Abstract
Background
Lenvatinib is regarded as the first-line therapy for patients with unresectable hepatocellular carcinoma (HCC). This study assessed the efficacy and safety of lenvatinib with or without immune checkpoint inhibitors (ICIs) in patients with unresectable HCC.
Methods
In this multicentric retrospective study, patients with unresectable HCC who treated with lenvatinib with or without ICIs would be enrolled. Overall survival, progression-free survival, objective response rate, and disease control rate were calculated to assess the antitumor response.
Results
Between January 2019 and August 2020, 65 patients received lenvatinib plus ICIs while other 45 patients received lenvatinib. The baseline characteristics were comparable between the two groups. Lenvatinib plus ICIs provided significantly higher overall survival (hazard ratio = 0.47, 95% CI 0.26–0.85; p = 0.013) and progression-free survival (hazard ratio = 0.35, 95% CI 0.20–0.63; p < 0.001) than lenvatinib monotherapy. Moreover, patients with lenvatinib plus ICIs had significantly higher objective response rate (41.5% vs 20.0%, p = 0.023) and disease control rate (72.3% vs 46.7%, p = 0.009) per RECIST v1.1 than those with lenvatinib. No treatment-related deaths were observed. Grade 3 or greater adverse events occurring in 10% or more of patients in either treatment group were hypertension [13 (20.0%) of 65 patients treated with lenvatinib plus ICIs vs 8 (17.8%) of 45 patients treated with lenvatinib], and palmar–plantar erythrodysesthesia [seven (10.8%) vs two (4.4%)].
Conclusions
In this real-world study, lenvatinib combined with ICIs showed significantly promising efficacy and manageable safety than lenvatinib alone in patients with unresectable HCC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03060-w.
Keywords: Hepatocellular carcinoma, Immune checkpoint inhibitors, Lenvatinib, Overall survival
Introduction
In the global cancer statistics 2020, hepatocellular carcinoma (HCC) is the seventh incident cancer and ranked as the third most common cancer-related death [1]. Due to the lack of significant symptoms and signs of early disease, many patients with HCC are diagnosed at advanced stage disease [2, 3]. For such patients, systemic treatment is the recommended therapy in official HCC guidelines [4–6]. The multikinase inhibitor sorafenib was the first and the only agent as a standard first-line systematic treatment to show extended overall survival (OS) in patients with advanced HCC in 2007–2017 [4–6]. All other randomized phase 3 trials have been negative with respect to OS when compared with sorafenib in a first-line setting. However, regorafenib [7], nivolumab with or without ipilimumab [8], pembrolizumab [9], cabozantinib [10], and ramucirumab [for patients with alpha fetoprotein (AFP) ≥ 400 ng/mL] [11] are proved as second-line systematic treatment in sorafenib-experienced patients with HCC. Until 2018, multikinase inhibitor lenvatinib was approved as the second promising first-line drug for HCC patients with unresectable HCC [12]. In the REFLECT trial, lenvatinib showed non-inferior to sorafenib in terms of OS, and clinically meaningful improvement in progression-free survival (PFS) and objective response rate (ORR), especially for Asian patients [12]. After that, some retrospective studies with small sample size in real-world clinical practice have confirmed the safety and efficacy of lenvatinib alone or combined with transarterial chemoembolization for patients with unresectable HCC [13–15].
As anti-PD-1 mAb agents, nivolumab and pembrolizumab, which are well tolerated by the majority of HCC patients and are potential for durable objective responses [8, 9], are immune checkpoint inhibitor (ICI). In recent years, more and more other ICIs are used in clinical trials [16, 17]. In 2020, combinations of atezolizumab (an anti-PD-L1 mAb agent) plus bevacizumab (a monoclonal antibody that targets VEGF) are also available to treat patients with unresectable HCC in the frontline [18]. In the phase Ib KEYNOTE-524 trial, lenvatinib plus pembrolizumab showed promising efficacy and manageable safety in treatment-naive unresectable HCC [19]. In 2021, a phase II trial (RESCUE) found camrelizumab (an anti-PD-1 mAb agent) combined with apatinib (a VEGFR-2 tyrosine kinase inhibitor) showed promising antitumor activity with a tolerable safety profile in patients with advanced HCC in both the first-line and the second-line setting [20]. However, the clinical benefit of combining lenvatinib with ICI for such patients remains uncertain in the real-world setting. Therefore, we performed this retrospective analysis to evaluate the efficacy and safety of combining lenvatinib with ICI for patients with unresectable HCC. Moreover, we also investigated whether the combining lenvatinib with ICI provides a greater survival benefit than lenvatinib alone in such patients.
Patients and methods
Study population
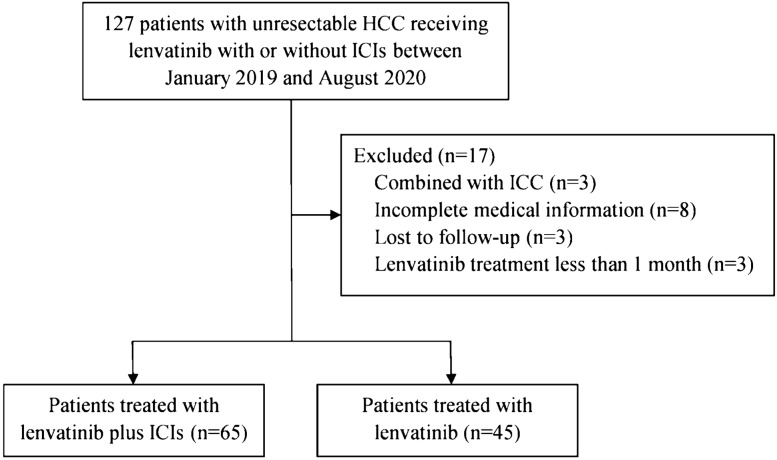
Consecutive patients were identified via the electronic medical records. Between January 2019 and August 2020, 127 patients received lenvatinib treatment for unresectable HCC at the Hepatobiliary Surgery Department II, Guangxi Medical University Cancer Hospital, Nanning, China, and the Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou, China. Of these, 110 patients were selected based on the following eligibility criteria: (1) patients with unresectable HCC not fit for hepatic resection, local ablation, or chemoembolization therapy, (2) received oral lenvatinib monotherapy or a combination of lenvatinib and ICIs, including those receiving other therapies (such as hepatic resection, transarterial chemoembolization, radiotherapy, or second- or third-line targeted therapy) after lenvatinib with or without ICIs treatment, (3) had at least 1 measurable lesion as defined by revised Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 [21], (4) had Child–Pugh class A or B liver function, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate organ function at the time of lenvatinib initiation, (5) dynamic enhanced computerized tomography or magnetic resonance imaging study performed within 1 month prior to initiation of lenvatinib and ≥ 1 months after initiation of lenvatinib to evaluate the treatment response, (6) a treatment interval of ≥ 1 months since previous sorafenib or regorafenib treatment, and (7) an observation period of ≥ 1 months. Patients receiving lenvatinib treatment for intrahepatic cholangiocarcinoma, with incomplete medical information or lost to follow-up, were excluded. Moreover, treatment duration of lenvatinib less than 1 month was also excluded.
