Abstract
Introduction
Although recent clinical trials have demonstrated the efficacy of CD19-directed chimeric antigen receptor (CAR) T-cell therapy for refractory or relapsed B acute lymphoblastic leukemia (r/r B-ALL), most trials exclude patients with high-burden CNS leukemia (CNSL) to avoid the risk of severe neurotoxicity. There were only sparse cases describing the effect of CAR T cells on low-burden CNSL, and the safety and effectiveness of CAR T cells in high-burden CNSL remains unknown.
Methods
Here, we retrospectively analyzed the results of CD19 CAR T-cell therapy in 12 pediatric patients that had low (Blasts < 20/μL in CSF) or high-burdens (Blasts or intracranial solid mass) of CNS B-ALL, that are enrolled in three clinical trials and one pilot study at Beijing Boren Hospital
Results
Eleven patients (91.7%) achieved complete remission (CR) on day 30, and one patient got CR on day 90 after infusion. Most patient experienced mild cytokine-release syndrome. However, of the five patients who retained > 5/μL blasts in CSF or a solid mass before CAR T-cell expansion, four developed severe (grade 3–4) neurotoxicity featured by persistent cerebral edema and seizure, and they fully recovered after intensive managements. Sustained remission was achieved in 9 of the 12 patients, resulted in a 6-month leukemia-free survival rate of 81.8% (95% CI 59.0–100). Only one patient has CNS relapse again.
Conclusion
Our study demonstrates that CAR T cells are effective in clearing both low- and high-burden CNSL, but a high CNSL burden before CAR T-cell expansion may cause severe neurotoxicity requiring intense intervention.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-020-02829-9.
Keywords: CD19 CAR T, B-ALL, CNSL, High-burden, Immunotherapy
Introduction
The presence of central nervous system (CNS) involvement at the time of diagnosis is uncommon (about 3–7%) in pediatric acute lymphoblastic leukemia (ALL), and most of CNS leukemia (CNSL) have good prognosis with the help of CNS-directed therapies, such as cranial irradiation, intrathecal injection (IT) with chemotherapeutic drugs and systemic chemotherapy [1,2]. Approximately 1% of the patients have still CNS relapsed [3]. With re-induction of CNS-directed therapy, the event-free survival is more than 50% without hematopoietic stem cell transplantation (allo-HCT), but for patients with CNSL that is refractory to CNS-directed therapies, there were few treatment options [4]. Allo-HCT has been considered for combined bone marrow and CNS relapsed patients [2], while it has not been recommended for patients with positive minimal residual disease (MRD+) leukemia in CNS [5]. Therefore, it is urgent to develop new treatments for these patients.
CD19-directed chimeric antigen receptor (CAR) T cells have shown dramatic effect in refractory or relapsed B-ALL in clinical trials throughout the world. However, most trials have excluded patients with high-burden CNSL to avoid the risk of severe neuronal adverse events, and there were only sparse reported cases of B-ALL patients with CNSL that were treated with CD19 CAR T cells [4–12]. Lee et al. and Shannon et al. independently reported two B-ALL patients with CNS2 (blasts < 5/μL in cerebrospinal fluid, CSF) that have no obvious neurotoxicity and achieved MRD− in CSF after CAR T-cell therapy [4, 6]. He et al. reported three CNS B-ALL patients who were enrolled at CNS3 status (blasts > 5/μL), but had been reduced to a low-burden with chemotherapy before CAR T-cell infusion, and all achieved complete remission (CR) 2 weeks post CAR T infusion, accompanied with mild neurotoxicity featured by reduction of consciousness or mild convulsion and cognitive impairment [7]. Dai et al. reported that two B-ALL adults with refractory but low-burden CNS3 became MRD− 1 month after CAR T-cell infusion and achieved an leukemia-free survival (LFS) of > 20 months, and one of them experienced mild numbness and stiffness of lower limbs and abdominal skin (grade 1) [8]. These reports overall supported the feasibility of CAR T therapy for CNS B-ALL. However, these cases were limited and sparse, and the toxicities and long-term effectiveness of this strategy need to be evaluated in more patients. Furthermore, in most of these studies, only patients with low-burden leukemia in CNS (< 20/μL in CSF) were involved, but the feasibility, safety and efficacy of CD19 CAR T cells in patients with high-burden (> 20/μL in CSF or solid mass) CNSL before or during CAR-cell infusion remains unexplored.
Here, we report the results of CD19 CAR T-cell therapy for 7 r/r B-ALL pediatric patients that had high-burden CNSL (blasts > 20/μL in CSF or intracranial mass lesions) in a pilot study, and 5 patients that had low-burden CNSL (blasts < 20/μL in CSF) and have been enrolled in the clinical trials, at Beijing Boren Hospital. Our data demonstrated that CD19 CAR T cells were effective in inducing MRD− remission in all patients, and the remission is durable in most (9/12) patients with a median follow-up of 7 months. Severe neurotoxicity was observed in 4 patients who had high-burden CNSL or solid mass before CAR T-cell expansion, but these patients fully recovered with intensive interventions. Our results are among the early studies to evaluate the feasibility, toxicity and efficacy of CD19 CAR T-cell therapy in refractory high-burden CNSL in children.
