Abstract
Neutrophils exert either pro- or anti-tumor activities. However, few studies have focused on neutrophils at the tumor initiation stage. In this study, we unexpectedly found a subcutaneous nodule in the groin areas of mice inoculated with tumor cells. The nodule was developed 24 h after the inoculation, filled with tumor cells and massively recruited neutrophils, being designated as tumor nodules. 22% of the neutrophils in tumor nodules are surface TLR9 (sTLR9) expressing neutrophils (sTLR9+ neutrophils). With tumor progression, sTLR9+ neutrophils were sustainably increased in tumor nodules/tumor tissues, reaching to 90.8% on day 13 after inoculation, with increased expression of IL-10 and decreased or no expression of TNFα. In vivo administration of CpG 5805 significantly reduced sTLR9 expression of the sTLR9+ neutrophils. The reduction of sTLR9 on neutrophils in tumor nodules contributed to the induction of an anti-tumor microenvironment conductive to the inhibition of tumor growth. Overall, the study provides insights for understanding the role of sTLR9+ neutrophils in the tumor development, especially in the early stage.
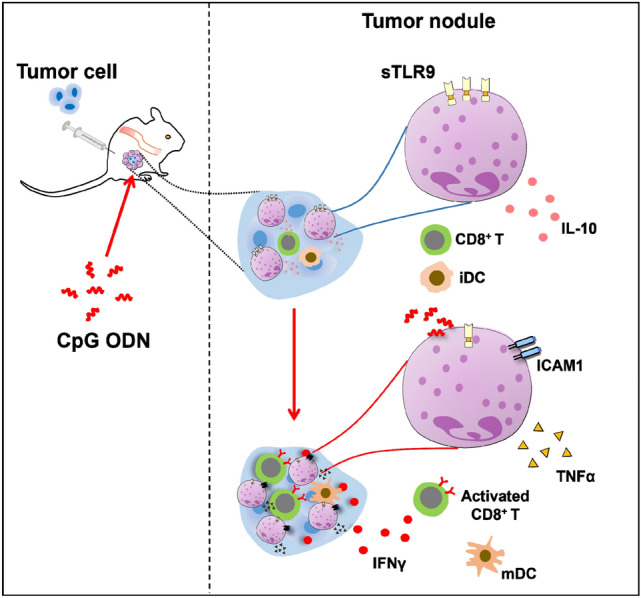
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03451-1.
Keywords: Subcutaneous nodule, Tumor cell inoculation, Surface Toll-like receptor 9, Neutrophils, Tumor development
Introduction
Infiltration of immune cells is a hallmark of solid tumors [1]. At the beginning of tumor development, innate immune cells, including monocytes and neutrophils, are recruited into local tissues where tumor cells arise [2]. The neutrophils infiltrated in various solid tumors are known as tumor-associated neutrophils (TANs) [3–9], constituting a cell population in the tumor microenvironment (TME). In the TME, the TANs can be reprogramed into anti-tumoral TAN1 or pro-tumoral TAN2 [10, 11]. Type I interferons (IFNs) promote the generation of TAN1 [12]. Transforming growth factor-β (TGF-β) is linked to the differentiation of TAN2 [10]. These two immune phenotypes may mark the end point of a continuum of functional state of neutrophils [13]. TAN1 and TAN2 are primarily defined by their functions rather than surface markers [14]. In certain circumstances, TAN1 may express high levels of immunostimulatory factors, including tumor necrosis factor α (TNFα) and intercellular adhesion molecule 1 (ICAM-1, CD54) [10, 15], and TAN2 may express high levels of immunosuppressive cytokines, such as IL-10 [16, 17]. So far, TANs are primarily investigated in established or advanced solid tumors. Few studies are about neutrophils at the stage of tumor initiation.
Human and mouse neutrophils express Toll-like receptor 9 (TLR9) [18, 19]. The TLR9 is migratory [20–22]. After being produced in the endoplasmic reticulum (ER), the TLR9 is transported by chaperone uncoordinated 93 homolog B1 (UNC93B1) to endosomes, being an endosomal TLR9 (eTLR9) or to the cell surface, being a surface TLR9 (sTLR9) [23–25]. sTLR9 expressing neutrophils (sTLR9+ neutrophils) could bind certain TLR9 ligands and then be activated to secrete cytokines [18]. The sTLR9 can be relocated to endosomes, mediated by UNC93B1 recruited adaptor protein 2 (AP-2) complexes [26]. Conventionally, the eTLR9 is activated by unmethylated CpG motifs present in pathogen-derived DNA, as well as by CpG ODN (oligodeoxynucleotide that contains unmethylated CpG dinucleotides) [18]. The activation consequently initiates myeloid differentiation factor 88 (MyD88)-dependent signaling, leading to the increased expression of its downstream transcription factors like interferon regulatory factor (IRF) 5, followed by the production of type I IFN [22, 27]. Type I IFN activates the migration of CD8+ T cells into the TME [28, 29] and induces the upregulation of intercellular adhesion molecule (ICAM-1) on TANs [30]. ICAM-1 binds to lymphocyte function associated antigen-1 (LFA-1) on CD8+ T cells. The CD8+ T cells could be activated by ICAM-1 expressing neutrophils to eliminate tumor cells [31, 32]. Comparatively, sTLR9 is less studied and few researches are about sTLR9 expressing neutrophils (sTLR9+ neutrophils). In our previous work, we found that sTLR9 could negatively regulate the antibody response of B cells to vaccines [33] and that sTLR9+ neutrophils attenuated the deadly systemic hyperinflammatory responses in mice [34–36]. However, it remains elusive whether and how the sTLR9+ neutrophils are involved in the development of solid tumors.
In this study, we unexpectedly noticed the formation of a nodule at the site of tumor cell inoculation in mice. The nodules, occurred within 24 h post-inoculation, are rich in sTLR9+ neutrophils. Curiously, we probed the roles of sTLR9+ neutrophils in tumor initiation and progression.
Materials and methods
Cells and cell culture
BALB/c mice-derived CT26 colon carcinoma cells (CT26 cells), BALB/c mice-derived H22 liver carcinoma cells (H22 cells), C57BL/6 mice-derived B16 melanoma cells (B16 cells) (American Type Culture Collection) were maintained in RPMI 1640 medium supplemented with 10% (V/V) fetal bovine serum (FBS) (GIBCO) and antibiotics (100 IU/mL of penicillin and streptomycin) (RPMI 1640/10% FBS). All cells were cultured at 37 °C in a 5% CO2 humidified incubator. All cell lines were subjected to mycoplasma testing using a MycAway™-Color One-step mycoplasma detection Kit by Yeasen Biotech Co. Ltd., Shanghai city, China.
To detect cellular components in tumor nodules, the tumor nodules were collected from mice inoculated with CT26 cells, H22 cells or B16 cells. Tumor nodule cells were released by grinding a collected tumor nodule in 1 mL RPMI 1640/10% FBS in a sterile petri plate. After filtration through a 300-mesh filter and centrifugation at 350×g for 5 min, the cells were resuspended in PBS containing 2% FBS for detecting CD45+ leucocytes, Ly6G+ neutrophils, F4/80+ macrophages, CD11c+ DCs, CD3+ T cells and CD19+ B cells.
