Abstract
Bovine herpesvirus 1 (BHV-1) induces immune suppression, but the mechanisms for suppression are not well identified. We examined the induction and activity of BHV-1-specific cytolytic CD4+ T lymphocytes (CTL) by stimulating peripheral blood mononuclear cells (PBMC) of cattle immunized with attenuated live BHV-1. Cytolytic effector cells were primarily CD4+ T lymphocytes and lysed autologous, but not allogeneic, macrophages infected with BHV-1 or pulsed with BHV-1 polypeptides. Apoptosis of BHV-1-expressing target cells was observed in CD4+ CTL assays by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) analysis. To determine if apoptosis was mediated by a perforin- or Fas-mediated pathway, EGTA, a known selective inhibitor of the perforin pathway, was used. EGTA did not inhibit CD4+-T-cell-mediated cytotoxic activity, but it did limit the NK cell cytotoxicity of virus infected cells. These findings support the concept that CD4+ CTL lyse macrophages pulsed with BHV-1 polypeptides through a Fas-mediated lytic pathway by inducing apoptosis in the target cells. The prominent cytotoxicity mediated by CD4+ CTL suggests a mechanism of selective removal of viral antigen-associated antigen-presenting cells.
Bovine herpesvirus 1 (BHV-1) is a member of the Alphaherpesvirinae subfamily. Other members of the subfamily include herpes simplex virus (HSV), pseudorabies virus, and equine herpesvirus. Like these viruses, BHV-1 afflicts both the respiratory and genital tracts (15). Because herpesvirus persists in the presence of specific antiviral antibody and because a cell-to-cell viral transfer pathway of infection occurs (39), cell-mediated immunity is considered an important mechanism in viral elimination (5, 9). This idea is supported by cytotoxic T-lymphocyte (CTL) activity against viral surface glycoproteins gB, gC, and gD in HSV-1 and BHV-1 infection (14) and by protection from lethal HSV-1 challenge in mice by adoptive transfer of immune T cells (30).
Interestingly, BHV-1, as well as HSV, is known to down-regulate the expression of major histocompatibility complex (MHC) class I molecules (25, 32). Inhibition of MHC class I expression on the surface of infected cells would probably interfere with the protective function of CD8+ CTL. Although bovine CD8+ CTL have been reported as a mechanism to lyse BHV-1-infected cells (14), these CTL cells are difficult to demonstrate in vitro (37). Mechanisms other than CD8+ CTL may play an important role in controlling viral infection.
In HSV infection, CD4+ CTL activity has been proposed as one of the major immune mechanisms to control HSV recurrence in herpesvirus stromal keratitis in humans (10), and mice (16). In fact, early efforts to clone CTL specific for HSV-1 and HSV-2 resulted in CD4+ rather than CD8+ CTL clones (46, 47). In HSV infection, both CD4+ and CD8+ HSV-specific CTL were induced with UV-inactivated HSV or live HSV stimulation (35, 40). In contrast to HSV, little is known about the protective mechanisms against BHV-1.
CTL trigger target cell death though T-cell receptor–peptide–MHC complex involvement. CD8+ CTL can lyse target cells through either Fas- or perforin-mediated cell death (27). The Fas-dependent pathway may play a more prominent role in CD4+-, rather than, CD8+-CTL-mediated lysis. CD4+ T cells may even lyse exclusively through the Fas-mediated lytic pathway (24, 27). However, perforin-mediated cytotoxicity by human CD4+ CTL has been found in a CD4+-T-cell clone and in peripheral CD4+ T cells (43, 45), although perforin expression is confined mainly to CD8+ T cells, natural killer (NK) cells, and γδ T cells. Recently, bovine Fas cDNA was isolated and its nucleotide sequence was determined (48), providing support for the notion that this Fas lytic mechanism may exist in cattle. The identification of bovine Fas provides support for the notion that CD4+ CTL may lyse target cells through the Fas-mediated lytic pathway in cattle (48). In humans, CD4+ CTL lyse antigen-pulsed target macrophages by inducing apoptosis (11).
Our work focused on the possible mechanisms of bovine CD4+ CTL lysis of cells expressing BHV-1 peptides. Defining the mechanism(s) of cellular protection in BHV-1 infection is a critical step to understanding viral pathogenesis and controlling viral spread. The present study characterizes the phenotype and functional aspects of CTL detectable in peripheral blood mononuclear cells (PBMC) of BHV-1-immunized cattle, using autologous macrophages pulsed with BHV-1 polypeptides or infected with live BHV-1. The CTL could serve as a major immune mechanism against BHV-1 infection, as well as a possible role in selective elimination of virus-infected antigen-presenting cells.
MATERIALS AND METHODS
Animals, cells, and virus strains.
Normal healthy cattle (Jersey, Holstein, or Guernsey), 2 to 8 years old, were housed at the University of Wisconsin—Madison Dairy Cattle Center and were used for all experiments. Cattle were immunized annually with a strain of temperature-sensitive modified live bovine rhinotracheitis-parainfluenza 3 vaccine (SmithKline Beecham, Philadelphia, Pa.) and were seropositive for BHV-1. Blood was taken for experiments throughout the year irrespective of the date of immunization. Culture supernatant containing BHV-1 (Cooper strain ATCC VR-864) was harvested after infection of MDBK cells (ATCC CCL 22) for 3 days. The MDBK cells were cultured in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (FBS), 100 U of penicillin per ml, and 100 μg of gentamicin per ml. The BHV-1 infectivity titer was determined by limiting-dilution titer determination in MDBK cells. BHV-1 (McIntyre) was kindly provided by Judd Aiken (University of Wisconsin—Madison). Bovine leukemia virus (BLV) was recovered from the BL3 cell line (ATCC CRL 8037) by ultracentrifugation at 140,000 × g for 2.5 h. Autologous adherent macrophages and the canine osteosarcoma cell line D17 (ATCC CCL183) were used as target cells.
Peptide preparation.
BHV-1 virions from culture supernatant were precipitated by ultracentrifugation at 140,000 × g for 2.5 h. The virus pellet was dissolved in 70% formic acid, and 500 mg of cyanogen bromide (Sigma Chemical Co., St. Louis, Mo.) was added to the virus suspension overnight at room temperature (31). The solvent was evaporated under a stream of nitrogen overnight, and the residue was suspended in water. The nitrogen-treated BHV-1 residue was purified twice on a Sephadex G-75 column, and the fractions containing polypeptides were collected and lyophilized. The BHV-1 polypeptides were assessed by sodium dodecyl sulfate (SDS)-gel electrophoresis, and no intact BHV-1 remained in the cyanogen bromide-treated BHV-1 preparations. Ovalbumin (OVA) (grade VI; Sigma), BLV, and HSV-1 were prepared similarly to BHV-1 to obtain polypeptides.
Antibodies and immunofluorescent staining.
