Abstract
Immunotherapy has gained great interest in thoracic malignancies in the last decade, first in non-small cell lung cancer (NSCLC), but also more recently in small-cell lung cancer (SCLC) and malignant pleural mesothelioma (MPM). However, while 15–20% of patients will greatly benefit from immune checkpoint blockers (ICBs), a vast majority will rapidly exhibit resistance. Reasons for this are multiple: non-immunogenic tumors, immunosuppressive tumor microenvironment or defects in immune cells trafficking to the tumor sites being some of the most frequent. Current progress in adoptive cell therapies could offer a way to overcome these hurdles and bring effective immune cells to the tumor site. In this review, we discuss advantages, limits and future perspectives of adoptive cell therapy (ACT) in thoracic malignancies from lymphokine-activated killer cells (LAK), cytokine-induced killer cells (CIK), natural killer cells (NK), dendritic cells (DC) vaccines and tumor-infiltrating lymphocytes (TILs) to TCR engineering and CARs. Trials are still in their early phases, and while there may still be many limitations to overcome, a combination of these different approaches with ICBs, chemotherapy and/or radiotherapy could vastly improve the way we treat thoracic cancers.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03142-3.
Keywords: Adoptive cell therapies, Thoracic malignancies, CART cells, Lymphokine activated killer cells, Dendritic cell vaccines, Tumor infiltrating lymphocytes
Introduction
Thoracic malignancies are a very frequent cancer type. They are the first cause of deaths by cancer worldwide [1]. One of the greatest advances in lung cancer and malignant pleural mesothelioma (MPM) treatment these past decades has been the growing use of immunotherapy. Immune checkpoint blockers (ICBs) have revolutionized the prognosis of non-small cell lung cancer (NSCLC) [2], while also exhibiting good results in small-cell lung cancer (SCLC) [3] and MPM [4, 5]. However only 15–20% of them achieve response on ICBs, others either displaying primary resistance or secondary resistance. Resistance can be explained by different immune phenotypes in tumors. Three immune phenotypes have been defined with different mechanisms of resistance: “immune desert” with no immune reaction, “immune-inflamed” with an abundant infiltration of immune cells, and “altered” with the tumor and its microenvironment blocking an efficient T cell infiltration [6]. “Hot tumors” correspond to an “immune-inflamed” phenotype and “cold tumors” to an “immune desert” phenotype. Response to ICBs is often positively associated with a “hot” phenotype and is very rare in “cold” phenotypes [7]. Interestingly, tumors can be heterogeneous with hot and cold immune regions, driving resistance to ICBs through selection of “cold” subclones [8].
These different phenotypes will translate into different outcomes to ICBs and other immunotherapies. Resistance in “immune desert” tumors is driven by non-immunogenic tumor cells with a low TMB, a high neoantigen intratumor heterogeneity (defined by a high fraction of subclonal neoantigens) resulting in a higher probability of selecting subclones with a poor immunogenicity [9], a lower clonal T cell arsenal [10], and/or a lack of recruitment of antigen-presenting cells like dendritic cells. In “immune-inflamed” tumors, resistance can be driven by an overexpression of immunosuppressive cells and cytokines. Finally, in “altered” tumors resistance can be explained by either an immunosuppressive environment or the impossibility for T cells to infiltrate the tumor, for example, through an epigenetic silencing of genes involved in T cell trafficking to the TME [11], a modification in the secretion of chemokines [12], stromal fibroblasts obstructing T cell infiltration [13]. Some patients also develop secondary resistance to ICBs through different mechanisms [14]: loss of neoantigen, acquired defects in antigen presentation (HLA molecules, β2-microglobulin, etc.), downregulation of IFN-γ pathway, upregulation of alternate immune checkpoint inhibiting receptors leading to T cell exhaustion.
ICBs are the more widely used immunotherapies [15], but many other anticancer immunotherapies are also being explored: adoptive cell transfers, tumor-targeting monoclonal antibodies, immunogenic cell death inducers, co-stimulatory monoclonal antibodies, anticancer vaccines, oncolytic virotherapies, pattern recognition receptors (PRR) agonists, inhibitors of immunosuppressive metabolism (IDO or adenosine inhibitors), immunostimulatory cytokines [16, 17].
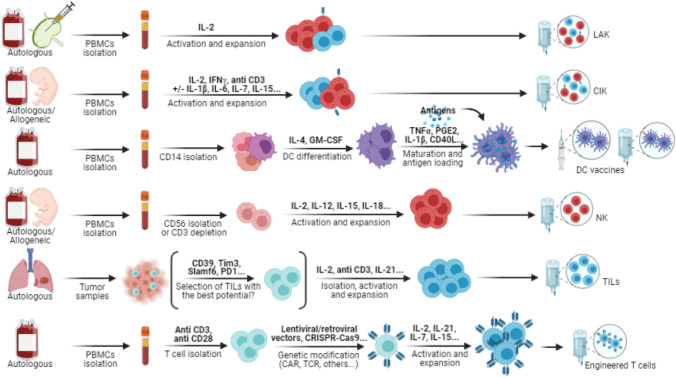
Adoptive cell therapies (ACT) are of particular interest, which is underlined by the growing number of registered clinical trials on the topic [18]. They have already displayed promising results in hematological malignancies and some solid tumor types like melanoma. Immune cells are isolated from patients or donors for ex vivo expansion, stimulation, and sometimes specific engineering before infusion or reinfusion into the patient to increase immune infiltration and cytotoxicity against tumor cells (see Fig. 1). In cases of “immune desert” tumors, adoptive T cell therapy might yield better results than targeting immune checkpoints, especially if T cells have been engineered to react against tumor antigens and/or traffic in the tumor, since the problem is not only exhausted T cells but also the lack of specific T cells and/or trafficking of these T cells. Dendritic cell (DC) vaccines could also recruit new antigen-specific T cells and help turn a “cold” tumor nonresponding to ICBs into a “hot” tumor more sensitive to treatment [7].
Fig. 1.
Adoptive therapies preparation. Preparation methods for ACT are quite similar. PBMCs are obtained from autologous or allogeneic peripheral blood (mainly by leukapheresis), cord blood or non-metastatic lymph nodes. In the particular case of TILs, they are obtained from tumor samples (mainly resected specimens but also biopsy samples). For non-mixed therapies, cells are often isolated depending on the intended therapy based on their CD markers: CD14 + cells for DC vaccines, CD56 + or CD3- cells for NK cells, CD3 + and CD28 + cells for T cells, etc. In every case, cytokines are then used for activation and expansion with variations depending on the type of expanded cells. Some therapies require additional steps. For DC vaccines, antigens have to be added to pulse the DCs (peptides, tumor lysate, mRNA, etc.) after isolation, differentiation and maturation. T cells can be engineered with gene therapies: CARs or TCRs are added through lentiviral or retroviral transduction, CRISPR-Cas9 gene editing, electroporation, etc. Other genes can also be inserted, like chemokine receptors or cytokines. Preparations are then injected into the patients. Different routes are available: intravenous, intranodal, subcutaneous, or intradermal. In addition to T-cell-based therapies, lymphodepleting chemotherapy and IL-2 infusions are often used to increase in vivo persistence. Figure created with BioRender.com
Theoretically, ACT could reach patients than ICBs cannot. Tumor vaccines with DC increase recruitment, activation, and expansion of effector cells, while infusions of different cells of the innate or adaptive immune response, such as NK, NKT or T cells, increase the pool of cells with an antitumoral activity. Unfortunately, ACT still faces many limitations in solid tumors such as thoracic malignancies, and convincing clinical trials are still lacking. In this review, we expose what has already been explored in thoracic malignancy and what is currently under investigation, thus providing an overview of what can be achieved with ACT in NSCLC, SCLC and MPM.
Non-engineered adoptive therapies
Lymphokine-activated killer cells (LAK)
LAK cells have been one of the first adoptive therapies to be tested in patients. They are autologous lymphocytes isolated from non-metastatic regional lymph nodes or peripheral blood that are amplified and activated in vitro using recombinant human interleukin 2 (IL-2). This leads to a slight predominance of NK cells, mixed with T cells and NKT cells, with a limited expansion ability in vitro and a low cytotoxic activity in vivo [19]. Administration of high doses of intravenous or subcutaneous IL-2 to the patient is required after infusion of LAK cells and leads to severe toxicities such as pulmonary edema and respiratory failure, limiting the use of LAK cells in clinical practice.
One of the first clinical trial testing LAK cells in combination with intravenous IL-2 in multi-treated metastatic cancer patients was published by Rosenberg et al. in 1985 [20]. Complete regression was observed in one case of melanoma, and partial responses were observed in 10 of 25 patients with diverse cancer types (including one lung adenocarcinoma). Side effects consisted primarily of fluid retention due to IL-2. More complete results in 1987 showed cases of complete response principally in melanomas and renal-cell cancers. Efficacy has not been proven in lung cancer, whether in the adjuvant context, where contradictory results were noted [21, 22], or metastatic context. Advances in the comprehension of the immune system and cytokine use lead to the discovery of more effective and less toxic cell therapies.
Cytokine-activated killer cells (CIK)
Improvements in the preparation of lymphocytes lead to the use of CIK cells, with a first published study in 1991 [23]. Since then, they have been largely tested in clinical trials and positive results have been observed [24, 25]. IL-2 is still a primordial part of the activation and expansion mix, but IFNγ, anti-CD3 monoclonal antibodies and IL-1β have been added and IL-2 support is no longer required after infusion. Strictly speaking, CIK cells refer to cells with a mixed T cell- and NK-cell-like phenotype, which can be considered as a subset of NKT cells. They have both an HLA-restricted specific activity like T cells with an invariant TCR and a non-HLA-restricted direct cytotoxicity like NK cells with expression of NK receptors such as NKG2D or DNAM-1. Flow cytometry reveals that CIK preparations usually contain a varying proportion of CD3 + CD56 + cells with a median around 35% [24], a majority of CD3 + CD56-T cells (mostly CD8 +) and a small proportion of CD3-CD56 + NK cells. NKT cells usually account for less than 5% of peripheral blood mononuclear cells (PBMCs) in patients. In clinical trials, research teams generally aim for over 20–30% CD3 + CD56 + cells in the infusion after expansion (accounting for NKT cells and some activated T cells expressing CD56).
To prepare CIK cells, PBMCs are generally obtained from peripheral blood of patients or donors, but they can also be obtained from cord blood. For in vitro activation and amplification, they are cultured with IFNγ added on day 0 and anti-CD3 monoclonal antibody and IL-2 added the next day. IFNγ is important for expansion of CD56 + cells, while anti-CD3 antibodies serve as a mitogenic stimulus and IL-2 promotes the expression of essential molecules involved in the cytolytic activity [26]. IL-1β is also frequently added to the preparation for better expansion and activation. Several studies showed that stimulation or transfection with IL-6, IL-7, IL-12, or IL-15 could also improve proliferation and/or cytotoxicity [27–29]. They all have different effects on CIK cells preparation. IL-15, for example, might have a lesser expansion ability than IL-2 but generates CIK cells with a greater cytotoxic activity [29].
Numerous clinical trials have been published on CIK cells with methodologies of heterogeneous quality. An international registry on CIK cells (IRCC) has been created to overview advances in this domain. It implemented quality criteria for CIK cell clinical trials and published reports every 5 years approximately. The most recent one accounts for 106 clinical trials published between 1999 and 2019, lung cancer being the most investigated cancer type with 26.4% of the included publications [24]. The vast majority of these trials uses autologous CIK cells [30–32], although several studies use allogeneic CIK or cord blood derived CIK cells [33]. Studies seem to suggest that at least 6 × 10*6 CIK cells are required to obtain a satisfactory clinical response, authors even preferring to use up to 10*10 cells. However, in some heavily pretreated, immunodeficient, or elderly patients, expansion is insufficient and does not meet these requirements [25] and hence the need for allogeneic CIK cells. They are considered to have less alloreactivity than T cells, but the IRCC still reports cases of graft-versus-host disease in studies using allogeneic preparations [24]. In terms of outcomes, repeated CIK cell infusions are significantly associated with longer OS and progression free survival (PFS) in 27 of the included studies without any severe toxicity. Main side effects are grade 1 or 2 fever, chills, fatigue, headache, and skin rash. Cytopenia is rare. Most studies focus on advanced and/or metastatic lung cancer. Some of them examine the association of CIK cells and radiotherapy and show benefits in OS and response rate compared to control groups [34].
In many studies, CIK cells are combined with dendritic cells (DCs) due to their synergic activities. Mostly, they use isolated mature DCs, but in some cases they use DCs pulsed with lung cancer antigens [35] or tumor lysate [36]. Synergy can be explained in vitro through interaction between the two cell types. DCs increase the proliferation and the antitumoral activity of CIK cells by increasing IL-12 secretion and decreasing T regulator lymphocytes (Tregs) [25]. In vivo it can be explained through two complementary mechanisms of action: CIK cells directly target tumor cells, while DCs prime effector T cells, increasing T cell infiltration and therefore the antitumoral effect. All in all, CIK cells might be a promising option in lung cancer but also in other cancer types. Large phase-III clinical trials with a robust methodology are, however, needed before drawing any conclusions.
