Abstract
Background
Despite the remarkable clinical advance of immune checkpoint inhibitors (ICIs) in the treatment of lung cancer, there are limited studies focused on evaluating efficacy of ICIs for patients with human epidermal growth factor receptor 2 (HER2)-mutant lung adenocarcinoma.
Methods
We conducted a multicenter retrospective study of patients with HER2-mutant lung adenocarcinoma who received ICIs therapy at Shanghai Pulmonary Hospital, Shanghai Chest Hospital and the First Affiliated Hospital of Wenzhou Medical University between 2016 and 2021. Response was defined with reference to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.
Results
Among the 26 patients enrolled in our study, the overall objective response rate (ORR) was 38.5%, disease control rate (DCR) was 84.6% and median progression-free survival (PFS) was 7.4 months. Majority of patients were treated with immunochemotherapy combination regimens (16/26, 61.5%), with a median PFS of 8.4 months. Among the 9 patients receiving ICIs-based therapy as first-line treatment, 5 patients had partial response (PR) and 4 patients had stable disease (SD), with a median PFS of 9.1 months. Of the entire cohort, 5 patients who received ICIs before epidermal growth factor receptor (EGFR)/HER2-targeting drugs achieved a median PFS of 8.4 months.
Conclusion
Our retrospective study provides clinical evidence that front line of ICIs-based therapy is also worth considering for the treatment to improve survival outcomes of patients with HER2-mutant lung adenocarcinoma.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03100-5.
Keywords: HER2-mutant, Immune checkpoint inhibitors, Non-small cell lung cancer, Efficacy
Introduction
Over the last ten years, oncogene driver-based therapies have improved outcomes for advanced non-small cell lung cancer (NSCLC), such as targeting epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) and ROS proto-oncogene 1 (ROS1) [1]. However, it remains unavoidable development of resistance and tumor recurrence in this setting. Moreover, there are limited targeted therapies for patients with rare oncogenic drivers. Similar to EGFR, human epidermal growth factor receptor 2 (HER2) is a member of the human epidermal growth factor receptor (HER/EGFR/ERBB) family. Patients harboring HER2 mutations account for 1% to 4% in lung adenocarcinoma[2–5]. Nevertheless, despite HER2-targeting drugs such as pyrotinib and trastuzumab deruxtecan (T-DXd), have revealed promising efficacy in advanced NSCLC with HER2 mutations[6, 7], there are no targeted drugs approved for this patient population.
Besides these molecularly targeted therapies, Immune checkpoint inhibitors (ICIs) like programmed cell death protein 1 (PD-1) inhibitors and programmed cell death ligand 1 (PD-L1) inhibitors have been approved as a standard of care in treatment for advanced NSCLC. Yet, the efficacy of ICIs in oncogene driven NSCLC remains uncertain, as most clinical trials were conducted without patients harboring known oncogenic mutations[8, 9]. There are limited number of studies reporting the clinical benefits of ICIs in HER2-positive NSCLC[10].
In this real-world retrospective cohort study, our efforts focused on investigating the potential benefits of ICIs treatment in patients with lung adenocarcinoma who harboring HER2 mutation.
Material and methods
Patients
Records for patients with HER2-mutant lung adenocarcinoma who were treated with PD-1/PD-L1 inhibitors in Shanghai Pulmonary Hospital, Shanghai Chest Hospital and the First Affiliated Hospital of Wenzhou Medical University between 2016 and 2021 were reviewed. Patients were staged according to the eighth edition of the TNM staging system. Patients who had eligible imaging information for objective responses were enrolled. All of them received PD-1/PD-L1 inhibitors as monotherapy or in combination with chemotherapy or anti-angiogenesis or EGFR/HER2-targeting drugs regardless of treatment lines. Radiographic partial response (PR), stable disease (SD), and progression disease (PD) were defined with reference to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 [11]. The objective response rate (ORR) and disease control rate (DCR) were defined as complete response (CR) plus PR, and CR plus PR plus SD, respectively. Participating centers were in charge of obtaining informed patient consent and institutional approval.