Monoclonal antibodies 16-1E10 (anti-CD2), and biotin-labeled C5B6 (anti-CD11c) were produced in our laboratories (18, 19). Additional monoclonal antibodies were SBU-T8 (anti-CD8; University of Melbourne, Parkville, Victoria, Australia), CC15 (specific for the WC1 molecule on bovine γδ T cells; Chris Howard, Institute for Animal Health, Compton, England), IL-A12 (anti-CD4; C. Baldwin) (3), 33 (anti-immunoglobulin M [IgM]; Klaus Nielsen, Animal Research Institute, Nepean, Ontario, Canada), P1.17 (an isotype control, ATCC TIB10), W6/32 (anti-MHC class I; Accurate Chemical & Scientific Corp, Westbury, N.Y.), and H4 (anti-MHC class II; One Lambda, Los Angeles, Calif.). One-color immunofluorescence was performed with fluorescein-conjugated goat anti-mouse IgG (heavy plus light chains) (Jackson ImmunoResearch Laboratories, Inc., Avondale, Pa.). Monoclonal antibody CAM36A (anti-CD14) (Veterinary Medical Research and Development, Pullman, Wash.) was biotin labeled as specified by the instructions accompanying the EZ-Link NHS-LC-biotinylation kit (Pierce, Rockford, Ill.). The percentage of fluorescent cells was determined by flow cytometry (EPICS; Coulter, Hialeah, Fla.).
Activation of virus-specific cytotoxic cells.
PBMC were recovered from the interface following centrifugation (25°C for 30 min at 1,800 × g) of venous blood through Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). After being washed three times in 1× phosphate-buffered saline (PBS), recovered cells were resuspended in RPMI 1640 (Sigma) supplemented with 2 mM l-glutamine, 25 mM HEPES, 5 × 10−5 M 2-mercaptoethanol, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% FBS (Equitech-Bio, Ingram, Tex.). Isolated PBMC were cultured in RPMI 1640 in 25-cm2 tissue culture flasks (2 × 107 cells/flask) with UV-inactivated BHV-1 at a multiplicity of infection (MOI) of 2 and 50 U of recombinant human interleukin-2 (rhIL-2; Boehringer Mannheim). Virus inactivation following UV irradiation was confirmed by a lack of viral replication in MDBK cells. BHV-1-exposed cytotoxic effector cells were generated after 7 to 10 days of culture at 37°C under 5% CO2.
CTL assay.
In CD4+-CTL assays, the PBMC cultured with UV-inactivated BHV-1 were used as effector cells while the adherent macrophages pulsed with BHV-1 polypeptides were used as target cells. Briefly, macrophages isolated from PBMC were cultured in six-well cell culture plates as target cells in RPMI 1640–10% FBS after 1 week at 37°C in a humidified 5% CO2 incubator. Each well of six-well plates contained 5 × 105 to 10 × 105 adherent macrophages, and BHV-1 polypeptides or live BHV-1 (MOI, 10) was added 2 or 12 h before the CTL assays. The target cells were labeled with 250 μCi of 51Cr for 1 h, trypsinized, washed three times with 1× PBS, and incubated with the effector cells in triplicate at 5 × 103 cells in round-bottom microtiter wells at 37°C for 6 h. Effector cells were washed twice and added in triplicate to the plated target cells at various effector-to-target ratios. The total volume was 200 μl/well. The assay mixture was incubated for 4 h in a humidified 5% CO2 incubator at 37°C, and following culture, 100 μl of supernatant/well was counted in a gamma counter. Samples were counted as positive when the mean exceeded the control by 3 standard deviations in 51Cr release. Data were used only if the spontaneous release was less than 25% of the maximum release. The percent specific 51Cr release was calculated from the following formula: percent specific lysis = 100 × (cpm experimental − cpm spontaneous lysis)/(cpm total release − cpm spontaneous lysis). NK-like cytotoxicity was determined with freshly isolated PBMC as effector cells and D17 cells infected with BHV-1 at an MOI of 10 for 2 h as target cells. NK-like assay mixtures were incubated for 18 h at 37°C under 5% CO2. The chemical inhibitors EGTA, MgCl2, or CaCl2 were added at the beginning of selected CTL or NK-like assays.
DNA apoptosis analysis.
Standard CTL assays were performed, except that the target cells were not labeled with Na51CrO4. After 4 h of incubation, medium control and experimental cells were transferred into 1.8-ml tubes. All cells were incubated with biotinylated anti-CD11c and anti-CD14 antibodies for 30 min at 4°C. After being washed with 1× PBS, the cells were treated with phycoerythrin-conjugated streptavidin (1:100) (Jackson ImmunoResearch Laboratories) for 30 min at 4°C. After two washes with 1× PBS, all the cells were treated as specified by the protocol of the In Situ Cell Death kit (Fluorescein) (Boehringer Mannheim, Indianapolis, Ind.). The cells were fixed with 4% paraformaldehyde. After two washes with 1× PBS, the cells were permeabilized (0.1% Triton X-100 in 0.1% sodium citrate) for 2 min on ice. Adherent macrophages were collected and washed twice with 1× PBS. The macrophages serving as a positive control were treated with 10 U of RQ-DNase (Promega, Madison, Wis.) for 10 min at room temperature. Following two washes with 1× PBS, the cells (including positive control macrophages) were incubated with the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) reaction mixture (terminal deoxynucleotidyltransferase, fluorescein-labeled nucleotide mixture). The macrophages serving as a negative control were incubated with the fluorescein-labeled nucleotide mixture without terminal transferase. Target cells were analyzed by flow cytometry and gated by a combination of forward and 90° light scatter.
Depletion of CD4+ and CD8+ T-lymphocyte subsets.
Effector cells (2 × 107 cells) were depleted of CD4+ or CD8+ T lymphocytes after 7 to 8 days in culture by using anti-CD4 (IL-12A) or anti-CD8 (SBU-T8) antibodies, washed twice, and treated with 1:5 rabbit complement (Cedarlane Laboratories, Hornby, Ontario, Canada) on ice for 30 min. The effector cells were washed twice before being reconstituted in RPMI 1640 with 10% FBS, and an aliquot of treated effector cells was analyzed by flow cytometry for cell phenotype.
CD4+-T-lymphocyte enrichment.
In the effector cell population, CD8+ T cells, γδ+ T cells, and B cells were selectively depleted by using anti-CD8 (SBU-T8), anti-WC1 (CC15), and anti-IgM (33) antibodies with an AIS MicroCELLector (Applied Immune Sciences, Inc., Menlo Park, Calif.) as recommended by the manufacturer. By using this selection technique, CD4+ T cells were enriched to greater than 95% as confirmed by flow cytometry.
MHC class II typing.