For detecting F4/80+ macrophages, Ly6G+ neutrophils, CD3+ T cells and CD19+ B cells, cells were isolated from tumor-draining lymph nodes (TDLNs) and spleens of the mice inoculated with CT26 colon carcinoma cells. The TDLN cells or splenocytes were released by grinding the isolated lymph nodes or spleens in 1 mL RPMI 1640/10% FBS in a sterile petri plate. The TDLN cells were filtered through a 300-mesh filter and centrifuged at 350×g for 5 min before being resuspended in phosphate-buffered saline (PBS) containing 2% FBS. The released splenocytes were centrifuged at 350×g at 4 °C for 5 min. The cell pellets from the spleen were re-suspended in 3 mL ammonium-chloride-potassium (ACK) buffer (150 mM NH4Cl, 10 mM KHCO3, 10 mM EDTA, pH 7.2–7.4) to lyse red blood cells. After 10 min, the suspensions were centrifuged at 350×g at 4 °C for 5 min. After removing erythrocytes, the splenocytes were resuspended with RPMI 1640/10% FBS. The isolated TDLN cells and splenocytes were kept on ice for further use.
Mice
Female BALB/c and C57BL/6 mice, 6–8-week-old, were purchased from Yisi Laboratory Animal Technology Co., Ltd., Changchun, China, maintained in specific pathogen-free conditions at the Laboratory Animal Center of Jilin University and used for the mouse experiments in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. During the experiments, mice were given free access to food and water. The mouse experiments were approved by the Ethics Committee of the College of Basic Medical Sciences of Jilin University.
Antibodies and reagents
The following antibodies were obtained from BD Biosciences (Franklin Lakes, NJ, USA): anti-mouse monoclonal antibodies (mAbs) Ly6G (FITC-conjugated) (551460), F4/80 (PE-conjugated) (565410), CD3 (APC-conjugated) (561826), CD4 (FITC-conjugated) (553651), CD8a (FITC-conjugated) (553031), CD19 (FITC-conjugated) (553785), CD86 (APC-conjugated) (558703), CD11c (FITC-conjugated) (561067), CD80 (PE-conjugated) (553769), IFN-γ (PE-conjugated) (554412), CD45 (APC-conjugated) (561018), ICAM1 (PE-conjugated) (553253), TNFα (PE-Cy™7-conjugated) (557644) and IL-10 (PE-conjugated) (561060). TLR9 (APC-conjugated, FAB7960R) was purchased from R&D Systems (MN, USA), and 4-1BB (APC-conjugated, 106109) was purchased from BioLegend (San Diego, CA). Antibodies for detecting tumor cell specific HSP70 (Heat shock protein 70) [37] were purchased from Abcam. Alexa-Fluor 488 fluorescent secondary antibody was from Servicebio (Wuhan, China). 4′,6-diamidino-2 phenylindole (DAPI) was from Invitrogen. Saponin (S-4521) was purchased from Sigma-Aldrich (MO, USA). 4% tissue fixative (G1101-500 mL) was purchased from Servicebio (Wuhan, China).
Oligodeoxynucleotides (ODNs)
Two single-stranded oligodeoxynucleotides of CpG 5805 [33] and CCT ODN [38] were synthesized, fully phosphorothioate-modified and purified by Sangon Biotech (Sangon Biotech, Shanghai, China). The ODNs were diluted in PBS and had no detectable endotoxin tested by the Limulus amebocyte lysate assay (Associates of Cape Cod, Inc., MA, USA).
Establishment of mouse tumor nodule models
CT26 cells, H22 cells or B16 cells were subcutaneously (s.c.) inoculated in the groin areas of recipient mice in single-cell suspension with 2 × 106 cells in 0.1 mL of PBS per mouse. After 24 h, a subcutaneous nodule was observed the injection site of tumor cells. Nodule volume = length × width2 × 0.5.
Immunofluorescence analysis
For identifying the CT26 cells, the isolated tumor nodules were fixed in formalin-embedded paraffin and sectioned. 4 μm sections of tumor nodule were blocked in goat serum for 30 min and incubated with monoclonal anti-Hsp70 antibody (1:100) overnight at 4 °C. After adding Alexa Fluor 488 secondary antibody (1:200), the sections were incubated for 1 h at 37 °C, followed by DAPI staining for 5 min. Stained slides were digitally scanned by a Pannoramic SCAN scanning system (3dhistech CaseViewer, 1141 Budapest, Hungary). The acquired images were processed using the slideviewer software (2.1v, 3dhistech, Budapest, Hungary).
Treatment of mouse CT26 or B16 tumor model
CT26 cells or B16 cells were subcutaneously inoculated in the groin areas of BALB/c or C57BL/6 mice (n = 6/group). At 24 h post inoculation, the treatment was started by injecting the CpG 5805 or CCT ODN at 10 μg in 0.1 mL of PBS in the tumor cell inoculation site. The survival of the tumor bearing mice was recorded. The tumor volume was calculated based on the formula of Tumor volume = length × width2 × 0.5.
Flow cytometry
Approximately 1 × 106 cells were stained with antibodies in each condition. For surface staining, cells were stained with fluorescent labeled mAbs against Ly6G, TLR9, F4/80, CD45, CD3, CD4, CD8a, CD19, 4-1BB, CD80, CD86, CD11c, ICAM1 and corresponding isotype controls at 4 °C in the dark for 30 min, and then washed twice in PBS before detection. For indirect staining, cells were collected, fixed with 4% paraformaldehyde, permeabilized with 0.1% saponin, stained with fluorescent labeled mAbs against TLR9, IFNγ, TNFα, IL-10 and corresponding isotype controls at 4 °C in dark for 30 min, then washed twice in PBS before detection. All stained cells were analyzed using an Accuri C6 flow cytometer (BD Biosciences).
Statistical analysis
Data are indicated as mean ± SD. Comparisons between groups were performed by unpaired t-tests. Survival curves of mice were estimated and compared using the Kaplan–Meier method and log-rank test, respectively. Statistically significance was set at p < 0.05. Statistical analyses were performed using GraphPad Prism software (version 8.0).
Results
Subcutaneous neutrophil infiltrated nodules induced by inoculating tumor cells
To study the early development characteristics of tumor implanted in vivo, 2 × 106 CT26 colon carcinoma cells (CT26 cells) were subcutaneously (s.c.) inoculated into the groin area of BALB/c mice, and the local changes of the tumor cells were observed. Unexpectedly, at 24 h post inoculation, an obvious nodule in each mouse was noticed at the inoculation site. The nodules, with a size about 4 mm × 2.5 mm (length × width), were located in the inner lining of the skin, being coated with an intact and transparent membrane (Fig. 1a). For gaining an insight into the nodule, cells in the nodule were harvested and analyzed. The inoculated CT26 cells, recognized by anti-Hsp70 antibodies [37], were dispersed among the nodule cells (Fig. 1b). More precisely, the cellular composition within the nodule was analyzed using flow cytometry. CD45+ leukocytes constituted 64.5% of the nodule cells. Among the CD45+ leukocytes, 98%–99% are Ly6G+ neutrophils, and 1%–2% are F4/80+ macrophages (Fig. 1c). The results revealed that inoculating tumor cells could induce a rapidly growing subcutaneous nodule at inoculation site of mice. The nodules were massively infiltrated by neutrophils, and therefore designated as neutrophil infiltrated tumor nodules.
Fig. 1.