Diagnosis and staging of HCC
The diagnosis of HCC was based on the image analysis using enhanced computerized tomography and/or magnetic resonance imaging, with or without AFP of at least 400 ng/mL [4, 22]. HCC staging was specified according to the Barcelona Clinical Liver Cancer (BCLC) staging system [4–6].
Agent treatment and assessment of adverse events
Patients with unresectable HCC received lenvatinib (Levima®, Eisai, Tokyo, Japan) orally at a dose of either 8 mg/day for patients < 60 kg or 12 mg/day for those ≥ 60 kg [12]. In this retrospective study, ICIs included pembrolizumab [9] (KEYTRUDA, Merck Sharp & Dohme Co., Inc.), camrelizumab [20] (SHR-1210, Jiangsu HengRui Medicine Co., Ltd.), toripalimab [23] (triprizumab, JS001, Shanghai Junshi Biosciences Co., Ltd), tislelizumab [24] (BGB-A317, BeiGene), and sintilimab [25] (IBI308, Innovent Biologics [Suzhou] Co. Ltd.). Dose and duration of ICIs were according to the guidelines provided by the manufacturer. Although ICIs were recommended to all patients with unresectable HCC in this cohort, many of them refused ICIs therapy due to frequent hospitalizations and high costs.
If a patient develops grade ≥ 3 severe adverse events (AEs) or any unacceptable grade 2 drug related AEs, the drug dose should be reduced or the treatment interrupted according to the guidelines for administration of lenvatinib. Moreover, dose reduction or temporary interruption would be maintained until the symptom released to either grade 1 or 2. However, if significantly clinical tumor progression was observed or serious AEs occurred, lenvatinib and ICIs treatment would be discontinued. At this case, second- or third-line targeted therapy and second-line ICIs would be recommended to patients. ICI switching was permitted in the combination group. AEs were assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 [26].
Data collection and study objectives
The following data were collected and analyzed: age, gender, ECOG performance status score, Child–Pugh liver function score, positive of hepatitis B surface antigen, positive of hepatitis C antibody, AFP level, platelet count, total bilirubin, alanine aminotransferase, albumin, prothrombin time, tumor size, tumor number, absence or presence of extrahepatic metastasis and macrovascular invasion, BCLC stage, and previous therapy. All imaging data were independently assessed by one radiologist (W.W) who was blinded to the clinical data and one hepatologist (J.-H.Z or L.L). Discrepancies between these two examiners would be resolved by another more experienced radiologist who was also blinded to the clinical data.
The primary endpoint was OS, defined as the time from the first dose of lenvatinib to death from any cause. The secondary endpoints included PFS, ORR, and disease control rate (DCR). PFS was defined as the time from the first dose of lenvatinib to radiographic disease progression according to the RECIST criteria or death from any cause, whichever occurred first. ORR was defined as the proportion of patients with complete response or partial response. Moreover, complete or partial response should be maintained for at least 4 weeks from the first radiological confirmation of that case. DCR was defined as the proportion of patients with ORR plus stable disease. The treatment response was evaluated according to RECIST v1.1 [21] and modified RECIST (mRECIST) [27], respectively.
Statistics analysis
The statistical package used to perform the analyses was IBM SPSS software (ver. 26.0 SPSS Inc., Chicago, IL, USA). Continuous variables were converted to categorical variables. Categorical data were expressed as number and percentage. The distributions of the categorical variables were compared with Pearson’s Chi-square test or Fisher’s exact test. OS and PFS were calculated with the Kaplan–Meier method, with the values compared using the log-rank test. The median OS and PFS were estimated, and the corresponding 95% confidence intervals (CIs) were calculated using the Brookmeyer and Crowley method. To identify the risk factors associated with OS or PFS after the first dose of lenvatinib, multivariate analyses were performed using a Cox proportional hazards model. Any factors that were statistically significant at p value < 0.10 in the univariate analysis were entered into a stepwise Cox regression analysis. A two-tailed p value < 0.05 was considered to indicate statistically significant.
Results
Patient demographic and baseline clinical characteristics
Between 15 January 2019 and 25 August 2020, 110 patients were included based on the criteria for inclusion and exclusion in this study: 45 patients received lenvatinib monotherapy and 65 patients received combination therapy with lenvatinib plus ICIs (Fig. 1). The baseline characteristics were comparable between the two groups (Table 1). The median age was 53 (30–75) years. Most of the study population was male (88.2%) and the main etiology of HCC was hepatitis B virus (79.1%). All patients with positive hepatitis B antigen received entecavir or tenofovir antiviral therapy. The proportion of patients with extrahepatic metastases was 32.7% and 81 (73.6%) patients were with macrovascular invasion. No patient was with BCLC 0 or A disease. Seven (6.4%) patients had received other targeted therapy before the first dose of lenvatinib. Only 22 (20%) patients had not received local or systematic therapies before the first dose of lenvatinib. The detailed treatments before lenvatinib are shown in online suppl. Table 1.
