Abstract
Stimulator of interferon genes (STING) contributes to anti-tumor immunity by activating antigen-presenting cells and inducing mobilization of tumor-specific T cells. A role for tumor-migrating neutrophils in the anti-tumor effect of STING-activating therapy has not been defined. We used mouse tumor transplantation models for assessing neutrophil migration into the tumor triggered by intratumoral treatment with STING agonist, 2′3′-cyclic guanosine monophosphate-adenosine monophosphate (cGAMP). Intratumoral STING activation with cGAMP enhanced neutrophil migration into the tumor in an NF-κB/CXCL1/2-dependent manner. Blocking the neutrophil migration by anti-CXCR2 monoclonal antibody impaired T cell activation in tumor-draining lymph nodes (dLNs) and efficacy of intratumoral cGAMP treatment. Moreover, the intratumoral cGAMP treatment did not show any anti-tumor effect in type I interferon (IFN) signal-impaired mice in spite of enhanced neutrophil accumulation in the tumor. These results suggest that both neutrophil migration and type I interferon (IFN) induction by intratumoral cGAMP treatment were critical for T-cell activation of dLNs and the anti-tumor effect. In addition, we also performed in vitro analysis showing enhanced cytotoxicity of neutrophils by IFN-β1. Extrinsic STING activation triggers anti-tumor immune responses by recruiting and activating neutrophils in the tumor via two signaling pathways, CXCL1/2 and type I IFNs.
Supplementary Information
The online version of this article (10.1007/s00262-021-02864-0) contains supplementary material, which is available to authorized users.
Keywords: Stimulator of interferon genes, cGAMP, Neutrophils, CXCR2, Type I interferons, Cancer immunity cycle
Introduction
Immunotherapy has become the fourth option for cancer treatment, complementing surgery, chemotherapy, and radiotherapy [1]. During the past few decades, multiple cancer immunotherapies have been successfully applied in clinical practice including oncolytic viruses [2], chimeric antigen receptor T cells [3], bispecific antibodies [4], and immune checkpoint inhibitors [5]. Many clinical trials are in progress testing potential synergistic effects of treatments combining immunotherapy with other therapies because some patients are non-responders to monotherapy of cancer immunotherapy. This will likely mean that potent combinations of immunotherapies will be required to efficiently modulate the cancer-immunity cycle, which includes seven critical steps for achieving anti-tumor immune responses [6].
Neutrophils are the predominant circulating leukocyte population in humans. They play a well-established role in host defense. When pathogens invade a host tissue, neutrophils extravasate from the circulation and enter the tissue to phagocytose and kill them by releasing activating cytokines (e.g., tumor necrosis factor-α, interleukin-1, interferons (IFNs), etc.) and defensins, along with toxic substances and reactive oxygen species (ROS) [7]. Although neutrophils have been defined in vivo as pro-tumor cells, similar to immune suppressive cells, in the context of tumor immunology [8], the exact role of neutrophils in the tumor cell microenvironment is controversial. Many patients with advanced cancer show neutrophilia, which is associated with worse prognosis in many cancers [9, 10]. In contrast, some studies have reported that a high neutrophil count in the tumor tissue is associated with a favorable prognosis [11]. Thus, whether neutrophils play a pro-tumor or anti-tumor role in the tumor microenvironment is not fully understood.
Stimulator of interferon genes (STING) is activated by second messenger cyclic dinucleotides such as 2′3′-cyclic guanosine monophosphate-adenosine monophosphate (cGAMP), which are synthesized by cyclic GMP-AMP synthase. STING activates multiple transcription factors including interferon regulatory factor (IRF) 3, IRF7, signal transducer and activator of transcription 6 (STAT6), and NF-κB to induce inflammation responses for host defense [12]. Our previous studies showed that STING is spontaneously activated in tumor tissues and contributes to anti-tumor immunity and its agonists are effective adjuvants for cancer immunotherapy [13, 14]. Furthermore, intratumoral cGAMP administration recruits CD11b+Ly6C+ inflammatory monocytes/macrophages to the tumor site, and these monocytes/macrophages contribute to the anti-tumor effects of intratumoral cGAMP treatment [15].
In the current study, we demonstrate that intratumoral cGAMP treatment also recruited CD11b+Ly6G+ neutrophils to the tumor site. The neutrophil accumulation in the tumor site was dependent on the STING/NF-κB/CXCL1/2/CXCR2 axis. cGAMP treatment also activated cytotoxicity of neutrophils via the IRF3/7/type I IFN pathway-dependent ROS production. Blocking neutrophil accumulation using anti-CXCR2 mAb impaired the T-cell activation in the draining lymph nodes and efficacy of cGAMP treatment, suggesting that accumulation of neutrophils in the type I IFN-inflamed tumor microenvironment may be essential for the anti-tumor effect mediated by STING-targeting immunotherapy, and that neutrophils would serves as a bridge between innate and adaptive immunity under the inflammation condition.
Methods
Cell lines and mice
Mouse breast cancer cell line E0771 and mouse lung cancer cell line 3LL were purchased from CH3 BioSystems and JCRB Cell Bank, respectively. Mouse melanoma cell line B16F10 was kindly donated by Dr. Kitamura (Hokkaido University). All cell lines were maintained in RPMI-1640 medium (Nacalai Tesque) supplemented with 10% fetal bovine serum (Biowest, #S1650), penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37 °C in a 5% CO2 incubator. C57BL/6 J and STING-deficient (gt) mice (C57BL/6 J-Tmem173gt/) were obtained from Charles River Japan and the Jackson Laboratory, respectively. IRF3/IRF7 double knockout (DKO) mice were generated by crossing IRF3-KO (B6;129S6-Bcl2l12/Irf3 < tm1Ttg > /TtgRbrc) and IRF7-KO (B6;129P2-Irf7 < tm1Ttg > /TtgRbrc) mice, which were provided by Dr. T. Taniguchi (Tokyo University). Type I IFN α/β receptor 1-deficient (IFNAR-KO) mice were generated using CRISPR/Cas9 technology. Six- to 10-week-old female mice were used for experiments and maintained and handled in accordance with the regulations of the animal facility at the Asahikawa Medical University.