Occurrence of subcutaneous nodules in mice inoculated with tumor cells. Mice were s.c. inoculated with syngeneic tumor cells in groin areas, then observed the occurrence of subcutaneous nodules. a The nodules induced by 2 × 106 CT26 cells in BALB/c mice at 24 h post inoculation. b CT26 cells (green color) in the CT26 cell induced nodule. CT26 cells in the harvested nodule cells were identified with monoclonal anti-Hsp70 and Alexa 488 anti-mouse antibodies (green). Nuclei of the CT26 cells were stained with DAPI (blue). c Immune cells in the CT26 cell-induced nodule. The collected nodule cells were analyzed via flow cytometry, using anti-CD45 antibody for leukocytes, anti-Ly6G antibody for neutrophils (Neu) and anti-F4/80 antibody for macrophages (Mφ). d Correlations of the number of inoculated tumor cells with the nodule formation. BALB/c mice were inoculated with CT26 cells at various numbers (upper) and then observed for the nodule occurrence kinetically (lower). e The nodules induced by 2 × 106 B16 cells or H22 cells. C57BL/6 mice or BALB/c mice were inoculated with B16 cells or H22 cells. At 24 h post inoculation, the nodules were observed (upper) and the immune cells in the collected nodule cells were analyzed (lower) as those described in (c). The tumor cell-induced nodules were indicated with circled-black dotted lines. Scale bars = 5 mm
To find correlations of the number of inoculated tumor cells and tumor nodule formation, we s.c. inoculated the mice (n = 5 per group) with 2 × 104, 2 × 105 and 2 × 106 CT26 cells respectively, and checked the tumor nodule formation up to 13 days (Fig. 1d, upper). At 24 h post inoculation, visible tumor nodules developed in all of the five mice inoculated with 2 × 106 CT26 cells. At day 3 or day 7 post inoculation, the similar tumor nodules occurred in two out of five mice received 2 × 104 CT26 cells, and in four out of five mice received 2 × 105 CT26 cells, respectively (Fig. 1d, lower). The result indicated that the number of tumor cells inoculated was positively correlated with the early formation of tumor nodules. Based on the result, CT26 cells at 2 × 106/inoculation were used to carry out the following experiments.
To explore the universality of various tumor cells in inducing the tumor nodules, we inoculated B16 melanoma cells (B16 cells) and H22 hepatocellular carcinoma cells (H22 cells) in groin areas of mice respectively, and observed the tumor nodule occurrence. Consistently, the B16 and H22 cells also induced obvious tumor nodules at 24 h post inoculation (Fig. 1e), indicating that short-term induction of tumor nodule formation by the inoculation of tumor cells was a universal phenomenon.
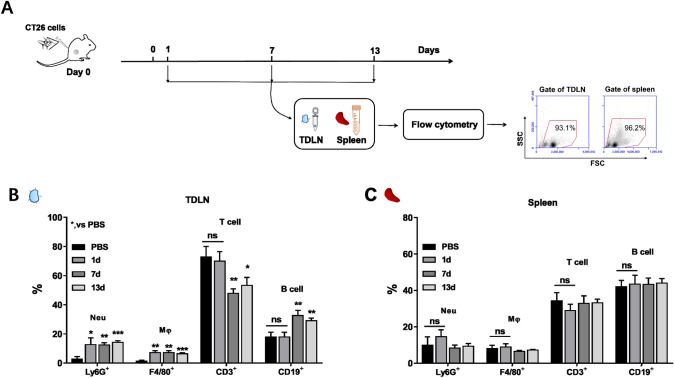
Changes of immune cells outside the tumor nodules
Following the observation of massive recruitment of neutrophils in tumor nodules, we wanted to see if the mice inoculated with tumor cells had changes in immune cells outside the tumor nodules. We inoculated BALB/c mice with 2 × 106 CT26 cells in groin areas, and then harvested the tumor draining lymph nodes (TDLNs) and spleens in the mice followed by analyzing their immune cells dynamically (Fig. 2a). The analysis showed that the number of Ly6G+ neutrophils and F4/80+ macrophages in TDLN cells increased by 10% and 5% on day 1 after CT26 cell inoculation, while the number of T cells and B cells did not change obviously. The increase of Ly6G+ neutrophils and F4/80+ macrophages was maintained at day 7 and day 13 (Fig. 2b). Notably, CD3+ T cells were decreased in the TDLNs, from 73% at day 1 to 58% at day 13. Accompanying with the decrease of the proportion of CD3+ T cells, the percentage of CD19+ B cells were increased in the TDLNs, from 18% at day 1 to 29% at day 13 (Fig. 2b). The decrease of CD3+ T cells could be attributed to the movement of the activated T cells from TDLNs to the local tumors [39] since the decrease was accompanied with significant increase of CD3+ T cells in the tumor tissues developed from the tumor nodules (Fig. S1). No significant changes in the number of myeloid or lymphoid cells were detected in splenocytes with the tumor progression (Fig. 2c). Together, the results suggested that the recruitment of neutrophils in tumor nodules of mice were in parallel with apparent changes in neutrophils and macrophages in TDLNs.
Fig. 2.
The percentage of different immune cells in lymphoid organs of mice inoculated with tumor cells. BALB/c mice (n = 6, at each time point) were s.c. inoculated with 2 × 106 CT26 cells in the groin areas on day 0 followed by collecting tumor draining lymph nodes (TDLNs) and spleens for analysis of different immune cells on day 1, 7 and 13 post inoculation by flow cytometry. a The experiment procedure. b The percentages of different immune cells in TDLNs. c The percentages of different immune cells in spleens. Note: Neutrophil (Neu), Ly6G+; Macrophage (Mφ), F4/80+; T cell, CD3+; B cell, CD19+. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
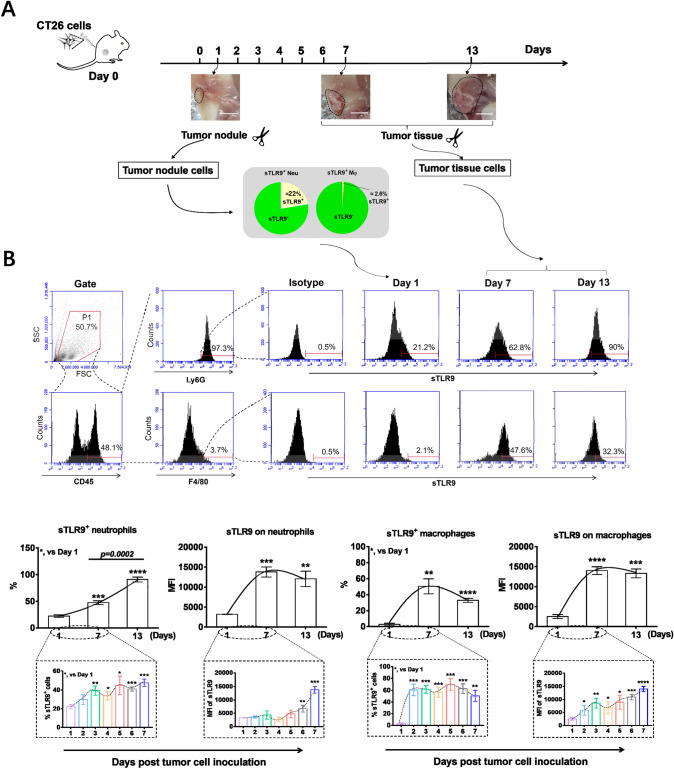
sTLR9 expressing neutrophils in tumor nodules/tumor tissues
Considering that neutrophils express surface Toll like receptor 9 (sTLR9) [18, 19] and sTLR9 expressing neutrophils (sTLR9+ neutrophils) are in traumatized tissues and the site of inflammatory responses [34–36], we detected sTLR9+ neutrophils in the tumor nodules or tumor tissues developed from the inoculated CT26 cells. On day 7 and 13 post tumor cell inoculation, the tumor nodules induced by CT26 cells in mice developed into tumors with a size of about 100–150 mm3 and 1000 mm3 (Fig. 3a). At day 1 post inoculation with CT26 cells, approximate 22% neutrophils in the tumor nodule were sTLR9+ neutrophils (Fig. 3a). Comparatively, at day 7 or day 13 post inoculation, 47.5% or 90.8% of Ly6G+ neutrophils in tumor tissues were sTLR9+ neutrophils, respectively (Fig. 3b, lower and left). In parallel, sTLR9+ F4/80+ macrophages constituted 2.6%, 50.4% or 32.9% of the F4/80+ macrophages in the tumor nodules /tumor tissues at day 1, 7 and 13 post inoculation, respectively (Fig. 3b, lower and right). Similar changes in sTLR9+ neutrophils and sTLR9+ macrophages were also detected in the tumor nodules or tumor tissues induced by B16 cells or H22 cells (Fig. S2). The observations imply the sTLR9+ neutrophils are involved in the initiation and progression of tumors.
