Abstract
Recent studies have shown that tumor-derived exosomes participate in the communication between tumor cells and their microenvironment and mediate malignant biological behaviors including immune escape. In this study, we found that gastric cancer (GC) cell-derived exosomes could be effectively uptaken by Vγ9Vδ2 T cells, decrease the cell viability of Vγ9Vδ2 T cells, induce apoptosis, and reduce the production of cytotoxic cytokines IFN-γ and TNF-α. Furthermore, we demonstrated that exosomal miR-135b-5p was delivered into Vγ9Vδ2 T cells. Exosomal miR-135b-5p impaired the function of Vγ9Vδ2 T cells by targeting specificity protein 1 (SP1). More importantly, blocking the SP1 function by Plicamycin, an SP1 inhibitor, abolished the effect of stable miR-135b-5p knockdown GC cell-derived exosomes on Vγ9Vδ2 T cell function. Collectively, our results suggest that GC cell-derived exosomes impair the function of Vγ9Vδ2 T cells via miR-135b-5p/SP1 pathway, and targeting exosomal miR-135b-5p/SP1 axis may improve the efficiency of GC immunotherapy based on Vγ9Vδ2 T cells.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02991-8.
Keywords: Gastric cancer, Exosome, Vγ9Vδ2 T cell, miR-135b-5p, SP1
Introduction
Gastric cancer (GC) is one of the most prevalent malignancies of the digestive system. In 2018, there were more than 1 million new cases and an estimated 783,000 deaths, making it the fifth most common cancer and the third leading cause of cancer death worldwide [1]. In China, GC accounts for 10.6% of all cancers, and the mortality rate is higher than the incidence rate [2]. Tumor immunotherapy has received significant attention in the treatment of multiple cancers including GC in recent years [3, 4]. Unfortunately, the results of GC immunotherapy were not satisfactory [5–7].
As a subtype of T cell, gamma delta (γδ) T cells express a T cell receptor composed of γ and δ chains and exert an important role in innate and adaptive immune surveillance [8]. Andrew et al. used the CIBERSORT, a computational approach for inferring leukocyte representation in bulk tumor transcriptomes, to demonstrate that intra-tumoral γδ T cells emerged as the significant favorable prognostic signatures across human malignancies, including GC [9]. Vγ9Vδ2 T cells represent a major subset of γδ T cells and have shown potent anti-tumor activity in vivo or in vitro [10]. Combined allogeneic human Vγ9Vδ2 T cell immunotherapies and chemotherapy could efficiently regulate the development of human epithelial ovarian cancer cells in vivo [11]. Adoptive transfer of Vγ9Vδ2 T cells in combination with zoledronic acid reduced the tumor burden of secondary pulmonary metastases and decreased osteolysis in a murine model of osteolytic breast cancer [12]. In addition, intraperitoneal injection of in vitro expanded Vγ9Vδ2 T cells in combination with zoledronate could result in local control of malignant ascites in patients with GC [13]. Thus, a better understanding of Vγ9Vδ2 T cell regulation in the tumor microenvironment will improve the efficiency of Vγ9Vδ2 T cell immunotherapy in cancers.
Exosomes, small vesicles released by a variety of cells, contain cell-specific bioactive substances such as DNA, mRNA, protein, and miRNA, which could mediate communication between cells [14]. Emerging evidence shows that exosomes play an important role in the regulation of diverse biological processes of cancers, including immune evasion [15–17]. For instance, GC-derived exosomes induced the production of PD1-positive tumor-associated macrophages, which produced large amounts of IL-10 and impaired the function of CD8 + T cells [18]. Ren et al. reported that GC-derived exosomes could promote the proliferation of myeloid-derived suppressor cells and induce their immunosuppressive function by increasing the expression of ARG1 [19]. Breast cancer cell-derived exosomal lncRNA SNHG16 promoted the activation of the TGF-β1/SMAD5 pathway and resulted in the conversion of γδ1 T cells into the CD73 + immunosuppressive subtype [20]. Moreover, tumor-derived exosomes could orchestrate an anti- and pro-tumoral γδ T cell equilibrium under different oxygen pressures in the tumor microenvironment [21]. However, the effect of tumor-derived exosomes on Vγ9Vδ2 T cell function is poorly explored in the tumor microenvironment of GC.
MiRNAs are endogenous single-stranded noncoding RNAs that bind to the 3’ untranslated region (3’-UTR) of specific target mRNAs, resulting in mRNA destabilization and/or translational inhibition [22, 23]. Accumulating studies show that miRNAs can be secreted into the extracellular environment through exosomes and modulate various biological processes [24, 25]. In this study, we identified that GC-derived exosomal miR-135b-5p could be transferred to Vγ9Vδ2 T cells and thereby impaired the function of Vγ9Vδ2 T cells by targeting specificity protein 1 (SP1).
Materials and methods
Cell culture
The human GC cell lines SGC7901, MGC803 and MKN45 were cultured in DMEM (Biological Industries, Israel) supplemented with 10% fetal bovine serum (FBS, Gibco, USA, #10,099,141) and 1% penicillin–streptomycin (Beyotime, Shanghai, China, #C0222). The human gastric mucosal epithelial cell line GES-1 was cultured in RPMI 1640 (Biological Industries, Israel) supplemented with 10% FBS and 1% penicillin–streptomycin. All cells were cultured in a humidified incubator at 37 °C in 5% CO2.
Cell transfection and infection
MGC803 cells were transfected with equal amounts (100 pmol) of miRNA mimics, inhibitors, or negative control RNA (GenePharma, Suzhou, China) using Lipofectamine 2000 (Invitrogen, California, USA) according to the manufacturer’s protocols. The lentivirus anti-miR-135b-5p (LV-miR-135b-IN) and lentivirus negative control (LV-NC) were obtained from GenePharma. For lentivirus infection, MGC803 cells and MKN45 cells were infected with LV-miR-135b-IN or LV-NC at a multiplicity of infection (MOI) of 60. The efficiency of infection was confirmed by counting GFP-positive cells under a fluorescence microscope.
Human Vγ9Vδ2 T cell preparation
Human peripheral blood samples were collected from healthy donors at the First Affiliated Hospital of Soochow University. The Institutional Review Board of the First Affiliated Hospital of Soochow University approved the study protocol (reference number: 2021071). Peripheral blood mononuclear cells (PBMCs) were separated from the peripheral blood samples of healthy individuals via differential density gradient centrifugation process using Lymphocyte Separation Medium (TBD, Tianjin, China, #LTS1077) according to the manufacturer’s instructions. To expand Vγ9Vδ2 T cells, PBMCs were cultured in RPMI 1640 supplemented with 10% FBS, 1% penicillin–streptomycin, zoledronic (5 μM, Abcam, #ab141980), recombinant human interleukin-2 (150 U/mL, PeproTech, USA, #200–02), β-mercaptoethanol (50 μM, Sigma-Aldrich, Germany, #M3148), MEM with nonessential amino acids (1:100, Gibco, USA, #11,140,050) and L-glutamine (1:100, Gibco, USA, #25,030,081) at a density of 1.5 × 106 cells per milliliter for 14 days. The culture medium was replaced by fresh culture medium without zoledronic every 2–3 days. The status and phenotype of γδ T cells were evaluated by flow cytometry. The purity of γδT cells was greater than 90%. In some experiments, the Vγ9Vδ2 T cells were purified by the anti-TCR γδ MicroBead Kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer’s instructions.
Human CD4 + T cell and CD8 + T cell isolation
Human CD4 + T cells and CD8 + T cells were isolated from PBMCs using Anti-PE MicroBeads (Miltenyi Biotec) and PE-conjugated anti-human CD4 (Biolegend, #300,508) or PE-conjugated anti-human CD8 (Biolegend, #301,008) according to the manufacturer's protocol.
Conditioned medium (CM)
GES-1 cells, MGC803 cells or MKN45 cells (8 × 105) were cultured in 6-well plates for 4 h. Then, the medium was replaced with FBS-free culture medium. After 24 h, the conditioned medium (CM) was collected.