Generation of Ifnar1 knockout (IFNAR1KO) mice
For generating of Ifnar1 knockout mice, CRISPR technology was utilized. In vitro fertilization (IVF) was performed as previously described [16]. Briefly, HTF (Human tubal fluid) medium (ARK Resource Co., Ltd.) was used for mouse sperm preincubation, IVF, and embryo transfer. For sperm preincubation, a 200 μl droplet was used. For oocyte collection and IVF, a 100 μl volume droplet was used. Embryos were washed by passing through four such droplets. Each droplet was placed on a 35 mm culture dish (Corning® Cat. No. 430588, Thermo Fisher Scientific), covered with liquid paraffin oil (Nacalai Tesque), and kept at 37 °C under 5% CO2 in humidified air overnight. After IVF, fertilized embryos were subjected to electroporation to inject Cas9 protein, tracrRNA and crRNA [17]. For electroporation, a platinum block electrode (Cat. No. LF501PT1-10, BEX Co., Ltd.; length: 10 mm, width: 1 mm, height: 0.5 mm, gap: 1 mm) was used. The electrode was connected to a CUY21EDIT II (BEX Co., Ltd.) electroporator, and set under a stereoscopic microscope (SZX2-ZB16, Olympus). The collected fertilized embryos cultured in HTF medium were washed with Opti-MEM™ I (Thermo Fisher Scientific) three times to remove the serum-containing medium. The embryos were then placed in a line in the electrode gap filled with 500 ng/μl Guide-it™ Recombinant Cas9 (Electroporation-Ready) protein (Takara Bio), 300 ng/μl tracrRNA and 200 ng/μl crRNA-containing Opti-MEM™ I solution (total 5 μl volume), and electroporation was performed. The electroporation conditions were 25 V (3 ms ON + 97 ms OFF) ± 3 repeats. After electroporation, the embryos were immediately collected from the electrode chamber and subjected to three washes with M2 medium (ARK Resource) followed by two washes with KSOM medium (ARK Resource). The embryos were then cultured in KSOM medium at 37 °C and 5% CO2 in humidified air overnight, and 2-cell embryos were transferred to pseudopregnant recipient ICR female mice (Japan SLC).
The sequence targeting Ifnar1 gene was designed using CRISPRdirect (https://crispr.dbcls.jp/) [18] and was as follows: Ifnar1 (5′-TGT GCC AGG AAA TCT CCA AG-3′). The tracrRNA and crRNA were purchased from FASMAC Co. Ltd. in dry form, dissolved in Opti-MEM™ I to 1 μg/μl and stored at –30 °C until use.
Validation of IFNAR1KO mice
Splenocytes were collected from WT and IFNAR1KO mice stained with PE-conjugated anti-IFNAR1 mAb (MAR1-5A3) and mouse IgG1,k (MOPC-21), and analyzed by the BD Accuri C6 Plus Flow Cytometer. Bone marrow-derived macrophages (BMDM) were generated from WT and IFNAR1KO mice by cultured in the presence of 20 ng/ml of M-CSF (PeproTech) for 7 days. BMDM derived from WT (WT-BMDM) and IFNAR1KO mice (IFNAR1-BMDM) were stimulated with 2000 U/ml of IFN-β1 (BioLegend) for 2 and 24 h to evaluate gene expression levels of Mx1, Oasl1, Cxcl9, and Cxcl10 and cell surface expression levels of H-2 kb, I-Ab, CD80, and CD86, respectively. Gene expression levels were assessed by real-time PCR and cell surface expression levels were analyzed by CytoFLEX flow cytometer (Beckman coulter).
Tumor-bearing animal model
E0771 cells (2 × 105 cells) were injected into the mammary fat pads of mice, and B16F10 cells (2 × 105 cells), and 3LL cells (3 × 105 cells) were intradermally injected into mice. On day 7 after tumor implantation, tumor-bearing mice received intratumoral injections of either cGAMP (InvivoGen; 1–2.5 μg/25 μl/dose) or mock treatment with the vehicle (phosphate-buffered saline (PBS)). In some experiments, anti-CXCR2 monoclonal antibody (mAb) (R&D Systems), its isotype control rat IgG2a mAb (2A3, Bio X cell), or an inhibitor of NF-κB (BAY 11–7082; FUJIFILM Wako Chemicals) was simultaneously injected intraperitoneally into tumor-bearing mice in addition to intratumoral cGAMP treatment on day 7. The tumor area (mm2) was measured with calipers at the indicated time points. Mice with tumors > 300 mm2 were sacrificed. All protocols were approved by the Asahikawa Medical University Institutional Animal Care and Use Committee (approval no. 19111). This study was performed in accordance with the Declaration of Helsinki.
Analysis of tumor-infiltrating leukocytes (TILs)
Tumor-bearing mice received intratumoral injection of PBS or cGAMP (0.5–2.5 μg in 25 μl PBS/dose) on day 7. After 4 h, tumor tissues were dissociated enzymatically and mechanically using a GentleMACS Dissociator (Miltenyi Biotec), as per the manufacturer’s guidelines. The dissociated samples were centrifuged at 1800 rpm for 3 min, and the supernatant was discarded. The cell pellets were resuspended in the above-mentioned medium and passed through a 35-μm pore nylon mesh strainer. To elucidate the cell population in the TILs, single-cell suspension was stained with anti-CD45 (I3/2.3), anti-CD11b (M1/70), anti-Ly6C (HK1.4), and anti-Ly6G (1A8) mAbs following treatment with TruStain FcX Anti-mouse CD16/CD32 antibody (clone, 93). All antibodies were obtained from BioLegend. The stained cells were analyzed using the BD Accuri C6 Plus Flow Cytometer (BD Biosciences).
RNA extraction and quantification of mRNA levels
Total RNA was extracted using the RNeasy Mini Kit (Qiagen) and was reverse-transcribed to cDNA using the PrimeScript 1st strand cDNA Synthesis Kit (Takara Bio). These samples were amplified using LightCycler 480 Probes Master (Roche Diagnostics) and TaqMan probes purchased from Applied Biosystems (Waltham) as follows: Cxcl1 (Mm04207460_m1), Cxcl2 (Mm00436450_m1), Ifnb1 (Mm00439552_s1), Mx1 (Mm00487796_m1), Oasl1 (Mm00455081_m1), Cxcl9 (Mm00434946_m1), Cxcl10 (Mm00445235_m1), and Gapdh (Mm99999915_g1). mRNA expression was measured with quantitative real-time PCR using the LightCycler 480 System and Software (Roche Diagnostics). Gapdh was used as an internal control and to normalize each mRNA expression level. Relative expression levels compared with control samples were calculated in each experiment using the ΔΔCt method.
