Abstract
Background
Analysis of hepatocellular carcinoma (HCC) single-cell sequencing data was conducted to explore the role of tumor-associated neutrophils in the tumor microenvironment.
Methods
Analysis of single-cell sequencing data from 12 HCC tumor cores and five HCC paracancerous tissues identified cellular subpopulations and cellular marker genes. The Cancer Genome Atlas (TCGA) and the Gene Expression Omnibus (GEO) databases were used to establish and validate prognostic models. xCELL, TIMER, QUANTISEQ, CIBERSORT, and CIBERSORT-abs analyses were performed to explore immune cell infiltration. Finally, the pattern of tumor-associated neutrophil roles in tumor microenvironmental components was explored.
Results
A total of 271 marker genes for tumor-associated neutrophils were identified based on single-cell sequencing data. Prognostic models incorporating eight genes were established based on TCGA data. Immune cell infiltration differed between the high- and low-risk groups. The low-risk group benefited more from immunotherapy. Single-cell analysis indicated that tumor-associated neutrophils were able to influence macrophage, NK cell, and T-cell functions through the IL16, IFN-II, and SPP1 signaling pathways.
Conclusion
Tumor-associated neutrophils regulate immune functions by influencing macrophages and NK cells. Models incorporating tumor-associated neutrophil-related genes can be used to predict patient prognosis and immunotherapy responses.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03567-4.
Keywords: HCC, Single-cell, CellChat, Tumor-associated neutrophils, Immunotherapy
Background
Hepatocellular carcinoma (HCC) has one of the worst prognoses among solid tumors [1]. Many aspects of the etiology of HCC vary worldwide. However, the incidence of hepatocellular carcinoma is increasing yearly [2]. The life of patients diagnosed with HCC early can be prolonged after radical surgery [3]. However, a limited clinical benefit is seen in patients with advanced HCC [4].
The complex composition and dynamics of the tumor microenvironment in HCC lead to extremely poor outcomes for patients. However, the advent of single-cell sequencing technology has expanded the understanding of cellular components and gene expression features in the tumor microenvironment [5]. Single-cell sequencing analysis allows us to study gene expression differences and intercellular mechanistic pathways at the cellular level on a single-cell basis [6].
Neutrophils play an indispensable role in the tumor microenvironment of HCC [7]. Tumor-associated neutrophils have been shown to promote tumor progression by secreting cytokines, exosomes, and other factors [8, 9].
B cells and NK cells have been reported in the tumor microenvironment of HCC [10, 11]. However, the roles of neutrophils in the tumor microenvironment and immunotherapy response of HCC are understudied. This study revealed the role of tumor-associated neutrophils in HCC patients based on single-cell sequencing data from tumor core and paracancerous tissues of HCC.
Methods
Research materials
This study was performed with single-cell sequencing data and transcriptome data from patients with HCC. Single-cell sequencing data from the tumor cores of 12 patients with HCC and five paracancerous tissues were obtained from GSE189903 [12] (https://www.ncbi.nlm.nih.gov/geo/). Transcriptome data and clinical information of patients with HCC represented in The Cancer Genome Atlas (TCGA) (https://portal.gdc.cancer.gov/) and GSE14520 [13] (https://www.ncbi.nlm.nih.gov/geo/) were used in the study.
Data processing
R software and related functional packages were used to read 10 × Genomics single-cell sequencing data and create Seurat objects. The Harmony package was used to eliminate batch effects between samples. The “FindNeighbors” function was employed to characterize different cell subpopulations at a resolution of 2.5. The uniform manifold approximation and projection (UMAP) dimensionality reduction technique was used to visualize the clustered cells on a two-dimensional map. The “SingleR” function and previous literature reports [14–16] were used to identify cell subpopulations. The Wilcoxon rank-sum was used to identify genes that were differentially expressed between different cell subpopulations. Genes with |log2 (fold change)|> 1 and adjusted P value < 0.01 were regarded as marker genes.
Establishment and validation of models of tumor-associated endothelial cells
Marker genes for tumor-associated endothelial cells were selected for inclusion in univariate Cox regression analysis. Prognosis-related genes were subjected to LASSO regression analysis to generate a penalty function to compress the coefficients of the variables to prevent overfitting of the model. The results of multivariate Cox regression analysis were used to construct the risk score model. The high-risk and low-risk groups were assigned based on the median value of the risk score.
The area under the receiver operating characteristic (ROC) curve was applied to evaluate the prognostic ability of the model. Kaplan‒Meier survival analysis was performed to assess differences in prognosis. The GEO dataset is an independently validated dataset.
