Abstract
The hemoglobin-haptoglobin scavenger receptor CD163 is present in both a membrane-bound form on monocytes and macrophages (mCD163) and a shed soluble circulating form (sCD163). CD163 is a well-described marker of M2-like tumor-associated macrophages, but in patients with metastatic renal cell carcinoma (mRCC), monocyte mCD163 and serum sCD163 levels have not previously been investigated and associated with patient overall survival (OS). Here, we report mCD163 expression on peripheral blood monocytes, as well as sCD163 serum levels, in samples from 89 patients newly diagnosed with mRCC and 20 healthy controls. We found that in mRCC patients, compared to healthy controls, monocyte mCD163 levels were reduced (P < 0.001) whereas serum sCD163 levels were increased (P = 0.004). Moreover, an inverse correlation between mCD163 and sCD163 levels (P = 0.04) was shown. In survival analyses, intermediary levels of monocyte mCD163 were associated with longest OS, compared to both lower and higher mCD163 levels, which were both associated with worse outcomes (P < 0.01). Further, higher levels of sCD163 at diagnosis were associated with poor OS in both univariate (P < 0.001) and multivariate analysis (HR = 1.28; 95%CI 1.09–1.50, P = 0.002). Importantly, stratification by low vs. high sCD163 was able to separate patients with International Metastatic RCC Database Consortium (IMDC) intermediate risk (IMDCINT) into two subgroups with different OS (P = 0.03): IMDCINT-sCD163LOW showed survival similar to IMDCFAV patients, and IMDCINT-sCD163HIGH showed survival similar to IMDCPOOR patients. Thus, baseline sCD163 is a novel independent biomarker of OS in mRCC, and using sCD163 as an add-on biomarker may improve prognostic value for patients in the heterogenous IMDC intermediate group.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03266-6.
Keywords: CD163, Soluble CD163, Monocyte, Renal cell carcinoma, Biomarker
Introduction
Renal cell carcinoma (RCC) represents around 90% of all renal neoplasms with clear cell RCC being the most common histological subtype [1, 2]. Nearly 20% of patients have distant metastases at the time of diagnosis, and an additional 20–30% develop metastatic RCC (mRCC) following nephrectomy. Despite recent therapeutic improvements for patients with mRCC, including tyrosine kinase inhibitors (TKI) and immune checkpoint inhibitors (ICI), the prognosis for the majority of patients with mRCC remains poor [3]. For prognostic stratification, the Memorial Sloan Kettering Cancer Center (MSKCC) and the International Metastatic RCC Database Consortium (IMDC) risk score systems have consistently separated patients into three distinct risk groups. Both risk score systems, however, allocate almost half of patients into the intermediate risk group with a heterogeneous prognosis, highlighting the need for additional prognostic biomarkers [4].
In many malignant tumors, the microenvironment is characterized by chronic inflammation [5]. Tumor-associated macrophages (TAMs) are key players in the link between inflammation and cancer [6] and are considered of major importance in tumor development and progression [7]. In general, TAMs are “alternatively activated”, express so-called M2-associated markers including CD163, and have been shown to promote tumor progression by supporting angiogenesis, tissue remodelling, and suppression of anti-tumor immunity [8, 9]. CD163 is the hemoglobin-haptoglobin scavenger receptor, and its expression is highly restricted to cells of the monocyte/macrophage lineage [10, 11]. Besides hemoglobin scavenging, CD163 may have a role in anti-inflammatory signalling [12], and may serve as a gateway for monocyte/macrophage-targeted drug delivery [13]. A high density of TAMs has been associated with poor outcomes in several cancers including clear cell renal cell carcinoma (ccRCC) [14, 15]. Further, in recent studies on ccRCC, high tumor infiltration by CD163-expressing TAMs was associated with higher TNM stage, higher Fuhrman nuclear grade, and was independently associated with poor outcome [16, 17].
Circulating monocytes can differentiate into macrophages and contribute to the population of TAMs [18]. Based on the expression levels of CD14 and CD16, monocytes can be divided into three functionally and phenotypically different subsets: the classical (CD14+CD16−), the intermediate (CD14+CD16+), and the non-classical (CD14dimCD16++) monocytes [19]. CD163 is expressed mainly on classical monocytes, with lower and negligible expression on intermediate and non-classical monocytes, respectively [20]. In patients with malignancies, circulating monocytes have been shown to be altered with regard to both gene expression profile and distribution within the monocyte subsets [21, 22]. Further, in a number of diseases including cancer, the frequency of CD163-expressing circulating monocytes has been found elevated [23–25].
Due to ectodomain shedding by the enzyme ADAM17 [26], CD163 is also present as a soluble protein (sCD163) in serum, and other body fluids [11, 27]. Elevated serum levels of sCD163 have been associated with poor outcomes in several malignant diseases e.g. malignant melanoma [28], ovarian cancer [29], and multiple myeloma [30], and it has been suggested that the levels in serum reflect the activity of CD163+ tissue macrophages, including TAMs, as well as circulating CD163+ monocytes [30]. Recently, sCD163 levels were reported for a cohort of patients with suspected RCC, showing higher levels in patients with malignant versus benign tumors, and further increased levels in the small subgroup of patients with metastatic disease [31].
Here we describe, for the first time, levels of monocyte CD163 expression (mCD163) and serum sCD163 in a cohort of mRCC patients and healthy controls, and demonstrate that both biomarkers were independently associated with patient outcomes.
Materials and methods
Patients
Eighty-nine patients diagnosed with clear cell mRCC were enrolled in a phase II clinical trial (Danish Renal Cancer Group (DaRenCa) Study-1 between 2009 and 2015 at the Department of Oncology, Aarhus University Hospital, Aarhus, Denmark [32]. Patients were stratified into either favorable (MSKCCFAV, n = 47) or intermediate (MSKCCINT, n = 42) risk groups [33]; patients with a poor prognosis according to MSKCC were not included in the DaRenCa-1 study. Included patients were randomized to treatment with Interleukin-2/Interferon-α ± bevacizumab. The clinical outcome of the DaRenCa-1 study has been published previously [32].
Blood samples were collected at diagnosis, 5 weeks, 9 months, and/or progression, for isolation of peripheral blood mononuclear cells (PBMCs) as well as serum. PBMCs were isolated from heparinized whole blood by Ficoll-Paque Plus (Amersham Biosciences, Amersham, UK) density gradient centrifugation according to manufacturer’s instructions. Serum samples and PBMCs were stored at − 80 °C and − 150 °C, respectively. The study was approved by the local ethical committee (M-20070190) and all patients provided signed consent forms before inclusion. The IMDC risk scores were calculated post-hoc, as described by Heng et. al. [4], based on data collected as part of the clinical trial. Total corrected calcium was calculated from total calcium and albumin by the following formula: Corrected Calcium [mmol/L] = 0.02 * (Normal Albumin (40 g/L)—measured Albumin) + Calcium. Upper limit used for dichotomization in regard to IMDC was 2.55 mmol/L.
