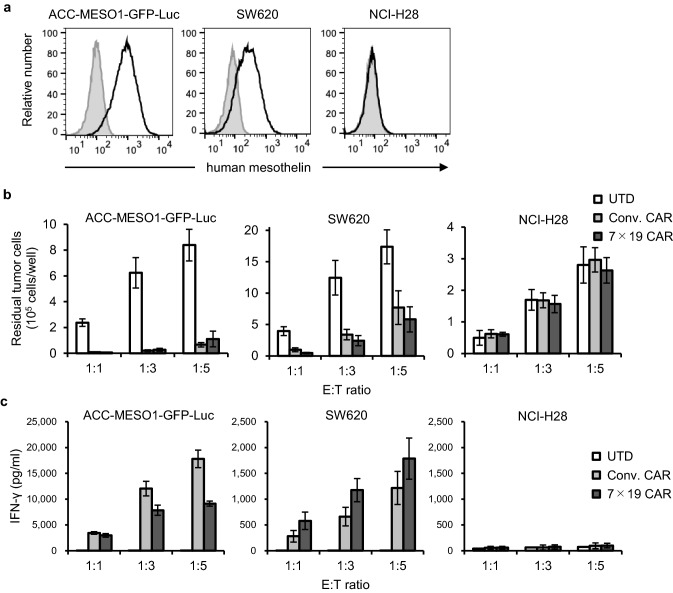
We next examined the immunological functions of Conv. and 7 × 19 CAR-T cells in response to human tumor cell lines expressing endogenous mesothelin on their cell surface. As a target tumor, we used ACC-MESO1-GFP-Luc, a malignant mesothelioma cell line with high expression of endogenous mesothelin (Fig. 2a) and exogenously transfected them with GFP and luciferase. SW620, a colorectal cancer cell line with moderate expression of endogenous mesothelin, was also used. NCI-H28, a malignant mesothelioma cell line lacking mesothelin expression, was used as a negative control. When co-cultured with these tumor cells, both Conv. CAR and 7 × 19 CAR-T cells significantly reduced the number of residual mesothelin-positive tumor cells compared to UTD T cells. Further, the ACC-MESO1-GFP-Luc cells were more susceptible to the killing than SW620 cells (Fig. 2b). Conv. CAR and 7 × 19 CAR-T cells did not exhibit any killing activity against the mesothelin-negative NCI-H28 cells, confirming the specificity of CAR. In addition, the secretion of IFN-γ by Conv. CAR and 7 × 19 CAR-T cells, but not UTD T cells, was detected during co-culture with mesothelin-positive tumor cells, while considerably higher level of IFN-γ was induced by ACC-MESO1-GFP-Luc compared to SW620 cells (Fig. 2c). Negligible level of IFN-γ was detected during co-culture with NCI-H28. Interestingly, 7 × 19 CAR-T cells made more IFN-γ compared to Conv. CAR-T cells in response to SW620, but not to the ACC-MESO1-GFP-Luc cells. To validate this observation, we employed two additional tumor cell lines, NCI-H2052 and HCT116, which express mesothelin at high and low levels, respectively. It was found that 7 × 19 CAR-T cells produced more IFN-γ in response to HCT116, but not NCI-H2052 (Supplementary Fig. 1), similarly to the results in Fig. 2c. Regarding these findings, one possible explanation is that decreased intensity of CAR signal in response to antigen-low target cells can be complemented by IL-7 receptor signal in 7 × 19 CAR-T cells, leading to the enhanced production of IFN-γ.
Fig. 2.