Immune cell infiltration analysis
Differences in immune cell activity and proportions between the high- and low-risk groups were estimated. Single-sample GSEA (ssGSEA) was used to evaluate differences in immune cell activity and immune pathways between the high- and low-risk groups. An estimation algorithm was used to predict differences in immune scores, interstitial scores, and total scores between the two groups.
Prediction of the response to immunotherapy
A variety of methods can be used to predict the outcomes of patients in the high-risk and low-risk groups treated with immunotherapy. The TIDE score predicts the response to immunotherapy in tumor patients through multiple perspectives. A lower TIDE score indicates a lower probability of immune escape in the patient (http://tide.dfci.harvard.edu). Immune checkpoint inhibitor therapy is an increasingly crucial strategy in HCC. This study evaluated the relationships between the model genes and 47 immune checkpoint inhibitory genes.
Pathway enrichment analysis
The differences in cellular pathway activity between tumor-associated and normal cells were determined by considering differences in the expression of genes in the cells. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway gene sets were downloaded for gene set enrichment analysis (GSEA). The “AUCell” function was used to calculate the proportion of cells in each cluster with enrichment of genes in the input KEGG gene set from the area under the curve values. The gene expression of cells was extracted from annotated Harmony objects. Differences in pathway enrichment between tumor-associated neutrophils and normal tissue-resident neutrophils were identified based on the “AUCell” method.
Trajectory analysis
Cells in the tumor microenvironment are in a state of dynamic change. Tumor-associated neutrophils were isolated for analysis of their differentiation trajectory during tumor progression using Monocle (version 2.24.0). Genes used for trajectory analysis were screened for an average expression ≥ 0.1. The “DDRTree” function and a setting of max components = 2 parameters were used to downscale cell data. Major differences in important signaling pathways were explored to identify branching. Heatmaps were plotted and grouped based on the expression of trajectory branching genes.
Analysis of intercellular communication
Cells in the tumor microenvironment interact with each other. Ligands and receptors on the cellular surface are the main conduits for intercellular signal transduction. Networks of intercellular interactions can be predicted based on gene expression levels in cells. CellChatDB is a reference database for receptor‒ligand interactions. The expression matrix and classification information were extracted from the Harmony objects, and the “createCellChat” package was applied to create a CellChat object. Ligand‒receptor pairs for the identified overexpressed genes were filtered, and the results were used to construct a PPI network. The parameter minimum cell = 3 was used to screen for communication relationships between low-quality cells.
Real-time quantitative PCR
Fifteen total RNA samples from liver cancer tissue and paired paracancerous tissue samples were treated with an RNA separator total RNA extraction reagent (Vazyme). cDNA was synthesized from total RNA using NovoScript® plus an all-in-one first-strand cDNA synthesis kit (Novoprotein). GAPDH was used as an internal control. Real-time quantitative PCR (RT-qPCR) analysis was performed with different primer sequences using the SYBR Green Master Mix (TaKaRa). The primers used for RT-qPCR are in Supplemental Table 1.
Statistical analysis
The Wilcoxon rank-sum test was used as a backup to analyze differences between two groups. Univariate Cox regression analysis, least absolute shrinkage and selection operator (LASSO) regression analysis, and multivariate Cox regression analysis were performed to screen for prognosis-related genes. The Kaplan‒Meier method and the log-rank test were used for survival analysis. All data analyses were conducted using R (4.1.2, https://www.r-project.org/) software. A two-sided P value of < 0.05 was considered to indicate a statistically significant difference (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Results
Identification of tumor-associated endothelial cell marker genes
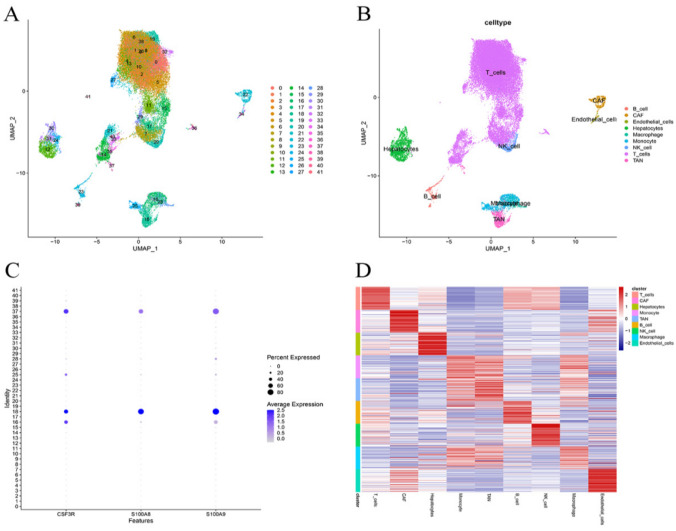
The single-cell sequencing GSE189903 dataset containing data for 12 tumor cores of HCC and five paracancerous tissues was included in this study. A total of 41,403 cells from the 12 patients with HCC were included in the study after removal of low-quality cells and Harmony integration. A total of 43,592 cells from the five paracancerous tissues were included in the study. There were 41 cell clusters after UMPA downscaling. After SingleR and reference to the previous literature, Clusters 18 and 37 were identified as tumor-associated neutrophils. The Wilcoxon-Mann‒Whitney test identified a total of 271 marker genes for tumor-associated neutrophils (Fig. 1).
