Abstract
Purpose
Upper tract urothelial carcinoma (UTUC) is relatively rare in Western countries. The impact of programmed death-ligand 1 (PD-L1) expression on UTUC remains unclear because previous studies have focused on bladder UC. We investigated the association of PD-L1 expression with clinicopathological features and prognosis in patients with UTUC.
Methods
We retrospectively reviewed the patients with UTUC that we treated at our institute from 2013 to 2018. In total, 105 patients with UTUC undergoing radical nephroureterectomy were analyzed to evaluate the PD-L1 expression on representative whole-tissue sections using the Combined Positive Score (CPS; Dako 22C3 pharmDx assay). A PD-L1 CPS ≥ 10 was considered positive.
Results
Among the 105 UTUC cases, 17.1% exhibited positive PD-L1 expression. A CPS ≥ 10 was significantly associated with higher tumor stage (≥ T2, p = 0.034) and lymph node invasion at diagnosis (p = 0.021). A multivariable analysis indicated that a CPS ≥ 10 was an independent prognostic predictor of shorter cancer-specific survival (hazard ratio [HR] = 4.59, 95% confidence interval [CI] = 1.66 − 12.7, p = 0.003) and overall survival (HR = 2.51, 95% CI = 1.19 − 5.27, p = 0.015).
Conclusions
A PD-L1 CPS ≥ 10 in UTUC was associated with adverse pathological features and independently predicted worse cancer-specific and overall survival.
Supplementary Information
The online version of this article 10.1007/s00262-021-02890-y.
Keywords: Combined positive score, Immune checkpoint inhibitor, Programmed death-ligand 1, Survival, Upper tract urothelial carcinoma
Introduction
Upper tract (renal pelvis and ureter) urothelial carcinoma (UTUC) is uncommon in Western countries and constitutes 5% to10% of all UCs [1]. However, the incidence of UTUC is relatively high in Taiwan, and several studies have reported that some risk factors such as ingestion of aristolochic acid-containing Chinese herbs or arsenic-contaminated artesian-well water may be responsible for the high incidence [2, 3]. Kang et al. [4] reported that the recurrence rates after primary UTUC in the bladder and contralateral upper tract were 31.2% and 5.8%, respectively.
Programmed death-ligand 1 (PD-L1), a cell surface glycoprotein, serves as a negative checkpoint in immunity and interacts with its receptor, programmed death-1 (PD-1), to protect normal cells from excessive immune responses and tissue damage [5]. PD-L1 has been reported in UCs and its expression in tumor cells is associated with advanced tumor stage and poor prognosis [6, 7].
The impact of PD-L1 expression on UTUC remains unclear because previous studies have mainly concentrated on bladder UC. Two meta-analysis studies have demonstrated that PD-L1 positivity in UC was correlated with more advanced tumor stage and poorer outcomes, especially in bladder UC [8, 9]. However, some differences have been observed in etiology, gene expression and response to chemotherapy between UTUC and bladder UC [10]. No evidence has indicated that these findings in bladder UC can be applied to UTUC. In the present study, we investigated the association of PD-L1 expression with the clinicopathological features and clinical prognosis of patients with UTUC to further understand the significance of PD-L1 in the era of immunotherapy.
Patients and methods
Study population
From 2013 to 2018, 640 patients were diagnosed with UTUC at our institute. To minimize the specimen heterogeneity, only patients undergoing radical nephroureterectomy (RNU) were retrospectively reviewed. Exclusion criteria included patients with neoadjuvant systemic treatment or distant metastasis at the time of diagnosis, muscle-invasive bladder UC before or after RNU, or lack of PD-L1 staining. Finally, a total of 105 patients met the criteria and were included in this study. The specimens were retrieved from the surgical pathology database of Kaohsiung Chang Gung Memorial Hospital (KCGMH). Lymph node staging was determined by radiologists based on imaging or by the treating physicians who decided the final lymph node status. The pathologic tumor stage was determined according to the 2017 American Joint Committee on Cancer TNM staging for renal pelvis and ureter cancer. Locoregional recurrence was defined by recurrent lesion at previous operation site or retroperitoneal lymph node enlargement observed on computed tomography scans during the follow-up period. Distant metastasis was defined as any suspicious lesion evidenced by radiological images and clinical symptoms based on chart review. Additionally, recurrent bladder cancer was determined according to postoperative cystoscopic follow-up and pathological confirmation. Furthermore, previous bladder cancer history and the cause of death during the follow-up period were determined by the treating physicians, chart review, or death certificates. This study was approved by the institutional review board of KCGMH (No. 2002050051).
Immunochemistry
The surgical tissue samples were fixed in formalin. Serial slides, with a thickness of 4 μm, were sectioned from the blocks of tumor specimens for immunohistochemical analysis. Dako mouse monoclonal anti − PD-L1 antibody (clone: 22C3), purchased from Agilent Technologies Inc. (Santa Clara, CA, USA), was used for the immunohistochemical analysis with EnVision FLEX Visualization System for Dako Autostainer Link 48 according to the manufacturer’s instructions.