Fig. 1.
Patient selection flow. HCC hepatocellular carcinoma, ICC intrahepatic cholangiocarcinoma, ICIs immune checkpoint inhibitors
Table 1.
Patient baseline demographic and clinical characteristics
| Variables | REFLECT trial | KEYNOTE 524 | Present study | ||
|---|---|---|---|---|---|
| Lenvatinib, n = 478 (%) | Lenvatinib plus pembrolizumab, n = 100 (%) | Lenvatinib, n = 45 (%) | Lenvatinib + ICIs, n = 65 (%) | p | |
| Age, yrs | |||||
| < 65 | 270 (56.5) | 38 (38) | 37 (82.2) | 59 (90.8) | 0.246 |
| ≥ 65 | 208 (43.5) | 62 (62) | 8 (17.8) | 6 (9.2) | |
| Gender | |||||
| Male | 405 (84.7) | 81 (81) | 42 (93.3) | 55 (84.6) | 0.233 |
| Female | 73 (15.3) | 19 (19) | 3 (6.7) | 10 (15.4) | |
| ECOG performance status | |||||
| 0 | 304 (63.6) | 62 (62) | 29 (64.4) | 43 (66.2) | 1.000 |
| 1 | 174 (36.4) | 38 (38) | 16 (35.6) | 22 (33.8) | |
| Child–Pugh score | |||||
| A | 475 (99.4) | 98 (98) | 31 (68.9) | 43 (66.2) | 0.838 |
| B | 3 (0.6) | 2 (2) | 14 (31.9) | 22 (33.8) | |
| Hepatitis B surface antigen | |||||
| Negative | 227 (47.5) | 81 (81) | 10 (22.2) | 13 (20.0) | 0.815 |
| Positive | 251 (52.5) | 19 (19) | 35 (77.8) | 52 (80.0) | |
| Hepatitis C virus antibody | |||||
| Negative | 387 (81.0) | 64 (64) | 43 (95.6) | 61 (93.8) | 1.000 |
| Positive | 91 (19.0) | 36 (36) | 2 (4.4) | 4 (6.2) | |
| α-fetoprotein concentration | |||||
| < 200 ng/ml | 255 (53.3) | 61 (61) | 20 (44.4) | 24 (36.9) | 0.553 |
| ≥ 200 ng/ml | 222 (46.4) | 36 (36) | 25 (55.6) | 41 (63.1) | |
| Missing | 1 (0.2) | 3 (3) | 0 | 0 | |
| Extrahepatic metastases | |||||
| Absent | 187 (39.1) | 48 (48) | 30 (66.7) | 44 (67.7) | 1.000 |
| Present | 291 (60.9) | 52 (52) | 15 (33.3) | 21 (32.3) | |
| Macrovascular invasion | |||||
| Absent | 369 (77.2) | 80 (80) | 15 (33.3) | 14 (21.5) | 0.191 |
| Present | 109 (22.8) | 20 (20) | 30 (66.7) | 51 (78.5) | |
| BCLC stage | |||||
| B | 104 (21.8) | 29 (29) | 11 (24.4) | 8 (12.3) | 0.125 |
| C | 374 (78.2) | 71 (71) | 34 (75.6) | 57 (87.7) | |
| Tumor size | – | ||||
| < 5 cm | – | – | 10 (22.2) | 13 (20.0) | 0.815 |
| ≥ 5 cm | – | – | 35 (77.8) | 52 (80.0) | |
| Tumor number | – | ||||
| Single | – | – | 15 (33.3) | 25 (38.5) | 0.688 |
| Multiple | – | – | 30 (66.7) | 40 (61.5) | |
| Platelet count | – | ||||
| < 100 × 109/L | – | – | 5 (11.1) | 9 (13.8) | 0.776 |
| ≥ 100 × 109/L | – | – | 40 (88.9) | 56 (86.2) | |
| Total bilirubin | – | ||||
| ≤ 1 ULN | – | – | 34 (75.6) | 40 (61.5) | 0.150 |
| > 1 ULN | – | – | 11 (24.4) | 25 (38.5) | |
| Alanine aminotransferase | – | ||||
| < 40U/L | – | – | 31 (68.9) | 36 (55.4) | 0.170 |
| ≥ 40U/L | – | – | 14 (31.1) | 29 (44.6) | |
| Albumin | – | ||||
| < 30 g/ml | – | – | 6 (13.3) | 11 (16.9) | 0.790 |
| ≥ 30 g/ml | – | – | 39 (86.7) | 54 (83.1) | |
| Prothrombin time, median (IQR) | – | – | |||
| < 13 s | – | – | 27 (60.0) | 42 (64.6) | 0.690 |
| ≥ 13 s | – | – | 18 (40.0) | 23 (35.4) | |
Data are median (IQR) or N (%)
ECOG Eastern Cooperative Oncology, BCLC Barcelona Clinic Liver Cancer, HBV, Hepatitis B virus, HCV Hepatitis C virus, ULN upper limit of normal
The median duration of lenvatinib therapy was 5.3 (1.1–19.6) months in the lenvatinib group and 6.7 (1.2–21.5) months in the combination group, respectively. Five types of ICIs were used as first-line ICI, including pembrolizumab (n = 5), camrelizumab (n = 31), sintilimab (n = 21), toripalimab (7), and tislelizumab (n = 1). In the combination group, 65 patients were treated with a total of 459 cycles of ICIs (median 7, range 1–16). Fourteen (21.5%) of them received second-line ICIs due to tumor progression or severe adverse events. In addition, some patients received subsequent transarterial chemoembolization (n = 8), regorafenib (n = 4), and sorafenib (n = 1). In the lenvatinib group, 5 patients received subsequent treatments, including hepatectomy (1), transarterial chemoembolization (n = 1), regorafenib (n = 2), and radiotherapy (n = 1) (online suppl. Table 1).