Natural killer cells (NK)
Other immune cells can be specifically expanded from PBMCs, such as NK cells, which have been attracting increasing attention in immune-based therapies [37]. They are innate lymphoid cells characterized by a CD3-CD56 + phenotype. Depletion of CD3 + T cells is an essential step to their successful expansion from PBMCs, as opposed to LAK and CIK cells (see Fig. 1). NK cell infiltration is associated with improved OS in most solid cancers, although results are contradictory in lung cancer [38]. This could partly be explained by a decreased efficacy of infiltrating NK cells in the strong immunosuppressive TME that is often associated with lung cancer. They are known for their cytolytic function through exocytosis of perforin and granzyme granules, their production of cytokines such as IFNγ which increases antigen presentation and activation of effector T cells, their expression of death-inducing ligands and their antibody-dependent cellular cytotoxicity (ADCC) [37]. Contrary to effector T cells, they do not need antigen sensitization to target tumor cells. Loss of HLA expression in tumor cells increases their susceptibility to NK cells instead of rendering them resistant. Indeed, NK cells recognize stressed cells that have lost their HLA-I molecules or overexpress stress-induced ligands (MICA, MICB, RAET1E, B7-H6, etc.). These ligands bind to NK cell receptors (DNAM1, NKG2D, NCRs, CD16, etc.) and overcome inhibitory signals, inducing the cytotoxic activity of NK cells against stressed cells like tumor cells. The CD16 receptor also binds the constant fraction of tumor antigen-specific immunoglobulins, inducing ADCC [39]. It is the only receptor not needing cytokine pre-activation to exert its action. For activation of the other receptors, cytokines are needed, the most used being IL-2 [40]. Many other cytokines have been studied to improve expansion and activation of NK cells in vivo or in vitro. IL-15 promotes activation, proliferation and survival [41]. It seems to yield better results than IL-2, notably regarding a better selection of NK cells. Indeed, IL-2 also expands T cells, including Treg, as was shown with LAK and CIK cells. Preclinical studies suggest that a combination of IL-12, IL-15 and IL-18 could be better than IL-15 or IL-2 used alone [42, 43]. IL-18 can induce memory-like NK cells and IL-21 is also known to increase NK cell proliferation, cytotoxicity, and IFNγ production [44]. Ex vivo stimulation of NK cells is essential before treatment because they are often in a resting state in advanced solid tumors [45]. Recent studies suggest that the activity of unexpanded NK cells is limited due to the immunosuppressive characteristics of the tumor microenvironment but that expanded NK cells overcome this immunosuppression and rescue exhausted tumor-infiltrated lymphocytes through IFNγ [46]. They also upregulate PDL1 expression, acting in synergy with PD1-blockade therapy.
NK cell preparation starts with PBMCs isolation from peripheral blood (PBMCs can be separated from whole blood or through leukapheresis), umbilical cord blood, or postpartum placenta. NK cells can also be differentiated from embryonic stem cells, possibly leading to more active NK cells [47], but embryonic stem cells’ manipulation is still controversial. PBMCs are then depleted of CD3 + T cells and expanded/activated through different means before infusion into the patient: culture with cytokines mentioned above (IL-2, IL-15, IL-18), culture with stimulatory cells or gene transduction with membrane-bound cytokines to induce autocrine stimulation [39]. Contrary to adoptive T cell therapy, allogeneic treatment is possible with haploidentical NK cells but should be preceded by lymphodepleting therapy using fludarabine and cyclophosphamide to prevent rejection by the patient’s immune system and therefore increase their persistence [48]. Robust expansion should generate enough NK cells to treat more than 20 patients from one leukapheresis. In case of autologous infusion, lymphodepletion could also be beneficial through depletion of immunosuppressive myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Treg).
In one of the first phase-I trial in lung cancer patients in 2010, Iliopoulou et al. [49] used haploidentical KIR-mismatched NK cells. The reasoning behind the use of these allogeneic NK cells is to use their donor-versus-recipient alloreactivity to fight against T cells. Inhibitory receptors on NK cells (KIR) usually bind HLA-I ligands on tumor cells. When there is a mismatch between the inhibitory receptors and the HLA molecules presented by the tumor cells, NK cells are not inhibited and can induce apoptosis of cancer cells in a more potent way than autologous NK cells. In their study, Iliopoulou et al. used repetitive administrations of allogeneic NK cells in locally advanced or metastatic NSCLC, showing no toxicity, and particularly no graft-versus-host disease. NK cells were isolated based on CD56 expression and expanded with IL-15 and hydrocortisone. Out of 15 patients, two achieved partial response and 6 stable disease. Lin et al. [50] also used haploidentical KIR-mismatched NK cells in 110 advanced pre-treated NSCLC patients but added an anti-PD1 checkpoint blocker (pembrolizumab) as a co-treatment. No difference in the incidence of adverse events was observed between the group receiving NK cell therapy versus the group that was not. There was a decrease in circulating tumor cells after treatment with NK cells compared to the control group, and objective response rate (ORR) was of 36.4% in the NK group versus 18.5% in the control group. PFS and OS were also longer in the NK group. They obtained similar positive results in a previous study combining NK cell therapy and percutaneous cryoablation in NSCLC [51].
To overcome expansion difficulties, some teams prefer to use allogeneic immortalized cell lines with a good activity and expansion potential. The most frequent is the NK-92 cell line, which is a cell line derived from a 50-year-old male patient with non-Hodgkin lymphoma [52]. Advantages are a good expansion potential and a lack of most inhibitory KIR receptors. However, there are also disadvantages: they must be irradiated prior to infusion to avoid tumorigenesis, they need IL-2 for their survival, and they lack the CD16 receptor, which is essential to ADCC. These two last limits can, however, be overcome with some modifications in the cell line [52]. A phase I study in 2013 showed a good safety profile with promising results in 3 out of 4 lung cancer patients [53]. Further trials were probably disappointing since no clinical trial is currently registered on clinicaltrials.gov using NK-92 in solid tumors other than glioblastoma.
Autologous NK cells are another option. Multhoff et al. [54] used Hsp70-targeting autologous NK cells in Hsp70-positive advanced NSCLC patients after radio-chemotherapy. Hsp70 is a stress-inducible protein overexpressed in cancers but not in normal cells that NK cells recognize when they have been pre-stimulated with IL-2 and an Hsp70-derived peptide called TKD. Radio- and chemotherapy increase the density of its membrane-bound form, motivating the use of the Hsp70 NK cells after radio-chemotherapy in this phase II study. Interestingly, culture with TKD and IL-2 resulted in only 6–23% of CD3-CD56 + cells in the infusion product, reinforcing the idea that IL-2 is not enough to expand only NK cells. Disease control seemed better in the treatment group, but no statistically significant difference was observed. No severe adverse event was attributed to NK cell therapy either.
Overall, trials about NK cell adoptive therapy have been scarce in thoracic malignancy (see Table 1). This can partly be explained by difficulties encountered to expand NK cells to therapeutic doses. New preparation methods are under investigation. Several current studies use SNK01, which is a novel mix allowing for expansion and activation of NK cells, but results are still pending [55, 56]. In conclusion, while NK cells could be a good option in theory, clinical outcomes are disappointing. Further progress could be made in identifying better methods to improve expansion, activation and persistence. Combinations with other immune therapies could also be a solution, since the TME might have a role in inactivating infused NK cells [45].
Table 1.
Selected clinical trials using CIK (without associated DCs) and NK cells adoptive therapy
| Reference | Study type | Patients | Treatment | Source | Dose-administered | Preparation | Efficacy | Toxicity |
|---|---|---|---|---|---|---|---|---|
| Wu, 2008 [30] | Randomized controlled trial | 59 NSCLC, stage IIIB–IV | CIK + CT vs CT | Autologous PBMCs | 1 × 10*9 × 5 (every 48 h), 5d after CT |
IL-1α, γIFN, IL-2, anti-CD3 |
ORR 45% vs 43%, DCR 90% vs 66%, mPFS 6.7 m vs 4.7 m, mOS 15 m vs 11 m |
Temporary fever and headache |
| Niu, 2011 [33] | Randomized controlled trial | 40 advanced solid tumors: 15 NSCLC | CIK + 2nd line CT vs 2nd line CT | Cord-blood | 9 × 10*9 × 6 (within 12d), 1w after CT | γIFN, IL-2, IL-1, anti-CD3 | ORR 30% vs 15%, DCR 80% vs 70%, mPFS 3.5 m vs 2.0 m, mOS 11.2 m vs 7.5 m | Grade 1–2 flu-like symptoms |
| Li, 2012 [31] | Phase II | 100 NSCLC stage I–IIA, 74 NSCLC stage IIIB-IV | CIK + CT vs CT | Autologous PBMCs | 1 × 10*10 on d15 and d17 of each CT cycle |
IL-1α, γIFN, IL-2, anti-CD3 |
Early-stage: mOS 73 m vs 53 m Advanced stage: mOS 24 m vs 10 m, mPFS 13 m vs 6 m |
No data |
| Jin, 2014 [32] | Randomized controlled trial | 943 NSCLC, stage I–III | CIK + standard treatment vs standard treatment | Autologous PBMCs | 2–6 × 10*9 × 3 consecutive days, 6 courses (18 m) | γIFN, IL-2, anti-CD3 | mOS 48 m in the CIK group vs 36 m, 56.2% recurrence vs 78.6% | No data |
| Iliopoulou, 2010 [49] | Phase I | 16 NSCLC, stage IIIB–IV | NK + 1st or 2nd line CT | Allogeneic (haploidentical -KIR mismatch relative donors), peripheral blood | 0.2–2 × 10*6/kg (× 2–4 doses) | CD56 isolation (microbeads), IL-15, HC | 2 PR, 6 SD, 7 PD, mPFS 5.5 m, mOS 15 m | No side effect |
| Tonn, 2013 [53] | Phase I | 13 advanced or metastatic solid tumors: 3 SCLC, 1 NSCLC | NK | Allogeneic, NK-92 cell line | 1–10 × 10*9 cells/m2, 2 infusions, 48 h apart | IL-2 | Lung cancer: 2 PR, 1 SD (2y), 1 PD | No side effect |
| Lin, 2020 [50] | Randomized controlled trial | 109 NSCLC, stage IIIB-IV, PDL1 ≥ 1%, PD after CT/ TKI | NK + Pembrolizumab vs Pembrolizumab without NK | Allogeneic (haploidentical -KIR mismatch), peripheral blood | 3 × 10*9 (3 infusions/2 weeks, 2–6 cycles) | “HANK cell culture medium” | ORR 36.4% (20 PR, 30 SD, 5 PD) vs 18.5% (10 PR, 29 SD, 15 PD), reduction in CTCs, mPFS 6.5 m (HR 0.58), mOS 15.5 m (HR 0.60) | Adverse events attributable to Pembrolizumab (no difference between the 2 groups) |
| Multhoff, 2020 [54] | Phase II | 16 NSCLC, stage IIIA/B, after RCT, mHsp70 + | NK | Autologous, peripheral blood | 10*8–10*9 (/2–6 weeks, 4 cycles) | TKD peptide, IL-2 | 1 CR, 1 PR, 2 SD, 2 PD in the treatment group vs 1 PR, 1 SD, 5 PD in the control group | No adverse event attributed to NK cells |
| Chawla, 2020 [55] | Phase I, ongoing | 1 NSCLC, 5 sarcomas, 1 CRC | NK | Autologous | 1–2 × 10*9 (/week, 5 cycles) | SNK01 preparation | SD as best overall response in the first 3 patients | No adverse event in the first 3 patients |
| Kim, 2020 [56] | Phase I/II, ongoing | 14 NSCLC, stage IV, PDL1 + | NK + Pembrolizumab vs Pembrolizumab without NK | Autologous | 2–4 × 10*9 (/week, 6 cycles) | SNK01 preparation | 4 PR in the first 6 patients in the treatment group vs 3 PD in the control group | No adverse event in the first 6 patients |
CT chemotherapy, TKI tyrosine kinase inhibitor, RCT adiochemotherapy, CRC colorectal cancer, HC hydrocortisone, CR complete response, PR partial response, SD stable disease, PD progressive disease, mOS = median overall survival, mPFS median progression-free survival, CTCs circulating tumor cells, anti-CD3 anti-CD3 monoclonal antibody
Tumor-infiltrating lymphocytes (TILs)
Development of TILs has been concomitant to the development of LAK cells [57], but whereas LAK cells have been abandoned in favor of more developed therapies like CIK cells or NK cells, TILs have proven their efficacy in melanoma patients [58, 59] and are still under investigation in many ongoing clinical trials. Promising results have been shown in renal cell cancer and now also NSCLC, but results are still pending in other solid tumors. Because of its high TMB (and the high potentiality for neoantigens it offers) and the known specificity of TILs for neo-antigens, NSCLC has always been thought to be a good potential target for adoptive therapy with TILs. Advantages compared to LAK cells are their specificity toward the patient’s tumor cells and the fact that they can be expanded with a dose of IL-2 100 times lower, inducing less toxicity.
