Abstract
Introduction
Immune checkpoint inhibitors (ICIs) became the standard of care for several solid tumors. A limited fraction of patients (pts) achieves a long-term benefit. Plasmatic and intracellular cholesterol levels have emerged as promising biomarkers. The aim of the present study was to determine whether cholesterol efflux capacity (CEC), mediated by serum transporters (ABCA1 and ABCG1) and passive diffusion (PD), impacts on clinical outcome of advanced non-small cell lung cancer (NSCLC) and metastatic renal cell carcinoma (mRCC) pts treated with ICIs.
Material and methods
We retrospectively enrolled advanced NSCLC and mRCC pts consecutively treated with ICIs between October 2013 and October 2018. CEC and cholesterol loading capacity (CLC) were assessed by well-established specific cell models. As primary endpoint, CEC, PD and CLC were correlated with overall survival (OS) while the effects of these parameters on progression-free survival (PFS) and clinical benefit (CB), defined as complete/partial response or stable disease, represented secondary endpoints.
Results
NSCLC accounted for 94.2% of 70 enrolled cases, and serum sample suitable for CEC and PD determination was available in 68. Blood cholesterol and serum ABCA1, ABCG1, PD and CLC were associated with outcomes (OS, PFS and CB) at univariate analysis. At the multivariate analysis, only PD confirmed its positive prognostic value in terms of OS, PFS and CB.
Conclusion
The favorable impact of cholesterol PD on clinical outcome might reflect its main conformation in mature HDL particles which potentially shape an inflamed context, ultimately promoting ICI efficacy. Further prospective studies are needed to support our findings and uncover targetable pathways.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03398-3.
Keywords: Cholesterol efflux capacity, ABCA1, ABCG1, Passive diffusion, Cholesterol loading capacity, Immunotherapy
Introduction
Immune checkpoint inhibitors (ICIs) have reshaped the treatment landscape of several solid tumors, including metastatic renal cell carcinoma (mRCC) and non-small-cell lung cancer (NSCLC), with immunotherapy-driven strategies replacing the standard targeted- or chemo-based therapies [1, 2]. However, only a limited fraction of patients achieves a long-term benefit from ICIs, underlining the need to identify prognostic and predictive biomarkers.
Several tissue and circulating factors, reflecting the immune-inflammatory, metabolic and genetic status of mRCC and NSCLC patients, have been proposed as potential determinants of ICI response [3].
In particular, cholesterol metabolism emerged as promising and attractive biomarker. This view is supported by various studies documenting the prognostic impact of cholesterol blood levels on the outcome of ICI-treated patients [4, 5]. Moreover, at intracellular level, cholesterol is able to affect the activity of tumor infiltrating lymphocytes (TILs), and the spatial organization in lipid rafts confers to cholesterol the ability to segregate bioactive molecules and adaptor proteins, ultimately fine tuning in a dynamic fashion multiple signaling involved in oncogenic and immune pathways [4, 6].
Connecting essential mediators of cholesterol efflux to mRCC and NSCLC biology and therapeutics are an area of intense research, although still largely uncovered [7]. There are four ways by which cholesterol could be effluxed from the cell: (i) passive diffusion (PD), (ii) Scavenger Receptor Class B Type I (SR-BI) SR-BI-facilitated diffusion, (iii) active efflux by ATP-binding cassette transporter A1 (ABCA1)-, and (iv) ABCG1-mediated efflux to apoA-1 and high-density lipoprotein (HDL). While evidence on SR-BI involvement in biomolecular cancer processes is scarce, recent findings demonstrated that ABCA1-/G1-dependent cholesterol efflux is critically implicated in modulating immune function through T cells activation [8]. Specifically, the regulatory properties of HDL are independent of their plasma concentration and may be assessed for each individual as cholesterol efflux capacity (CEC) [9]. The interaction of HDL with different membrane transporters (ABCA1/ABCG1), functional to remove cholesterol from cells (CEC), not only limits foam cell formation, but also patrols key intracellular inflammatory pathways [10, 11]. The link between cholesterol metabolism and cancer-immune interplay was recently strengthened by evidence of the impact of HDL-ABCA1 interaction and ABCG1 activity on T cell activation and cancer development, respectively [12, 13]. This observation has prompted new and partly investigated hypotheses on the relationship between lipid metabolism and immune functions [14].
Herein, we report the results of a retrospective study investigating the association between ABCA1- and ABCG1-mediated cholesterol efflux capacity (CEC), passive diffusion (PD) and loading capacity (CLC) with clinical outcome of advanced cancer patients treated with immunotherapy.
Materials and methods
Patient eligibility
The present study included advanced cancer patients, consecutively treated from October 2013 to October 2018 with single anti-PD-1, anti-PD-L1 or anti-CTLA-4 agent or with anti-PD-1 plus anti-CTLA-4 combination, regardless of the treatment line, at the Medical Oncology Unit of the University-Hospital of Parma (Italy).