Methods
Study design and patients
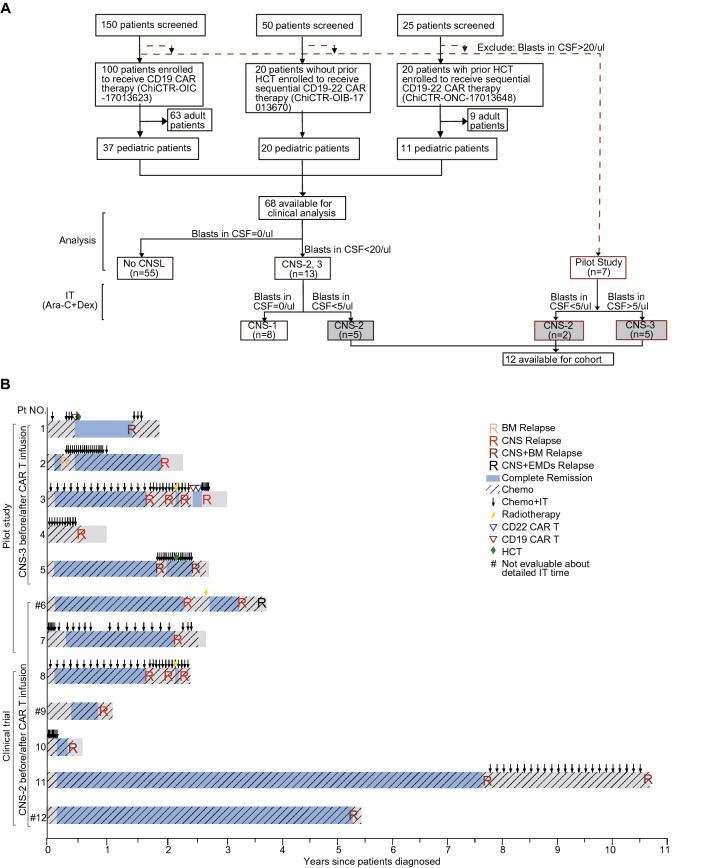
We retrospectively analyzed 12 refractory or relapsed B acute lymphoblastic leukemia (B-ALL) children who had CNSL before the time of CD19 chimeric antigen receptor (CAR) T-cell infusion, in three clinical trials (ClinicalTrials#: ChiCTR-OIC-17013623/ ChiCTR-ONC-17013648/ ChiCTR-OIB-17013670) and a pilot study conducted between December 10, 2017 and March 30, 2020 at Beijing Boren Hospital. The seven patients (No. 1–7) in the pilot study had > 20/µL blasts in CSF or intracranial solid mass at enrollment, and after bridging chemotherapy, two had < 5/µL blasts in CSF, and other 5 still retained > 5/µL blasts in CSF or intracranial solid mass before CAR T-cell expansion (Fig. 1a). Among the 68 pediatric patients enrolled in the three clinical trials, 12 had < 20/µL blasts in CSF at enrollment, and 5 (No. 8–12) retained > 0 but < 5/μL blasts in CSF after conditioning chemotherapy and were thus included in our analysis.
Fig. 1.
Patient flowchart and treatment overview. a Flowchart showing the inclusion of patients in this study. b The treatment history of each enrolled patient. Chemo chemotherapy, IT intrathecal chemotherapy, FC fludarabine and cyclophosphamide, CNSL central nervous system leukemia, TL testicular leukemia, EMDs extramedullary diseases, HCT hematopoietic stem cell transplantation, PR partial response, CR complete remission, R relapse, BM bone marrow
All the treatments have been approved by the institutional review board of Beijing Boren Hospital, and informed consent was obtained in accordance with the Declaration of Helsinki. The detailed protocols were in Supplemental Methods pp 2–7. There was no significant difference in most clinical features between patients in the clinical trials and the pilot study (Table S1 in Supplemental Methods pp 10), excepting that patients in the pilot study had higher leukemia burden in CNS than those in the clinical trials (P = 0.017).
Design of CAR and manufacture of CAR T-cells
A lentiviral vector carrying a CD19 CAR with a 4-1BB costimulatory domain and a CD3-zeta signaling domain was constructed as previously described [12–14]. The CD19 recognition domain was composed of a single-chain fragment variable region derived from the FMC63 monoclonal antibody. Cytotoxicity of CD19 CAR T cells have been validated previously [11, 12]. The detailed procedure and CD22 CAR T manufacture procedure were detailed in Supplemental Methods pp7.
Clinical procedures and assessment and management of adverse events
After leukapheresis, patients received lymphodepleting chemotherapy composing of Fludarabine at 30 mg/m2/day and Cyclophosphamide at 250 mg/m2/day on days -5 to -3 before CD19 CAR T-cell infusion (day 0). Patients in the pilot study received intrathecal bridging chemotherapy with cytarabine (Ara-C) at 25 mg (age < 10) or 35 mg (age ≥ 10) and dexamethasone (Dex) at 5 mg every other day before CD19 CAR T-cell infusion. Extra systemic bridging chemotherapy was also given to patients who had isolated mass before infusion: patient 4 received darubicin (IDA) at 10 mg for 1 day and Dex at 30 mg for another 2 days and patient 5 received VLD consisting of Vindesine (VDS) at 1 mg, Dex 14 at mg and L-Asparaginase (L-ASP) at 10,000 U for 3 days.
Cytokine-release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were graded through ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells [15]. To manage ICANS, mannitol (2.5 ml/kg/dose) and furosemide (1 mg/kg/dose) were used intravenously to prevent or control intracranial hypertension. Intrathecal injection with Dex at 5 mg/dose was conducted when patients had severe or progressive ICANS. For patients who developed severe ICANS with status epilepticus, benzodiazepine, Midazolam and diazepam were used intravenously as acute management, and Clonazepam and Dilantin were given until all symptoms were relieved. For patients who developed severe cerebral edema, intravenous injection of steroids (dexamethasone or methylprednisolone, 2–15 mg/kg/d) was added besides intrathecal injection of Dex. The details of the managements of CRS and ICANS and other supportive cares are in Supplemental Methods pp8–9.
Statistical analysis
Exact methods (Clopper-Pearson, 95% confidence intervals and Fisher’s exact tests) were used for categorical variables. Difference between two groups was analyzed by Wilcoxon rank-sum test or Chi-square test. Spearman correlation coefficient was used to evaluate the association of different factors with CRS or ICANS grade, respectively. The Kaplan–Meier approach was performed to estimate time-to-event analyses. All statistical analyses were performed using NCSS Statistical and Data Analysis version 12.0.2, and all the P values of < 0.05 were considered significant.