Lymphocytes were typed for BoLA-D by restriction fragment length polymorphism (12, 36). Briefly, chromosomal DNA from donor PBMC was isolated with the TurboGen-Genomic DNA purification kit (Invitrogen, San Diego, Calif.). Genomic DNA (20 μg) was digested with PvuII and TaqI (Promega), and the digests were electrophoresed in 1% Tris-borate-EDTA (TBE)–agarose gels followed by capillary transfer to Magna NT nylon transfer membranes (MSI, Westboro, Mass.). The membranes were prehybridized and hybridized with formamide hybridization solutions at 42°C (50% formamide, 1% SDS, 10% dextran sulfate, 200 μg of salmon sperm DNA per ml) for 4 h and overnight, respectively. Human HLA-DRα, HLA-DRβ, HLA-DQα, and HLA-DQβ cDNAs (ATCC 57392, ATCC 57080, ATCC 57390, and ATCC 57082, respectively) were used as hybridization probes. The cDNAs were isolated from their plasmids and 32P-labeled by the random-primer method (Life Technologies Inc., Gaithersburg, Md.). Following hybridization, the membranes were washed in 0.4 M NaOH at 45°C for 30 min and subsequently twice in 0.2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS–0.2 M Tris (pH 7.5) at room temperature for 10 min to remove the probes. Then the transfer membranes were washed twice in 2× SSC–0.5% SDS for 10 min and once in 1.0× SSC–0.5% SDS at 60°C for 40 min. The membranes were exposed to autoradiographic film at −70°C for 1 to 5 days.
RESULTS
CTL effector cell phenotype.
We have previously shown that T lymphocytes from BHV-1-immunized donors proliferated in response to recombinant herpesvirus proteins (28). To determine the phenotype of responding cells, PBMC cultured with UV-inactivated BHV-1 for 7 to 10 days were incubated with MAbs and analyzed by flow cytometry. After 7 days of culture, CD4+ T lymphocytes increased as the dominant cell subpopulation, ranging from 70 to 90%, in contrast to the situation for PBMC cultured without BHV-1 (Table 1). The percentage of CD8+ T cells as well as that of γδ+ T cells remained relatively unchanged following culture of the cells with virus for 7 days. Typically, 2.5 × 107 to 3.7 × 107 viable cells/flask were recovered following culture. The increase in the number of CD4+ T lymphocytes suggests that these cells are BHV-1 responsive. To determine the function of these cultured cells, we tested BHV-1-specific T-lymphocyte activity.
TABLE 1.
PBMC cultured with or without UV-inactivated BHV-1a
| Phenotype | % of T-cell type inb:
|
Monoclonal antibody (isotype) | ||
|---|---|---|---|---|
| Fresh PBMC | 10-day-cultured PBMC
|
|||
| With inactivated BHV | Without inactivated BHV | |||
| CD2+ | 42.2 ± 3.6 | 83.8 ± 3.7 | 47.9 ± 2.2 | 16-1E10-B3 (IgG2a) |
| CD4+ | 24.6 ± 6.2c | 76.1 ± 3.3 | 25.6 ± 3.7 | IL-A12 (IgG2a) |
| CD8+ | 14.7 ± 20.7 | 14.8 ± 11.0 | 19.6 ± 10.5 | SBUT8 (IgG2a) |
| IgM+ | 5.0 ± 9.2 | 4.1 ± 0.6 | 1.2 ± 1.7 | 33 (IgG1) |
| WC1+d | 7.5 ± 27.2 | 11.4 ± 8.8 | 20.4 ± 17.4 | cc15 (IgG2a) |
PBMC were obtained from attenuated BHV-1 immunized cattle and cultured with UV-inactivated BHV-1 for 10 days.
The percentage of cells identified does not equal 100 because the CD2 molecule is present on CD4- and CD8-staining cells.
CD4+ T lymphocytes increased as the dominant cell population, ranging from 70 to 90% in five different experiments.
WC1 is a cell surface molecule that is expressed on bovine γδ T cells.
BHV-1-specific CTL and antigen dose-dependent CTL activity.
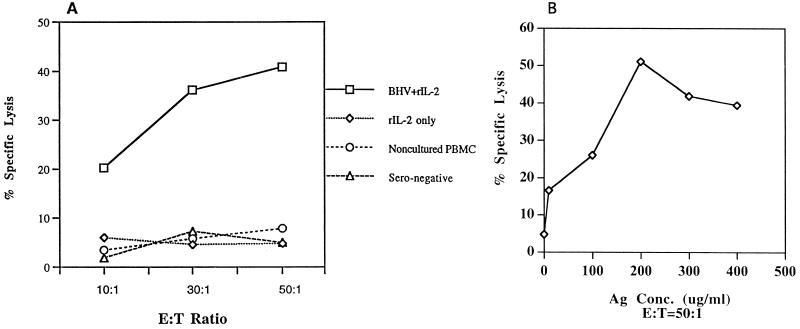
In the murine system, exogenous peptides can be presented through both exogenous and endogenous pathways and can be expressed with MHC class I and II molecules on the cell surface (6, 31). Because macrophages are infected with BHV-1 (21), we used autologous macrophages infected with BHV-1 or pulsed with BHV-1 polypeptides as target cells for BHV-1-specific CTL. The results of a CTL assay representative of 12 assays are shown in Fig. 1A. By this criterion, BHV-1 seronegative cattle were uniformly negative. CTL assays were carried out on cells stimulated with UV-inactivated BHV-1 in vitro for 7 to 10 days in the presence or absence of exogenous rhIL-2. It is unlikely that the observed BHV-1-specific cytolysis is an artifact of prolonged culture or exogenous cytokines, since cytokines alone were unable to induce detectable antigen-specific CTL activity (Fig. 1A). rhIL-2 at 80 U per ml improved the consistency of cytolysis among different CTL assays. However, increased rhIL-2 concentrations greater than 100 U/ml in the effector cell culture lead to nonspecific effector cell lysis of both nonpulsed and antigen-pulsed target cells (data not shown). Since macrophages pulsed with BHV-1 polypeptides served as target cells, antigen concentrations were titrated to determine the minimal antigen quantity for optimal CTL activity (Fig. 1B). At an effector-to-target-cell ratio of 50:1, BHV-1-specific lysis increased as the BHV-1 polypeptide concentration increased to 200 μg/ml.
FIG. 1.
(A) BHV-1-specific CTL were induced in PBMC bulk cultures stimulated with UV-inactivated BHV-1 (MOI, 10). The effector cells were PBMC isolated from BHV-1 immunized cattle and stimulated with UV-inactivated BHV-1 and rhIL-2 at 80 U/ml for 7 to 10 days. Target cells were macrophages recovered from PBMC and pulsed for 2 h with BHV-1 polypeptides and 1 h with 51Cr before the CTL assays. The CTL assay mixtures were incubated for 6 h. Effector cells from a nonimmunized seronegative animal served as a control. This result is representative of 12 separate experiments. (B) BHV-1 polypeptide antigen (Ag) concentration was titrated for CTL activity. At an effector-to-target (E:T) ratio of 50:1, BHV-1-specific lysis increased as the BHV-1 polypeptide concentration increased. Samples were counted as positive when the mean lysis exceeded the medium control by 3 standard deviations. The spontaneous release was less than 25% of the maximum release. This experiment was repeated once with similar results.
