Abstract
Tumor mutation burden (TMB) predicts response to immunotherapy in non-small cell lung cancer (NSCLC). The current TMB evaluation is expensive and not satisfactory. Here, novel tumor mutation score (TMS) was defined as the number of genes with mutations in candidate genes and compared with TMB and PD-L1 in 240 NSCLC patients and validated in 34 NSCLC patients. Eighteen genes were significantly associated with longer progression-free survival (PFS) or better response. The number of mutated genes within 18 favorable genes were defined as TMS18. TMS18 (HR = 0.307, P < 0.001) had smaller hazard ratio and P value than TMB (HR = 0.455, P = 0.004) and PD-L1 expression (HR = 0.403, P = 0.005) in survival analysis. Moreover, TMS18 had significantly higher AUC than TMB and TMS18 combined with PD-L1 improved the accuracy. Universal cutoff of TMS18 enriched more patients with benefits. These findings were largely consistent in the validation cohort. Taken together, TMS18 was more powerful than TMB in predicting response of ICIs in NSCLC. Selective TMS was more feasible and cost-effective than unselective TMB. TMS18 combined with PD-L1 might yield better efficiency in predicting response of ICIs in NSCLC with future validation in larger cohorts.
Supplementary Information
The online version contains supplementary material available at (10.1007/s00262-021-02868-w).
Keywords: Tumor mutation score, Tumor mutation burden, Immunotherapy, Biomarker, Lung cancer
Introduction
Immune checkpoint inhibitors (ICIs) are emerging as the state of the art for cancer treatment and have brought tremendous change in the therapeutic landscape for advanced cancer patients, especially in non-small cell lung cancer (NSCLC) [1, 2]. There are urgent needs for clinically practical biomarkers for identifying patients who may benefit from ICIs. To date, PD-L1 expression [3] and tumor mutation burden (TMB) [4] have been approved by Food and Drug Administration (FDA) of USA as predictive biomarkers in NSCLC. High TMB represents genomic instability and might trigger immunogenicity improvement with neoantigen derived from mutations in cancer cells [5]. The golden standards for TMB testing are by whole-genome sequencing (WGS) or whole-exome sequencing (WES) [1, 6].
Currently, TMB evaluation by targeted sequencing with cancer gene panels is widely accepted since mounting evidences proved that TMB by targeted sequencing was highly consistent with TMB by WES [7]. Representative gene panels including Memorial Sloan Kettering Cancer Center’s Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) testing and FoundationOne CDx (F1CDx) testing, have been approved by FDA of USA [1]. These gene panels usually contain 300–500 genes and cover around one megabase of coding DNA and larger gene panels are being developed [1, 8]. However, many genes within these gene panels are proven to be unnecessary or even negatively correlated with response to ICIs [1]. Mutations in genes including EGFR and STK11 were not correlated with clinical benefit of ICIs or even predicted primary resistance to ICIs [9–11]. ICIs did not improve the overall survival compared with docetaxel [12] in NSCLC patients with EGFR mutations, which might be due to negative correlation between PD-L1 expression and EGFR mutations [9]. Similarly, less tumor-infiltrating lymphocytes and PD-L1 expression were also detected in STK11-mutant NSCLC [13], although STK11 mutations were positively correlated with high TMB. STK11 alterations were reported to be the most prevalent genomic driver of primary resistance to PD-1 axis inhibitors in KRAS-mutant lung adenocarcinoma [10]. TMB was defined as the total number of mutations found in the DNA of cancer cells. Non-selective TMB included all nonsynonymous somatic mutations in the calculation, which we believed would significantly attenuate the power of TMB as biomarker for ICI.
Moreover, although TMB could serve as a candidate biomarker of clinical outcome from ICIs [14, 15] in advanced cancer including melanoma [16, 17], lung cancer [18–21], and urothelial carcinoma [22], recent study reported that there may not be one universal definition of high TMB [1, 7, 23] since TMB might differ across different platforms or gene panels. High cost and without universal definition of high TMB also greatly hampered its application in clinic [23, 24]. Therefore, we developed tumor mutation score (TMS) [25, 26], defined as number of genes with nonsynonymous somatic mutations among certain candidate genes. Compared with TMB, novel TMS excluded the adverse mutations and even unnecessary mutations and would not be affected across platforms. In this study, we compared TMS and TMB as predictive biomarker for ICIs in the large MSK-IMPACT cohort [7, 27] and validated in Rizvi cohort [19].