Eligible patients must fulfill the following criteria: histologically or cytologically confirmed diagnosis of locally advanced or metastatic NSCLC or RCC, at least one ICI administration, stored serum sample collected before starting immunotherapy, and plasmatic cholesterol level assessed within one month prior to immunotherapy. All patients provided written informed consent to receive treatment with ICI. All the patients who were alive at the time of the data collection provided an informed consent to be included in the study. The procedures followed were in accordance with the Declaration of Helsinki. The study was approved by the local ethical committee (Comitato Etico Area Vasta Emilia Nord; protocol number 37649, approved on September 21, 2021).
Study design
We conducted a retrospective, monocenter study aimed at investigating the prognostic value of active mediated CEC and cholesterol PD and CLC in advanced cancer patients treated with ICIs. The serum samples were collected prospectively due to inclusion of these patients also in another observational study active in our center.
The primary objective was to test the correlation of CEC (mediated by ABCA-1 and ABCG-1), cholesterol PD and CLC with overall survival (OS, primary endpoint). The secondary objectives were the associations of active CEC, cholesterol PD and CLC with progression-free survival (PFS) and clinical benefit (CB).
OS was defined as the time from immunotherapy initiation until death from any cause. PFS was defined as the time from immunotherapy initiation to the first documented tumor progression or death, whichever occurred first. Patients without event occurrence at the data cut-off of 31 December 2021 were considered as censored at the time of the last follow-up. CB was defined as the proportion of patients experiencing an objective response (either complete or partial response) or stable disease as best response to immunotherapy according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) [15].
Cholesterol efflux capacity (CEC) determination
HDL-CEC was examined using a standardized isotope technique, through the use of well-established cell models [16, 17]. CEC evaluation accounts as a metric of the HDL interaction with ABCA1 and ABCG1 active transporters, counteracting the unspecific process mediated by PD. For the evaluation of PD cholesterol efflux pathway, J774 murine macrophages (J774 A.1, from ATCC) were used in basal conditions, while cell incubation in the presence of 0.3 mM of a cAMP analog (cpt-cAMP, Sigma-Aldrich, Milan, Italy) allowed the induction of ABCA1 expression and was used as a study model for total CEC as previously described [18, 19]. The difference between total CEC and PD-mediated CEC made possible to identify the net contribution of cholesterol efflux through ABCA1 [19]. Moreover, to identify ABCG1-mediated cholesterol efflux, Chinese hamster ovary (CHO) cells were used. CHO cells have been transfected or not with human ABCG1 gene. The difference between CEC of transfected and non-transfected cells enabled to evaluate the contribution of ABCG1 [20, 21]. Cells were initially radio labeled by 24 h exposure to (1,2-3H)-cholesterol (PerkinElmer, Milan, Italy) in the presence of an Acyl-coenzyme A: cholesterol acyltransferase (ACAT) inhibitor allowing the maintenance of cholesterol in an unesterified form. After labeling, cells went through an equilibration time in culture medium with 0.2% BSA (BSA, Sigma-Aldrich). Finally, to assess ABCA1- and PD-mediated CEC, cells were incubated with 2% (v/v) serum for 4 h, while for the evaluation of ABCG1-mediated CEC, cells were incubated for 6 h with 1% (v/v) serum [21]. CEC values were expressed as percentage ratio between the radioactivity released in the medium and the total radioactivity incorporated by the cells. In each experiment, a pool of normolipidemic human sera was used as the reference standard. CEC values of these standard sera were used to normalize the different experiments so that inter-assay variability could be corrected [22]. Another pool of normolipidemic human sera was used in each experiment as the reference standard 2, and its CEC value, following normalization, was considered an index of intra-assay variability. These analyses were performed at Department of Food and Drug, University of Parma endowed with long-term experience on these laboratory procedures.
Cholesterol loading capacity (CLC) determination
To evaluate CLC, we employed THP-1 cells, seeded in 24-well plates at a density of 500,000 cells per well. To induce macrophage differentiation, cells were incubated for 72 h in RPMI 1640 culture medium supplemented by 10% fetal bovine serum (FBS) and phorbol 12-myristate 13-acetate (PMA, 100 ng/mL). Cells were then exposed to 5% (v/v) patients’ serum, who acts as cholesterol donors, for 8 h. At the end, after washing in PBS, macrophages were lysed in a solution of 1% cholic acid and DNA-asi (50 U/mL) and left to stir overnight. CLC was evaluated as μg of cholesterol per mg of protein.
Amplex Red Cholesterol Assay Kit (Molecular Probes, Life Technologies), a fluorimetric assay, was used for the quantification of cholesterol content in the lysates, while to evaluate the protein content in the lysates, we adopted the bicinchoninic acid method.
