Abstract
A new provirus clone of feline leukemia virus (FeLV), which we named FeLV-A (Rickard) or FRA, was characterized with respect to viral interference group, host range, complete genome sequence, and in vivo pathogenicity in specific-pathogen-free newborn cats. The in vitro studies indicated the virus to be an ecotropic subgroup A FeLV with 98% nucleotide sequence homology to another FeLV-A clone (F6A/61E), which had also been fully sequenced previously. Since subgroup B polytropic FeLVs (FeLV-B) are known to arise via recombination between ecotropic FeLV-A and endogenous FeLV (enFeLV) env elements, the in vivo studies were conducted by direct intradermal inoculation of the FRA plasmid DNA so as to eliminate the possibility of coinoculation of any FeLV-B which may be present in the inoculum prepared by propagating FeLV-A in feline cell cultures. The following observations were made from the in vivo experiments: (i) subgroup conversion from FeLV-A to FeLV-A and FeLV-B, as determined by the interference assay, appeared to occur in plasma between 10 and 16 weeks postinoculation (p.i.); (ii) FeLV-B-like recombinants (rFeLVs), however, could be detected in DNA isolated from buffy coats and bone marrow by PCR as early as 1 to 2 weeks p.i.; (iii) while a mixture of rFeLV species containing various amounts of N-terminal substitution of the endogenous FeLV-derived env sequences were detected at 8 weeks p.i., rFeLV species harboring relatively greater amounts of such substitution appeared to predominate at later infection time points; (iv) the deduced amino acid sequence of rFeLV clones manifested striking similarity to natural FeLV-B isolates, within the mid-SU region of the env sequenced in this work; and (v) four of the five cats, which were kept for determination of tumor incidence, developed thymic lymphosarcomas within 28 to 55 weeks p.i., with all tumor DNAs harboring both FeLV-A and rFeLV proviruses. These results provide direct evidence for how FeLV-B species evolve in vivo from FeLV-A and present a new experimental approach for efficient induction of thymic tumors in cats, which should be useful for the study of retroviral lymphomagenesis in this outbred species.
Feline leukemia virus (FeLV), a member of the retrovirus family, is a naturally occurring virus found in the domestic cat population (16, 35). Since its first isolation in 1964 (20), various studies of FeLV have led to a better understanding of contagiously transmitted retroviral diseases in natural environments (2, 14, 28, 43). Three horizontally transmitted FeLV subgroups, termed FeLV subgroup A (FeLV-A), FeLV-B, and FeLV-C, have been defined by viral interference assays that detect genetic sequence variation in the viral surface glycoprotein (SU) moiety of the envelope (env) gene (45, 46). FeLV-A is an ecotropic virus which is present in all natural isolates; FeLV-B is a polytropic virus that is found with FeLV-A and is overrepresented in cats with lymphosarcomas relative to infected but otherwise healthy cats; and FeLV-C, which is also polytropic, is found infrequently but in association with FeLV-A or FeLV-A plus FeLV-B and is known to induce fatal aplastic anemia in cats (1, 8, 15, 19, 24, 39, 40). There is evidence to support an origin of FeLV-B viruses by recombination in SU between FeLV-A and endogenous FeLV (enFeLV) env elements (9, 21, 32, 33, 42, 47, 48, 52). It has been speculated that FeLV-C might also be a variant of FeLV-A due to mutational events (28). In this regard, FeLV-A is consistently associated with all FeLV-related proliferative and antiproliferative diseases in the domestic cat population.
Although several biological isolates of FeLV-A are available, only a few have been molecularly cloned. The list includes molecular clones FeLV-A/Glasgow-1 (pFGA) (52), G1(L) (23), F6A and F3A (7), and GMA-3-2 (54), of which only one FeLV-A clone, F6A, has been completely sequenced. In this report, we describe the molecular cloning and biological properties of another clone of FeLV-A (FRA), for which we also present the complete genome sequence. In an attempt to seek evidence for in vivo derivation of other FeLV subgroups, as well as to determine the pathogenicity of this newly isolated FeLV-A molecular clone, we examined FRA-infected cats over a prolonged period of observation. Although previous studies addressed the issue of in vivo derivation of FeLV-B species from a FeLV-A molecular clone (4, 42), administration of an inoculum prepared by propagating the virus in feline cell cultures could not rule out the possibility of introducing rFeLVs along with the parental virus. Noting the success of establishing a retroviral infection in vivo by direct delivery of proviral DNA into animals by either intramuscular or intradermal injection (22, 36, 55, 56), we studied the infection, virus evolution, and pathogenicity of FRA by direct intradermal injection of the pFRA plasmid into specific-pathogen-free (SPF) newborn kittens. In this report, we present data demonstrating in vivo generation of FeLV-B species from FeLV-A FRA molecular clone as well as the high efficiency of lymphoma induction in cats by the approach of direct inoculation of the proviral plasmid DNA.
MATERIALS AND METHODS
Subgenomic cloning.
Genomic DNA was prepared from the thymic tumor tissue of cat 4746-1, which was cochallenged with an FeLV-A Rickard plasma preparation and a mixture of in vitro-generated rFeLVs (33, 48). A subgenomic library was constructed by using 8- to 20-kb EcoRI digestion fragments ligated into the λ DASH II phagemid (Stratagene, La Jolla, Calif.) vector. Proviral clones were identified by screening with probe exU3 (27), which is specific for the U3 region of all known exogenous FeLV long terminal repeat (LTR) sequences, and then subcloned into the pBluescript (Stratagene) vector. One of the clones with an insert of 11.4 kb was determined to be similar to FeLV-A by PCR amplification of its env gene with FeLV-A-specific primer sets (47, 48). After establishing its infection in the feline embryo fibroblast cell line H927 (38) by plasmid DNA transfection, we tentatively designated the clone FeLV-A (Rickard), or FRA.
Viral interference assay.
Viral interference assays to identify FeLV-A, FeLV-B, and FeLV-C were performed as previously described (45). FeLV pseudotypes of murine sarcoma virus were generated with virus stocks derived from molecular clones FeLV-A/Glasgow-1 (pFGA) (52), FeLV-B/GA (pBHM-1) (9), and FeLV-C/Sarma (pFSC) (39).
Host range analyses.
The H927 cells were transfected with pFRA by the calcium phosphate transfection method as described previously (34). Viral stocks were prepared from cell culture supernatant fluids and subsequently used to test their infectivity in the following cell lines: feline T-lymphoid tumor 3201B (49) and large granular lymphoma-derived MCC (6); human fibrosarcoma HT1080 (ATCC CCL-121), T-lymphoblastic leukemia CEM (ATCC CCL-119), and B-lymphoid tumor Raji (ATCC CCL-86); canine osteogenic sarcoma D-17 (ATCC CCL-183); mouse fibroblast NIH 3T3; and mink lung cell line (ATCC CCL-64). The cell lines were maintained under culture conditions as described previously (6, 34) or as recommended by the American Type Culture Collection. After initial virus infection in the presence of DEAE-dextran, the cells were maintained for 2 weeks during which period culture supernatants were monitored by the enzyme-linked immunosorbent assay (ELISA) for FeLV capsid antigen detection (Virachek FeLV ELISA; Synbiotics, San Diego, Calif.).