Number of metastatic sites was calculated as the sum of the numbers for organ-specific metastasis (dichotomized data, as shown in Table 1).
Table 1.
Baseline clinical and paraclinical data on included patients
| Variable | All Patients | sCD163 at baseline | ||
|---|---|---|---|---|
| Low (< 2.18 mg/L) | High (≥ 2.18 mg/L) | P-value | ||
| N | 89 | 43 | 45 | |
| Sex – N (%) | 0.71 | |||
| Female | 22 (25) | 10 (23) | 12 (27) | |
| Male | 67 (75) | 33 (77) | 33 (73) | |
| Age – median (range) | 57 (28–69) | 57 (37–67) | 58 (28–69) | 0.89 |
| MSKCC – N (%) | 0.38 | |||
| Favorable | 47 (53) | 25 (58) | 22 (49) | |
| Intermediate | 42 (47) | 18 (42) | 23 (51) | |
| IMDC – N (%) | 0.44 | |||
| Favorable | 22 (25) | 13 (31) | 9 (20) | |
| Intermediate | 47 (54) | 22 (52) | 24 (55) | |
| Poor | 18 (21) | 7 (17) | 11 (25) | |
| Kidney Tumor – N (%) | 13 (15) | 7 (16) | 6 (13) | 0.70 |
| Metastases – N (%) | ||||
| Lung | 71 (80) | 33 (77) | 37 (82) | 0.52 |
| Lymph node | 56 (63) | 23 (53) | 32 (71) | 0.09 |
| Liver | 14 (16) | 3 (7) | 11 (24) | 0.04 |
| Bone | 23 (26) | 9 (21) | 14 (31) | 0.28 |
| Soft Tissue | 17 (19) | 11 (26) | 6 (13) | 0.15 |
| Adrenal Glands | 13 (15) | 4 (9) | 9 (20) | 0.23 |
| Smoking – N (%) | 0.65 | |||
| Never Smoked | 29 (33) | 13 (30) | 16 (36) | |
| Previous Smoked | 41 (47) | 20 (47) | 21 (48) | |
| Current Smoker | 18 (20) | 10 (23) | 7 (16) | |
| Paraclinical – median (IQR) | ||||
| LDH (mg/L) | 168 (145–196) | 163 (139–189) | 174 (146–196) | 0.51 |
| Haemoglobin (mmol/L) | 8.3 (7.5–9) | 8.5 (7.8–9.1) | 8.1 (7.2–9) | 0.12 |
| Total, corrected calcium (mmol/L) | 2.37 (2.30–2.50) | 2.34 (2.28–2.44) | 2.42 (2.33–2.52) | 0.06 |
| Albumin (g/L) | 38 (35–41) | 39 (37–42) | 37 (35–39) | 0.02 |
| C-reactive protein (mg/L) | 9.84 (2.53–41.25) | 4.38 (1.32–20.8) | 13.94 (4.89–63.01) | 0.002 |
| Neutrophils (109/L) | 4.34 (3.17–5.37) | 4.37 (2.97–5.50) | 4.34 (3.24–5.74) | 0.83 |
| Monocytes (109/L) | 0.63 (0.52–0.85) | 0.59 (0.51–0.75) | 0.65 (0.57–0.95) | 0.04 |
| Platelets (109/L) | 272 (231–348) | 261 (231–305) | 300 (232–380) | 0.06 |
Binary and categorical data are shown as N (numbers) with percentage of the total cohort of patients in parentheses
Continuous data are shown as median values and IQR (interquartile range). Furthermore, the same parameters are shown after stratification of the patients by the median value of sCD163 (2.18 mg/L). P-values were calculated from the relevant statistical test between the low versus high sCD163 groups. Significant differences are highlighted by bold font
Note that the present cohort does not include patients with MSKCC poor risk score, since such patients were excluded from the DaRenCa-1 study
MSKCC Memorial Sloan Kettering Cancer Center, IMDC International Metastatic RCC Database Consortium, LDH Lactate dehydrogenase
A control group of 20 age- and sex-matched healthy controls from the local blood bank, Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark were anonymously included in the study.
Serum sCD163 ELISA analysis
Serum concentrations of sCD163 were measured using a validated in-house sandwich ELISA assay [27]. Serum concentrations of C-reactive protein (CRP) were measured on a Roche cobas® 6000 analyzer (Roche Diagnostics) according to the clinical standard procedure at the Department of Clinical Biochemistry, Aarhus University Hospital, Aarhus, Denmark.
Multiparameter flow cytometry
Samples of PBMCs were thawed, washed, and labelled with an antibody cocktail containing anti-CD56 (B159) V450, anti-CD14 (MΦP9) V500 (BD Biosciences), anti-CD16 (3G8) PerCP, Live/Dead near-IR fixable dye (Life Technologies) and anti-CD163 (Mac2-158) PE (Trillium Diagnostics). All antibodies were titrated for optimal performance. For compensation, OneComp eBeads (eBiosciences) were used for all antibodies except for anti-CD16 PerCP, for which BD Comp Beads Plus (BD Biosciences) were used. For the Live/Dead near-IR dye, Amine Reactive Compensation (ArC) beads (Life Technologies) were used. Samples were run on a BD LSRFortessa flow cytometer (BD Biosciences), see Supplementary Fig. 1 for optical configuration of the LSRFortessa. The cytometer settings were calibrated and adjusted each day by cytometer setup and tracking (CST) beads using FACS Diva and application settings (BD Biosciences). At least 100,000 events were acquired for all samples and at least 30,000 for compensation controls. Data were compensated and analyzed using FlowJo 10.0.7 for Mac (Tree Star Inc., OR, USA). On each day of the experiment, Sphero 8-peak beads (BD Biosciences) were run along with the samples to document cytometer stability over time (Supplementary Fig. 2).
The gating strategy used for data analysis is shown in Supplementary Fig. 3.
Blocking with purified human IgG (100 µg/mL, Beriglobin, CSL Behring) was used to alleviate non-specific antibody binding as described previously [34] for the majority of samples since this procedure was introduced in our lab during the study period.
Two different lots of anti-CD163 PE antibody were available during the study. These two lots were compared side-by-side showing good concordance in monocyte CD163 MFI levels (Supplementary Fig. 4).
Statistics
Gaussian distribution of data was assessed by Q-Q plots before analysis. Data not showing Gaussian distribution were log-transformed and reassessed by Q-Q plots. Equal variance was tested by either Variance Ratio test or Bartlett’s test.
Comparisons of continuous data between two groups were performed by Student’s t-test for data showing a Gaussian distribution and having equal variance, otherwise, the Mann–Whitney U test was used. Comparison of mCD163 MFI levels between monocyte subsets was performed using a paired t-test.
Comparisons of continuous data, with more than two groups, were performed by oneway-ANOVA for data showing a Gaussian distribution and having equal variance, otherwise, the Kruskal–Wallis test was performed.