Analysis of immune cells in lymph nodes
Lymph nodes were first chopped into small pieces and passed through a 35-μm pore nylon mesh strainer. The resulting cell suspension was washed and resuspended in the above-mentioned medium. Axillary and inguinal LNs were analyzed as tumor-draining LNs (dLNs) in B16F10 or 3LL-bearing and E0771-bearing mice, respectively. Cognate LNs in opposite side of dLNs were analyzed as distant LNs (disLNs). To elucidate the cell population in the LNs, single-cell suspension was stained with the following mAbs that were conjugated to fluorescein isothiocyanate, phycoerythrin, phycoerythrin combined with a cyanine dye (PE-Cy7), or allophycocyanin (BioLegend): anti-CD3 (17A2), anti-CD4 (RM4-5), anti-CD8a (53–6.7), anti-CD69 (H1.2F3), anti-CD11b (M1/70), anti-CD11c (N418), anti-CD80 (16-10A1), anti-CD86 (GL-1), anti-H-2 kb (AF6-88.5), anti-I-Ab (AF6-120.1), rat IgG2b,k (RTK4530), and mouse IgG2a,k (MOPC-173). The stained cells were analyzed using the BD Accuri C6 Plus Flow Cytometer or CytoFLEX. The supernatant was collected after 24 h, and IFN-γ levels were assessed with an enzyme-linked immunosorbent assay (ELISA) (BD Bioscience) according to the manufacturer’s instructions. Absorbance was read with a GloMax Discover Microplate Reader (Promega) at 450 nm.
Hematoxylin and eosin (H&E) staining and immunohistochemistry
Tumor tissue samples were fixed in 10% formalin overnight. Tissues were dehydrated in ethanol, embedded in paraffin, sectioned at 5 μm, and stained with H&E according to standard protocols. For immunohistochemistry, tumor sections were stained and visualized with diaminobenzidine according to standard procedures. Rabbit polyclonal antibody for cleaved caspase 3 (#9661; Cell Signaling TECHNOLOGY) was used as the primary antibody at a dilution of 1:200 overnight at 4 °C. SignalStain® Boost IHC Detection Reagent (HRP, Rabbit) (1:400 #8114; Cell Signaling TECHNOLOGY) was used for detection. Images were acquired and analyzed using the BZ-X700 fluorescence microscope with BZ-H3 software (KEYENCE).
Cell sorting and cytotoxicity assay
Mouse spleens were collected from wild type (WT) mice or IFNAR1KO mice. The excised spleens were gently passed through a 100-μm pore nylon mesh strainer and rinsed with the above-mentioned medium. Before sorting, CD4-positive and CD19-positive cells were excluded by MACS (magnetic-activated cell sorting) (Miltenyi Biotech). The remaining splenocytes were stained with anti-CD45 (I3/2.3), anti-CD11b (M1/70), anti-Ly6C (HK1.4), and anti-Ly6G (1A8) mAbs following treatment with TruStain FcX Anti-mouse CD16/CD32 antibody (clone, 93). All antibodies were obtained from BioLegend. The CD45+CD11b+Ly6C−Ly6G+ neutrophil fraction was isolated using BD FACSAria II (BD Biosciences). The neutrophils were co-cultured with E0771 cells labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (ThermoFisher SCIENTIFIC) at effector:target cell ratios of 5:1 or 0:1 in PBS or IFN-β (2000 U/ml). In some experiments, 10 μM diphenyleneiodonium chloride (DPI) (Sigma-Aldrich) was added to the co-culture to inhibit ROS production. After 2.5 h, cells were harvested and stained with 7-AAD viability staining solution (BioLegend). The cells were analyzed using the BD Accuri C6 Plus Flow Cytometer.
Statistical analyses
The statistical significance of differences between two groups was determined with the unpaired t test. Two-way analysis of variance (ANOVA) was conducted for multiple group comparisons. The log-rank (Mantel–Cox) test was used to determine statistically significant differences in survival among indicated groups. The difference between the means was considered significant at p < 0.05. GraphPad Prism7 was used for the analyses.
Results
Intratumoral injection of cGAMP leads to accumulation of Ly6G+ neutrophils in the tumor site in a STING-dependent manner
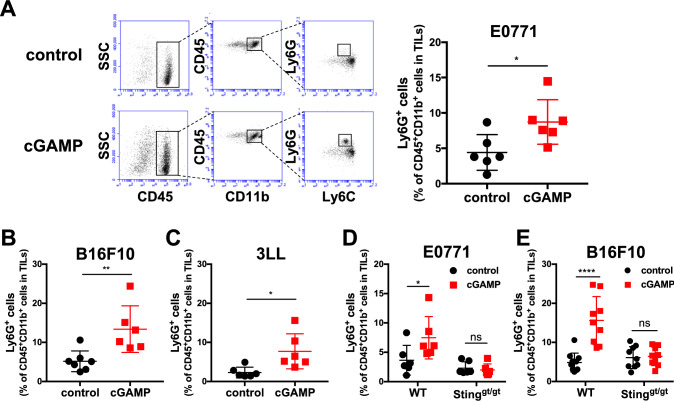
We first examined the effect of intratumoral injection of cGAMP on CD45+ TILs. On day 0, E0771 murine breast cancer cells were injected into the mammary fat pad of C57BL/6 mice. On day 7, we intratumorally injected cGAMP (1 μg/25 μl/dose) or PBS (as a control) into the E0771-bearing mice. After 4 h of treatment, CD11b+Ly6G+ neutrophils were significantly increased in the cGAMP-treated group compared to the control group (Fig. 1A). Furthermore, we also detected enhanced infiltration of neutrophils into the tumor site following intratumoral cGAMP treatment of mice bearing B16F10 murine melanoma cells and 3LL murine lung cancer cells (Fig. 1b and c). Because intratumoral injection of cGAMP enhanced accumulation of neutrophils in the tumor site in WT mice but not in STINGgt/gt mice, the accumulations of Ly6G+ cells in the tumor by intratumoral cGAMP treatment are dependent on a STING signaling (Fig. 1d and e).