Nucleotide sequencing and primers.
Primers in the env gene region used for pFRA sequencing were as follows. Primers oriented in the sense direction included RB59 (47); RB38 (24); RB27, F6A sequence 7069 to 7088 (GACTGTTCCTAAGACCCACC); RB60, F6A sequence 7473 to 7488 (CAATTAGTGCCTTAGA); and RB43, F6A sequence 7647 to 7665 (GAGAAAGACTAAAACAGCG). Antisense primers were RB52 (47); RB16 (24); RB17 and RB39 (5); RB62, complementary to the published F6A sequence 8028 to 8009 (GTAATTTTCCATGCCTTGTG); and H17, complementary to F6A 7704 to 7685 (CCTTCAAACCATCCCTGTTG). Multiple primers encompassing the gag and pol genes and based on the published sequence of the F6A clone (7) were synthesized to completely sequence the FRA plasmid. In addition, RB400, complementary to F6A 488 to 474 (CCCAAATGAAAGACC), and RB46, corresponding to F6A sequence 7922 to 7938 (TGTATGATTCCATTTAG), were used to sequence the 5′ and 3′ LTRs, respectively. Both the manual dideoxynucleotide termination method (44) and automated cycle sequencing were performed with the above primers. Automated fluorescence-based cycle sequencing was conducted with the ABI Prism 377 DNA sequencer (Perkin-Elmer, Foster City, Calif.) and the ABI Prism Dye Terminator cycle-sequencing kit (P/N 402080) as specified by the manufacturer. Nucleotide sequences from automated sequencing were initially read and analyzed with the ABI Prism DNA sequencer analysis software, version 3.0. Subsequent analyses and comparisons of all sequence data were performed with GeneJockey software. The complete env gene and a portion of the pol gene in which a notable sequence variation with F6A occurred were sequenced in both directions. The remainder of the provirus sequence, although sequenced in a single direction, was confirmed by at least two independent sequencing reactions.
pFRA plasmid challenge.
Thirteen SPF kittens were inoculated intradermally with 50 μg of pFRA plasmid DNA combined with a cationic lipid compound (DOTAP; Boehringer Mannheim) at 24 h postpartum (56). The kittens remained with their own dams until weaning at 10 weeks of age and then were separated by sex into one or two animals per cage. Once paired, cage mates were not changed throughout the remainder of the study. All inoculations and subsequent blood specimen collections were performed under ketamine anesthesia (25 mg/kg).
Antigen and antibody detection.
FeLV antigenemia was measured by antigen capture enzyme-linked immunosorbent assay (Petchek FeLV ELISA; Synbiotics, San Diego, Calif.) for the detection of p27 capsid protein in plasma and tissues. Anti-FeLV antibody responses were detected by indirect immunofluorescence on the FL-74 cat T-lymphoma cell line chronically infected with FeLVs representing each of the A, B, and C subgroups (11). At necropsy, hematopoietic and various other tissues were collected for histopathologic examination as well as for the detection of FeLV antigen in tissue sections by immunofluorescence assay.
PCR analysis.
Genomic DNA was isolated from bone marrow and buffy coat samples at various postinfection (p.i.) time points with a tissue genomic DNA isolation kit (Qiagen, Santa Clarita, Calif.). Nested PCR was performed with 250 ng of genomic DNA in the first round of amplification (35 cycles), using the following primer set: H18, the 5′ sense primer, corresponding to F6A sequence 5840 to 5860 (ACATATCGTCCTCCTGACCAC) at the pol/env junction which is conserved among all exogenous FeLVs; and H20, the 3′ antisense primer, complementary to the exogenous U3 sequence in the LTR (F6A 8210 to 8189, GAAGGTCGAACTCTGGTCAACT). A 1-μl volume of PCR product from the first round of amplification was used in second-round amplification with another 35 cycles and with primers RB53 and RB17 as reported previously (33). Genomic DNA was also isolated from the terminal thymic tumors from four cats (cats 5022 to 5025) as well as from a lymph node metastasis of cat 5023 as described above. Direct PCR was carried out with 250 ng of DNA, using primer sets RB59 and RB17 for FeLV-A-specific amplification and RB53 and RB17 for FeLV-B-specific amplification. PCR was also performed to amplify a region of LTR by using primers RB84, F6A sequence 8025 to 8045 (TTACTCAAGTATGTTCCCATG), and RB42, complementary to F6A sequence 8324 to 8306 (GGTCAAGTCTCAGCAAAGA). Total RNA was prepared with an RNA isolation kit (Clontech, Palo Alto, Calif.) from plasma samples at various time points. Nested reverse transcriptase PCR (RT-PCR) was performed as previously described (33) with the same sets of primers as listed above. pFRA and FeLV-B/GA (pBHM-1) served as controls in these PCRs.
TA cloning and characterization of clones.
By using the entire 50-μl PCR volume, the desired PCR product bands were purified from 1% (or 2% for LTR amplification) agarose gels and cloned into the TA cloning vector (pCR2.1) (Invitrogen, Carlsbad, Calif.). For each tissue sample analyzed for the 3′ crossover site, 6 to 12 clones were selected and nucleotide sequencing was performed as described above. The 3′ recombination crossover junctions were determined by sequence alignment to both enFeLV CFE-6 (21) and FRA. A total of 41 clones isolated from various tissues of four different cats at different time points were further sequenced and analyzed for nucleotide sequence similarity to reported sequences of natural FeLV-B isolates in a ca. 600-bp region of the SU portion of the rFeLV env gene. Three clones derived from a PCR product of the LTR U3 region of a tumor DNA, which was larger than the expected FRA product, were also sequenced in both directions to define the changes.
Nucleotide sequence accession number.
The complete FRA provirus sequence reported here was deposited in GenBank under accession no. AF052723.
RESULTS
Biological and biochemical characterization of the FRA molecular clone.
To determine the interference pattern of FRA, feline embryonic fibroblast (FEA) cells were transfected by pFRA and used as initiators. FeLV pseudotypes of murine sarcoma virus representing subgroups A, B, or C were used for superinfection. The results (not shown) indicated that FRA infection of FEA cells could block subgroup A virus-mediated but not subgroup B or C virus-mediated morphologic transformation. This subgroup A phenotype was consistent with the ecotropic host range of FRA. While FRA failed to infect cells of heterologous species such as human fibrosarcoma, B-lymphoid, and T-lymphoid cells, mouse fibroblasts, or mink lung cells, it could readily infect the feline cell lines tested, namely, FEA and H927 fibroblasts, and 3201B and MCC lymphoid cells (data not shown). However, like the F6A virus, FRA could cause a slight infection in the D-17 canine cells, which did not increase over the period of observation.
The entire FRA proviral genome (8,448 bp) encompassing 5′-LTR-gag-pol-env-LTR-3′ was sequenced. The comparison of the nucleotide sequence with that of another FeLV-A provirus clone, F6A (8,440 bp), revealed an overall 98% homology. The eight additional nucleotides in FRA relative to F6A were found as one extra nucleotide in each of the two LTRs and six extra nucleotides over a 55-bp region in the middle of the pol gene. It was noteworthy that all of these extra nucleotides were present in the corresponding sequence of the enFeLV CFE-6 clone (21).