Mesothelin-specific responses of Conv. CAR and 7 × 19 CAR-T cells in vitro . a Surface expression of endogenous mesothelin was assessed in three human solid cancer cell lines using flow cytometry. Filled and open histograms indicate unstained and stained samples, respectively. b Conv. CAR-T, 7 × 19 CAR-T, or UTD T cells were co-cultured with the indicated tumor cells at effector to target (E:T) ratio of 1:1, 1:3, and 1:5 for 2 days. The number of residual tumor cells was analyzed using flow cytometry. Data are shown as mean ± SD of triplicate samples. c The supernatants of co-cultured cells described in b were harvested and the concentration of IFN-γ was assessed using ELISA. Data are shown as mean ± SD of triplicate samples. Representative data from three independent experiments are shown
Enhanced anti-tumor efficacy of 7 × 19 CAR-T cells in an orthotopic model of human mesothelioma
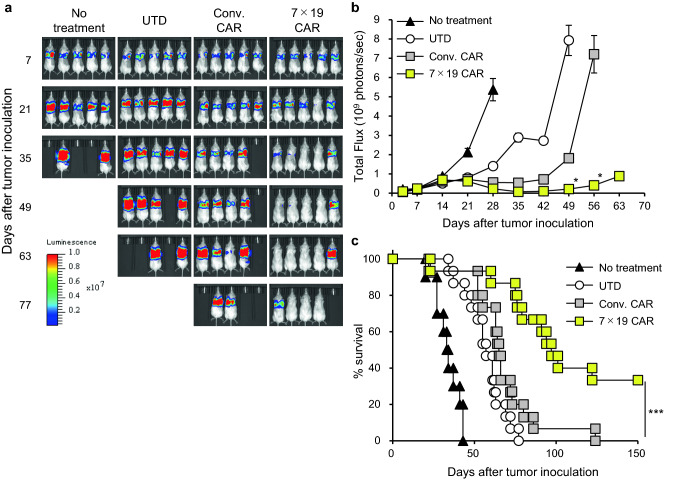
To investigate the anti-tumor activity of 7 × 19 CAR-T cells in vivo, we established an orthotopic model of human malignant mesothelioma. Immunodeficient NSG mice were intrapleurally inoculated with ACC-MESO1-GFP-Luc tumor cells on day 0, and then treated with i.v. injection of Conv. CAR-T, 7 × 19 CAR-T, or UTD T cells on day 10, or left untreated. The growth of the mesothelioma cells was evaluated through bioluminescence imaging assessed using the IVIS. Treatment with Conv. CAR-T and 7 × 19 CAR-T cells inhibited the growth of the mesotheliomas and the anti-tumor effect of 7 × 19 CAR-T cells was significantly more potent and sustainable compared to that of Conv. CAR-T cells (Fig. 3a and b). UTD T cells showed a modest inhibition of tumor growth, possibly due to allogeneic responses of UTD T cells against the tumors. Survival of the mice was significantly prolonged following treatment with 7 × 19 CAR-T cells compared to Conv. CAR-T or UTD T cells (Fig. 3c). Treatment with 7 × 19 CAR-T cells achieved complete tumor regression in 33% of the mice, whereas all the mice eventually died from the tumor in the groups treated with Conv. CAR-T or UTD T cells. Thus, 7 × 19 CAR-T cells demonstrated potent therapeutic effect in the orthotopic model of human malignant mesothelioma. To reveal the functional phenotypes of 7 × 19 CAR-T cells in the tumor microenvironment, we examined the ability of TILs to express cytokines and effector molecules, and found that significant increases of TNF-α and Granzyme B, as well as a trend of increased IFN-γ, in 7 × 19 CAR-T cells (Supplementary Fig. 2). It was also revealed that Ki-67 expression on 7 × 19 CAR-T cells was upregulated compared to that of non-CAR-T cells (data not shown). Taken together, 7 × 19 CAR-T cells appear to have potent effector functions with an increased proliferative activity in this model.
Fig. 3.
Anti-tumor effects of 7 × 19 CAR-T cells in orthotopic model of human malignant mesothelioma Immunodeficient NSG mice were intrapleurally inoculated with 2 × 106 ACC-MESO1-GFP-Luc cells on day 0, followed by i.v. injection of 1 × 105 Conv. CAR-T, 7 × 19 CAR-T, or UTD T cells on day 10, or left untreated. Tumor growth was assessed using IVIS every week. a Representative bioluminescence images of the mice are shown. b Total flux of whole-body bioluminescence measured by IVIS is shown as mean ± standard error of the mean (SEM). Data from two independent experiments are combined (n = 10 mice for no treatment group; n = 15 for UTD T and Conv. CAR-T cell treatment groups; n = 14 for 7 × 19 CAR-T cells treatment group). *P < 0.05 by one-way ANOVA. c Mouse survival of each group is shown. ***P < 0.001 by log-rank test (comparison between Conv. CAR-T and 7 × 19 CAR-T cells)
Capability of 7 × 19 CAR-T cells to prevent relapse of malignant mesothelioma
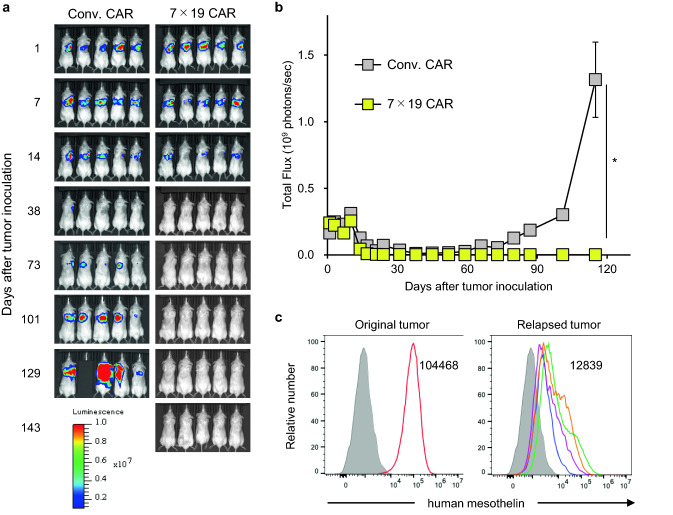