Fig. 1.
Intratumoral injection of cGAMP induces accumulation of Ly6G+ neutrophils in the tumor site in a STING-dependent manner. a E0771-, b B16F10-, or c 3LL-bearing WT mice received intratumoral injection of PBS or cGAMP (0.5–2 μg) on day 7. After 4 h of treatment, tumor tissues were resected, and TILs were collected for flow cytometric analysis using anti-CD45, anti-CD11b, anti-Ly6C, and anti-Ly6G mAbs. Representative flow cytometric histograms are shown (a). The percentages of the increased fraction (CD45+CD11b+Ly6C−Ly6G+ cells) in TILs treated with control or cGAMP are depicted. Data were pooled from two independent experiments, each with three mice per group. Statistical significance levels were determined with the unpaired t test; *p < 0.05; **p < 0.01. Bars and error bars indicate the mean and standard deviation (SD), respectively. d E0771- or e B16F10-bearing WT or STING-deficient (gt/gt) mice were treated and analyzed according to the same protocol as in (a-c). The percentages of the increased fraction (CD45+CD11b+Ly6C−Ly6G+ cells) in TILs are depicted. Data were pooled from two independent experiments, each with three to five mice per group. Statistical significance levels were determined with two-way ANOVA; ns, not significant; *p < 0.05; **p < 0.01; ****p < 0.0001. Bars and error bars indicate the mean and SD, respectively
STING-triggered neutrophil accumulation in the tumor is dependent on the NF-κB/CXCR2 axis
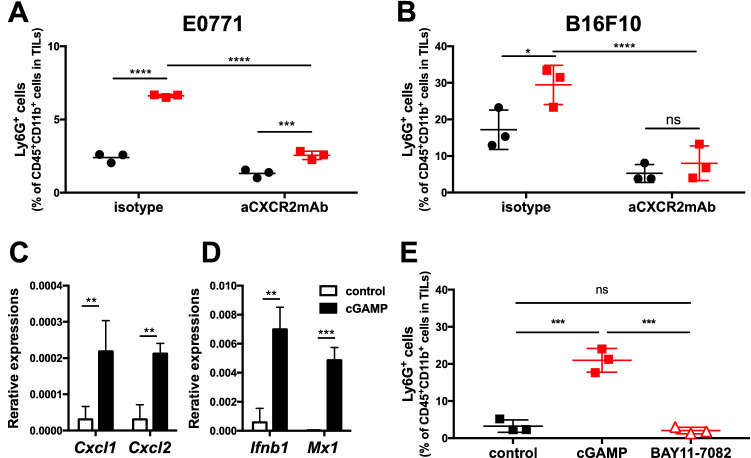
The chemokine receptor, CXCR2, is a receptor for CXCL1/CXCL2 and is crucial for the migration of neutrophils to sites of inflammation. We therefore addressed whether CXCR2 is involved in the migration of neutrophils to the tumor site following intratumoral injection of cGAMP. E0771- or B16F10-bearing mice received intratumoral injections of cGAMP or PBS on day 7, simultaneously with intraperitoneal injection of anti-CXCR2 mAb or isotype control mAb. The accumulation of neutrophils was suppressed by treatment with anti-CXCR2 mAb compared to controls (Fig. 2a and b). In accordance with the result, gene expression levels of Cxcl1 and Cxcl2 were enhanced by cGAMP treatment as well as Ifnb1 and Mx1 (Fig. 2c and d). Because STING activates NF-κB to induce CXCL1/2 production [19], we assessed if NF-κB signaling contributes to STING-triggered neutrophil migration using its inhibitor, BAY 11-7082. Simultaneous treatment with BAY 11-7082 and cGAMP inhibited STING-triggered neutrophil accumulation (Fig. 2e). These results indicate that intratumoral STING activation with cGAMP leads to accumulation of neutrophils in the tumor site in an NF-κB/CXCR2-dependent manner.
Fig. 2.
STING-triggered neutrophil accumulation in the tumor is dependent on the NF-κB/CXCR2 axis. a E0771- or b B16F10-bearing WT mice simultaneously received intraperitoneal injection of isotype or anti-CXCR2 mAb (50 μg) with intratumoral treatment with cGAMP (0.5 or 2.0 μg) on day 7. After 4 h of cGAMP treatment, tumor tissues were resected, and TILs were collected for flow cytometric analysis using anti-CD45, anti-CD11b, anti-Ly6C, and anti-Ly6G mAb. The percentages of the increased fraction (CD45+CD11b+Ly6C−Ly6G+ cells) in TILs are depicted. B16F10-bearing WT mice received intratumoral cGAMP treatment on day 7, and tumor tissues were collected for gene expression analysis after 3 h of treatment. Expression levels of Cxcl1 and Cxcl2 (C) and Ifnb1 and Mx1 (d) were analyzed with quantitative real-time PCR. Statistical significance levels were determined with the unpaired t test; ns, not significant; **p < 0.01; ***p < 0.001. Error bars indicate the SD. e E0771-bearing mice simultaneously received intraperitoneal injection of control or BAY 11–7082 (10 μM) with intratumoral treatment with cGAMP (0.5 μg) on day 7. After 4 h of cGAMP treatment, tumor tissues were resected, and TILs were collected for flow cytometric analysis as in a and b. Representative data are shown. Statistical significance levels were determined with two-way ANOVA; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Bars and error bars indicate the mean and SD, respectively
The accumulation of neutrophils in the tumor site contributes to the anti-tumor effect mediated by intratumoral injection of cGAMP
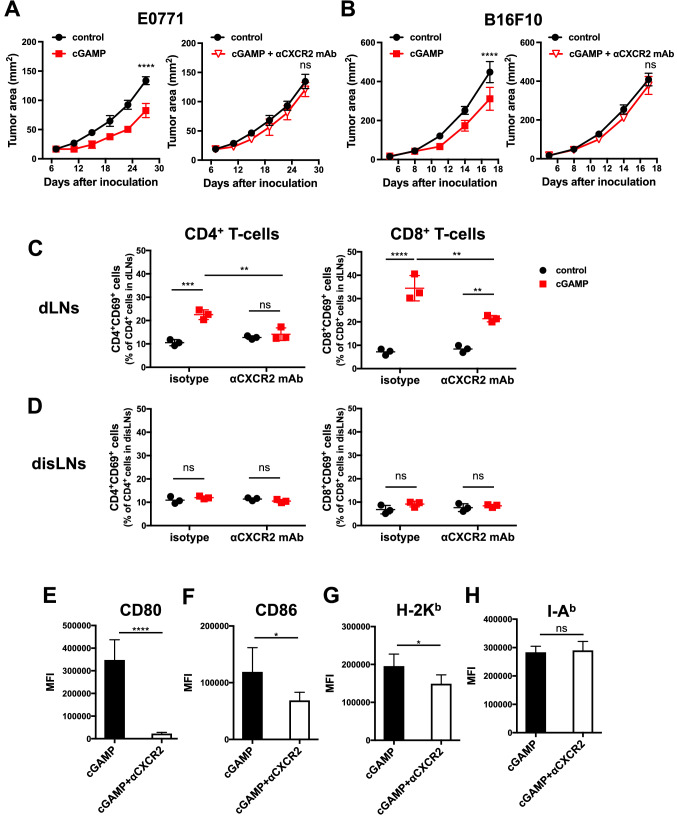
We assessed whether the STING-triggered tumor-infiltrating Ly6G+ neutrophils contribute to the anti-tumor effect. When anti-CXCR2 mAb was simultaneously injected into the mice, the efficacy of cGAMP therapy was totally abrogated (Fig. 3a and b). Furthermore, the frequencies of CD69+ activating T-cells in dLNs, but not in disLNs, were decreased following treatment with anti-CXCR2 mAb (Fig. 3c and d). Furthermore, we found that CD11b+CD11c+ DCs in dLNs of mice treated with anti-CXCR2 mAb showed lower expression levels of H-2 Kb, CD80, and CD86 than those of mice treated with the isotype control mAb. No significant changes were found in the expression level of I-Ab between mice treated with anti-CXCR2 mAb and those treated with the isotype control mAb (Fig. 3 E–H). These findings indicate that STING-triggered neutrophil accumulation in the tumor contributes to anti-tumor immunity via activating acquired immunity in dLNs.
Fig. 3.