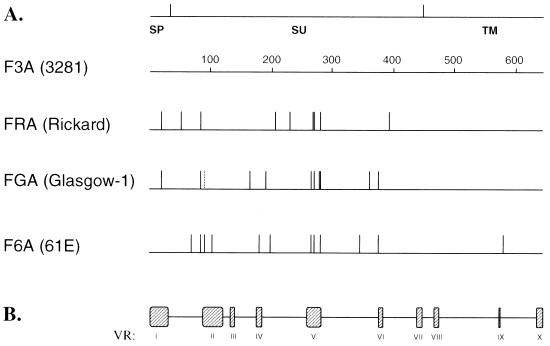
Attempts were made to compare the deduced amino acid sequence of the env gene of FRA with the reported sequences of other FeLV-A isolates, F6A, F3A, and FGA. While there was extensive homology among the env gene of all these isolates, FRA exhibited the highest homology to F3A, with a difference of only nine amino acids over the entire 643-amino-acid region of the env gene (Fig. 1). The sequence divergence appeared to be the highest between FRA and F6A representing 14 amino acids alterations scattered in the SU domain and one occurring in TM region.
FIG. 1.
Comparison of the deduced amino acid sequence of the env gene of FRA with other FeLV-A isolates. (A) The top line indicates the relative positions of signal peptide (SP), surface glycoprotein (SU), and transmembrane protein (TM) regions within the env gene. Numbers on the diagram indicate amino acid numbering starting from SP (34). Sequences are depicted as horizontal lines, and all sequences are compared with that of F3A. Vertical lines indicate sites of amino acid substitutions compared to F3A. The dashed vertical line in the FGA sequence indicates an amino acid change that was unique to the FGA isolate. (B) Locations of the previously characterized FeLV variable regions (VR) within the env gene (21).
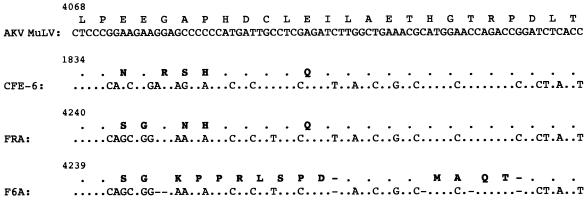
As stated above, a higher level of homology was detected between the FRA sequence and the enFeLV CFE-6, compared to the homology to F6A, in the pol gene, specifically in the portion encoding the middle region of the RT gene product. This is illustrated in Fig. 2. It appeared that in this region, FRA and enFeLV CFE-6 nucleotide sequences had more similarity to the reported sequence of the murine leukemia virus (AKV MuLV) (17) than did the F6A sequence. A total of six nucleotide deletions scattered over the 55-bp region of the F6A sequence resulted in a reading frameshift to produce 12 amino acid substitutions and 2 amino acid deletions which were apparently unique to F6A. The last of the six nucleotide deletions at position 4299 of F6A seemed to return the F6A sequence back in frame with those of AKV MuLV, enFeLV CFE-6, and FRA sequences.
FIG. 2.
Nucleotide and deduced amino acid sequence variations in the mid-pol region of FeLV clones. The indicated sequences, residing within the RT gene, are presented in reference to AKV MuLV sequence (17). The single-letter designation for the amino acid sequence is shown above the nucleotide sequence. Identity to the MuLV sequence is indicated by dots, and amino acid changes are shown in boldface type. To maintain sequence alignment, gaps were introduced into the F6A sequence (indicated by dashes). The number at the top left corner of each sequence indicates the position of the starting nucleotide corresponding to GenBank accession no. J01998 (for AKV MuLV), L06140 (for enFeLV, CFE-6), AF052723 (for FRA), and M18247 (for F6A).
A comparison of the LTR sequence of FRA with other FeLV isolates did not reveal any significant differences. Like all mammalian simple-genome oncoviruses (13), the U3 region of FRA LTR contained a binding site for leukemia virus factor b, a viral core-like element, the motif for nuclear factor-1 (NF-1), and the glucocorticoid response element. In addition, a novel protein binding site termed FLV-1, which is found in the FeLV LTR downstream of the enhancer domain (2), was present in the FRA U3. All of these binding sites were present as one copy in the FRA LTR.
In vivo infectivity and pathogenicity of FRA.
The FRA plasmid DNA was inoculated intradermally into 13 1-day-old cats. While all the cats which were kept beyond 4 weeks of observation developed chronic FeLV antigenemia, the antigenemia was detected at only 34 weeks p.i. in one cat (cat 5026) (Table 1). Detection of antigenemia beginning between 3 and 5 weeks p.i. in most of the cats was, however, comparable to that in studies involving an uncloned Rickard FeLV-A challenge (33).
TABLE 1.
Summary of results of antigenemia and subgroup analysis of all FRA-challenged cats
| Cat | Viremia status at following time (wk) p.i.a
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 28 | 34 | 37 | 43 | 50 | 52 | 55 | 65 | >65 | |
| 5020 | − | − | − | − | − | + | + | + | + | + (A) | + | + | + (AB) | + | + (AB) | + | + (AB) | + (A) | + (A) | + (AB) | + | + (AB) | + (AB) | NDb | + (ABC) | Anemia |
| 5022 | − | − | − | − | + | + | + | + | + | + (A) | + | + | + (A) | + | + (AB) | + | + (AB) | + (AB) | Thymic LSAb | |||||||
| 5023 | − | − | − | − | − | + (A) | + | + | + | + (A) | + | + | + (AB) | + | + (AB) | + | + (AB) | + (AB) | + (AB) | + (AB) | Thymic LSA | |||||
| 5024 | − | − | − | + | + | + (A) | + | + | + | + (A) | + | + | + (AB) | + | + (AB) | + | + (AB) | + (AB) | + (AB) | + (AB) | + (AB) | Thymic LSA | ||||
| 5025 | − | − | − | − | + | + (A) | + | + | + | + (A) | + | + | + (A) | + | + (A) | + | + (A) | + (A) | + (A) | + (A) | + (AB) | + (A) | + (A) | + (A) | Thymic LSA | |
| 5029 | − | − | − | Euthanized | ||||||||||||||||||||||
| 5033 | − | − | − | |||||||||||||||||||||||
| 5028 | − | − | − | − | − | Euthanized | ||||||||||||||||||||
| 5032 | − | − | − | − | + | |||||||||||||||||||||
| 5030 | − | − | − | + | + | + | + | + | + | + | + | + | Euthanized | |||||||||||||
| 5031 | − | − | − | − | + | + | + | + | + | + | + | + | ||||||||||||||
| 5026 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | Euthanized | ||||||
| 5027 | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||||
Viremia status is depicted as − or +; subgroup determinations are based on the interference assay, and the results are shown as A, AB, or ABC where such test was performed.
LSA, lymphosarcoma; ND, not done.