Treatment efficacy
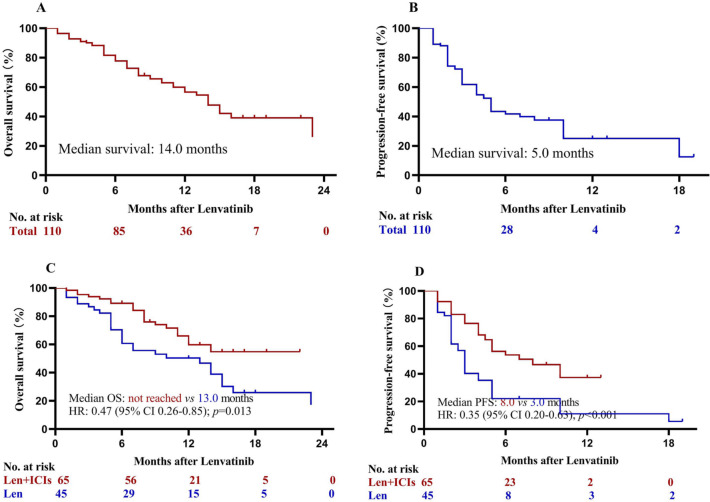
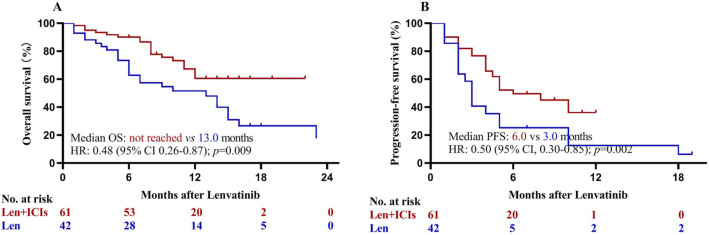
The follow-up was finished on April 2, 2021. The median follow-up was 17.0 months in the lenvatinib group and 12.5 months in the lenvatinib plus ICIs group. At the time of analysis, 28 (62.2%) deaths occurred in the lenvatinib group and 22 (33.8%) deaths occurred in the lenvatinib plus ICIs group. The median OS was 14.0 months for the total population (Fig. 2a). In addition, 34 (75.6%) and 31 (47.7%) patients had disease progression or death in lenvatinib group and lenvatinib plus ICIs group, respectively. The median PFS was 5.0 months for the total population (Fig. 2b). The Kaplan–Meier estimated 12-month OS rates were 59.8% in the lenvatinib plus ICIs group and 50.4% in the lenvatinib group. Patients with lenvatinib plus ICIs had statistically significant higher OS than those with lenvatinib [hazard ratio (HR) = 0.47; 95% CI, 0.26–0.85; p = 0.013; Fig. 2c]. The corresponding median PFS was 8.0 months compared with 3.0 months (HR = 0.35; 95% CI, 0.20–0.63; p < 0.001; Fig. 2d). Sensitivity analysis after excluding patients with previous targeted therapy (n = 7) confirmed that lenvatinib plus ICIs provided significant higher OS and PFS than lenvatinib (all p < 0.05; Fig. 3).
Fig. 2.
Kaplan–Meier analysis of overall and progression-free survival. a Overall survival for the total population, b progression-free survival for the total population, c overall survival for lenvatinib plus ICIs and lenvatinib monotherapy groups, and d progression-free survival for lenvatinib plus ICIs and lenvatinib monotherapy groups. HR hazard ratio, ICIs immune checkpoint inhibitors, Len lenvatinib, OS overall survival, PFS progression-free survival
Fig. 3.
Kaplan–Meier analysis of overall (a) and progression-free survival (b) after excluding patients with previous targeted therapy (n = 7). ICIs immune checkpoint inhibitors, Len lenvatinib, HR hazard ratio, OS overall survival, PFS progression-free survival
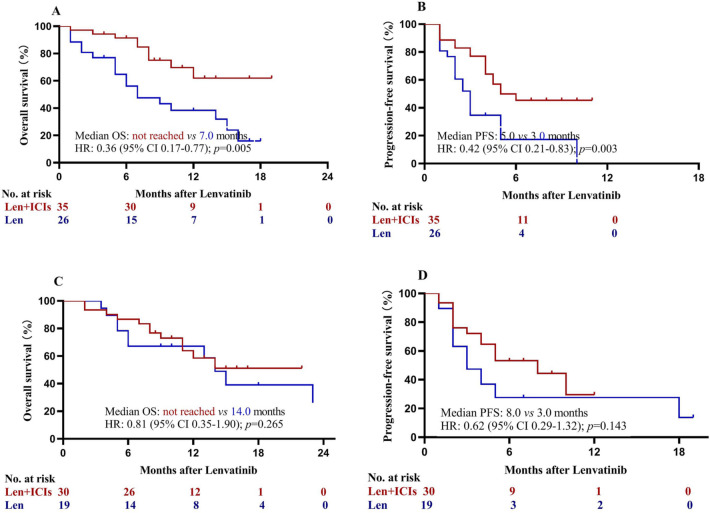
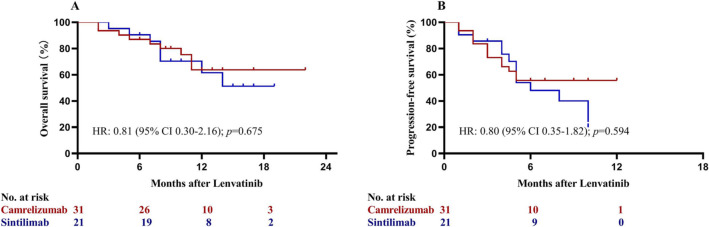
Patients achieved a partial response had significant better OS than those with stable or progressive disease (p < 0.001; Fig. 4). Subgroup analysis based on serum AFP concentrations was performed. Patients with lenvatinib plus ICIs had statistically significant higher OS (HR = 0.36; 95% CI, 0.17–0.77; p = 0.005; Fig. 5a) and PFS (HR = 0.42; 95% CI, 0.21–0.83; p = 0.003; Fig. 5b) than those with lenvatinib only among patients with AFP concentrations of at least 400 ng/ml. Among patients with AFP concentrations of less than 400 ng/mL, however, patients in the two groups had similar OS (HR = 0.81; 95% CI, 0.35–1.90; p = 0.265; Fig. 5c) and PFS (HR = 0.62; 95% CI, 0.29–1.32; p = 0.143; Fig. 5d). Subgroup analysis comparing the efficacy of camrelizumab (n = 31) and sintilimab (n = 21) showed patients receiving lenvatinib plus camrelizumab or sintilimab had similar OS and PFS (all p > 0.05; Fig. 6).