Fig. 3.
sTLR9 expressing neutrophils in tumor nodules or tumor tissues. BALB/c mice (n = 6, at each time point) were s.c. inoculated with 2 × 106 CT26 cells in groin areas at day 0. At corresponding time points post inoculation, sTLR9+ neutrophils and sTLR9+ macrophages in tumor nodules or tumor tissues were detected by flow cytometry. a The experiment procedure. b The percentage of sTLR9+ neutrophils/macrophages and the expression of sTLR9 on neutrophils/macrophages. The tumor nodules/tumor tissues were indicated with circled-black dotted lines. Scale bars = 5 mm. MFI, mean fluorescence intensity. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
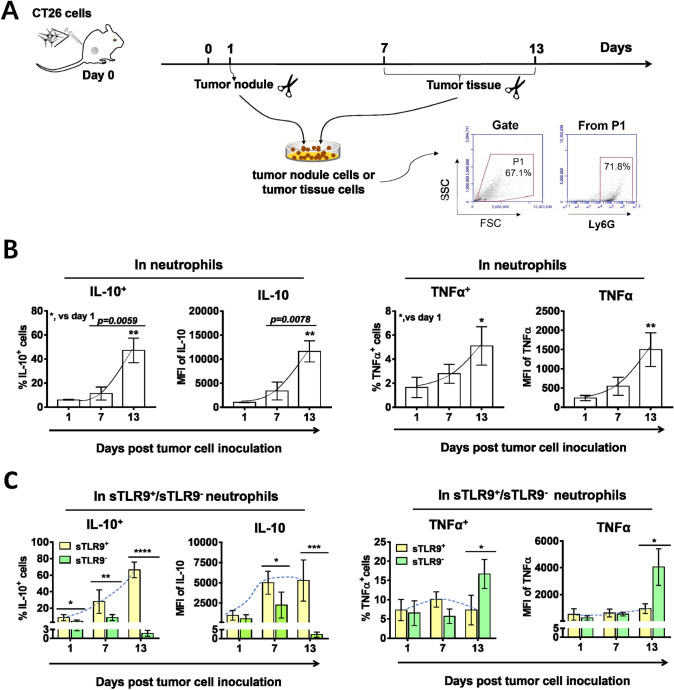
Correlation between sTLR9 expression and IL-10/TNFα production in neutrophils in tumor nodules/tumor tissues
Illuminated by the studies showing that in TME, neutrophils could express IL-10, favoring tumor progression [16, 17], or TNFα, limiting tumor progression [10, 15], we detected the expression of IL-10 or TNFα in neutrophils of tumor nodules /tumor tissues in groin areas on day 1, 7 and 13 post inoculation with 2 × 106 CT26 cells, and the percentage of IL-10 expressing neutrophils (IL-10+ neutrophils) or TNFα expressing neutrophils (TNFα+ neutrophils) by flow cytometry (Fig. 4a). The results showed that the percentage of IL-10+/TNFα+ neutrophils and the expression level of IL-10/TNFα in neutrophils all increased with the tumor progression from day 1 to day 13 post tumor cell inoculation. By the 13th day after tumor cell inoculation, the percentage of IL-10+ neutrophils or TNFα+ neutrophils increased by 7.8 times or 3 times compared with that in the first day (Fig. 4b). It indicated that neutrophils expressing IL-10 gradually became the dominant subgroup of neutrophils in TME with tumor progression. To determine the relationship between the expression of IL-10/TNFα and sTLR9 in neutrophils of tumor nodules /tumor tissues, we analyzed the expression of IL-10/TNFα in sTLR9+ neutrophils and sTLR9− neutrophils by gating the double positive cells of IL-10/TNFα and sTLR9. We found that sTLR9+ neutrophils were mainly IL-10 expressing neutrophils. At day 1, 7 and 13 post tumor cell inoculation, 17%, 30% and 70% of sTLR9+ neutrophils were IL-10+ neutrophils, respectively. The expression level of IL-10 in sTLR9+ neutrophils also increased gradually with the tumor progression, and more than doubled on day 7 and 13 compared with that on the 1st day (Fig. 4c, left). However, there was low expression of TNFα in sTLR9+ neutrophils, and TNFα+ neutrophils were mainly neutrophils that did not express sTLR9. In sTLR9− neutrophils, there was a threefold increase in the percentage of TNFα+ neutrophils on day 13 compared to that on day 1 after tumor cell inoculation (from 6% to 18%), and also a threefold increase in TNFα levels on these cells (from 1000 MFI to 4000 MFI) (Fig. 4c, right). Together, the results imply that IL-10+sTLR9+neutrophils or TNFα+sTLR9− neutrophils could be in a pro-tumor or anti-tumor phenotype in the tumor nodules/tumor tissues, respectively.
Fig. 4.