Immunohistochemical analysis
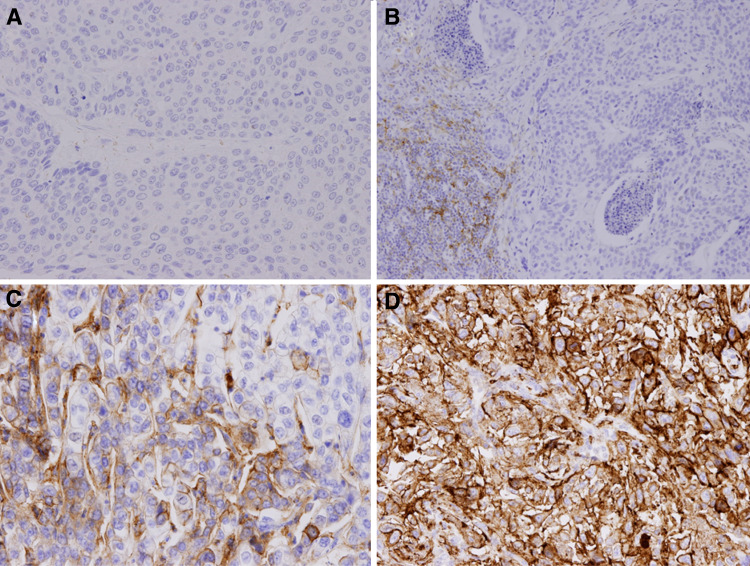
The results of the immunohistochemical analysis were interpreted by a genitourinary pathologist (MTS) based on the manufacturer’s algorithm. Any convincing partial or complete linear membrane staining of viable tumor cells and any convincing membrane and/or cytoplasmic staining of lymphocytes and macrophages within tumor nests and/or immediately adjacent supporting stroma were considered PD-L1 staining and were included in scoring. PD-L1 protein expression in UC was determined using the Combined Positive Score (CPS), which corresponds to the number of PD-L1 staining cells (tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells, multiplied by 100. To assess the best discriminative cutoff value of PD-L1 CPS for UTUC cancer-specific and overall survival, an SAS macro (%FINDCUT) was used to dichotomize patients [11]. Our data revealed that the PD-L1 CPS cutoff of 10 was the best discriminator for UTUC cancer-specific and overall survival (data not shown). Therefore, PD-L1 was considered to have no expression when the specimen had a CPS of less than 10. Specimens with a CPS equal to or over 10 were regarded as containing PD-L1 expression [12]. Figure 1 illustrates the negative expression and positive expression of PD-L1 as evidenced through immunohistochemical staining.
Fig. 1.
Immunohistochemical stain for PD-L1 expression in UTUC samples by clone 22C3. The CPS was used to evaluate PD-L1 expression. a and b represent PD-L1 negative expression, whereas c and d represent PD-L1 positive expression. a CPS = 0; b CPS < 10; c CPS = 60; d CPS = 100
Statistical analysis
The relationships between PD-L1 expression, clinical characteristics and pathological features were examined with Fisher’s exact test. The Kaplan − Meier method was applied to evaluate the survival, and differences were tested using the log-rank test. Univariable and multivariable Cox proportional hazard regression models were used to assess prognostic indicators, including age, PD-L1 expression, TNM classification, and other clinicopathological characteristics. Variables significant on univariable analysis were included to perform multivariable analysis by forward stepwise selection. The level of significance was set at p < 0.05. All statistical computations were performed using SPSS version 20.
Results
Patient characteristics
Patient demographic characteristics are listed in Table 1. This study included 105 patients with a consistent distribution of gender. Among the 105 patients, 63 (60%) were older than 65 years, and the median age was 68 years. Fifteen (14.3%) patients had enlarged retroperitoneal lymph node before RNU and none of them received neoadjuvant chemotherapy or immunotherapy.
Table 1.