Fig. 1.
Single-cell sequencing analysis. A UMAP plot colored by various cell clusters. B UMAP plot colored by subpopulation of cells after annotation. C The cell types identified by marker genes. D Heatmap showing the top ten marker genes in each cell cluster
Prognostic models of tumor-associated endothelial cells
There were 70 overlapping genes between genes identified as overexpressed in TCGA and tumor-associated neutrophil marker genes (Supplementary Table 2). These seventy genes were subjected to univariate Cox regression analysis, LASSO regression analysis and multivariate Cox regression analysis to establish a prognostic model with marker genes for tumor-associated neutrophils in HCC. A final set of eight genes was used to establish the prognostic model (Fig. S1).
Analysis of immune cell infiltration
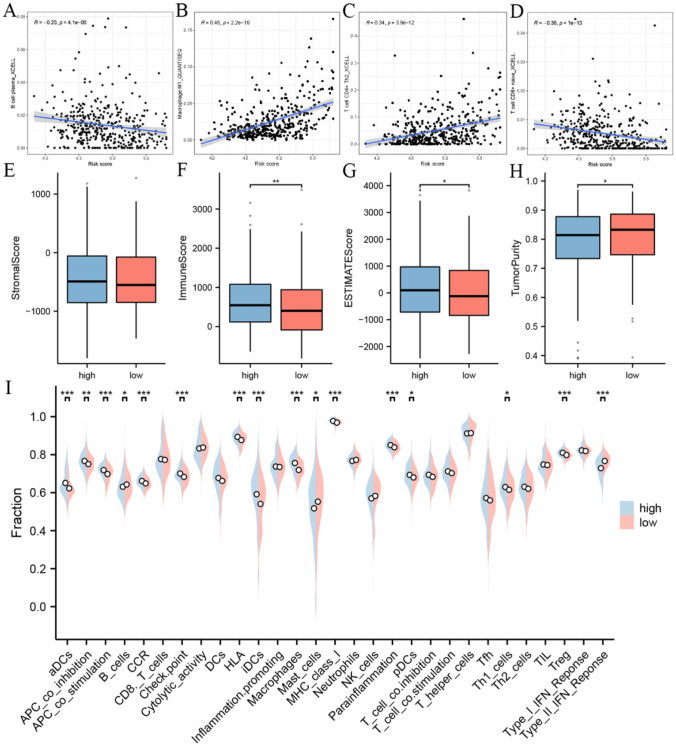
Analysis based on the risk model showed strong correlations with CD8 + cells, CD4 + cells, B cells, and NK cells. The ssGSEA results indicated that dendritic cells, B cells, macrophages, mast cells, Th1 cells, and Tregs showed different activities in the high- and low-risk groups. The estimation algorithm demonstrated that the immune score, total score, and tumor purity score differed between the high-risk group and the low-risk group (Fig. 2).
Fig. 2.
Tumor immune microenvironment analysis. A–D Immune cell and risk model correlation analysis. Tumor microenvironment score differences compared based on ESTIMATE algorithm, E stromal score, F immune score, G estimate score, and H tumorpurity score. I Results of the ssGSEA analysis
Immunotherapy response prediction
Patients in the high-risk group had higher TIDE scores than those in the low-risk group, indicating a higher degree of immune escape in patients in the high-risk group (Fig. 3A). Both the T-cell exclusion and CAF scores were higher in the high-risk group than in the low-risk group (Fig. 3B–D). This finding also showed that patients in the low-risk group had better outcomes with immunotherapy. The majority of immune checkpoint blockade-related genes were differentially expressed between the high- and low-risk groups. Further correlation analysis showed high correlations between the expression of the model genes and that of most immune checkpoint genes (Fig. 3E, F).