Exosomes’ isolation
Exosomes were isolated from CM using an exoEasy Maxi Kit (Qiagen, Hilden, Germany, #76,064) according to the manufacturer’s instructions. Exosomes were quantified by measuring protein concentration (Enhanced BCA Protein Assay Kit, Beyotime, #P0010). The morphology and size of exosomes identified by transmission electron microscopy (TEM) or Nanoparticle Tracking Analysis (NTA) were performed in Wuhan Microscopic Biotechnology Co., Ltd (Wuhan, China).
Exosomes uptake
Exosomes were labeled with Dil (Beyotime, #C1991s) according to the manufacturer’s instructions. Vγ9Vδ2 T cells were seeded at 1.5 × 106 cells per well and treated with Dil-labeled exosomes (30 μg) for 6 h at 4 ℃ or 37 ℃. Dil-positive Vγ9Vδ2 T cells were then detected by flow cytometry.
Inhibition of exosomes release
MGC803 cells or MKN45 cells (8 × 105) were cultured in 6-well plates for 4 h. Then, the medium was replaced with FBS-free culture medium with or without GW4869 (10 μM, MedChemExpress, #HY-19363), an inhibitor of exosome generation. After 24 h, the conditioned medium was collected for exosome isolation.
CCK-8 assay
Vγ9Vδ2 T cells were seeded at 1 × 105 cells per well in a 96-well plate and incubated with exosomes or Plicamycin (200 ng/ml, MedChemExpress, Monmouth Junction, # HY-A0122) for 24 h. Subsequently, Cell Counting Kit-8 (CCK-8) reagent (10ul, NCM Biotech, #C6005) was added to each well and incubated for 3 h, and the optical density (OD) at 450 nm was measured. WST-8 in CCK-8 reagent can be reduced to formazan by dehydrogenase in the mitochondria of living cells, and the output is proportional to the number of living cells. The difference in cell viability of Vγ9Vδ2 T cells was analyzed by comparing the results of OD450 in different groups.
Apoptosis assay
To evaluate the apoptosis of Vγ9Vδ2 T cells treated with CM, exosome, GW4869, or Plicamycin, a PE Annexin-V Apoptosis Detection Kit I (BD Biosciences, NJ, USA, #559,763) was used according to the manufacturer’s instructions. To detect the apoptosis of CD4 + T cells or CD8 + T cells after treatment with CM, cells were stained with FTIC-conjugated Annexin-V (BD Pharmingen, #556,420) and 7-AAD. After staining, the cells were analyzed using a FACSAriaII flow cytometer (Beckman Coulter, Brea, CA, USA). Annexin-V + /7-AAD- cells and Annexin-V + /7-AAD + cells were considered to have undergone apoptosis.
Flow cytometry
To analyze the level of IFN-γ or TNF-α in Vγ9Vδ2 T cells treated with CM, exosome, GW4869, or Pilcamycin, the cells were incubated with Cell Activation Cocktail (Biolegend, #423,304) for 10 h. Subsequently, cells were stained with PC7-conjugated anti-CD3 (BioLegend, #344,816), and FITC-conjugated anti-γδ T (BioLegend, #331,208) at 4 ℃ for 20 min. Then, cells were fixed and permeabilized with a Fixation/Permeabilization Kit (BD Biosciences, #554,714) according to the manufacturer’s instructions and followed by staining with PE-conjugated antibody against IFN-γ or TNF-α (BioLegend, #506,507, #502,909). After staining, the cells were analyzed by flow cytometry.
Real-time quantitative PCR (RT-qPCR)
Total RNA from cells was extracted using Trizol (Beyotime, #R0016), and RNA from exosomes was isolated using a miRNeasy Serum/Plasma Kit (Qiagen, #217,184) according to the manufacturer’s instructions. cDNA was synthesized using miRNA first-strand cDNA Synthesis Kit (Vazyme, Nanjing, China, #MR101-02) or MonScript RTIII Super Mix (Monad, China, #MR05201M). PCRs were performed on a CFX96 Touch™ real-time PCR system (Bio-Rad, CA, USA) using miRNA Universal SYBR qPCR Master Mix (Vazyme, #MQ101-02) or MonAmp ChemoHS qPCR Mix (Monad, China, #MQ00401S). The cycling conditions were as follows: one cycle at 95 °C for 5 min, 40 cycles of amplification at 95 °C for 10 s, and 60 °C for 30 s. RT-qPCR for each sample was performed three times. U6 was used as an internal control for cellular miRNAs, while miR-16 was used as an internal control for exosomal miRNAs. β-actin was used to normalize for individual gene expression. All primers used for RT-qPCR are listed in Supplementary Table 1.
Western blotting
Cells and exosomes were lysed in RIPA buffer (Beyotime, #P0013D) containing protease inhibitors (Beyotime, #P1045-1) and phosphatase inhibitors (Beyotime, #P1045-2). The protein concentrations were measured using an Enhanced BCA Protein Assay Kit (Beyotime, #P0010) according to the manufacturer’s instructions. Total protein (20 μg) was separated by 10% SDS-PAGE (Beyotime, #P0012A) and transferred onto 0.45 µm PVDF membranes (GE Healthcare Life science, Germany). The membranes were blocked with 5% BSA (Fcmacs, Nanjing, China, #FMS-WB021) for 1 h and then incubated with anti-SP1 (Beyotime, #AF8040), anti-HSP70 (Beyotime, #AF1156), anti-CD63 (Proteintech, #25,682–1-AP), or anti-β-actin (Immunoway, #YM3028) at 4 °C overnight. The membranes were incubated with the corresponding HRP-conjugated secondary antibodies for 1 h at room temperature. Finally, the membranes were visualized with ECL reagents (NCM Biotech, Suzhou, China, #10,100) using a Chemi DocTM MP Imaging System (Bio-Rad). The samples were normalized to β-actin.
Luciferase reporter assay
We obtained pmirGLO vectors containing the wild-type (WT) or mutant (Mut) SP1 3’UTR from Realgene Biotech (Nanjing, China). For luciferase reporter assay, MGC803 cells were seeded in a 12-well plate. When cells reached 50% confluence, pmirGLO vectors and miR-135b-5p mimic or miR-135b-5p inhibitor were co-transfected into the cells using Lipofectamine 2000. After 24 h, luciferase activity was examined using a DualLuciferase Reporter Assay System (Promega, Madison, USA, #E1910) according to the manufacturer’s instructions.
Statistical analysis
All statistical analysis was performed by GraphPad Prism 7.0. Student’s t test was used to compare the differences between the two groups. A one-way ANOVA test was used for multiple comparisons. All values are presented as the mean ± SD. Data are representative of results from at least 3 independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 was considered as statistically significant.
Results
The conditioned medium of GC cells inhibits the function of Vγ9Vδ2 T cells.