Association of PD-L1 expression with clinicopathological characteristics in 105 patients with UTUC
| PD-L1 CPS ≥ 10 N (%) | PD-L1 CPS < 10 N (%) | p value | |
|---|---|---|---|
| Patient number | 18 (17.1) | 87 (82.9) | |
| Follow duration | 14.1 ± 12.3 (mo) | 27.3 ± 16.5 (mo) | 0.002* |
| Age | |||
|
> 65 yr ≤ 65 yr |
9 (50) 9 (50) |
54 (62.1) 33 (37.9) |
0.43 |
| Gender | |||
| Male | 11 (61.1) | 41 (47.1) | 0.311 |
| Female | 7 (38.9) | 46 (52.9) | |
| Pathologic stage | |||
| pTa/is | 0 (0) | 26 (29.9) | 0.02* |
| pT1 | 3 (16.7) | 13 (14.9) | |
| pT2 | 5 (27.8) | 16 (18.4) | |
| pT3 | 8 (44.4) | 26 (29.9) | |
| pT4 | 2 (11.1) | 6 (6.9) | |
| Muscle-invasive tumor | |||
| < T2 | 3 (16.7) | 39 (44.8) | 0.034* |
| ≥ T2 | 15 (83.3) | 48 (55.2) | |
| Lymph node status | |||
| N0/Nx | 12 (66.7) | 78 (89.7) | 0.021* |
| N1/N2 | 6 (33.3) | 9 (10.3) | |
| Papillary feature | |||
| Absent | 8 (44.4) | 23 (26.4) | 0.158 |
| Present | 10 (55.6) | 64 (73.6) | |
| Tumor grade | |||
| Low | 0 (0) | 11 (12.6) | 0.205 |
| High | 18 (100) | 76 (87.4) | |
| Lymphovascular invasion | |||
| Absent | 10 (55.6) | 61 (70.1) | 0.272 |
| Present | 8 (44.4) | 26 (29.9) | |
| Concomitant CIS | |||
| Absent | 7 (38.9) | 35 (40.2) | 1.000 |
| Present | 11 (61.1) | 52 (59.8) | |
| Squamous differentiation | |||
| Absent | 11 (61.1) | 67 (77) | 0.234 |
| Present | 7 (38.9) | 20 (23) | |
| Margin positive | |||
| Negative | 16 (88.9) | 77 (88.5) | 1.000 |
| Positive | 2 (11.1) | 10 (11.5) | |
| Tumor necrosis | |||
| Absent | 7 (38.9) | 54 (62.1) | 0.114 |
| Present | 11 (61.1) | 33 (37.9) | |
| Multifocal tumor | |||
| Solitary | 13 (72.2) | 60 (69) | 1.000 |
| Multifocal | 5 (27.8) | 27 (31) | |
| Bladder recurrence | |||
|
Yes No |
0 (0) 18 (100) |
25 (28.7) 62 (71.3) |
0.006* |
| Locoregional recurrence | |||
|
Yes No |
7 (38.9) 11 (61.1) |
18 (20.7) 69 (79.3) |
0.128 |
| Distant metastasis | |||
|
Yes No |
5 (27.8) 13 (72.2) |
17 (19.5) 70 (80.5) |
0.525 |
| Adjuvant chemotherapy | |||
|
Yes No |
1 (5.6) 17 (94.4) |
7 (8) 80 (92) |
1.000 |
| Palliative chemotherapy | |||
|
Yes No |
2 (11.1) 16 (88.9) |
7 (8) 80 (92) |
0.65 |
| Palliative immunotherapy | |||
|
Yes No |
5 (27.8) 13 (72.2) |
3 (3.4) 84 (96.6) |
0.004* |
| Overall death | 10 (55.6) | 45 (51.7) | |
| Death due to UTUC | 8 (44.4) | 18 (20.7) | |
CIS carcinoma in situ
*indicates p < 0.05
Association of PD-L1 expression with clinicopathological characteristics
Among the 105 patients, 18 (17.1%) had a PD-L1 CPS ≥ 10 (Table 1). The follow-up duration was significantly shorter in patients with a PD-L1 CPS ≥ 10 (14.1 ± 12.3 months vs. 27.3 ± 16.5 months, p = 0.002, Table 1). Patients with PD-L1 CPS ≥ 10 were significantly associated with higher T stage (≥ T2, p = 0.034), lymph node invasion at diagnosis (p = 0.021), and less bladder recurrence (p = 0.006) (Table 1).
Association of PD-L1 expression with cancer-specific survival and overall survival
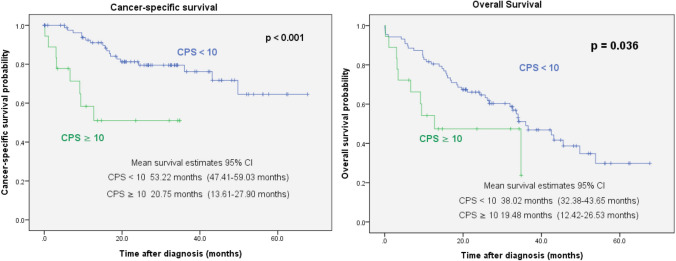
Among the 105 patients, the median follow-up time after initial treatment was 23.5 months (range, 1 − 67.6 months). During this period, 55 patients died, and 26 patients died of UTUC-related causes. A Kaplan–Meier analysis revealed that a PD-L1 CPS ≥ 10 was significantly associated with shorter cancer-specific survival (CSS) (p < 0.001) and overall survival (OS) (p = 0.036) (Fig. 2). Due to the few events, the number of variables that could be included on multivariable analysis was limited to established predictors of oncologic outcomes (such as T and N stages) to find the predictors and improve the statistical accuracy. On the multivariable analysis by forward stepwise selection for OS, PD-L1 CPS ≥ 10 was an independent predictor of shorter OS (hazard ratio [HR] = 2.51, 95% confidence interval [CI] = 1.19 − 5.27, p = 0.015) in addition to age > 65 years and higher T stage (Table 2). To evaluate the factors predicting CSS, the multivariable analysis also indicated that PD-L1 CPS ≥ 10 (HR = 4.59, 95% CI = 1.66 − 12.7, p = 0.003), higher T stage, and lymph node invasion at diagnosis were independent predictors of shorter CSS (Table 2).