Results
Patient enrollment and characteristics
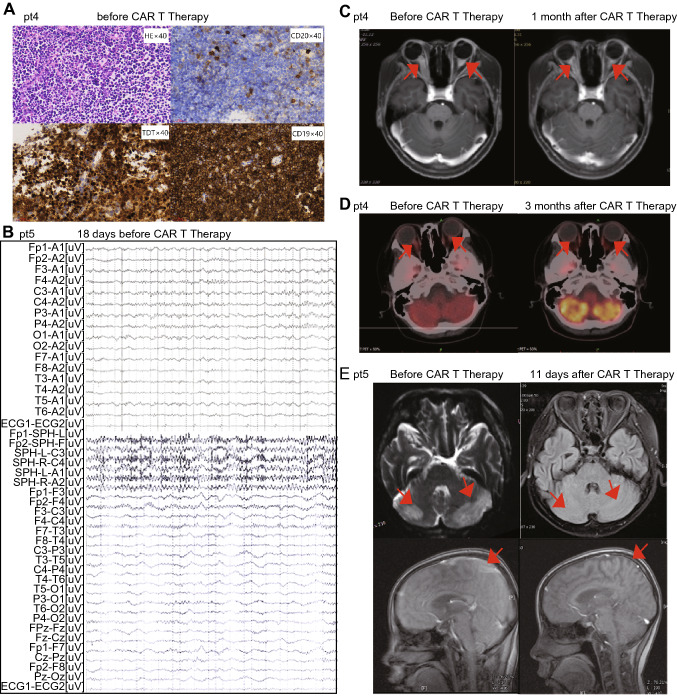
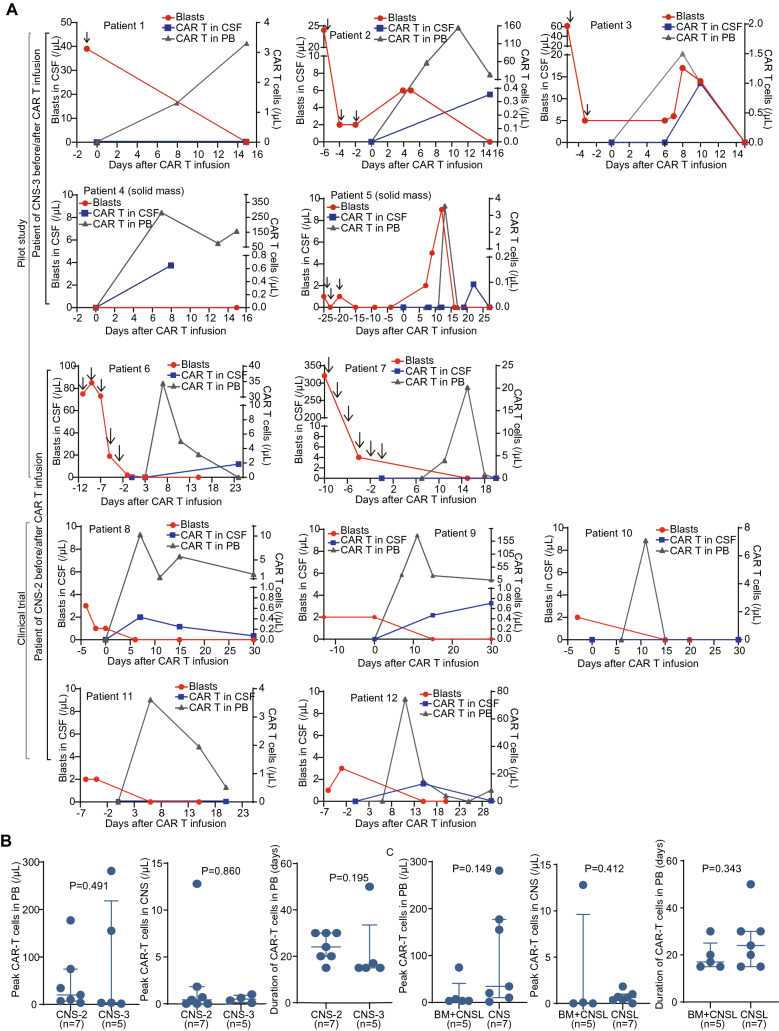
From 3 clinical trials and one pilot study, we retrospectively analyzed 12 pediatric patients, who aged 1–12 years and had refractory B-ALL in CNS after failing from 8–35 cycles of triple intrathecal chemotherapies. Six (50%) of the 12 patients (No. 2, 3, 5, 6, 8 and 11) had ≥ 2 times of CNS relapse (Fig. 1b and Table 1). Three (25%) patients (No. 3, 6 and 8) underwent cranial radiotherapy previously. Five (50%) patients (No. 1, 5, 10–12) had combined BM and CNS relapse and two (No. 1 and 5) of them relapsed after transplantation. Two patients (No. 1 and 3) had failed previous CD19 CAR T-cell therapies. Combined extramedullary diseases (EMDs) were observed in 2 patients (17%) including patient 1 with kidney involvement and patient 6 with testicular leukemia. The details of individual patients were shown in Table 1. All the 7 patients in the pilot study (No. 1–7) had obvious neuronal symptoms at enrollment (Table S2 in supplemental methods pp11). Patient 1 had facial paralysis and paraplegia. Patient 4 had vision disorder due to occupying in optic nerve (Fig. 2a), and his visual acuity was evaluated as binocular no light perception. Patient 5 had facial paralysis and occupying lesions in temporal and occipital lobe region detected by MRI and quickly developed grand malepileptic seizure 18 days before infusion (Fig. 2b). Other patients had severe headache and positive reflex of Babinski’s sign. The neuronal symptoms were not prominent in the 5 patients (No. 8–12) from clinical trials, and usually manifested as mild headache. Only two of 7 patients in pilot study (No. 6 and 7) achieved < 5/µL in CSF without further progression. CNS disease was reduced transiently but progressed to > 5/µL blasts in 3 patients before CAR T-cell substantially expanded, indicating the very refractory nature of the diseases. No CNS disease progression was observed in patients enrolled in trials. The peak blast counts in CSF were significantly different between the patients from clinical trial as 2 (range 1–4)/μL and those from the pilot study as 13 (range 6–39)/μL after chemotherapy and before CAR T-cell expansion (P = 0.006). No blasts were detected in the CSF of patient 4 who had mass lesions (Fig. 3a).
Table1.