Expression of IL-10/TNFα in neutrophils or in sTLR9+/sTLR9− neutrophils in tumor nodules/tumor tissues. BALB/c mice were (n = 6, at each time point and each group) s.c. inoculated with 2 × 106 CT26 cells in groin areas on day 0. On day 1, 7 and 13, cells isolated from tumor nodules or tumor tissues were analyzed in a flow cytometry, using Ly6G-FITC, IL-10-PE, TNFα-PerCP and TLR9-APC antibodies. a The experiment procedure. b The expression of IL-10/TNFα in neutrophils. c The expression of IL-10/TNFα in sTLR9+ neutrophils /sTLR9− neutrophils. MFI, mean fluorescence intensity. *p<0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
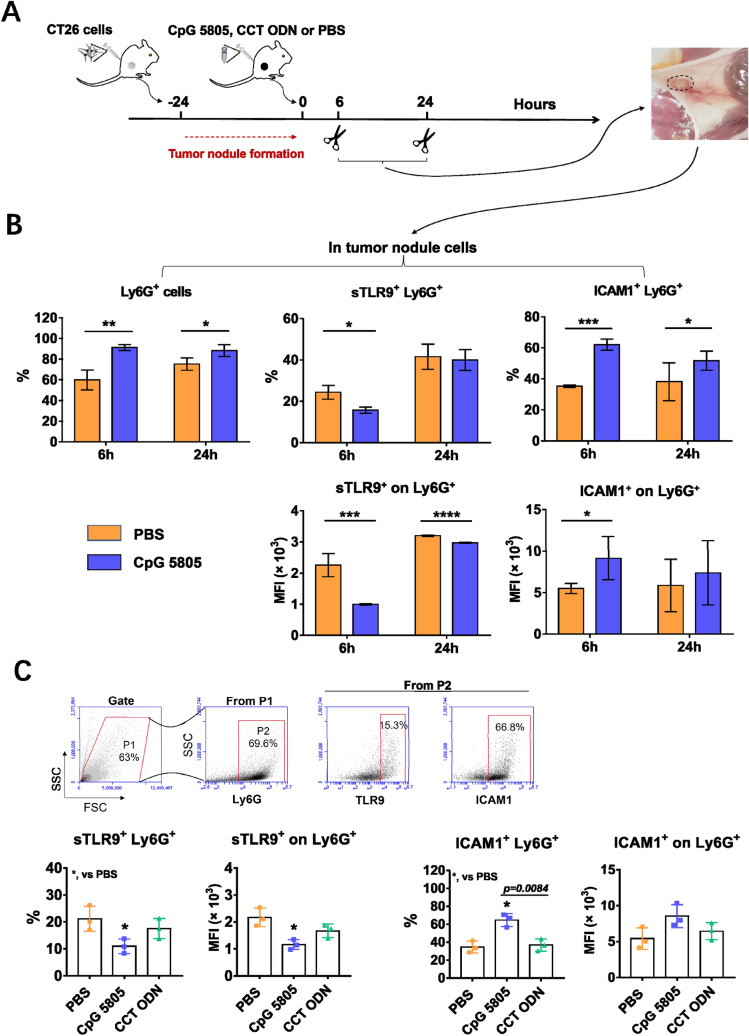
Downregulation of sTLR9 on neutrophils promotes the induction of an anti-tumor microenvironment in tumor nodules which contributing to the inhibition of tumor growth
To study whether the downregulation of sTLR9 on neutrophils in tumor nodules was beneficial to the induction of anti-tumor immune microenvironment, considering that CpG ODN as TLR9 agonist could downregulate the sTLR9 level on B cell to activate it [33] and the ICAM1 expressing neutrophils could activate CD8+ T cells [22, 30], we used CpG 5805 as a research tool in vivo to regulate the expression of sTLR9 and ICAM1 on neutrophils in tumor nodules. In the experiment in mice (Fig. 5a), we found that CpG 5805 injection induced a clear increase of the proportion of neutrophils in tumor nodules at 6 h and 24 h, respectively. The percentages of neutrophils in tumor nodules were 60%–80% within 24 h after PBS injection, and CpG 5805 made this proportion reach more than 90% (Fig. 5b, left). This means that the neutrophil is the absolute dominant immune cell in the early tumor nodule and the space for analysis of CpG 5805 to regulate the sTLR9 on other immune cells in the nodule is very small. Therefore, we focused on testing the sTLR9 expression and activation of neutrophils in the early tumor nodules. We found that among the increased neutrophils, the percentage of sTLR9+ neutrophils was obviously reduced from 25% to 15% at 6 h while the percentage of ICAM1+ neutrophils markedly increased from 35% to 60% at 6 h and from 40% to 50% at 24 h, respectively, after CpG 5805 injection. Similarly, at 6 h after CpG 5805 injection, sTLR9 levels on the neutrophil were downregulated to half the levels in the PBS group, while ICAM1 levels were doubled at the same time point (Fig. 5b, right). To confirm the finding above, we selected an inhibitory ODN with CCT repeats (CCT ODN) as control to inhibit TLR9 migration [38] to see if the sTLR9 and ICAM1 on neutrophils were affected. The result revealed that CpG 5805 could still downregulate the sTLR9 and upregulate the ICAM1 on neutrophils in tumor nodules at 6 h post CpG 5805 injection.However, after CCT ODN injection, there was neither decrease in sTLR9 or increase in ICAM1 on the neutrophils in tumor nodules (Fig. 5c). The result suggests that lowering sTLR9 on neutrophils can make the neutrophils enter into active state, which may be beneficial to the induction of anti-tumor microenvironment in tumor nodules at the early stage of tumor development.
Fig. 5.
The percentage of sTLR9+ and ICAM1+ neutrophils and the expression of sTLR9 and ICAM1 on neutrophils in tumor nodules of mice treated by CpG 5805 or CCT ODN. BALB/c mice were s.c. inoculated with 2 × 106 CT26 cells in groin areas. 24 h later, the mice were injected with a single dose of CpG 5805, CCT ODN or PBS followed by detecting the sTLR9 and ICAM1 on neutrophils in tumor nodules using flow cytometry. a The experiment procedure. b The percentages of neutrophils and sTLR9+/ICAM1+ neutrophils and the expression levels of sTLR9 and ICAM1 on neutrophils at 6 h and 24 h post CpG 5805 injection. c The percentage of sTLR9+/ICAM1+ neutrophils and the expression levels of sTLR9 and ICAM1 on neutrophils at 6 h post CpG 5805 or CCT ODN injection. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
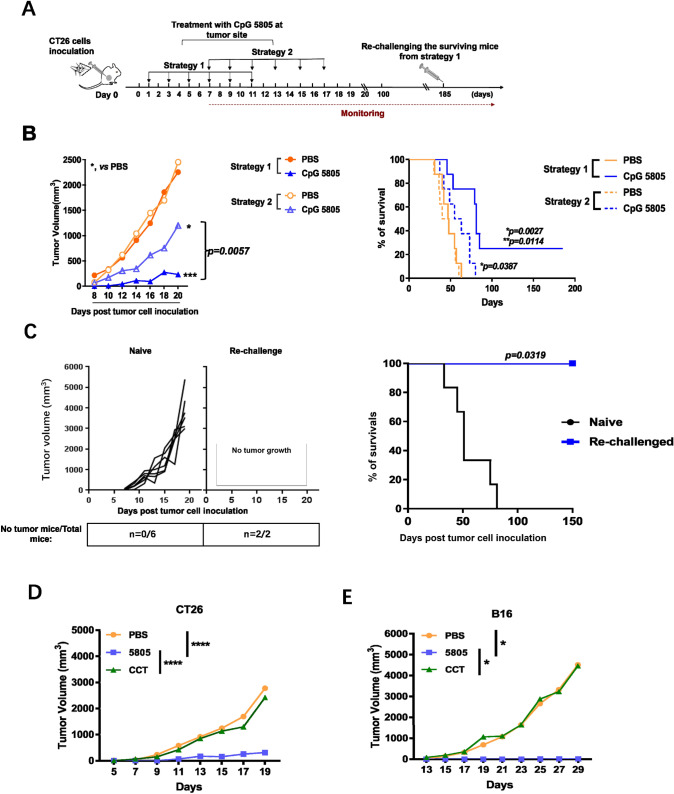
In order to verify the hypothesis that the downregulation of sTLR9 levels on neutrophils in tumor nodules at the early stage of tumor development contributed to the inhibition of tumor growth, two strategies were adopted to administer CpG 5805 treatment locally at the site of CT26 cell inoculation, and then the tumor growth and survival of tumor-bearing mice were dynamically monitored (Fig. 6a). We found that the treatment with CpG 5805 in both strategies all significantly inhibited the tumor growth compared with PBS treatment. Comparatively, CpG 5805 injection at strategy 1 was more efficient, manifested by that mice received strategy 1 treatment developed fivefold smaller tumors than that in mice received strategy 2 treatment on day 20 post tumor cell inoculation and prolonged the survival of tumor-bearing mice to a greater extent (p = 0.0027) (Fig. 6b). By day 185, two mice received CpG 5805 based on strategy 1 treatment were still tumor-free and survived. To see if a specific antitumor effect had been induced by the treatment of CpG 5805 under the strategy 1 in long-lived mice, we inoculated the same CT26 cells into these two surviving mice. The result showed that two surviving mice were tumor-free for 150 days post re-challenge inoculation and remained alive (Fig. 6c). To prove the findings above related to the reduction of sTLR9 on neutrophils in tumor nodules, CCT ODN, that could inhibit TLR9 migration [38] and not reduce the sTLR9 on neutrophils shown in Fig. 5c, was used in tumor suppressive experiment in mice. Under the strategy 1 treatment with CCT ODN or CpG 5805 in mice inoculated with CT26 cells, we found that CCT ODN treatment could not inhibit the tumor growth while CpG 5805 still exhibited a significant inhibitory effect on tumor growth (Fig. 6d). By the same way in B16-bearing model mice, CCT ODN also had no effect on inhibiting the tumor growth (Fig. 6e). Together, the results suggest that the downregulation of sTLR9 on neutrophils in tumor nodules at the early stage of tumor development contributes to the establishment of an anti-tumor microenvironment and thus to the inhibition of tumor growth.