Fig. 2.
Association of PD-L1 expression with cancer-specific survival and overall survival in 105 patients with UTUC
Table 2.
Univariable and multivariable analyses by forward stepwise selection of clinicopathological features for the prediction of CSS and OS in patients with UTUC
| Cancer-specific survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable* | Univariable | Multivariable* | |||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age (year) | ||||||||
| > 65 vs. ≤ 65 | 2.45 (1.02 − 5.87) | 0.044 | 3.97 (2.04 − 7.73) | < 0.001 | 4.10 (2.06 − 8.17) | < 0.001 | ||
| PD-L1 expression | ||||||||
| CPS ≥ 10 vs. < 10 | 4.33 (1.82 − 10.33) | 0.001 | 4.59 (1.66 − 12.7) | 0.003 | 2.09 (1.04 − 4.23) | 0.04 | 2.51 (1.19 − 5.27) | 0.015 |
| T stage | < 0.001 | 0.001 | 0.001 | 0.004 | ||||
| Ta/is | Referent | Referent | Referent | Referent | ||||
| T1 | 1.87 (0.26 − 13.39) | 0.531 | 1.08 (0.15 − 7.98) | 0.943 | 2.02 (0.73 − 5.60) | 0.175 | 1.55 (0.56 − 4.33) | 0.399 |
| T2 | 3.52 (0.64 − 19.49) | 0.15 | 2.45 (0.42 − 14.3) | 0.318 | 2.95 (1.15 − 7.53) | 0.024 | 2.37 (0.92 − 6.10) | 0.073 |
| T3 | 7.29 (1.58 − 33.71) | 0.011 | 4.30 (0.85 − 21.7) | 0.077 | 4.03 (1.69 − 9.64) | 0.002 | 2.40 (0.97 − 5.93) | 0.058 |
| T4 | 30.42 (5.91 − 156.5) | < 0.001 | 20.6 (3.49 − 121.4) | 0.001 | 8.64 (2.93 − 25.5) | < 0.001 | 7.98 (2.66 − 23.9) | < 0.001 |
| Lymph node status | ||||||||
| N1/N2 vs. N0/Nx | 6.43 (2.87 − 14.4) | < 0.001 | 3.00 (1.18 − 7.67) | 0.022 | 2.62 (1.34 − 5.14) | 0.005 | ||
| Adjuvant chemotherapy | ||||||||
| Yes vs. No | 3.22 (1.19 − 8.70) | 0.021 | 1.97 (0.83 − 4.67) | 0.126 | ||||
| Palliative chemotherapy | ||||||||
| Yes vs. No | 3.10 (1.24 − 7.72) | 0.015 | 1.29 (0.55 − 3.02) | 0.553 | ||||
| Palliative immunotherapy | ||||||||
| Yes vs. No | 2.27 (0.67 − 7.67) | 0.186 | 1.05 (0.33 − 3.39) | 0.935 | ||||
*Variables significant on univariable analysis were included to perform multivariable analysis by forward stepwise selection
Subset analysis with groups of similar follow-up time
Further subset analysis was done in a subgroup of 74 patients (70.5% of the total cohort, 16 patients in PD-L1 CPS ≥ 10 group and 58 patients in PD-L1 CPS < 10 group) with similar follow-up time. Using NCSS 10 Statistical Software (LLC, Kaysville, Utah, USA), the greedy method was used for matching unequal size in the two groups. The results of subset analysis are shown in supplementary Tables 1 and 2. In subset analysis, patients with PD-L1 CPS ≥ 10 have higher percentages of higher T stage and lymph node invasion at diagnosis (but there was no statistically significant difference, supplementary Table 1). PD-L1 CPS ≥ 10 was not an independent predictor of shorter CSS and OS (supplementary Table 2).
Discussion
Immune checkpoint inhibitors, particularly against the PD-L1/PD-1 pathway, have demonstrated promising anti-tumor ability and improved outcomes in UC [13–16]. These results highlight the association between PD-L1 expression and clinicopathological characteristics as well as oncological outcomes. To date, most published studies have focused on bladder UC [7, 17–21], whereas only few studies addressing UTUC have been published [22–26], as summarized in Table 3. To our knowledge, this is the first study to evaluate the association of PD-L1 expression with clinicopathological features and prognosis using a PD-L1 CPS of 10 or more as a biomarker in patients with UTUC. Our study indicated that a PD-L1 CPS ≥ 10 was associated with higher tumor stage (≥ T2), lymph node invasion and less bladder recurrence (0 vs. 28.7%). The latter case was likely due to greater hazard risk of CSS and OS in patients with PD-L1 CPS ≥ 10. This study demonstrated the impact of PD-L1 expression on adverse pathological features and poor prognosis.
Table 3.