The pattern of seroconversion in these newborn cats was determined by fluorescent antibody titers reacting to FL-74 cell membrane antigens (the feline lymphoid tumor cell line FL-74 chronically produces all three A, B and C subgroups of FeLV). First assayed at 8 weeks p.i., most cats, in general, showed an antibody response (data not shown). The antibody titers, expressed as the highest serum dilution with ≥50% positive cells (11), varied from 10 to 160. For two cats (cats 5020 and 5023), the titers decreased to <10 at 20 and 24 weeks p.i., respectively, while in one cat (cat 5026), the titer increased to 320 by 22 weeks p.i. This last cat, which rapidly developed a strong antibody response, escaped viremia until the last time point of 34 weeks p.i. (Table 1).
Four of the five pFRA-challenged cats kept for monitoring tumor development exhibited thymic lymphosarcoma between 28 and 55 weeks p.i. The fifth cat developed nonregenerative anemia and was euthanized at 65 weeks p.i.
Selected plasma samples collected from individual animals were tested for the FeLV subgroup by the interference assay. At 10 weeks p.i., all plasma samples were positive for subgroup A and negative for subgroups B and C (Table 1). By 20 weeks p.i., four of the five cats had converted to an AB subgroup phenotype. In cat 5020, one of those four, no subgroup B virus was recovered for weeks 28 and 34 although by week 50 subgroup B was once again detected (Table 1). This cat eventually converted to subgroup ABC and died of severe nonregenerative anemia. Except for one sample at week 43, which was positive for both subgroups A and B, plasma samples from cat 5025 were of subgroup A throughout the observation period.
To determine the generation of recombinant FeLVs in vivo in various tissues early in infection, one group of cats was euthanized at 2, 4, 14, and 34 weeks after pFRA injection. For this study, bone marrow, buffy coat, and plasma samples from some of these cats as well as such samples from some of the cats kept for tumor induction were further analyzed for the presence of the recombinant env gene.
PCR detection of recombinant viral products in tissues of the FRA-infected cats.
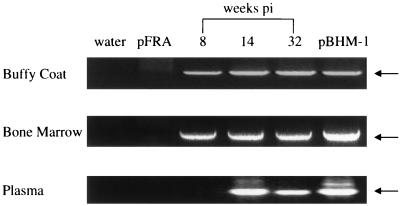
The DNA extracted from bone marrow and buffy coat specimens collected from different infected cats at different time points was examined for the presence of recombinant env proviruses by nested PCR. Serial plasma samples collected at 2, 4, 6, 8, 14, and 32 weeks p.i. from a single cat (cat 5023) were also examined for the presence of recombinant viral RNA species by RT-PCR. A representative analysis is depicted in Fig. 3, and the data obtained from the tests of bone marrow and buffy coat samples are summarized in Table 2. It was interesting that the recombinants evolved rapidly in the inoculated cats, since bone marrow specimen collected as early as 2 weeks p.i. were positive for env recombinant provirus. Most of the buffy coat samples tested were also positive, and one of the two such samples collected at 1 week p.i. was determined to contain recombinant proviruses. There was, however, a delay in the appearance of detectable rFeLVs in the plasma. In the plasma of cat 5023, rFeLV RNA was detected at 14 weeks p.i. by RT-PCR but not at 8 weeks p.i. or earlier time points (Fig. 3). This plasma analysis of a single cat was consistent with the results of an interference assay of the plasma of this and other cats (Table 1), in which most of the cats exhibited subgroup conversion at about 16 weeks p.i.
FIG. 3.
Detection of rFeLVs in buffy coat, bone marrow, and plasma samples from cat 5023 by nested PCR. Analyses of samples at 8, 14, and 32 weeks p.i. are displayed. While cell DNA was amplified for buffy coat and bone marrow specimens, plasma RNA was analyzed following the reverse transcription reaction. Plasma samples at earlier time points (2, 4, and 6 weeks p.i.) were determined to be negative and are not shown. The arrows mark the correct PCR product of ∼0.9 kb. Water and pFRA were used as negative controls in all experiments, while the FeLV-B/GA clone (pBHM-1) was used as positive control as indicated. The reverse transcription negative control with water at the reverse transcription step followed by nested PCR was also negative and is not shown.
TABLE 2.
Detection of env gene recombinants in tissues of FRA-infected cats
| Specimen | Time point at collection (wk p.i.) | Cat | No. positive/total no. tested |
|---|---|---|---|
| Bone marrow | 2 | 5029, 5033 | 2/2 |
| 8 | 5020, 5022, 5023, 5025 | 4/4 | |
| 14 | 5020, 5022, 5030, 5031 | 4/4 | |
| Buffy coat | 1 | 5029, 5033 | 1/2 |
| 2 | 5029, 5033 | 2/2 | |
| 8 | 5020, 5022, 5023 | 3/3 | |
| 14 | 5020, 5022, 5030, 5031 | 3/4 |
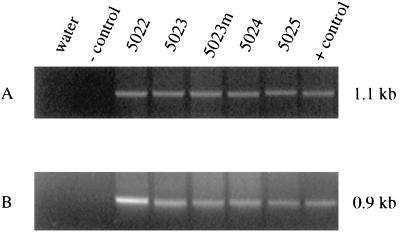
Because of tumor cell clonal proliferation, it was not necessary to use nested PCR to detect rFeLV proviruses in the tumor tissues. Direct PCR with the tumor DNAs revealed the expected size of PCR products for the recombinant env gene fragment (Fig. 4). All four primary thymic tumors, as well as a metastatic lymph node deposit of one tumor (in cat 5023), were uniformly positive for the existence of the recombinant proviruses in addition to the parental FRA-like proviruses.
FIG. 4.
Detection of both FeLV-A and rFeLV proviruses in primary tumor samples of four cats by direct PCR. (A) FeLV-A-specific primers produced a PCR product of the expected size (1.07 kb). Water and pBHM-1 were used as negative controls, while pFRA served as a positive control. (B) FeLV-B-specific primers amplified a product of the expected size (∼0.9 kb). Water and pFRA were negative controls, and pBHM-1 was the positive control. 5023m denotes a lymph node metastasis in cat 5023.
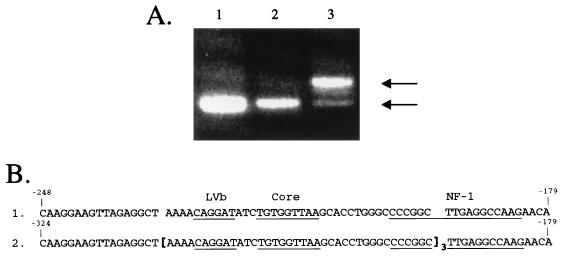
Since FeLV-related naturally occurring T-cell tumors were reported to harbor proviruses with enhancer duplication in the LTR (12, 25), we wished to examine the four FRA-induced experimental thymic tumors as well as the metastatic specimen described above for potential changes in the LTR enhancer region. Unlike the natural tumors, only one of the four experimental tumors exhibited a PCR product in the U3 region that was larger than the expected product from FRA LTR. This is illustrated in Fig. 5. The most prominent larger product of the tumor DNA of cat 5025 was molecularly cloned, and three individual clones were sequenced. While there were one to four nucleotide differences between the clones in the entire 375-bp region we cloned, these clones were identical in the 38-bp perfect triplication from the LVb-binding site (51) to the middle of the NF-1 site (12) in the U3 region. The scattered nucleotide differences were located downstream of the NF-1 site.