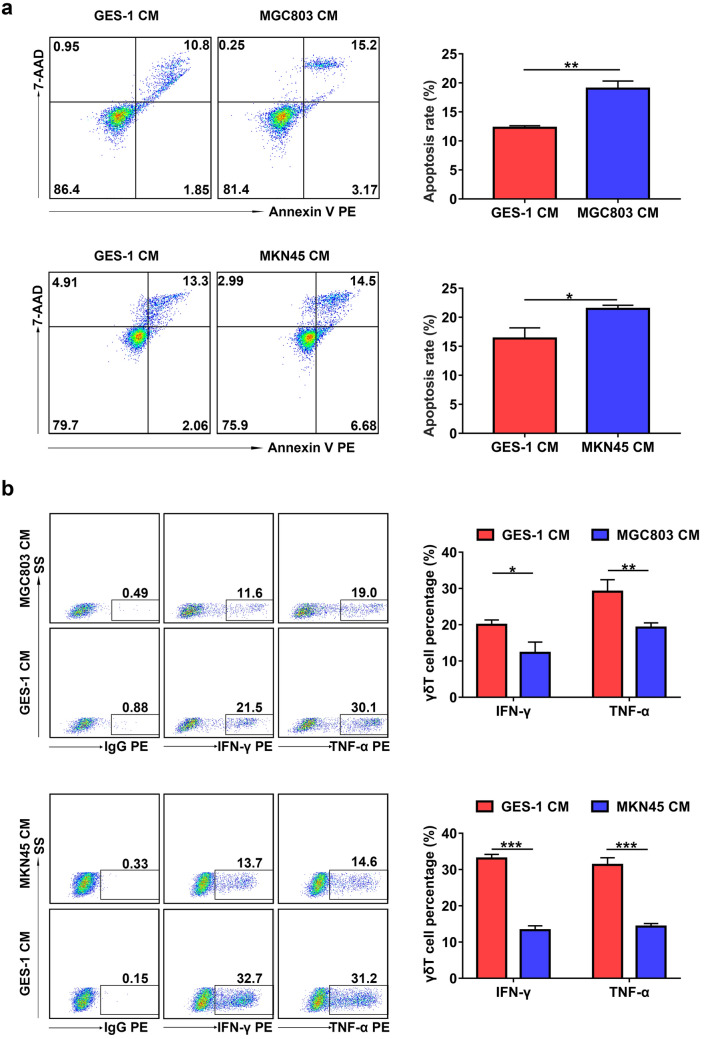
To determine the effect of GC cells on Vγ9Vδ2 T cell function, Vγ9Vδ2 T cells were treated with CM from GES-1, MGC803 or MKN45 cells. The apoptosis rate of Vγ9Vδ2 T cells treated with CM from MGC803 or MKN45 cells was significantly higher than that of cells treated with CM from GES-1 cells (Fig. 1a). In addition, the results of flow cytometry showed that both IFN-γ and TNF-α levels in Vγ9Vδ2 T cells treated with CM from MGC803 or MKN45 cells markedly decreased compared with that of cells treated with CM from GES-1 cells (Fig. 1b). These results suggest that GC cell-derived CM promotes apoptosis of Vγ9Vδ2 T cells and suppresses the expression of IFN-γ and TNF-α.
Fig. 1.
GC cell-derived conditioned medium induced apoptosis and inhibited cytokine production of Vγ9Vδ2 T cells. a The apoptosis rate of Vγ9Vδ2 T cells treated with conditioned medium (CM) from GES-1 cells (GES-1 CM), MGC803 cells (MGC803 CM) or MKN45 cells (MKN45 CM) for 24 h was detected by flow cytometry. b The levels of intracellular IFN-γ and TNF-α in Vγ9Vδ2 T cells treated with GES-1 CM, MGC803 CM or MKN45 CM for 24 h were detected by flow cytometry. Values are expressed as means ± SD. Data are representative of results from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001
GC cell-derived exosomes inhibit the function of Vγ9Vδ2 T cells in vitro.
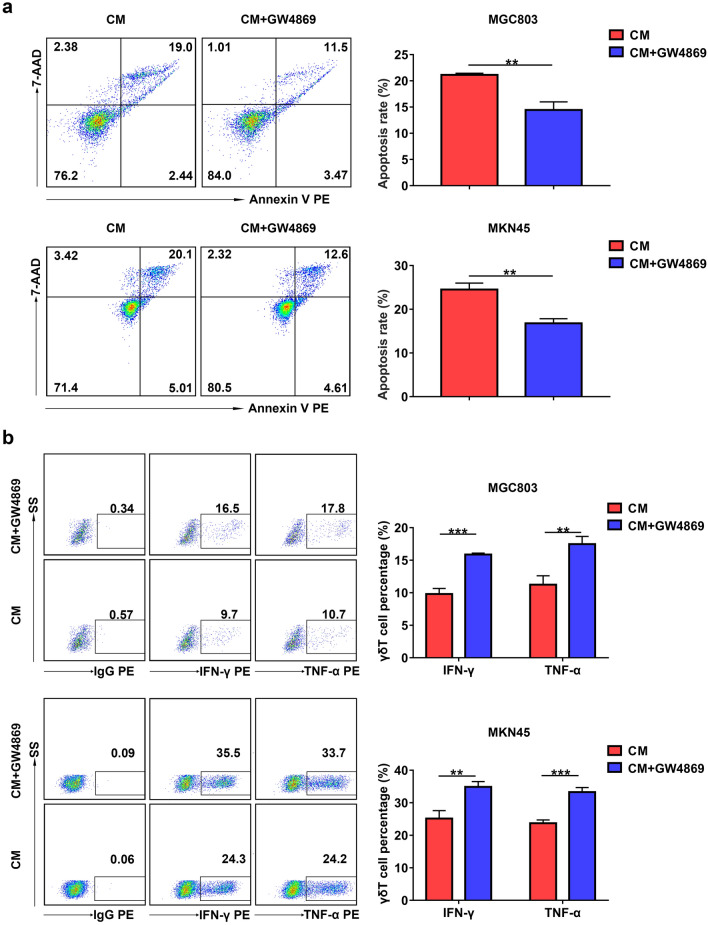
Given that GC cells can release exosomes to modulate immune cells [18, 26], we hypothesized that exosomes derived from GC cells were involved in the regulation of Vγ9Vδ2 T cell function. To verify this hypothesis, we used GW4869, which has been used to block the secretion of exosomes, to inhibit exosome generation in MGC803 and MKN45 cells [27, 28]. As shown in Supplementary Fig. 1a, treatment with GW4869 markedly attenuated the generation of exosomes in MGC803 cells, as evidenced by reduced protein concentrations. CM from MGC803 cells and MKN45 cells pretreated with GW4869 significantly decreased the apoptosis rate of Vγ9Vδ2 T cells (Fig. 2a). Moreover, CM from MGC803 cells and MKN45 cells pretreated with GW4869 obviously increased the proportions of IFN-γ + and TNF-α + Vγ9Vδ2 T cells (Fig. 2b). These results indicate that suppressing the generation of exosomes in GC cells inhibits the apoptosis of Vγ9Vδ2 T cells and increases the expression of IFN-γ and TNF-α.
Fig. 2.
GC cell-derived conditioned medium modulated Vγ9Vδ2 T cell function via exosomes. a The apoptosis rate of Vγ9Vδ2 T cells treated with CM from MGC803 and MKN45 cells pretreated by GW4869 was detected by flow cytometry. b The levels of intracellular IFN-γ and TNF-α in Vγ9Vδ2 T cells treated with CM from MGC803 and MKN45 cells pretreated by GW4869 were detected by flow cytometry. Values are expressed as means ± SD. Data are representative of results from three independent experiments. **p < 0.01, ***p < 0.001
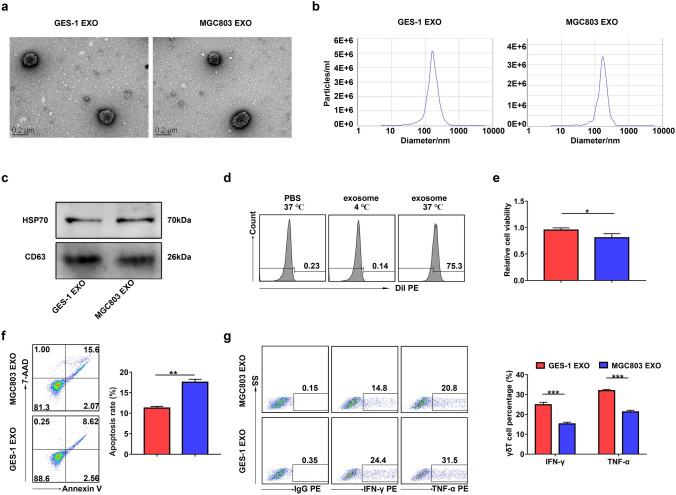
Then, we purified exosomes from the culture supernatant of GES-1, MGC803, or MKN45 cells. Purified exosomes were identified by TEM, NTA, and western blotting (Fig. 3a–c). HSP70 and CD63 are known as exosomal markers. In order to prove that exosomes could be taken up by Vγ9Vδ2 T cells, we incubated Vγ9Vδ2 T cells with Dil-labeled exosomes that were isolated from CM of MGC803 cells. Examination using flow cytometry confirmed the uptake of Dil-labeled exosomes by Vγ9Vδ2 T cells at 37 ℃ (Fig. 3d). We next demonstrated the important effect of GC cell-derived exosomes on Vγ9Vδ2 T cell function. As shown in Fig. 3e and Supplementary Fig. 2a, both MGC803 and MKN45 cell-derived exosomes significantly reduced the cell viability of Vγ9Vδ2 T cells compared with GES-1 cell-derived exosomes. Consistently, the apoptosis rate of Vγ9Vδ2 T cells treated with MGC803 or MKN45 cell-derived exosomes was distinctly higher than that of cells treated with GES-1 cell-derived exosomes (Fig. 3f and Supplementary Fig. 2b). Moreover, exosomes derived from MGC803 or MKN45 cells reduced the IFN-γ and TNF-α production in Vγ9Vδ2 T cells compared with GES-1 cell-derived exosomes (Fig. 3g and Supplementary Fig. 2c). Together, these results suggest that GC cell-derived exosomes can regulate the function of Vγ9Vδ2 T cells.