Review of the current literature on PD-L1 expression and associated outcomes in patients with UTUC
| First author and year | Patient number | Clone | Cutoff for PD-L1 ( +) | Tissue analysis | Impact on pathological feature | Impact on prognosis |
|---|---|---|---|---|---|---|
| Skala, 2017 [23] | 149 | 5H1 | ≥ 5% of TCs | Whole-tissue sections | Higher grade, T stage and LVI | Worse CSS at TPS ≥ 50% |
| Krabbe, 2017 [22] | 423 | E1L3N | ≥ 1% of TCs | TMA | Lower T stage | Better RFS and OS in OCD |
| Zhang, 2017 [24] | 162 | E1L3N | ≥ 5% of TCs | Whole-tissue sections | N.S | PD-L1 ( +) on tumor cells = > shorter CSS |
| Miyama, 2018 [25] | 271 | SP263 | ≥ 5% of TCs | TMA | Higher T stage and LVI | Shorter MFS and OS at high platelet count |
| Arriola, 2019 [26] | 72 | E1J2J | ≥ 1% of TCs | Whole-tissue sections | Higher T stage, squamous differentiation | N.S |
| Present study | 105 | 22C3 | CPS ≥ 10 | Whole-tissue sections | Higher T stage, LN invasion at diagnosis | Shorter CSS and OS |
TCs tumor cells; CPS combined positive score; TMA tissue microarray; LVI lymphovascular invasion; N.S not significant; CSS cancer-specific survival; RFS recurrence-free survival; OS overall survival; MFS metastasis-free survival; OCD organ confined disease; LN lymph node
Conflicting evidence exists regarding the role of PD-L1 expression in the pathological features and prognosis of UTUC. Skala et al. [23] examined the whole-tumor sections of 149 patients with UTUC by defining PD-L1 positivity (clone 5H1) with a cutoff value of 5%. In their study, 23.5% of primary UTUC cases tested positive for PD-L1. PD-L1 expression was correlated with higher tumor grade, T stage, and lymphovascular invasion. CSS was significantly associated only with PD-L1 expression based on a cutoff value of 50%. Zhang et al. [24] used different PD-L1 clones with the same positivity cutoff value of 5% to examine the whole-tissue sections of 162 UTUC cases. They discovered no significant association between PD-L1 expression and tumor stage or adverse pathological features. However, PD-L1 positivity in tumor cells was an independent predictor of shorter CSS, and PD-L1 positivity in tumor-infiltrating mononuclear cells was associated with longer CSS. Taken together, the results from these studies indicate that PD-L1 expression has the potential to predict adverse clinicopathological features and poor prognosis, which is in accordance with our findings.
However, Krabbe et al. [22] reported an opposite result for PD-L1 expression using tissue microarrays (TMAs) in 423 UTUC cases. In their cohort, 26.2% of patients exhibited PD-L1 positivity in tumor cells based on a cutoff value of 1%, and PD-L1 positivity predicted favorable prognosis among patients with organ-confined tumor. Various elements can explain these contradictory results, such as differences in the ethnicity of the study cohort, PD-L1 antibody clones used, cutoff value selected to indicate positivity, or staining protocols adopted. In addition, the tumor tissues were analyzed using TMAs in their study, which might have resulted in underestimated heterogeneity and limited accuracy of PD-L1 positivity in tissues [27]. By contrast, in the present study, we used whole-tissue sections to minimize the heterogeneity.
The definition of PD-L1 positivity in the present study is determined using the CPS (in tumor cells and immune cells). However, in aforementioned studies [22–26], they defined PD-L1 positivity as expression in tumor cells only (Table 3). Heterogeneity varies among several PD-L1 staining methods because testing for PD-L1 has not been standardized yet. Over the past years, increasing attention has been paid on the significance of PD-L1 expression in tumor-infiltrating immune cells. Bellmunt et al. [19] reported that PD-L1 positivity in tumor-infiltrating immune cells was associated with longer OS in patients with metastatic bladder UC. By comparing four PD-L1 immunohistochemistry assays (22C3, 28–8, SP142, and SP263), Hirsch et al. [28] discovered that a higher variability of results in tumor-infiltrating immune cell staining than in tumor cell staining because of the lack of training in immune cell scoring. Based on a clinical trial [16], incorporating tumor-associated inflammatory cells into the PD-L1 positivity was more helpful when selecting responders than using tumor cells alone. This finding was also observed in a meta-analysis study [9], which indicated that PD-L1 expression obtained using the CPS offered a clearer dichotomy between responders and non-responders receiving immune checkpoint inhibitors. In addition, the CPS can be determined easily without the need to calculate tumor and immune cells separately. Therefore, the CPS scoring system could be a good way to predict tumor prognosis and identify which patients will benefit the most from PD-1/PD-L1 − targeted therapies.