FIG. 5.
Analysis of the exogenous LTR U3 region in tumor specimens from FRA-infected cats. (A) PCR amplifying a 300-bp region from the DNA of two thymic tumors. Lanes: 1, pFRA as control; 2, tumor from cat 5024; 3, tumor from cat 5025. The upper arrow indicates the larger product, whereas the lower arrow indicates the normal-sized product. (B) Sequences encompassing the region of triplication are compared between FRA (lane 1) and tumor DNA from cat 5025 (lane 2). The triplicate sequence is shown within the brackets. Structural motifs are underlined and identified above the FRA sequence. Numbers at the ends of the sequences mark the relative distance from the CAP site.
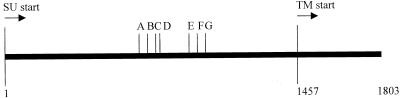
Structural features of env gene recombinants.
Our previous studies with the in vitro-derived rFeLV pool showed that they contained multiple recombination structural motifs with various 3′ crossover sites in the env gene which were designated recombination junction sites A through G (33, 47, 48). These junction sites represented various amounts of enFeLV substitution starting near the 5′ end of the rFeLV env such that recombination junction site A had the smallest amount of enFeLV-derived sequence (CFE-6 nucleotide env 746) and junction site G had the greatest enFeLV substitution (CFE-6 nucleotide env 1016) (48). In the present study, we cloned the approximately 0.9-kb PCR product encompassing the recombination sites from bone marrow and buffy coat specimens of one pFRA-inoculated cat (cat 5022). These samples were collected at various time points during the infection period, ranging from 8 to 28 weeks p.i. We also cloned the PCR products from the terminal thymic tumor samples from all four cats (cats 5022, 5023, 5024, and 5025). The results of the analysis of recombination sites are summarized in Table 3, the top portion of which provides a map of the recombination sites relative to SU and TM start positions. In bone marrow and buffy coat samples from cat 5022, recombinant species with 3′ crossover sites E, F, and G or >G were relatively more abundant at later time points than at earlier time points. In tumor samples from all four cats, the recombinants with greater amounts of endogenously derived sequences were also the predominant species.
TABLE 3.
Summary of 3′ recombination junction sites observed in env gene of in vivo-derived rFeLV clones from four cats
| Cat | Specimen | Time (wk) p.i. | No. of clones at crossover site:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| <A or A | B | C | D | E | F | G or >G | |||
| 5022 | Bone marrow | 8 | 2 | 1 | 8 | ||||
| 14 | 1 | 2 | 9 | ||||||
| 28 | 4 | 7 | |||||||
| Buffy coat | 8 | 3 | 1 | 6 | |||||
| 14 | 7 | 2 | |||||||
| 28 | 1 | 6 | 3 | ||||||
| Tumor | 28 | 4 | 1 | 1 | |||||
| 5023 | Tumor | 37 | 4 | 4 | 1 | 3 | |||
| 5024 | Tumor | 43 | 5 | 1 | |||||
| 5025 | Tumor | 55 | 2 | 3 | 1 | ||||
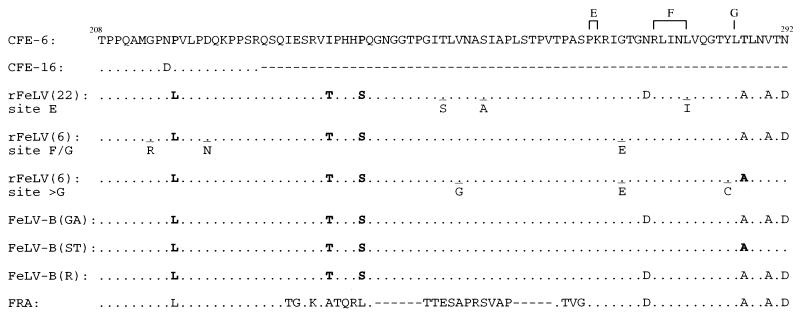
In similar experiments, when we aligned the deduced amino acid sequences of the same amplified region encompassing the recombination sites with sequences of the natural FeLV-B isolates, namely, FeLV-B/GA (29), FeLV-B/ST (29), and FeLV-B/Rickard (10), as well as those for enFeLV CFE-6 and CFE-16 (21) and the parental FRA, four amino acid differences relative to the CFE-6 sequence were invariably noted in all the rFeLV clones examined which had 3′ recombination sites downstream of site D. These included 2 clones from the 8-week-p.i. bone marrow sample from cat 5022, 3 clones from the 8-week-p.i. bone marrow sample from cat 5023, and a total of 24 clones from four terminal thymic tumor samples from cats 5022, 5023, 5024, and 5025. In addition, the five clones we analyzed of the 1-week-p.i. buffy coat sample from cat 5033 harbored all four amino acid changes. The sequences of these clones representative of different recombination sites (E, F, G, and >G) are presented in Fig. 6. The first amino acid change relative to the enFeLV background was the conversion of MGPNP or MGPDP to MGPNL epitope that is conserved in all exogenous FeLV irrespective of subgroups (33). The next two consistent amino acid changes downstream of this epitope appeared to resemble the amino acid sequences of all previously characterized FeLV-B isolates but different from either the parental FeLV-A or the putative enFeLV partners. The last consistent amino acid difference was present in FRA and all FeLV-B isolates. The noted amino acid differences were the result of single-nucleotide variations from the corresponding background sequence of the CFE-6 clone (21). All were transition mutations: two C to T, one T to C, and one A to G.
FIG. 6.
Deduced amino acid sequence comparison of the mid-region of the SU gene of the in vivo-derived rFeLV clones with those of parental enFeLVs, various FeLV-B isolates, and FRA. Clones representative of recombination sites E, F/G, and >G (sites marked above the CFE-6 sequence) are shown. Numbers in parentheses indicate the total number of such clones examined. Amino acid sequences are presented relative to CFE-6, with dots indicating identity. Four consistent amino acid sequence differences, observed in all in vivo-derived rFeLV clones as well as three isolates of FeLV-B, GA, ST, and Rickard (R), are highlighted by boldface type (with the exception of the last position, for which only clones of recombinant site >G are highlighted) under the corresponding amino acid in CFE-6 (highlighted in gray). Other scattered amino acid changes that were detected in a few clones are also listed underneath the corresponding consensus sequence. Those positions are underlined. Numbers at both ends of the CFE-6 sequence depict the relative positions of these amino acids from the start point of the mature SU peptide. CFE-16 has a truncated SU peptide sequence because of a natural deletion (21). The reference FRA sequence is shown at the bottom, with gaps (dashes) introduced to maintain the sequence alignment.
DISCUSSION
The interest of this work is twofold. First, direct evidence has been obtained to indicate that polytropic FeLV-B species can arise rapidly in vivo in cats infected with a single molecular species of ecotropic FeLV-A. The structural analyses of the env gene of recombinant viruses evolved in vivo provide a scenario of selection of recombinant species over the time course of infection to represent viral species which closely mimic the previously characterized natural isolates of the FeLV-B subgroup. Second, evidence is presented to demonstrate that thymic lymphosarcomas can be induced with high frequency over a latency period of 28 to 55 weeks in SPF newborn cats by intradermal administration of proviral DNA of an FeLV subgroup A virus. These issues are discussed below.