Repeated measures were analysed by mixed-effects analysis due to missing values.
Comparisons of categorial data between two groups were performed by chi2 or Fisher's exact test (for outcomes with less than 5 events). Comparisons of categorial data with more than two groups were performed by chi2 (R × C). Correlations were assessed using Pearson correlation.
Kaplan–Meier plots and logrank tests (or logrank test for trend when > 2 groups) were used for survival analyses. Overall survival was calculated from date of randomization until death or last follow-up. This was performed both in the total patient cohort and after stratification based on MSKCC prognostic groups. Cut-off values equal to the 25th, 50th, and 75th percentile of mCD163 and sCD163 levels were investigated. The cut-off values were determined in the individual analysed groups. The established sCD163 upper reference value of the age group 50–74 years (3.76 mg/L) [27] was used as an additional cut-off value.
Cox proportional hazards regression models were applied in uni- and multivariate survival analyses. This was done after checking that all assumptions for this method were met, including that of proportional hazards. Since there was a non-linear relationship between the log(hazards) and monocyte mCD163 PE MFI (not meeting the required assumptions), this parameter was included in a multivariate Cox regression model using restricted cubic splines functions [35].
Prism 9 for Mac (Graphpad Software, San Diego, CA) was used to create graphs, Kaplan–Meier plots as well as mixed effect analysis. STATA v. 15 for Mac or Windows (StataCorp LLC, TX) was used for the statistical analyses and to create the graphs shown in Fig. 4.
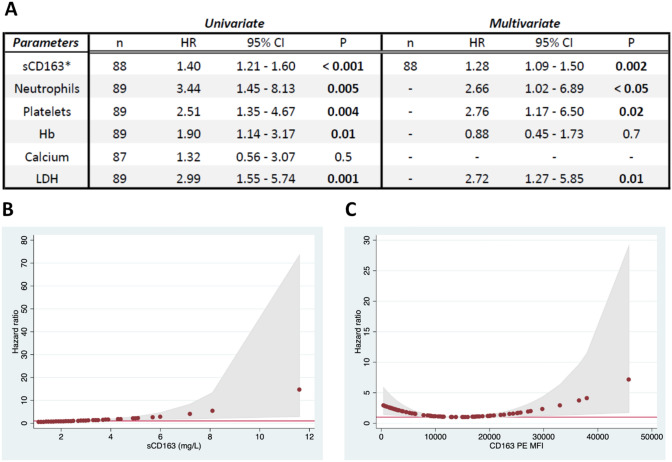
Fig. 4.
Survival analyses by Cox Proportional Hazards regression analysis. A Uni- and multivariate Cox regression analyses of sCD163 and known prognostic factors in mRCC. *sCD163 data were included as a continuous variable in Cox regression analyses, whereas data on the other factors were categorized into two groups according to the cut-off used in the MSKCC/IMDC scores: LDH > 1.5 × upper limit of normal; corrected calcium, platelets, and neutrophils > normal range, and hemoglobin < normal range. Thus, a hazard ratio (HR) of 1.4 for sCD163 denotes a 1.4 times increased hazard (risk of death) with each increase of one unit in serum sCD163 (mg/L). For the categorical parameters, the HR denotes the hazard difference between patients with abnormal vs. normal values as stated above. Data on albumin-corrected calcium did not reach statistical significance in the univariate analysis, and was not included in the multivariate analysis. It is seen that increased sCD163, neutrophils, platelets, and LDH were all independent prognostic factors in this cohort of mRCC patients. LDH: Lactate dehydrogenase. B Cox regression-modeled association between serum sCD163 and HR, showing an increased HR with higher sCD163. C Cox regression-modeled association between monocyte mCD163 MFI and HR. Intermediate mCD163 MFI levels (around the mean value of 12,500) were associated with the most favorable outcome, whereas both very low or very high CD163 MFI levels were associated with a worse outcome. These data did not fulfil the model assumptions for Cox proportional hazards regression. MFI: Median fluorescence intensity. HR equal to 1 is marked by a solid red line. Gray areas show 95%-CI for HR
Results
Baseline characterization of the patient cohort and healthy controls
Patients in the present study were originally included in the DaRenCa-1 clinical trial, which enrolled only patients classified as favorable or intermediate risk according to the MSKCC risk model [32]. Baseline characteristics of the patients, including a stratification by median serum sCD163 levels, are shown in Table 1. The median age was 57 years and 75% were male. The IMDC prognostic score was favorable, intermediate, and poor in 25%, 54%, and 21%, respectively. The majority of patients had lung (80%) and lymph node (63%) metastases, 26% had bone and 16% liver metastases. Stratified by median sCD163, most baseline factors were well balanced, but patients with low sCD163 had lower levels of liver metastases (P = 0.04), albumin (P = 0.02), CRP (P = 0.002) and circulating monocytes (P = 0.04). Smoking status was not associated with differences in baseline levels of either sCD163 (P = 0.65) or mCD163 (P = 0.99).
High levels of serum sCD163 were correlated with high metastatic burden; patients with ≥ 4 metastatic sites had higher levels of serum sCD163 compared to the group with one (P = 0.001) and three (P = 0.04) metastatic sites (Supplementary Fig. 5).
The included healthy controls matched the mRCC patients by sex (P = 0.97) and age (P = 0.18).
Decreased monocyte mCD163 and increased serum sCD163 levels in mRCC patients compared to healthy controls.
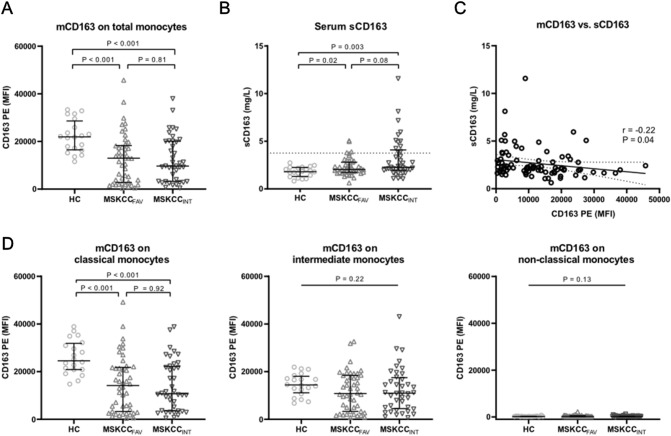
Monocyte CD163 expression (mCD163) was assessed by flow cytometry, for both the total monocyte population and for the three major monocyte subsets (see Supplementary Fig. 3 for gating strategy). Patients with mRCC showed lower expression levels of mCD163 on the total population of circulating monocytes, compared to healthy controls (P < 0.001), but with no difference between MSKCCFAV and MSKCCINT patients (P = 0.81, Fig. 1A).
Fig. 1.