Fig. 3.
Prediction of response to immunotherapy. A, B, C, D Results of the TIDE predictive analysis. E Results of correlation analysis of immune checkpoint inhibitor genes. F Results of immune checkpoint inhibition gene expression analysis
Pathway enrichment analysis
Single-cell sequencing data provides information about gene expression in each cell. Genes expressed in neutrophils in tumor tissues and in normal tissues were scored by “AUCell” for enrichment utilizing the gene set downloaded from GSEA. The enrichment scores of genes expressed in tumor-associated neutrophils in the KEGG B-cell receptor signaling pathway, KEGG glycolysis gluconeogenesis, KEGG jak stat signaling pathway, KEGG lysosome, KEGG MAPK signaling pathway, and KEGG regulation of autophagy pathways were significantly higher than those in the normal group (Fig. 4). Transcriptome data analysis confirmed differences in glycolytic gene expression between the normal and tumor groups (Fig. 5).
Fig. 4.
A–J KEGG Pathway AUCell Score Results. K Results of differential analysis of KEGG pathway tumor tissues and paracancerous tissues
Fig. 5.
Analysis of glycolysis-related genes. A, B Expression correlation analysis of model genes and glycolysis-related genes in normal and tumor tissues. C Differential expression of glycolysis-related genes in high- and low-risk groups
Trajectories of neutrophil maturation in HCC
Pseudotime analysis was performed on tumor-associated neutrophils based on the Monocle package. The two clusters of tumor-associated neutrophils could be classified into four mid-developmental stages. Tumor-associated neutrophils in stages 1, 4, and 5 were at earlier developmental stages. Tumor-associated neutrophils in stage 3 were at later developmental stages. Differences in the expression of the model genes appeared at different developmental stages in neutrophils. The expression of ALDH2, APLP2, ATP6V0B, ETS2, S100A10, and SPP1 gradually decreased during the development of tumor-associated neutrophils. However, SLC16A3 and TPP1 showed a trend of decreasing and then increasing. BEAM was utilized to find genes that were regulated in a branch-dependent manner. The heatmap shows (left to right) the results of clustering based on genes in glycolytic pathways, the KEGG B-cell receptor signaling pathway and the KEGG glycolysis/gluconeogenesis signaling pathway in tumor-associated neutrophils during cellular development (Fig. 6).
Fig. 6.
Trajectory analysis of tumor-associated neutrophils. A–D Differentiation trajectory results for tumor-associated neutrophils. E Variation in the expression of model genes in tumor-associated neutrophil differentiation trajectories. F, G Glycolysis gluconeogenesis signaling pathway and B cell receptor signaling pathway differentially expressed gene along the pseudotime were hierarchically clustered into five subclusters
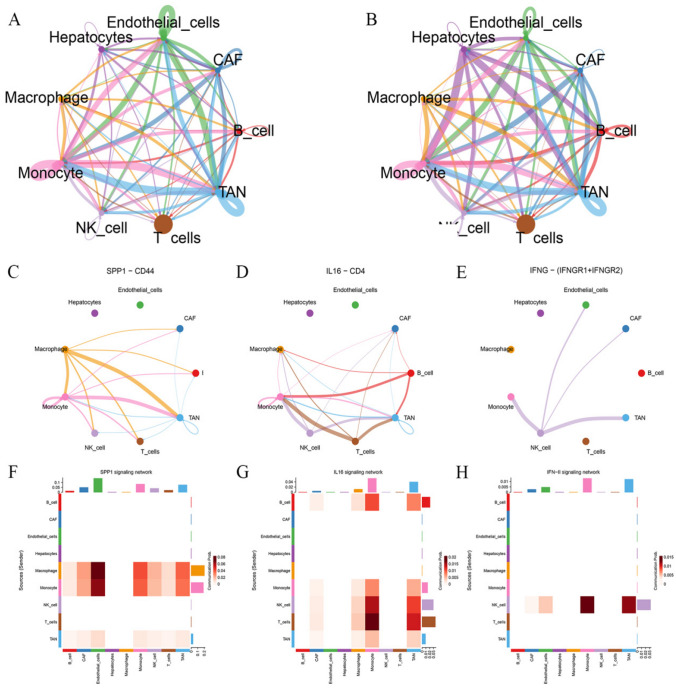
Analysis of cell‒cell interactions
The weights and probabilities of intercellular signaling pathways in the tumor microenvironment of HCC were evaluated based on the “CellChat” algorithm. Tumor-associated neutrophils play a considerable role in intercellular communication. Macrophages and NK cells communicate closely with tumor-associated neutrophils. The SPP1, IL16, and IFN-II signaling pathways were the predominant signaling pathways involved in the interactions between tumor-associated neutrophils and other cells (Fig. 7).