Fig. 3.
GC cell-derived exosomes regulated the function of Vγ9Vδ2 T cells. a–c GES-1 cell-derived exosomes (GES-1 EXO) and MGC803 cell-derived exosomes (MGC803 EXO) were identified by TEM a, NTA b and western blotting c. The expression of exosomal markers HSP70 and CD63 was analyzed by western blotting. (d) The proportions of Dil-positive Vγ9Vδ2 T cells among Vγ9Vδ2 T cells treated with Dil-labeled exosomes at 4 ℃ or 37 ℃ were detected by flow cytometry. e The cell viability of Vγ9Vδ2 T cells treated with exosomes from GES-1 (GES-1 EXO) or MGC803 (MGC803 EXO) cells was analyzed by CCK-8 assay. f, g The apoptosis rate (f) and the IFN-γ and TNF-α production g of Vγ9Vδ2 T cells treated with exosomes from GES-1 (GES-1 EXO) orMGC803 (MGC803 EXO) cells were detected by flow cytometry. Values are expressed as means ± SD. Data are representative of results from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001
GC cell-derived exosomal miR-135b-5p is involved in the γδ T cell dysfunction
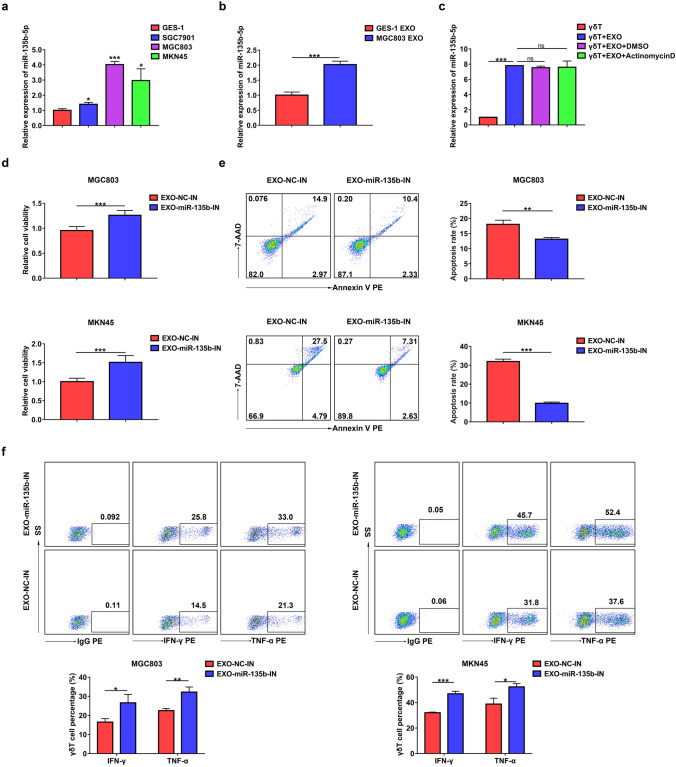
Exosomal miRNAs have been reported to be involved in cell–cell communication and modulate the biological functions of recipient cells [29]. Previous studies showed that upregulated miR-135b-5p in GC tissues was related to a variety of malignant biological behaviors of GC, including angiogenesis, proliferation, migration, and invasion [30–32]. Therefore, we hypothesized that GC cell-derived exosomes modulate Vγ9Vδ2 T cell functions that are miR-135b-5p dependent. Through RT-qPCR verification, we found that the expression level of miR-135b-5p was frequently upregulated in the GC cell lines (SGC7901, MGC803, and MKN45) compared to the GES-1 cells (Fig. 4a). Furthermore, the expression level of miR-135b-5p in MGC803 cell-derived exosomes was distinctly higher than that in GES-1 cell-derived exosomes (Fig. 4b). We next investigated whether exosomes could deliver miR-135b-5p into Vγ9Vδ2 T cells. As shown in Fig. 4c, MGC803 cell-derived exosomes markedly increased the levels of miR-135b-5p in Vγ9Vδ2 T cells. Moreover, the addition of Actinomycin D, a transcription inhibitor, did not modify this effect (Fig. 4c), suggesting that MGC803 cell-derived exosomes mediated miR-135b-5p shuttling. To investigate the effect of GC cell-derived exosomal miR-135b-5p on Vγ9Vδ2 T cell functions, stable miR-135b-5p knockdown MGC803 or MKN45 cell lines were established and displayed decreased miR-135b-5p expression (Supplementary Fig. 3a and 3b). Importantly, miR-135b-5p knockdown obviously reduced the miR-135b-5p levels in MGC803 and MKN45 cell-derived exosomes (Supplementary Fig. 3c). Furthermore, compared with Vγ9Vδ2 T cells after treatment with exosomes derived from control cells, Vγ9Vδ2 T cells treated with exosomes derived from stable miR-135b-5p knockdown MGC803 or MKN45 cells showed increased cell viability, decreased apoptosis rate, and elevated IFN-γ and TNF-α production (Fig. 4d–f). These data suggest that GC cell-derived exosomal miR-135b-5p is involved in the Vγ9Vδ2 T cell dysfunction.
Fig. 4.