Critical role of neutrophil accumulation in the tumor site in the anti-tumor effect mediated by intratumoral injection of cGAMP. a E0771- or b B16F10-bearing WT mice (n = 5/group) simultaneously received intraperitoneal injection of isotype or anti-CXCR2 mAb (50 μg) with intratumoral treatment with cGAMP (0.5–2.0 μg) on day 7. After 18 h of treatment with cGAMP, c draining lymph nodes (dLNs) and d distant lymph nodes (disLNs) were collected and stained with antibodies against CD4, CD8, CD3, and CD69 to assess the T-cell activation status. To address the activation status of antigen-presenting cells, the median fluorescence intensity (MFI) of CD80 (e), CD86 (f), H-2 Kb (g), or I-Ab (h) was measured in CD11b+CD11c+ cells of dLNs with flow cytometry. Statistical significance levels were determined with the unpaired t test; ns, not significant, *p < 0.05; ***p < 0.001; ****p < 0.0001. Bars and error bars indicate the mean and SD, respectively
Impaired T-cell activation in cGAMP-treated type I IFN-deficient mice despite neutrophil accumulation
Because STING stimulation induced type I IFN signaling (Fig. 2C), we investigated whether type I IFNs are also involved in the accumulation of neutrophils in the tumor site following intratumoral injection of cGAMP by using knockout mice for both IRF3 and IRF7 (DKO) and IFNAR1 which is a receptor for type I IFNs. IFNAR1KO mice were confirmed no expression of IFNAR1 and unresponsiveness to IFN-β1 in BMDM (Fig. 4a and b and Fig. S1). As a result, neutrophils accumulated in the tumor site in both DKO and IFNAR1KO mice after cGAMP treatment as well as in WT mice (Fig. 4c and d). In contrast, there were no activating T-cells in the dLNs of these type I IFN-deficient mice, but not WT mice (Fig. 4e and f). In accordance with the impaired T-cell activation, the anti-tumor effect of the cGAMP treatment was totally abrogated in IFNAR1KO mice (Fig. 4g and h). These results indicate that neutrophil accumulation in the tumor site is not sufficient for activating acquired immunity and eradicating tumor.
Fig. 4.
Involvement of type I IFNs in the cytotoxic activity of neutrophils and efficacy of cGAMP treatment. a Bone marrow-derived macrophages (BMDM) were generated in WT and IFNAR1KO mice and expression levels of IFNAR1 on their cell surface by flow cytometer. Gray: isotype control, Red: anti-IFNAR1mAb. b WT- and IFNAR1-BMDM were stimulated with control or IFN-β1 (2000 U/mL) for 24 h and expression levels of H2-Kb on their cell surface by flow cytometer. Gray: isotype control, Blue: control treatment, Red: IFN-β1 treatment. c E0771- or d B16F10-bearing WT, IRF3/IRF7 double knockout (DKO), or interferon (IFN)-alpha receptor 1 knockout (IFNAR1KO) mice received intratumoral injection of PBS or cGAMP (0.5 or 2.0 μg) on day 7. After 4 h of cGAMP treatment, tumor tissues were resected, and TILs were collected for flow cytometric analysis using anti-CD45, anti-CD11b, anti-Ly6C, and anti-Ly6G mAb. The percentages of the increased fraction (CD45+CD11b+Ly6C−Ly6G+ cells) in TILs are depicted. e, f E0771-bearing WT, DKO, or IFNAR1KO mice received intratumoral injection of PBS or cGAMP on day 7. After 4 h of treatment, dLNs were collected and analyzed using the same procedure as in Fig. 3c and d. The proportions of CD4+CD69+ cells in CD4+ T-cells (e) and CD8+CD69+ cells in CD8+ T-cells (f) in dLNs are depicted. B16F10-bearing WT or IFNAR1KO mice (n = 6/group) received intratumoral injections of cGAMP or PBS on day 7. g The tumor area (mm2) and h survival of individual mice were monitored. Statistical significance levels were determined with two-way ANOVA; ns, not significant, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Bars and error bars indicate the mean and SD, respectively (c-f). Statistical significance levels were determined with the unpaired t test; ns, not significant; ****p < 0.0001. Error bars indicate the SD (g). Statistical significance levels were determined with the log-rank (Mantel-Cox) test; ns, not significant; *p < 0.05 (h)
Involvement of type I IFNs in the cytotoxic activity of neutrophils and efficacy of cGAMP treatment
We hypothesized that neutrophils would require type I IFNs to promote anti-tumor immunity via activating immune cells because intratumoral cGAMP treatment did not activate T-cells in DKO and IFNAR1KO mice despite neutrophil accumulation in the tumor site. To address the question, we assessed tissue damage mediated by intratumoral cGAMP treatment by pathological analysis. High-power microscopic examination following H&E staining revealed that tumor cell adhesion appeared to be weaker and the number of apoptotic cells was increased at the edge of the tumor in WT mice compared to IFNAR1KO mice. Furthermore, cleaved caspase 3 was found to be detected in larger areas in WT mice than in IFNAR1KO mice (Fig. 5a). Although these results indicate that type I IFNs are important for tissue damages mediated by cGAMP treatment, the cGAMP-mediated anti-tumor effect appeared to be due to the response of host immune cells, not the tumor itself, because cGAMP treatment did not induce any immune responses in STING-deficient mice (Fig. 1e) [15]. Thus, both neutrophil migration and type I IFN induction in the tumor site are crucial for the anti-tumor effect of intratumoral cGAMP treatment.