In our study, PD-L1 CPS positivity was associated with higher tumor stage and worse prognosis. Binding of PD-L1 to PD-1 may deliver an inhibitory signal to suppress the activation of cytotoxic T cells, which helps tumor cells evade the host immune attack [29]. In addition to PD-1, PD-L1 also interacts with B7.1 to block its ability to activate T cells. The PD-L1 expression in tumors has been linked to tumor progression and unfavorable prognosis [29]. This may explain why PD-L1 expression (CPS ≥ 10) was absent in lower T stage (pTa/is) in our study. In bladder UC, previous studies have demonstrated that patients with higher primary T stage had higher percentages of PD-L1 expression [7, 17]. With respect to UTUC, higher percentages of PD-L1 expression were also observed in patients with higher T stage [23, 25]. Therefore, there seems to be an association between higher tumor stage and higher PD-L1 expression in UC. This finding raised the question of whether neoadjuvant or adjuvant systemic therapy can achieve pathologic downstaging or survival benefit in patients with UTUC and a CPS ≥ 10. The impact of neoadjuvant chemotherapy on high-risk or locally advanced UTUC remains controversial. Two meta-analysis studies [30, 31] suggested that neoadjuvant chemotherapy group had higher downstaging rate, better overall survival, recurrence-free survival and cancer-specific survival when compared to controls. Recently, a multi-institutional retrospective study [32] showed the complete response rate of 10.1% and pathologic downstaging rate of 44.9% in 267 UTUC patients receiving neoadjuvant chemotherapy. In addition, pathologic downstaging is a strong predictor of survival outcomes [32]. However, these studies were all retrospective, and there was a possibility of patient selection bias. Further prospective randomized studies are needed to provide better evidence. Interestingly, the PURE-01 study [33] reported a promising complete response rate of 54.3% in cases with muscle-invasive bladder UC and a CPS ≥ 10, receiving neoadjuvant pembrolizumab treatment. Whether the results observed from bladder UC could be applicable to UTUC remains unclear. Further prospective and randomized trials are warranted to investigate the efficacy of neoadjuvant PD-1/PD-L1 inhibitors or chemotherapy in patients with UTUC and a CPS ≥ 10.
In our study, the regimens for adjuvant and first-line palliative chemotherapy were gemcitabine and cisplatin in most patients. For patients with impaired renal function, dose-reduction or split-dose administration of cisplatin was used. One patient received gemcitabine alone for adjuvant chemotherapy due to old age and comorbidities. One patient received gemcitabine alone for first-line palliative chemotherapy due to chronic kidney disease stage 5. For palliative immunotherapy, pembrolizumab was used in most patients; other regimens were nivolumab and atezolizumab. Based on 2020 National Comprehensive Cancer Network (NCCN) guidelines, adjuvant chemotherapy is considered for patients with UTUC ≥ pT2 and/or pN + . In this retrospective study, however, adjuvant chemotherapy is not routinely given for patients with UTUC ≥ pT2 and/or pN + due to impaired renal function or other comorbidities. Higher percentage of patients receiving adjuvant chemotherapy in the PD-L1 CPS < 10 group may have potential impact on survival data. Therefore, we incorporated adjuvant chemotherapy into univariable and multivariable analyses, and the result still indicated that PD-L1 CPS ≥ 10 is a statistically significant predictor of worse survival. Further prospective and large series of study will be needed to draw the solid conclusion.
In our study, the difference in follow-up duration of the two groups is partly due to the nature of retrospective study and partly due to higher percentage of cancer death in patients with CPS ≥ 10. In subset analysis, the results are different from those in total cohort. After subset selection with similar follow-up time, the further smaller sample size naturally limits the power of analysis and might have other selection bias, resulting in the missing significance of predicting CSS and OS.
The strengths of this study include the comprehensive evaluation of PD-L1 expression using whole-tissue sections and a validated anti − PD-L1 antibody in both tumor and immune cells. Whole-tissue sections minimize the heterogeneity further than TMAs do. Our study has some limitations. First, this was a retrospective study with a short median follow-up duration of 23.5 months. Not all specimens were included during the study period. Second, lymph node dissection (LND) is not routinely performed during RNU because the survival benefit related to the procedure remains unclear. In our institute, LND is only performed in patients with suspicion of nodal invasion evident in imaging. Most patients’ unknown nodal status was determined on the basis of imaging, which might have resulted in some nodal metastasis being occulted. Third, this study lacks a central pathological review. However, immunohistochemistry interpretation was performed by an experienced genitourinary pathologist blinded to clinical outcomes. Moreover, additional studies are necessary to evaluate whether PD-L1 positivity can be used in conjunction with mutational load, gene expression subtypes, or other biomarkers as effective predictors or prognosticators in the era of personalized medicine [13, 33]. In addition, due to our sample size and few events, we could not perform analysis incorporating all known independent predictors of survival to see how PD-L1 CPS ≥ 10 would improve prognostic ability. We limited the number of variables incorporated into univariable and multivariable analyses due to the limited number of events. However, we used the prognostic variables with the most impact (such as T and N stages).
Conclusions
Our data indicate that PD-L1 CPS positivity with a cutoff value of 10 occurred in 17.1% of UTUC cases. A PD-L1 CPS ≥ 10 was significantly associated with adverse pathological features, including higher tumor stage and lymph node invasion at diagnosis. More importantly, a PD-L1 CPS ≥ 10 was an independent prognosticator of shorter CSS and OS in patients with UTUC.
Supplementary Information
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Author contributions
CHC and MYT wrote the manuscript. CHC, EMT, PHC designed the experiments. CHC, MYT, and PCC collected and analyzed the data. MTS performed the interpretation of histological staining. HLL and JLS supported the detail of experiments. EMT and PHC supervised the work. All authors read and approved the final manuscript.