Fig. 4.
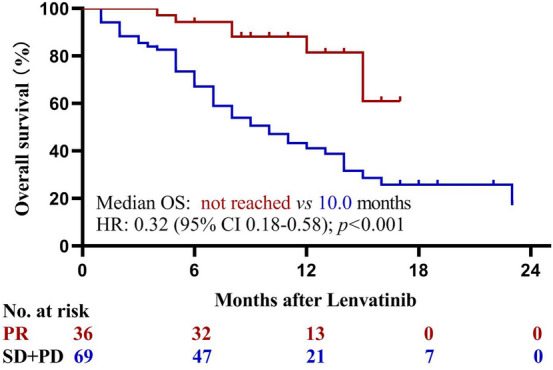
Kaplan–Meier analysis of overall survival stratified by tumor response which was assessed with Response Evaluation Criteria in Solid Tumors v1.1. HR hazard ratio, OS overall survival, PR partial response, SD stable disease, PD progressive disease
Fig. 5.
Kaplan–Meier analysis of overall and progression-free survival stratified by alpha fetoprotein concentrations. a Overall survival among patients with ≥ 400 ng/mL; b progression-free survival among patients with ≥ 400 ng/mL; c overall survival among patients with < 400 ng/mL; and d progression-free survival among patients with < 400 ng/mL. HR hazard ratio, ICIs immune checkpoint inhibitors, Len lenvatinib, OS overall survival, PFS progression-free survival
Fig. 6.
Kaplan–Meier analysis of overall (a) and progression-free survival (b) in subgroup analysis comparing the efficacy of camrelizumab and sintilimab. HR hazard ratio
Multivariate analyses showed that lenvatinib plus ICIs treatment and serum albumin level (> 30 g/ml) were independent protect factors for both OS and PFS (online suppl. Table 2).
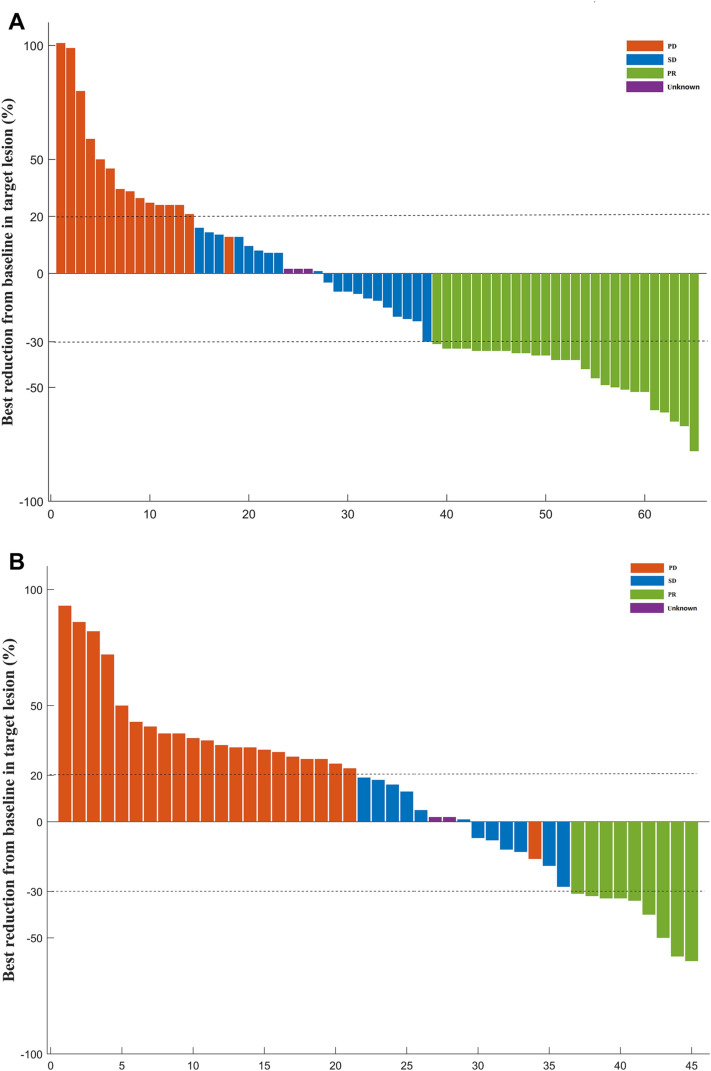
The tumor responses are presented in Table 2. Based on the RECIST v1.1 criteria, the ORR and DCR were significantly higher in patients with lenvatinib plus ICIs than those with lenvatinib (41.5% vs 20.0%, p = 0.023; 72.3% vs 46.7%, p = 0.009; respectively). None patient achieved complete response according to the RECIST v1.1 criteria, while four (3.6%) patients achieved complete response according to the mRECIST criteria. A waterfall plot was constructed to show the best change from baseline in the sum of the longest target lesion size per patient in the two groups (Fig. 7).
Table 2.