Although CAR-T cells demonstrate notable therapeutic effect in hematological malignancies, one potential issue is the late-phase relapse of tumors following temporal clinical response [24]. In this regard, we previously reported that mouse 7 × 19 CAR-T cells induced a long-lasting anti-tumor memory response [17]. Thus, we next investigated whether the human 7 × 19 CAR-T cells offer the similar advantage of protecting from tumor relapse in the orthotopic tumor model. NSG mice were inoculated intrapleurally with ACC-MESO1-GFP-Luc tumor cells on day 0, and then treated with i.v. injection of Conv. CAR-T or 7 × 19 CAR-T cells on day 1. In this model, treatment with Conv. CAR-T cells induced a transient remission of malignant mesothelioma by day 38, followed by a late-phase tumor relapse and eventual death of all mice by day 143 (Fig. 4a, b). In sharp contrast, the mice treated with 7 × 19 CAR-T cells rejected the tumor and maintained tumor-free condition without relapse over 143 days. We found that a residual number of 7 × 19 CAR-T cells was higher than that of Conv. CAR-T cells on day 39, when both treatments similarly rejected the tumor cells (data not shown). In addition, majority of residual T cells in the mice survived over 140 days by 7 × 19 CAR-T cell therapy demonstrated CD45RA-negative, CCR7-negative effector memory phenotype (Supplementary Fig. 3). To further explore the potential mechanisms of tumor relapse observed in the Conv. CAR-T cell-treated mice, we next examined the cell surface expression of mesothelin in the relapsed tumors. All the relapsed tumors showed a marked downregulation of mesothelin expression on the cell surface compared to the original tissue-cultured tumor cells prior to inoculation (Fig. 4c). Taken together these results, 7 × 19 CAR-T cells have a feature quantitatively and qualitatively superior to Conv. CAR-T cells in our model, which leads to a long-lasting therapeutic effect by preventing the persistence or emergence of tumors with downregulated target antigens.
Fig. 4.
Prevention of orthotopic tumor relapse by the treatment with 7 × 19 CAR-T cells Immunodeficient NSG mice were intrapleurally inoculated with 2 × 106 ACC-MESO1-GFP-Luc cells on day 0, followed by i.v. injection of 1 × 105 Conv. CAR-T or 7 × 19 CAR-T cells on day 1. Tumor growth was assessed using IVIS every week. a Representative bioluminescence images of the mice are shown. b Total flux of whole-body bioluminescence measured by IVIS is shown as mean ± SEM (representative data from two independent experiments). c Relapsed ACC-MESO1-GFP-Luc tumor cells were harvested from the chest cavity of the mice, which were treated with Conv. CAR-T cells and euthanized on day 79–126 due to massive tumor relapse, and individually assessed for the expression of endogenous mesothelin using flow cytometry (right panel, open histograms with four distinct colors indicating four individual relapsed tumors). As a control, the expression level of endogenous mesothelin in the original ACC-MESO1-GFP-Luc tumor cells was also examined (left panel, open histogram). The filled histogram indicates an unstained control. The numbers in the histogram indicate the mean fluorescence intensity of mesothelin expression in the original and relapsed tumors (average of four individual relapsed tumors in right panel)
Potent anti-tumor effects of 7 × 19 CAR-T cells against pancreatic cancer in PDX model
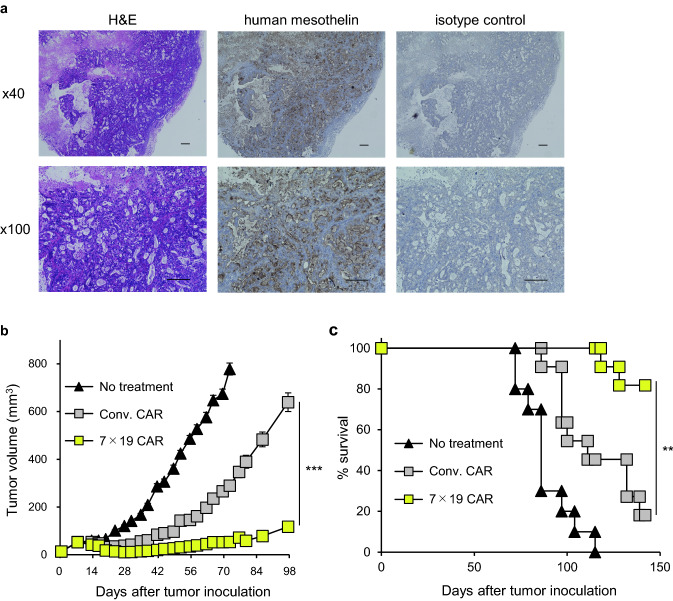
Inoculation of suspended tumor cells from in vitro culture conditions does not completely mimic the actual heterogeneous structure and microenvironment of solid tumors. To closely replicate the characteristics of original human tumors, we used a PDX model to examine the anti-tumor effects of 7 × 19 CAR-T cells. Mesothelin-positive human pancreatic tumor blocks were implanted s.c. into NSG mice and confirmed to establish a solid tumor mass expressing high level of mesothelin (Fig. 5a). The mice were then treated with an i.v. injection of Conv. CAR-T or 7 × 19 CAR-T cells or left untreated. Following treatment with Conv. CAR-T cells, ten out of eleven mice developed PDX tumor masses, of which nine mice died by day 147 (Fig. 5b and c). In sharp contrast, nine out of eleven mice treated with 7 × 19 CAR-T cells survived beyond 147 days, of which five mice achieved complete regression of the PDX tumor. These results confirmed the potent anti-tumor effects of 7 × 19 CAR-T cells in the PDX model of human pancreatic tumor.