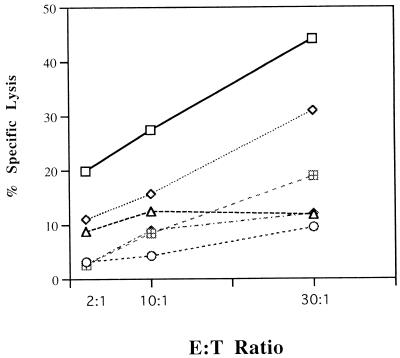
Freshly isolated PBMC or unstimulated, cultured lymphocytes from BHV-immunized or nonimmunized donors did not show appreciable lysis of autologous targets (Fig. 1A). Different target antigens were used to determine the viral specificity of CTL (Fig. 2). Antigen-specific CTL activity confirmed that macrophages pulsed with BHV polypeptides were lysed by BHV-1-stimulated PBMC while autologous macrophages pulsed with polypeptides of OVA, BLV, or the related HSV-1 were not lysed by BHV-stimulated PBMC, similar to the medium control (Fig. 2). The success of finding BHV-specific CD4+ CTL was confirmed repeatedly in BHV-1-immunized cattle of Holstein, Jersey, and Guernsey breeds (data not shown).
FIG. 2.
The antigen specificity of bovine CTL was determined by using different target antigens in CTL assays. BHV-1, OVA, BLV, and HSV-1 were treated with cyanogen bromide (CNBr) to produce polypeptides. Autologous macrophages were lysed by effector cells only when BHV-1 polypeptides were added. Similar results were obtained in three additional experiments. Symbols: □, CNBr plus BHV-1; ◊, live BHV-1; ○, CNBr plus HSV; ▵, CNBr plus OVA; ⊞, CNBr plus BLV;  , no antigen.
, no antigen.
The CTL response is mediated by CD4+ cells.
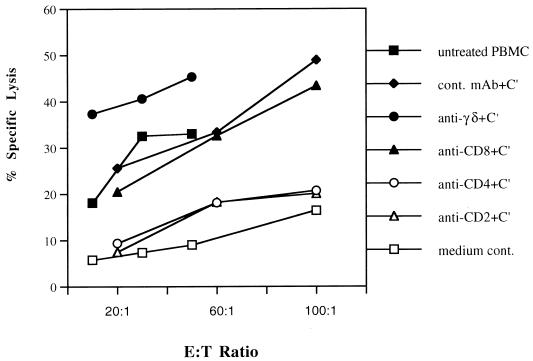
To identify the phenotype of the effector cell population, subpopulations of cultured PBMC were negatively selected by specific antibodies plus complement. CD4+, CD8+, and γδ+ T lymphocytes were selectively removed from cultured PBMC before CTL assays by using anti-CD4, anti-CD8, anti-γδ, and isotype control monoclonal antibodies plus rabbit complement. Flow cytometry confirmed that greater than 95% CD4+, CD8+, and γδ+ T lymphocytes were depleted by using the respective antibodies (data not shown). Effector cells treated with the IgG2a isotype control monoclonal antibody (P1.17) plus rabbit complement showed a similar cell phenotype to nontreated effector cells. Following antibody and complement treatment, effector cells were used in CTL assays (Fig. 3). CD4+-T-cell depletion led to a marked loss of CTL activity against autologous macrophages pulsed with BHV-1 polypeptide. In contrast, neither CD8+ T-cell nor γδ+ T-cell depletion correlated with the loss of BHV-1-specific cytolysis. Thus, CD4+-T-cell depletion with the resulting absence of BHV-1-specific cytolysis strongly suggested that BHV-1-specific CTLs are CD4+ T lymphocytes which were generated from PBMC stimulated in vitro with UV-inactivated BHV-1 (Fig. 3).
FIG. 3.
The BHV-1-specific CTL response is mediated by CD4+ T cells. Individual cell populations of BHV-1-infected cultured PBMC were negatively selected by specific antibodies plus complement. The CD2+- or CD4+-T cell deletion led to the absence of BHV-1-specific cytolysis. cont., control; C′, complement.
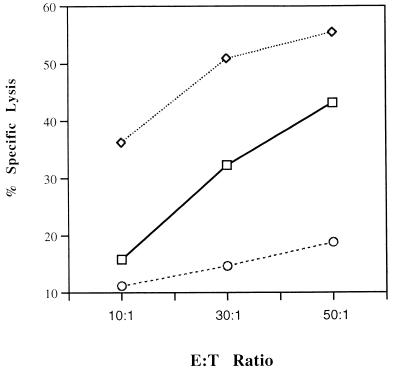
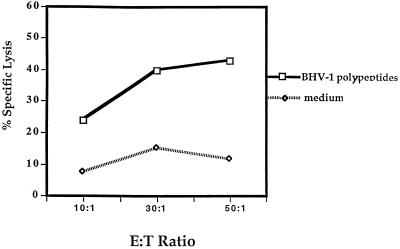
To further confirm the importance of BHV-1-specific CD4+-T-cell cytolysis, individual effector cell subpopulations were enriched. Isolated CD4+ T cells were approximately 95% of the cell population after negative selection by a panning technique (data not shown). CTL assays performed with these enriched CD4+ T cells lysed macrophages pulsed with BHV-1 polypeptides (Fig. 4). Similar attempts were made to enrich CD8+ T lymphocytes and γδ+ T lymphocytes; however, insufficient CD8+ and γδ+ T cells were obtained for CTL assays. Positive CD4+-T-cell selection was not performed since unrestricted cytolytic effector cells are produced by cross-linking CD4 molecules during positive selection (7).
FIG. 4.
Enriched CD4+ T lymphocytes mediated the lysis of BHV-1 polypeptide-pulsed target cells. The enriched CD4+ CTL activity paralleled the nonenriched effector cell population. Symbols: □, PBMC effector cells; ◊, enriched CD4+ T cells; ○, medium control.
CD4+ CTLs are MHC restricted.