TILs are a heterogeneous population composed of terminally differentiated T cells with many inhibitory receptors and of stem cell-like CD8 + T cells. There is cause for believing that exhausted dysfunctional TILs represent the real antigen-specific population while other T cells are merely bystanders that can be characterized by a CD39- phenotype [60, 61]. To produce TILs for adoptive therapy, lymphocytes are isolated from tumor tissue. They are expanded and activated in vitro with cytokines and feeder cells or antibody-coated beads (anti-CD3). IL-2 is the most used cytokine but a downside of IL-2-based expansion is that it leads to negative selection of the exhausted cancer-specific T cells that have become less responsive to IL-2 [62]. Research is underway to rapidly identify and select the tumor-reactive cells before IL-2 expansion [63]. For example, an enhanced sorting strategy based on CD39, Tim3, Slamf6 and PD1 expression could improve in vivo persistence by selecting T cells of interest [64]. Another way to improve reactivity towards tumor cells is to incubate TILs not only with cytokines but also with predicted HLA-I neoantigens, thus increasing the proportion of neoantigen-specific CD8 + TILs [62]. The use of IL-21 could also be beneficial by promoting memory T cells instead of dysfunctional terminally differentiated T cells, increasing persistence [61].
A lymphodepleting regimen is often administered before reinfusion into the patient, followed by subsequent IL-2 infusions. Lymphodepletion with fludarabine and cyclophosphamide is used to decrease Treg and other immunosuppressive cells and to decrease endogenous T cells, increasing efficacy and proliferation of TILs in their stead [65]. Due to this lymphodepleting regimen, adoptive T cells can represent up to 80% of circulating CD8 + T cells several months after infusion [66].
Extraction and culture of TILs ex vivo allow for reactivation and expansion before reinfusion into the patient, on the hypothesis that a larger number of T cells will overcome more easily immunosuppression after this treatment. It relies on the assumption that the patient presents an immunosuppressed phenotype, but an immunogenic tumor with active lymphocytes. It cannot work if there are not enough TILs to amplify, if they are too strongly altered, or if they do not recognize tumor antigens. It is of interest to note that natural TCR with a high affinity for self-antigens are negatively selected in the thymus [67]. By a matter of consequence, TILs against self-tumor-associated antigens have lower affinity TCRs and weaker activity than TILs against neoantigens.
Studies on TILs ACT have been ongoing for several decades in NSCLC (see Table 2). Kradin et al. in 1989 [68] tested them on melanoma, renal cell cancer and advanced or metastatic NSCLC patients, but TILs were mostly effective in melanoma and renal cell cancer patients with around 25% objective tumor response, whereas no response or progressive disease was observed in NSCLC. Ratto et al. [69] found a survival benefit after surgery and radio-chemotherapy in a large randomized controlled trial in 1996, mainly driven by the stage IIIB subgroup. No other study confirmed these results, and they might be different with our actual standard of care. Not much has been published since. Studies are currently ongoing in advanced or metastatic NSCLC, in association with PD-1/PDL-1 checkpoint blockers [70] or as standalone therapy [71]. Creelan et al. just published the results of a phase I study [72] focusing on TILs therapy in patients progressing on nivolumab in advanced NSCLC. They expanded TILs from resected metastases (mainly pleural nodes and supraclavicular lymph nodes) before starting nivolumab and added TILs, a lymphodepleting regimen and IL-2 if the patient had progressive disease. Only one patient had insufficient TILs after expansion, and 16 patients were treated with ACT. Two complete responses were noted and persisted after 18 months (interestingly enough, one of them carried an EGFR mutation) and 11 patients had a reduction in tumor burden with a median of best change around -35%. Two patients stopped treatment because of toxicities, mainly attributed to IL-2 infusion and lymphodepleting regimen. These results are therefore quite promising for the use of TILs in NSCLC.
Table 2.
Selected clinical trials using TILs
| Reference | Study type | Patients | Treatment | Source | Dose-administered | IL2 / FC | Efficacy | Toxicity |
|---|---|---|---|---|---|---|---|---|
| Kradin, 1989 [68] | Phase II | 8 NSCLC, 13 melanoma, 7 renal cell carcinomas | TILs | Autologous, tumor biopsy samples | 10*10 | IL2 | 5 PR (melanoma and renal cell carcinoma), 10 SD, 13 PD | Correlated with IL-2 dose: fever, local rash and superficial thrombophlebitis, nausea, weight gain, supraventricular tachycardia, rise in bilirubin and creatinine |
| Ratto, 1996 [69] | Randomized controlled trial | 113 NSCLC, stage II–IIIB, post-surgery | RT ± Cisplatin Vinblastine ± TILs | Autologous, resected tumor tissue | 4–70 × 10*9 | IL2 | mOS 22.4 m in the TIL group vs 14.1 m in the control group | Correlated with IL-2 dose: fever, chills, nausea |
| Chesney, 2019 [70] | Phase II, ongoing | 135 advanced melanomas, HNSCC or NSCLC | TILs (Lifileucel) ± Pembrolizumab or Nivolumab + Ipilimumab | Autologous, resected tumor tissue | IL2 + FC | No published result yet | No published result yet | |
| Massarelli, 2021 [71] | Phase II, ongoing | 95 NSCLC, stage III–IV, pre-treated | TILs | Autologous, resected tumor tissue | IL2 + FC | No published result yet | No published result yet | |
| Creelan, 2021 [72] | Phase I | 20 NSCLC, stage IV—16 received TILs | Nivolumab, then TILs if PD | Autologous, resected metastases | 4.3–175 × 10*9 | IL2 + FC | 2 CR after 1.5 years, 11 reductions in tumor burden | Grade 4 cytopenia and in 1 patient pulmonary oedema |
HNSCC head and neck squamous cell cancer, RT radiotherapy, FC fludarabine and cyclophosphamide, PR partial response, SD stable disease, PD progressive disease, mOS median overall survival
Since a large number of clinical trials testing TILs ACT have been carried out in melanoma patients, there are some data available on prognosis markers of efficacy. TILs are often a mix of CD4 + and CD8 + T cells, but a higher proportion of CD8 + T cells in the infused TILs seems to be predictive of a better response [73]. Interestingly, when comparing TILs expanded from lung cancer patients and melanoma patients, Ben-Avi et al. found a significative lower proportion of CD8 + T cells in lung cancer patients than in melanoma [74], even though they have similar expansion capacities. A higher peripheral blood lymphocyte count also seems to be an indicator of response [68], as is HLA-I expression. Indeed, loss of HLA expression is logically correlated with a lower T cell activity since T cells then become incapable of recognizing tumor cells [75]. Loss of HLA expression or defects in the antigen-processing machinery are a common escape mechanism in cancers. It is particularly frequent in NSCLC and is associated with a decreased T cell infiltration [76]. The only ways to overcome this are immune cells not necessarily needing HLA expression to exert their activity like NK, NKT, or CAR-T-cells or new treatments that could reestablish HLA presentation. In tumor murine models, Fhit gene transduction induces the restoration of HLA-I expression and induces a T cell-mediated immune rejection of the tumor [77]. Whole-exome and transcriptome sequencing show that high HLA-I antigen processing and presentation score is associated with better outcomes [78]. The same study also focused on neoantigen prediction and concluded that higher mutation and neoantigen load are associated with better OS and PFS in adoptive T cell therapy.
Another mechanism of resistance of cancers toward T cells is the immunosuppression exerted by the TME. However, preclinical trials suggest that activated adoptive T cells might overcome MDSCs and M2 tumor-associated macrophages (TAMs) immunosuppression in vivo [79]. This probably depends on the avidity of TCR for HLA-presented antigens, since high-avidity T cells recognizing antigens with a high-affinity for HLA-I proteins seem to overcome this immunosuppression, whereas low-avidity T cells or low-affinity antigens do not [80]. It would therefore be logical to prime or engineer T cells toward specific antigens to improve efficacy like with DC vaccines, TCR-engineered cells, and CAR-T-cells.
Tumor vaccines using dendritic cells (DCs) and dendritic cell-derived exosomes (DEX)
In opposition to the previous ACT that can be considered as passive and rely on the antitumoral effect of cells that are injected into the patient, another option is to educate the patient’s immune system. The goal is to expand and activate T cells targeting relevant tumor antigens within the patient to kill cancer cells and acquire an immunological memory for future relapses [81]. This can be achieved through what has been called tumor vaccines. Many different forms exist, for example injection of peptides, mRNAs, DCs pulsed with antigens. DCs are a particularly attractive option in terms of efficacy [82]. Indeed, they are the most potent antigen-presenting cells (APCs) of our organism. They offer the advantage of stimulating both the adaptive and innate immunity. They prime lymphocytes, but they also have a more global action on antitumoral response. For example, through cell-to-cell contact, they can promote NK cell activity [83]. Development of DC vaccines led to an approved treatment for prostate cancer (Sipuleucel-T) but regrettably DCs vaccines have been rather disappointing in thoracic malignancies [84] (see Supplementary Table 1).
Dendritic cells are obtained through leukapheresis and isolation of monocytes/dendritic cells. It is important to note that isolation of monocytes through the purification of CD14 + cells also isolate myeloid-derived suppressor cells (MDSCs) and that this can limit DC maturation in patients with a high proportion of MDSCs [85]. Isolated cells are then cultured in vitro with IL-4 and GM-CSF to induce differentiation into DCs. Activating factors are added to promote maturation, like TNFα, IL-1β, IL-6 and prostaglandin E2, but the use of TriMix (electroporation with mRNA encoding CD40 ligand, CD70 and a constitutively active TLR4) has proven to be more rapid and efficient [86]. Indeed, TNFα, IL-1β, IL-6 and prostaglandin E2 could induce dysfunctional DCs with a high expression of PDL-1 [81]. After differentiation and maturation, DCs are loaded with antigens, which can be proteins, peptides, mRNA, cancer cell line lysate or tumor lysate. Of note, peptides are HLA-restricted and cannot be used with every patient, whereas other preparations such as mRNA or tumor lysates do not exhibit this issue. Finally, DCs are injected into the patient to activate antigen-specific T cells. Methods of injections vary between studies. Subcutaneous (SC) and intradermal (ID) injections are often used because it is easy and safe, but the majority of DCs will never reach the lymph nodes and will stay at the site of injection. Biodistribution is better with intranodal (IN) or intravenous (IV) administrations. Intravenous administration results in biodistribution into the pulmonary vascular bed where numerous resident T cells can be encountered and activated [81]. Several studies use different routes of administration at the same time (for example, intradermal and intravenous). Dose and rhythm of administration are subject to discussion. A strong expansion and activation of T cells with a high single-dose could result in exhaustion, whereas lower repeated stimulation could boost central memory T cells and lead to a more long-term efficient response.
One of the main challenges of this approach is to determine immunogenic antigens, especially in lung cancer patients who do not share the same antigens. Targetable antigens are divided into five different categories: neoantigens (due to single-nucleotide variations and indels in tumor cells), cancer-germline antigens (epigenetically suppressed except in gonads, placenta and some cancers), self-tumor-antigens (shared between tumor cells and their tissue of origin), overexpressed shared antigens (present in normal tissue but overexpressed in tumor cells), and viral oncogenes (in some cancers induced by viruses). Neoantigens are particularly interesting because of their tumor cell specificity and their potential for strong avidity. Indeed, due to self-tolerance, self-tumor-antigens have a lower avidity for T cells [67]. There is supposedly no risk of targeting healthy cells when targeting neoantigens, in opposition to overexpressed oncogenic proteins or oncofetal antigens that might be expressed by other cells. Oncoviral antigens would also be an optimal target but are not involved in thoracic malignancies.
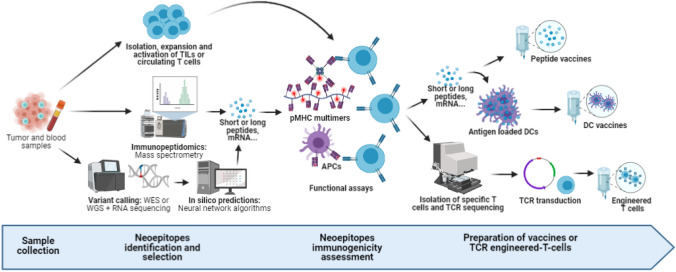
Principal challenges in identifying relevant neoantigens consist in difficulties to infer protein expression levels, HLA presentation and TCR affinity from RNA sequencing or whole-exome data. In the last 10 years, advances in bioinformatics have led to the creation of pipelines predicting the most interesting neoantigens presented by the tumor [62]. Neoantigens and their clonality are inferred from the analysis of whole-exome sequencing and RNA sequencing of tumor cells and normal cells. Algorithms can then be used to predict neoantigens with the best potential to undergo HLA presentation and TCR recognition [87]. Mass-spectrometry-based immunopeptidomics is another way to select candidate neoepitopes. Immunogenicity is then tested in the presence of T cells recognizing the neoepitopes presented on pMHC multimers or antigen-presenting cells. Finally, the selected antigens can either be pulsed into dendritic cells in vitro or directly injected into the patient to boost a specific adaptive immune response (see Fig. 2). Despite high cost and analysis time, this technique yields good results in melanoma and seems feasible in clinical practice [88]. Ding et al. [89] recently published a study evaluating this technique in advanced lung cancer patients. Eighteen patients were included. One was excluded because of insufficient material for high throughput sequencing and 5 others were excluded due to insufficient numbers of actionable neoepitopes, loss of heterozygosity in HLA or death from rapid tumor progression during preparation time, underlining several limits of the method. Thirteen to 30 peptide-based neoantigens were selected for the remaining patients and loaded into autologous DCs. Tolerance was good with only minor reactions at the site of subcutaneous injection. Partial response was seen in 3 patients and stable disease in 6 patients (DCR 75%), with better outcomes in patients continuing ICBs to which they had previously become resistant (4 patients), showing promising results for this technique.
Fig. 2.