Fig. 5.
Improved anti-tumor effects of 7 × 19 CAR-T cells in PDX model of human pancreatic cancer . a NSG mice were implanted s.c. with human pancreatic tumor blocks on day 0, and the established solid tumor masses were harvested on day 99. Images of H&E and immunohistochemical staining with anti-human mesothelin or isotype control Ab are shown. Scale bar indicates a length of 200 μm. b and c NSG mice were implanted s.c. with human pancreatic tumor blocks on day 0, and then treated with i.v. injection of 3 × 106 Conv. CAR-T or 7 × 19 CAR-T cells on day 9 or left untreated. Thereafter, the tumor size (b) and mouse survival (c) were assessed. In (b), the tumor volume is shown as mean ± SEM. Representative data from two independent experiments are shown. ***P < 0.001 by one-way ANOVA. In (c), data from two independent experiments are combined (n = 10 for untreated group; n = 11 for Conv. CAR-T, and 7 × 19 CAR-T cell-treated groups). **P < 0.01 by log-rank test
Mechanistic analyses of the enhanced anti-tumor effects of 7 × 19 CAR-T cells in PDX pancreatic tumor model
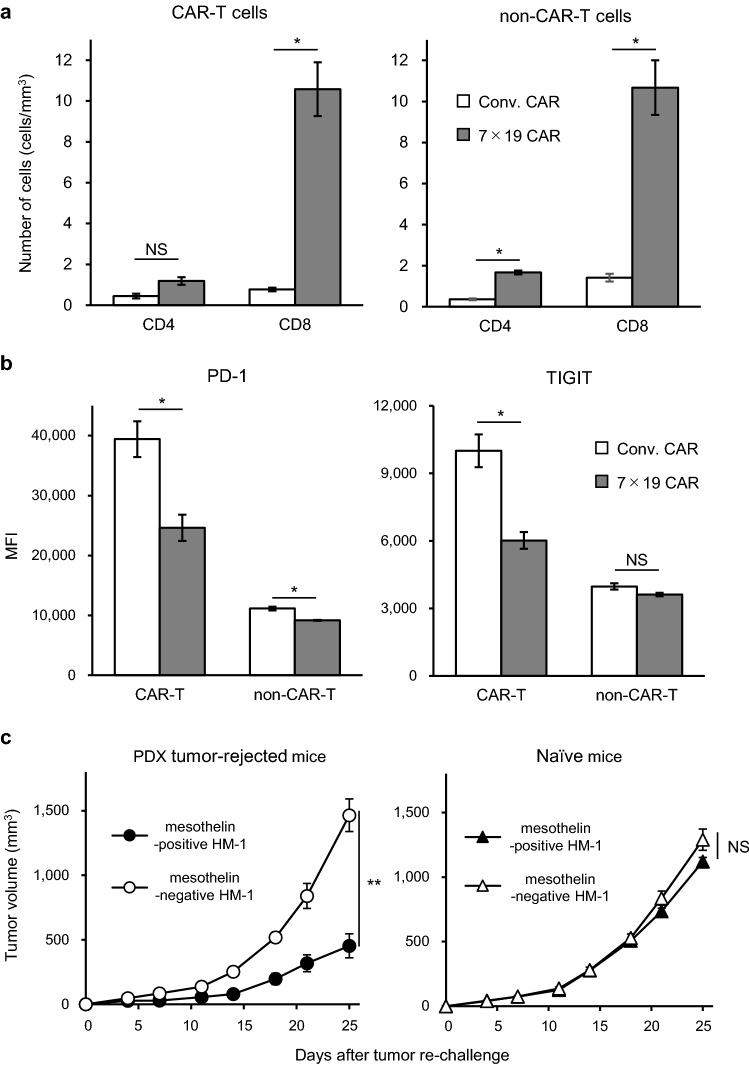
To explore the mechanism underlying the notable anti-tumor effects induced by 7 × 19 CAR-T cells, we examined the phenotypes of the tumor-infiltrating lymphocytes (TILs) in the PDX tumor. The number of CAR-T cells in the TILs harvested from the mice treated with 7 × 19 CAR-T cells was approximately eight times higher than those from the mice treated with Conv. CAR-T cells (Fig. 6a). Furthermore, the number of non-CAR-T cell in the TILs was also significantly increased in the mice treated with 7 × 19 CAR-T cells compared to those treated with Conv. CAR-T cells, even though the same number of non-CAR-T cells was injected. These results indicate that IL-7 and CCL19 produced by the 7 × 19 CAR-T cells enhanced the infiltration of the non-CAR-T cells as well as CAR-T cells, which was consistent with our previous findings with the mouse 7 × 19 CAR-T cells [17]. We also examined the exhaustion phenotype of the TILs from the PDX tumors and found that the expression level of both PD-1 and TIGIT were significantly downregulated in the CD8-positive CAR-T cells in the TILs of 7 × 19 CAR-T cell-treated mice, compared to those from Conv. CAR-T cell-treated mice (Fig. 6b). In addition, downregulation of PD-1 expression was also detected in CD8-positive non-CAR-T cells of TILs in PDX tumors treated by 7 × 19 CAR-T cells. These results suggested that 7 × 19 CAR-T cells induced potent anti-tumor efficacy in both quantity and quality, even in the immunosuppressive pancreatic cancer PDX model.