Fig. 5.
Necessity of type I IFNs for cytotoxic function of neutrophils. a E0771-bearing WT or IFNAR1KO mice received intratumoral injection of cGAMP on day 7. After 4 h of treatment, tumor tissues were resected and fixed in formalin. Representative images and their higher magnifications of tumor tissues stained with H&E and anti-cleaved caspase 3 mAb are shown in the left and right panels, respectively. b CD11b+Ly6G+ neutrophils were isolated from splenocytes of WT or IFNAR1KO mice with a cell sorter using anti-CD45, anti-CD11b, anti-Ly6C, and anti-Ly6G mAb. The sorted Ly6G+ cells and CFSE-labeled E0771 tumor cells were used as effector and target cells, respectively. The effector cells and target cells were co-cultured with or without IFN-β (2000 U/ml). After 2.5 h of co-culture, the cells were collected to evaluate the percentages of dead cells by using 7-AAD and flow cytometry. Representative cytotoxicity mediated by Ly6G+ neutrophils derived from WT (left panel) and IFNAR1KO (middle panel) mice are shown. DPI (10 μM) was added to the culture of WT neutrophils to suppress ROS production (right panel). Two independent experiments with similar results were performed. Statistical significance levels were determined with two-way ANOVA; ns, not significant; *p < 0.05; ****p < 0.0001. Bars and error bars indicate the mean and SD, respectively
To address the relationship between cell damage and neutrophils in conditions with high levels of type I IFNs, we assessed whether the cytotoxicity of Ly6G+ neutrophils was promoted by type I IFNs in vitro. Ly6G+ neutrophils sorted from splenocytes of WT or IFNAR1KO mice were stimulated with or without IFN-β1 (2000 U/ml) and then co-cultured with CFSE-labeled E0771 tumor cells. IFN-β1-stimulated neutrophils showed high cytotoxicity against E0771 tumor cells compared to control them in WT mice but not IFNAR1KO mice. Furthermore, when an inhibitor of NADPH oxidase DPI was added to the culture, the enhanced cytotoxicity of neutrophils by IFN-β1 was abrogated, suggesting that neutrophils require stimulation with type I IFNs for enhanced cytotoxicity (Fig. 5b). These results suggest that enhanced local immune responses by cGAMP treatment are dependent on type I IFN-triggered cytotoxic activity of neutrophils as well as their migration via CXCL1/2.
Discussion
In the present study, we revealed that intratumoral injection of cGAMP triggers anti-tumor immune responses in a STING-dependent manner due to accumulation and activation of neutrophils in the tumor site via induction of expression of CXCL1/2 and type I IFNs through activation of signaling pathways involving NF-κB and IRF3/IRF7, respectively. The anti-tumor effect of intratumoral cGAMP administration was impaired when migration of neutrophils into the tumor site was blocked by the anti-CXCR2 mAb. Furthermore, intratumoral injection of cGAMP did not inhibit tumor growth or T-cell activation in the dLNs in IFNAR1-deficient mice, although neutrophils accumulated in the tumor. Neutrophils activated by type I IFNs in vitro showed improved cytotoxicity due to enhanced ROS production. These results suggest that neutrophils in the tumor site that are activated by type I IFNs are critical for the anti-tumor effects of intratumoral cGAMP treatment.
We found that STING-mediated accumulation of Ly6G+ neutrophils in the tumor site was suppressed by introducing the anti-CXCR2 mAb, suggesting that accumulation is dependent on CXCR2. In a previous report, Weiss et al. revealed that STING activation by 5,6-dimethylxanthenone-4-acetic acid, a ligand of STING, induces infiltration of neutrophils, monocytes, and CD8+ T cells and that these immune cell subsets cooperate in inducing tumor regression. However, the detailed mechanisms of neutrophil infiltration into the tumor site and the role of neutrophils in the anti-tumor effect have not been clarified [20].
We demonstrated that intratumoral STING activation by cGAMP induced high expression levels of Cxcl1 and Cxcl2 in the tumor site. The STING signaling pathway is initiated by binding its ligand (cyclic dinucleotides), followed by activation of TANK-binding kinase 1 (TBK1), which phosphorylates STING. Phosphorylated STING induces phosphorylation of IRF3 and IRF7 by TBK1 and activates transcription of type I IFNs. Phosphorylated STING also induces phosphorylation of STAT6. Phosphorylated STAT6 results in production of chemokines, such as CCL2, CCL20, and CCL26 [12, 21, 22]. In our current study, we found that intratumoral cGAMP administration induced accumulation of neutrophils in the tumor site in both IRF3/IRF7-deficient (DKO) mice and IRF3/IRF7/STAT6-deficient (TKO) mice as well as WT mice. Additionally, both types of knockout mice showed high expression levels of Cxcl1 and Cxcl2 in response to intratumoral cGAMP treatment. These results suggest that the STING-induced accumulation of neutrophils in the tumor site via CXCL1/CXCL2/CXCR2 is dependent on STING downstream pathways with the exception of IRF3/IRF7 or STAT6.