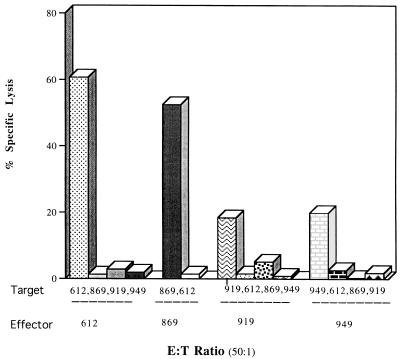
To determine the role of MHC in peptide recognition by CD4+ CTL, BoLA class II alleles were characterized by restriction fragment length polymorphism (RFLP) (2, 36). Each of the animals possessed unrelated class II HLA-DQβ alleles (data not shown) based on RFLPs reported for the nine classes of class II genes found in cattle (1, 12). Additional RFLP studies further elucidated that the animals possessed unrelated BoLA class II HLA-DRα and HLA-DRβ (data not shown). Thus, the cattle were MHC class II unrelated in DR and DQ alleles. After BoLA typing, autologous and allogeneic CTL assays were performed to evaluate CD4+ CTL MHC restriction. As expected, BHV-stimulated lymphocytes exhibited virus-specific lysis against only autologous targets and no lysis against mismatched allogeneic macrophages presenting viral peptides (Fig. 5). These data indicate that the BHV-1-specific immune response can be mediated by CD4+, MHC class II-restricted T cells in attenuated BHV-1-vaccinated cattle. Inhibition of BHV-1-specific cytolysis was attempted by using anti-MHC class I (W6/32), anti-MHC class II (H4), anti-CD4 (IL-A12), or anti-CD8 (SBU-T8) antibodies added to effector cells 30 min before the CTL assays. Minimal inhibition of CTL activity was observed in any monoclonal antibody combination in assays repeated two or three times (data not shown).
FIG. 5.
BHV-1-specific CD4+ CTL are MHC restricted. BHV-1 stimulated CTL from immunized cattle exhibited no virus-specific lysis activity against mismatched allogeneic macrophages pulsed with BHV polypeptides. Antigen-specific CD4+ CTL lysed only autologous macrophages pulsed with BHV-1 polypeptides. This result is representative of six separate experiments.
CD4+-CTL-mediated cellular cytotoxicity is not blocked by Mg2+ and EGTA.
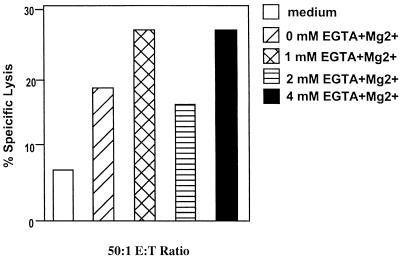
To evaluate the perforin or Fas pathway involvement in CD4+ CTL lysis of macrophages pulsed with BHV-1 polypeptides, EGTA and MgCl2 were added to the CTL assay mixture as a Ca2+ chelator to remove free Ca2+ in the medium at the beginning of the assays. In the presence of 4 mM EGTA, antigen-specific CD4+-CTL activity was not inhibited, suggesting that CD4+ CTL activity is not sensitive to the absence of free Ca2+ in the medium of the assays (Fig. 6). Since the perforin-mediated lytic pathway is sensitive to the absence of calcium while the Fas-mediated lytic pathway is not (27), these results suggested that CD4+ CTL may involve the Fas-mediated lytic pathway.
FIG. 6.
CD4+ CTL are not Ca2+ dependent and may use the Fas-mediated lytic pathway. Addition of selected concentrations of Mg2+ and EGTA (Ca2+ chelator) to the CD4+ CTL assay mixtures did not inhibit BHV-specific cytolytic activity, suggesting that antigen-specific CD4+ CTL are not calcium dependent. Medium was added to macrophage target cells as a negative control for miscellaneous lytic activity in CTL assays. Similar results were obtained in five separate experiments.
NK-like cytotoxicity is blocked by Mg2+ and EGTA.
NK-cell cytotoxicity assays were performed as a control for CD4+-CTL activity since NK cells in general lyse their target cells through a perforin-mediated lytic pathway (9, 27). D17 cells infected with BHV-1 served as target cells and noncultured PBMC served as effector cells in an 18-h cytotoxicity assay. NK-cell cytolytic activities were inhibited by 1.5 mM EGTA plus MgCl2 and could be partially recovered by calcium addition (data not shown). This finding suggests that NK-cell cytotoxicity requires free calcium for lytic function and parallels the requirements of the lytic pathway.
Apoptosis of target cells is mediated by CD4+ CTL.
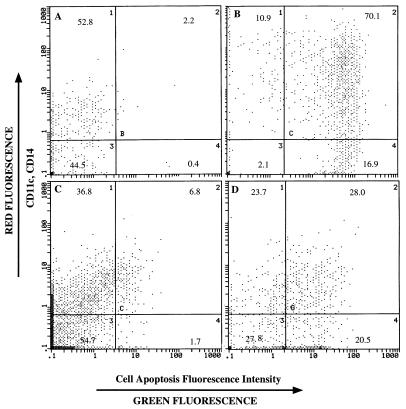
The macrophage target cells were gated for size and complexity by flow cytometry and analyzed for fluorescence by the TUNEL method. Approximately 30% of target cell macrophages pulsed with BHV-1 polypeptides exhibited programmed cell death after the 4-h CTL assay compared to 7% of macrophages in the medium control (Fig. 7).
FIG. 7.
Apoptosis of macrophages pulsed with viral polypeptides induced by CD4+ CTL. All macrophages were treated with anti-macrophage antibodies (anti-CD11c and anti-CD14) and were gated by forward and low-angle light scatter as large and complex cells. The anti-CD11c and anti-CD14 antibodies were labeled with phycoerythrin (red fluorescence) to identify macrophages, while the fragmented DNA of apoptotic cells were labeled with fluorescein isothiocyanate-dUTP by terminal deoxynucleotidyltransferase (green fluorescence). Intact macrophages were either not labeled with fluorescein isothiocyanate-dUTP (negative control) (A) or not labeled with fluorescein isothiocyanate-dUTP (positive control) (B). Compared to the target cells without viral polypeptide treatment (medium control) (C), about 30% of target cells pulsed with BHV-1 polypeptides exhibited apoptosis in CTL assays (D).
Cells were treated with biotin-labeled anti-CD11c and anti-CD14 antibodies, specific for leukocyte function antigen-1 and the lipopolysaccharide receptors, respectively, present on macrophages and were then treated with phycoerythrin-conjugated streptavidin. Then the large, complex cells were gated as candidates for apoptosis evaluation. In viral polypeptide-specific CTL assays, 30% of target cells were positive by TUNEL analysis, compared to about 7% of target cells not treated with viral peptides, suggesting that CD4+ CTL lysed autologous macrophages through an apoptotic pathway. Parallel 51Cr cytotoxicity assays indicated that CD4+ CTL lysed 40% of autologous macrophages pulsed with BHV-1 polypeptides (Fig. 8).
FIG. 8.
A standard 51 Cr CTL assay was performed in parallel with the TUNEL CTL assay in Fig. 7, demonstrating CD4+ CTL lysis of autologous macrophages pulsed with BHV-1 polypeptides. The “medium” curve indicates macrophages cultured without viral polypeptides. Antigen-specific lysis of the 51Cr-releasing CTL assay (indicated by the “BHV-1 polypeptides” curve) was within the same range as the apoptosis observed in Fig. 3D.