Fig. 6.
Mechanistic analyses of the enhanced anti-tumor effects of 7 × 19 CAR-T cells in the PDX tumor model (a and b) NSG mice were implanted s.c. with human pancreatic tumor blocks on day 0, and then treated with i.v. injection of 3 × 106 Conv. CAR-T or 7 × 19 CAR-T cells on day 9. On day 14, the tumor mass was extracted and enzymatically digested to a obtain single-cell suspension, followed by flow cytometric analysis. Expression level of human CD3, CD4, CD8, and EGFP (as markers for CAR expression), and that of PD-1 and TIGIT was examined. a The number of CAR-T and non-CAR-T cells in the TILs are shown (as per tumor volume). Data are expressed as the mean ± SEM. *P < 0.05 calculated using one-way ANOVA. NS: not significant. b The expression level of PD-1 (left) and TIGIT (right) on CD8-positive CAR-T cells and non-CAR-T cells are shown. Data are expressed as the mean ± SEM. *P < 0.05 calculated using one-way ANOVA. NS: not significant. (c) NSG mice which had rejected PDX tumor and survived over 147 days following treatment with 7 × 19 CAR-T cells as described in Fig. 5c, or naïve NSG mice, were inoculated s.c. with 1 × 106 cells of mesothelin-positive and negative HM-1 on the right and left flanks, respectively. Tumor sizes were periodically measured using a caliper. Data are expressed as the mean ± SEM. **P < 0.01 by one-way ANOVA. NS: not significant
To further explore the anti-tumor memory response, we re-challenged the NSG mice which had previously rejected the PDX tumors and survived over 147 days following the treatment with 7 × 19 CAR-T cells with mesothelin-positive or negative tumors. To this end, mouse ovarian cancer cell line, HM-1, which was originally mesothelin-negative, was genetically modified to stably express human mesothelin. The PDX tumor-rejected NSG mice were inoculated s.c. with mesothelin-positive and negative HM-1 on the right and left flank, respectively. As a control, naïve NSG mice were also inoculated s.c. with these tumor cells in a similar manner. We found that the growth of the human mesothelin-positive tumors was significantly inhibited in the PDX-rejected mice, but not in naïve mice (Fig. 6c). These results suggested that 7 × 19 CAR-T cells were engrafted in the PDX tumor-rejected NSG mice and maintained antigen-specific memory functions to protect from relapse.
Discussion
In this study, we engineered human CAR-T cells to produce human IL-7 and CCL19 and evaluated their anti-tumor potential against solid tumors in mouse models of orthotopic malignant mesothelioma and PDX pancreatic cancer. Consistent with our previous findings with mouse 7 × 19 CAR-T cells [17], the concomitant production of IL-7 and CCL19 by the human CAR-T cells significantly augmented their therapeutic effects on solid tumors and their potential to protect against tumor relapse, compared to that by conventional CAR-T cells, and induced a long-term memory response specific to the CAR target. Analyses of the TILs indicated that the anti-tumor effects of human 7 × 19 CAR-T cells was caused, at least in part, by their improved ability to infiltrate and/or proliferate in the tumor tissues, as well as their property of exhaustion resistance.