Statistical analysis
Due to the observational retrospective nature of the study and since the primary and secondary objectives are aimed to explore the correlations between active CEC pathways, cholesterol PD and CLC with clinical outcomes, the sample size was not determined by a formal statistical estimation.
Baseline characteristics were summarized by using descriptive statistical metrics as median and interquartile range (IQR) and absolute and relative frequencies for quantitative and categorical variables, respectively. Boxplot and Kaplan–Meier curves were employed to graphically show the distribution of the cholesterol parameters and the 1-year patient survival outcomes (OS and PFS). A Pearson correlation matrix was developed to simultaneously show the distribution and the correlation among cholesterol parameters.
Univariable and multivariable semi-parametric Cox regression models were implemented to measure associations between cholesterol parameters and survival outcome; the logistic regression model was used to assess the association with the CB outcome. Stepwise backward selection approach was performed to identify the most parsimonious model through Akaike information criterion (AIC). Furthermore, adjusting hazard ratio estimation for age, sex and ECOG PS variables was evaluated.
All statistical analyses were be performed by using R Statistical Software v. 4.0.3.
Results
Patients characteristics
Overall, 70 patients were enrolled in the study. Two patients were excluded due to inadequate serum sampling for biochemical determinations. Therefore, 68 patients were included in the correlation analysis. At the data cut-off of 31 December 2021, 60 patients died and 61 patients experienced disease progression.
Baseline patient characteristics are summarized in Table 1.The median age was 72 years with male predominance (67.1%). Most primary tumors were NSCLC (94.2%). Twenty-seven patients (38.5%) had ≤ 2 metastatic sites, while 43 (61.5%) had more than 2 metastatic sites. The majority of patients received immunotherapy as second or more advanced lines (84.3%), while only eleven (15.7%) received immunotherapy as first-line treatment. No difference was found in baseline characteristics according to PD cholesterol level (Supplementary Table 1).
Table 1.
Baseline characteristics
| Clinicopathological variables | Patients, n = 70 (100%) |
|---|---|
| Age | 72 (41–84) |
| Sex | |
| Male | 47 (67.1) |
| Female | 23 (32.9) |
| Smoking status | |
| Current | 21 (30.0) |
| Former | 28 (40.0) |
| Never | 21 (30.0) |
| ECOG PS | |
| 0 | 23 (32.9) |
| 1 | 38 (54.3) |
| 2 | 9 (12.8) |
| Primary tumors | |
| Kidney | 4 (5.8) |
| NSCLC | 66 (94.2) |
| N. of Metastatic sites involved | |
| 1 | 5 (7.1) |
| 2 | 22 (31.4) |
| 3 | 20 (28.6) |
| 4 | 8 (11.4) |
| ≥ 5 | 15 (21.4) |
| N. of previous lines of therapy | |
| < 1 | 11 (15.7) |
| ≥ 1 | 59 (84.3) |
| ICI administered | |
| anti-PD-1 | 62 (88.6) |
| anti-PD-L1 | 6 (8.6) |
| anti-PD-1/PD-L1 + anti-CTLA-4 | 2 (2.8) |
| Drug administered | |
| Nivolumab | 48 (68.6) |
| Pembrolizumab | 14 (20.0) |
| Atezolizumab | 6 (8.6) |
| Nivolumab + ipilimumab | 2 (2.8) |
| History of hypercholesterolemia | |
| Yes | 24 (34.3) |
| No | 46 (65.7) |
| History of hypertriglyceridemia | |
| Yes | 11 (15.7) |
| No | 58 (82.8) |
| NA | 1 (1.5) |
| Statin therapy | |
| Yes | 21 (30.0) |
| No | 49 (70.0) |
| BMI | |
| < 25 | 43 (61.4) |
| ≥ 25 | 27 (38.6) |
ECOG PS Eastern Cooperative Oncology Group Performance status, NSCLC Non-small cell lung cancer, ICI Immune checkpoint inhibitor, PD-1 Programed death-1, PD-L1 Programed death ligand-1, CTLA-4 Cytotoxic T-lymphocyte antigen 4, BMI Body mass index, NA Not available
The quantitative distribution of plasmatic cholesterol, serum ABCA1- and ABCG1-mediated CEC, cholesterol PD and CLC is reported in Supplementary Fig. 1.