Monocyte mCD163 and serum sCD163 levels in mRCC patients and healthy controls. A Monocyte mCD163 MFI (PE) levels for healthy controls, and for mRCC patients with MSKCC favorable (MSKCCFAV) and intermediate (MSKCCINT) risk score measured by flow cytometry. B Serum sCD163 levels for healthy controls and mRCC patients measured by ELISA. Dotted line = 3.76 mg/L (upper reference value of sCD163 in the age group of 50–74 years, see ref. 27). C Correlation of mCD163 MFI and sCD163 for all mRCC patients. P- and r-values by Pearson correlation using ln(sCD163) and ln(mCD163). Best fitted line is shown (with 95% CI as dotted lines). D mCD163 MFI levels for classical, intermediate, and non-classical monocytes shown for healthy controls and each of the two MSKCC risk groups. Error bars show median with interquartile range. MFI: median fluorescence intensity
When looking at mCD163 expression for the three monocyte subsets, a clear pattern was seen for the healthy controls: Classical monocytes (CD14+CD16−) showed the highest mCD163 expression, intermediate monocytes (CD14+CD16+) had intermediary mCD163 expression, whereas non-classical monocytes (CD14dimCD16++) expressed almost no mCD163 (Fig. 1D, and Supplementary Fig. 3H). The mRCC patients showed more variable monocyte mCD163 levels, with higher mCD163 expression on classical vs. intermediate monocytes (P = 0.01), and with negligible mCD163 expression on non-classical monocytes (Fig. 1D). It is seen that the lower mCD163 expression in mRCC patients vs. controls, was mainly due to significantly lower mCD163 levels on classical monocytes.
The concentration of sCD163 in serum showed a pattern opposite to mCD163, with higher levels in mRCC patients compared to healthy controls: the median concentration of sCD163 was 2.18 mg/L for all mRCC patients vs. 1.81 mg/L for healthy controls (P = 0.004). Further, compared to the controls, sCD163 was significantly higher in both MSKCCFAV (P = 0.02) and MSKCCINT (P = 0.003) mRCC patients, but there was only a trend towards a higher sCD163 in MSKCCINT versus MSKCCFAV patients (P = 0.08, Fig. 1B). The range of sCD163 levels was clearly increased in mRCC patients, with sCD163 elevated above the reference range in 4 (9%) of MSKCCFAV patients and 12 (29%) of MSKCCINT patients (but none of the healthy controls).
We observed a weak inverse correlation between mCD163 MFI (of all monocytes) and serum sCD163 (r = −0.22, P = 0.04, Fig. 1C). This correlation was −0.12 (N = 47, P = 0.41) for MSKCCFAV patients, and −0.34 (N = 41, P = 0.03) for MSKCCINT patients. No significant correlation was observed for healthy controls (P = 0.94).
Further, since increased levels of sCD163 have been linked to inflammation, we analysed the relationship between sCD163 and CRP. In the patients, a positive correlation between sCD163 and CRP was observed (r = 0.46, P < 0.001), which was seen in both MSKCC risk groups. However, this positive correlation in the patients was at least to some extent driven by a few patients having particularly elevated levels of both sCD163 and CRP (Supplementary Fig. 6).
Dynamics of serum sCD163 during treatment
No differences were observed in sCD163 concentrations between baseline, 5 weeks, and 9 months of treatment or at progression (P = 0.28, Supplementary Fig. 7A). This was also the case when separately analyzing patients randomized to ± bevacizumab (Supplementary Fig. 7B). Furthermore, the relative change in serum sCD163 was compared between patients with objective response (complete or partial response), stable disease, or progressive disease. Again, no significant differences were found (Supplementary Fig. 7C–E).
Monocyte mCD163 as a prognostic biomarker: Kaplan–Meier analyses
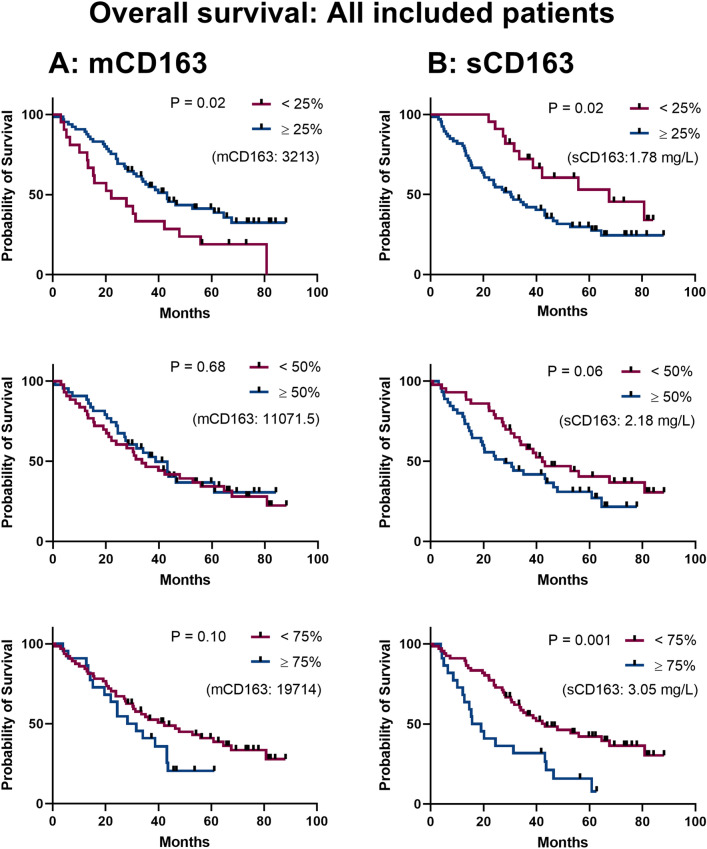
We evaluated the prognostic value of mCD163, initially for all included patients, and then after stratification by MSKCC risk group. Since monocyte mCD163 MFI has not previously been investigated as a prognostic marker in RCC patients, we examined the 25th, 50th, and 75th percentiles as cut-offs for survival analyses. Using the 25th percentile cut-off, low mCD163 was associated with poor outcomes (median 21.1 vs. 43.2 months, P = 0.02, Fig. 2A) while the 50th or 75th percentile cut-off showed no statistically significant difference in survival (P = 0.68 and P = 0.10, respectively, Fig. 2A).
Fig. 2.