It has been documented that introduction and expression of FeLV-A genetic material into feline cells in culture could give rise to FeLV-B species from recombinational events between ecotropic FeLV-A and enFeLV-derived env sequences (21, 32, 47, 48). However, it is the same fact that FeLV-B could emerge in FeLV-A-infected feline cell cultures that complicates the analysis of in vivo genesis of FeLV-B species when FeLV-A species propagated in feline cells are used as the inoculum to infect cats (33, 42). To avoid such a problem, we carried out the present study by intradermal administration of an FeLV-A provirus, namely, FRA, which we have molecularly cloned in the laboratory. By using cloned plasmid DNA as the inoculum, we eliminated the step of propagating the ecotropic FeLV-A in feline cell cultures, which is known to facilitate recombinational events. The outcome of this study conclusively demonstrates that FeLV-B-like rFeLVs can be generated rapidly and easily from FeLV-A and enFeLV in tissues of the experimental cats. The recombinant species could be detected in buffy coat and bone marrow cells of the animals as early as 1 to 2 weeks p.i. The systemic distribution of the recombinant species is, however, relatively slow, since the rFeLVs could not be detected in the plasma samples until about 14 weeks p.i., as assayed by RT-PCR.
While examining the sites of recombination within the SU region of the env gene of the in vivo-formed rFeLVs, we found that recombinants with relatively greater amounts of enFeLV-derived N-terminal SU substitutions (those with 3′ crossover sites of E, F, G, and >G) were generally the predominant species observed at later time points during the course of infection. As we mentioned previously (33), it reinforces the idea that recombinants with more endogenously derived SU sequence may have an in vivo selective advantage. In this regard, it is noteworthy that a recent report described that a chimeric FeLV construct containing the FeLV-B sequence at approximately 50% of the N-terminal SU (resembling a recombination structural motif similar to the natural FeLV-B/GA isolate) was able to recognize human Pit1 (HuPit1), HuPit2, and hamster Pit2 (HaPit2) receptors while another chimeric construct, which contained only one-third of the N-terminal FeLV-B SU sequence, was able to recognize HuPit1 better than HuPit2 (3). The authors of that report suggested that Pit2 recognition might be an important in vivo selection factor. Another potential contributing factor could be that the presence of a larger endogenously derived moiety of the SU protein confers some resistance to host immune surveillance. While the extent of contributions by these or other factors remains to be determined, it is clear that rFeLVs emerging as the majority species in the infected cats in this study do harbor relatively greater amounts of enFeLV-derived SU sequence exhibiting structural motifs which resemble those present in natural FeLV-B isolates.
Another point of interest is that all rFeLV clones examined with recombination sites downstream of site D reveal certain consistent amino acid differences in the mid-SU region compared to the known sequence of enFeLV elements which are present as proviral DNA (21) or expressed as mRNAs in feline thymic cells (26). If this known type of enFeLV transcripts participated in the de novo recombination events with FRA mRNA during reverse transcription (18, 53), one has to propose that single nucleotide mutations in the background of the enFeLV frame will be required to result in sequence similarity to natural FeLV-B isolates. Alternatively, the possibility exists that there is an unidentified enFeLV proviral element whose sequence is more similar to FeLV-B mRNA than to CFE-6. Irrespective of the models, i.e., recombination of FRA with a hitherto unknown enFeLV or recombination with prototype CFE-6 enFeLV followed by evolution through mutation, the amino acid differences observed here, which were present at the earliest time point of analysis, may represent functional and structural constraints required for the efficient propagation of rFeLVs in vivo.
The observation that in addition to the newly generated FeLV-B species, one of the cats (cat 5025) contained FeLV-C species at a late stage (65 weeks p.i.) is quite intriguing. Repeated analysis of this plasma sample confirmed the presence of FeLV-C-like viruses, and the cat, in fact, succumbed to severe anemia known to be induced by FeLV-C species (24, 39). It will now be important to isolate this FeLV-C-like virus to examine the mechanism by which it might have originated.
In vivo studies with FeLV-A preparations by the conventional route of intravenous or intraperitoneal inoculation have shown a low frequency of thymic tumor induction; only 4 of 28 cats developed tumors (30, 31, 37, 42). In contrast to the past experiments, we found a much higher incidence of tumor induction when we introduced FRA intradermally in DNA form, since four of the five cats developed thymic lymphosarcoma and the fifth died of anemia. A logical question is thus raised, i.e., whether the determinants of pathogenicity are specific for the FRA clone or whether they are related to the approach by which the viral material was delivered to the animals. Since FRA has as high as 98% nucleotide sequence homology to F6A, which was previously used to study tumor induction in vivo (31, 42), it is unlikely that minor sequence divergences detected in either the pol or env gene could be the discriminating factors. However, it cannot be ruled out at this time whether even such minimal changes may be responsible for increased recombinogenicity or any other functional attributes of the FRA virus. Parallel studies with pFRA and pF6A are necessary to evaluate the role of minor nucleotide variations in FeLV-A pathogenesis. The promoter-enhancer region of FRA does not appear to harbor any unique alterations that may distinguish it from other FeLVs, and this region is not naturally duplicated in the FRA virus genome, although enhancer duplication has been implicated with increased leukemogenicity (2). While naturally occurring FeLV-related T-lymphoid tumors have been associated with enhancer duplication or triplication in proviral sequences present in tumors (12, 25), we found that only one of the four experimental tumors contained enhancer triplication in these integrants.
Considering the above, it seems likely that the route of administration of the proviral DNA may be important in tumorigenesis. Parental and recombinant viruses may be more readily accessible to the putative target cells when intradermally induced. These issues, however, remain to be addressed in future experiments. Although ours is the first report of FeLV infection of cats by direct inoculation of the viral genetic material, we note that after the completion of this work, intramuscular or intradermal inoculation of feline immunodeficiency virus DNA was successfully used to establish feline immunodeficiency virus infection in cats (41, 50). Thus, it appears that cloned feline retrovirus DNA inoculation rather than inoculation by virion preparations will be increasingly useful for the study of retrovirus-mediated pathogenesis in domestic cats.
In conclusion, our results have confirmed that rFeLV could be generated rapidly in vivo from parental FeLV-A infection and that the rFeLVs thus formed undergo a selection process during the course of infection to yield populations enriched in species with larger N-terminal SU substitution from the endogenous elements. We also demonstrate the efficiency of FeLV-A infection by DNA inoculation and suggest that such an approach may be valuable in obtaining additional clues to the mechanisms of retrovirus-induced hematopoietic malignancies in this outbred species.
ACKNOWLEDGMENTS
We thank Lily Li for technical assistance with automated sequencing.
This work was supported by Public Health Service grant CA51485 from the National Cancer Institute.