Neoepitopes selection and use in adoptive therapies. Identification of immunogenic neoepitopes is a crucial step to creating personalized adoptive therapies. Several methods exist. Tumor and blood samples can be sequenced (through whole genome or whole exome sequencing) to identify variants specific to the tumor. Expression is then validated by RNA sequencing, and neural network algorithms are used to predict neoepitopes with the best avidity and stability for TCR recognition. Another way to identify these presented neoepitopes is through mass spectrometry of the tumor samples. Neoepitopes are then synthetized (mainly through the form of short peptides or mRNA) to be tested for immunogenicity. They are either presented by isolated autologous or haploidentical allogeneic antigen-presenting cells or by pMHC multimers to expanded T cells. Immunogenicity is evaluated by functional assays such as activation of co-stimulatory markers (4-1BB/CD137) or IFNγ secretion. Reactive T cells can then be isolated in order to sequence the specific TCR, which can then be transduced into effector cells before reinfusion into the patient. Neoepitopes can also be directly injected into the patient or pulsed into DCs to stimulate the adaptive response of the patient. Figure created with BioRender.com
An easier, less specific, and more frequently used option for targeting multiple tumor-specific antigens is to incubate dendritic cells with tumor lysate or dead tumor cells. Two phase-I studies with autologous tumor lysate demonstrate safety but no efficacy signal [90, 91]. In MPM, Aerts and his team use tumor lysate to pulse DCs. In their first trials, they used autologous tumor lysate [92], but now they prefer to use allogeneic tumor lysate to facilitate implementation. In a phase I study, 9 patients were treated with DCs pulsed with allogeneic tumor lysate extracted from 5 mesothelioma cell lines [93]. Two patients experienced partial response while the remaining 7 had stable disease (half of the patient were in the adjuvant context). PFS was 8.8 months. The authors are currently leading phase II/III trials to expand on these promising results [94, 95].
As in other adoptive therapies, a limitation faced by DC vaccination in lung cancer is the strong immunosuppressive TME, explaining poor results in clinical trials outside the premalignant or minimal residual disease setting. Indeed, vaccines will induce a T cell response, but T cells and DCs might not be able to act against the immunosuppression they are faced with. Tregs might also be expanded and block effector cells after vaccination [96]. Chemokines like CCL4 are often downregulated, decreasing the recruitment of DCs into the tumor. VEGF, TGFβ, IL-6 and IL-10 secretion inhibits differentiation and maturation of DCs. The lack of nutrients and oxygen in the tumor alters their metabolism. Other strong limitations are the process of cancer immunoediting and tumor escape through HLA loss. Whereas highly immunogenic antigens are expressed in the early stages of cancer, T cell immunoselection leads to the selection of tumor cells lacking these high affinity antigens [97], and as described with TILs, tumor cells can acquire resistance through loss of antigen presentation, becoming invisible to TCR-mediated immunity. Rosenthal et al. evaluated RNA sequencing data of tumor cells depending on TIL ratio and showed that immune-infiltrated regions exhibit ongoing immunoediting as opposed to non-infiltrated regions [98]. They noted loss of heterozygosity in HLA antigens and depletion of neoantigens, showing the negative selection that occurs when facing an efficient immune microenvironment.
In thoracic malignancies, DC vaccines have been explored in different indications and with different methods [81]. The first study in 2001 used DCs pulsed with a peptide derived from an HLA-10201-specific peptide of the carcinoembryonic antigen (CEA) in metastatic or recurrent NSCLC or colorectal cancer (CRC) with elevated serum CEA [99]. Tolerance was acceptable with 5 out of 12 cases of mild diarrhea and complete response was observed in 2 patients, however only colorectal cancers. Other studies on CEA-pulsed DCs showed similar results, with at best stable disease [100, 101]. Shared overexpressed antigens have also been investigated in NSCLC with a first clinical trial on TAA mucin 1 (MUC1)-loaded DCs in 2003 [102]. Patients with advanced or metastatic breast or lung cancer were included, and partial response was observed in 7 out of 9 MUC1-positive patients with no severe side effects. Due to difficulties pertaining to a stronger immunosuppressive environment, not many studies have been carried out in SCLC. Antonia et al. [103] looked into p53 DC vaccines. Indeed, tumor-suppressor gene TP53 is mutated in more than 90% of SCLC cases. Tolerance was good, even after repeated infusions. Interestingly, while DC vaccines achieved mitigated responses during treatment, second-line chemotherapy yielded better ORR in the 41.8% of patients with a p53-specific T cell response after initial DCs infusions (78,6% vs 33,3% in patients with a negative immune response). Whether this is related to innate immune characteristics of the patient or if there is a causal link remains to be determined. The idea of loading DCs with multiple antigens has also been investigated but in very preliminary studies [104].
In the adjuvant context, DCs pulsed with apoptotic bodies of an allogeneic cell line have been tested in NSCLC with a good safety profile but no control group to compare efficacy [105]. MUC1-pulsed dendritic cells with siRNA-silencing of suppressor of cytokine signaling A (SOCS1), which downregulates DC activation, showed promising results in resected NSCLC [106].
DCs can also be used to activate immune cells in a TCR-independent manner. Ishikawa et al. [107] published a phase I study using α-galactosylceramide (α-GalCer)-pulsed DCs. It is a type of glycolipid that specifically activates NKT cells and increases their cytotoxic activity and their IFNγ and IL-4 secretion. Treatment response was disappointing with at best stable disease. However, overall survival seemed promising and a phase II/III study is ongoing [108]. DCs have also been used to attract other immune cells. In a phase I study, Lee et al. [109] transduced the CCL21 chemokine into DCs instead of pulsing DCs with antigens to increase T cell infiltration. DCs were then injected directly into the tumor through CT-guidance or bronchoscopy. CD8 + T cell infiltration and PDL1 expression increased, but there was no signal towards improved clinical outcomes.
Some studies have explored another way to use DCs for vaccines without having to infuse the actual DCs. They prefer to use DC-derived exosomes (Dex). Dex are 50–150-nm-diameter secreted extracellular vesicles displaying a molecular composition that bestows them with potent immunostimulatory properties. Dex maintains the key functions of DCs in their ability to present tumor-associated antigens (TAA) and to activate TAA-specific immune responses [110, 111] as well as triggering NK cells response [112]. Three clinical trials using autologous TAA-loaded Dex have been previously completed in cancer patients [113–115]. These studies highlighted the feasibility of large-scale Dex production and confirmed an excellent safety profile for Dex administration in patients. In NSCLC patients, a first study on 9 patients with advanced MAGE+ NSCLC received MAGE3.A1-loaded Dex. Although only minimal increases in peptide-specific T cell activity were detected, NK cell lytic activity was upregulated. Observed stability of disease in some of the immunized patients was observed [114]. In a second study in NSCLC patients, “second generation” of Dex (derived from IFNγ-matured DC) was used [116]. The results of this phase II trial indicated that IFN-γ-Dex was well tolerated and that these “second generation” of Dex could boost NKp30-dependent NK cell functions while having no detectable induction of antigen-specific T cell responses. Twenty-two patients were evaluated for clinical responses according to RECIST criteria after injections of IFN-γ-Dex. The median PFS and OS for all 22 patients were 2.2 and 15 months, respectively. There was no objective tumor response according to RECIST criteria [115].
In terms of prognostic markers of response for DC vaccines in general, retrospective studies suggest that the adenocarcinoma subtype, an erythema reaction with a diameter superior or equal to 30 mm at the injection [117], the development of immune-related side effects, or a percentage of lymphocytes superior to 20% prior to vaccination [118] are all associated with better outcomes. They also underline better outcomes in case of repeated injections, but their retrospective and uncontrolled designs limit their interpretation.
As was mentioned with CIK cells, combinations between DC and CIK cells yield better results than any of these therapies alone. While ORR-to-DC vaccines in lung cancer is about 9.3 and 3.0% in SCLC, DC vaccination combined with CIK cell therapy and/or chemotherapy is around 31.2% [81].
Engineered adoptive therapies
TCR engineering
The most used TCR-engineered immune cells are T cells. Contrary to TILs, adoptive therapy using engineered T cells does not require the presence of many active and specific T cells in the tumor and could possibly be achieved in tumors with a “cold” phenotype. The question is no longer how to harvest enough cells to reach clinical efficacy, but how to select high-avidity TCRs and how to expand this engineered population without inducing dysfunction. T cells are obtained from the peripheral blood of patients, mainly by leukapheresis, and are transduced with a TCR against tumor antigens before expansion and reinfusion, principally through lentiviral or retroviral transduction, but newer techniques like CRISPR-Cas9 editing can also be used. Stimulation usually occurs with anti-CD3 and anti-CD28 antibodies, followed by expansion in the presence of IL-2 [119]. However, IL-2 expansion might lead to the development of dysfunctional terminally differentiated T cells. To prevent this, a short culture time is recommended, as well as the use of memory-phenotype promoting factors like IL-21 [61] or IL-7 and IL-15 without IL-2 [119]. These cytokines favor the expansion of stem-cell-like T cells rather than terminally differentiated T cells, which has proven to increase persistence. Further engineering of the T cells could also help improve trafficking and persistence, for example, by inducing cytokine secretion directly onto T cells [120]. This way cytokines will continue to exert their action after infusion into the patient. This will be discussed in more detail in a further paragraph. Lymphodepleting chemotherapy prior to infusion and high-dose IL-2 after infusion are globally linked with better outcomes compared to studies where no other action has been implemented to ensure persistence [121].
The main challenge is the same as for DC vaccines and remains a major limitation in lung cancer: there is no obvious optimal antigen that would target all tumor cells and preserve healthy cells. There is an important heterogeneity in tumor antigen expression between patients but also within the patients themselves. The variety of antigens used in clinical trials focusing on thoracic malignancies reflects this heterogeneity (see Supplementary Table 2). Some target antigens are common between cancer types, such as New York esophageal squamous cell carcinoma 1 (NY-ESO-1), MAGE-A10 and cancer testis antigen 2 (LAGE-1a). Clinical trials using engineered TCR against some of these antigens have shown an ORR above 50% in synovial sarcoma, multiple myeloma and metastatic melanoma [122, 123]. In the case of NY-ESO-1, there are surprisingly not as many on-target off-tumor side effects as could be expected of a shared antigen. Clinical trials are actually ongoing in advanced NSCLC, testing these T cells alone or in combination with pembrolizumab [124, 125]. However, on-target off-tumor toxicities are quite frequent with antigens shared between tumor cells and healthy tissue. For example, T cells targeting ACE cause severe colitis [126] and T cells targeting melanoma/melanocytes antigens cause uveitis and hearing loss [127].
Studies using bioinformatics to determine individualized relevant antigens in a patient are ongoing. Biotech companies are investigating target peptides with the best specificity, copy number and expression homogeneity. They created different T cell-based therapies based on this technology, like IMA101 and IMA201 for the company Immatics. IMA101 are T cells targeting antigens identified by an outsourced platform from a pool of predefined targets, which are currently being tested in relapsed or refractory solid tumors [128] and IMA201 are MAGEA4/8-TCR-engineered T cells [129]. In these clinical trials, they focus on tumor-associated antigens (TAAs). Tumor samples are screened for expression of frequent TAAs (MAGEA1, MAGEA4, MAGEA8 NY-ESO-1, etc.), and patients are included if their tumor expresses at least one of the TAAs for the first clinical trial and MAGEA4/8 for the second. In the first, T cells are then selected depending on their specificity toward up to four of these TAAs, activated and expanded with IL-21 before reinfusion into the patient. In the second, TCRs have been identified through the Immatics TCR discovery platform for their affinity and for their specificity toward TAAs without recognition of healthy cells. They are then transduced into the patient’s isolated PBMCs before reinfusion. Results regarding efficacy and toxicity are still pending. The next step under investigation for these platforms is the identification of high-affinity neoantigens and TCR engineering toward several of these neoantigens, as was previously described with vaccination (see Fig. 2). Clinical trials are currently ongoing to determine the feasibility, safety, and efficacy of these personalized adoptive T cell therapies (NCT03970382, NCT03891706). However, an important limitation compared to vaccination is the added time needed for the identification of T cells with a high-affinity neoantigen-specific TCR and their expansion [130, 131]. TCR identification can be shortened with the use of allogeneic T cells [132], but allogeneic TCRs are at risk for self-reactivity against healthy tissue. Once specific TCR are identified, they are transferred into T cells by different methods, like retro- or lentiviral transduction, or more recently CRISPR-Cas9 editing [133]. Pipelines predicting the best neoantigens in a patient are an innovative solution but are unfortunately patient-specific and do not allow for mass production of TCR-engineered T-cells for several patients.
CAR engineering
CAR-T cells (Chimeric Antigen Receptor T cells) have gained a lot of attention due to their promising results in hematological malignancies. Strong benefits have been observed in acute and chronic lymphoblastic leukemias and in non-Hodgkin lymphomas with anti-CD19 CAR-T-cells [134–136] and anti-CD20 CAR-T-cells [137], with up to 90% of study patients achieving complete remission.