Summary of best response
| RECIST v1.1 | mRECIST | |||||
|---|---|---|---|---|---|---|
| Combination therapy, n = 65 (%) | Monotherapy, n = 45 (%) | p value | Combination therapy, n = 65 (%) | Monotherapy, n = 45 (%) | p value | |
| CR | 0 (0) | 0 (0) | NA | 3 (4.6) | 1 (2.2) | 0.643 |
| PR | 27 (41.5) | 9 (20.0) | 0.023 | 29 (44.6) | 11 (24.4) | 0.043 |
| SD | 20 (30.8) | 12 (26.7) | 0.675 | 23 (35.4) | 15 (33.3) | 0.842 |
| PD | 15 (23.1) | 22 (48.9) | 0.007 | 7 (10.8) | 16 (35.6) | 0.004 |
| Unknown or not evaluable | 3 (4.6) | 2 (4.4) | 1.000 | 3 (4.6) | 2 (4.4) | 1.000 |
| ORR | 27 (41.5) | 9 (20.0) | 0.023 | 32 (49.2) | 12 (26.7) | 0.029 |
| DCR | 47 (72.3) | 21 (46.7) | 0.009 | 55 (84.6) | 27 (60.0) | 0.007 |
CR complete response, DCR disease control rate; mRECIST modified Response Evaluation Criteria in Solid Tumors, NA not assessable; ORR objective response rate, PD progressive disease, PR partial response, RECIST Response Evaluation Criteria in Solid Tumors, SD stable disease
Fig. 7.
The best change from baseline in sum of the longest target lesion diameter per patient receiving lenvatinib plus immune checkpoint inhibitors (a) or receiving lenvatinib monotherapy (b). Assessed with Response Evaluation Criteria in Solid Tumors v1.1 in patients with image measurements before and after treatment
Safety profile
Treatment-related adverse events, which occurred in ≥ 10% of patients, are shown in Table 3. No treatment-related deaths were observed in this study. Hypertension, palmar–plantar erythrodysesthesia, hyperbilirubinemia, and nausea/vomiting were the most frequent adverse events in the two groups. More patients in the combination group were observed with hyperbilirubinemia (38.5% vs 20.0%, p = 0.058), elevated alanine aminotransferase (36.9% vs 20.0%, p = 0.090), and proteinuria (29.2% vs 11.1%, p = 0.034). Most of the cases with hyperbilirubinemia and elevated alanine aminotransferase were mainly mild to moderate and returned to normal within 1 week after liver protection treatment in most patients. Two patients developed grade 3 immune-related hepatotoxicity with hyperbilirubinemia and elevated alanine aminotransferase occurred simultaneously (one in the third day after the first dose of toripalimab therapy while another in the fifth day after the four doses of camrelizumab therapy). After treatment with corticosteroids and suspending ICIs, these two patients recovered after 1.6 and 1.1 months, respectively. In addition, mild to moderate reactive cutaneous capillary endothelial proliferation (58.1%, 18/31) was only observed among patients using camrelizumab [20]. This AE was clinically tolerable, and disappeared after suspending camrelizumab.
Table 3.
Treatment-related adverse events
| Adverse event | Combination therapy, n = 65 (%) | Monotherapy, n = 45 (%) | p value | |||
|---|---|---|---|---|---|---|
| Any grade (%) | Grade 3–4 (%) | Any grade (%) | Grade 3–4 (%) | Any grade | Grade 3–4 | |
| Hypertension | 31 (47.7) | 13 (20.0) | 18 (40.0) | 8 (17.8) | 0.443 | 0.810 |
| Palmar–plantar erythrodysesthesia | 25 (38.5) | 7 (10.8) | 15 (33.3) | 2 (4.4) | 0.688 | 0.304 |
| Hyperbilirubinemia | 25 (38.5) | 2 (3.1) | 9 (20.0) | 0 (0) | 0.058 | 0.512 |
| Nausea or vomiting | 25 (37.3) | 3 (4.6) | 13 (29.5) | 1 (2.2) | 0.422 | 0.643 |
| Elevated alanine aminotransferase | 24 (36.9) | 5 (7.7) | 9 (20.0) | 2 (4.4) | 0.090 | 0.698 |
| Fatigue | 23 (35.4) | 1 (1.5) | 14 (31.1) | 0 (0) | 0.686 | 1.000 |
| Diarrhea | 20 (30.8) | 5 (7.7) | 11 (24.4) | 2 (4.4) | 0.523 | 0.698 |
| Proteinuria | 19 (29.2) | 0 (0) | 5 (11.1) | 0 (0) | 0.034 | – |
| Thrombocytopenia | 12 (18.5) | 2 (3.1) | 5 (11.1) | 0 (0) | 0.422 | 0.512 |
| Abdominal pain | 9 (13.8) | 1 (1.5) | 5 (11.1) | 0 (0) | 0.776 | 1.000 |
| Neutropenia | 9 (13.8) | 0 (0) | 6 (13.3) | 0 (0) | 1.000 | – |
| Rash | 7 (10.8) | 1 (1.5) | 5 (11.1) | 0 (0) | 1.000 | 1.000 |
| Reactive cutaneous capillary endothelial proliferation | 18 (27.7) | 0 (0) | 0 (0) | 0 (0) | < 0.001 | 0 (0) |
Discussion
Few studies investigating the clinical benefit of combination therapy with tyrosine kinase inhibitors and ICIs for patients with unresectable HCC were reported in the real-world setting. The present observational study showed significantly better OS (not reached vs 13.0 months), PFS (8.0 vs 3.0 months), and ORR (41.5% vs 20.0% according to the RECIST v1.1 criteria; 49.2% vs 26.7% according to the mRECIST criteria) with lenvatinib plus ICIs than with lenvatinib in patients with unresectable HCC. In the multivariate analysis, the type of treatment and albumin were the independent factors for both OS and PFS. In addition, both groups were found to have a manageable toxicity profile. This benefit confirms previous phase 1b findings that showed promising efficacy of lenvatinib plus pembrolizumab in patients with unresectable HCC who had received no previous systemic treatment [19]. Most (80%) patients in the present study had received one or more therapies for HCC before receiving lenvatinib, including 41.8% had received potential curative hepatic resection or radiofrequency ablation. However, most of these patients had tumor progression or tumor recurrence before they were treated lenvatinib. Due to the fact that lenvatinib in combination with ICIs has not been approved as standard treatment worldwide and the high cost, this population included a subset of particularly high-risk patients, 32.7% of whom had extrahepatic metastases, and 73.6% of whom had macrovascular invasion. Among patients with macrovascular invasion, most of them were with portal vein tumor thrombus involving main portal trunk or the portal vein contralateral branch. Compared to lenvatinib alone, lenvatinib plus ICIs reduced the risk of death by 53% and significantly prolonged OS due to an increase of 5.0 months in median PFS and a corresponding 65% reduction in the risk of disease progression or death.