Patient baseline characteristics, CAR T-cell parameters, and treatment outcome
| Patient no./gender/age | Prior treatment time (m) | Pre-chemo | Pre-IT | Pre-radio | Pre-HCT | Pre-CART | Times of relapse | EMDs | BM involvement | IT bridged to CAR T infusion | CSF:WBC before CAR-T expansion (/µL) | Dose of CAR T cells (× 106/kg) | CAR T-cell viability (%) | CAR T-cell transduction efficiency (%) | IT to ICANS | Response | Post-CAR treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/F/11 | 20 | + | 8 | − | + | CD19 | 1 | CNS + Kidney | + | 3 | 39 | 3.78 | 74.6 | 40.1 | 1 | CR/MRD- | – | CCR/7 |
| 2/M/13 | 27 | + | 20 | − | − | – | 2 | CNS | − | 3 | 6 | 4.60 | 85.8 | 28.2 | 3 | CR/MRD- | HCT | CCR/7 |
| 3/M/9 | 36 | + | 35 | + | − | CD19-22 | 4 | CNS | − | 2 | 17 | 2.02 | 76.5 | 54.1 | 4 | CR/MRD- | – | CCR/6 |
| 4(mass)/F/12 | 12 | + | 11 | − | − | – | 1 | CNS | − | 0 | – | 4.70 | 88.5 | 38.0 | 0 | PR | HCT | CCR/6 |
| 5(mass)/F/12 | 34 | + | 19 | − | + | – | 2 | CNS | + | 3 | 9 | 0.31 | 85.0 | 22.3 | 8 | CR/MRD- | CD22 | CCR/15 |
| 6/M/8 | 46 | + | # | + | − | – | 3 | CNS + TL | − | 5 | 2 | 1.42 | 92.4 | 45.3 | 0 | CR/MRD- | – | R/6 |
| 7/M/3 | 32 | + | 22 | − | − | – | 1 | CNS | − | 5 | 4 | 0.54 | 76.8 | 19.3 | 0 | CR/MRD- | – | CCR/1 |
| 8/M/9 | 27 | + | 28 | + | − | – | 3 | CNS | − | 0 | 3 | 1.05 | 93.2 | 57.5 | 1 | CR/MRD- | CD22 | R/8 |
| 9/F/1 | 13 | + | # | − | − | – | 1 | CNS | − | 0 | 2 | 0.50 | 91.7 | 82.0 | 0 | CR/MRD- | CD22/HCT | R(EMDs)/3 |
| 10/F/1 | 7 | + | 8 | − | − | – | 1 | CNS | + | 0 | 2 | 1.60 | 87.4 | 29.3 | 0 | CR/MRD- | CD22 | CCR/17 |
| 11/M/8 | 129 | + | 23 | − | − | – | 2 | CNS | + | 0 | 1 | 0.28 | 95.2 | 22.8 | 1 | CR/MRD- | HCT | CCR/10 |
| 12/M/7 | 65 | + | 20 | − | − | – | 1 | CNS | + | 0 | 1 | 1.00 | 94.9 | 48.2 | 0 | CR/MRD- | CD22 | CCR/12 |
Patient 1–5 had high-burden of CNSL and patient 6–12 had low-burden of CNSL before CAR T expansion
− no, + yes, M male, F female, MRD minimal residual disease, Pre previous, Chemo chemotherapy, IT intrathecal chemotherapy, CNS central nervous system, TL testicular leukemia, EMDs extramedullary diseases, HCT hematopoietic stem cell transplantation, PR partial response CR complete remission, R relapse, BM bone marrow
Fig. 2.
Response of CNS solid mass to CAR T-cell therapy in two patients. a Hematoxylin–eosin and other HIC markers (TdT, CD19, CD20) staining of a tissue specimen taken from the craniopharyngeal canal in sellar region shows blasts of patient 4 before CAR T infusion (original magnification × 400). b Electroencephalogram of patient 5 with seizure 18 days before CAR T infusion. c A leukemia lesion on the optic nerve of patient 4 before (left panel) and 1 month after CAR T-cell therapy (right panel), examined by magnetic resonance imaging (MRI). d A leukemia lesion on the optic nerve of patient 4 before (left panel) and 3 months after CAR T-cell therapy (right panel), examined by position-emission tomography (PET)/CT. e A leukemia lesion in the watershed area and cerebral cortex of patient 5 before (left panel) and 11 days after CAR T-cell therapy (right panel), examined by magnetic resonance imaging (MRI). Arrows indicate leukemia lesions
Fig. 3.
Monitoring of blasts and CAR T cells after CD19 CAR T-cell infusion. a Monitoring of CAR T cells in the peripheral blood (PB) and cerebrospinal fluid (CSF), and leukemic cells in CSF during CAR T-cell infusion. The brown arrows indicate intrathecal chemotherapy with cytarabine and dexamethasone. b Comparison of peak level and duration of CAR T cells in PB and CSF between patients with different CNS status. c Comparison of peak level and duration of CAR T cells in PB and CSF between patients with only CNS relapse and combined CNS and BM relapse. The P values are the Wilcoxon rank-sum tests values across groups
Clinical response and CAR T-cell expansion
The median infused dose of CD19 CAR T cells was 3.78 (range 0.31–4.70) × 106/kg. Along with CD19 CAR T-cell expansion, blasts in CSF of all patients decreased gradually (Fig. 3a and S1). Eleven (91.7%) patients achieved CR/CRi with all CNS symptoms alleviated on day 30 after infusion (Fig. 2e). Patient 4 achieved partial remission by MRI examination on day 30 but had no vision improvement, and she finally achieved CR 3 months later without other treatments (Fig. 2c and d). The five patients who had combined BM and CNS disease also achieved MRD− in BM on day 30. In the 2 patients who had combined EMDs, all lesions disappeared on day 30.
CD19 CAR T cells were readily detectable in the PB from all patients, indicating the efficient in vivo expansion. Before CAR T-cell obvious expansion, seven patients had < 5/µL blasts in CSF (namely CNS2), and 5 patients had > 5/µL blasts in CSF or a solid mass in CNS (namely CNS3). The CAR T expansion in CNS3 patients peaked from day 7–15 in PB after infusion (1.5–281-fold), and the median persistent duration of CAR T cells in PB detectable by FCM was 15 days (range 15–50). For CNS2 patients, the expansion peaked from day 7–15 in PB after infusion (3.6–177-fold), and the median persistent duration was 24 days (range 15–30). Eight (67%) patients had detectable CAR T cells in the CSF, the peak expansion of which was much lower than that in PB (Fig. 3a). Four patients without obvious neurotoxicities were evaluated for CNS status merely on day 30, and their blasts have been eliminated but CAR T cells were not detectable in CSF, which might be due to lower expansion or missing the appropriate detection time point. There was no significant difference between CNS2 and CNS3 groups in peak expansion and persistence of CAR T cells in PB (P = 0.491 and 0.195, Fig. 3b), and in peak expansion of CAR T cells in CSF (P = 0.860, Fig. 3b) (CAR T cells were not monitored in CSF after remission in most patients, so the persistent time of CAR T cells in CSF could not been evaluated). There was no significant difference in the peak expansion of CAR T cells (PB or CSF) or the persistent time of CAR T-cells (PB) between patients with isolated CNSL and those with combined BM and CNS leukemia (P = 0.149, 0.412 and 0.343, Fig. 3c). For patients with mild neurotoxicity, we did not evaluate CNS status usually within 30 days after infusion.