The difference between CARs and TCRs is that CARs are a completely synthetic receptor designed to recognize a specific antigen without HLA presentation. Antigens must be surface proteins, whereas TCR can target intracellular peptides that are presented by HLA molecules at the cell surface. Several generations of chimeric receptors now exist. They all are artificial fusion proteins with an extracellular antigen binding portion and a transmembrane domain. They differ by their intracellular co-signaling portions. First generation receptors only had a CD3ζ binding site, but the activation signal was insufficient to properly induce activation and proliferation. Second- and third-generation CARs have more co-stimulatory binding sites to increase clonal expansion, for example 4-1BB (also known as CD137) and CD28. Interestingly, 4-1BB and CD28 are not completely equivalent. It has been suggested that 4-1BB favors T-cell memory-associated genes, whereas CD28 leads to an exhausted phenotype more quickly [61]. Third-generation CARs differ from second-generation CARs by combining several co-stimulatory domains. Fourth-generation CARs are also called TRUCKs for “T cells redirected for antigen-unrestricted cytokine-initiated killing.” They differ from other CARs by the insertion of genes coding for cytokine like IL-12 or IL-18 into the CAR vector to improve persistence and cytotoxicity [138]. Safety genes can also be added into the construct [139, 140]. They are also called suicide genes and code for molecules expressed on CAR-T-cells (or less frequently TCR-engineered T cells) that lead to their death upon administration of a specific drug. For example, the Caspase9 system can be used. Administration of a synthetic molecule induces the dimerization of the chimeric protein coded by the safety gene, which induces apoptosis [140]. It allows for rapid clearing of CAR-T-cells when they cause severe adverse effects by binding antigens on healthy cells.
As with TCR engineering, T cells are harvested through leukapheresis. CARs are then transduced in vitro with a lentivirus or retrovirus, or other gene editing strategies such as TALEN or CRISPR-Cas9 before expansion. Transduction can have varying results depending on the patient, some patients being excluded from clinical trials due to insufficient expansion rate after transduction [141]. CAR mRNA electroporation into T cells for transient expression has also been studied [142], with the hypothesis that there would be lesser toxicities, but efficacy seems to be harder to demonstrate. Finally, they are reinfused into the patient, often following non-myeloablative lymphodepletion using cyclophosphamide and fludarabine. Allo-CAR-T-cells have also been described but have not been used in thoracic malignancies.
There are several limits to the use of CAR-T-cells. For one, important toxicities have been reported in hematological malignancies. A third of patients experience a cytokine release syndrome [143] with an increase in IFNγ and TNFα leading to the recruitment of macrophages and the release of cytokines such as IL-1, IL-6, and IL-10. Symptoms range from mild to severe, with fever, fatigue, myalgia, nausea, but also hypoxia, arterial hypertension and 40% glial lesions. Tocilizumab (an anti-IL-6 monoclonal antibody) is the treatment of choice. Another third of patients suffer from a cytokine-induced cerebral toxicity named ICANS [143], with symptoms of confusion, delirium and cerebral edema. Treatment consists of steroids. However, these two syndromes are not observed in early phase studies in solid tumors. This might be explained by the localization of tumor cells, which are generally not in the blood or lymphoid organs in solid malignancies contrary to hematological malignancies. Nevertheless, organ toxicity is possible if the target antigen is expressed on normal cells. This has been anticipated in some constructs, and suicide genes have been incorporated in the event of severe toxicities arising.
In solid tumors, there are more obstacles to overcome, mainly due to the TME [144]. It can have a barrier effect, preventing the penetration of CAR-T-cells. It can also lead to the anergy of the infused T cells if the proportion of immunosuppressive cells such as MDSCs, TAM or Treg is too important. In preclinical studies, CAR-T-cells that have succeeded in infiltrating the tumor rapidly lose their activity. They regain it if they are isolated from the tumor [145]. This could partly be explained by inhibitory receptors like PD-1, which is an argument for combining ICBs and adoptive T cell therapy. Another issue is the low persistence of CAR-T-cells, which is an important determinant of their efficacy [146]. Persistence can be improved by enhancing memory CAR-T-cell formation, for example, by using IL-7 and IL-15, which are cytokines preserving memory phenotypes during expansion [147].
Another important issue, as mentioned with vaccines and TCR-engineered cells, is to find optimal target antigens. EGFR has been studied as a target of CAR-T-cells in NSCLC due to its frequent overexpression. In a phase I study, Feng et al. [148] showed that it was safe in relapsed or refractory NSCLC, with only a transient grade 3–4 serum lipase increase. A median of 29.28% of the cells injected expressed the EGFR-CAR, and persistence was extremely variable, from 11 days to more than 37 weeks. Only 2 patients in 11 obtained partial response, and they were in a subgroup also receiving a cisplatin doublet. Even though no conclusion can be drawn from this phase I study, recent studies suggest that adding platins to the lymphodepleting regimen increases CAR-T-cell infiltration and response [149]. Indeed, even with low-dose fludarabine and cyclophosphamide, Specht et al. observed that anti-ROR-1 CAR-T-cells had limited tumor infiltration and T cell activity in NSCLC and triple-negative breast cancer (TNBC) [139]. PDL-1 has also been proposed as a target. It is a frequent target of checkpoint blockers and causes anergy in effector cells when binding PD-1. It is overexpressed not only on tumor cells but also on immunosuppressive immune cells such as MDSCs, and its expression is supposedly very low in vital organs. In theory, it should specifically target PDL-1-positive lung cancer cells and the immunosuppressive TME. Results have, however, been disappointing with the development of severe delayed pulmonary toxicity in the first patient included in a phase-I study [150]. Pneumonitis correlated with isolated high IL-6 and CRP levels without any argument for infection or typical cytokine release syndrome. Evolution was favorable after treatment with tocilizumab and corticosteroids.
Apart from NSCLC, CAR-T-cells have gained a growing interest in MPM (see Supplementary Table 2). Adusumili et al. and Haas et al. created anti-mesothelin CAR-T-cells, since mesothelin is a highly expressed cell-surface glycoprotein in MPM (with the exception of sarcomatoid mesotheliomas) that can also be found in metastatic lung, ovarian, pancreatic and breast cancers [140, 151] and at low levels on normal mesothelial cells. No toxicity was observed in the two phase I trials, and a phase II trial is currently recruiting. Lymphodepletion improved CAR-T-cells’ expansion by an tenfold ratio, but not persistence, which was under 28 days for most patients [151]. Of note, of 18 patients included in the phase I study by Adusumili et al., 14 received subsequent anti-PD1 therapy and 11 achieved disease control (2 complete responses, 5 partial responses and 4 stable disease). Hiltbrunner et al. published a phase-I clinical trial using intra-pleural injections of anti-FAP (fibroblast activation protein) CAR-T-cells after three chemotherapy cycles [141]. FAP is overexpressed on all MPM subtypes and on tumor stroma compared to healthy tissues. Systemic anti-FAP CAR-T-cells were detected at 21 days, proving systemic distribution, and no toxicity was observed. Targeting FAP has the advantage of weakening the tumor stroma and to increase tumor infiltration, allowing for better efficacy toward FAP-expressing tumor cells.
Many argue that TCR-based therapies offer more advantages than CAR-based therapies [131]. CARs need a higher amount of antigens per cell for activation [152], have more difficulties penetrating the tumor and, as mentioned above, only target membrane-bound antigens, whereas TCRs have access to a larger panel of antigens through HLA presentation. Neoantigens being of great interest in thoracic malignancies and being mostly intracellular, TCR-based therapies might be particularly interesting in lung cancer treatment. HLA-restriction can, however, be a limit to TCR-therapy, since most clinical trials only include HLA-A*02,011-positive patients, excluding large parts of the population [128, 129, 153]. Tumor escape through loss of antigen is a common problem to both CAR-based and TCR-based T cell therapies, but CAR-T-cells have the advantage of not being impacted by defects in HLA presentation since they are not HLA-dependent, contrary to TCR therapies. A partial solution to loss of antigen is to create therapies targeting several antigens [154]. Another solution already mentioned above is to add CARs to NK or CIK cells. While TCR and CAR-engineered T cells are of great interest, TCR- and CAR-engineered NK or CIK cells are an interesting alternative [155]. With NK cells, there is a lesser need for HLA compatibility, allowing the use of allogeneic NK cells for “off-the-shelf” manufacturing with reduced alloreactivity issues. They have less toxicity (no cytokine release syndrome), a better persistence, and they retain antitumoral effect through their innate cytotoxic activity in case of tumor escape through antigen loss [156]. Drawbacks are the difficulties encountered for isolation, expansion and transfection of NK cells. CAR-NK cells are under development with ongoing studies investigating CARs against PDL1, MUC1, mesothelin [52], etc. Research teams have also created CIK cells with a CEA-CAR for colon carcinoma [157] and CD44v6-CAR for soft tissue sarcomas [158], both appearing to yield better results than non-modified CIK cells. No study has been published as of yet in thoracic malignancy using CAR-NK or CAR-CIK cells.
Other modifications
To improve persistence and efficacy, T cells can be modified to express different molecules besides TCR or CAR engineering. To address the issue of T cells trafficking, T cells can be modified to express chemokine receptors. In preclinical models of MPM, adding the chemokine receptor CCR2b to anti-mesothelin CAR-T-cells led to a 12.5-fold increase in T-cell tumor infiltration, which in turn increased antitumor activity [159]. Trafficking can also be improved by regional delivery. Intrapleural administration of adoptive T cell therapy directly into the pleura for MPM bypasses pulmonary sequestration and increases infiltration rate [140, 160]. Efficacy of effector cells in the TME can also be improved through genetic modifications. For example, engineering IL-12 secretion into CD8 + T cells improves their efficacy in a melanoma mouse model [161]. IL-12 acts by reprogramming suppressive immune cells, attenuating the inhibiting effects of cells such as MDSCs on effector cells and leading to an increase in the percentage of active CD8 + T cells [162]. Another research team suggested that IL-12 can allow CAR-T-cells to retain an antitumoral activity even after tumor antigen loss through recruitment of macrophages with an antitumoral phenotype [120].
T cells can also be modified to be less susceptible to inhibition. For example, the PD-1 gene can be removed using CRISPR-Cas9 editing [163, 164]. In heavily pre-treated NSCLC (at least 3 lines of treatment) with PDL-1 expression on tumor cells, knock-out of PD-1 using CRISPR-Cas9 was judged safe with no grade 3 or 4 toxicity [164]. Grade 2 events consisted of lymphopenia and neutropenia and only occurred in one patient. However, there was no signal for efficacy either, even though persistence seemed good. Disease control rate at 8 weeks was 16.7%, and objective response rate was null. This study underlines preparation time and its limits. Indeed, preparation of T cells for reinfusion spanned from 17 to 40 days with a median time of 25 days, leading to 4 exclusions of patients either because of progressive disease or because of intercurrent acute pathology. Interestingly, of 22 enrolled patients, 5 had insufficient expansion of T cells for treatment, underlining another limit of adoptive therapies using autologous cells in clinical practice. Another noteworthy manipulation of PD-1 has been experimented in mice with a switch receptor [165]. A chimeric receptor has been transduced into CAR-T-cells with a truncated extracellular domain of PD-1 and a transmembrane and signaling domain of CD28, which is a stimulatory receptor. Binding with PDL-1 would then lead to stimulation instead of anergy.
The goal of these different methods is to solve the different limitations that adoptive therapies face, such as trafficking to the tumor site, persistence, maintaining an antitumoral activity, fighting against an immunosuppressive TME. They are often combined with TCR or CAR engineering [131].
Combinations
While all the adoptive therapies mentioned previously offer attractive options to improve the immune response toward cancer cells, they often have a disappointing efficacy in phase II studies. Improving one part of the immune response without acting on other opposing parts might not be enough. By way of example, adding T cells without inhibition of the PDL-1/PD-1 axis and/or immunosuppressive immune cells decreases their potential activity. With this reasoning in mind, many recent studies on adoptive therapies focus on combinations between different modalities of treatment.
Combinations between immune-based therapies
Melanoma is currently the solid tumor type with the most positive results in immunotherapy clinical trials, so it is a good example of what can be done with adoptive therapies, ICBs, and other kinds of immunotherapy. Many clinical trials are currently investigating combinations, and preliminary results indicate better results than any of these treatments taken alone [166]. However, close monitoring of adverse events is necessary, toxicity and autoimmunity increasing with combinations.
Combinations with immune checkpoint inhibitors are largely studied [50, 70, 72, 125], since the efficacy of adoptive T cell therapy might be compromised by the expression of suppressive immune checkpoints and conversely, immune checkpoint blockers alone might not have an effect in the absence of immune effector cells in sufficient quantity. Treatments to prime and recruit T cells like DC vaccines could benefit from the addition of molecules decreasing immunosuppression like ICBs [7].
Some adoptive therapies like DCs and CIK are already often used in association in clinical trials as was described above. Adoptive therapies using different kinds of immune cells can have synergistic results. DC vaccines increase antigen presentation and T-cell priming. T-cell adoptive therapies increase the pool of TILs and their cytotoxicity.
Many other therapies targeting the immune microenvironment exist and could be combined with adoptive therapies and ICBs depending on the tumor phenotype. Comprehensive reviews describe these different options [7].
Combinations with radiotherapy, chemotherapy, and targeted therapies
Combinations of “classic” cancer treatments with immunotherapies have now become the reference in lung cancer. Worldwide recommendations place a combination of chemotherapy and immune checkpoint blockers as first-line therapy in NSCLC and SCLC, and studies are currently investigating this option in MPM.