Survival analyses by mCD163 and sCD163 levels in all included mRCC patients. A Kaplan–Meier survival analyses using monocyte mCD163 MFI levels stratified by 25th, 50th, or 75th percentile in the total mRCC patient cohort. B Survival analyses as in A) using peripheral blood serum sCD163 data from the total patient cohort. P-values by logrank tests. The separator value for binomial categorization is stated in parentheses on each plot. Censored patients are annotated with a rectangle
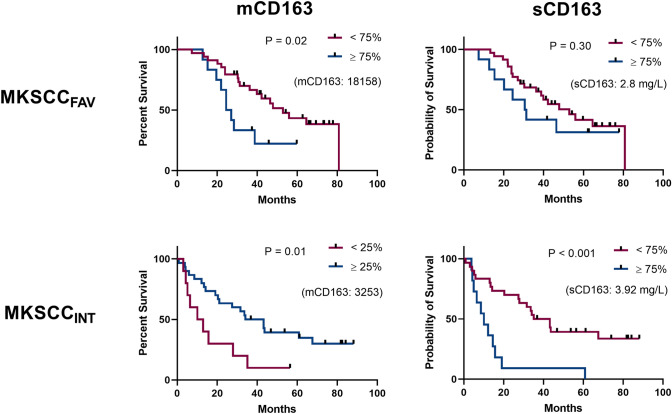
When also stratifying by MSKCC risk group, we observed that the association between low mCD163 and poor outcome (25th percentile cut-off) was especially pronounced in MSKCCINT patients. In contrast, for high mCD163 (75th percentile cut-off) there was an association with poor outcome only in MSKCCFAV patients (Fig. 3A & Supplementary Fig. 8).
Fig. 3.
Survival analyses by mCD163 and sCD163 levels, stratified by MSKCC prognostic groups. Analyses as shown in Fig. 2 were performed for MSKCCFAV and MSKCCINT patients, respectively. Here, we show data for the cut-off value yielding the lowest P-value for mCD163 and sCD163, respectively (see Supplementary Figs. 8 and 9 for all investigated cut-off values). P-values by logrank test. The separator value for binomial categorization is stated in parentheses on each plot. Censored patients are annotated with a rectangle
Serum sCD163 as a prognostic biomarker: Kaplan–Meier analyses
As for mCD163, survival analyses were performed to investigate the prognostic value of the serum levels of sCD163. Using the 25th, 50th, and 75th percentiles as cut-offs in Kaplan–Meier analyses, higher levels of sCD163 were associated with poor outcomes. The absolute difference in median OS between patients with low vs. high sCD163 was largest when using the 25th percentile (1.78 mg/L) cut-off: 67.6 vs. 30.3 months, respectively (P = 0.02). The same pattern was observed when using the 50th percentile (2.18 mg/L, 42.20 vs. 27.84 months, P = 0.06), and the 75th percentile (3.07 mg/L, 43.21 vs. 17.38 months, P = 0.001), as seen in Fig. 2B. A similar result was obtained by using the established upper reference range for the sCD163 assay (3.76 mg/L, 43.18 vs. 14.97 months, P = 0.001, data not shown).
Survival analyses also were performed after stratification by MSKCC risk group. Here, it was clear that the association of high sCD163 with poor outcome was most pronounced in MSKCCINT patients, with a clear separation of survival curves for all cut-off values, whereas for MSKCCFAV patients there were no significant differences in OS (Fig. 3B & Supplementary Fig. 9).
When investigating the prognostic value for sCD163 for patients randomized to ± bevacizumab as part of the DaRenCa-1 trial, there was no significant impact of this randomization (data not shown).
Multivariate analyses: serum sCD163 is an independent prognostic biomarker in mRCC patients
As the above Kaplan–Meier analyses indicated potential of both monocyte mCD163 and serum sCD163 as prognostic markers in mRCC patients, we next performed uni- and multivariate Cox regression survival analyses to further investigate the biomarker potential. These analyses included already established prognostic factors in mRCC, for which data was available (neutrophils, platelets, hemoglobin, corrected calcium, and LDH). Results of these analyses are shown in Fig. 4A for serum sCD163. It is seen that in univariate analyses, all the investigated biomarkers, except for serum calcium levels, showed statistically significant associations with outcome (including for sCD163: HR = 1.40, P < 0.001). In the multivariate analysis, sCD163 (HR = 1.28; 95%CI 1.09–1.50; P = 0.002) was an independent prognostic marker associated with mRCC patient overall survival.
Since sCD163 is a biomarker of inflammation, we also investigated the prognostic value of CRP levels, showing a significant association between increased CRP and poor OS in the univariate analysis (HR = 1.35, P < 0.001). However, when also including CRP data in the multivariate analysis, sCD163 remained an independent prognostic factor (HR = 1.24; 95%CI 1.04–1.47; P = 0.016).
It is seen that for serum sCD163 there was an approximately linear association between sCD163 levels and the HR (Fig. 4B). However, for monocyte mCD163 expression levels, our analyses showed a biphasic relationship between mCD163 expression and the HR (Fig. 4C), which was in accordance with the results from the Kaplan–Meier analyses described above. The monocyte mCD163 MFI data did not fulfil the assumptions for Cox proportional hazards regression analysis, and thus we do not report quantitative results on uni- and multivariate Cox regression analyses for the mCD163 parameter. However, using cubic-splines function statistics [35], we were able to include the monocyte mCD163 parameter in a multivariate Cox regression analysis with the same covariates as in Fig. 4A. The results may be interpreted with caution but showed that patients with monocyte mCD163 levels close to the mean value (PE MFI ~ 12,500) had the most favorable outcome, whereas both lower (P = 0.002) and higher (P = 0.001) mCD163 levels were associated with worse outcome.
sCD163 as a potential add-on biomarker to improve the IMDC prognostic score
As described above, the MSKCC score was used for inclusion of patients in the DaRenCa study-1, and overall survival curves by MSKCC risk groups can be seen in Supplementary Fig. 10. However, the IMDC risk model has now become the preferred risk assessment tool. Therefore, we investigated the performance of sCD163 as a biomarker supplement to the IMDC score.
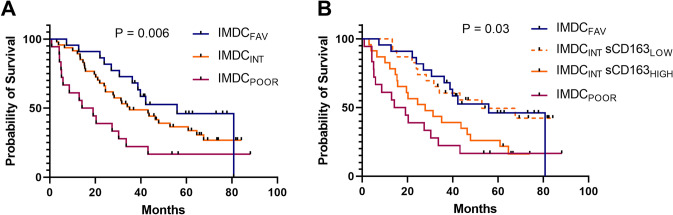
The prognostic value of the IMDC score is clearly demonstrated with good curve separation (P = 0.006, Fig. 5A). Interestingly, when dividing the IMDC intermediate (IMDCINT) patients into two groups based on the sCD163 median value (IMDCINT-sCD163LOW or IMDCINT-sCD163HIGH) a clear separation of the survival curves was seen (P = 0.03, Fig. 5B); IMDCINT-sCD163LOW showed survival similar to IMDCFAV (P = 0.85) and IMDCINT-sCD163HIGH patients showed survival similar to IMDCPOOR patients (P = 0.26).