Cytokine-release syndrome, neurotoxicity and managements
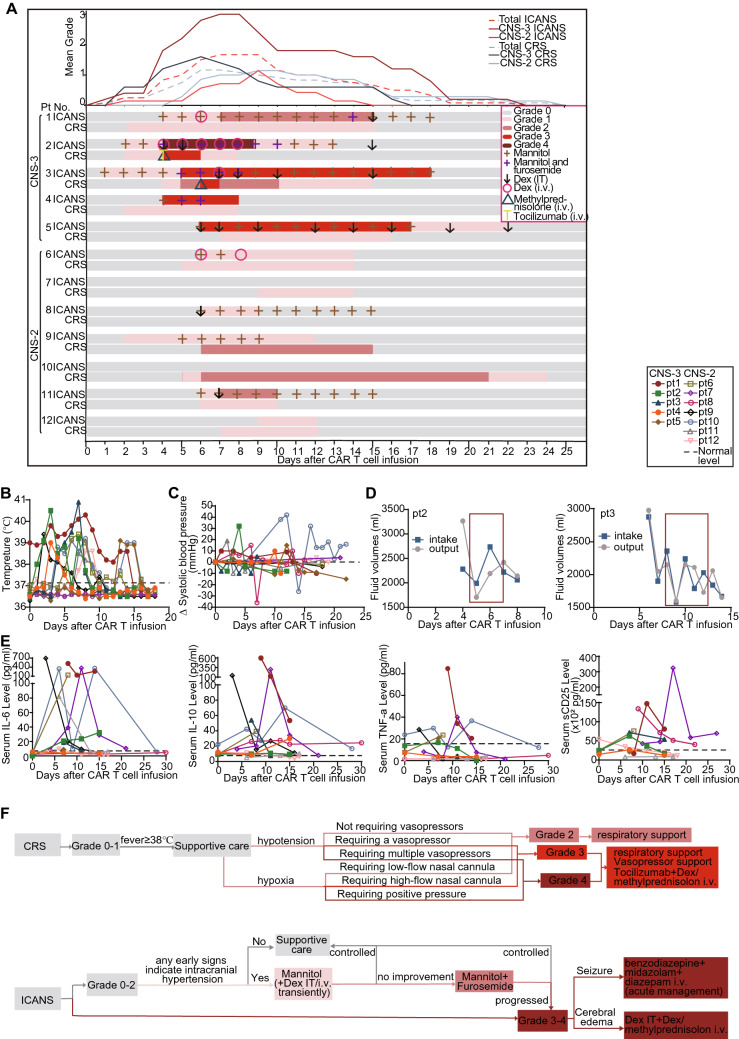
CRS and ICANS were routinely monitored after CAR T-cell infusion. The median onset of CRS occurred on day 5 (range 2–9), and the median time of the resolution was on day 14 (range 8–24) after infusion. Ten patients (83.3%) experienced no CRS or mild (grade 1–2) CRS mostly manifested as fever (< 40 °C) and hypoxia requiring low-flow nasal cannula (< 6 L/minute) and other supportive care including antipyretics, intravenous fluids and non-invasive respiratory support. Only two patients in CNS3 group developed grade 3 CRS manifested as high fever (> 40 °C) with hypoxia requiring high-flow nasal cannula (> 6 L/minute) (Fig. 4a and b), and the imbalance of volume intake and output indicated that they probably had vascular leakage syndrome (Fig. 4d).
Fig. 4.
Adverse events. a Management of patients with CRS and ICANS. Colors on the swimmer lane plot indicate the highest grade of any cytokine-release symptom and neurologic symptom recorded on each day for patients through the first 25 days after CAR T infusion (n = 12; 9 grade 1–2 CRS, 2 grade 3–4 CRS; 6 grade 1–2 ICANS, 4 grade 3–4 ICANS). The graph (top) shows the mean of the highest grade of neurotoxicity occurring in all patients on each day after CAR-T cell infusion. Dex Dexamethasone, i.v. intravenous, IT intrathecal injection. b Temperature monitoring after CD19 CAR T-cell infusion. c Changes of systolic blood pressure after CD19 CAR T-cell infusion. d Changes of fluid volumes after CD19 CAR T-cell infusion. e Monitoring of serum cytokine markers of systemic inflammation including interleukin-6 (IL-6), Tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), sCD25 after CD19 CAR T-cell infusion. f The flowchart showing the detailed treatment of CRS and ICANS
ICANS was obviously observed in 10/12 patients (83%). The median onset of ICANS, occurred on day 5 (range 2–9), with the median resolution time of day 14 (range 8–24) after infusion. The blood pressure increased in some patients (No. 1–5, 8, 10, 11), which might reflect the intracranial hypertension (Fig. 4c). Five patients with CNS2 (No. 6, 8, 9, 11, 12) and 1 patient with CNS3 (No. 1) responded to CAR T cells to developed grade 1–2 ICANS manifested as mild abnormality in orientation, naming, following command, writing and attention. Fontanel protuberance with unclosed fontanel occurred in two patients (No. 9 and 10), indicating an increase of intracranial pressure. Patient 9 also exhibited mild inattention on day 5 and was treated with mannitol for five days.
Severe ICANS (grade 3 or 4) was observed in 4 (80%) of the 5 patients in CNS3 group, manifested as cerebral edema and seizure, and the severest ICANS (grade 4) occurred in patient 2 and 3. Patient 2 developed mild inattention on day 2 and progressed to grade 4 ICANS on day 4 manifested as vomiting, blindness and neck rigidity, and mannitol and furosemide were given from days 2–13 to reduce intracranial pressure. Two cycles of Dex were intrathecal injected, while his symptoms still aggravated as life-threatening repetitive seizure and coma. Subsequently, methylprednisolone and tocilizumab were intravenously injected to control CRS and ICANS. This patient’s vision recovered after 2 days and other symptoms were alleviated finally. The duration of his treatments lasted for almost 2 weeks. Patient 3 had a fever of 40 °C on day 7 and developed grade 3 ICANS featured by severe vomiting and slightly positive neck rigidity on day 5. Furosemide and mannitol were continuously used from day 1–17. On day 7, he had severe convulsion and was treated with 4 cycles of intrathecal administration of Dex. He finally resolved on day 18. Patients 4 and 5 who had solid mass developed grade 3 ICANS. In patient 4, the left eyeball and the superior and inferior conjunctiva became red and swollen and the eyeball was bloated and tender, and she also had slightly positive neck rigidity on day 4. After 3 days’ treatment with mannitol and furosemide, the swelling of eyeball is obviously alleviated, and in the next day, her neck rigidity also eased. Patient 5 experienced headache and occasional convulsions in the corner of his mouth on day 6 and was treated with mannitol and intrathecal Dex, but she still subsequently developed sensory neuropathy in her left leg on the next day. After 12 days of treatment, her ICANS was reduced to grade 1, and finally resolved on day 22. The detailed information of ICANS was in Table 2, Table S2 and Fig. 4a. The detailed principle of ICANS management is in Fig. 4f.
Table 2.