To date, various obstacles in using CAR-T cells to eliminate solid tumors have been highlighted [25]. Such obstacles include insufficient migration and infiltration of the transferred CAR-T cells in the tumor tissues, immunosuppressive conditions in the tumor microenvironment, antigenic heterogeneity of the cancer cells, and complexity of the cellular components constituting the solid tumors. To address these obstacles, we developed 7 × 19 CAR-T cells and demonstrated their superior effect in the treatment of solid tumors in mouse syngeneic models [17]. Mechanistically, 7 × 19 CAR-T cells induced massive infiltration of both transferred CAR-T and endogenous immune cells including host T cells and DCs. The endogenous T cells as well as the transferred 7 × 19 CAR-T cells are indispensable for the potent anti-tumor efficacy, suggesting a possibility of epitope spreading following tumor cell killing by the CAR-T cells. While these findings support the translation of this technology into clinical application, further studies are necessary to confirm the efficacy and mechanisms using human 7 × 19 CAR-T cells in models that closely mimic clinical tumors. In particular, since subcutaneous inoculation of tumor cells has been employed for establishing the mouse syngeneic solid tumor models irrespective of the origin of tumors, further evaluation in orthotopic tumor models is necessary. In addition, the heterogeneity of human solid tumor cells and the surrounding stromal cells should be replicated with appropriate models such as the PDX tumors.
In this study, we first examined the therapeutic potential of human 7 × 19 CAR-T cells against mesothelin using orthotopic models of malignant mesothelioma, in which human tumor cells were inoculated intrapleurally into immunodeficient mice. Human 7 × 19 CAR-T cells not only demonstrated superior anti-tumor therapeutic effects compared to Conv. CAR-T cells but were also effective in relapse models of orthotopic tumor. In the latter model, Conv. CAR-T cells failed to circumvent the relapse of tumors with downregulated mesothelin expression, indicating the validity of our model, which replicates one of the crucial causes of the failure of CAR-T cell therapy in the clinic [26–28]. While the precise mechanism of antigenic escape in the tumors remains to be identified, it is possible that tumor cells develop de novo mutations to diminish tumor antigens in response to the attack by the CAR-T cells. It should be noted that, even in such a model, 7 × 19 CAR-T cells successfully prevented tumor relapse. One potential interpretation of this result is that the 7 × 19 CAR-T cells had the potential to fully eliminate tumor cells prior to the occurrence of the antigen-loss mutation. This interpretation is supported by our finding that 7 × 19 CAR-T cells showed an enhanced IFN-γ production in response to antigen-low tumor cells, compared to Conv. CAR-T cells, and that residual 7 × 19 CAR-T cell number was higher than Conv. CAR-T cells at the timing when the tumors became undetectable in this model. In addition, the decreased expression of PD-1 and TIGIT on 7 × 19 CAR-T cells also indicated their competent functionality without an exhaustion.
In the current study, we also examined whether 7 × 19 CAR-T cells show a superior therapeutic effect in PDX tumor, which was derived from unfractionated primary tumor tissue of cancer patients and, thus, maintains the heterogeneous nature of tumor antigenicity and cellular components within tumor tissues. We used PDX tumors derived from pancreatic cancer, which are known to be composed of abundant tumor-associated stroma cells and characterized by highly immunosuppressive tumor microenvironments [29]. Our study revealed that 7 × 19 CAR-T cells outperformed Conv. CAR-T cells in tumor growth suppression and prolongation of mouse survival even in the PDX model. Although several previous studies have shown the therapeutic effects of CAR-T cells in the PDX tumor model [30–37], the current study is the first, to the best of our knowledge, to demonstrate the superiority of cytokine/chemokine-producing CAR-T cells over Conv. CAR-T cells in PDX pancreatic tumors. Thus, our results revealed a promising feature of human 7 × 19 CAR-T cells for clinical application.
In tumor models involving the transfer of human CAR-T cells into immunodeficient mice, graft versus host disease caused by the xenogeneic T cell responses is inevitable [38]. In addition, since CAR-T cells were manufactured from healthy donor-derived T cells in this study, allogeneic responses of CAR-T cells against tumor tissues due to a disparity of MHC are also induced. Thus, the anti-tumor effects observed in our models may be the sum of both tumor-specific and non-specific responses, implying a possibility of overestimation. Nonetheless, the tumor re-challenge experiments clearly demonstrated that human 7 × 19 CAR-T cells established mesothelin-specific anti-tumor responses. Thus, xenogeneic or allogeneic responses caused by human CAR-T cells did not undermine the superiority of the 7 × 19 CAR-T cell technology over Conv. CAR-T cells. Results of the re-challenge experiments also manifested as long-term persistence of functional 7 × 19 CAR-T cells in vivo.
In conclusion, in this study, we demonstrated for the first time that human CAR-T cells producing human IL-7 and CCL19 can generate the potent therapeutic efficacy against solid tumors, similar to those observed with mouse 7 × 19 CAR-T cells. Although careful evaluation of its safety is still necessary, our current results are clearly encouraging and support investigation of the therapeutic advantage of 7 × 19 CAR-T cells in clinical settings to translate this technology to address the unmet medical need of improved therapies for patients with solid tumors.