Data collection
The recorded demographics and clinical characteristics included age, gender, smoking status, HER2 alteration types, PD-L1 status and treatment data. HER2 alteration was tested using amplification refractory mutation system (ARMS) and confirmed by DNA direct sequencing if necessary. PD-L1 status was assessed in formalin-fixed tumor samples according to local procedures. The follow-up end date was April 2021.
Statistical analysis
Progression-free survival (PFS) was calculated from initiation of ICIs treatment to disease progression or death from any causes. The Kaplan–Meier method was used to assess PFS for the entire cohort. Statistical analyses were performed with SPSS 23.0 (IBM, Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA).
Results
Patient characteristics
The analysis included 26 patients with HER2-mutant lung adenocarcinoma managed in 3 medical centers (Tables 1, 2). Patients received PD-1/PD-L1 inhibitors therapy, including monotherapy (n = 5), combination with chemotherapy (n = 16), combination with anti-angiogenesis therapy (n = 3), combination with chemotherapy plus anti-angiogenesis therapy (n = 1), and combination with chemotherapy plus HER2-targeting drugs (n = 1). Our cohort was presented with a median age of 55 years (range 38 to 69 years), a higher proportion of never-smokers (n = 18, 69.2%), and a slightly higher proportion of male (n = 14, 53.8%). Of the 15 patients whose PD-L1 status were available, 7 patients had a PD-L1 tumor proportion score greater than 1%. 3 patients had history of pulmonary resection for lung cancer. Besides, PD-1/PD-L1 inhibitors were treated as first-line treatment in 9 patients (34.6%), and as second-line or later-line treatment in 17 patients (65.4%).
Table 1.
Baseline characteristics of the entire cohort
| Variables | Treatment data | P | ||
|---|---|---|---|---|
| All (%) | Group A | Group B | ||
| (N = 26) | (N = 16) | (N = 10) | ||
| Median age, years (range) | 55 (38–69) | 58 (38–69) | 47.5 (40–68) | |
| Gender | 0.422 | |||
| Male | 14 (53.8) | 10 (62.5) | 4 (40) | |
| Female | 12 (46.2) | 6 (37.5) | 6 (60) | |
| Smoking history | 0.420 | |||
| Never smokers | 18 (69.2) | 10 (62.5) | 8 (80) | |
| Former or current smokers | 8 (30.8) | 6 (37.5) | 2 (20) | |
| ECOG PS | 0.138 | |||
| 0–1 | 24 (92.3) | 16 (100) | 8 (80) | |
| ≥ 2 | 2 (7.7) | 0 (0) | 2 (20) | |
| PD-L1 status | 0.474 | |||
| ≥ 50% | 1 (3.8) | 1 (6.3) | 0 (0) | |
| 1–49% | 6 (23.1) | 4 (25) | 2 (20) | |
| Negative | 8 (30.8) | 6 (37.5) | 2 (20) | |
| Unknown | 11 (42.3) | 5 (31.3) | 6 (60) | |
| History of pulmonary resection for lung cancer | 0.538 | |||
| Yes | 3 (11.5) | 1 (6.3) | 2 (20) | |
| No | 23 (88.5) | 15 (93.8) | 8 (80) | |
| Line of ICIs therapy | 0.087 | |||
| First-line | 9 (34.6) | 8 (50) | 1 (10) | |
| > Second-line | 17 (65.4) | 8 (50) | 9 (90) | |
| Best response to ICIs therapy | 0.203 | |||
| PR | 10 (38.5) | 6 (37.5) | 4 (40) | |
| SD | 12 (46.2) | 9 (56.3) | 3 (30) | |
| PD | 4 (15.4) | 1 (6.3) | 3 (30) | |
| Objective response rate, % | 38.5 | 37.5 | 40 | 1.000 |
| Disease control rate, % | 84.6 | 93.8 | 70 | 0.264 |
Group A: combination with chemotherapy; Group B: including monotherapy (n = 5), combination with anti-angiogenesis (n = 3), combination with chemotherapy plus anti-angiogenesis (n = 1), or combination with chemotherapy plus HER2-targeting drugs (n = 1)
ICIs, immune checkpoint inhibitors; ECOG PS, Eastern Cooperative Oncology Group performance status; PR, partial response; SD, stable disease; PD, progression disease
Table 2.