In the REFLECT and the KEYNOTE-524 trial, patients were excluded if they had with invasion at the main portal vein, clear invasion of the bile duct, 50% or higher liver occupation, with Child–Pugh class B or C liver function, or received prior systemic therapy for HCC [12, 19]. However, in this real-world observational cohort, 32.7% patients were with Child–Pugh class B, 51.8% with intrahepatic tumor larger than 10 cm, and 6.4% received prior systemic therapy for HCC. Moreover, significantly more patients were with macrovascular invasion (73.6%) than that in the REFLECT (22.8%) [12] or the KEYNOTE-524 trial (20%) [19] (Table 1). Due to the high cost of antitumor therapies and low socioeconomic status in most of the included patients, 67.7% patients in the combination group and 91.1% in the lenvatinib group did not receive subsequent antitumor therapy after tumor progression or severe AEs (online suppl. Table 1). These may account for the slightly lower 12-month OS rates in the present study (lenvatinib, 50.4% vs 56% in the REFLECT; lenvatinib plus ICIs, 59.8% vs 67.5% in the KEYNOTE-524 trial). However, these findings suggest that high-risk patients may still benefit from treatment with lenvatinib with or without ICIs.
Single-agent ICIs failed to show a survival benefit in patients with unresectable HCC when compared with sorafenib in the CheckMate 459 trial [28] or with placebo in the KEYNOTE-240 study [29]. The ORR was 15.0% in the CheckMate 459 trial [28] and 18.3% in the KEYNOTE-240 study [29] per RECIST v1.1. Several immunotherapy combinations showed promising results in several phase 1b to 3 trials. The combination of atezolizumab and bevacizumab provided significantly better PFS than with monotherapy with atezolizumab in phase 1b trial GO30140 [30] or sorafenib in phase 3 IMbrave150 study [18]. Therefore, atezolizumab plus bevacizumab is recommended as first-line treatment for patients with unresectable HCC in ASCO HCC guideline [31]. The phase Ib KEYNOTE-524 trial (n = 104) showed ORRs 36.0% per RECIST v1.1 and 46.0% per mRECIST with lenvatinib plus pembrolizumab [19]. Our findings in the real-world setting were consistent with the results of KEYNOTE-524 trial [19], with ORRs 41.5% per RECIST v1.1 and 49.2% per mRECIST with lenvatinib plus ICIs. In addition, the ORRs were only 20.0% with lenvatinib monotherapy in the present study, and 18.8% in REFLECT trial [12] per RECIST v1.1. These findings suggest that both lenvatinib and ICIs contribute to the overall treatment benefit of the combination in patients with unresectable HCC.
Although AFP has been recognized as both a diagnostic and prognostic biomarker for HCC, its role and biological function in HCC remain unclear. Subgroup analyses based on baseline concentration of AFP suggest that patients with elevated AFP are more likely to derive an OS and PFS benefit from lenvatinib plus ICIs. Similar trend is reported in KEYNOTE-524 trial that patients with AFP of at least 200 ng/mL had slightly higher ORR than those with AFP of less than 200 ng/mL (41.7% vs 34.4%) [19]. In the REACH trial, AFP concentration was identified as the only clinically relevant factor of OS with an interaction with ramucirumab or placebo [32]. Based on this finding, REACH-2 is the first positive trial in a biomarker-selected population of patients with HCC [11]. Preclinical models have found that tumor expression of AFP may indicate a biologically distinct HCC subtype associated with more stem cell-like features and poor prognosis [33, 34]. Together, these findings may help to select patients with unresectable HCC who would derive an OS and PFS benefit from lenvatinib plus ICIs. However, further investigation and confirmation are needed.
The spectrum, incidence, and severity of adverse events in the lenvatinib plus ICIs or the lenvatinib group were consistent with the known safety profile of each agent and those observed in other studies [12, 19, 23]. Although no treatment-related deaths occurred, 18.5% of patients with lenvatinib plus ICIs discontinued treatment owing to adverse events, as compared with 15.6% of patients with lenvatinib. Hepatic function abnormal was the most common reason for discontinuation in the two groups. Severe immune-related hepatotoxicity was observed in two patients (3.1%). Therefore, hematological tests, especially liver function, should be closely observed during the use of ICIs [35].
This retrospective study had several limitations. First, its retrospective design made it vulnerable to other potential biases, such as different socioeconomic status among patients. However, there was no difference in the baseline characteristics between the two groups. Second, subsequent treatments may also be a confounding factor [36, 37]. However, only a few patients (32.3% vs 11.1%, p = 0.012) in the two groups received subsequent treatments. Different proportion of subsequent therapies may be attributed to the difference of patients’ socioeconomic status or the effect of the combination therapy. Third, the follow-up duration was relatively short for OS, which lead to the lack of long-term survival data. However, the follow-up duration was sufficient to evaluate PFS and tumor response, which can be more accurately reflect efficacy than OS [38]. Fourth, five types of ICIs were used in this study. Until now, there are no clinical studies of head-to-head comparisons for ICIs that provide evidence of which ICI works best. Finally, some adverse events, such as hypothyroidism and cardiotoxicity, could not be observed in some patients due to the lack of standard hematological tests during the follow-up.