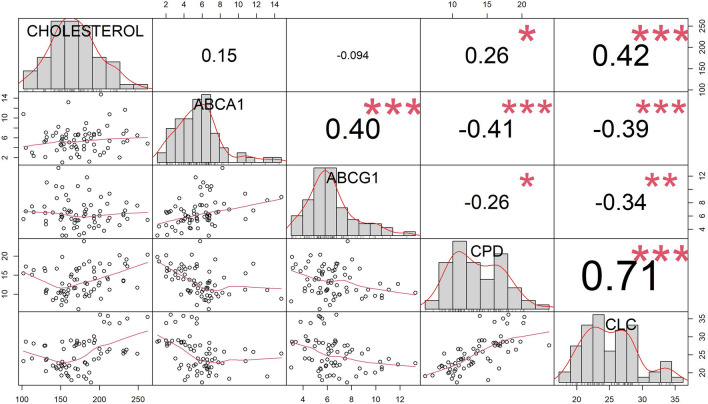
The distribution and correlations among the variables of interest (level of plasmatic cholesterol, serum ABCA1- and ABCG1-mediated CEC, cholesterol PD and CLC) are shown in Fig. 1. Blood cholesterol levels positively correlated with cholesterol PD and CLC (ρ = 0.26 and 0.42, respectively). Conversely, ABCA1- and ABCG1-mediated CEC did not show any significant association with cholesterolemia.
Fig. 1.
Correlation matrix about the distributions (on the diagonal line) and correlations (as scatter plots and Pearson’s correlation values in lower and upper triangle, respectively) among the variables of interest (level of plasmatic cholesterol, serum ABCA-1 and ABCG-1-mediated CEC, PD and CLC). ABCA-1 = ATP-binding cassette transporter A1; ABCG1 = ATP-binding cassette transporter G1; CEC = cholesterol efflux capacity; CPD = cholesterol passive diffusion; CLC = cholesterol loading capacity
As expected, a positive interaction was demonstrated between ABCA1 and ABCG1 transporters, while ABCA1-/ABCG1-mediated efflux was inversely correlated with CLC. Finally, a strong positive correlation was found between cholesterol PD efflux and CLC (ρ = 0.71).
Association between cholesterol parameters and clinical outcomes
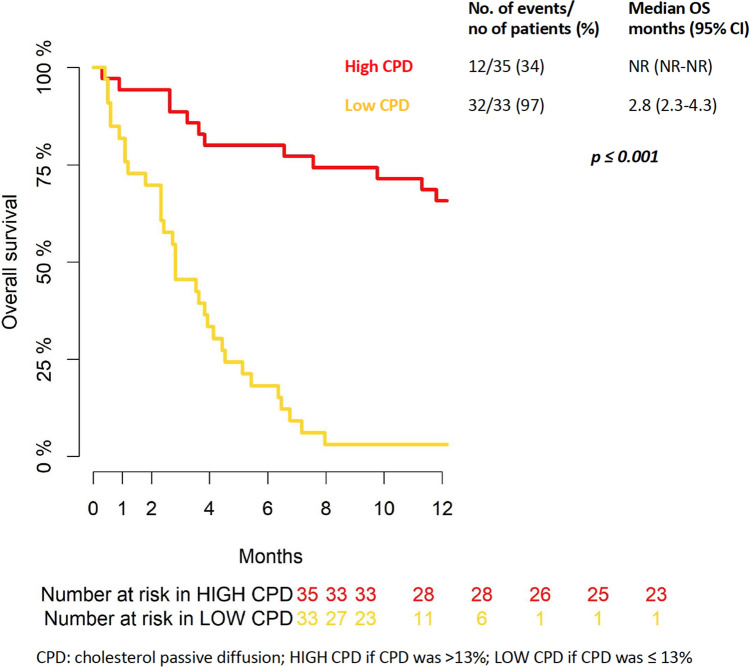
The median 2-year follow-up was 5.85 IQR months [2.63–19.81]. The rate of patients alive at 6 and 12 months was 50% and 36%, respectively (Supplementary Fig. 2). As reported in Table 2, all cholesterol parameters were associated with prolonged OS at the univariable analysis level; nonetheless, in the multivariable model based on Stepwise Backward selection, only cholesterol PD was confirmed as protection factors [HR 0.77 (95%CI 0.67–0.89), p < 0.001]. Although not statistically significant (p = 0.07), CLC seems to be a potential protection factor (upper 95 CI% was equal to 1.01). Given the absence of a validated cut-off in the literature, the median value of 13% was used to stratify the population according to high (equal or greater than 13%) and low cholesterol PD. The Fig. 2 described OS curves by high and low cholesterol PD, and a statistically significance difference was found (p ≤ 0.001). OS rate at 6 and 12 months was 80 and 65.7% in high cholesterol PD group, respectively. Instead, OS rate at 6 and 12 months was 18 and 3% in low cholesterol PD group.
Table 2.