Grading of adverse events among all 12 treated patients
| Patient No | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRS | ||||||||||||
| Total | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 0 | 2 | 2 | 1 | 1 |
| Fever | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Hypotension | ||||||||||||
| Hypoxia | 3 | 3 | 2 | 2 | ||||||||
| ICANS | ||||||||||||
| Total | 2 | 4 | 3 | 3 | 3 | 1 | 0 | 3 | 1 | 1 | 3 | 1 |
| ICE score | 2 | 1 | 1 | 1 | 1 | |||||||
| Depressed level of consciousness | 4 | |||||||||||
| Seizure | 4 | 3 | 3 | |||||||||
| Motor weakness | 4 | |||||||||||
| Elevated ICP/cerebral edema | 4 | 3 | 3 | 3 | 3 | |||||||
Grading according to the American Society for Transplantation and Cellular Therapy (ASTCT)
ICP intracranial pressure, ICE score immune effector cell-associated encephalopathy score, CRS cytokine-release symptom, ICANS immune effector cell-associated neurotoxicity syndrome
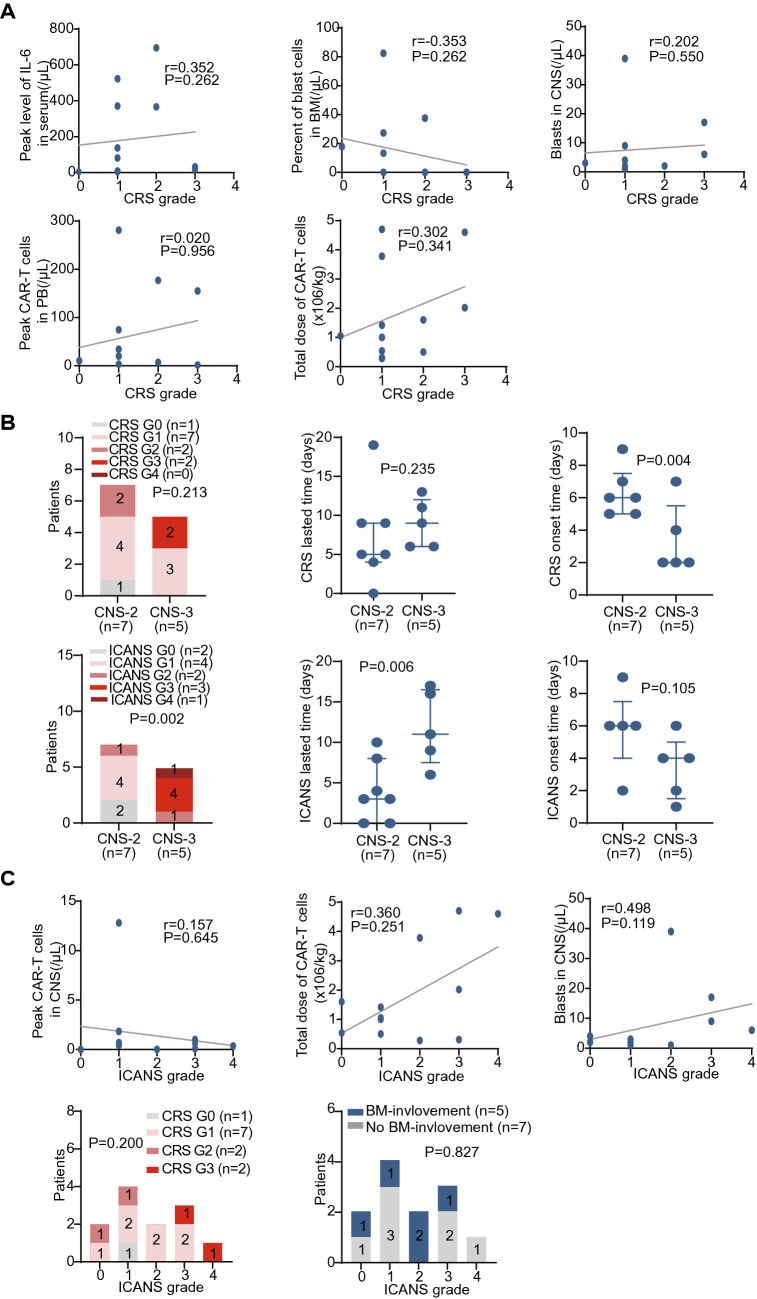
Serum cytokine markers of systemic inflammation including interleukin-6 (IL-6), interleukin-10 (IL-10), soluble CD25 (sCD25), and tumor necrosis factor-α (TNF-α) were elevated after infusion and reached peak levels around 10 days after infusion (Fig. 4e). Cytokine profiles in CSF were not routinely monitored. Possibly due to the low leukemia burden in BM, Severe CRS was not associated with baseline BM leukemia burden or peak level of IL-6 (P = 0.262 and 0.262, Fig. 5a), peak expansion of CAR T cells in PB, or infusion dose of CAR T-cells (P = 0.956 and 0.341, Fig. 5a). Although grade 3 CRS occurred in the two patients with CNS3, the CRS severity did not significantly correlate with CNSL burden, and there was no significant difference in severity or duration of CRS between CNS2 and CNS3 patients (P = 0.213 and 0.235, Fig. 5b). Nevertheless, patients in CNS3 group showed a significantly earlier onset of CRS after infusion (P = 0.004, Fig. 5b).
Fig. 5.
Influence of various factors on CRS and ICANS severity. a The correlation of peak level of serum IL-6, baseline disease burden in BM, leukemia burden in CSF, dose of CAR T cells infusion, or peak CAR T-cell expansion in PB with CRS grade. b Comparison of grade, persistent time and onset time of CRS and ICANS between patients with different CNS status. c The correlation of CRS grade, BM-involvement, leukemia burden in CSF, dose of CAR T cells infusion, or peak CAR T-cell expansion in CSF with ICANS grade. The P values are the Chi-square tests values and Spearman correlation coefficient tests values across groups
Notably, the ICANS in CNS3 patients was significantly severer and more persistent than that of CNS2 patients (P = 0.002 and 0.006, Figs. 4a and 5b), but there was no significant difference between these two groups in the onset time of ICANS (P = 0.105, Fig. 5b). We did not observe a severer neurotoxicity in patients with solid mass than those without, and the ICANS period seemed to be shorter in patient 4 who had solid mass in his two optic nerves but no blasts in CSF (P = 0.251, Fig. 5c). Patients 2 and 3 with grade 4 ICANS had higher peak expansions of CAR-T cells in CSF than the other patients, implying a potential association of CSF CAR T-cell expansion with neurotoxicity, but this difference did not achieve statistical significance possibly due to the limited number of participants (P = 0.645, Fig. 5c). Severe ICANS was not associated with higher infused cell dose, and severe ICANS were observed even in patient 5 who received a very low dose of CAR T cells (P = 0.251, Fig. 5c). Severe neurotoxicity was also not associated with severe CRS or bone marrow disease (P = 0.200 and 0.827, Fig. 5c).