Methods and Materials
Patients and data curation
The training cohort contained a total of 240 NSCLC patients from MSK-IMPACT cohort. All the patients received MSK-IMPACT testing and the sequencing data was reported in 2017 [27] and updated recently with response and follow-up data [4]. All patients were treated with at least one dose of ICIs (atezolizumab, avelumab, durvalumab, ipilimumab, nivolumab, pembrolizumab, or tremelimumab). The clinical and mutation profiles of the cohort were available at cBioPortal (http://www.cbioportal.org). The validation Rizvi cohort contained 34 NSCLC patients treated with pembrolizumab and all patients received WES testing [19]. The genomic and clinical data were available in the supplementary materials of the original article. The clinicopathological characteristics of the MSK-IMPACT cohort and Rizvi cohort are listed in Table 1.
Table 1.
Clinicopathological characteristics of 240 patients in MSK-IMPACT cohort and 34 patients in Rizvi cohort
| Characteristics | MSK-IMPACT /NO. (%) | Rizvi cohort/NO. (%) |
|---|---|---|
| Age median (range) | 66(22–92) | 63(41–80) |
| Sex | ||
| Male | 118 (49%) | 16(47%) |
| Female | 122 (51%) | 18(53%) |
| Histology | ||
| Adenocarcinoma | 186 (78%) | 30(88%) |
| Squamous | 34 (14%) | 3(9%) |
| Other | 20 (8%) | 1(3%) |
| Smoking | ||
| Ever | 193 (80%) | 28(82%) |
| Never | 47 (20%) | 6(18%) |
| Best overall response | ||
| CR | 3 (1%) | 0(0%) |
| PR | 46 (19%) | 12(35%) |
| SD | 83 (34%) | 10(20%) |
| PD | 108 (45%) | 12(35%) |
| Durable clinical benefit | ||
| YES | 69 (29%) | 14(41%) |
| NO | 158 (66%) | 17(50%) |
| NE | 13 (5%) | 3(9%) |
| Treatment | ||
| Anti-PD-(L)1 | 206(86%) | 34(100%) |
| Anti-PD-(L)1 + anti-CTLA-4 | 34(14%) | / |
| TMB median (range) | 7.37 (0.82–91.84) | 199(11–1192) WES |
| TMS median (range) | 7 (1–77) | / |
| TMS18 median (range) | 1 (0–9) | 1(0–7) |
| PD-L1 score (N = 84) median (range) | 1 (0–100) | / |
CR, complete response; PR, partial response; SD, stable disease; PD, progressed disease; DCB, durable clinical benefit (CR/PR or SD > 6 months); NE, not evaluable (follow-up < 6 months); WES, whole-exome sequencing
MSK-IMPACT and WES testing
Generally, MSK-IMPACT testing required tumor and matching blood sample and was capable of detecting alterations in genes in human genomic DNA obtained from tumor tissue [1, 7, 27]. WES testing was performed in the validation cohort to fully extract the matching genomic data in the MSK-IMPACT testing since different gene panels have different gene lists.
TMB and TMS calculation
In the training cohort, TMB was calculated according to the MSK-IMPACT workflow. The total number of somatic mutations identified was normalized to the exonic coverage of the panel in megabases (Mb). In the validation cohort, TMB by WES included the whole exomes while TMB by MSK-IMPACT only included genes in MSK-IMPACT panel.
Tumor mutation score (TMS) was defined as number of genes with nonsynonymous somatic mutations among certain candidate genes. Total TMS was the number of genes with nonsynonymous somatic mutations among all the genes sequenced, while TMS18 was number of genes with nonsynonymous somatic mutations among 18 favorable prognostic genes associated with better responses in patients treated with ICI.