STING also triggers the NF-κB signaling pathway via activation of the inhibitor of kappa B kinase [23]. We demonstrated that BAY 11–7082, an inhibitor of NF-κB, inhibited accumulation of neutrophils in the tumor site despite intratumoral administration of cGAMP. Because NF-κB mediates transcription of CXCL1 and CXCL2 [19], we presume that intratumoral STING activation by cGAMP enhances expression of CXCL1 and CXCL2 via activation of NF-κB, which subsequently causes accumulation of neutrophils in the tumor site. As well as STING, Toll-like receptors (TLRs) also activate NF-κB in their signal pathway. Therefore, we intratumorally administrated polyI:C, R837, and CpG, which were agonists for TLR3, TLR7, and TLR9 to address whether intratumoral stimulation of TLRs also induce neutrophil accumulation in the tumor site. Interestingly, stimulation with each agonist did not induce neutrophil accumulation in the tumor site (data not shown), suggesting that STING has a unique function to activate innate immunity.
Based on the current study, intratumoral STING activation by cGAMP induces anti-tumor immune responses via activation of several signaling pathways, including NF-κB and IRF3/IRF7. These pathways collaborate to induce cytotoxicity of neutrophils in the tumor site. The former effectively recruits neutrophils into the tumor site by inducing CXCL1/CXCL2, and the latter activates neutrophils by inducing type I IFNs.
In recent years, tumor-associated neutrophils (TANs) have been classified into an anti-tumor (N1) or pro-tumor (N2) phenotype similar to tumor-associated macrophages, which are polarized toward an anti-tumor (M1) or a pro-tumor (M2) phenotype [24]. Type I IFNs drive neutrophil differentiation into an N1 anti-tumor state [25–27]. Additionally, Takeshima et al. reported that N1 neutrophils improve the anti-tumor immune response due to ROS production [28]. In the present study, we revealed that enhanced cytotoxicity of neutrophils by IFN-β was suppressed by an inhibitor of ROS production. Thus, STING-mediated induction of CXCL1/2 and type I IFNs may cooperate in differentiating TANs into the N1 phenotype in the tumor site and effectively augmenting their anti-tumor effects.
The role of N1 neutrophils in the anti-tumor immune response is not only direct tumor killing because we found that co-administration of the anti-CXCR2 mAb with cGAMP diminished both neutrophil accumulation in the tumor site and activation of DCs and T cells in tumor dLNs. Furthermore, anti-tumor effects of intratumoral cGAMP treatment were also inhibited by blockade of the CXCL1/2 or type I IFN pathway. These findings imply that TANs act as an intermediary between innate and adaptive immunity in the tumor site inflamed by STING stimulation.
We hypothesize that DAMPs are involved in the activation of DCs in mice that received intratumoral cGAMP treatment because DAMPs are released by damaged or dying cells and are recognized by macrophages and DCs through pattern recognition receptors on their surface such as TLR4 [29, 30]. Although apoptosis has been hypothesized to be a poorly immunogenic and tolerogenic type of cell death [31], some dying apoptotic cells induce an immune response by releasing DAMPs, similar to cells undergoing necrosis and necroptosis [32, 33]. In the inflamed tumor tissue induced by STING activation, DAMPs are likely to be released by neutrophil cytolysis. Indeed, we found that systemic administration of a TLR4 inhibitor attenuated activations of DCs and T cells in dLNs, even though the inhibitor did not suppress neutrophil accumulation triggered by cGAMP treatment in the tumor site (data not shown). However, the involvement of DAMPs in the efficacy of cGAMP treatment still remains to be addressed. These results imply that type I IFN-exposed neutrophils in the tumor microenvironment induce apoptosis of tumor cells and probably surrounding normal cells via producing ROS, and subsequent anti-tumor immune responses. Although STING has an anti-tumor effect by stimulating antigen-presenting cells and leading to mobilization of tumor-specific T cells via production of type I IFNs, our current findings unveil a new role for STING in the first step of the cancer-immunity cycle through neutrophil activation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Dr. Tadatsugu Taniguchi for providing IRF3-KO and IRF7-KO and Mr. Hayakawa Toshiyuki and Ms. Hino Chihiro (at the Animal Laboratory for Medical Research, Center for Advanced Research and Education, Asahikawa Medical University) and Ms. Matsumoto Rie (at the Department of Pathology, Asahikawa Medical University) for devotedly maintaining the mice. This work was supported by the Akiyama Life Science Foundation to T. Ohkuri.
Abbreviations
- CD
Cluster of differentiation
- CXCL
Chemokine (C-X-C motif) ligand
- CXCR
CXC chemokine receptor
- mAb
Monoclonal antibody
- NF-κB
Nuclear factor-kappa B
Author contributions
MN, AK, TO, and HK designed and preformed experiments; MN and TO analyzed results and made the figures; JU and HF generated IFNAR1KO mice; MN, AK, TO, YY, SY, MO, KO, SH, RH, TK, TN, KO, NA, YH, CE, and H.K. discussed the results; MN, TO, and HK wrote the paper. All authors read and approved the final manuscript.
Funding
This study was supported by the grants from the Akiyama Life Science Foundation (T. Ohkuri).
Data availability
All data and materials generated during the current study are available from the corresponding authors upon reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Ethics approval
Animal use protocol was approved by the Asahikawa Medical University Institutional Animal Care and Use Committee (approval no. 19111). This study was performed in accordance with the Declaration of Helsinki.
Availability of data and material
All data and materials generated during the current study are available from the corresponding authors upon reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takayuki Ohkuri, Email: ohkurit@asahikawa-med.ac.jp.
Hiroya Kobayashi, Email: hiroya@asahikawa-med.ac.jp.