Clinical details of patients
| Patient number | Gender | Age | Smoking history | PD-L1 status | Patterns of HER2 Variants | Best response | Treatment lines | Combined therapy |
|---|---|---|---|---|---|---|---|---|
| P1 | Female | 68 | No | Unknown | A775_G776insYVMA | PR | 4 | Anti-angiogenesis therapy |
| P2 | Female | 60 | No | Unknown | L755P mutation | PR | 1 | Chemotherapy |
| P3 | Male | 58 | Yes | Unknown | A775_G776insYVMA | PD | 2 | Chemotherapy |
| P4 | Male | 65 | No | Negative | A775_G776insYVMA | PR | 1 | Chemotherapy |
| P5 | Female | 53 | No | Unknown | A775_G776insYVMA | SD | 3 | Chemotherapy |
| P6 | Male | 47 | Yes | Unknown | A775_G776insYVMA | SD | 4 | None |
| P7 | Female | 46 | No | Negative | A775_G776insYVMA | PD | 3 | None |
| P8 | Female | 66 | No | Unknown | Unknown | SD | 1 | Chemotherapy |
| P9 | Male | 62 | Yes | 1–49% | G776 > VC | SD | 4 | Chemotherapy |
| P10 | Female | 67 | No | Unknown | G778_P780dup | PR | 2 | None |
| P11 | Female | 50 | No | > 50% | Unknown | PR | 1 | Chemotherapy |
| P12 | Male | 40 | No | Unknown | G778_P780dup | PD | 3 | None |
| P13 | Male | 58 | Yes | 1–49% | A775_G776insYVMA | SD | 5 | Chemotherapy |
| P14 | Male | 38 | No | 1–49% | A775_G776insYVMA | SD | 4 | Chemotherapy |
| P15 | Female | 68 | No | Unknown | A775_G776insYVMA | SD | 1 | Anti-angiogenesis therapy |
| P16 | Female | 40 | No | 1–49% | Y772_A775dup | PD | 2 | Anti-angiogenesis therapy plus Chemotherapy |
| P17 | Male | 53 | No | Unknown | Y772_A775dup | PR | 2 | None |
| P18 | Male | 69 | No | Unknown | G776delinsVC | SD | 1 | Chemotherapy |
| P19 | Female | 44 | No | 1–49% | G776delinsVC | SD | 1 | Chemotherapy |
| P20 | Male | 48 | Yes | 1–49% | Y772_A775dup | SD | 2 | Anti-angiogenesis therapy |
| P21 | Male | 57 | No | Negative | Y772_A775dup | PR | 2 | Chemotherapy |
| P22 | Female | 58 | No | Negative | G776delinsVC | SD | 2 | Chemotherapy |
| P23 | Female | 45 | No | Negative | G776delinsVC | PR | 3 | Chemotherapy plus HER2-targeting drugs |
| P24 | Male | 52 | Yes | Negative | Y772_A775dup | SD | 3 | Chemotherapy |
| P25 | Male | 40 | Yes | Negative | Unknown | PR | 1 | Chemotherapy |
| P26 | Male | 63 | Yes | Negative | Unknown | PR | 1 | Chemotherapy |
PD-L1, programmed cell death ligand 1; PR, partial response; SD, stable disease; PD, progressive disease
Patterns of HER2 variants
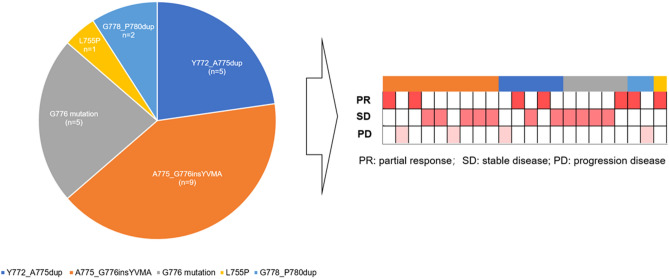
A total of 5 patterns of HER2 variants were available in the 22 patients (Fig. 1), and 4 patients were lack of details genetic characteristics. A775_G776insYVMA was the most common HER2 variant (n = 9), followed by Y772_A775dup (n = 5) and G776 mutation (n = 5, including G776 > VC and G776delinsVC). The other two patterns of HER2 variants were G778_P780dup and L755P mutation (n = 2, n = 1, respectively). Among these 22 patients, a majority of them achieved PR or SD as best response(n = 18).