Fig. 5.
sCD163 as a potential add-on biomarker to improve the IMDC prognostic score. A Overall survival of all patients according to their IMDC risk score at baseline. The reported P-value is by logrank test for trend. Censored patients are annotated with a rectangle. B The IMDC intermediate risk group (IMDCINT) was divided into two groups by the baseline serum sCD163 concentration (split on median: 2.25 mg/L). The reported P-value is by logrank test of difference in OS between IMDCINT-sCD163LOW vs. IMDCINT-sCD163HIGH. Further, there was no statistically significant difference in survival between IMDCINT-sCD163LOW vs. IMDCFAV patients (P = 0.85) or IMDCINT-sCD163HIGH vs. IMDCPOOR patients (P = 0.26). Censored patients are annotated with a rectangle
Discussion
This is the first report on mCD163 and sCD163 as biomarkers of OS in mRCC patients. We demonstrate that both monocyte membrane-bound CD163 (mCD163) and serum soluble CD163 (sCD163) levels in patients with mRCC were independent biomarkers of patient outcome. High sCD163 was an independent prognostic factor associated with poor outcomes, whereas, for monocyte mCD163, patients having either very low or very high levels experienced worse outcomes than patients with intermediary mCD163 levels.
Importantly, the level of sCD163 was able to separate patients with IMDCINT risk into two subgroups having survival similar to patients with IMDCFAV and IMDCPOOR, respectively. Hence, using sCD163 as an add-on to the IMDC risk score may improve the prognostic stratification in patients with mRCC. As both MSKCC and IMDC risk score systems allocate almost half of the patients into the intermediate risk group [4], the sCD163 biomarker may improve prognostic allocation and patient counselling. Since current treatment recommendations differ between IMDCFAV and IMDCINT-POOR patients [36], these results may be used to improve prognostic staging and treatment decisions in the future, if the results can be reproduced in larger prospective studies, with current standard of care.
The present study included patients with available serum/PBMC samples that were collected as part of the DaRenCa-1 clinical trial, where patients were treated with Interleukin-2 and Interferon-α (± bevacizumab) as frontline treatment and primarily TKI-based treatment at relapse as described previously [32].
The MSKCC risk score was used as inclusion criteria in the DaRenCa-1 trial with inclusion of only favorable and intermediate risk patients, and thus the MSKCC score was included in the present study for stratification by prognosis.
The prospective study design with long follow-up is a strength of the study, whereas limitations include the moderate number of included patients, the used treatment regimen in the clinical trial that differ from the current standard of care, and a patient cohort including only patients with MSKCC favorable and intermediate risk score.
Our analysis of monocyte subset mCD163 expression levels showed that in both healthy controls and mRCC patients the highest expression of CD163 was found on classical monocytes, with lower and negligible expression on intermediate and non-classical monocytes, respectively. This is in agreement with previous reports [20]. Monocyte mCD163 levels were generally decreased in mRCC patients, compared to healthy controls, whereas the opposite was seen for serum sCD163 levels that were increased in the patients. There were no statistically significant differences in mCD163 or sCD163 levels between MSKCCFAV and MSKCCINT patients. The difference in mCD163 and sCD163 between healthy controls and cancer patients differs between malignancies. Decreased mCD163 has also been reported in colorectal cancer [37] whereas no differences were observed in a study on multiple myeloma [38]. For sCD163, significantly increased levels in patients, compared to healthy controls, have also been reported for diffuse large B-cell lymphoma (DLBCL) [39], and hepatocellular cancer [40], whereas no differences were found in studies on colorectal cancers [37] and multiple myeloma [38].
Importantly, sCD163 remained a statistically significant independent prognostic factor in multivariate Cox regression analysis also including CRP as a co-variate, which indicates that sCD163 is not merely a bystander marker of inflammation. This is in accordance with previous studies highlighting sCD163 as an independent prognostic biomarker in multiple myeloma [30], diffuse large B-cell lymphoma [39], and hepatocellular carcinoma [40]. In studies on colorectal cancer [37], and epithelial ovarian cancer [29], no significant prognostic value was found in multivariate survival analyses (OS).
So far, the mechanism behind increased sCD163 reported for a number of cancers, as well as the prognostic value of sCD163, is not fully understood. It is known that sCD163 is mainly released from monocytes/macrophages by the enzyme ADAM17 [26], the expression of which is increased in various cancers [41], including renal cell carcinoma [42]. Further, infiltration of CD163-expressing TAMs in human tumors is high, which is associated with poor outcomes, including in RCC [13, 14]. This, together with increased ADAM17 activity likely contribute to increased sCD163 levels in cancer patients. We found a negative correlation between mCD163 and sCD163 which has been reported previously [43] and suggests that monocyte CD163 also contributes to the pool of circulating sCD163. However, this is still unresolved and a recent study found no correlation between monocyte mCD163 and sCD163 in patients with multiple myeloma [38].
We investigated dynamic changes of serum sCD163 during the course of treatment, showing no significant changes, irrespective of the observed response to treatment. This absence of association between sCD163 dynamics and treatment response may seem contradictory due to the prognostic value of sCD163. These results further highlight the need for future studies on the biological background for prognostic value of sCD163 in malignancies.
In the present study, the prognostic value of sCD163 was mainly observed in the MSKCCINT group. For monocyte mCD163, low levels were associated with poor outcomes in MSKCCINT patients and high levels were associated with poor outcomes in MSKCCFAV patients. This observation may indicate that the large group of MSKCCINT patients is more heterogenous in relation to monocyte-macrophage-related immune activation. In line with this, recent studies exploring the genetic signatures of RCC described several different subtypes [44, 45]; a common feature was two main subtypes based on angiogenic and immunogenic activity. Interestingly, the fraction of patients with the angiogenic subtype decreased, and the fraction of immunogenic subtype increased, when moving from the favorable to the intermediate risk group, and from the intermediate to the poor risk group (by both MKSCC and IMDC) [44]. Associations of these genetic signatures with monocyte mCD163 and serum sCD163 should be investigated in future studies. Such analyses may add to our biological understanding of the observed association between mCD163/sCD163 levels and patient outcomes.
Regarding the possibility of implementing the investigated biomarkers in routine clinic, there is a clear advantage of sCD163 over mCD163, since sCD163 is measured by a simple ELISA assay—whereas mCD163 is measured by flow cytometry yielding arbitrary outcome values, not easily standardized between laboratories.
In conclusion, both monocyte mCD163 and serum sCD163 showed value as prognostic biomarkers in mRCC patients, and high serum levels of sCD163 were an independent prognostic marker of poor overall survival. Using sCD163 as an add-on to the IMDC risk score may improve the prognostic stratification in patients with an IMDC intermediate risk score. This finding should be validated in larger prospective cohorts, as it may have potential impact on treatment strategies in the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
For excellent laboratory assistance, the authors would like to thank Helle Ryom from Department of Clinical Biochemistry, Aarhus University Hospital, Anni Skovbo from the Department of Biomedicine, Aarhus University, and the staff at the FACS Core Facility, Aarhus University, where all flow cytometry experiments were performed using a BD LSRFortessa flow cytometer. We thank Aparna Udupi, Section for Biostatistics, Department of Public Health, Aarhus University for assistance with Cox regression analyses.