Cox regression models results
| Univariable | Multivariable | Stepwise backward selection | |
|---|---|---|---|
| on OS | HR [95%CI], p value |
Full model HR [95%CI], p value |
Reduced model, AIC = 292.2 HR [95%CI], p value |
| Cholesterol (mg/dl) | 0.99 [0.98–0.99], 0.02 | 0.99 [0.98–1.01], 0.417 | Removed |
| ABCA1(%)* | 1.19 [1.09–1.29], < 0.001 | 1.07 [0.93–1.23], 0.326 | Removed |
| ABCG1 (%)* | 1.21 [1.06–1.38], 0.004 | 1.04 [0.91–1.20], 0.561 | Removed |
| Cholesterol PD (%)* | 0.72 [0.64–0.80], < 0.001 | 0.80 [0.69–0.94], 0.005 | 0.77 [0.67–0.89], < 0.001 |
| CLC (μg/mg) | 0.83 [0.77–0.89], < 0.001 | 0.92 [0.83–1.01], 0.09 | 0.92 [0.84–1.01], 0.07 |
| on PFS | HR [95%CI], p value |
Full model HR [95%CI], p value |
Reduced model, AIC = 340.8 HR [95%CI], p value |
| Cholesterol (mg/dl) | 0.99 [0.98–0.99], 0.01 | 0.99 [0.98–1.00], 0.139 | Removed |
| ABCA1 (%)* | 1.12 [1.02–1.23], 0.01 | 1.01 [0.88–1.16], 0.915 | Removed |
| ABCG1 (%)* | 1.19 [1.04–1.36], 0.01 | 1.09 [0.94–1.26], 0.269 | 1.10 [0.96–1.26], 0.167 |
| Cholesterol PD (%)* | 0.80 [0.73–0.88], < 0.001 | 0.85 [0.73–0.97], 0.014 | 0.81 [0.74–0.90], < 0.001 |
| CLC (μg/mg) | 0.88 [0.82–0.94], < 0.001 | 0.98 [0.89–1.07], 0.622 | Removed |
| on CB | OR [95%CI], p value |
Full model OR [95%CI], p value |
Reduced model, AIC = 79.2 OR [95%CI], p value |
| Cholesterol (mg/dl) | 1.02 [1.00–1.03], 0.045 | 1.00 [0.99–1.02], 0.67 | |
| ABCA1 (%)* | 0.82 [0.65–1.00], 0.068 | 1.04 [0.78–1.35], 0.80 | |
| ABCG1 (%)* | 0.67 [0.48–0.88], 0.009 | 0.75 [0.51–1.04], 0.11 | 0.78 [0.55–1.04], 0.12 |
| Cholesterol PD (%)* | 1.31 [1.13–1.57], 0.001 | 1.12 [0.90–1.42], 0.31 | |
| CLC (μg/mg) | 1.30 [1.14–1.54], < 0.001 | 1.16 [0.96–1.46], 0.16 | 1.26 [1.10–1.48], 0.003 |
OS Overall survival, ABCA1 ATP-binding cassette transporter A1, ABCG1 ATP-binding cassette transporter G1, PD Passive diffusion, CLC Cholesterol loading capacity, PFS Progression-free survival, CB Clinical benefit
*% = cholesterol efflux amount/overall cellular cholesterol
Fig. 2.
Kaplan-Meyer curve for overall survival
No remarkable change in HR estimation and their significance was observed by adjusting for age, sex, and ECOG PS (supplementary Table 2).
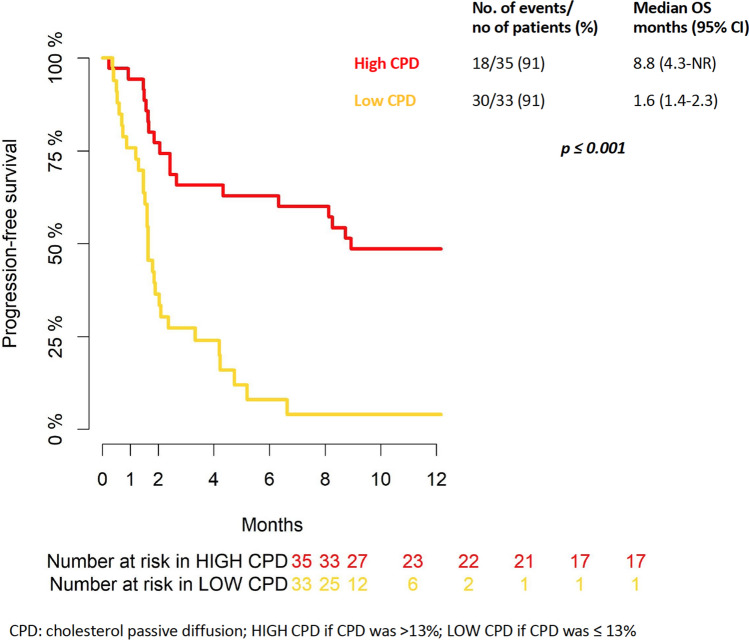
PFS rate at 6 and 12 months was 38% and 29%, respectively (Supplementary Fig. 3). Univariable and multivariable PFS analyses mainly paralleled the results observed for OS with the exception of cholesterol PD level that was confirmed as the only statistically significant protection factor [HR 0.81 (95%CI 0.74–0.90), p < 0.001] (Table 2). Using the cut-off to 13%, as above described, the Fig. 3 shown the PFS curves according to high and low cholesterol PD. PFS rate at 6 and 12 months was 63 and 49% in high cholesterol PD group, respectively. Instead, PFS rate at 6 and 12 months was 8 and 4% in low cholesterol.