Fig. 1.
Patterns of HER2 variants and tumor responses(n = 22)
There were no statistics differences with respect to PD-L1 status, tumor response or PFS between patients with A775_G776insYVMA and other HER2 variant (Supplementary Table 1, Supplementary Fig. 1).
Treatment and survival of whole cohort
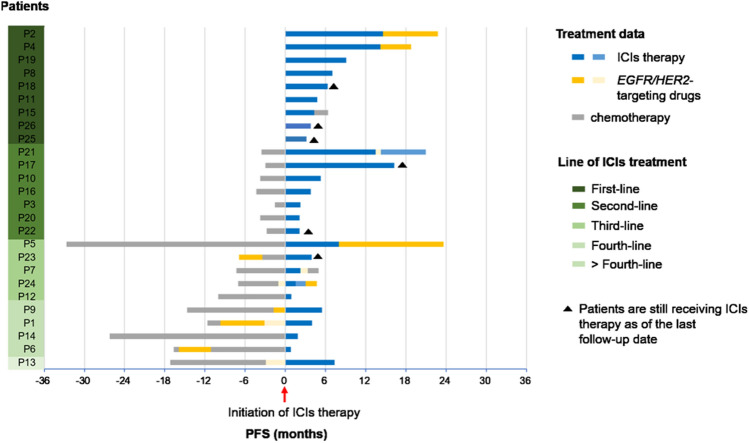
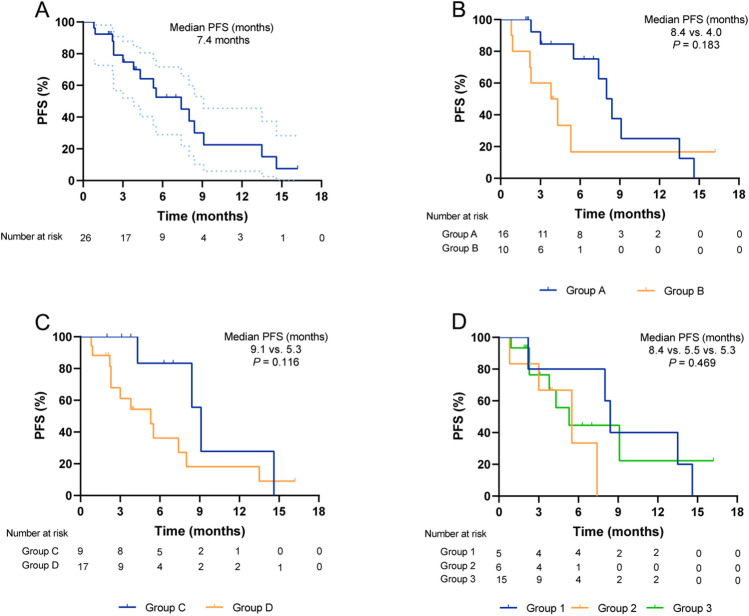
Among the 26 patients with HER2-mutant lung adenocarcinoma, the best response to PD-1/PD-L1 inhibitors is demonstrated in Tables 1, 2. Among the patients who received ICIs therapy, 6 patients are still receiving the treatment as of the last follow-up date (Fig. 2). About 10 out of 26 patients achieved PR and 12 patients achieved SD, achieving an ORR of 38.5% and DCR of 84.6%. As of data cutoff, 16 patients (61.5%, 16/26) had events of PFS, and median PFS was 7.4 months (95% confidence interval [CI], 4.4 to 10.4 months) (Fig. 3a). Meanwhile, patients who treated with ICIs therapy at earlier line tend to achieve longer PFS (Fig. 2). The median PFS from the initiation of first-line immunotherapy was 9.1 months (95% CI, 7.9–10.2) (Fig. 3c). In total of 17 patients (65.4%) received second or more lines of immunotherapy with a median PFS of 5.3 months (95% CI: 2.6–8.0) (Fig. 3c). Particularly, vast majority of patients were treated with first-line immunochemotherapy combination regimens (8/9, 88.9%) (Table 2). Of the entire cohort, 16 patients have been treated with immunochemotherapy combination regimens (16/26, 61.5%), achieving a median PFS of 8.4 months (95% CI: 7.1–9.6) (Fig. 3b, Table 1). Of interest, we observed a higher ORR (60%) and longer PFS (8.4 months) in patients receiving ICIs before EGFR/HER2-targeting drugs (Fig. 3d, Supplementary Table 2). However, there was no statistic difference of ORR or median PFS between those three groups (ORR: 60% versus 33.3% versus 33.3%, P = 0.545; PFS: 8.4 months versus 5.5 months versus 5.3 months, P = 0.469).