DISCUSSION
Our data indicated that BHV-specific CD4+ CTL precursors are present in peripheral blood of BHV-seropositive cattle, the natural host for this infection. BHV-1-specific cytolytic activity of CD4+ T cells can be induced after PBMC are cocultured with UV-inactivated virus. This observation supports the hypothesis that CD4+ CTL are preferentially activated by cell-free UV-inactivated BHV while CD8+ CTL are stimulated primarily by fixed BHV-infected fibroblasts (15). Such a differential activation has also been reported for cell-free HSV activating CD4+ T cells, whereas HSV-infected fibroblasts primarily stimulated CD8+ T cells (44).
Our findings indicate the effector cells produced in response to cell-free BHV-1 could lyse autologous macrophages pulsed with BHV-1 polypeptides. The cytolytic activities are BHV-1-antigen specific, since macrophages pulsed with OVA, BLV, and related HSV polypeptides or macrophages without antigen could not be lysed by similar effector cells. Similarly, live BHV-1-infected macrophages can serve as target cells lysed by CD4+ CTL. However, results obtained with pulsed BHV polypeptides were more consistent than those obtained with live virus for several possible reasons. First, the inconsistency observed with live virus could result from variation in macrophage-virus infection (21) or virus induced macrophage death. Second, live virus may induce apoptosis of CD4+ T cells, as observed in bovine CD4 T cells with BHV-1 infection (20) and in human CD4 T cells from cord blood with human immunodeficiency virus type 1 infection (26). Third, peptide-bound class II MHC molecule complexes are more heterogeneous than those produced by intracellular processing, indicating that peptide-pulsed macrophages may be recognized by a more diverse group of CTL than are virus-infected macrophages (42).
However, in BHV-1 infection, the role of CTL, important cells in many viral infections, has received minimal attention (37). BHV-1 infection in murine and bovine fibroblasts down-regulates MHC class I expression (25, 32), as well as class I transcription. Interfering with MHC class I expression, BHV could escape a CD8+ CTL immune response. Similar herpesviruses, e.g., HSV, Epstein-Barr virus, and cytomegalovirus, have confirmed mechanisms to evade the immune system by blocking viral antigen loading by transporters associated with antigen processing to MHC class I molecules (22, 29). Studies with BHV-1 have identified that modified live vaccines, as well as wild-type virus, can decrease MHC class I but not class II expression (32). The virus mediates this effect by interfering with the synthesis of class I heavy chain and the assembly/transport of class I molecules by 8 to 12 h. Although blocking viral antigen transfer to the endoplasmic reticulum has not been confirmed in BHV-1 infection, this unique herpesvirus evasive mechanism supports the importance of cytolytic CD4+ lymphocytes in herpesvirus infection. Therefore, activation of CD4+ CTL during BHV infection could compensate for the loss of BHV-1-specific CD8+ CTL activity caused by viral down-regulation of MHC class I antigen presentation.
Macrophages expressing MHC class II molecules are infected by BHV-1, and expression of MHC class II can be enhanced by a variety of stimuli, particularly gamma interferon (IFN-γ) and tumor necrosis factor alpha. Also, after IFN-γ treatment, class II molecules can be induced in other cell types, e.g., vascular endothelial cells, dermal fibroblasts, placental cells, and melanocytes (23). Activated T lymphocytes can express MHC class II molecules, and BHV-1 infection can induce local leukocyte infiltration and IFN-γ production by activated T lymphocytes (39). Therefore, cells other than macrophages and B cells might express MHC class II molecules and viral peptides during BHV-1 infection. In bovine Theileria parva infection, culture of infected αβ+ T cells, γδ+ T cells, and B-cell lines revealed high expression of MHC class II molecules. However, in vivo, MHC class II-negative parasitized lymphocytes progressively outnumbered MHC class II-positive parasitized cells, suggesting that host-mediated destruction or antigenic modulation of parasitized MHC class II-expressing cells was occurring (13). Similarly, BHV-1-specific CD4+ CTL may play an important role to control BHV-1 infection in vivo by killing virus infected cells transiently expressing MHC class II molecules.
The often massive infiltration of leukocytes in herpesvirus infection could result in extensive cytokine production. In herpetic stromal keratitis, human epidermal keratinocytes are induced to express HLA class II (DR) molecules by IFN-γ and HSV-infected are killed by both CD4+ T lymphocytes and NK cells (10, 16). Thus, depletion of certain antigen-presenting cells by CD4+ T cells could regulate the immune response in herpesvirus infection, potentially polarizing or influencing cytokine production without severe immunopathology (4).
In contrast, depletion of MHC class II-bearing macrophages by CD4+ CTL could also limit the local immune response to BHV-1 infection, suppressing immune surveillance and allowing the virus to escape. Macrophages play an important role in controlling virus spread during the initial stage of virus infection. The monocytes and macrophages obtained from BHV-1-infected animals produce IFN-α, function as cytotoxic cells, and express increased MHC class II molecules, serving as antigen-presenting cells for further activation of virus-specific immune response (39). Presumably, these activities are directed to limit virus spread; therefore, depletion of macrophages from infected animals could subvert immune functions that control viral infection. In this context, CD4+-CTL activity may be a major immune system mechanism for removal of virus-infected cells and control of virus spread, or it could be an immunosuppressive mechanism where CD4+ CTL help the virus evade immune responses. Further studies and direct evidence are needed to clarify the possibility of antigen-specific CD4+ CTL facilitating virus spread.
Interestingly, our parallel studies of apoptosis by using TUNEL analysis and standard 51Cr release CTL assays revealed comparable levels of cell death, an observation similar to others (8) supporting a mechanism of programmed cell death initiated by CD4+ CTL. CD4+ CTL are believed to predominantly use the Fas-mediated lytic pathway (17), although occasionally they use the perforin-mediated lytic pathway (27, 38, 45). Macrophages can express Fas (CD95) molecules on the cell surface, and there is evidence that Th1-type CD4+ CTL lyse target cells through Fas-mediated apoptosis (11, 33, 34). Perforin undergoes a calcium-induced conformational change, forming pores on the target cell membrane that are inhibited in the absence of free Ca2+ by the Ca2+ chelator Mg2+ and EGTA, while Fas-dependent cytotoxicity is only minimally inhibited in the presence of EGTA (27). In our CTL assays, cytotoxicity is not inhibited by EGTA, suggesting that CD4+ CTL lyse BHV-1-polypeptide-pulsed macrophages through the Fas-mediated lytic pathway. In contrast, NK-cell cytolytic activity, used for comparison, involves the perforin-mediated lytic pathway (41). The NK-cell cytotoxicity was inhibited in the absence of Ca2+, and addition of Ca2+ to the cytotoxicity assay mixtures restored the NK-cell cytotoxicity. The present studies suggest that NK cells may lyse BHV-1-infected target cells through the perforin-dependent pathway while CD4+ CTL mediate lysis through the Fas-dependent pathway. In contrast to the present observations, others have observed that HSV-1 could induce the apoptosis of CD4 T cells but apoptosis was not inhibited by anti-Fas or anti-FasL antibodies (26). These workers concluded that Fas-FasL was not the mechanism of apoptosis. However, virus-altered cells may use the same intracellular apoptotic pathway but not require surface Fas-FasL triggering for apoptosis induction. Additional studies to explore the role of the Fas-dependent pathway could utilize caspase inhibitors to block CTL activity, measuring caspase activity in target cells following effector cell treatment, or inhibiting Fas-FasL interaction by monoclonal antibody treatment. Presently, anti-bovine Fas or FasL antibodies are not available.