Fig. 7.
A The number of interaction in cell–cell communication network. B The weight of interaction in cell–cell communication network. C, D, E Cell–cell communication interaction in SPP1, IL16, and INF-II signaling pathway. F, G, H Heatmap display of intercellular communication weights
Independent verification
RT‒qPCR was performed to verify gene expression differences in fifteen patients from Renmin Hospital of Wuhan University. The results showed that TPP1, SPP1, SLC16A3, S100A10, ATP6V0B, and APLP2 were highly expressed in tumor tissues. ETS2 and ALDH2 were expressed at low levels in tumor tissues (Fig. 8). The protein expression data confirmed the above results (Fig. S2).
Fig. 8.
Analysis of gene expression. A–H Analysis of gene differential expression in TCGA database. I Verifying the expression of genes that constitute the risk model through RT-qPCR
Discussion
HCC is one of the most common cancers worldwide [17]. The majority of HCC patients are diagnosed in China [18]. However, the low rate of early diagnosis and drug resistance lead to enormous challenges in the treatment of HCC [19]. It is widely believed that the tumor microenvironment is a fertile ground for cancer cell growth and metastasis. Immune cells and stromal cells in the tumor microenvironment are the main cellular components that mediate immune tolerance and immune escape [20, 21]. Numerous studies have shown that tumor-associated neutrophils are involved in tumor growth and metastasis [22–24]. Moreover, neutrophils can suppress the immune functions of T cells, B cells, and NK cells by secreting cytokines [25, 26]. Single-cell sequencing was used to obtain cell-level expression data. In addition, a wide range of algorithms based on multiple resources were used for cell subpopulation classification, trajectory analysis, and pathway analysis, among other analyses [27, 28]. Advances in new technologies have accelerated the study of neutrophils heterogeneity in the tumor microenvironment of HCC [29]. Consequently, tumor-associated neutrophils are anticipated to be a new target for HCC immunotherapy.
Eight tumor neutrophil-related prognostic genes were identified on the basis of transcriptomic data and clinical information from patients. The secreted acidic glycoprotein SPP1 functions as a crucial mediator of cell-to-cell interactions [30]. SPP1 + macrophages are involved in immune escape in the tumor microenvironment. Knockdown of SPP1 was accompanied by increased CD8 + T cell infiltration in tumors [31]. Analysis of intercellular communication revealed a strong association of SPP1 and CD44 in macrophages and tumor-associated neutrophils [32]. It was suggested that tumor-associated neutrophils are involved in regulating cellular immune functions in the tumor microenvironment of HCC [33]. Overexpression of ALDH2 increased autophagy in HCC cells. Hu et al. further found that ALDH2 inhibited immune escape in HCC via the ROS pathway [34, 35]. In the present study, single-cell sequencing analysis further uncovered the function of ALDH2 in regulating the immune microenvironment by acting on stromal cells mainly through the ROS signaling pathway. The results of multiple transcriptome data analyses showed that APLP2 is an influential factor in hepatic immune cell infiltration and the immunotherapy response. Single-cell sequencing further confirmed that APLP2 regulates immune functions by affecting tumor-associated neutrophils [36, 37]. HCC occurs mostly in individuals with a verified history of liver disease [38]. Massive neutrophil infiltration is observed in inflammatory diseases [39]. S100A10, a member of the S100 family, participates in the calcium signaling pathway to promote neutrophil infiltration of tumors in the tumor microenvironment [40, 41]. LC16A3, ETS2, and TPP1 all demonstrated differential expression in tumor-associated neutrophils over time in a time course analysis. This result indicated that they were involved in the regulation of immune suppression by tumor-associated neutrophils. Unlike other genes, SLC16A3 demonstrated an extremely strong correlation with immunomodulatory genes. Mukai et al. found that SLC16A3 is involved in lactate transport [42]. This observation was consistent with the increased glycolysis observed in our tumor-associated neutrophil granules [26]. Consequently, the model gene for tumor-associated neutrophils is anticipated to be a potential target for enhancing immunocompetence in HCC.