Abbreviations
- ccRCC
Clear cell renal cell carcinoma
- CRP
C-reactive protein
- ELISA
Enzyme-linked immunosorbent assay
- HR
Hazard ratio
- ICI
Immune checkpoint inhibitor
- IMDC
International Metastatic RCC Database Consortium
- IMDCFAV
Favorable risk group by IMDC
- IMDCINT
Intermediate risk group by IMDC
- IMDCPOOR
Poor risk group by IMDC
- LDH
Lactate dehydrogenase.
- mCD163
Monocyte membrane-bound CD163
- MFI
Median fluorescence intensity
- mRCC
Metastatic renal cell carcinoma
- MSKCC
Memorial Sloan Kettering Cancer Center
- MSKCCFAV
Favorable risk group by MSKCC
- MSKCCINT
Intermediate risk group by MSKCC
- OS
Overall survival
- PBMCs
Peripheral blood mononuclear cells
- RCC
Renal cell carcinoma
- sCD163
Soluble CD163
- sCD163HIGH
sCD163 above or equal to the median value
- sCD163LOW
sCD163 below the median value
- TAMs
Tumor-associated Macrophages
- TKI
Tyrosine kinase inhibitor
Author contributions
KML, MH, FD and MNA: designed the study, analyzed and interpreted the data. KML and MNA: drafted the manuscript. HJM performed the ELISA measurements. SAK, KML, MH, and MNA: performed and analyzed the flow cytometry experiments. FD: recruited patients, performed clinical monitoring, and provided clinical expertise. All authors provided critical review and approved the final version of the manuscript.
Funding
The study was funded by the Department of Biomedicine, Aarhus University and Department of Clinical Biochemistry, Aarhus University Hospital. KML received a research scholarship from the Danish Cancer Society. FD received a research grant from the Central Denmark Region Health Research Foundation and SAK received a research grant from the Max and Inge Wørzner Memorial Foundation.
Data availability
The data supporting the results reported in this study can be made available upon reasonable request to the corresponding author.
Declarations
Conflict of interests
All authors declare no conflict of interest.
Ethics approval & consent to participate
The DaRenCa-1 study was approved by the local ethical committee (M-20070190) and all patients provided signed consent forms before inclusion. According to Danish law, the use of anonymous samples from blood donors as a control group does not require specific ethical committee approval.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Frede Donskov and Morten Nørgaard Andersen have contributed equally to this work.
References
- 1.Abe H, Kamai T. Recent advances in the treatment of metastatic renal cell carcinoma. Int J Urol. 2013;20(10):944–955. doi: 10.1111/iju.12187. [DOI] [PubMed] [Google Scholar]
- 2.Ko JJ, Xie W, Kroeger N, Lee J-l, Rini B, Knox JJ, et al. Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol. 2015;16(3):293–300. doi: 10.1016/S1470-2045(14)71222-7. [DOI] [PubMed] [Google Scholar]
- 3.Bosse D, Lin X, Simantov R, Lalani AA, Derweesh I, Chang SL, et al. Response of primary renal cell carcinoma to systemic therapy. Eur Urol. 2019;76(6):852–860. doi: 10.1016/j.eururo.2019.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Heng DYC, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Belgiovine C, D'Incalci M, Allavena P, Frapolli R. Tumor-associated macrophages and anti-tumor therapies: complex links. Cell Mol Life Sci. 2016;73(13):2411–2424. doi: 10.1007/s00018-016-2166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baird L, Terskikh A. The tumor microenvironment. Adv Exp Med Biol. 2010;671:67–73. doi: 10.1007/978-1-4419-5819-8_6. [DOI] [PubMed] [Google Scholar]
- 8.Bover LC, Cardó-Vila M, Kuniyasu A, Sun J, Rangel R, Takeya M, et al. A previously unrecognized protein-protein interaction between TWEAK and CD163: potential biological implications. J Immunol. 2007;178(12):8183–8194. doi: 10.4049/jimmunol.178.12.8183. [DOI] [PubMed] [Google Scholar]
- 9.Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev. 2016;99(Pt B):180–185. doi: 10.1016/j.addr.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 11.Moller HJ, Peterslund NA, Graversen JH, Moestrup SK. Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood. 2002;99(1):378–380. doi: 10.1182/blood.V99.1.378. [DOI] [PubMed] [Google Scholar]
- 12.Moestrup S, Møller H. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med. 2004;36(5):347–354. doi: 10.1080/07853890410033171. [DOI] [PubMed] [Google Scholar]
- 13.Andersen MN, Etzerodt A, Graversen JH, Holthof LC, Moestrup SK, Hokland M, et al. STAT3 inhibition specifically in human monocytes and macrophages by CD163-targeted corosolic acid-containing liposomes. Cancer Immunol Immunother. 2019;68(3):489–502. doi: 10.1007/s00262-019-02301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen H, Liu J, Chen S, Ma X, Ying Y, Li J, et al. Prognostic value of tumor-associated macrophages in clear cell renal cell carcinoma: a systematic review and meta-analysis. Front Oncol. 2021;11:657318. doi: 10.3389/fonc.2021.657318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larionova I, Tuguzbaeva G, Ponomaryova A, Stakheyeva M, Cherdyntseva N, Pavlov V, et al. Tumor-associated macrophages in human breast, colorectal, lung. Ovarian Prostate Cancers Front Oncol. 2020;10:566511. doi: 10.3389/fonc.2020.566511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama T, Saito K, Kumagai J, Nakajima Y, Kijima T, Yoshida S, et al. Higher serum C-reactive protein level represents the immunosuppressive tumor microenvironment in patients with clear cell renal cell carcinoma. Clin Genitourin Cancer. 2018;16(6):e1151–e1158. doi: 10.1016/j.clgc.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Hong TY, Wang YN, Peng G, Yu YW, Zhang J, et al. Combining UBR5 and CD163(+) tumor-associated macrophages better predicts prognosis of clear cell renal cell carcinoma patients. Cancer Immunol Immunother. 2021;70:2925–2935. doi: 10.1007/s00262-021-02885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017;17(6):349–362. doi: 10.1038/nri.2017.28. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol. 2013;4:23. doi: 10.3389/fimmu.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tippett E, Cheng WJ, Westhorpe C, Cameron PU, Brew BJ, Lewin SR, et al. Differential expression of CD163 on monocyte subsets in healthy and HIV-1 infected individuals. PLoS ONE. 2011;6(5):e19968. doi: 10.1371/journal.pone.0019968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schauer D, Starlinger P, Reiter C, Jahn N, Zajc P, Buchberger E, et al. Intermediate monocytes but not TIE2-expressing monocytes are a sensitive diagnostic indicator for colorectal cancer. PLoS ONE. 2012;7(9):e44450. doi: 10.1371/journal.pone.0044450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamm A, Prenen H, Van Delm W, Di Matteo M, Wenes M, Delamarre E, et al. Tumour-educated circulating monocytes are powerful candidate biomarkers for diagnosis and disease follow-up of colorectal cancer. Gut. 2016;65(6):990–1000. doi: 10.1136/gutjnl-2014-308988. [DOI] [PubMed] [Google Scholar]