Fig. 2.
Survival benefits based on treatment data and line of ICIs treatment (n = 26)
Fig. 3.
Clinical outcomes on ICIs-based treatment. a Progression-free survival (PFS) from ICIs therapy initiation for the entire cohort. Dotted lines indicate 95% confidence intervals. b Group A: combination with chemotherapy; Group B: including monotherapy (n = 5), combination with anti-angiogenesis (n = 3), combination with chemotherapy plus anti-angiogenesis (n = 1), or combination with chemotherapy plus HER2-targeting drugs (n = 1). c Group C: Patients received ICIs as first-line treatment; Group D: Patients received ICIs as second or later-line treatment. d Group 1: Patients received ICIs before EGFR/HER2-targeting drugs; Group 2: Patients received ICIs after EGFR/HER2-targeting drugs; Group 3: patients never treated with EGFR/HER2-targeting drugs until the follow-up end date
In this study, 7 patients (26.9%) were recorded to develop any-grade adverse events (AEs), including 3 patients (11.5%) with grade 3–4 AEs (one each neutropenia, thrombocytopenia and liver function test abnormality).
Discussion
This retrospective study included patients with HER2-mutant advanced lung adenocarcinoma treated with ICIs therapy. The proportion of male and never-smoker was higher, 53.8% and 69.2%, respectively. More than half of patients received PD-1/PD-L1 inhibitors combined with chemotherapy. Overall, patients who received ICIs-based therapy achieved a median PFS of 7.4 months and the ORR was 38.5%. Besides, the median PFS was 9.1 months among patients who received ICIs as first-line treatment. Patients who received ICIs before EGFR/HER2-targeting drugs achieved a median PFS of 8.4 months. A majority of the patients harbored with 12-bp exon 20 insertion (A775_G776insYVMA). Meanwhile, a large proportion of them had PR or SD as best response to ICIs therapy.