Supplementary Information
Acknowledgment
The authors would thank Drs. Daisuke Umezu, Jun Mori, Takahiro Sasaki, Yasunori Iida for excellent advices on the study. The authors would also thank Hiromi Kurosawa, Mihoko Ida, Nana Okada, Makiko Miyamoto, Satoshi Tatekabe, Nanami Nakamura, and Chisaki Mochida for their excellent technical supports.
Abbreviations
- CAR
Chimeric antigen receptor
- DCs
Dendritic cells
- E:T
Effector to target
- IL
Interleukin
- IFN
Interferon
- I.v.
Intravenously
- NSG
NOD.Cg-PrkdcscidIl2rgtm1Wjl/Szj
- PD-1
Programed cell death 1
- PDX
Patient-derived xenograft
- S.c.
Subcutaneously
- ScFv
Single-chain variable fragment
- TIGIT
T cell immunoreceptor with Ig and ITIM domains
- TILs
Tumor-infiltrating lymphocytes
Author contributions
S.G., Yu.S., K.A. and K.T. designed the study and interpreted the data; S.G. performed the experiments and analyzed the data; Yo.S. and S.Y. provided the materials; M.E. and K.T. supervised the study; S.G. and K.T. wrote the manuscript.
Funding
This study was supported by Practical Research for Innovative Cancer Control, and Project for Cancer Research and Therapeutic Evolution (P-CREATE) 16770206 (to K.T.) by Japan Agency for Medical Research and Development (AMED), and Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (C) Grant Number 19K07625 (to Yu.S.).
Availability of data and material
The datasets generated and analyzed during the current study are available from the corresponding author upon a reasonable request.
Compliance with ethical standards
Conflicts of interest
K.T. and Yu.S. hold stocks of Noile-Immune Biotech Inc., and receive remuneration from Noile-Immune Biotech Inc. Other authors declare no conflict of interest.
Ethical approval
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Yamaguchi University. Human PBMCs were derived from healthy volunteers who gave a written informed consent. Patient-derived xenograft tumor was derived from a pancreatic cancer patient who gave written informed consent. This study was approved by the ethics committee of the Yamaguchi University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY. Axicabtagene ciloleucel car t-cell therapy in refractory large b-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jager U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R, Chu J, Anak O, Salles G, Maziarz RT. Tisagenlecleucel in adult relapsed or refractory diffuse large b-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 5.June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 6.Guedan S, Ruella M, June CH. Emerging cellular therapies for cancer. Annu Rev Immunol. 2019;37:145–171. doi: 10.1146/annurev-immunol-042718-041407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majzner RG, Mackall CL. Tumor antigen escape from Car t-cell therapy. Cancer Discov. 2018;8(10):1219–1226. doi: 10.1158/2159-8290.CD-18-0442. [DOI] [PubMed] [Google Scholar]
- 8.Petty AJ, Heyman B, Yang Y. Chimeric antigen receptor cell therapy: overcoming obstacles to battle cancer. Cancers (Basel) 2020 doi: 10.3390/cancers12040842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25(2):268–276. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Martinez M, Moon EK. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019;10:128. doi: 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4(3):e994446. doi: 10.4161/2162402x.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shum T, Omer B, Tashiro H, Kruse RL, Wagner DL, Parikh K, Yi Z, Sauer T, Liu D, Parihar R, Castillo P, Liu H, Brenner MK, Metelitsa LS, Gottschalk S, Rooney CM. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 2017;7(11):1238–1247. doi: 10.1158/2159-8290.CD-17-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima M, Sakoda Y, Adachi K, Nagano H, Tamada K. Improved survival of chimeric antigen receptor-engineered T (CAR-T) and tumor-specific T cells caused by anti-programmed cell death protein 1 single-chain variable fragment-producing CAR-T cells. Cancer Sci. 2019;110(10):3079–3088. doi: 10.1111/cas.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu W, Zi Z, Jin Y, Li G, Shao K, Cai Q, Ma X, Wei F. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother. 2019;68(3):365–377. doi: 10.1007/s00262-018-2281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF, Maus MV, Fraietta JA, Zhao Y, June CH. Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther. 2018;26(7):1855–1866. doi: 10.1016/j.ymthe.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kagoya Y, Tanaka S, Guo T, Anczurowski M, Wang CH, Saso K, Butler MO, Minden MD, Hirano N. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018 doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018 doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 18.Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169(1):424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 19.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8(11):1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 20.Bergan L, Gross JA, Nevin B, Urban N, Scholler N. Development and in vitro validation of anti-mesothelin biobodies that prevent CA125/Mesothelin-dependent cell attachment. Cancer Lett. 2007;255(2):263–274. doi: 10.1016/j.canlet.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Lanitis E, Poussin M, Hagemann IS, Coukos G, Sandaltzopoulos R, Scholler N, Powell DJ., Jr Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol Ther. 2012;20(3):633–643. doi: 10.1038/mt.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, Davila E. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res: Off J Am Assoc Cancer Res. 2012;18(23):6436–6445. doi: 10.1158/1078-0432.CCR-12-1449. [DOI] [PubMed] [Google Scholar]