REFERENCES
- 1.Abkowitz J L. Retrovirus-induced feline pure red blood cell aplasia: pathogenesis and response to suramin. Blood. 1991;77:1442–1451. [PubMed] [Google Scholar]
- 2.Athas G B, Starkey C R, Levy L S. Retroviral determinants of leukemogenesis. Crit Rev Oncol. 1994;5:169–199. doi: 10.1615/critrevoncog.v5.i2-3.40. [DOI] [PubMed] [Google Scholar]
- 3.Boomer S, Eiden M, Burns C C, Overbaugh J. Three distinct envelope domains, variably present in subgroup B feline leukemia virus recombinants, mediate Pit1 and Pit2 receptor recognition. J Virol. 1997;71:8116–8123. doi: 10.1128/jvi.71.11.8116-8123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boomer S, Gasper P, Whalen L R, Overbaugh J. Isolation of a novel subgroup B feline leukemia virus from a cat infected with FeLV-A. Virology. 1994;204:805–810. doi: 10.1006/viro.1994.1597. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti R, Hofman F M, Pandey R, Mathes L E, Roy-Burman P. Recombination between feline exogenous and endogenous retroviral sequences generates tropism for cerebral endothelial cells. Am J Pathol. 1994;144:348–358. [PMC free article] [PubMed] [Google Scholar]
- 6.Cheney C M, Rojko J L, Kociba G J, Wellman M L, Di Bartola S P, Rezanka L J, Forman L, Mathes L E. A feline large granular lymphoma and its derived cell line. In Vitro Cell Dev Biol. 1990;26:455–463. doi: 10.1007/BF02624087. [DOI] [PubMed] [Google Scholar]
- 7.Donahue P R, Hoover E A, Beltz G A, Riedel N, Hirsch V M, Overbaugh J, Mullins J I. Strong sequence conservation among horizontally transmissible, minimally pathogenic feline leukemia viruses. J Virol. 1988;62:722–731. doi: 10.1128/jvi.62.3.722-731.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dornsife R E, Gasper P W, Mullins J I, Hoover E A. Induction of aplastic anemia by intra-bone marrow inoculation of a molecularly cloned feline retrovirus. Leuk Res. 1989;13:745–755. doi: 10.1016/0145-2126(89)90087-8. [DOI] [PubMed] [Google Scholar]
- 9.Elder J H, Mullins J I. Nucleotide sequence of the envelope gene of Gardner-Arnstein feline leukemia virus B reveals unique sequence homologies with a murine mink cell focus-forming virus. J Virol. 1983;46:871–880. doi: 10.1128/jvi.46.3.871-880.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elder J H, Mullins J I. Nucleotide sequence of the envelope genes and LTR of the subgroup B, Rickard strain of feline leukemia virus. In: Weiss R, Teich N, Varmus H, Coffin J, editors. RNA tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1985. pp. 1005–1010. [Google Scholar]
- 11.Essex M M, Klein G, Snyder S P, Harrold J B. Feline sarcoma virus (FeSV)-induced tumors: correlation between humoral antibody and tumor regression. Nature (London) 1971;233:195–197. doi: 10.1038/233195a0. [DOI] [PubMed] [Google Scholar]
- 12.Fulton R, Plumb M, Shield L, Neil J C. Structural diversity and nuclear protein binding sites in the long terminal repeats of feline leukemia virus. J Virol. 1990;64:1675–1682. doi: 10.1128/jvi.64.4.1675-1682.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golemis E A, Speck N A, Hopkins N. Alignment of U3 region sequences of mammalian type C viruses: identification of highly conserved motifs and implications for enhancer design. J Virol. 1990;64:534–542. doi: 10.1128/jvi.64.2.534-542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy W D., Jr . Feline oncoretroviruses. In: Levy J A, editor. The Retroviridae. Vol. 2. New York, N.Y: Plenum Press; 1993. pp. 109–180. [Google Scholar]
- 15.Hardy W D, Jr, Hess P W, MacEwen E G, McClelland A J, Zuckerman E E, Essex M, Cotter S M, Jarrett O. Biology of feline leukemia virus in the natural environment. Cancer Res. 1976;36:582–588. [PubMed] [Google Scholar]
- 16.Hardy W D, Jr, Old L J, Hess P W, Essex M, Cotter S. Horizontal transmission of feline leukaemia virus. Nature (London) 1973;244:266–269. doi: 10.1038/244266a0. [DOI] [PubMed] [Google Scholar]
- 17.Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984;49:471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu W S, Temin H M. Retroviral recombination and reverse transcription. Science. 1990;250:1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- 19.Jarrett O, Hardy W D, Jr, Golder M C, Hay D. The frequency of occurrence of feline leukemia virus subgroups in cats. Int J Cancer. 1978;21:334–337. doi: 10.1002/ijc.2910210314. [DOI] [PubMed] [Google Scholar]
- 20.Jarrett W F H, Crawford E M, Martin W B, Davie F A. A virus-like particle associated with leukemia (lymphosarcoma) Nature (London) 1964;202:567–569. doi: 10.1038/202567a0. [DOI] [PubMed] [Google Scholar]
- 21.Kumar D V, Berry B T, Roy-Burman P. Nucleotide sequence and distinctive characteristics of the env gene of endogenous feline leukemia provirus. J Virol. 1989;63:2379–2384. doi: 10.1128/jvi.63.5.2379-2384.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letvin N L, Lord C I, King N W, Wyand M S. Risks of handling HIV. Nature (London) 1991;349:573. doi: 10.1038/349573a0. [DOI] [PubMed] [Google Scholar]
- 23.Luciw P, Parker D, Potter S, Najarian R. Feline leukemia virus (FeLV), strains A/Glasgow-1 and C, env genes. In: Weiss R, Teich N, Varmus H, Coffin J, editors. RNA tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1985. pp. 1000–1004. [Google Scholar]
- 24.Mathes L E, Pandey R, Chakrabarti R, Hofman F M, Hayes K A, Stromberg P, Roy-Burman P. Pathogenicity of a subgroup C feline leukemia virus (FeLV) is augmented when administered in association with certain FeLV recombinants. Virology. 1994;198:185–195. doi: 10.1006/viro.1994.1021. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto Y, Momoi Y, Watari T, Goitsuka R, Tsujimoto H, Hasegawa A. Detection of enhancer repeats in the long terminal repeats of feline leukemia viruses from cats with spontaneous neoplastic and nonneoplastic diseases. Virology. 1992;189:745–749. doi: 10.1016/0042-6822(92)90598-j. [DOI] [PubMed] [Google Scholar]
- 26.McDougall A, Terry A, Tzavaras T, Rojko J, Cheney C, Neil J C. Defective endogenous viruses are expressed in feline lymphoid cells: evidence for a role in natural resistance to subgroup B feline leukemia viruses. J Virol. 1994;68:2151–2160. doi: 10.1128/jvi.68.4.2151-2160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullins J I, Chen C S, Hoover E A. Disease-specific and tissue-specific production of unintegrated feline leukemia virus variant DNA in feline AIDS. Nature (London) 1986;319:333–336. doi: 10.1038/319333a0. [DOI] [PubMed] [Google Scholar]
- 28.Neil J C, Fulton R, Rigby M, Stewart M. Feline leukemia virus: generation of pathogenic and oncogenic variants. Curr Top Microbiol Immunol. 1991;171:68–92. doi: 10.1007/978-3-642-76524-7_4. [DOI] [PubMed] [Google Scholar]