In our study, the clinical characteristics were as anticipated to a cohort of patients with HER2 alterations, including a higher percent of never-smokers[12, 13]. However, there was a lower proportion of female in our cohort (12/26, 46.2%). One possible reason is the differences in selection of patient population for receiving ICIs treatment in clinical practice. As for HER2-mutant type, we observed 5 patterns of HER2 variant, including A775_G776insYVMA, Y772_A775dup, G776 mutation, G778_P780dup and L755P mutation. It was consistent with the other report that A775_G776insYVMA is the most common variant[14]. Among the patients with available patterns of HER2 variant, we observed an encouraging clinical response to ICIs therapy, in which most of them had PR or SD as best responses (18/22).
As for our cohort, we observed longer PFS in patients undergoing ICIs-based therapy than was reported in prior studies[13, 15, 16], in which PFS was only 2.2–3.6 months for receiving single-agent ICIs or combination with cytotoxic T-lymphocyte antigen 4 (CTLA-4) inhibitors. Of particular interest, 9 of 26 patients treated with ICIs in the first-line setting, in which 5 patients had PR as best response. It might be correlated to the higher percent of patients received PD-1/PD-L1 inhibitors combined with chemotherapy (n = 16, 61.5%) and 8 patients were treated in first-line setting (8/16, 50%). A recent study observed a median PFS of 6 months among NSCLC patients with HER2-mutation, who received ICIs in combination with chemotherapy as first-line treatment[17]. Similarly, a case series indicated that the combination of ICIs and chemotherapy may be a promising first-line treatment choice, with a median PFS of 8 months among NSCLC patients with HER2-alterations[18]. Chemotherapeutic agents may enhance the strength of effector T cells by upregulating the expression of co-stimulatory molecules (B7-1) or downregulating co-inhibitory molecules (PD-L1) on cancer cells[19]. Alternatively, some chemotherapy drugs can stimulate the release of tumor antigens and potentially upregulate the expression of major histocompatibility complex (MHC) class I molecules, which might enhance tumor antigen presentation and then enhance the response to ICIs therapy[19, 20]. Although the mechanisms underlying the response to ICIs therapy among HER2 mutation remain elusive, our findings do not support inferior outcomes of ICIs-based therapy, including immunochemotherapy combination regimens, in patients with HER2-mutant lung adenocarcinoma.
Despite our clinically relevant findings based on a multicenter patient population, the conclusion is limited by the retrospective nature and small number of patients enrolled in this cohort that causes bias in patient selection. Moreover, one of the strengths about our study is the enrollment of a real-word cohort composed of patients with HER2 mutations receiving ICIs therapy, a rare population in clinical trials. Taken together, our findings provide a new clinical therapeutic insight for lung adenocarcinoma harbored with HER2-mutant.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Tianqing Chu, Jinyan Ye, Xing Li, Chao Zhao and Xiangling Chu collected the relevant data; Xiangling Chu, Huiping Qiang and Mengqing Xie drafted the manuscript text; Xiangling Chu and Xing Li performed statistical analyses; Jing Zhao, Yan Wu, Juan Zhou and Chaonan Han gave critical comments; Chunxia Su designed this study; Chunxia Su and Tianqing Chu revised the paper. All authors approved the final version of the manuscript.
Abbreviations
- CI
Confidence interval
- DCR
Disease control rate
- HER2
Human epidermal growth factor receptor 2
- ICIs
Immune checkpoint inhibitors
- PD-L1
Programmed cell death ligand 1
- NSCLC
Non-small-cell lung cancer
- ORR
Objective response rate
- PD-1
Programmed cell death protein 1
- PFS
Progression-free survival
Funding
This work was supported by the National Natural Science Foundation of China (grant number: 82072568, 81874036), Shanghai Hospital Development Center (grant number: SHDC12020110) and Shanghai Anticancer Association (grant number: SACA-CY19B06).
Data availability material
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Conflict of interest
The authors declare no potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiangling Chu, Huiping Qiang and Mengqing Xie have contributed equally.
Contributor Information
Tianqing Chu, Email: tianqing_chu@126.com.
Chunxia Su, Email: susu_mail@126.com.