Our experiments revealed the presence of CD4+ CTL precursors in animals immunized by attenuated BHV-1 vaccine. The successful induction of CD4+ CTL from bovine peripheral blood showed the long-anticipated scenario of CD4+ CTL involvement in cell-mediated immunity against BHV-1 infection. CD4+ CTL may induce the apoptosis of macrophages through Fas-mediated lysis, suggesting BHV-1-specific CD4+ CTL probably compensate for the loss of CD8+ CTL since BHV-1 can down-regulate MHC class I expression in infected cells (25, 32). However, since CD4+ CTL also lysed virus-infected antigen-presenting cells, we predict that CD4+ CTL might actually limit a local immune response; therefore, in vivo, CD4+ CTL may control viral spread and limit the local immune response to viral infection.
ACKNOWLEDGMENTS
This work is supported by grants USDA 93-37204-9205 and 96-35204-3670.
We thank Yi Gao, Linda Eskra, and Jerome Harms for providing critical reagents and for many helpful discussions.
REFERENCES
- 1.Andersson L, Davies C J. The major histocompatibility complex. In: Goddeeris B M L, Morrison W I, editors. Cell-mediated immunity in ruminants. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 37–57. [Google Scholar]
- 2.Andersson L B J, Rask L, Anderson P A. Genomic hybridization of bovine class II major histocompatibility genes. 1. Extensive polymorphism of DQα and DQβ genes. Anim Genet. 1986;17:95–112. doi: 10.1111/j.1365-2052.1986.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin C L, Teale A J, Naessens J G, Goddeeris B M, MacHugh N D, Morrison W I. Characterization of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J Immunol. 1986;136:4385–4391. [PubMed] [Google Scholar]
- 4.Braakman E, Rotteveel F T M, Bleek G V, Seventer G A V, Lucas C J. Are MHC class II-restricted cytotoxic T lymphocytes important? Immunol Today. 1987;8:265–267. doi: 10.1016/0167-5699(87)90185-X. [DOI] [PubMed] [Google Scholar]
- 5.Campos M, Griebel P, Bielefeldt Ohmann H, Babiuk L A. Cell-mediated cytotoxic responses in lungs following a primary bovine herpes virus type 1 infection. Immunology. 1992;75:47–52. [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone F R, Moore M W, Sheil J M, Bevan M J. Induction of cytotoxic T lymphocytes by primary in vitro stimulation with peptide. J Exp Med. 1988;167:1767–1779. doi: 10.1084/jem.167.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi S H, Splitter G A. Induction of MHC-unrestricted cytolytic CD4+ T cells against virus infected target cells by cross-linking CD4 molecules. J Immunol. 1994;153:3874–3881. [PubMed] [Google Scholar]
- 8.Cohen J J. Apoptosis. Immunol Today. 1993;14:126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- 9.Cook C G, Letchworth G J, Splitter G A. Bovine naturally cytolytic cell activation against bovine herpes virus type 1-infected cells does not require late viral glycoproteins. Immunology. 1989;66:565–569. [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham A L, Noble J R. Role of keratinocytes in human recurrent herpetic lesions. Ability to present herpes simplex virus antigen and act as target for T lymphocyte cytotoxicity in vitro. J Clin Invest. 1989;83:490–496. doi: 10.1172/JCI113908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalit A, Song X, Lacy E, Nikolic-Zugic J, Friedman S M, Elkon K B. Th1 CD4+ lymphocytes delete activated macrophages through the Fas/APO-1 antigen pathway. Proc Natl Acad Sci USA. 1995;92:11223–11229. doi: 10.1073/pnas.92.24.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies C J, Andersson L, Joosten I, Mariani P, Gasbarre L C, Hensen E J. Characterization of bovine MHC class II polymorphism using three typing methods: serology, RFLP, and IEF. Eur J Immunogenet. 1992;19:253–262. doi: 10.1111/j.1744-313x.1992.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 13.DeMartini J C, MacHugh N D, Naessens J, Teale A J. Differential in vitro and in vivo expression of MHC class II antigens in bovine lymphocytes infected by Theileria parva. Vet Immunol Immunopathol. 1993;35:253–273. doi: 10.1016/0165-2427(93)90038-6. [DOI] [PubMed] [Google Scholar]
- 14.Denis M, Slaoui M, Keil G, Babiuk L A, Ernst E, Pastoret P-P, Thiry E. Identification of different target glycoproteins for bovine herpes virus type 1-specific cytotoxic T lymphocytes depending on the method of in vitro stimulation. Immunology. 1993;78:7–13. [PMC free article] [PubMed] [Google Scholar]
- 15.Denis M, Splitter G, Thiry E, Pastoret P-P, Babiuk L A. Infectious bovine rhinotracheitis (bovine herpesvirus 1): helper T cells, cytotoxic T cells, and NK cells. In: Goddeeris B M L, Morrison W I, editors. Cell-mediated immunity in ruminants. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 157–172. [Google Scholar]
- 16.Doymaz M Z, Foster C M, Destephano D, Rouse B T. MHC II-restricted, CD4+ cytotoxic T lymphocytes specific for herpes simplex virus-1: implications for the development of herpetic stromal keratitis in mice. Clin Immunol Immunopathol. 1991;61:398–409. doi: 10.1016/s0090-1229(05)80011-x. [DOI] [PubMed] [Google Scholar]
- 17.El-Khatib M, Stanger B Z, Dogan H, Cui H, Ju S-T. The molecular mechanism of FasL-mediated cytotoxicity by CD4+ Th1 clones. Cell Immunol. 1995;163:237–244. doi: 10.1006/cimm.1995.1122. [DOI] [PubMed] [Google Scholar]
- 18.Eskra L, O’Reilly K L, Splitter G A. Effect of monoclonal antibodies on in vitro function of T-cell subsets. Vet Immunol Immunopathol. 1991;27:215–222. doi: 10.1016/0165-2427(91)90103-j. [DOI] [PubMed] [Google Scholar]
- 19.Eskra L, O’Reilly K L, Splitter G A. The bovine p150/95 molecule (CD11c/CD18) functions in primary cell-cell interaction. Vet Immunol Immunopathol. 1991;29:213–227. doi: 10.1016/0165-2427(91)90015-5. [DOI] [PubMed] [Google Scholar]