Funding
None.
Data availability
All data generated or analyzed during this study are included in this published article.
Compliance with ethical standards
Conflict of interest
The author declares that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chien-Hsu Chen, Mu-Yao Tsai, Ping-Chia Chiang and Ming-Tse Sung have contributed equally to this work and serve as co-first authors.
Contributor Information
Eing-Mei Tsai, Email: tsaieing@kmu.edu.tw.
Po-Hui Chiang, Email: tuoa480713@yahoo.com.tw.
References
- 1.Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, Cowan NC, Bohle A, Van Rhijn BW, Kaasinen E, Palou J, Shariat SF. European association of urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol. 2015;68(5):868–879. doi: 10.1016/j.eururo.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Yang MH, Chen KK, Yen CC, Wang WS, Chang YH, Huang WJ, Fan FS, Chiou TJ, Liu JH, Chen PM. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology. 2002;59(5):681–687. doi: 10.1016/s0090-4295(02)01529-7. [DOI] [PubMed] [Google Scholar]
- 3.Lai MN, Wang SM, Chen PC, Chen YY, Wang JD. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl Cancer Inst. 2010;102(3):179–186. doi: 10.1093/jnci/djp467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang CH, Yu TJ, Hsieh HH, Yang JW, Shu K, Huang CC, Chiang PH, Shiue YL. The development of bladder tumors and contralateral upper urinary tract tumors after primary transitional cell carcinoma of the upper urinary tract. Cancer. 2003;98(8):1620–1626. doi: 10.1002/cncr.11691. [DOI] [PubMed] [Google Scholar]
- 5.Dai S, Jia R, Zhang X, Fang Q, Huang L. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290(1):72–79. doi: 10.1016/j.cellimm.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, Fradet Y, Lacombe L, Kwon ED. PD-L1 (B7–H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109(8):1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7–H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother CII. 2007;56(8):1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen Y, Chen Y, Duan X, Zhu W, Cai C, Deng T, Zeng G. The clinicopathological and prognostic value of PD-L1 in urothelial carcinoma: a meta-analysis. Clin Exp Med. 2019;19(4):407–416. doi: 10.1007/s10238-019-00572-9. [DOI] [PubMed] [Google Scholar]
- 9.Ding X, Chen Q, Yang Z, Li J, Zhan H, Lu N, Chen M, Yang Y, Wang J, Yang D. Clinicopathological and prognostic value of PD-L1 in urothelial carcinoma: a meta-analysis. Cancer Manag Res. 2019;11:4171–4184. doi: 10.2147/CMAR.S176937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel N, Arya M, Muneer A, Powles T, Sullivan M, Hines J, Kelly J. Molecular aspects of upper tract urothelial carcinoma. Urol Oncol. 2014;32(1):28e11–20. doi: 10.1016/j.urolonc.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Meyers J, Mandrekar J (2015) Cutpoint determination methods in survival analysis using SAS®: updated% FINDCUT macro. In: Proc SAS Glob Forum
- 12.Hodgson A, Slodkowska E, Jungbluth A, Liu SK, Vesprini D, Enepekides D, Higgins K, Katabi N, Xu B, Downes MR. PD-L1 Immunohistochemistry assay concordance in urothelial carcinoma of the bladder and hypopharyngeal squamous cell carcinoma. Am J Surg Pathol. 2018;42(8):1059–1066. doi: 10.1097/pas.0000000000001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/s0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, Savage MJ, Perini RF, Keefe SM, Bajorin D, Bellmunt J. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi: 10.1016/s1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 15.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF, Investigators K. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. New Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plimack ER, Bellmunt J, Gupta S, Berger R, Chow LQM, Juco J, Lunceford J, Saraf S, Perini RF, O'Donnell PH. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18(2):212–220. doi: 10.1016/s1470-2045(17)30007-4. [DOI] [PubMed] [Google Scholar]
- 17.Boorjian SA, Sheinin Y, Crispen PL, Farmer SA, Lohse CM, Kuntz SM, Leibovich BC, Kwon ED, Frank I. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14(15):4800–4808. doi: 10.1158/1078-0432.ccr-08-0731. [DOI] [PubMed] [Google Scholar]
- 18.Xylinas E, Robinson BD, Kluth LA, Volkmer BG, Hautmann R, Kufer R, Zerbib M, Kwon E, Thompson RH, Boorjian SA, Shariat SF. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2014;40(1):121–127. doi: 10.1016/j.ejso.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Bellmunt J, Mullane SA, Werner L, Fay AP, Callea M, Leow JJ, Taplin ME, Choueiri TK, Hodi FS, Freeman GJ, Signoretti S. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2015;26(4):812–817. doi: 10.1093/annonc/mdv009. [DOI] [PubMed] [Google Scholar]