GC cell-derived exosomal miR-135b-5p was involved in the Vγ9Vδ2 T cell dysfunction. a The expression of miR-135b-5p in GES-1 cells, SGC7901 cells, MGC803 cells or MKN45 cells was analyzed by RT-qPCR. b The expression of miR-135b-5p in GES-1 cell-derived exosomes (GES-1 EXO) or MGC803 cell-derived exosomes (MGC803 EXO) was analyzed by RT-qPCR. c The expression of miR-135b-5p in Vγ9Vδ2 T cells co-treated with MGC803 exosome and Actinomycin D was analyzed by RT-qPCR. d The cell viability of Vγ9Vδ2 T cells treated with exosomes from MGC803 cells or MKN45 cells infected with LV-miR-135b-IN (EXO-miR-135b-IN) or LV-NC (EXO-NC-IN) was analyzed by CCK-8 assay. e, f The apoptosis rate e and the IFN-γ and TNF-α production f of Vγ9Vδ2 T cells treated with EXO-miR-135b-IN or EXO-NC-IN were detected by flow cytometry. Values are expressed as means ± SD. Data are representative of results from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001
SP1 is a functional target of miR-135b-5p in Vγ9Vδ2 T cells
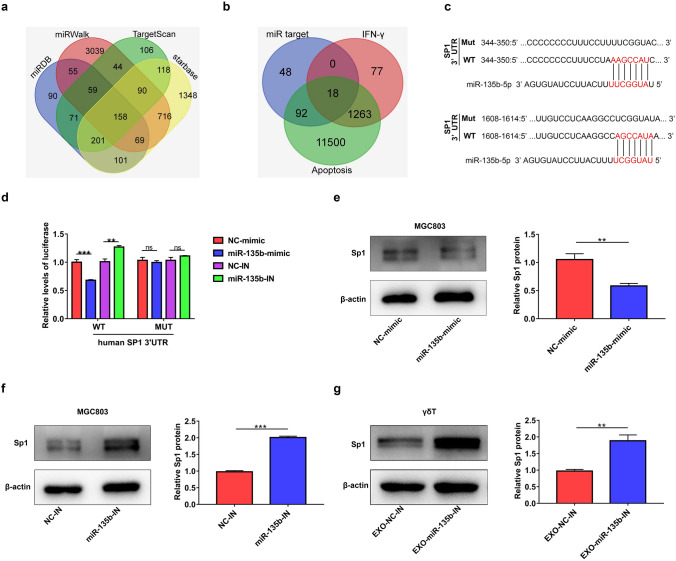
To explore how miR-135b-5p could modulate the function of Vγ9Vδ2 T cells, we utilized four mRNA target-predicting algorithms (miRDB, miRWalk, Targetscan, and starbase) to identify the potential downstream targets of miR-135b-5p (Fig. 5a). One hundred and fifty-eight co-targets of miR-135b-5p were used to compare with apoptosis- or IFN-γ- associated genes downloaded from GeneCards (http://www.genecards.org). As shown in Fig. 5b, there were 18 genes in common (Fig. 5b and Supplementary Table 2). Among these 18 genes, SP1 has been reported to promote the production of IFN-γ and inhibit the apoptosis of cells [33–36]. As shown in Supplementary Fig. 4a, treatment with Plicamycin, an inhibitor of SP1, obviously decreased the mRNA expression of T-bet, which is involved in regulating the expression of IFN-γ in Vγ9Vδ2 T cells [37]. Moreover, the inhibition of SP1 markedly increased the apoptosis rate of Vγ9Vδ2 T cells (Supplementary Fig. 4b). Therefore, we hypothesized that GC cell-derived exosomal miR-135b-5p may regulate Vγ9Vδ2 T cell function through targeting SP1. The hybridization models between the 3’-UTR of human SP1 and miR-135b-5p are shown in Fig. 5c. Luciferase reporter assay confirmed that miR-135b-5p overexpression reduced, while miR-135b-5p knockdown elevated, the activity of luciferase reporter containing wild-type (WT) SP1 3’-UTR (Fig. 5d). Mutagenesis of the miR-135b-5p binding sites abolished all miR-135b-5p mediated regulatory effects (Fig. 5d). In addition, the results of western blotting showed that the upregulation of miR-135b-5p obviously reduced the protein expression of SP1 in MGC803 cells, whereas knockdown of miR-135b-5p enhanced the levels of SP1 protein in this cell type (Fig. 5e and f). Moreover, exosomes derived from stable miR-135b-5p knockdown MGC803 cells markedly increased the protein levels of SP1 in Vγ9Vδ2 T cells (Fig. 5g).
Fig. 5.
SP1 is a functional target of miR-135b-5p. a The targets of miR-135b-5p were predicted by TargetScan, miRDB, miRWalk and starbase. b The Venn diagram of targets of miR-135b-5p and apoptosis or IFN-γ-associated with genes downloaded from GeneCards. c The binding position between miR-135b-5p and SP1 was predicted by TargetScan. d The luciferase activity of MGC803 cells co-transfected with the human SP1 wild-type (WT) 3ˈUTR or the SP1 mutant (MUT) 3ˈUTR and miR-135b-5p mimic (miR-135b-mimic), miR-135b-5p inhibitor (miR-135b-IN), control RNA mimic (NC-mimic) or control RNA inhibitor (NC-IN) was detected. e, f The protein expression of SP1 in MGC803 cells treated with NC-mimic, miR-135b-mimic, NC-IN, or miR-135b-IN was analyzed by western blotting. g The protein expression of SP1 in Vγ9Vδ2 T cells treated with exosomes from MGC803 cells infected with LV-miR-135b-IN (EXO-miR-135b-IN) or MGC803 cells infected with LV-NC (EXO-NC-IN) was analyzed by western blotting. Values are expressed as means ± SD. Data are representative of results from three independent experiments. **p < 0.01, ***p < 0.001
Blocking SP1 function abolishes the effect of exosomes derived from stable miR-135b-5p knockdown GC cells on Vγ9Vδ2 T cell function
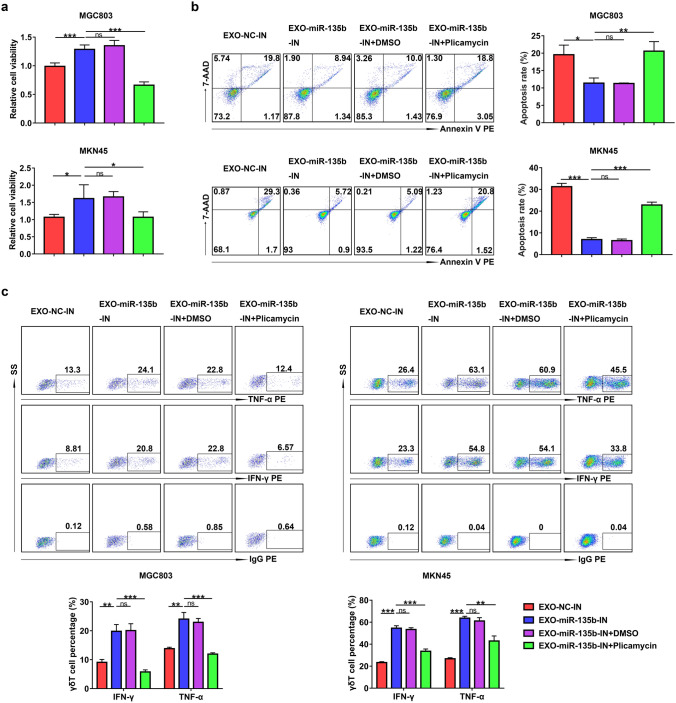
We next wondered whether the inhibition of Vγ9Vδ2 T cell function by miR-135b-5p was related to SP1. We found that the treatment with Plicamycin could abolish the effect of exosomes derived from stable miR-135b-5p knockdown MGC803 or MKN45 cells on Vγ9Vδ2 T cell viability, apoptosis, and IFN-γ and TNF-α production (Fig. 6a-c). These results indicate that GC cell-derived exosomal miR-135b-5p inhibits the function of Vγ9Vδ2 T cells via SP1.
Fig. 6.
Exosomal miR-135b-5p modulated Vγ9Vδ2 T cell function via SP1. a The cell viability of Vγ9Vδ2 T cells co-treated with EXO-miR-135b-IN and Plicamycin was analyzed by CCK-8 assay. b, c The apoptosis rate b and the IFN-γ and TNF-α production (c) of Vγ9Vδ2 T cells co-treated with EXO-miR-135b-IN and Plicamycin were detected by flow cytometry. Values are expressed as means ± SD. Data are representative of results from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
In recent years, tumor immunotherapy based on γδ T cells shows an attractive prospect due to multiple favorable anti-tumor characteristics of this cell type, such as recognizing malignant cells, infiltrating tumors, and depicting strong cytotoxic and pro-inflammatory activity [38–40]. However, the γδ T cell immunotherapy had limited effects in the treatment of solid tumors [41–43]. To improve the effectiveness of γδ T cells in cancer immunotherapy, many strategies including breaking the suppressive tumor microenvironment have been proposed and explored [40, 43]. In our present study, we found that the CM of GC cells could induce the apoptosis of Vγ9Vδ2 T cells and reduce the production of IFN-γ and TNF-α. These results suggest that there may be one or more factors in the microenvironment of GC that can inhibit the immune function of Vγ9Vδ2 T cells. Therefore, it is vital to figure out what causes the γδ T cell dysfunction in the tumor microenvironment. Herein, we reported that GC cells modulated the function of Vγ9Vδ2 T cells via exosomes, as evidenced by the decreasing apoptosis of Vγ9Vδ2 T cells and the increasing IFN-γ and TNF-α expression after incubation with CM from GC cells pretreated with GW4869. However, we could not exclude the possibility that other factors including immune checkpoint molecules may be involved in the regulation of Vγ9Vδ2 T cells in the tumor microenvironment. In addition, a published study noted that tumor-derived immunosuppressive exosomes induced a suppressor phenotype in CD8 + T cells [44].