Chemotherapy such as docetaxel are known to decrease MDSCs. This is particularly interesting when taking into the account the fact that the infusion of non-myeloablative chemotherapeutic agents often used before infusion of adoptive therapy is followed by a rapid Treg and MDSC reconstitution with an enhanced immunosuppressive activity. Docetaxel could improve the efficacy of adoptive T cell transfer, CIK cells and DC immunotherapy [167]. Chemotherapy is also known to increase antigen presentation [168], and studies suggest that chemotherapies such as gemcitabine induce MICA/MICB expression on tumor cells. This can potentially have a synergistic effect with adoptive therapies using NK cells or NKG2D-expressing T cells [169].
Radiotherapy is known to increase the penetrance of immune cells into the tumor and antigen presentation [168]. Indeed, the growing use of radiotherapy and ICBs had led to the observation of better outcomes on ICB treatment when radiotherapy was used. And this not only in the irradiated region but in the whole body, which is called the “abscopal effect.” One of the main hypothesis is that radiotherapy increases neoantigen presentation, hence increasing T cell stimulation, since the main predictors for abscopal effect are an increased serum IFNβ after radiation and early dynamic changes of T cell clones [170].
Targeted therapies against oncogenes known to induce an immunosuppressive TME such as EGFR could also improve the outcome of adoptive therapies. Monoclonal antibodies like trastuzumab, rituximab or cetuximab can also pave the way for NK or CIK cell therapy. Indeed, through their CD16 receptors these cells can recognize the Fab portion of the antibodies and induce antibody-dependent cell-mediated cytotoxicity (ADCC). Combinations between already used specific antibodies and adoptive therapy with NK or CIK cells could therefore improve clinical response [25].
Conclusion
Adoptive therapies are a promising treatment that could be particularly useful in patients who are resistant to ICBs. DC vaccines and adoptive T cell therapy, engineered or not, increase the pool of reactive T cells, with the possibility to target selected specific high-avidity antigens. CIK and NK cells increase the cytotoxicity against tumor cells, potentially overcoming a common escape mechanism that is loss of antigen expression.
However, results are disappointing in solid tumors like lung cancer and MPM for now, underlining the need for optimization before clinical application. It is a costly and time-consuming technique that would require a better selection of patients most susceptible to respond. Predictive markers of response are therefore sorely needed. Adoptive cells face many hurdles depending on the tumor immune phenotype: low persistence, low trafficking to tumor sites, inhibition of effector functions, global immunosuppression due to the tumor microenvironment, etc. Methods for cell selection, activation, expansion, and engineering are constantly evolving to overcome all these obstacles. Advances in gene editing and bioinformatics are a great step towards more efficient adoptive therapies, and results of trials using these new technologies (neoantigen selection, gene editing in engineered cells to not only express high-avidity TCRs or CARs but also to improve homing and persistence) are greatly awaited. Special attention should also be given to combination therapies as was already established for ICBs. Simultaneously increasing antigen presentation, effector cell infiltration, and decreasing immunosuppression would yield better results than any of these strategies used alone, placing adoptive therapies as a promising future cornerstone of cancer treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Footnotes
Précis: Research on adoptive cell therapies (ACT) has been ongoing for several decades, yielding varying results. We provide an overview of the state of ACT in thoracic malignancies with results from clinical trials and new leads to improve its efficacy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schabath MB, Cote ML (2019) Cancer progress and priorities: lung cancer. Cancer Epidemiol Prev Biomark. Am Assoc Cancer Res 28:1563–1579. [DOI] [PMC free article] [PubMed]
- 2.Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, et al. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25:4592–4602. doi: 10.1158/1078-0432.CCR-18-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remon J, Aldea M, Besse B, Planchard D, Reck M, Giaccone G et al (2021) Small cell lung cancer: a slightly less orphan disease after immunotherapy. Ann Oncol Off J Eur Soc Med Oncol. [DOI] [PubMed]
- 4.Scherpereel A, Mazieres J, Greillier L, Lantuejoul S, Dô P, Bylicki O, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20:239–253. doi: 10.1016/S1470-2045(18)30765-4. [DOI] [PubMed] [Google Scholar]
- 5.Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet Lond Engl. 2021;397:375–386. doi: 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Wang S, Zhou Q (2020) The resistance mechanisms of lung cancer immunotherapy. Front Oncol [Internet]. Frontiers; 2020 [cited 2021 Marcs 21];10. 10.3389/fonc.2020.568059/full [DOI] [PMC free article] [PubMed]
- 7.Galon J, Bruni D (2019) Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 18:197–218. [DOI] [PubMed]
- 8.AbdulJabbar K, Raza SEA, Rosenthal R, Jamal-Hanjani M, Veeriah S, Akarca A, et al. Geospatial immune variability illuminates differential evolution of lung adenocarcinoma. Nat Med. 2020;26:1054–1062. doi: 10.1038/s41591-020-0900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bindea G, Mlecnik B, Galon J. Expand to shield: IL-15 and in situ lymphocytic proliferation. Oncoimmunology. 2021;10:1886726. doi: 10.1080/2162402X.2021.1886726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domen A, Quatannens D, Zanivan S, Deben C, Van Audenaerde J, Smits E et al (2021) Cancer-associated fibroblasts as a common orchestrator of therapy resistance in lung and pancreatic cancer. Cancers, 13. [DOI] [PMC free article] [PubMed]
- 14.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koulouris A, Tsagkaris C, Nikolaou M (2021) Real impact of novel immunotherapy drugs in cancer. The Experience of 10 Last Years. Toxins, p 13. [DOI] [PMC free article] [PubMed]
- 16.Gaissmaier L, Christopoulos P (2020) Immune modulation in lung cancer: current concepts and future strategies. Respir Int Rev Thorac Dis, pp 1–27. [DOI] [PubMed]
- 17.Kim JY, Lee KH, Eisenhut M, van der Vliet HJ, Kronbichler A, Jeong GH et al (2019) Efficacy of cancer immunotherapy: an umbrella review of meta-analyses of randomized controlled trials. Cancers [Internet]. 2019 [cited 2021 Mar 18];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6895783/ [DOI] [PMC free article] [PubMed]
- 18.Yu JX, Hubbard-Lucey VM, Tang J (2019) The global pipeline of cell therapies for cancer. Nat Rev Drug Discov 18:821–822. [DOI] [PubMed]
- 19.Pittari G, Filippini P, Gentilcore G, Grivel J-C, Rutella S. Revving up natural killer cells and cytokine-induced killer cells against hematological malignancies. Front Immunol. 2015;6:230. doi: 10.3389/fimmu.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 21.Kimura H, Yamaguchi Y. A phase III randomized study of interleukin-2 lymphokine-activated killer cell immunotherapy combined with chemotherapy or radiotherapy after curative or noncurative resection of primary lung carcinoma. Cancer. 1997;80:42–49. doi: 10.1002/(SICI)1097-0142(19970701)80:1<42::AID-CNCR6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Yano T, Sugio K, Yamazaki K, Kase S, Yamaguchi M, Ondo K, et al. Postoperative adjuvant adoptive immunotherapy with lymph node-LAK cells and IL-2 for pathologic stage I non-small cell lung cancer. Lung Cancer Amst Neth. 1999;26:143–148. doi: 10.1016/S0169-5002(99)00082-3. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Schmidt-Wolf IGH. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J Cell Physiol. 2020;235:9291–9303. doi: 10.1002/jcp.29827. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Mi Y, Guo N, Xu H, Xu L, Gou X, et al. Cytokine-induced killer cells as pharmacological tools for cancer immunotherapy. Front Immunol. 2017;8:774. doi: 10.3389/fimmu.2017.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garofano F, Gonzalez-Carmona MA, Skowasch D, Schmidt-Wolf R, Abramian A, Hauser S et al (2019) Clinical trials with combination of cytokine-induced killer cells and dendritic cells for cancer therapy. Int J Mol Sci [Internet]. 2019 [cited 2021 Mar 31];20. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6747410/ [DOI] [PMC free article] [PubMed]
- 27.Lin G, Wang J, Lao X, Wang J, Li L, Li S, et al. Interleukin-6 inhibits regulatory T cells and improves the proliferation and cytotoxic activity of cytokine-induced killer cells. J Immunother Hagerstown Md. 1997;2012(35):337–343. doi: 10.1097/CJI.0b013e318255ada3. [DOI] [PubMed] [Google Scholar]
- 28.Zoll B, Lefterova P, Ebert O, Huhn D, Von Ruecker A, Schmidt-Wolf IG. Modulation of cell surface markers on NK-like T lymphocytes by using IL-2, IL-7 or IL-12 in vitro stimulation. Cytokine. 2000;12:1385–1390. doi: 10.1006/cyto.2000.0733. [DOI] [PubMed] [Google Scholar]
- 29.Iudicone P, Fioravanti D, Cicchetti E, Bonanno G, Pandolfi A, Scocchera R, et al. Cytotoxic potential of interleukin-15 stimulated cytokine induced killer (CIK) against epithelial cancer cell lines. Cytotherapy. 2014;16:S23. doi: 10.1016/j.jcyt.2014.01.070. [DOI] [PubMed] [Google Scholar]
- 30.Wu C, Jiang J, Shi L, Xu N. Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer. Anticancer Res. 2008;28:3997–4002. [PubMed] [Google Scholar]
- 31.Li R, Wang C, Liu L, Du C, Cao S, Yu J, et al. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother CII. 2012;61:2125–2133. doi: 10.1007/s00262-012-1260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin C, Li J, Wang Y, Chen X, Che Y, Liu X, et al. Impact of cellular immune function on prognosis of lung cancer patients after cytokine-induced killer cell therapy. Asian Pac J Cancer Prev APJCP. 2014;15:6009–6014. doi: 10.7314/APJCP.2014.15.15.6009. [DOI] [PubMed] [Google Scholar]
- 33.Niu Q, Wang W, Li Y, Qin S, Wang Y, Wan G, et al. Cord blood-derived cytokine-induced killer cells biotherapy combined with second-line chemotherapy in the treatment of advanced solid malignancies. Int Immunopharmacol. 2011;11:449–456. doi: 10.1016/j.intimp.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Xiao Z, Wang C-Q, Zhou M-H, Li N-N, Liu S-Y, He Y-J, et al. Clinical efficacy and safety of CIK plus radiotherapy for lung cancer: a meta-analysis of 16 randomized controlled trials. Int Immunopharmacol. 2018;61:363–375. doi: 10.1016/j.intimp.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Xu Z, Chen X, Duan Y, Chen Z, Sun J. Clinical benefits of Livin peptide-loaded DCs/CIKs combined with chemotherapy in advanced non-small cell lung cancer. Am J Cancer Res. 2019;9:406–414. [PMC free article] [PubMed] [Google Scholar]
- 36.Shi S-B, Tang X-Y, Tian J, Chang C-X, Li P, Qi J-L. Efficacy of erlotinib plus dendritic cells and cytokine-induced killer cells in maintenance therapy of advanced non-small cell lung cancer. J Immunother Hagerstown Md. 1997;2014(37):250–255. doi: 10.1097/CJI.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 37.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16:7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 38.Nersesian S, Schwartz SL, Grantham SR, MacLean LK, Lee SN, Pugh-Toole M et al (2021) NK cell infiltration is associated with improved overall survival in solid cancers: a systematic review and meta-analysis. Transl Oncol 14:100930. [DOI] [PMC free article] [PubMed]
- 39.Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19:200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 40.Bryceson YT, March ME, Ljunggren H-G, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33:74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209:2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol Baltim Md. 1950;1999(162):4511–4520. [PubMed] [Google Scholar]
- 44.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 45.Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol. 2014;44:1582–1592. doi: 10.1002/eji.201344272. [DOI] [PubMed] [Google Scholar]
- 46.Poznanski SM, Ritchie TM, Fan IY, El-Sayes A, Portillo AL, Ben-Avi R et al (2021)Expanded human NK cells from lung cancer patients sensitize patients’ PDL1-negative tumors to PD1-blockade therapy. J Immunother Cancer, p 9. [DOI] [PMC free article] [PubMed]
- 47.Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 49.Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother CII. 2010;59:1781–1789. doi: 10.1007/s00262-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin M, Luo H, Liang S, Chen J, Liu A, Niu L, et al. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J Clin Invest. 2020;130:2560–2569. doi: 10.1172/JCI132712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin M, Liang S-Z, Wang X-H, Liang Y-Q, Zhang M-J, Niu L-Z, et al. Clinical efficacy of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced non-small cell lung cancer. Immunol Res. 2017;65:880–887. doi: 10.1007/s12026-017-8927-x. [DOI] [PubMed] [Google Scholar]
- 52.Franks SE, Wolfson B, Hodge JW (2020) Natural born killers: NK cells in cancer therapy. Cancers [Internet]. 2020 [cited 2021 Apr 4];12. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7465121/ [DOI] [PMC free article] [PubMed]
- 53.Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–1570. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 54.Multhoff G, Seier S, Stangl S, Sievert W, Shevtsov M, Werner C, et al. Targeted natural killer cell-based adoptive immunotherapy for the treatment of patients with NSCLC after radiochemotherapy: a randomized Phase II Clinical Trial. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26:5368–5379. doi: 10.1158/1078-0432.CCR-20-1141. [DOI] [PubMed] [Google Scholar]
- 55.Chawla SP, Kim KM, Chua VS, Jafari O, Song PY. Phase I study of SNK01 (autologous non-genetically modified natural killer cells with enhanced cytotoxicity) in refractory metastatic solid tumors. J Clin Oncol. 2020;38:e15024–e15024. doi: 10.1200/JCO.2020.38.15_suppl.e15024. [DOI] [Google Scholar]
- 56.Kim EJ, Ko D-H, Ji W, Rho JK, Lee JC, Song PY, et al. A randomized phase I/IIa study to evaluate the safety and efficacy of SNK01 (non-genetically modified autologous natural killer cells with enhanced cytotoxicity) plus pembrolizumab in patients with stage IV non-small cell lung cancer. J Clin Oncol. 2020;38:3037–3037. doi: 10.1200/JCO.2020.38.15_suppl.3037. [DOI] [Google Scholar]
- 57.Topalian SL, Solomon D, Avis FP, Chang AE, Freerksen DL, Linehan WM, et al. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Clin Oncol Off J Am Soc Clin Oncol. 1988;6:839–853. doi: 10.1200/JCO.1988.6.5.839. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res Off J Am Assoc Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dafni U, Michielin O, Lluesma SM, Tsourti Z, Polydoropoulou V, Karlis D, et al. Efficacy of adoptive therapy with tumor-infiltrating lymphocytes and recombinant interleukin-2 in advanced cutaneous melanoma: a systematic review and meta-analysis. Ann Oncol Off J Eur Soc Med Oncol. 2019;30:1902–1913. doi: 10.1093/annonc/mdz398. [DOI] [PubMed] [Google Scholar]
- 60.Simoni Y, Becht E, Fehlings M, Loh CY, Koo S-L, Teng KWW, et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 61.Janelle V, Delisle J-S (2021) T-Cell Dysfunction as a limitation of adoptive immunotherapy: current concepts and mitigation strategies. Cancers [Internet]. 2021 [cited 2021 Apr 5];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7913380/ [DOI] [PMC free article] [PubMed]
- 62.Morotti M, Albukhari A, Alsaadi A, Artibani M, Brenton JD, Curbishley SM et al (2021) Promises and challenges of adoptive T-cell therapies for solid tumours. Br J Cancer, pp 1–18. [DOI] [PMC free article] [PubMed]
- 63.Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9:2724. doi: 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez-Usatorre A, Carmona SJ, Godfroid C, Yacoub Maroun C, Labiano S, Romero P. Enhanced Phenotype definition for precision isolation of precursor exhausted tumor-infiltrating CD8 T cells. Front Immunol. 2020;11:340. doi: 10.3389/fimmu.2020.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, et al. Increased intensity lymphodepletion and adoptive immunotherapy–-how far can we go? Nat Clin Pract Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pedersen SR, Sørensen MR, Buus S, Christensen JP, Thomsen AR. Comparison of vaccine-induced effector CD8 T cell responses directed against self- and non-self-tumor antigens: implications for cancer immunotherapy. J Immunol Baltim Md. 1950;2013(191):3955–3967. doi: 10.4049/jimmunol.1300555. [DOI] [PubMed] [Google Scholar]
- 68.Kradin RL, Kurnick JT, Lazarus DS, Preffer FI, Dubinett SM, Pinto CE, et al. Tumour-infiltrating lymphocytes and interleukin-2 in treatment of advanced cancer. Lancet Lond Engl. 1989;1:577–580. doi: 10.1016/S0140-6736(89)91609-7. [DOI] [PubMed] [Google Scholar]
- 69.Ratto GB, Zino P, Mirabelli S, Minuti P, Aquilina R, Fantino G, et al. A randomized trial of adoptive immunotherapy with tumor-infiltrating lymphocytes and interleukin-2 versus standard therapy in the postoperative treatment of resected nonsmall cell lung carcinoma. Cancer. 1996;78:244–251. doi: 10.1002/(SICI)1097-0142(19960715)78:2<244::AID-CNCR9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 70.Chesney JA, Lutzky J, Thomas SS, Nieva JJ, Munoz Couselo E, Martin-Liberal J et al (2019) A phase II study of autologous tumor infiltrating lymphocytes (TIL, LN-144/LN-145) in patients with solid tumors. J Clin Oncol 37:TPS2648–TPS2648.