Fig. 3.
Kaplan-Meyer curve for progression-free survival
Thirty-two patients displayed CB as best response to the ICI treatment (partial response 21.4%, stable disease 24.3%, no complete responses). The variables directly associated with CB were: plasmatic cholesterol level, serum cholesterol PD efflux and CLC, while ABCA1- (borderline significant, p = 0.068) and ABCG1-mediated efflux showed an inverse correlation with CB. Although in the multivariable model, no cholesterol parameters were associated with CB, the Stepwise Backward selection identified a bivariable model in which the CLC was confirmed as factor positively associated with CB outcome [HR 1.26 (1.10–1.48) CI95%, p < 0.003] (Table 2).
No remarkable changes in HR estimations and their levels of significance were observed by adjusting for age, sex, and ECOG PS (supplementary Table 2).
Discussion
In the present study, we sought to investigate the correlation between CEC, cholesterol PD and CLC processes with the clinical outcome of patients affected by advanced cancer undergoing ICIs. To the best of our knowledge, our report represents the first in-depth investigation focused on the impact of cholesterol-related parameters on cancer patient prognosis.
Our previous data demonstrated a positive prognostic role of high blood cholesterol (i.e., > 200 mg/dl) in the same setting of patients [4]. Thus, to dissect the biological and clinical implications of blood cholesterol in this context, we addressed our attention to cholesterol efflux modalities from cells (active CEC and cholesterol PD) as indirect measure of quality of the total plasma cholesterolemia as well as to the type of lipoprotein involved in these processes [17, 23].
In our research, active efflux processes mediated by ABCA1 and ABCG1 transporters resulted inversely associated with cholesterol efflux PD and CLC. This evidence reflects the different type of lipoproteins which drive cholesterol efflux processes. In fact, while ABCA1 and ABCG1 active efflux is mainly mediated by small and lipid-poor HDL, PD and CLC are mediated by mature, cholesterol-rich HDL lipoproteins, usually containing apoB [17, 23].
Our results documented a positive correlation between both PFS and OS with cholesterol PD. It is a widely accepted notion that small and lipid-poor HDL particles are able to interact with ABCA1 and ABCG1 transporters leading to activation of signaling pathway resulting in anti-inflammatory and anti-antioxidant effect [24]. Hence, given the complementary role of ABCA1-/ABCG1-mediated CEC and cholesterol PD, we can speculate that passive diffusion might reflect the presence of more mature HDL particles and more inflamed context likely explaining the better response to immunotherapy. Our observation is in line with the documented ability of HDL to reduce cellular inflammatory pathways trough the interaction with active membrane transporters ABCA1 and ABCG1 [24, 25]. Moreover, it has been widely reported that HDL interacting with these active transporters is not involved in cholesterol PD efflux [17]. On the other hand, lipoprotein metabolism is a complex and multifaceted process, and apoB-containing lipoproteins which mediate CLC are linked to mature and cholesterol-rich HDL able to promote a slow cholesterol PD efflux [23, 26]. Our data suggest a closely relationship between CLC and CB: given the strong association between CLC and cholesterol PD, this observation is in accordance with the correlation between cholesterol PD and better clinical outcome. Recently, Goossens P and colleagues demonstrated that suppression of active cholesterol efflux ABCA1- and ABCG1-mediated pathways reverts the tumor promoting functions of TAMs and reduces tumor progression [27]. In addition, stratifying the population according to high and low cholesterol PD level, an encouraging difference was found between the two groups in terms of PFS and OS, underling the potential prognostic role of the cholesterol biomarker efflux in patients treated with immunotherapy.
Our data support the hypothesis, already tested in autoimmune disease, that the simple evaluation of the serum lipid profile and in particular total blood cholesterol level is not sufficient to provide information on the functional characteristics of circulating lipoproteins [25].
Some limitations of the present study have to be acknowledged, including the retrospective design, the small sample size, the potential selection bias related to the availability of a stored serum as inclusion criteria and the heterogeneity of our patient population in terms of primary tumors and immunotherapy line of treatment.
In conclusion, the positive association of cholesterol PD with both PFS and OS might be attributed to more mature HDL particles shaping a rather inflamed immune context which likely conditions a better response to ICI. Further analyses are already ongoing, aimed at exploring the interplay between cholesterol quality and the inflammatory cytokine network in the same patient population.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (Supplementary Figure 1. Graphical representation (boxplot) of the values distribution of plasmatic cholesterol, serum ABCA1- and ABCG1-mediated CEC, cholesterol PD and CLC. ABCA-1 = ATP-binding cassette transporter A1; ABCG1 = ATP-binding cassette transporter G1; CEC = cholesterol efflux capacity; CPD = cholesterol passive diffusion; CLC = Cholesterol Loading Capacity. Unit of measurement: cholesterol = mg/dl; ABCA-1, ABCG-1, CEC, CPD and CLC: % value)
Acknowledgements
Dr Alessio Cortellini acknowledges the support provided by the NIHR Imperial BRC.