References
- 1.Zhou C, Wu Y-L, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 2.Stephens P, Hunter C, Bignell G, et al. Lung cancer: Intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431:525–526. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- 3.Sonobe M, Manabe T, Wada H, et al. Lung adenocarcinoma harboring mutations in the ERBB2 kinase domain. J Mol Diagn. 2006;8(3):351–356. doi: 10.2353/jmoldx.2006.050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shigematsu H, Takahashi T, Nomura M, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65:1642–1646. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- 5.Pillai RN, Behera M, Berry LD, et al. HER2 mutations in lung adenocarcinomas: a report from the lung cancer mutation consortium. Cancer. 2017;123(21):4099–4105. doi: 10.1002/cncr.30869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou C, Li X, Wang Q, et al. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: a multicenter, open-label, single-arm. Phase II Study J Clin Oncol. 2020;38(24):1753–2761. doi: 10.1200/JCO.20.00297. [DOI] [PubMed] [Google Scholar]
- 7.Tsurutani J, Iwata H, Krop I, et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 2020;10(5):688–701. doi: 10.1158/2159-8290.CD-19-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mok. TSK, Wu. Y-L, Kudaba. I, et al., (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial Lancet, 393(1083): 1819–1830. [DOI] [PubMed]
- 9.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 10.Addeo A, Passaro A, Malapelle U, et al. Immunotherapy in non-small cell lung cancer harbouring driver mutations. Cancer Treat Rev. 2021;96:102179. doi: 10.1016/j.ctrv.2021.102179. [DOI] [PubMed] [Google Scholar]
- 11.E.A. Eisenhauer PT, J. Bogaerts, L.H. Schwartz, D. Sargent, R. Ford, J. Dancey, S. Arbuck, S. Gwyther, M. Mooney, L. Rubinstein, L. Shankar, L. Dodd, and R. Kaplan DL, J. Verweij, (2009) New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer, 45(2): 228-47 [DOI] [PubMed]
- 12.Mazières J, Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol. 2016;27(2):281–6. doi: 10.1093/annonc/mdv573. [DOI] [PubMed] [Google Scholar]
- 13.Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30(8):1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolfo C, Russo A. HER2 mutations in non-small cell lung cancer: a herculean effort to hit the target. Cancer Discov. 2020;10(5):643–645. doi: 10.1158/2159-8290.CD-20-0225. [DOI] [PubMed] [Google Scholar]
- 15.Guisier F, Dubos-Arvis C, Vinas F, et al. Efficacy and Safety of Anti-PD-1 immunotherapy in patients with advanced NSCLC With BRAF, HER2, or MET mutations or RET translocation: GFPC 01–2018. J Thorac Oncol. 2020;15(4):628–636. doi: 10.1016/j.jtho.2019.12.129. [DOI] [PubMed] [Google Scholar]
- 16.Lau SCM, Fares AF, Le LW, et al. Subtypes of EGFR- and HER2-mutant metastatic NSCLC influence response to immune checkpoint inhibitors. Clin Lung Cancer. 2021;22(4):253–259. doi: 10.1016/j.cllc.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Saalfeld FC, Wenzel C, Christopoulos P, et al., (2021) Brief Report: Efficacy of immune checkpoint inhibitors alone or in combination with chemotherapy in NSCLC harboring ERBB2 mutations. J Thorac Oncol,. [DOI] [PubMed]
- 18.Zhao S, Xian X, Tian P, et al. Efficacy of combination chemo-immunotherapy as a first-line treatment for advanced non-small-cell lung cancer patients with HER2 alterations: a case series. Front Oncol. 2021;11:633522. doi: 10.3389/fonc.2021.633522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–443. doi: 10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang MY, Jiang XM, Wang BL, et al. Combination therapy with PD-1/PD-L1 blockade in non-small cell lung cancer: strategies and mechanisms. Pharmacol Ther. 2021;219:107694. doi: 10.1016/j.pharmthera.2020.107694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability material
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.