Fig. 6.
Tumor growth and survival of tumor-bearing mice treated with CpG 5805 at the site of tumor cell inoculation with two different strategies. a The experiment procedure. BALB/c mice were s. c. inoculated with CT26 cells on day 0 and then injected with CpG 5805 or PBS at one of two treatment strategies followed by monitoring the tumor growth and survivals of mice (n = 8/group). The survival mice were rechallenged with same CT26 cells. b The curves of tumor growth and survivals of mice treated with CpG 5805 or PBS in two strategies. In the result of tumor growth, *p < 0.05, ***p < 0.001. In the result of survivals, *, vs PBS group; **, vs CpG 5805 group in strategy 2. c The tumor growth and survival of rechallenged mice which survived from strategy 1 treatment of CpG 5805 or naive mice. The two mice survived from CpG 5805 treatment based on strategy 1 were re-challenged with CT26 cells and naive mice (n = 6) were inoculated parallel as control. d The tumor growth of mice inoculated with CT26 tumor cells from strategy 1 treatment of CCT ODN or CpG 5805. e The tumor growth of mice inoculated with B16 tumor cells from strategy 1 treatment of CCT ODN or CpG 5805. *p < 0.05, ****p < 0.0001
Downregulating the sTLR9 on neutrophils in tumor nodules contributes to the establishment of anti-tumor immune microenvironment
To determine whether downregulating the sTLR9 on neutrophils in tumor nodules contributed to the establishment of an anti-tumor immune microenvironment, based on the success of tumor suppression experiments in vivo, and considering the fact that specific anti-tumor effects had been demonstrated but CpG 5805 might be also regulate other immune cells besides neutrophils, we examined the percentage and activation of neutrophils, dendritic cells (DCs) and CD8+ T cells in tumor nodules of CT26 cell inoculated mice post 24 h of three injections of CpG 5805 under strategy 1. We found that Ly6G+ neutrophils were markedly increased and CD8+ T cells were increased by 2.9% in tumor nodules of mice treated with CpG 5805 (Fig. 7a). Among the increased Ly6G+ neutrophils, sTLR9 expression was obviously reduced and the percentage of ICAM1+ neutrophils and the ICAM1 expression were markedly upregulated (Fig. 7b). Compared with PBS treatment, the percentage of CD80+/CD86+ DCs was doubled and the expression of CD80/86 on CD11c+ DCs was tribled in the tumor nodules after CpG 5805 treatment (Fig. 7c). Meanwhile, in the tumor nodule cells of mice treated with CpG 5805, the number of CD8+ T cells significantly increased and the percentage of 4-1BB+CD8+ T cells and IFNγ+ cells increased by about 1/3, respectively, and the level of IFNγ had a tendency to increase (Fig. 7d, e). The result reveals that CpG 5805 is not specifically targeting the sTLR9 on neutrophils because it also results in the activation of other immune cells such as DCs and CD8+ T cells in tumor nodules. Even though, the reduction of sTLR9 on neutrophils in tumor nodules can indeed induce the formation of an anti-tumor immune microenvironment.
Fig. 7.
Downregulating the sTLR9 on neutrophils and detecting the tumor immune microenvironment in tumor nodules. BALB/c mice (n = 5) were s.c inoculated with CT26 cells in groin areas. 24 h later, the treatment was started by injecting CpG 5805 or PBS in inoculation sites, once every other day. At 24 h after the third injection, tumor nodules were isolated and detected for their cellular components. a The components of immune cells in tumor nodules. Neutrophil (Neu), Ly6G+; Macrophage (Mφ), F4/80+; Dendritic cell (DC), CD11c+; T cell, CD3+; B cell, CD19+. b The counts of neutrophils, the precentage of sTLR9+/ICAM1+ neutrophils and the expression of sTLR9 and ICAM1 on neutrophils in tumor nodules. c The percentage of CD80+/CD86+ DCs and the expression of CD80/CD86 on CD11c+ DCs. d The count of CD8+ T cells, the percentage of 4-1BB+CD8+ T cells and the expression of 4-1BB on CD8+ T cells. e The percentage of IFNγ+ cells and the expression of IFNγ in tumor nodule cells. MFI, mean fluorescence intensity. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Discussion
In recent years, innate immune cells infiltrated in solid tumors have attracted attentions [40]. Here, we report the finding of the formation of neutrophil-infiltrated nodules induced by tumor cell inoculation in mice. The nodules, with a milky-white appearance in the inner lining of the skin, were clearly observed at 24 h after inoculation. The nodule formation is a universal phenomenon happened in mice inoculated with various syngeneic malignant cells. Characteristically, the nodules contain abundant neutrophils with a number overwhelmingly surpassed the tumor cells. Several reasons may contribute to the neutrophil recruitment. The tumor cells, after being implanted, could produce vascular endothelial growth factor (VEGF) and various chemokines, such as CXCL1, CXCL2, CXCL5, CXCL6, CXCL8 (IL-8), and migration inhibitory factor (MIF). The VEGF renders nearby vessels hyperpermeable and thereby allow the leakage of plasma into local tissues [41], where the plasma proteins form fibrin gels around tumor cells. The gels provide a favorable substrate for the attachment and migration of neutrophils [42]. The chemokines attract neutrophils from the blood circulation into the local tissues [43, 44]. The neutrophils, interacted with the tumor cells, produce CCL17 which attract regulatory T cells (Tregs) [45]. The Tregs produce IL-8, which recruit more neutrophils, involving in creating a positive loop for the neutrophil infiltration [46]. Evolutionally, in mouse or human body, neutrophils are selectively developed as rapidly migratory responders to eliminate/fight tiny foreign intruders, such as continuously existed-extracellular bacteria that often break the skin or other barriers of human body. Obviously, mouse or human body, without/lacking the experience of suddenly exposed big amount of inoculated tumor cells, are trying to use the same evolutionally acquired machinery to deal with a swarm of the inoculated tumor cells they have never encountered. Thus, we may conjecture that the rapidly recruited neutrophils around the inoculated tumor cells could be intended to eliminate or fight the tumor cells to maintain the homeostasis of a host.