Limitations of transcriptome sequencing approaches have hindered the exploration of expression differences in neutrophils [43]. However, neutrophils demonstrate a high degree of heterogeneity in HCC [44]. In the microenvironment of solid tumors, metabolic alterations induce cell polarization [45, 46]. One of the key features of the tumor microenvironment in HCC is hypoxia. Several studies have found that products of glycolysis promote tumor immune escape and growth [47, 48]. The results of our study revealed significantly increased glycolytic activity in tumor-associated neutrophils. Additionally, tumor-associated neutrophils can enhance their communication with immune cells through the SPP1, IF16, and UFN-II signaling pathways. Tumor-associated neutrophils can serve as a marker of poor prognosis and a new target for immunotherapy [49]. Immune cell infiltration analysis revealed differences between the high- and low-risk groups in the infiltration of cells such as macrophages, NK cells, T cells, and B cells [25]. Differences in the expression of the majority of immune checkpoint inhibitory genes were found between the two groups. The TIDE immunotherapy scores indicated that patients in the low-risk group derived a greater benefit from immunotherapy.
In this study, the role of tumor-associated neutrophils in HCC was identified by analysis of single-cell sequencing data. However, this study still has limitations. First, the findings lack validation in cells, tissues, and animals. Second, the tumor microenvironment of HCC comprises a complex series of components, and multiple elements can influence the immune environment.
Conclusion
In conclusion, tumor-associated neutrophils regulate immune functions by influencing macrophages, NK cells, etc. Models incorporating tumor-associated neutrophil-related genes can be used to predict patient prognosis and immunotherapy responses. However, the findings require further validation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None
Abbreviations
- GEO
Gene expression omnibus
- GSEA
Gene set enrichment analysis
- HCC
Hepatocellular carcinoma
- KEGG
Kyoto encyclopedia of genes and genomes
- LASSO
Least absolute shrinkage and selection operator
- RT-qPCR
Real-time quantitative PCR
- ROC
Receiver operating characteristic
- ssGSEA
Single-sample GSEA
- TCGA
The cancer genome atlas program
- UMAP
Uniform manifold approximation and projection
Authors contributions
DY contributed to the conceptualization, JS, BY, and YS were involved in data curation, Yu Zhou and Yanbing Zhang contributed to the formal analysis, DY and Yu Zhou were involved in writing—original draft, and YD and KZ contributed to writing—review and editing.
Funding
This study was funded by the Hubei Provincial Fund Committee (2022CFB122).
Data availability
Publicly available datasets were analyzed in this study.
Declarations
Conflict of interest
None.
Ethical approval
This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University. Written informed consent was obtained from all patients to participate in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dashuai Yang, Yu Zhou and Yanbing Zhang have contributed equally to this work.
Contributor Information
Kailiang Zhao, Email: zhaokl1983@qq.com.
Youming Ding, Email: dingym@whu.edu.cn.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34(7):969–77.e2. doi: 10.1016/j.cmet.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Jiang Y, Yuan H, Fang Q, Cai N, Suo C, et al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the global burden of disease study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70(4):674–683. doi: 10.1016/j.jhep.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Liu CY, Chen KF, Chen PJ. Treatment of liver cancer. Cold Spring Harb Perspect Med. 2015;5(9):a021535. doi: 10.1101/cshperspect.a021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grady WM, Yu M, Markowitz SD. Epigenetic alterations in the gastrointestinal tract: current and emerging use for biomarkers of cancer. Gastroenterology. 2021;160(3):690–709. doi: 10.1053/j.gastro.2020.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhury MMH, Salazar CJJ, Nurunnabi M. Recent advances in bionanomaterials for liver cancer diagnosis and treatment. Biomaterials science. 2021;9(14):4821–4842. doi: 10.1039/D1BM00167A. [DOI] [PubMed] [Google Scholar]