Long-term follow-up
To the observation endpoint, four patients did not receive any further treatments, and among them, three (75%) (No. 7, 3 and 1) remained in remission at 1, 6 and 7 months after infusion, and one (No. 6) had a relapse at 6 months. Three (25%) (No. 2, 4 and 11) patients were bridged to transplantation as consolidation and remained in remission at 7, 6 and 10 months after CAR T-cell infusion. Five (42%) patients received a secondary CD22 CAR T-cell infusion as consolidation, and among them, patient 5, 10, 12 remained in remission at 15, 17 and 12 months, and patients 8 and 9 had a relapse at 8 and 3 months. Of the three relapsed patients, only 1 was CNS relapse (No. 8) and 2 were CD19-relapse (No. 6 and 9). In a median follow-up time of 7 months (range 1–17), the 6-month LFS rate was 81.8% (95% CI 59.0–100) and one-year overall survival (OS) rate was 87.5% (95% CI 64.6–100, Fig. 6a). LFS was not significantly different between subgroups divided by different consolidation therapies (P = 0.558). There was no significant difference of LFS between CNS2 and CNS3 subgroups (P = 0.155, Fig. 6b) and between subgroups according to other clinicogenetic factors (Figure S2). Seven of the 8 patients who were not bridged to transplantation did not have immunoglobulin or non-malignant B-cell recovery at the endpoint (Fig. 6c), suggesting the long-lasting surveillance of CD19 CAR T cells in vivo. The detailed outcomes of patients are listed in Table 1.
Fig. 6.
Long-term outcome. a Kaplan–Meier curves for leukemia-free survival, overall survival and CNS leukemia-free survival of all patients who received CAR T-cell therapy. b Kaplan–Meier curves for leukemia-free survival for patient subgroups according to different leukemia burden after CAR T-cell therapy. c Recovery of immunoglobulin and normal B cells in PB among 12 patients, determined by immunoturbidimetry and flow cytometry, respectively. MRD minimal residual disease, HCT hematopoietic stem cell transplantation, PR partial response, CR complete remission. Numbers in parentheses show number of patients with event/number of patients per group. The P values are the log-rank tests values across groups
Discussion
CAR T cells may provide an opportunity for patients who had CNSL that are refractory to current therapeutic regimens. Despite the recent reports of CD19 CAR T-cell treatment of r/r B-ALL patients with CNSL, the number of these cases was still very small, and rarely involved high-burden CNSL. The feasibility, toxicity and effectiveness of CAR T cells in CNSL need to be evaluated in more patients. Here, we demonstrated that anti-CD19 CAR T cells were very effective in inducing remission of 12 pediatric patients with different burdens or forms of refractory CNSL. Although CAR T cells rapidly clear blasts in CSF within 1 month, it might take longer time (2–3 months) to eliminate solid mass in CNS. The long-term anti-CNSL efficacy was dramatic since only one patient had CNS relapse again.
Neurotoxicity was a major adverse event of CAR T therapy regardless the presence of CNSL, [16] and the impact of CNSL on neurotoxicity triggered by CAR T cells was not clear. Similar with other studies, [4–9] CD19 CAR T cells merely induced mild (grade 1–2) neurotoxicity in patients with low burden of CNSL (blasts < 5/μL in CSF) before CAR T-cell expansion. In a recent study of Tisagenlecleucel in adult secondary CNS lymphoma, of eight patients with solid mass in CNS, three patients had also blasts in CSF, no severe neurotoxicity has been observed. [17] Different from this study, high-burden (blasts > 5/μL in CSF) or solid-mass CNSL patients mostly developed severe and persistent neurological symptoms in our study. Whether the severer neurotoxicity occurs in children due to immature central nervous system, different disease features or CAR constructs should be further studied. Encephaledema and intracranial hypertension were commonly observed on the initial phase of ICANS in our patients. CD19 CAR T-cell therapy should not be necessarily feasible for patients with high-burden CNSL except no other options to reduced leukemia burden. To avoid mortality from severe ICANS, intensive interventions were conducted. Mannitol was usually effective in patients who had symptoms of intracranial hypertension, and in patients without improvement after mannitol use, furosemide was further added. Shah NN, et al. reported intrathecal chemotherapy for management of steroid-refractory CAR-T-cell-associated neurotoxicity syndrome, [18] we also found that intrathecal administration of Dex was very effective for patients who had progressive ICANS and developed seizures but did not hamper the efficacy of CAR T cells. All patients recovered after intensive interventions. These results indicate that a high burden of CNSL before CAR T-cell expansion may predispose to severe neurotoxicity, which need to be intensively monitored and managed. In addition, to reduce CNSL burden before CAR T-cell treatment may help lower the risk of severe ICANS.
Nine (75%) of the 12 patients treated with CAR T cells remained in remission until the observation endpoint, and 8 achieved an LFS of over 6 months. Intriguingly, of the three relapsed patients, only one had a CNS relapse. Consolidations with allo-HCT or CD22 CAR T cells have been conducted in 6 patients after primary infusion, but we have not observed an association of consolidation with favorable outcome, probably due to the limited number of patients. The LFS of patients was not associated with most clinicogenetic factors including leukemia burden in BM or CNS. Most patients without transplantation had active CAR T surveillance as manifested by low serum Ig levels and B-cell aplasia. These results collectively indicate that CD19 CAR T cells have dramatic long-term efficacy against CNSL.
Irreversible long-term neural damages were serious problems associated with CNS-targeted therapy for leukemia. A report indicated that radiotherapy based on FTBI-regimen caused the reduction of subcortical structure volumes and decline in cognitive performance [19]. Several other studies also reported the late neurotoxicity after chemotherapy in ALL survivors. Indeed, some neurotoxic drugs, such as high-dose methotrexate (MTX), were associated with long-term impairments in memory, attention, processing speed, and executive function [20–23]. A previous report [8]] showed a case of low-burden CNSL that developed long-term neurotoxicity manifested as numbness and stiffness of lower limbs and abdominal skin 4 weeks after two rounds of infusion of CD19 CAR T cells, which lasted for 8 weeks. In contrast, our study did not show any delayed irreversible neuronal impairments within the follow-up period, suggesting that as a more targeted therapy, CAR T cells may have less permanent side effects on CNS. Even though, extended observation periods were needed to confirm the long-term impact of CAR T cells on structural and functional status of CNS.