- 20.Faraj SF, Munari E, Guner G, Taube J, Anders R, Hicks J, Meeker A, Schoenberg M, Bivalacqua T, Drake C, Netto GJ. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology. 2015;85(3):703.e701–706. doi: 10.1016/j.urology.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pichler R, Heidegger I, Fritz J, Danzl M, Sprung S, Zelger B, Brunner A, Pircher A. PD-L1 expression in bladder cancer and metastasis and its influence on oncologic outcome after cystectomy. Oncotarget. 2017;8(40):66849–66864. doi: 10.18632/oncotarget.19913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krabbe LM, Heitplatz B, Preuss S, Hutchinson RC, Woldu SL, Singla N, Boegemann M, Wood CG, Karam JA, Weizer AZ, Raman JD, Remzi M, Rioux-Leclercq N, Haitel A, Rapoport LM, Glybochko PV, Roscigno M, Bolenz C, Bensalah K, Sagalowsky AI, Shariat SF, Lotan Y, Xylinas E, Margulis V. Prognostic value of PD-1 and PD-L1 expression in patients with high grade upper tract urothelial carcinoma. J Urol. 2017;198(6):1253–1262. doi: 10.1016/j.juro.2017.06.086. [DOI] [PubMed] [Google Scholar]
- 23.Skala SL, Liu TY, Udager AM, Weizer AZ, Montgomery JS, Palapattu GS, Siddiqui J, Cao X, Fields K, Abugharib AE, Soliman M, Hafez KS, Miller D, Lee CT, Alva A, Chinnaiyan AM, Morgan TM, Spratt DE, Jiang H, Mehra R. Programmed death-ligand 1 expression in upper tract urothelial carcinoma. Eur Urol Focus. 2017;3(4–5):502–509. doi: 10.1016/j.euf.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Yu W, Feng X, Zhao Z, Fan Y, Meng Y, Hu S, Cui Y, He Q, Zhang H, Li D, He Z, Zhou L, Jin J, Han W. Prognostic significance of PD-L1 expression on tumor cells and tumor-infiltrating mononuclear cells in upper tract urothelial carcinoma. Med Oncol (Northwood, London, England) 2017;34(5):94. doi: 10.1007/s12032-017-0941-2. [DOI] [PubMed] [Google Scholar]
- 25.Miyama Y, Morikawa T, Miyakawa J, Koyama Y, Kawai T, Kume H, Fukayama M. The prognostic value of PD-L1 expression in upper tract urothelial carcinoma varies according to platelet count. Cancer Med. 2018;7(9):4330–4338. doi: 10.1002/cam4.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arriola AGP, Farahani SJ, Bhargava HK, Guzzo TJ, Brooks JSJ, Lal P. PD-L1 expression reveals significant association with squamous differentiation in upper tract urothelial carcinoma. Am J Clin Pathol. 2019;151(6):561–573. doi: 10.1093/ajcp/aqz002. [DOI] [PubMed] [Google Scholar]
- 27.Li C, Huang C, Mok TS, Zhuang W, Xu H, Miao Q, Fan X, Zhu W, Huang Y, Lin X, Jiang K, Hu D, Chen X, Huang P, Lin G. Comparison of 22C3 PD-L1 expression between surgically resected specimens and paired tissue microarrays in non-small cell lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2017;12(10):1536–1543. doi: 10.1016/j.jtho.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P, Hanks D, Vennapusa B, Mistry A, Kalamegham R, Averbuch S, Novotny J, Rubin E, Emancipator K, McCaffery I, Williams JA, Walker J, Longshore J, Tsao MS, Kerr KM. PD-L1 Immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2017;12(2):208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 29.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18(24):6580–6587. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 30.Kim DK, Lee JY, Kim JW, Hah YS, Cho KS. Effect of neoadjuvant chemotherapy on locally advanced upper tract urothelial carcinoma: a systematic review and meta-analysis. Crit Rev Oncology/hematology. 2019;135:59–65. doi: 10.1016/j.critrevonc.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Li K, Xie W, Gao L, Huang G, Zhou J, Mei B, Chen J. Impact of neoadjuvant chemotherapy on survival prognosis and pathological downstaging in patients presenting with high-risk upper tract urothelial carcinoma. Medicine. 2020;99(18):e20184. doi: 10.1097/md.0000000000020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foerster B, Abufaraj M, Petros F, Azizi M, Gupta M, Schweitzer D, Margulis V, Iwata T, Kimura S, Shabsigh A, Briganti A, Ku JH, Muilwijk T, Kassouf W, Matin SF, Spiess PE, Pierorazio PM, Hendricksen K, Shariat SF, Collaboration U. Efficacy of preoperative chemotherapy for high risk upper tract urothelial carcinoma. J Urol. 2020;203(6):1101–1108. doi: 10.1097/JU.0000000000000737. [DOI] [PubMed] [Google Scholar]
- 33.Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Luciano R, Colecchia M, Giannatempo P, Mortarini R, Bianchi M, Fare E, Monopoli F, Colombo R, Gallina A, Salonia A, Messina A, Ali SM, Madison R, Ross JS, Chung JH, Salvioni R, Mariani L, Montorsi F. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol Off J Am Soc Clin Oncol Jco1801148. 2018;36:3353–3360. doi: 10.1200/jco.18.01148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.