- 7.Zhao C, Liu S, Gao F, Zou Y, Ren Z, Yu Z. The role of tumor microenvironment reprogramming in primary liver cancer chemotherapy resistance. Front Oncol. 2022;12:1008902. doi: 10.3389/fonc.2022.1008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polidoro MA, Mikulak J, Cazzetta V, Lleo A, Mavilio D, Torzilli G, et al. Tumor microenvironment in primary liver tumors: a challenging role of natural killer cells. World J Gastroenterol. 2020;26(33):4900–4918. doi: 10.3748/wjg.v26.i33.4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang QY, Ho DW, Tsui YM, Ng IO. Single-cell transcriptomics of liver cancer: hype or insights? Cell Mol Gastroenterol Hepatol. 2022;14(3):513–525. doi: 10.1016/j.jcmgh.2022.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotsiliti E, Leone V, Schuehle S, Govaere O, Li H, Wolf MJ, et al. Intestinal B cells license metabolic T-cell activation in NASH microbiota/antigen-independently and contribute to fibrosis by IgA-FcR signalling. J Hepatol. 2023;79(2):296–313. doi: 10.1016/j.jhep.2023.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin R, Zhao H, He Q, Li F, Li Y, Zhao H. Advances in single-cell sequencing technology in the field of hepatocellular carcinoma. Front Genet. 2022;13:996890. doi: 10.3389/fgene.2022.996890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma L, Heinrich S, Wang L, Keggenhoff FL, Khatib S, Forgues M, et al. Multiregional single-cell dissection of tumor and immune cells reveals stable lock-and-key features in liver cancer. Nat Commun. 2022;13(1):7533. doi: 10.1038/s41467-022-35291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao N, Dang H, Ma L, Martin SP, Forgues M, Ylaya K, et al. Intratumoral γδ T-cell infiltrates, chemokine (C-C Motif) ligand 4/chemokine (C-C Motif) ligand 5 protein expression and survival in patients with hepatocellular carcinoma. Hepatology (Baltimore, MD) 2021;73(3):1045–1060. doi: 10.1002/hep.31412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheiner B, Pinato DJ. Tumor-infiltrating neutrophils: gatekeepers in liver cancer immune control. Gastroenterology. 2023;164(7):1338–1339. doi: 10.1053/j.gastro.2023.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Wu F, Fan J, He Y, Xiong A, Yu J, Li Y, et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat Commun. 2021;12(1):2540. doi: 10.1038/s41467-021-22801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue R, Zhang Q, Cao Q, Kong R, Xiang X, Liu H, et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature. 2022;612(7938):141–147. doi: 10.1038/s41586-022-05400-x. [DOI] [PubMed] [Google Scholar]
- 17.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (London, England) 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 18.Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ (Clinical Research Ed) 2020;371:m3544. doi: 10.1136/bmj.m3544. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Oura K, Morishita A, Tani J, Masaki T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: a review. Int J Mol Sci. 2021;22(11):5801. doi: 10.3390/ijms22115801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Hassan W, Jabeen Q, Khan GJ, Iqbal F. Interdependent and independent multidimensional role of tumor microenvironment on hepatocellular carcinoma. Cytokine. 2018;103:150–159. doi: 10.1016/j.cyto.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 22.Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133(20):2159–2167. doi: 10.1182/blood-2018-11-844548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng MSF, Tan L, Wang Q, Mackay CR, Ng LG. Neutrophils in cancer-unresolved questions. Sci China Life Sci. 2021;64(11):1829–1841. doi: 10.1007/s11427-020-1853-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Zhang W, Yuan X, Fu M, Qian H, Xu W. Neutrophils in cancer development and progression: roles, mechanisms, and implications (Review) Int J Oncol. 2016;49(3):857–867. doi: 10.3892/ijo.2016.3616. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Zhou XH, Li JR, Zheng TH, Yao FB, Gao B, et al. Neutrophils: driving inflammation during the development of hepatocellular carcinoma. Cancer Lett. 2021;522:22–31. doi: 10.1016/j.canlet.2021.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Geh D, Leslie J, Rumney R, Reeves HL, Bird TG, Mann DA. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022;19(4):257–273. doi: 10.1038/s41575-021-00568-5. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Wang Z, Ding Y, Qin Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front Immunol. 2023;14:1133308. doi: 10.3389/fimmu.2023.1133308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XY, Shen Y, Zhang L, Guo X, Wu J. Understanding initiation and progression of hepatocellular carcinoma through single cell sequencing. Biochim Biophys Acta. 2022;1877(3):188720. doi: 10.1016/j.bbcan.2022.188720. [DOI] [PubMed] [Google Scholar]