- 23.Goodale D, Phay C, Brown W, Gray-Statchuk L, Furlong P, Lock M, et al. Flow cytometric assessment of monocyte activation markers and circulating endothelial cells in patients with localized or metastatic breast cancer. Cytometry B Clin Cytom. 2009;76(2):107–117. doi: 10.1002/cyto.b.20449. [DOI] [PubMed] [Google Scholar]
- 24.Hou J, Wang X, Zhang M, Wang M, Gao P, Jiang Y. Circulating CD14(+)CD163(+)CD209(+) M2-like monocytes are associated with the severity of infection in helicobacter pylori-positive patients. Mol Immunol. 2019;108:13–22. doi: 10.1016/j.molimm.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Zhang B, Cao M, He Y, Liu Y, Zhang G, Yang C, et al. Increased circulating M2-like monocytes in patients with breast cancer. Tumor Biol. 2017;39(6):101042831771157. doi: 10.1177/1010428317711571. [DOI] [PubMed] [Google Scholar]
- 26.Etzerodt A, Maniecki MB, Moller K, Moller HJ, Moestrup SK. Tumor necrosis factor alpha-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J Leukoc Biol. 2010;88(6):1201–1205. doi: 10.1189/jlb.0410235. [DOI] [PubMed] [Google Scholar]
- 27.Moller HJ. Soluble CD163. Scand J Clin Lab Invest. 2012;72(1):1–13. doi: 10.3109/00365513.2011.626868. [DOI] [PubMed] [Google Scholar]
- 28.Jensen TO, Schmidt H, Moller HJ, Hoyer M, Maniecki MB, Sjoegren P, et al. Macrophage markers in serum and tumor have prognostic impact in american joint committee on cancer stage I/II melanoma. J Clin Oncol. 2009;27(20):3330–3337. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- 29.No JH, Moon JM, Kim K, Kim YB. Prognostic significance of serum soluble CD163 level in patients with epithelial ovarian cancer. Gynecol Obstet Invest. 2013;75(4):263–267. doi: 10.1159/000349892. [DOI] [PubMed] [Google Scholar]
- 30.Andersen MN, Abildgaard N, Maniecki MB, Moller HJ, Andersen NF. Monocyte/macrophage-derived soluble CD163: a novel biomarker in multiple myeloma. Eur J Haematol. 2014;93(1):41–47. doi: 10.1111/ejh.12296. [DOI] [PubMed] [Google Scholar]
- 31.Davidsson S, Huotilainen S, Carlsson J, Sundqvist P. Soluble levels of CD163, PD-L1, and IL-10 in renal cell carcinoma patients. Diagnostics (Basel) 2022;12(2):336. doi: 10.3390/diagnostics12020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donskov F, Jensen NV, Smidt-Hansen T, Brondum L, Geertsen P. A randomized phase II trial of interleukin-2 and interferon-alpha plus bevacizumab versus interleukin-2 and interferon-alpha in metastatic renal-cell carcinoma (mRCC): results from the danish renal cancer group (DaRenCa) study-1. Acta Oncol. 2018;57(5):589–594. doi: 10.1080/0284186X.2018.1433324. [DOI] [PubMed] [Google Scholar]
- 33.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 34.Andersen MN, Al-Karradi SN, Kragstrup TW, Hokland M. Elimination of erroneous results in flow cytometry caused by antibody binding to Fc receptors on human monocytes and macrophages. Cytometry A. 2016;89(11):1001–1009. doi: 10.1002/cyto.a.22995. [DOI] [PubMed] [Google Scholar]
- 35.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Meth Prog Bio. 1997;54(3):201–208. doi: 10.1016/S0169-2607(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 36.Kotecha RR, Motzer RJ, Voss MH. Towards individualized therapy for metastatic renal cell carcinoma. Nat Rev Clin Oncol. 2019;16(10):621–633. doi: 10.1038/s41571-019-0209-1. [DOI] [PubMed] [Google Scholar]
- 37.Krijgsman D, De Vries NL, Andersen MN, Skovbo A, Tollenaar R, Moller HJ, et al. CD163 as a biomarker in colorectal cancer: the expression on circulating monocytes and tumor-associated macrophages, and the soluble form in the blood. Int J Mol Sci. 2020;21(16):5925. doi: 10.3390/ijms21165925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kvorning SL, Nielsen MC, Andersen NF, Hokland M, Andersen MN, Moller HJ. Circulating extracellular vesicle-associated CD163 and CD206 in multiple myeloma. Eur J Haematol. 2020;104:409–419. doi: 10.1111/ejh.13371. [DOI] [PubMed] [Google Scholar]
- 39.Vajavaara H, Ekeblad F, Holte H, Jorgensen J, Leivonen SK, Berglund M, et al. Prognostic impact of soluble CD163 in patients with diffuse large B-cell lymphoma. Haematologica. 2021;106(9):2502–2506. doi: 10.3324/haematol.2020.278182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazankov K, Rode A, Simonsen K, Villadsen GE, Nicoll A, Moller HJ, et al. Macrophage activation marker soluble CD163 may predict disease progression in hepatocellular carcinoma. Scand J Clin Lab Invest. 2016;76(1):64–73. doi: 10.3109/00365513.2015.1099722. [DOI] [PubMed] [Google Scholar]
- 41.Sinnathamby G, Zerfass J, Hafner J, Block P, Nickens Z, Hobeika A, et al. ADAM metallopeptidase domain 17 (ADAM17) is naturally processed through major histocompatibility complex (MHC) class I molecules and is a potential immunotherapeutic target in breast, ovarian and prostate cancers. Clin Exp Immunol. 2011;163(3):324–332. doi: 10.1111/j.1365-2249.2010.04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roemer A, Schwettmann L, Jung M, Roigas J, Kristiansen G, Schnorr D, et al. Increased mRNA expression of ADAMs in renal cell carcinoma and their association with clinical outcome. Oncol Rep. 2004;11(2):529–536. [PubMed] [Google Scholar]
- 43.Davis BH, Zarev PV. Human monocyte CD163 expression inversely correlates with soluble CD163 plasma levels. Cytometry B Clin Cytom. 2005;63(1):16–22. doi: 10.1002/cyto.b.20031. [DOI] [PubMed] [Google Scholar]
- 44.Motzer R, Banchereau R, Hamidi H, Powles T, Mcdermott D, Atkins MB, et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell. 2020;38(6):803–817. doi: 10.1016/j.ccell.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Costa NM, Cina D, Shrestha R, Bell RH, Lin YY, Asghari H, et al. Identification of gene signature for treatment response to guide precision oncology in clear-cell renal cell carcinoma. Sci Rep. 2020;10(1):2026. doi: 10.1038/s41598-020-58804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the results reported in this study can be made available upon reasonable request to the corresponding author.