- 24.Ruella M, Maus MV. Catch me if you can: leukemia escape after CD19-directed T Cell immunotherapies. Comput Struct Biotechnol J. 2016;14:357–362. doi: 10.1016/j.csbj.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Garcia A, Palazon A, Noguera-Ortega E, Powell DJ, Jr, Guedan S. CAR-T cells hit the tumor microenvironment: strategies to overcome tumor escape. Front Immunol. 2020;11:1109. doi: 10.3389/fimmu.2020.01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng J, Zhao L, Zhang Y, Qin Y, Guan Y, Zhang T, Liu C, Zhou J. Understanding the mechanisms of resistance to CAR T-Cell therapy in malignancies. Front Oncol. 2019;9:1237. doi: 10.3389/fonc.2019.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, Naranjo A, Starr R, Wagner J, Wright C, Zhai Y, Bading JR, Ressler JA, Portnow J, D'Apuzzo M, Forman SJ, Jensen MC. Bioactivity and safety of IL13Ralpha2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res: Off J Am Assoc Cancer Res. 2015;21(18):4062–4072. doi: 10.1158/1078-0432.Ccr-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinezlage M, Brem S, Maloney E, Shen A, Isaacs R, Mohan S, Plesa G, Lacey SF, Navenot JM, Zheng Z, Levine BL, Okada H, June CH, Brogdon JL, Maus MV. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung J, Seol HS, Chang S. The generation and application of patient-derived xenograft model for cancer research. Cancer Res Treat. 2018;50(1):1–10. doi: 10.4143/crt.2017.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byrd TT, Fousek K, Pignata A, Szot C, Samaha H, Seaman S, Dobrolecki L, Salsman VS, Oo HZ, Bielamowicz K, Landi D, Rainusso N, Hicks J, Powell S, Baker ML, Wels WS, Koch J, Sorensen PH, Deneen B, Ellis MJ, Lewis MT, Hegde M, Fletcher BS, St Croix B, Ahmed N. TEM8/ANTXR1-specific CAR T cells as a targeted therapy for triple-negative breast cancer. Cancer Res. 2018;78(2):489–500. doi: 10.1158/0008-5472.CAN-16-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng C, Zhao J, Zhou S, Dong J, Cao J, Gao J, Bai Y, Deng H. The vascular disrupting agent CA4P improves the antitumor efficacy of CAR-T cells in preclinical models of solid human tumors. Mol Ther. 2020;28(1):75–88. doi: 10.1016/j.ymthe.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Z, Zheng X, Jiao D, Zhou Y, Sun R, Wang B, Tian Z, Wei H. LunX-CAR T cells as a targeted therapy for non-small cell lung cancer. Mol Ther Oncolytics. 2020;17:361–370. doi: 10.1016/j.omto.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang H, Song B, Wang P, Shi B, Li Q, Fan M, Di S, Yang J, Li Z. Efficient growth suppression in pancreatic cancer PDX model by fully human anti-mesothelin CAR-T cells. Prot Cell. 2017;8(12):926–931. doi: 10.1007/s13238-017-0472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Z, Jiang X, Chen S, Lai Y, Wei X, Li B, Lin S, Wang S, Wu Q, Liang Q, Liu Q, Peng M, Yu F, Weng J, Du X, Pei D, Liu P, Yao Y, Xue P, Li P. Anti-GPC3-CAR T cells suppress the growth of tumor cells in patient-derived xenografts of hepatocellular carcinoma. Front Immunol. 2016;7:690. doi: 10.3389/fimmu.2016.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang N, Cheng C, Zhang X, Qiao M, Li N, Mu W, Wei XF, Han W, Wang H. TGF-beta inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight. 2020 doi: 10.1172/jci.insight.133977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei X, Lai Y, Li J, Qin L, Xu Y, Zhao R, Li B, Lin S, Wang S, Wu Q, Liang Q, Peng M, Yu F, Li Y, Zhang X, Wu Y, Liu P, Pei D, Yao Y, Li P. PSCA and MUC1 in non-small-cell lung cancer as targets of chimeric antigen receptor T cells. Oncoimmunology. 2017;6(3):e1284722. doi: 10.1080/2162402X.2017.1284722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao R, Cheng L, Jiang Z, Wei X, Li B, Wu Q, Wang S, Lin S, Long Y, Zhang X, Wu Y, Du X, Pei D, Liu P, Li Y, Cui S, Yao Y, Li P. DNAX-activating protein 10 co-stimulation enhances the anti-tumor efficacy of chimeric antigen receptor T cells. Oncoimmunology. 2019;8(1):e1509173. doi: 10.1080/2162402X.2018.1509173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, Shultz LD. Humanized mouse models of clinical disease. Annu Rev Pathol. 2017;12:187–215. doi: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon a reasonable request.