- 20.Eskra L, Splitter G A. Bovine herpesvirus-1 infects activated CD4+ lymphocytes. J Gen Virol. 1997;78:2159–2166. doi: 10.1099/0022-1317-78-9-2159. [DOI] [PubMed] [Google Scholar]
- 21.Forman A J, Babiuk L A. Effect of infectious bovine rhinotracheitis virus infection on bovine alveolar macrophage function. Infect Immun. 1982;35:1041–1047. doi: 10.1128/iai.35.3.1041-1047.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert P M, Tampe R, Peterson P A, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 23.Glimcher L H, Kara C J. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- 24.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 25.Hariharan M J, Nataraj C, Srikumaran S. Down regulation of murine MHC class I expression by bovine herpesvirus 1. Viral Immunol. 1993;6:273–284. doi: 10.1089/vim.1993.6.273. [DOI] [PubMed] [Google Scholar]
- 26.Ito M, Koide W, Watanabe M, Kamiya H, Sakurai M. Apoptosis of cord blood T lymphocytes by herpes simplex virus type 1. J Gen Virol. 1997;78:1971–1975. doi: 10.1099/0022-1317-78-8-1971. [DOI] [PubMed] [Google Scholar]
- 27.Kagi D, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 28.Leary T P, Splitter G A. Recombinant herpesviral proteins produced by cell-free translation provide a novel approach for the mapping of T lymphocyte epitopes. J Immunol. 1990;145:718–723. [PubMed] [Google Scholar]
- 29.Levitskaya J, Coram M, Levitsky V, Imreh S, Steigerwald-Mullen P M, Klein G, Kurilla M G, Masucci M G. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 30.Manickan E, Rouse R J D, Yu Z, Wire W S, Rouse B T. Genetic immunization against herpes simplex virus-protection is mediated by CD4+ T lymphocytes. J Immunol. 1995;155:259–265. [PubMed] [Google Scholar]
- 31.Moore M W, Carbone F R, Bevan M J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 32.Nataraj C, Eidmann S, Hariharan M J, Sur J H, Perry G A, Srikumaran S. Bovine herpesvirus 1 downregulates the expression of bovine MHC class I molecules. Viral Immunol. 1997;10:21–34. doi: 10.1089/vim.1997.10.21. [DOI] [PubMed] [Google Scholar]
- 33.Rahelu M, Williams G T, Kumraratne D S, Eaton G C, Gaston J S H. Human CD4+ cytolytic T cells kill antigen-pulsed target T cells by induction of apoptosis. J Immunol. 1993;150:4856–4866. [PubMed] [Google Scholar]
- 34.Ramsdell F, Seaman M S, Miller R E, Picha K S, Kennedy M K, Lynch D H. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Int Immunol. 1994;6:1545–1553. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 35.Schmid D S. The human MHC-restricted cellular response to herpes simplex virus type 1 is mediated by CD4+, CD8− T cells and is restricted to the DR region of the MHC complex. J Immunol. 1988;1:3610–3616. [PubMed] [Google Scholar]
- 36.Sigurdardottir S, Lunden A, Andersson L. Restriction fragment length polymorphism of DQ and DR class II genes of the bovine major histocompatibility complex. Anim Genet. 1988;19:133–150. doi: 10.1111/j.1365-2052.1988.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 37.Splitter G A, Eskra L, Abruzzini A F. Cloned bovine cytolytic T cells recognize bovine herpes virus-1 in a genetically restricted, antigen-specific manner. Immunology. 1988;63:145–150. [PMC free article] [PubMed] [Google Scholar]
- 38.Stalder T, Hahn S, Erb P. Fas antigen is the major target molecule for CD4+ T cell-mediated cytotoxicity. J Immunol. 1994;152:1127–1133. [PubMed] [Google Scholar]
- 39.Tikoo S K, Campos M, Babiuk L A. Bovine herpesvirus 1 (BHV-1): biology, pathogenesis, and control. Adv Virus Res. 1995;45:191–223. doi: 10.1016/s0065-3527(08)60061-5. [DOI] [PubMed] [Google Scholar]
- 40.Torpey D J, Lindsley M D, Rinaldo C R., Jr HLA-restricted lysis of herpes simple virus-infected monocytes and macrophages mediated by CD4+ and CD8+ T lymphocytes. J Immunol. 1989;142:1325–1332. [PubMed] [Google Scholar]
- 41.Van den Broek M F, Kagi D, Zinkernagel R M, Hengartner H. Perforin dependence of natural killer cell-mediated tumor control in vivo. Eur J Immunol. 1995;25:3514–3516. doi: 10.1002/eji.1830251246. [DOI] [PubMed] [Google Scholar]
- 42.Viner N J, Nelson C A, Deck B, Unanue E R. Complexes generated by the binding of free peptides to class II MHC molecules are antigenically diverse compared with those generated by intracellular processing. J Immunol. 1996;156:2365–2368. [PubMed] [Google Scholar]
- 43.Williams N S, Engelhard V H, Beirne B. Identification of a population of CD4+ CTL that utilizes a perforin-rather than a Fas ligand-dependent cytotoxic mechanism. J Immunol. 1996;156:153–159. [PubMed] [Google Scholar]
- 44.Yasukawa M, Instsuki A, Kobayashi Y. Differential in vitro activation of CD4+CD8− and CD8+CD4− herpes simplex virus-specific human cytotoxic T cells. J Immunol. 1989;143:2051–2057. [PubMed] [Google Scholar]
- 45.Yasukawa M, Yakushijin Y, Fujita S. Two distinct mechanisms of cytotoxicity mediated by herpes simplex virus-specific CD4+ human cytotoxic T cell clones. Clin Immunol Immunopathol. 1996;78:70–76. doi: 10.1006/clin.1996.0010. [DOI] [PubMed] [Google Scholar]
- 46.Yasukawa M, Zarling J M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984a;133:422–427. [PubMed] [Google Scholar]
- 47.Yasukawa M, Zarling J M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. II. Bifunctional clones with cytotoxic and virus-induced proliferative activities exhibit herpes simplex virus type 1 and 2 specific or type common reactivities. J Immunol. 1984b;133:2736–2742. [PubMed] [Google Scholar]
- 48.Yoo J, Stone R T, Beattie C W. Cloning and characterization of the bovine Fas. DNA Cell Biol. 1996;15:227–234. doi: 10.1089/dna.1996.15.227. [DOI] [PubMed] [Google Scholar]