Author contributions
All the authors contributed to the study conception and design. Cholesterol analysis was performed by EF, AR and AR. Data collection was performed by FP and RS. Statistical analysis was performed by GM. The first draft of the manuscript was written by FP and SB, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available in order to protect patient’s privacy.
Declarations
Conflict of interest
Alessio Cortellini received speaker fees and grant consultancies by AstraZeneca, MSD, BMS, Roche, Novartis and EISAI. Luca Cantini is granted by ESMO with an ESMO Translational Research Fellowship. Any views, opinions, findings, conclusions or recommendations expressed in this material are those solely of the author(s) and do not necessarily reflect those of ESMO. Melissa Bersanelli received research funding (Institutional) from Roche S.p.A., Pfizer, Seqirus UK, Novartis, Bristol Myers Squibb (BMS), AstraZeneca, Sanofi Genzyme; honoraria as a speaker at scientific events and for advisory role (personal fees) from BMS, MSD, Novartis, AstraZeneca, Pierre Fabre, Pfizer, IPSEN; personal fees for copyright transfer from SciClone Pharmaceuticals, IPSEN, Pierre Fabre, MSD, Sanofi Genzyme. Marcello Tiseo: He received honoraria for advisory boards and/or speakers’ fee for AstraZeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Otsuka, Takeda, Pierre Fabre, Amgen and Merck, Sanofi. Research Grants from AstraZeneca and Boehringer Ingelheim. Sebastiano Buti: He received honoraria as a speaker at scientific events and advisory role by BMS, Pfizer, MSD, Ipsen, AstraZeneca and Novartis; he also received research funding from Novartis. The remaining authors have no conflicts of interest to declare.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of individual’s details.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Area Vasta Emilia Nord protocol (Date September 21, 2021/No37649).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Quhal F, Mori K, Bruchbacher A, Resch I, Mostafaei H, Pradere B, Schuettfort VM, Laukhtina E, Egawa S, Fajkovic H, Remzi M, Shariat SF, Schmidinger M. First-line immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Eur Urol Oncol. 2021;4:755–765. doi: 10.1016/j.euo.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat Rev Clin Oncol. 2021;18:625–644. doi: 10.1038/s41571-021-00520-1. [DOI] [PubMed] [Google Scholar]
- 3.Vafaizadeh V, Barekati Z. Immuno-oncology biomarkers for personalized immunotherapy in breast cancer. Front Cell Dev Biol. 2020;8:162. doi: 10.3389/fcell.2020.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrone F, Minari R, Bersanelli M, Bordi P, Tiseo M, Favari E, Sabato R, Buti S. The prognostic role of high blood cholesterol in advanced cancer patients treated with immune checkpoint inhibitors. J Immunother. 2020;43:196–203. doi: 10.1097/CJI.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 5.Tong J, 3rd, Mao Y, Yang Z, Xu Q, Zheng Z, Zhang H, Wang J, Zhang S, Rong W, Zheng L., 3rd Baseline serum cholesterol levels predict the response of patients with advanced non-small cell lung cancer to immune checkpoint inhibitor-based treatment. Cancer Manag Res. 2021;13:4041–4053. doi: 10.2147/CMAR.S304022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Zhao W, Li X, He Y. Cholesterol metabolism as a potential therapeutic target and a prognostic biomarker for cancer immunotherapy. Onco Targets Ther. 2021;14:3803–3812. doi: 10.2147/OTT.S315998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maslyanko M, Harris RD, Mu D. Connecting cholesterol efflux factors to lung cancer biology and therapeutics. Int J Mol Sci. 2021;22:7209. doi: 10.3390/ijms22137209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favari E, Chroni A, Tietge QJF, Escolà-Gil JC, Bernini F. Cholesterol efflux and reverse cholesterol transport. Springer International Publishing; 2015. pp. 181–206. [DOI] [PubMed] [Google Scholar]
- 10.Ye D, Lammers B, Zhao Y, Meurs I, Van Berkel TJ, Van Eck M. ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: important targets for the treatment of atherosclerosis. Curr Drug Targets. 2011;12:647–660. doi: 10.2174/138945011795378522. [DOI] [PubMed] [Google Scholar]