- 71.Massarelli E, Goldberg Z, Cacovean A, Yadav B, Chen G, Jagasia M, et al. 188TiP A phase II multicenter study of autologous tumor infiltrating lymphocytes (TIL, LN-145) cell therapy in patients with metastatic non-small cell lung cancer (mNSCLC) J Thorac Oncol. 2021;16:S800. doi: 10.1016/S1556-0864(21)02030-X. [DOI] [Google Scholar]
- 72.Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021;27:1410–1418. doi: 10.1038/s41591-021-01462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Besser MJ, Itzhaki O, Ben-Betzalel G, Zippel DB, Zikich D, Kubi A, et al. Comprehensive single institute experience with melanoma TIL: Long term clinical results, toxicity profile, and prognostic factors of response. Mol Carcinog. 2020;59:736–744. doi: 10.1002/mc.23193. [DOI] [PubMed] [Google Scholar]
- 74.Ben-Avi R, Farhi R, Ben-Nun A, Gorodner M, Greenberg E, Markel G, et al. Establishment of adoptive cell therapy with tumor infiltrating lymphocytes for non-small cell lung cancer patients. Cancer Immunol Immunother CII. 2018;67:1221–1230. doi: 10.1007/s00262-018-2174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carretero R, Romero JM, Ruiz-Cabello F, Maleno I, Rodriguez F, Camacho FM, et al. Analysis of HLA class I expression in progressing and regressing metastatic melanoma lesions after immunotherapy. Immunogenetics. 2008;60:439–447. doi: 10.1007/s00251-008-0303-5. [DOI] [PubMed] [Google Scholar]
- 76.Perea F, Sánchez-Palencia A, Gómez-Morales M, Bernal M, Concha Á, García MM, et al. HLA class I loss and PD-L1 expression in lung cancer: impact on T-cell infiltration and immune escape. Oncotarget. 2018;9:4120–4133. doi: 10.18632/oncotarget.23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pulido M, Chamorro V, Romero I, Algarra I, S-Montalvo A, Collado A et al (2020) Restoration of MHC-I on tumor cells by fhit transfection promotes immune rejection and acts as an individualized immunotherapeutic vaccine. Cancers, 12. [DOI] [PMC free article] [PubMed]
- 78.Lauss M, Donia M, Harbst K, Andersen R, Mitra S, Rosengren F, et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat Commun. 2017;8:1738. doi: 10.1038/s41467-017-01460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arina A, Schreiber K, Binder DC, Karrison TG, Liu RB, Schreiber H. Adoptively transferred immune T cells eradicate established tumors despite cancer-induced immune suppression. J Immunol Baltim Md. 1950;2014(192):1286–1293. doi: 10.4049/jimmunol.1202498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arina A, Bronte V. Myeloid-derived suppressor cell impact on endogenous and adoptively transferred T cells. Curr Opin Immunol. 2015;33:120–125. doi: 10.1016/j.coi.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 81.Stevens D, Ingels J, Van Lint S, Vandekerckhove B, Vermaelen K (2020) Dendritic cell-based immunotherapy in lung cancer. Front Immunol 11:620374. [DOI] [PMC free article] [PubMed]
- 82.Zhang R, Yuan F, Shu Y, Tian Y, Zhou B, Yi L, et al. Personalized neoantigen-pulsed dendritic cell vaccines show superior immunogenicity to neoantigen-adjuvant vaccines in mouse tumor models. Cancer Immunol Immunother CII. 2020;69:135–145. doi: 10.1007/s00262-019-02448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 84.Thomas A, Giaccone G. Why has active immunotherapy not worked in lung cancer? Ann Oncol Off J Eur Soc Med Oncol. 2015;26:2213–2220. doi: 10.1093/annonc/mdv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laborde RR, Lin Y, Gustafson MP, Bulur PA, Dietz AB. Cancer vaccines in the world of immune suppressive monocytes (CD14(+)HLA-DR(lo/neg) Cells): the gateway to improved responses. Front Immunol. 2014;5:147. doi: 10.3389/fimmu.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bonehill A, Van Nuffel AMT, Corthals J, Tuyaerts S, Heirman C, Francois V, et al. Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2009;15:3366–3375. doi: 10.1158/1078-0432.CCR-08-2982. [DOI] [PubMed] [Google Scholar]
- 87.Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 88.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ding Z, Li Q, Zhang R, Xie L, Shu Y, Gao S, et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct Target Ther. 2021;6:26. doi: 10.1038/s41392-020-00448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang G-C, Lan H-C, Juang S-H, Wu Y-C, Lee H-C, Hung Y-M, et al. A pilot clinical trial of vaccination with dendritic cells pulsed with autologous tumor cells derived from malignant pleural effusion in patients with late-stage lung carcinoma. Cancer. 2005;103:763–771. doi: 10.1002/cncr.20843. [DOI] [PubMed] [Google Scholar]
- 91.Um S-J, Choi YJ, Shin H-J, Son CH, Park Y-S, Roh MS, et al. Phase I study of autologous dendritic cell tumor vaccine in patients with non-small cell lung cancer. Lung Cancer Amst Neth. 2010;70:188–194. doi: 10.1016/j.lungcan.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 92.Cornelissen R, Hegmans JPJJ, Maat APWM, Kaijen-Lambers MEH, Bezemer K, Hendriks RW, et al. Extended tumor control after dendritic cell vaccination with low-dose cyclophosphamide as adjuvant treatment in patients with malignant pleural mesothelioma. Am J Respir Crit Care Med. 2016;193:1023–1031. doi: 10.1164/rccm.201508-1573OC. [DOI] [PubMed] [Google Scholar]
- 93.Aerts JGJV, de Goeje PL, Cornelissen R, Kaijen-Lambers MEH, Bezemer K, van der Leest CH, et al. Autologous dendritic cells pulsed with allogeneic tumor cell lysate in mesothelioma: from mouse to human. Clin Cancer Res. 2018;24:766–776. doi: 10.1158/1078-0432.CCR-17-2522. [DOI] [PubMed] [Google Scholar]
- 94.on behalf of the DENIM team*, Belderbos RA, Baas P, Berardi R, Cornelissen R, Fennell DA et al (2019) A multicenter, randomized, phase II/III study of dendritic cells loaded with allogeneic tumor cell lysate (MesoPher) in subjects with mesothelioma as maintenance therapy after chemotherapy: DENdritic cell Immunotherapy for Mesothelioma (DENIM) trial. Transl Lung Cancer Res 8:280–285. [DOI] [PMC free article] [PubMed]
- 95.de Boer NL, van Kooten JP, Burger JWA, Verhoef C, Aerts JGJV, Madsen EVE (2019) Adjuvant dendritic cell based immunotherapy (DCBI) after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal mesothelioma, a phase II single centre open-label clinical trial: rationale and design of the MESOPEC trial. BMJ Open 9:e026779. [DOI] [PMC free article] [PubMed]
- 96.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosenthal R, Cadieux EL, Salgado R, Bakir MA, Moore DA, Hiley CT, et al. Neoantigen-directed immune escape in lung cancer evolution. Nature. 2019;567:479–485. doi: 10.1038/s41586-019-1032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci U S A. 2001;98:8809–8814. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Itoh T, Ueda Y, Kawashima I, Nukaya I, Fujiwara H, Fuji N, et al. Immunotherapy of solid cancer using dendritic cells pulsed with the HLA-A24-restricted peptide of carcinoembryonic antigen. Cancer Immunol Immunother CII. 2002;51:99–106. doi: 10.1007/s00262-001-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ueda Y, Itoh T, Nukaya I, Kawashima I, Okugawa K, Yano Y, et al. Dendritic cell-based immunotherapy of cancer with carcinoembryonic antigen-derived, HLA-A24-restricted CTL epitope: clinical outcomes of 18 patients with metastatic gastrointestinal or lung adenocarcinomas. Int J Oncol. 2004;24:909–917. [PubMed] [Google Scholar]
- 102.Kontani K, Taguchi O, Ozaki Y, Hanaoka J, Sawai S, Inoue S, et al. Dendritic cell vaccine immunotherapy of cancer targeting MUC1 mucin. Int J Mol Med. 2003;12:493–502. [PubMed] [Google Scholar]
- 103.Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 104.Perroud MW, Honma HN, Barbeiro AS, Gilli SC, Almeida MT, Vassallo J, et al. Mature autologous dendritic cell vaccines in advanced non-small cell lung cancer: a phase I pilot study. J Exp Clin Cancer Res CR. 2011;30:65. doi: 10.1186/1756-9966-30-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirschowitz EA, Foody T, Kryscio R, Dickson L, Sturgill J, Yannelli J. Autologous dendritic cell vaccines for non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22:2808–2815. doi: 10.1200/JCO.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 106.Ge C, Li R, Song H, Geng T, Yang J, Tan Q, et al. Phase I clinical trial of a novel autologous modified-DC vaccine in patients with resected NSCLC. BMC Cancer. 2017;17:884. doi: 10.1186/s12885-017-3859-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 108.Saka H, Kitagawa C, Ichinose Y, Takenoyama M, Ibata H, Kato T, et al. A randomized phase II study to assess the effect of adjuvant immunotherapy using α-GalCer-pulsed dendritic cells in the patients with completely resected stage II-IIIA non-small cell lung cancer: study protocol for a randomized controlled trial. Trials. 2017;18:429. doi: 10.1186/s13063-017-2103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee JM, Lee M-H, Garon E, Goldman JW, Salehi-Rad R, Baratelli FE, et al. Phase I trial of intratumoral injection of ccl21 gene-modified dendritic cells in lung cancer elicits tumor-specific immune responses and CD8+ T-cell infiltration. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23:4556–4568. doi: 10.1158/1078-0432.CCR-16-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pitt JM, Charrier M, Viaud S, André F, Besse B, Chaput N, et al. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J Immunol Baltim Md. 1950;2014(193):1006–1011. doi: 10.4049/jimmunol.1400703. [DOI] [PubMed] [Google Scholar]
- 111.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Viaud S, Terme M, Flament C, Taieb J, André F, Novault S et al (2009) Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PloS One 4:e4942. [DOI] [PMC free article] [PubMed]
- 113.Escudier B, Dorval T, Chaput N, André F, Caby M-P, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D et al (2016) Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 5:e1071008. [DOI] [PMC free article] [PubMed]
- 116.Viaud S, Ploix S, Lapierre V, Théry C, Commere P-H, Tramalloni D, et al. Updated technology to produce highly immunogenic dendritic cell-derived exosomes of clinical grade: a critical role of interferon-γ. J Immunother Hagerstown Md. 1997;2011(34):65–75. doi: 10.1097/CJI.0b013e3181fe535b. [DOI] [PubMed] [Google Scholar]
- 117.Takahashi H, Shimodaira S, Ogasawara M, Ota S, Kobayashi M, Abe H, et al. Lung adenocarcinoma may be a more susceptive subtype to a dendritic cell-based cancer vaccine than other subtypes of non-small cell lung cancers: a multicenter retrospective analysis. Cancer Immunol Immunother CII. 2016;65:1099–1111. doi: 10.1007/s00262-016-1872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Teramoto K, Ozaki Y, Hanaoka J, Sawai S, Tezuka N, Fujino S, et al. Predictive biomarkers and effectiveness of MUC1-targeted dendritic-cell-based vaccine in patients with refractory non-small cell lung cancer. Ther Adv Med Oncol. 2017;9:147–157. doi: 10.1177/1758834016678375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Manfredi F, Cianciotti BC, Potenza A, Tassi E, Noviello M, Biondi A et al (2020)TCR Redirected T Cells for cancer treatment: achievements, hurdles, and goals. Front Immunol [Internet]. 2020 [cited 2021 Apr 6];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7494743/ [DOI] [PMC free article] [PubMed]
- 120.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 121.Rath JA, Arber C (2020) Engineering strategies to enhance TCR-based adoptive T cell therapy. Cells [Internet]. 2020 [cited 2021 Apr 5];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7349724/ [DOI] [PMC free article] [PubMed]
- 122.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Creelan BC, Gainor JF, Govindan R, Hardy NM, Heymach J, Mudad R et al (2017) Two phase I/II open label clinical trials evaluating the safety and efficacy of autologous T cells expressing enhanced TCRs specific for NY-ESO-1 or MAGE-A10 in subjects with stage IIIb or stage IV non-small cell lung cancer (NCT02588612/NCT02592577). J Clin Oncol 35:TPS3096–TPS3096.