Melanoma-derived exosomes downregulated CD8 + T cell responses through reduced T cell receptor (TCR) signaling and diminished cytokine and granzyme B production [45]. Liu et al. showed that exosome-mediated miRNA-451 derived from GC cells increased the Th17-polarized differentiation of these infiltrated T cells [46]. In our present study, we found that CM from GC cells could induce the apoptosis of CD4 + T cells and CD8 + T cells (Supplementary Fig. 5a and 5b). Both our and others’ studies suggested that tumor-derived exosomes played important roles in the regulation of γδ T cells and αβ T cells in the tumor microenvironment. However, the regulatory effects of GC-derived exosomes on CD4 + T cells and CD8 + T cells need further to be explored.
As mediators of cell–cell communication within the tumor microenvironment, exosomes have been proved to be critically involved in tumor immune response [47, 48]. For example, hypoxia-induced tumor exosomes influenced macrophage recruitment and promoted M2-like polarization and miRNA-mediated metabolic shift [49]. In addition, nasopharyngeal carcinoma-derived exosomes facilitated Treg T cell recruitment and induced overexpression of cell markers associated with Treg phenotype [50]. Furthermore, tumor-derived exosomes orchestrated an anti- and pro-tumoral γδ T cell equilibrium under different oxygen pressures in the tumor microenvironment [21]. Herein, we found that GC cell-derived exosomes inhibited the function of Vγ9Vδ2 T cells via the miR-135b-5p/SP1 axis.
MiR-135b-5p has been reported to be highly expressed in GC tissues and is tightly associated with the poor prognosis of GC patients [51]. MiR-135b-5p induced by Interleukin 1 promoted inflammation-associated gastric carcinogenesis in mice [52]. Chen et al. reported that miR-135b-5p overexpression enhanced the viability, proliferation, invasion, and migration of GC cells by targeting Krüppel-like factor 4 (KLF4) [30]. Also, miR-135b-5p induced by helicobacter pylori suppressed apoptosis and increased cisplatin resistance in GC [53]. Moreover, exosomal miR-135b-5p derived from gastric tumors inhibited the FOXO1 protein expression and enhanced the growth of blood vessels [32]. In summary, these studies suggest that miR-135b-5p plays a crucial role in the occurrence and progression of GC. However, the role of miR-135b-5p in the regulation of GC immune response has not been elucidated. In this study, we unveiled that GC-secreted miR-135b-5p could be delivered to Vγ9Vδ2 T cells via exosomes, then decreased Vγ9Vδ2 T cell viability, increased apoptosis rate, and reduced IFN-γ and TNF-α production. To date, it has been reported that exosomal miRNAs, such as miR-1290, miR-21-5p, miR-423-5p, miR-501, miR-130a, and miR-155, play important roles in the control of proliferation, metastasis, drug resistance, and angiogenesis in GC [54–59]. Although our results indicated that GC cell-derived exosomal miR-135b-5p is an important regulator of Vγ9Vδ2 T cells, other exosomal miRNAs participated in the regulation of Vγ9Vδ2 T cells need further to be identified.
Additionally, we explored the possible mechanism by which exosomal miR-135b-5p may modulate the apoptosis of Vγ9Vδ2 T cells and IFN-γ and TNF-α production. With bioinformatic approaches, we found that among these targets of miR-135b-5p, SP1, a member of the SP transcription factor family [60], is associated with both cell apoptosis and IFN-γ production [33–36]. MAML1 knockdown inhibited the proliferation of T cell acute lymphoblastic leukemia cells and induced apoptosis through SP1-dependent inactivation of TRIM59 [33]. Bortezomib could downregulate the expression of Notch1 via degradation of SP1 to elicit the cytotoxicity in T-ALL cells in vitro and in vivo [35]. In addition, SP1 has been reported to be involved in the transcriptional control of T-bet expression, resulting in increased production of IFN-γ in NK cells and T cells [36]. Wang R et al. found that TGF-β1 could promote chondrocyte proliferation by downregulating SP1 through MSC-exosome-derived miR-135b [61]. Herein, in vitro luciferase assays indicated that SP1 was a direct target of miR-135b-5p. Moreover, miR-135b-5p overexpression reduced, while miR-135b-5p knockdown enhanced, the protein expression of SP1 in MGC803 cells. Also, exosomes derived from stable miR-135b-5p knockdown MGC803 cells markedly increased the protein levels of SP1 in Vγ9Vδ2 T cells. Most importantly, we observed that Plicamycin treatment abolished the effect of exosomes derived from stable miR-135b-5p knockdown MGC803 or MKN45 cells on Vγ9Vδ2 T cell viability, apoptosis, and IFN-γ and TNF-α production. All the above results indicate that the effect of exosomal miR-135b-5p on γδ T cell dysfunction is SP1 dependent. Previous studies have shown the potent anti-tumor activity of γδ T cells in multiple cancers, such as epithelial ovarian cancer and breast cancer [11, 12]. Given that both exosomes and miR-135b-5p played important roles in modulating tumor progression in various cancers, we inferred that the exosomal miR-135b-5p/SP1 pathway might be involved in the regulation of γδ T cell function in other types of cancer. Therefore, it may be valuable to explore the role of exosomal miR-135b-5p/SP1 pathway in regulating γδ T cell function in cancers in our future study.
In summary, our data in this study clarified that GC cells could impair the function of Vγ9Vδ2 T cells via the exosomal miR-135b-5p/SP1 pathway (Fig. 7). Targeting the exosomal miR-135b-5p/SP1 axis represents a promising approach that may be valuable for GC immunotherapy based on Vγ9Vδ2 T cells.
Fig. 7.
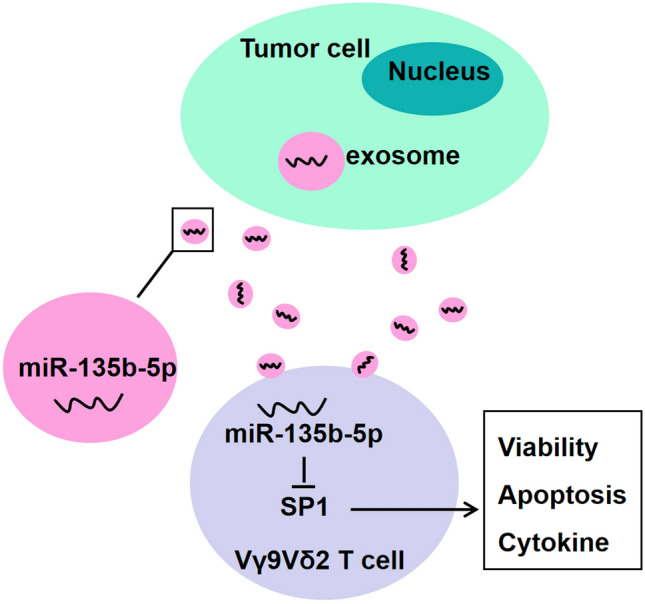
The outlined mechanism underlying GC cell-derived exosomal miR-135b-5p modulating the function of Vγ9Vδ2 T cells
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81802843, 82073156, 81672372); Suzhou Science & Technology plan project (SYS2019035, SS2019077).
Authors’ contributions
JL, TS, and WC designed the experiments; JL, LS, YC, and JZ performed most of the experiments; JS, JW, and GZ assisted with experiments and analysis of the data; MW and YG contributed to provide clinical samples; JL, TS, and WC wrote the manuscript.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tongguo Shi, Email: shitg@suda.edu.cn.