Noticeably, about 22% neutrophils in the tumor nodules express sTLR9 at 24 h post tumor cell inoculation, being sTLR9+ neutrophils. In contrast, at day 7 or day 13, the sTLR9+ neutrophils constitute up to 47.5% or 90.8% of the neutrophils in the tumor tissues. The observation implies that sTLR9+ neutrophils may contribute to the formation of a favorable microenvironment for tumor progression. With tumor progression, IL-10 are increasingly expressed in sTLR9+ neutrophils, meanwhile, TNFα is increasingly expressed in sTLR9− neutrophils (Fig. 4). Thus, tentatively, the sTLR9− neutrophils and sTLR9+ neutrophils could be demarcated as TAN1 and TAN2, respectively. TAN1s are characterized by anticancer activity and expressing high levels of TNFα and ICAM-1 [10, 15]. TNFα is a cytotoxic cytokine toward tumor cells [47, 48]. TAN2s are the IL-10+ neutrophils which suppress antitumor functions of T cells [16, 17]. Our previous work showed that IL-10-producing sTLR9+ neutrophils negatively regulate the production of pro-inflammatory cytokines [34], and reducing sTLR9 on B cells enhances antibody responses of the B cells in mice [33]. Presumably, sTLR9 could act as a check point regulator which reduces antibody responses of B cells [33] and negatively regulates anti-tumor activities of neutrophils.
Suggestively, if sTLR9 is a molecule capable of negatively regulating anti-tumor activities of neutrophils, reducing sTLR9 on neutrophils could promote the phenotype transformation of sTLR9+ neutrophils to sTLR9− neutrophils. To verify this, we used CpG 5805 to reduce sTLR9 expression on neutrophils in tumor nodules. The CpG 5805 was demonstrated to mobilize sTLR9 from the surface to the endosome in B cells [33]. In vivo application of CpG 5805 significantly reduced the sTLR9 expression on neutrophils in the tumor nodules induced by CT26 cells (Fig. 5). Even though CpG 5805 is not specifically downregulating the sTLR9 on neutrophils because of the activation of DCs and CD8+ T cells happened at the same time, the reduction of sTLR9 on neutrophils was accompanied with the dramatic inhibition of the tumors developed from CT26 cells (Fig. 6). Thus, the data imply that reducing sTLR9 on neutrophils contributed to the anti-tumor effect of CpG 5805. The phenomenon should rely on the type conversion of neutrophils instead of having or not, because along with the reduction of sTLR9 on neutrophils, the ICAM1 expression obviously increased. There are reports that intratumoral application of CpG ODN promotes the migration of anti-tumoral neutrophils into tumor tissues followed by DC activation and maturation for inducing the T cell-mediated anti-tumor effect [49]. After the depletion of neutrophils, CD8+ T cells entering the tumor tissues obviously decrease and their cytotoxicity also reduce [50]. Therefore, depleting the neutrophil would not prove the neutrophil’s role in the anti-tumor effect of CpG 5805. The reduction of sTLR9 on neutrophils contributing to anti-tumor immune responses is possibly in two ways. One is that reducing sTLR9, a presumed check point molecule of innate immune responses, could transform pro-tumor sTLR9+ neutrophils to anti-tumor sTLR9− neutrophils. Another is that the reduced sTLR9 could be internalized to endosomes, becoming an endosomal TLR9 (eTLR9). The neutrophils with less sTLR9 and more eTLR9 could produce more Type I IFN, a downstream cytokine of the eTLR9-IRF5 signaling pathway [22, 27]. The autocrine Type I IFN induces ICAM1 expression on neutrophils [30]. The ICAM1 expressing neutrophils activate CD8+ T cells through binding lymphocyte function associated antigen-1 (LFA-1) [31, 32]. We observed the upregulation of the ICAM1 on neutrophils, and the 4-1BB+ on CD8+ T cells in the tumor nodules of mice after receiving CpG 5805 (Fig. 7d). IFNγ is able to substantially upregulate ICAM1 on neutrophils [30]. Thus, a positive-feedback loop could exist in the tumor nodules, bolstering the proliferation and activation of tumor cell-specific T cells. Additionally, the CpG 5805-induced reduction of sTLR9 on neutrophils was accompanied with the activation of DCs in the tumor nodules (Fig. 7c). The activated DCs could present tumor antigens to prime tumor cell-specific T cells.
Meaningfully, we found the rapidly formed-subcutaneous nodules induced by inoculating tumor cells in mice. The nodules are massively infiltrated with both of sTLR9+ neutrophils with a pro-tumoral phenotype and sTLR9− neutrophils with an anti-tumoral phenotype. The sTLR9 could function as a checkpoint molecule in innate immune cells, negatively regulating the anti-tumor activity of neutrophils in TME. Reducing the sTLR9 on neutrophils promote the adaptive immune responses against tumor cells. One of the underlying mechanisms about how CpG ODN displays anti-tumor activities is that CpG ODN could reduce sTLR9 on neutrophils. Lastly, the mice with tumor nodules could be used as a useful animal model to study the roles of neutrophils in tumor development, especially in tumor initiation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Yunpeng Yao, Feiyu Lu, Yangeng Wang, Shujun Liu for technical assistance through the year. This study is financially supported by the National Natural Science Foundation of China (31670937).
Author contributions
MK was the main researcher for this study including the experiment design and operation, data analysis, and manuscript writing; WL, MZ, and KQ participated in operation for some experiments including flow cytometry and mouse experiments. YY and LW provided research ideas, funds, and the writing and revising of the manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liying Wang, Email: wangliy@jlu.edu.cn.