- 11.Thurm C, Schraven B, Kahlfuss S. ABC transporters in T cell-mediated physiological and pathological immune responses. Int J Mol Sci. 2021;22:9186. doi: 10.3390/ijms22179186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sag D, Cekic C, Wu R, Linden J, Hedrick CC. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat Commun. 2015;6:6354. doi: 10.1038/ncomms7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castella B, Kopecka J, Sciancalepore P, Mandili G, Foglietta M, Mitro N, Caruso D, Novelli F, Riganti C, Massaia M. The ATP-binding cassette transporter A1 regulates phosphoantigen release and Vγ9Vδ2 T cell activation by dendritic cells. Nat Commun. 2017;8:15663. doi: 10.1038/ncomms15663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhivaki D, Kagan JC. Innate immune detection of lipid oxidation as a threat assessment strategy. Nat Rev Immunol. 2022;22:322–330. doi: 10.1038/s41577-021-00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favari E, Thomas MJ, Sorci-Thomas MG. High-density lipoprotein functionality as a new pharmacological target on cardiovascular disease: unifying mechanism that explains high-density lipoprotein protection toward the progression of atherosclerosis. J Cardiovasc Pharmacol. 2018;71:325–331. doi: 10.1097/FJC.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 18.Bortnick AE, Rothblat GH, Stoudt G, Hoppe KL, Royer LJ, McNeish J, Francone OL. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J Biol Chem. 2000;275:28634–28640. doi: 10.1074/jbc.M003407200. [DOI] [PubMed] [Google Scholar]
- 19.Favari E, Calabresi L, Adorni MP, Jessup W, Simonelli S, Franceschini G, Bernini F. Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry. 2009;48:11067–11074. doi: 10.1021/bi901564g. [DOI] [PubMed] [Google Scholar]
- 20.Zimetti F, Favari E, Cagliero P, Adorni MP, Ronda N, Bonardi R, Gomaraschi M, Calabresi L, Bernini F, Guardamagna O. Cholesterol trafficking-related serum lipoprotein functions in children with cholesteryl ester storage disease. Atherosclerosis. 2015;242:443–449. doi: 10.1016/j.atherosclerosis.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Zimetti F, De Vuono S, Gomaraschi M, Adorni MP, Favari E, Ronda N, Ricci MA, Veglia F, Calabresi L, Lupattelli G. Plasma cholesterol homeostasis, HDL remodeling and function during the acute phase reaction. J Lipid Res. 2017;58:2051–2060. doi: 10.1194/jlr.P076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanotti I, Favari E, Bernini F. Cellular cholesterol efflux pathways: impact on intracellular lipid trafficking and methodological considerations. Curr Pharm Biotechnol. 2012;13:292–302. doi: 10.2174/138920112799095383. [DOI] [PubMed] [Google Scholar]
- 23.Di Costanzo A, Ronca A, D'Erasmo L, Manfredini M, Baratta F, Pastori D, Di Martino M, Ceci F, Angelico F, Del Ben M, Pavanello C, Turri M, Calabresi L, Favari E, Arca M. HDL-mediated cholesterol efflux and plasma loading capacities are altered in subjects with metabolically- but not genetically driven non-alcoholic fatty liver disease (NAFLD) Biomedicines. 2020;8:625. doi: 10.3390/biomedicines8120625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prosser HC, Ng MKC, Bursill CA. The role of cholesterol efflux in mechanisms of endothelial protection by HDL. Curr Opin Lipidol. 2012;23:182–189. doi: 10.1097/MOL.0b013e328352c4dd. [DOI] [PubMed] [Google Scholar]
- 25.Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, Zimetti F, Adorni MP, Bernini F, Meroni PL. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2014;73:609–615. doi: 10.1136/annrheumdis-2012-202914. [DOI] [PubMed] [Google Scholar]
- 26.Ying Q, Ronca A, Chan DC, Pang J, Favari E, Watts GF. Effect of a PCSK9 inhibitor and a statin on cholesterol efflux capacity: a limitation of current cholesterol-lowering treatments? Eur J Clin Invest. 2022;16:e13766. doi: 10.1111/eci.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goossens P, Rodriguez-Vita J, Etzerodt A, Masse M, Rastoin O, Gouirand V, Ulas T, Papantonopoulou O, Van Eck M, Auphan-Anezin N, Bebien M, Verthuy C, Vu Manh TP, Turner M, Dalod M, Schultze JL, Lawrence T. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. 2019;29:1376–1389. doi: 10.1016/j.cmet.2019.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 (Supplementary Figure 1. Graphical representation (boxplot) of the values distribution of plasmatic cholesterol, serum ABCA1- and ABCG1-mediated CEC, cholesterol PD and CLC. ABCA-1 = ATP-binding cassette transporter A1; ABCG1 = ATP-binding cassette transporter G1; CEC = cholesterol efflux capacity; CPD = cholesterol passive diffusion; CLC = Cholesterol Loading Capacity. Unit of measurement: cholesterol = mg/dl; ABCA-1, ABCG-1, CEC, CPD and CLC: % value)
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available in order to protect patient’s privacy.