References
- 1.Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Seminars Oncol. 2015;42(4):587–600. doi: 10.1053/j.seminoncol.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107(10):1373–1379. doi: 10.1111/cas.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu S, Li A, Liu Q, Li T, Yuan X, Han X, Wu K. Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol. 2017;10(1):78. doi: 10.1186/s13045-017-0444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu S, Liu Q, Han X, Qin S, Zhao W, Li A, Wu K. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Exp Hematol Oncol. 2017;6:31. doi: 10.1186/s40164-017-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, Wu K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17(1):129. doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 8.Mishalian I, Bayuh R, Levy L, Zolotarov L, Michaeli J, Fridlender ZG. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother. 2013;62(11):1745–1756. doi: 10.1007/s00262-013-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J, von der Maase H. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93(3):273–278. doi: 10.1038/sj.bjc.6602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, Mayaud C, Milleron B, Baud L, Cadranel J. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998;152(1):83–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Caruso RA, Bellocco R, Pagano M, Bertoli G, Rigoli L, Inferrera C (2002) Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in northern Italy. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 15 (8):831–837. 10.1097/01.Mp.0000020391.98998.6b [DOI] [PubMed]
- 12.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohkuri T, Ghosh A, Kosaka A, Zhu J, Ikeura M, David M, Watkins SC, Sarkar SN, Okada H. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol Res. 2014;2(12):1199–1208. doi: 10.1158/2326-6066.Cir-14-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harabuchi S, Kosaka A, Yajima Y, Nagata M, Hayashi R, Kumai T, Ohara K, Nagato T, Oikawa K, Ohara M, Harabuchi Y, Ohkuri T, Kobayashi H. Intratumoral STING activations overcome negative impact of cisplatin on antitumor immunity by inflaming tumor microenvironment in squamous cell carcinoma. Biochem Biophys Res Commun. 2020;522(2):408–414. doi: 10.1016/j.bbrc.2019.11.107. [DOI] [PubMed] [Google Scholar]
- 15.Ohkuri T, Kosaka A, Ishibashi K, Kumai T, Hirata Y, Ohara K, Nagato T, Oikawa K, Aoki N, Harabuchi Y, Celis E, Kobayashi H. Intratumoral administration of cGAMP transiently accumulates potent macrophages for anti-tumor immunity at a mouse tumor site. Cancer Immunol Immunother. 2017;66(6):705–716. doi: 10.1007/s00262-017-1975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeo T, Nakagata N (2018) In vitro fertilization in mice. Cold Spring Harbor protocols, (6). 10.1101/pdb.prot094524 [DOI] [PubMed]
- 17.Hashimoto M, Takemoto T. Electroporation enables the efficient mRNA delivery into the mouse zygotes and facilitates CRISPR/Cas9-based genome editing. Sci Reports. 2015;5:11315. doi: 10.1038/srep11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naito Y, Hino K, Bono H, Ui-Tei K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics (Oxford, England) 2015;31(7):1120–1123. doi: 10.1093/bioinformatics/btu743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke SJ, Lu D, Sparer TE, Masi T, Goff MR, Karlstad MD, Collier JJ. NF-kappaB and STAT1 control CXCL1 and CXCL2 gene transcription. Am J Physiol Endocrinol Metab. 2014;306(2):E131–149. doi: 10.1152/ajpendo.00347.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss JM, Guérin MV, Regnier F, Renault G, Galy-Fauroux I, Vimeux L, Feuillet V, Peranzoni E, Thoreau M, Trautmann A, Bercovici N. The STING agonist DMXAA triggers a cooperation between T lymphocytes and myeloid cells that leads to tumor regression. OncoImmunology. 2017;6(10):e1346765. doi: 10.1080/2162402X.2017.1346765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suschak JJ, Wang S, Fitzgerald KA, Lu S. A cGAS-Independent STING/IRF7 Pathway Mediates the Immunogenicity of DNA Vaccines. J Immunol. 2016;196(1):310. doi: 10.4049/jimmunol.1501836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Sun H, You F, Sun W, Zhou X, Chen L, Yang J, Wang Y, Tang H, Guan Y, Xia W, Gu J, Ishikawa H, Gutman D, Barber G, Qin Z, Jiang Z. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell. 2011;147(2):436–446. doi: 10.1016/j.cell.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Balka KR, Louis C, Saunders TL, Smith AM, Calleja DJ, D'Silva DB, Moghaddas F, Tailler M, Lawlor KE, Zhan Y, Burns CJ, Wicks IP, Miner JJ, Kile BT, Masters SL, De Nardo D. TBK1 and IKKepsilon act redundantly to mediate STING-induced NF-kappaB responses in myeloid cells. Cell Rep. 2020;31(1):107492. doi: 10.1016/j.celrep.2020.03.056. [DOI] [PubMed] [Google Scholar]
- 24.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120(4):1151–1164. doi: 10.1172/jci37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33(5):949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 27.Jablonska J, Wu CF, Andzinski L, Leschner S, Weiss S. CXCR2-mediated tumor-associated neutrophil recruitment is regulated by IFN-β. Int J Cancer. 2014;134(6):1346–1358. doi: 10.1002/ijc.28551. [DOI] [PubMed] [Google Scholar]
- 28.Takeshima T, Pop LM, Laine A, Iyengar P, Vitetta ES, Hannan R. Key role for neutrophils in radiation-induced antitumor immune responses: Potentiation with G-CSF. Proc Natl Acad Sci U S A. 2016;113(40):11300–11305. doi: 10.1073/pnas.1613187113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol. 2015;16(6):329–344. doi: 10.1038/nrm3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matzinger P. The danger model: a renewed sense of self. Science (New York, NY) 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 31.Bellamy CO, Malcomson RD, Harrison DJ, Wyllie AH. Cell death in health and disease: the biology and regulation of apoptosis. Semin Cancer Biol. 1995;6(1):3–16. doi: 10.1006/scbi.1995.0002. [DOI] [PubMed] [Google Scholar]
- 32.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32(4):157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Spencer DM, Mobarrez F, Wallén H, Pisetsky DS. The expression of HMGB1 on microparticles from Jurkat and HL-60 cells undergoing apoptosis in vitro. Scand J Immunol. 2014;80(2):101–110. doi: 10.1111/sji.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials generated during the current study are available from the corresponding authors upon reasonable request.