- 29.Arvanitakis K, Mitroulis I, Germanidis G. Tumor-associated neutrophils in hepatocellular carcinoma pathogenesis, prognosis, and therapy. Cancers. 2021;13(12):2899. doi: 10.3390/cancers13122899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Zhang R, Deng J, Dai X, Zhu X, Fu Q, et al. Construction of TME and Identification of crosstalk between malignant cells and macrophages by SPP1 in hepatocellular carcinoma. Cancer Immunol Immunother : CII. 2022;71(1):121–136. doi: 10.1007/s00262-021-02967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S, et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol. 2023;78(4):770–782. doi: 10.1016/j.jhep.2023.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Hu J, Zhang L, Xia H, Yan Y, Zhu X, Sun F, et al. Tumor microenvironment remodeling after neoadjuvant immunotherapy in non-small cell lung cancer revealed by single-cell RNA sequencing. Genome Medicine. 2023;15(1):14. doi: 10.1186/s13073-023-01164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eun JW, Yoon JH, Ahn HR, Kim S, Kim YB, Lim SB, et al. Cancer-associated fibroblast-derived secreted phosphoprotein 1 contributes to resistance of hepatocellular carcinoma to sorafenib and lenvatinib. Cancer Commun (London, England) 2023;43(4):455–479. doi: 10.1002/cac2.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu J, Yang L, Peng X, Mao M, Liu X, Song J, et al. ALDH2 hampers immune escape in liver hepatocellular carcinoma through ROS/Nrf2-mediated autophagy. Inflammation. 2022;45(6):2309–2324. doi: 10.1007/s10753-022-01694-1. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Fu L. The role of ALDH2 in tumorigenesis and tumor progression: targeting ALDH2 as a potential cancer treatment. Acta pharmaceutica Sinica B. 2021;11(6):1400–1411. doi: 10.1016/j.apsb.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su Y, Xue C, Gu X, Wang W, Sun Y, Zhang R, et al. Identification of a novel signature based on macrophage-related marker genes to predict prognosis and immunotherapeutic effects in hepatocellular carcinoma. Front Oncol. 2023;13:1176572. doi: 10.3389/fonc.2023.1176572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao Z, Huang J, Li J. (2023) Comprehensive intratumoral heterogeneity landscaping of liver hepatocellular carcinoma and discerning of APLP2 in cancer progression. Environ Toxicol [DOI] [PubMed]
- 38.Marengo A, Rosso C, Bugianesi E. Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu Rev Med. 2016;67:103–117. doi: 10.1146/annurev-med-090514-013832. [DOI] [PubMed] [Google Scholar]
- 39.Tang J, Yan Z, Feng Q, Yu L, Wang H. The roles of neutrophils in the pathogenesis of liver diseases. Front Immunol. 2021;12:625472. doi: 10.3389/fimmu.2021.625472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Huang H, Sze KM, Wang J, Tian L, Lu J, et al. S100A10 promotes HCC development and progression via transfer in extracellular vesicles and regulating their protein cargos. Gut. 2023;72(7):1370–1384. doi: 10.1136/gutjnl-2022-327998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, Shi M, Cao J, Yuan T, Yu G, Chen Y, et al. S100 calcium binding protein A10, A novel oncogene, promotes the proliferation, invasion, and migration of hepatocellular carcinoma. Front Genet. 2021;12:695036. doi: 10.3389/fgene.2021.695036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukai Y, Yamaguchi A, Sakuma T, Nadai T, Furugen A, Narumi K, et al. Involvement of SLC16A1/MCT1 and SLC16A3/MCT4 in l-lactate transport in the hepatocellular carcinoma cell line. Biopharm Drug Dispos. 2022;43(5):183–191. doi: 10.1002/bdd.2329. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Z, Zhou X, Li R, Michal JJ, Zhang S, Dodson MV, et al. Whole transcriptome analysis with sequencing: methods, challenges and potential solutions. Cellular Mol Life Sci : CMLS. 2015;72(18):3425–3439. doi: 10.1007/s00018-015-1934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei R, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 2020;13(1):3. doi: 10.1186/s13045-019-0836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du D, Liu C, Qin M, Zhang X, Xi T, Yuan S, et al. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharmaceutica Sinica B. 2022;12(2):558–580. doi: 10.1016/j.apsb.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sin SQ, Mohan CD, Goh RMW, You M, Nayak SC, Chen L, et al. (2022) Hypoxia signaling in hepatocellular carcinoma: challenges and therapeutic opportunities. Cancer metastasis reviews [DOI] [PubMed]
- 47.Wang L, Liu Y, Dai Y, Tang X, Yin T, Wang C, et al. Single-cell RNA-seq analysis reveals BHLHE40-driven pro-tumour neutrophils with hyperactivated glycolysis in pancreatic tumour microenvironment. Gut. 2023;72(5):958–971. doi: 10.1136/gutjnl-2021-326070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia S, Pan Y, Liang Y, Xu J, Cai X. The microenvironmental and metabolic aspects of sorafenib resistance in hepatocellular carcinoma. EBioMedicine. 2020;51:102610. doi: 10.1016/j.ebiom.2019.102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng Q, Klein C, Caruso S, Maille P, Laleh NG, Sommacale D, et al. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. 2022;77(1):116–127. doi: 10.1016/j.jhep.2022.01.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study.