- 125.Reckamp KL, Akerley W, Edelman MJ, Halmos B, He K, Johnson M et al (2019) Abstract CT225: A Phase Ib/IIa randomized pilot study to investigate the safety and tolerability of autologous T-cells with enhanced T-cell receptors specific to NY-ESO-1/LAGE-1a (GSK3377794) alone, or in combination with pembrolizumab, in advanced non-small cell lung cancer. Clin Trials [Internet]. American Association for Cancer Research; 2019 [cited 2021 Mar 26]. p. CT225–CT225. Available from: http://cancerres.aacrjournals.org/lookup/doi/10.1158/1538-7445.AM2019-CT225
- 126.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan D-AN, Feldman SA et al (2011)T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther J Am Soc Gene Ther 19:620–626. [DOI] [PMC free article] [PubMed]
- 127.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tsimberidou A-M, Ma H, Stewart C, Schoor O, Maurer D, Mendrzyk R et al (2018) Phase I adoptive cellular therapy trial with ex-vivo stimulated autologous CD8+ T-cells against multiple targets (ACTolog® IMA101) in patients with relapsed and/or refractory solid cancers. Ann Oncol 29:viii411.
- 129.George R. Blumenschein AB (2021) The University of Texas MD Anderson Cancer Center H, Immatics US I, MD Anderson Cancer Center H. Phase I trial evaluating genetically modified autologous T cells (ACTengine IMA201) expressing a T-cell receptor recognizing a cancer/germline antigen in patients with squamous NSCLC or HNSCC. [cited 2021 Mar 29]; Available from: https://meetinglibrary.asco.org/record/156705/abstract
- 130.Arnaud M, Duchamp M, Bobisse S, Renaud P, Coukos G, Harari A. Biotechnologies to tackle the challenge of neoantigen identification. Curr Opin Biotechnol. 2020;65:52–59. doi: 10.1016/j.copbio.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 131.Gaissmaier L, Elshiaty M, Christopoulos P (2020) Breaking bottlenecks for the TCR therapy of cancer. Cells, 9. [DOI] [PMC free article] [PubMed]
- 132.Ali M, Foldvari Z, Giannakopoulou E, Böschen M-L, Strønen E, Yang W, et al. Induction of neoantigen-reactive T cells from healthy donors. Nat Protoc. 2019;14:1926–1943. doi: 10.1038/s41596-019-0170-6. [DOI] [PubMed] [Google Scholar]
- 133.Roth TL, Puig-Saus C, Yu R, Shifrut E, Carnevale J, Li PJ, et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018;559:405–409. doi: 10.1038/s41586-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K et al (2014) Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6:224ra25. [DOI] [PMC free article] [PubMed]
- 136.Sj S, Mr B, Cs T, Ek W, P B, Jp M et al (2019) Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med [Internet]. N Engl J Med; 2019 [cited 2021 Mar 25];380. Available from: https://pubmed.ncbi.nlm.nih.gov/30501490/ [DOI] [PubMed]
- 137.Klein C, Jamois C, Nielsen T. Anti-CD20 treatment for B-cell malignancies: current status and future directions. Expert Opin Biol Ther. 2021;21:161–181. doi: 10.1080/14712598.2020.1822318. [DOI] [PubMed] [Google Scholar]
- 138.Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15:1145–1154. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 139.Specht JM, Lee S, Turtle C, Berger C, Veatch J, Gooley T et al (2018) Phase I study of immunotherapy for advanced ROR1+ malignancies with autologous ROR1-specific chimeric antigen receptor-modified (CAR)-T cells. J Clin Oncol 36:TPS79–TPS79.
- 140.Adusumilli PS, Zauderer MG, Rusch VW, O’Cearbhaill R, Zhu A, Ngai D, et al. Regional delivery of mesothelin-targeted CAR T cells for pleural cancers: Safety and preliminary efficacy in combination with anti-PD-1 agent. J Clin Oncol. 2019;37:2511–2511. doi: 10.1200/JCO.2019.37.15_suppl.2511. [DOI] [Google Scholar]
- 141.Hiltbrunner S, Britschgi C, Schuberth P, Bankel L, Nguyen-Kim TDL, Gulati P, et al. Local delivery of CAR T cells targeting fibroblast activation protein is safe in patients with pleural mesothelioma: first report of FAPME, a phase I clinical trial. Ann Oncol Off J Eur Soc Med Oncol. 2021;32:120–121. doi: 10.1016/j.annonc.2020.10.474. [DOI] [PubMed] [Google Scholar]
- 142.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Riaz IB, Zahid U, Kamal MU, Husnain M, McBride A, Hua A, et al. Anti-CD 19 and anti-CD 20 CAR-modified T cells for B-cell malignancies: a systematic review and meta-analysis. Immunotherapy. 2017;9:979–993. doi: 10.2217/imt-2017-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Long KB, Young RM, Boesteanu AC, Davis MM, Melenhorst JJ, Lacey SF, et al. CAR T cell therapy of non-hematopoietic malignancies: detours on the road to clinical success. Front Immunol. 2018;9:2740. doi: 10.3389/fimmu.2018.02740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moon EK, Wang L-C, Dolfi DV, Wilson CB, Ranganathan R, Sun J, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20:4262–4273. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jafarzadeh L, Masoumi E, Fallah-Mehrjardi K, Mirzaei HR, Hadjati J. Prolonged Persistence of Chimeric Antigen Receptor (CAR) T cell in adoptive cancer immunotherapy: challenges and ways forward. Front Immunol. 2020;11:702. doi: 10.3389/fimmu.2020.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McLellan AD, Ali Hosseini Rad SM (2019) Chimeric antigen receptor T cell persistence and memory cell formation. Immunol Cell Biol 97:664–674. [DOI] [PubMed]
- 148.Feng K, Guo Y, Dai H, Wang Y, Li X, Jia H, et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci. 2016;59:468–479. doi: 10.1007/s11427-016-5023-8. [DOI] [PubMed] [Google Scholar]
- 149.Srivastava S, Furlan SN, Jaeger-Ruckstuhl CA, Sarvothama M, Berger C, Smythe KS, et al. Immunogenic chemotherapy enhances recruitment of CAR-T cells to lung tumors and improves antitumor efficacy when combined with checkpoint blockade. Cancer Cell. 2021;39:193–208.e10. doi: 10.1016/j.ccell.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu H, Ma Y, Yang C, Xia S, Pan Q, Zhao H et al (2020) Severe delayed pulmonary toxicity following PD-L1-specific CAR-T cell therapy for non-small cell lung cancer. Clin Transl Immunol 9:e1154. [DOI] [PMC free article] [PubMed]
- 151.Haas AR, Tanyi JL, O’Hara MH, Gladney WL, Lacey SF, Torigian DA, et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol Ther J Am Soc Gene Ther. 2019;27:1919–1929. doi: 10.1016/j.ymthe.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harris DT, Kranz DM. Adoptive T cell therapies: a comparison of T cell receptors and chimeric antigen receptors. Trends Pharmacol Sci. 2016;37:220–230. doi: 10.1016/j.tips.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ellis JM, Henson V, Slack R, Ng J, Hartzman RJ, Katovich Hurley C (2000) Frequencies of HLA-A2 alleles in five U.S. population groups. Predominance Of A*02011 and identification of HLA-A*0231. Hum Immunol 61:334–40. [DOI] [PubMed]
- 154.Kaluza KM, Kottke T, Diaz RM, Rommelfanger D, Thompson J, Vile R. Adoptive transfer of cytotoxic T lymphocytes targeting two different antigens limits antigen loss and tumor escape. Hum Gene Ther. 2012;23:1054–1064. doi: 10.1089/hum.2012.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mensali N, Dillard P, Hebeisen M, Lorenz S, Theodossiou T, Myhre MR, et al. NK cells specifically TCR-dressed to kill cancer cells. EBioMedicine. 2019;40:106–117. doi: 10.1016/j.ebiom.2019.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J (2020) CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine 59:102975. [DOI] [PMC free article] [PubMed]
- 157.Schlimper C, Hombach AA, Abken H, Schmidt-Wolf IGH. Improved activation toward primary colorectal cancer cells by antigen-specific targeting autologous cytokine-induced killer cells. Clin Dev Immunol. 2012;2012:1–8. doi: 10.1155/2012/238924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Leuci V, Casucci M, Grignani G, Rotolo R, Rossotti U, Vigna E et al (2018) CD44v6 as innovative sarcoma target for CAR-redirected CIK cells. OncoImmunology 7:e1423167. [DOI] [PMC free article] [PubMed]
- 159.Moon EK, Carpenito C, Sun J, Wang L-CS, Kapoor V, Predina J et al (2011) Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res Off J Am Assoc Cancer Res 17:4719–4730. [DOI] [PMC free article] [PubMed]
- 160.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J et al (2014) Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 6:261ra151. [DOI] [PMC free article] [PubMed]
- 161.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Steding CE, Wu S, Zhang Y, Jeng M-H, Elzey BD, Kao C. The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology. 2011;133:221–238. doi: 10.1111/j.1365-2567.2011.03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E et al (2020) CRISPR-engineered T cells in patients with refractory cancer. Science, p 367. [DOI] [PMC free article] [PubMed]
- 164.Lu Y, Xue J, Deng T, Zhou X, Yu K, Deng L, et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med. 2020;26:732–740. doi: 10.1038/s41591-020-0840-5. [DOI] [PubMed] [Google Scholar]
- 165.Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, et al. A Chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 2016;76:1578–1590. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Foley KC, Nishimura MI, Moore TV. Combination immunotherapies implementing adoptive T-cell transfer for advanced-stage melanoma. Melanoma Res. 2018;28:171–184. doi: 10.1097/CMR.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kodumudi KN, Weber A, Sarnaik AA, Pilon-Thomas S. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J Immunol Baltim Md. 1950;2012(189):5147–5154. doi: 10.4049/jimmunol.1200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kershaw MH, Devaud C, John LB, Westwood JA, Darcy PK (2013) Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. Oncoimmunology 2:e25962. [DOI] [PMC free article] [PubMed]
- 169.Morisaki T, Onishi H, Koya N, Kiyota A, Tanaka H, Umebayashi M, et al. Combinatorial cytotoxicity of gemcitabine and cytokine-activated killer cells in hepatocellular carcinoma via the NKG2D-MICA/B system. Anticancer Res. 2011;31:2505–2510. [PubMed] [Google Scholar]
- 170.Formenti SC, Rudqvist N-P, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24:1845–1851. doi: 10.1038/s41591-018-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.