Weichang Chen, Email: weichangchen@126.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 68 (6):394–424. doi:10.3322/caac.21492 [DOI] [PubMed]
- 2.Feng R, Zong Y, Cao S, Xu R. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer communications (London, England) 2019;39(1):22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coutzac C, Pernot S, Chaput N, Zaanan A. Immunotherapy in advanced gastric cancer, is it the future? Crit Rev Oncol Hematol. 2019;133:25–32. doi: 10.1016/j.critrevonc.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Olino K, Park T, Ahuja N. Exposing hidden targets: combining epigenetic and immunotherapy to overcome cancer resistance. Semin Cancer Biol. 2020;65:114–122. doi: 10.1016/j.semcancer.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Matsueda S, Graham D. Immunotherapy in gastric cancer. World J Gastroenterol. 2014;20(7):1657–1666. doi: 10.3748/wjg.v20.i7.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim T, da Silva E, Coit D, Tang L. Intratumoral immune response to gastric cancer varies by molecular and histologic subtype. Am J Surg Pathol. 2019;43(6):851–860. doi: 10.1097/pas.0000000000001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones J, Smyth E. Gastroesophageal cancer: Navigating the immune and genetic terrain to improve clinical outcomes. Cancer Treat Rev. 2020;84:101950. doi: 10.1016/j.ctrv.2019.101950. [DOI] [PubMed] [Google Scholar]
- 8.Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol. 2006;126(1):25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 9.Gentles A, Newman A, Liu C, Bratman S, Feng W, Kim D, Nair V, Xu Y, Khuong A, Hoang C, Diehn M, West R, Plevritis S, Alizadeh A. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher J, Anderson J. Engineering approaches in human gamma delta T cells for cancer immunotherapy. Front Immunol. 2018;9:1409. doi: 10.3389/fimmu.2018.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joalland N, Lafrance L, Oullier T, Marionneau-Lambot S, Loussouarn D, Jarry U, Scotet E. in vivoCombined chemotherapy and allogeneic human Vγ9Vδ2 T lymphocyte-immunotherapies efficiently control the development of human epithelial ovarian cancer cells. Oncoimmunology. 2019;8(11):e1649971. doi: 10.1080/2162402x.2019.1649971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zysk A, DeNichilo M, Panagopoulos V, Zinonos I, Liapis V, Hay S, Ingman W, Ponomarev V, Atkins G, Findlay D, Zannettino A, Evdokiou A. Adoptive transfer of ex vivo expanded Vγ9Vδ2 T cells in combination with zoledronic acid inhibits cancer growth and limits osteolysis in a murine model of osteolytic breast cancer. Cancer Lett. 2017;386:141–150. doi: 10.1016/j.canlet.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada I, Matsushita H, Noji S, Mori K, Yamashita H, Nomura S, Shimizu N, Seto Y, Kakimi K. Intraperitoneal injection of in vitro expanded Vγ9Vδ2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med. 2014;3(2):362–375. doi: 10.1002/cam4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 15.Qu J, Qu X, Zhao M, Teng Y, Zhang Y, Hou K, Jiang Y, Yang X, Liu Y. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2009;41(12):875–880. doi: 10.1016/j.dld.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Liu D, Li C, Trojanowicz B, Li X, Shi D, Zhan C, Wang Z, Chen L. CD97 promotion of gastric carcinoma lymphatic metastasis is exosome dependent. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2016;19(3):754–766. doi: 10.1007/s10120-015-0523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y, Wang M, Zhu W, Qian H, Xu W. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell cycle (Georgetown, Tex) 2015;14(15):2473–2483. doi: 10.1080/15384101.2015.1005530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Li B, Wei Y, Zhao Y, Wang L, Zhang P, Yang J, He W, Chen H, Jiao Z, Li Y. Tumor-derived exosomes induce PD1 macrophage population in human gastric cancer that promotes disease progression. Oncogenesis. 2018;7(5):41. doi: 10.1038/s41389-018-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren W, Zhang X, Li W, Feng Q, Feng H, Tong Y, Rong H, Wang W, Zhang D, Zhang Z, Tu S, Zhu X, Zhang Q. Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer. Cancer management and research. 2019;11:4023–4040. doi: 10.2147/cmar.S198886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni C, Fang Q, Chen W, Jiang J, Jiang Z, Ye J, Zhang T, Yang L, Meng F, Xia W, Zhong M, Huang J. Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73+γδ1 Treg cells. Signal Transduct Target Ther. 2020;5(1):41. doi: 10.1038/s41392-020-0129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Cao B, Liang X, Lu S, Luo H, Wang Z, Wang S, Jiang J, Lang J, Zhu G. Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes. Oncogene. 2019;38(15):2830–2843. doi: 10.1038/s41388-018-0627-z. [DOI] [PubMed] [Google Scholar]
- 22.Feng C, She J, Chen X, Zhang Q, Zhang X, Wang Y, Ye J, Shi J, Tao J, Feng M, Guan W, Xia H, Zhang W, Xu G. Exosomal miR-196a-1 promotes gastric cancer cell invasion and metastasis by targeting SFRP1. Nanomedicine (Lond) 2019;14(19):2579–2593. doi: 10.2217/nnm-2019-0053. [DOI] [PubMed] [Google Scholar]
- 23.O'Connell R, Rao D, Chaudhuri A, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10(2):111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 24.Xie Y, Dang W, Zhang S, Yue W, Yang L, Zhai X, Yan Q, Lu J. The role of exosomal noncoding RNAs in cancer. Mol Cancer. 2019;18(1):37. doi: 10.1186/s12943-019-0984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi M, Xu L, Jiao Y, Luo S, Li A, Wu K. The role of cancer-derived microRNAs in cancer immune escape. J Hematol Oncol. 2020;13(1):25. doi: 10.1186/s13045-020-00848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Y, Zhang J, Mao Z, Jiang H, Liu W, Shi H, Ji R, Xu W, Qian H, Zhang X. Extracellular vesicles from gastric cancer cells induce pd-l1 expression on neutrophils to suppress T-cell Immunity. Front Oncol. 2020;10:629. doi: 10.3389/fonc.2020.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C, Luo F, Liu X, Lu L, Xu H, Yang Q, Xue J, Shi L, Li J, Zhang A, Liu Q. NF-kB-regulated exosomal miR-155 promotes the inflammation associated with arsenite carcinogenesis. Cancer Lett. 2017;388:21–33. doi: 10.1016/j.canlet.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Yuan W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17(1):147. doi: 10.1186/s12943-018-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, Gao Y, Gao S, Song D, Feng Y. MiR-135b-5p promotes viability, proliferation, migration and invasion of gastric cancer cells by targeting Krüppel-like factor 4 (KLF4) Archives of medical science : AMS. 2020;16(1):167–176. doi: 10.5114/aoms.2019.87761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Mao H, Zhang S, Sun L, Zhang W, Chen Q, Wang L, Liu H. lncRNA PCAT18 inhibits proliferation, migration and invasion of gastric cancer cells through miR-135b suppression to promote CLDN11 expression. Life Sci. 2020;249:117478. doi: 10.1016/j.lfs.2020.117478. [DOI] [PubMed] [Google Scholar]
- 32.Bai M, Li J, Yang H, Zhang H, Zhou Z, Deng T, Zhu K, Ning T, Fan Q, Ying G, Ba Y. miR-135b delivered by gastric tumor exosomes inhibits FOXO1 expression in endothelial cells and promotes angiogenesis. Molecular therapy : the journal of the American Society of Gene Therapy. 2019;27(10):1772–1783. doi: 10.1016/j.ymthe.2019.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Cheng H, Chen L, Hu X, Qiu H, Xu X, Gao L, Tang G, Zhang W, Wang J, Yang J, Huang C. Knockdown of MAML1 inhibits proliferation and induces apoptosis of T-cell acute lymphoblastic leukemia cells through SP1-dependent inactivation of TRIM59. J Cell Physiol. 2019;234(4):5186–5195. doi: 10.1002/jcp.27323. [DOI] [PubMed] [Google Scholar]