Yongli Yu, Email: ylyu@jlu.edu.cn.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zha C, Meng X, Li L, et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol Med. 2020;17:154–168. doi: 10.20892/j.issn.2095-3941.2019.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu W, Jiang X, Guan C, Gu M. The prognostic and predictive value of tumor infiltrating Macrophage and Neutrophil in patient with clear cell renal cell carcinoma: Tumor infiltrating lymphocytes in renal cell carcinoma. Medicine (Baltimore) 2020;99:e23181. doi: 10.1097/MD.0000000000023181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen TO, Schmidt H, Møller HJ, et al. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer. 2012;118:2476–2485. doi: 10.1002/cncr.26511. [DOI] [PubMed] [Google Scholar]
- 6.Li YW, Qiu SJ, Fan J, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497–505. doi: 10.1016/j.jhep.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 7.Rao HL, Chen JW, Li M, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients' adverse prognosis. PLoS ONE. 2012;7:e30806. doi: 10.1371/journal.pone.0030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen SR, Strøbech JE, Horton ER, et al. Suppression of tumor-associated neutrophils by lorlatinib attenuates pancreatic cancer growth and improves treatment with immune checkpoint blockade. Nat Commun. 2021;12:3414. doi: 10.1038/s41467-021-23731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari SM, Fallahi P, Galdiero MR, et al. Immune and inflammatory Cells in thyroid cancer microenvironment. Int J Mol Sci. 2019;20:4413. doi: 10.3390/ijms20184413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol. 2016;37:41–52. doi: 10.1016/j.it.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andzinski L, Kasnitz N, Stahnke S, et al. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer. 2016;138:1982–1993. doi: 10.1002/ijc.29945. [DOI] [PubMed] [Google Scholar]
- 13.Sionov RV. Leveling up the controversial role of neutrophils in cancer: When the complexity becomes entangled. Cells. 2021;10:2486. doi: 10.3390/cells10092486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizuno R, Kawada K, Itatani Y, et al. The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci. 2019;20:529. doi: 10.3390/ijms20030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaul ME, Levy L, Sun J, et al. Tumor-associated neutrophils display a distinct N1 profile following TGF-β modulation: a transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology. 2016;5:e1232221. doi: 10.1080/2162402X.2016.1232221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Santo C, Arscott R, Booth S, et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol. 2010;11:1039–1046. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TT, Zhao YL, Peng LS, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66:1900–1911. doi: 10.1136/gutjnl-2016-313075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindau D, Mussard J, Wagner BJ, et al. Primary blood neutrophils express a functional cell surface Toll-like receptor 9. Eur J Immunol. 2013;43:2101–2113. doi: 10.1002/eji.201142143. [DOI] [PubMed] [Google Scholar]
- 19.Miyake K, Onji M. Endocytosis-free DNA sensing by cell surface TLR9 in neutrophils: rapid defense with autoimmune risks. Eur J Immunol. 2013;43:2006–2009. doi: 10.1002/eji.201343882. [DOI] [PubMed] [Google Scholar]
- 20.Avalos AM, Kirak O, Oelkers JM, et al. Cell-specific TLR9 trafficking in primary APCs of transgenic TLR9-GFP mice. J Immunol. 2013;190:695–702. doi: 10.4049/jimmunol.1202342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukui R, Saitoh S, Kanno A, et al. Unc93B1 restricts systemic lethal inflammation by orchestrating Toll-like receptor 7 and 9 trafficking. Immunity. 2011;35:69–81. doi: 10.1016/j.immuni.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Marongiu L, Gornati L, Artuso I, et al. Below the surface: the inner lives of TLR4 and TLR9. J Leukoc Biol. 2019;106:147–160. doi: 10.1002/JLB.3MIR1218-483RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YM, Brinkmann MM, Paquet ME, et al. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 24.Pelka K, Bertheloot D, Reimer E, et al. The chaperone UNC93B1 regulates Toll-like receptor stability independently of endosomal TLR transport. Immunity. 2018;48:911–922.e7. doi: 10.1016/j.immuni.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tatematsu M, Funami K, Ishii N, et al. LRRC59 regulates trafficking of nucleic acid-sensing TLRs from the endoplasmic reticulum via association with UNC93B1. J Immunol. 2015;195:4933–4942. doi: 10.4049/jimmunol.1501305. [DOI] [PubMed] [Google Scholar]
- 26.Lee BL, Moon JE, Shu JH, et al. UNC93B1 mediates differential trafficking of endosomal TLRs. Elife. 2013;2:e00291. doi: 10.7554/eLife.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takaoka A, Yanai H, Kondo S, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 28.Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, et al. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17:2619–2627. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]
- 29.Sikora AG, Jaffarzad N, Hailemichael Y, et al. IFN-alpha enhances peptide vaccine-induced CD8+ T cell numbers, effector function, and antitumor activity. J Immunol. 2009;182:7398–7407. doi: 10.4049/jimmunol.0802982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. 2012;82:296–309. doi: 10.1016/j.critrevonc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Chen T, Goldstein JS, O'Boyle K, et al. ICAM-1 co-stimulation has differential effects on the activation of CD4+ and CD8+ T cells. Eur J Immunol. 1999;29:809–814. doi: 10.1002/(SICI)1521-4141(199903)29:03<809::AID-IMMU809>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 32.Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol. 2005;17:251–258. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Lu W, Cui C, Wang Y, et al. CpG ODN as an adjuvant arouses the vigor of B cells by relieving the negative regulation of surface TLR9 to enhance the antibody response to vaccine. Appl Microbiol Biotechnol. 2021;105:4213–4224. doi: 10.1007/s00253-021-11316-9. [DOI] [PubMed] [Google Scholar]
- 34.Meng X, Sun W, Ren Y, et al. Protective role of surface Toll-like receptor 9 expressing neutrophils in local inflammation during systemic inflammatory response syndrome in mice. Mol Immunol. 2017;90:74–86. doi: 10.1016/j.molimm.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Ren Y, Hua L, Meng X, et al. Correlation of surface Toll-like receptor 9 expression with IL-17 production in neutrophils during septic peritonitis in mice induced by E. coli. Mediat Inflamm. 2016;2016:3296307. doi: 10.1155/2016/3296307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Y, Lu W, Li X, et al. An oligodeoxynucleotide with AAAG repeats significantly attenuates burn-induced systemic inflammatory responses via inhibiting interferon regulatory factor 5 pathway. Mol Med. 2017;23:166–176. doi: 10.2119/molmed.2016.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stangl S, Gehrmann M, Dressel R, et al. In vivo imaging of CT26 mouse tumours by using cmHsp70.1 monoclonal antibody. J Cell Mol Med. 2011;15:874–887. doi: 10.1111/j.1582-4934.2010.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Sun W, Wu X, et al. An oligodeoxynucleotide with CCT repeats restrains CpG ODN-induced TLR9 trafficking. Curr Pharm Biotechnol. 2014;15(9):780–789. doi: 10.2174/1389201015666141031114708. [DOI] [PubMed] [Google Scholar]
- 39.Connolly KA, Kuchroo M, Venkat A, et al. A reservoir of stem-like CD8+ T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci Immunol. 2021;6:eabg7836. doi: 10.1126/sciimmunol.abg7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 41.Dvorak HF, Orenstein NS, Carvalho AC, et al. Induction of a fibrin-gel investment: an early event in line 10 hepatocarcinoma growth mediated by tumor-secreted products. J Immunol. 1979;122:166–174. doi: 10.4049/jimmunol.122.1.166. [DOI] [PubMed] [Google Scholar]
- 42.Ciano PS, Colvin RB, Dvorak AM, et al. Macrophage migration in fibrin gel matrices. Lab Invest. 1986;54:62–70. [PubMed] [Google Scholar]
- 43.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16:133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueha S, Shand FH, Matsushima K. Myeloid cell population dynamics in healthy and tumor-bearing mice. Int Immunopharmacol. 2011;11:783–788. doi: 10.1016/j.intimp.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Mishalian I, Bayuh R, Eruslanov E, et al. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17–a new mechanism of impaired antitumor immunity. Int J Cancer. 2014;135:1178–1186. doi: 10.1002/ijc.28770. [DOI] [PubMed] [Google Scholar]
- 46.Himmel ME, Crome SQ, Ivison S, et al. Human CD4+ FOXP3+ regulatory T cells produce CXCL8 and recruit neutrophils. Eur J Immunol. 2011;41:306–312. doi: 10.1002/eji.201040459. [DOI] [PubMed] [Google Scholar]
- 47.Comen E, Wojnarowicz P, Seshan VE, et al. TNF is a key cytokine mediating neutrophil cytotoxic activity in breast cancer patients. NPJ Breast Cancer. 2016;2:16009. doi: 10.1038/npjbcancer.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finisguerra V, Di Conza G, Di Matteo M, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522(7556):349–353. doi: 10.1038/nature14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humbert M, Guery L, Brighouse D, et al. Intratumoral CpG-B promotes antitumoral neutrophil, cDC, and T-cell cooperation without reprograming tolerogenic pDC. Cancer Res. 2018;78(12):3280–3292. doi: 10.1158/0008-5472.CAN-17-2549. [DOI] [PubMed] [Google Scholar]
- 50.Stoppacciaro A, Melani C, Parenza M, et al. Regression of an established tumor genetically modified to release granulocyte colony-stimulating factor requires granulocyte-T cell cooperation and T cell-produced interferon gamma. J Exp Med. 1993;178(1):151–161. doi: 10.1084/jem.178.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.