In conclusion, treatment with lenvatinib plus ICIs was associated with significantly better OS and PFS outcomes than lenvatinib in patients with unresectable HCC, especially for those with alpha fetoprotein concentrations of at least 400 ng/mL. Life-threatening hepatotoxicity was noted in 3.1% (2/65) of the patients who received ICIs. Lenvatinib combined with ICIs might represent a novel treatment option for these patients. However, our findings need randomized controlled trials with large sample size and long follow-up to verify.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
J.-H.Z conceived the study; all authors participated in the acquisition of the data; K.C and J.-H.Z analyzed data; K.C, W.W and J.-H.Z assessed tumor response; J.-H.Z drafted and revised the manuscript; all authors have read and approved the final version to be published.
Funding
J.-H.Z is in part supported by the National Natural Science Foundation of China (82060510), “Guangxi BaGui Scholars” Special Fund (2019AQ20), the Natural Science Foundation of Guangxi Province (2018GXNSFBA138018 and 2020GXNSFAA159022), the Graduate Course Construction Project of Guangxi Medical University (YJSA2017014), and the Guangxi Undergraduate Training Program for Innovation and Entrepreneurship (202110598178 and 202110598073). B.-D.X is in part supported by the “High-level innovation team and outstanding scholar program in Guangxi Colleges and Universities.”
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Ethical statement
All the procedures were carried out in accordance with the 1975 Declaration of Helsinki. This study was approved by the institutional review board of Guangxi Medical University Cancer Hospital (number LW2021026) and the Fourth Affiliated Hospital of Guangxi Medical University (number LW2021003).
Consent to participate
Patient consent was waived due to the retrospective nature of the study.
Footnotes
Kang Chen, Wei Wei, Lei Liu these authors contributed equally to this work.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Le-Qun Li, Email: xitongpingjia@163.com.
Jian-Hong Zhong, Email: zhongjianhong@gxmu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Farinati F, Vitale A, Spolverato G, Pawlik TM, Huo TL, Lee YH, et al. Development and validation of a new prognostic system for patients with hepatocellular carcinoma. PLoS Med. 2016;13:e1002006. doi: 10.1371/journal.pmed.1002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong JH, Peng NF, You XM, Ma L, Xiang X, Wang YY, et al. Tumor stage and primary treatment of hepatocellular carcinoma at a large tertiary hospital in China: a real-world study. Oncotarget. 2017;8:18296–18302. doi: 10.18632/oncotarget.15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 6.Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:871–873. doi: 10.1093/annonc/mdy510. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 8.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 10.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 12.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 13.Ando Y, Kawaoka T, Suehiro Y, Yamaoka K, Kosaka Y, Uchikawa S, et al. Analysis of post-progression survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Oncology. 2020;98:787–797. doi: 10.1159/000509387. [DOI] [PubMed] [Google Scholar]
- 14.Casadei-Gardini A, Scartozzi M, Tada T, Yoo C, Shimose S, Masi G, et al. Lenvatinib versus sorafenib in first-line treatment of unresectable hepatocellular carcinoma: an inverse probability of treatment weighting analysis. Liver Int. 2021;41:1389–1397. doi: 10.1111/liv.14817. [DOI] [PubMed] [Google Scholar]
- 15.Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15:663–675. doi: 10.1007/s12072-021-10184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–580. doi: 10.1016/S1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 17.Shek D, Read SA, Nagrial A, Carlino MS, Gao B, George J, et al. Immune-checkpoint inhibitors for advanced hepatocellular carcinoma: a synopsis of response rates. Oncologist. 2021;26:e1216–e1225. doi: 10.1002/onco.13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 19.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II Trial. Clin Cancer Res. 2021;27:1003–1011. doi: 10.1158/1078-0432.CCR-20-2571. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition) Liver Cancer. 2020;9:682–720. doi: 10.1159/000509424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He MK, Liang RB, Zhao Y, Xu YJ, Chen HW, Zhou YM, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. doi: 10.1177/17588359211002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen L, Guo J, Zhang Q, Pan H, Yuan Y, Bai Y, et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer. 2020;8:e000437. doi: 10.1136/jitc-2019-000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoy SM. Sintilimab: first global approval. Drugs. 2019;79:341–346. doi: 10.1007/s40265-019-1066-z. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Division of Cancer Treatment and Diagnosis. Cancer therapy evaluation program. Adverse events/CTCAE ]. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 16 Apr 2021
- 27.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 28.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. CheckMate 459: a randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30(Suppl 5):v874–v875. doi: 10.1093/annonc/mdz394.029. [DOI] [Google Scholar]
- 29.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 30.Lee MS, Ryoo BY, Hsu C, Numata K, Stein S, Verret W, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808–820. doi: 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 31.Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38:4317–4345. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 32.Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68:1451–1461. doi: 10.1158/0008-5472.CAN-07-6013. [DOI] [PubMed] [Google Scholar]
- 34.Shan YF, Huang YL, Xie YK, Tan YH, Chen BC, Zhou MT, et al. Angiogenesis and clinicopathologic characteristics in different hepatocellular carcinoma subtypes defined by EpCAM and α-fetoprotein expression status. Med Oncol. 2011;28:1012–1016. doi: 10.1007/s12032-010-9600-6. [DOI] [PubMed] [Google Scholar]
- 35.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudo M. Sequential therapy for hepatocellular carcinoma after failure of atezolizumab plus bevacizumab combination therapy. Liver Cancer. 2021;10(2):85–93. doi: 10.1159/000514312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng ZJ, Li L, Teng YX, Zhang YQ, Zhang YX, Liu HT et al (2021) Treatments of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus: Current Status and Controversy. J Clin Transl Hepatol. 10.14218/JCTH.12021.00179. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 38.Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology. 2021;73(Suppl 1):158–191. doi: 10.1002/hep.31327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.