- 34.Yao Y, Hu J, Shen Z, Yao R, Liu S, Li Y, Cong H, Wang X, Qiu W, Yue L. MiR-200b expression in breast cancer: a prognostic marker and act on cell proliferation and apoptosis by targeting Sp1. J Cell Mol Med. 2015;19(4):760–769. doi: 10.1111/jcmm.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koyama D, Kikuchi J, Hiraoka N, Wada T, Kurosawa H, Chiba S, Furukawa Y. Proteasome inhibitors exert cytotoxicity and increase chemosensitivity via transcriptional repression of Notch1 in T-cell acute lymphoblastic leukemia. Leukemia. 2014;28(6):1216–1226. doi: 10.1038/leu.2013.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu J, Wei M, Boyd Z, Lehmann E, Trotta R, Mao H, Liu S, Becknell B, Jaung M, Jarjoura D, Marcucci G, Wu L, Caligiuri M. Transcriptional control of human T-BET expression: the role of Sp1. Eur J Immunol. 2007;37(9):2549–2561. doi: 10.1002/eji.200737088. [DOI] [PubMed] [Google Scholar]
- 37.Lu H, Shi T, Wang M, Li X, Gu Y, Zhang X, Zhang G, Chen W. B7–H3 inhibits the IFN-γ-dependent cytotoxicity of Vγ9Vδ2 T cells against colon cancer cells. Oncoimmunology. 2020;9(1):1748991. doi: 10.1080/2162402x.2020.1748991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher J, Heuijerjans J, Yan M, Gustafsson K, Anderson J. γδ T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology. 2014;3(1):e27572. doi: 10.4161/onci.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lafont V, Sanchez F, Laprevotte E, Michaud H, Gros L, Eliaou J, Bonnefoy N. Plasticity of γδ T Cells: Impact on the Anti-Tumor Response. Front Immunol. 2014;5:622. doi: 10.3389/fimmu.2014.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoeres T, Smetak M, Pretscher D, Wilhelm M. Improving the efficiency of Vγ9Vδ2 T-cell immunotherapy in cancer. Front Immunol. 2018;9:800. doi: 10.3389/fimmu.2018.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicol A, Tokuyama H, Mattarollo S, Hagi T, Suzuki K, Yokokawa K, Nieda M. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer. 2011;105(6):778–786. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noguchi A, Kaneko T, Kamigaki T, Fujimoto K, Ozawa M, Saito M, Ariyoshi N, Goto S. Zoledronate-activated Vγ9γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy. 2011;13(1):92–97. doi: 10.3109/14653249.2010.515581. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Niu C, Cui J. Gamma-delta (γδ) T cells: friend or foe in cancer development? J Transl Med. 2018;16(1):3. doi: 10.1186/s12967-017-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maybruck B, Pfannenstiel L, Diaz-Montero M, Gastman B. Tumor-derived exosomes induce CD8 T cell suppressors. J Immunother Cancer. 2017;5(1):65. doi: 10.1186/s40425-017-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vignard V, Labbé M, Marec N, André-Grégoire G, Jouand N, Fonteneau J, Labarrière N, Fradin D. MicroRNAs in tumor exosomes drive immune escape in melanoma. Cancer Immunol Res. 2020;8(2):255–267. doi: 10.1158/2326-6066.Cir-19-0522. [DOI] [PubMed] [Google Scholar]
- 46.Liu F, Bu Z, Zhao F, Xiao D. Increased T-helper 17 cell differentiation mediated by exosome-mediated microRNA-451 redistribution in gastric cancer infiltrated T cells. Cancer Sci. 2018;109(1):65–73. doi: 10.1111/cas.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whiteside T. Exosomes and tumor-mediated immune suppression. J Clin Investig. 2016;126(4):1216–1223. doi: 10.1172/jci81136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y, Liu K, Li Q, Yao Y, Wang Y. Exosomes function in tumor immune microenvironment. Adv Exp Med Biol. 2018;1056:109–122. doi: 10.1007/978-3-319-74470-4_7. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, Liu B, Su L, Qiu Z. Hypoxic tumor-derived exosomal mir-301a mediates m2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Can Res. 2018;78(16):4586–4598. doi: 10.1158/0008-5472.Can-17-3841. [DOI] [PubMed] [Google Scholar]
- 50.Mrizak D, Martin N, Barjon C, Jimenez-Pailhes A, Mustapha R, Niki T, Guigay J, Pancré V, de Launoit Y, Busson P, Moralès O, Delhem N. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst. 2015;107(1):363. doi: 10.1093/jnci/dju363. [DOI] [PubMed] [Google Scholar]
- 51.Wu Y, Hu G, Wu R, Gong N. High expression of miR-135b predicts malignant transformation and poor prognosis of gastric cancer. Life Sci. 2020;257:118133. doi: 10.1016/j.lfs.2020.118133. [DOI] [PubMed] [Google Scholar]
- 52.Han T, Voon D, Oshima H, Nakayama M, Echizen K, Sakai E, Yong Z, Murakami K, Yu L, Minamoto T, Ock C, Jenkins B, Kim S, Yang H, Oshima M. Interleukin 1 Up-regulates MicroRNA 135b to promote inflammation-associated gastric carcinogenesis in mice. Gastroenterology. 2019;156(4):1140–1155.e1144. doi: 10.1053/j.gastro.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 53.Shao L, Chen Z, Soutto M, Zhu S, Lu H, Romero-Gallo J, Peek R, Zhang S, El-Rifai W. Helicobacter pylori-induced miR-135b-5p promotes cisplatin resistance in gastric cancer. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2019;33(1):264–274. doi: 10.1096/fj.201701456RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang J, Shen M, Yan M, Cui Y, Gao Z, Meng X. Exosome-mediated transfer of miR-1290 promotes cell proliferation and invasion in gastric cancer via NKD1. Acta Biochim Biophys Sin. 2019;51(9):900–907. doi: 10.1093/abbs/gmz077. [DOI] [PubMed] [Google Scholar]
- 55.Li Q, Li B, Li Q, Wei S, He Z, Huang X, Wang L, Xia Y, Xu Z, Li Z, Wang W, Yang L, Zhang D, Xu Z. Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis. 2018;9(9):854. doi: 10.1038/s41419-018-0928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang H, Fu H, Wang B, Zhang X, Mao J, Li X, Wang M, Sun Z, Qian H, Xu W. Exosomal miR-423-5p targets SUFU to promote cancer growth and metastasis and serves as a novel marker for gastric cancer. Mol Carcinog. 2018;57(9):1223–1236. doi: 10.1002/mc.22838. [DOI] [PubMed] [Google Scholar]
- 57.Liu X, Lu Y, Xu Y, Hou S, Huang J, Wang B, Zhao J, Xia S, Fan S, Yu X, Du Y, Hou L, Li Z, Ding Z, An S, Huang B, Li L, Tang J, Ju J, Guan H, Song B. Exosomal transfer of miR-501 confers doxorubicin resistance and tumorigenesis via targeting of BLID in gastric cancer. Cancer Lett. 2019;459:122–134. doi: 10.1016/j.canlet.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 58.Yang H, Zhang H, Ge S, Ning T, Bai M, Li J, Li S, Sun W, Deng T, Zhang L, Ying G, Ba Y. Exosome-derived miR-130a activates angiogenesis in gastric cancer by targeting c-myb in vascular endothelial cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2018;26(10):2466–2475. doi: 10.1016/j.ymthe.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Zhou Z, Zhang H, Deng T, Ning T, Liu R, Liu D, Bai M, Ying G, Ba Y. Exosomes carrying MicroRNA-155 target forkhead box O3 of endothelial cells and promote angiogenesis in gastric cancer. Molecular therapy oncolytics. 2019;15:223–233. doi: 10.1016/j.omto.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Black A, Black J, Azizkhan-Clifford J. Sp1 and krüppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188(2):143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 61.Wang R, Xu B, Xu H (2018) TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell cycle (Georgetown, Tex) 17 (24) doi:10.1080/15384101.2018.1556063 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.