- 29.Nunberg J H, Williams M E, Innis M A. Nucleotide sequences of the envelope genes of two isolates of feline leukemia virus subgroup B. J Virol. 1984;49:629–632. doi: 10.1128/jvi.49.2.629-632.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overbaugh J, Donahue P R, Quackenbush S L, Hoover E A, Mullins J I. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988;239:906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- 31.Overbaugh J, Hoover E A, Mullins J I, Burns D P W, Rudensey L, Quackenbush S L, Stallard V, Donahue P R. Structure and pathogenicity of individual variants within an immuno-deficiency disease-inducing isolate of FeLV. Virology. 1992;188:558–569. doi: 10.1016/0042-6822(92)90510-v. [DOI] [PubMed] [Google Scholar]
- 32.Overbaugh J, Reidel N, Hoover E A, Mullins J I. Transduction of endogenous envelope genes by feline leukemia virus in vitro. Nature (London) 1988;332:731–734. doi: 10.1038/332731a0. [DOI] [PubMed] [Google Scholar]
- 33.Pandey R, Bechtel M K, Su Y, Ghosh A K, Hayes K A, Mathes L E, Roy-Burman P. Feline leukemia virus variants in experimentally induced thymic lymphosarcomas. Virology. 1995;214:584–592. doi: 10.1006/viro.1995.0069. [DOI] [PubMed] [Google Scholar]
- 34.Pandey R, Ghosh A K, Kumar D V, Bachman B A, Shibata D, Roy-Burman P. Recombination between feline leukemia virus subgroup B or C and endogenous env elements alters the in vitro biological activities of the viruses. J Virol. 1991;65:6495–6508. doi: 10.1128/jvi.65.12.6495-6508.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen N C, Theilen G, Keane M A, Fairbanks L, Mason T, Orser B, Chen C-H, Allison C. Studies of naturally transmitted feline leukemia virus infection. Am J Vet Res. 1977;38:1523–1531. [PubMed] [Google Scholar]
- 36.Portis J L, McAtee F J, Kayman S C. Infectivity of retroviral DNA in vivo. J Acquired Immune Defic Syndr. 1992;5:1272–1277. doi: 10.1097/00126334-199212000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Quackenbush S L, Donahue P R, Dean G A, Myles M H, Ackley C D, Cooper M D, Mullins J I, Hoover E A. Lymphocyte subset alterations and viral determinants of immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J Virol. 1990;64:5465–5474. doi: 10.1128/jvi.64.11.5465-5474.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasheed S, Gardner M B. Characterization of cat cell cultures for expression of retrovirus, FOCMA and endogenous sarc genes. In: Hardy W D Jr, Essex M, McClelland A J, editors. Proceedings of the Third International Feline Leukemia Virus Meeting. New York, N.Y: Elsevier/North-Holland Publishing Co.; 1980. pp. 393–400. [Google Scholar]
- 39.Riedel N, Hoover E A, Gasper P W, Nicolson M O, Mullins J I. Molecular analysis and pathogenesis of the feline aplastic anemia retrovirus, FeLV-C-Sarma. J Virol. 1986;60:242–260. doi: 10.1128/jvi.60.1.242-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riedel N, Hoover E A, Dornsife R E, Mullins J I. Pathogenic and host range determinants of the feline aplastic anemia retrovirus. Proc Natl Acad Sci USA. 1988;85:2758–2762. doi: 10.1073/pnas.85.8.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rigby M A, Hosie M J, Willett B J, Mackay N, McDonald M, Cannon C, Dunsford T, Jarrett O, Neil J C. Comparative efficiency of feline immunodeficiency virus infection by DNA inoculation. AIDS Res Hum Retroviruses. 1997;13:405–412. doi: 10.1089/aid.1997.13.405. [DOI] [PubMed] [Google Scholar]
- 42.Rohn J L, Linenberger M L, Hoover E A, Overbaugh J. Evolution of feline leukemia virus variant genomes with insertions, deletions, and defective envelope genes in infected cats with tumors. J Virol. 1994;68:2458–2467. doi: 10.1128/jvi.68.4.2458-2467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roy-Burman P. Endogenous env elements: partners in generation of pathogenic feline leukemia viruses. Virus Genes. 1996;11:147–161. doi: 10.1007/BF01728655. [DOI] [PubMed] [Google Scholar]
- 44.Sanger F, Coulson A R, Barrell B G, Smith A J H, Roe B A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980;143:161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- 45.Sarma P S, Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973;54:160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- 46.Sarma P S, Log T. Viral interference in feline leukemia-sarcoma complex. Virology. 1971;44:352–358. [PubMed] [Google Scholar]
- 47.Sheets R L, Pandey R, Jen W-C, Roy-Burman P. Recombinant feline leukemia virus genes detected in naturally occurring feline lymphosarcomas. J Virol. 1993;67:3118–3125. doi: 10.1128/jvi.67.6.3118-3125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheets R L, Pandey R, Klement V, Grant C K, Roy-Burman P. Biologically selected recombinants between feline leukemia virus (FeLV) subgroup A and an endogenous FeLV element. Virology. 1992;190:849–855. doi: 10.1016/0042-6822(92)90924-e. [DOI] [PubMed] [Google Scholar]
- 49.Snyder H W J, Hardy W D, Jr, Zuckerman E E, Fleissner E. Characterisation of a tumour-specific antigen on the surface of feline lymphosarcoma cells. Nature (London) 1978;275:656–658. doi: 10.1038/275656a0. [DOI] [PubMed] [Google Scholar]
- 50.Sparger E E, Louie H, Ziomeck A M, Luciw P A. Infection of cats by injection with DNA of a feline immunodeficiency virus molecular clone. Virology. 1997;238:157–160. doi: 10.1006/viro.1997.8787. [DOI] [PubMed] [Google Scholar]
- 51.Speck N A, Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987;7:1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart M A, Warnock M, Wheeler A, Wilkie N, Mullins J I, Onions D E, Neil J C. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence for the recombinational origin of subgroup B viruses. J Virol. 1986;58:825–834. doi: 10.1128/jvi.58.3.825-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stuhlmann H, Berg P. Homologous recombination of copackaged retrovirus RNAs during reverse transcription. J Virol. 1992;66:2378–2388. doi: 10.1128/jvi.66.4.2378-2388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tzavaras T, Stewart M, McDougall A, Fulton R, Testa N, Onions D E, Neil J C. Molecular cloning and characterization of a defective recombinant feline leukemia virus associated with myeloid leukemia. J Gen Virol. 1990;71:343–354. doi: 10.1099/0022-1317-71-2-343. [DOI] [PubMed] [Google Scholar]
- 55.Willems L, Kettmann R, Dequiedt F, Portetelle D, Voneche V, Cornil I, Kerkhofs P, Burny A, Mammerickx M. In vivo infection of sheep by bovine leukemia virus mutants. J Virol. 1993;67:4078–4085. doi: 10.1128/jvi.67.7.4078-4085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willems L, Portetelle D, Kerkhofs P, Chen G, Burny A, Mammerickx M, Kettmann R. In vivo transfection of bovine leukemia provirus into sheep. Virology. 1992;189:775–777. doi: 10.1016/0042-6822(92)90604-n. [DOI] [PubMed] [Google Scholar]