In summary, our study demonstrated that CD19 CAR T cells are feasible and effective in treating both low-burden and high-burden central nervous system B-ALL that is refractory to current regimens, and the disease remission after this therapy is sustainable. Severe and persistent neurotoxicity, which were usually associated with a high leukemia burden in CNS, may occur during the treatment. Although it is manageable with timely and intensive interventions, this treatment should not be directly considered for patients with high-burden CNSL. To maximally reduce the leukemia burden before CAR T-cell, infusion may be helpful for lowering the risk of the development of severe neurotoxicity. Future clinical trials with larger cohort of CNSL patients will further validate and optimize this strategy to fulfill the unmet needs of children with incurable refractory CNSL.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients and their families who participated in this study. And we also thank all physicians, nurse, and other patient care providers involved in the care of these patients.
Abbreviations
- Allo-HCT
Allo-hematopoietic stem-cell transplant
- Ara-C
Cytarabine
- CAR
Chimeric antigen receptor
- CNS
Central nervous system
- CNSL
Central nervous system leukemia
- CR
Complete remission
- CRS
Cytokine release syndrome
- CSF
Cerebrospinal fluid
- Dex
Dexamethasone
- EMDs
Extramedullary diseases
- ICANS
Immune effector cell-associated neurotoxicity syndrome
- IDA
Darubicin
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- IT
Intrathecal injection
- L-ASP
L-Asparaginase
- LFS
Leukemia-free survival
- MRD
Minimal residual disease
- MTX
Methotrexate
- OS
Overall survival
- r/r B-ALL
Refractory or relapsed B-cell acute lymphoblastic leukemia
- sCD25
Soluble CD25
- TNF-α
Tumor necrosis factor-α
- VDS
Vindesine
Author contributions
YT and JP contributed to data collection, data analyses, data interpretation. BD and AHC contributed to CAR T-cell manufacture. JP, ZL, WS, JX, JD and ZW contributed to clinical protocol. XY was responsible for leukemic cell immunophenotyping. YT and JP were responsible for all statistical analyses. YT, JP and XF wrote the manuscript. JP and XF directed the study and had final responsibility to submit for publication. The authors read and approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (2019YFA0110200), the National Natural Science Foundation of China (81870090), the Tianjin Science Funds for Distinguished Young Scholars (17JCJQJC45800) and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2018PT32034).
Compliance with ethical standards
Conflict of interest
AHC is also a founding member of Shanghai YaKe Biotechnology Ltd. The remaining authors declare no conflict of interest.
Ethical approval and consent to participate
The study protocols were approved by the institutional review board at Beijing Boren hospital, and the patients provided written informed consent. This clinical investigation was conducted according to the principles of the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yue Tan and Jing Pan contributed equally to this study.
Contributor Information
Jing Pan, Email: panj@borenhospital.com.
Alex H. Chang, Email: alexhchang@yahoo.com
Xiaoming Feng, Email: fengxiaoming@ihcams.ac.cn.
References
- 1.Lazarus HM, Richards SM, Chopra R, et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRCUKALL XII/ECOG E2993. Blood. 2006;108(2):465–472. doi: 10.1182/blood-2005-11-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown P, Inaba H, Annesley C, et al. NCCN clinical practice guidelines in oncology: pediatric acute lymphoblastic leukemia, version 1.2020. Accessed date: 30 May 2019. [DOI] [PubMed]
- 3.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG2993 study. Blood. 2007;109(3):944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 4.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peter B, Hermann K, Gunter HR, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: The ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377–384. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 6.Shannon LM, Noelle F, Pamela AS, et al. Chimeric antigen receptor t cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He X, Xiao X, Qing L, et al. Anti-CD19 CAR-T as a feasible and safe treatment against central nervous system leukemia after intrathecal chemotherapy in adults with relapsed or refractory B-ALL. Leukemia. 2019;33(8):2102–2104. doi: 10.1038/s41375-019-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai H, Zhang W, Li X, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Onco Immunol. 2015;4(11):e1027469. doi: 10.1080/2162402X.2015.1027469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pullen J, Boyett J, Shuster J, et al. Extended triple intrathecal chemotherapy trial for prevention of CNS relapse in good-risk and poor-risk patients with B-progenitor acute lymphoblastic leukemia: a Pediatric Oncology Group study. J Clin Oncol. 1993;11:839–849. doi: 10.1200/JCO.1993.11.5.839. [DOI] [PubMed] [Google Scholar]
- 10.Pan J, Niu Q, Deng B, et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019;33(12):2854–2866. doi: 10.1038/s41375-019-0488-7. [DOI] [PubMed] [Google Scholar]
- 11.Pan J, Zuo S, Deng B, et al. Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood. 2020;135:387–391. doi: 10.1182/blood.2019003293. [DOI] [PubMed] [Google Scholar]
- 12.Pan J, Yang JF, Deng BP, et al. High efficacy and safety of low dose CD19 directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia. 2017;31(12):2587–2593. doi: 10.1038/leu.2017.145. [DOI] [PubMed] [Google Scholar]
- 13.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen recepatientor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel WL, Bianca DS, Frederick LL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212–2221. doi: 10.1182/blood-2018-12-893396. [DOI] [PubMed] [Google Scholar]
- 17.Matthew JF, Jorg D, Maria ML, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019;134(11):860–866. doi: 10.1182/blood.2019001694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nirav NS, Bryon DJ, Timothy SF, et al. Intrathecal chemotherapy for management of steroid-refractory CAR T-cell–associated neurotoxicity syndrome. Blood Adv. 2020;4(10):2119–2122. doi: 10.1182/bloodadvances.2020001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zając SO, Pawlak MA, Karmelita KK, et al. Long-term brain status and cognitive impairment in children treated for high-risk acute lymphoblastic leukemia with and without allogeneic hematopoietic stem cell transplantation: a single-center study. Pediatr Blood Cancer. 2020;67(6):e28224. doi: 10.1002/pbc.28224. [DOI] [PubMed] [Google Scholar]
- 20.Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32(9):949–959. doi: 10.1200/JCO.2013.53.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung YT, Sabin ND, Reddick WE, et al. Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of child-hood acute lymphoblastic leukaemia treated with chemotherapy: a longitudinal analysis. Lancet Haematol. 2016;3(10):456–466. doi: 10.1016/S2352-3026(16)30110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halsey C, Buck G, Richards S, et al. The impact of therapy for childhood acute lymphoblastic leukaemia on intelligence quotients; results of the risk-stratified randomized central nervous system treatment trial MRC UKALL XI. J Hematol Oncol. 2011;4:42. doi: 10.1186/1756-8722-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson EM, Nagaraja S, Ocampo A, et al. Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell. 2019;176(1–2):43–55.e13. doi: 10.1016/j.cell.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.