Favorable gene identification and biomarker comparison
Integrated analysis was performed in identification of favorable genes associated with better responses in NSCLC patients treated with ICI. Progression-free survival (PFS) counted from the day of ICI administration. Firstly, survival analysis of PFS was performed among sequenced genes by dividing patients into non-mutant group and mutant group. Then, efficacy was assessed by Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST1.1). Gene alterations were compared among patients with durable clinical benefit (DCB, complete response [CR]/partial response [PR] or stable disease [SD] that lasted > 6 months) and no durable benefit (NDB, progressed disease [PD] or SD that lasted ≤ 6 months). Lastly, gene alterations were also compared among patients with different best overall responses (BOR), which were CR/PR/SD Vs. PD groups. Taken together, gene alterations with significant association with longer PFS, DCB, or better BOR were identified as favorable genes in NSCLC after immunotherapy.
Eighteen favorable genes were identified and TMS18 based on these genes ranged from 0 to 18 and was divided into three groups by universal cutoff values (TMS18 = 0, 1 ≤ TMS18 < 3, and TMS18 ≥ 3) while TMB was divided into three groups by quantiles as reported (Bottom 80%, Top10-20%, Top10%) [7]. For 84 patients with evaluation of PD-L1 expression, high, moderate, and low expressions were defined as PD-L1 = 0%, 1% ≤ PD-L1 < 50%, and PD-L1 ≥ 50% based on the percentage of tumor cells with membranous staining as recommended. Thus, TMS18 was further compared with TMB and PD-L1 expression by survival analysis and differential analysis.
The 18 favorable genes were: ARID1B, ATRX, ERBB4, FGFR4, INPP4B, MGA, POLE, PTPRD, PTPRT, TERT, TET1, TP53, KDM5C, FAT1, AR, TSHR, MLL3, and BCOR.
Statistical analysis
Differences in TMS18, TMB, and PD-L1 expressions were examined by the Mann–Whitney U test for two-group comparisons or the Kruskal–Wallis exact test for three-group comparisons. The Fisher’s exact test was used to compare proportions. Log-rank test for difference in PFS was determined and hazard ratio (HR) value was determined from a COX model using the survival package (version 2.43–3). Correlations were examined by the Spearman rank correlation coefficients. Receiver operating characteristic curves (ROC) were assessed by generating the area under the curve (AUC). Statistical analysis was performed in R (version 3.5.1) and all reported P values were two-sided.
Results
TMS18 based on 18 favorable genes was significantly associated with TMB
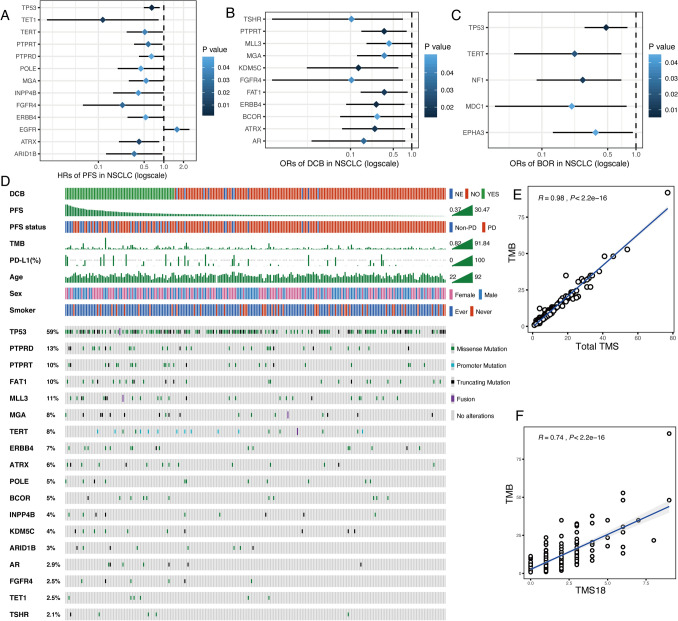
The main difference between TMS and TMB is that TMS only includes favorable gene alterations while TMB takes all nonsynonymous somatic mutations into account. Favorable gene alterations were defined as gene mutations which were significantly associated with longer PFS, DCB, or better BOR in the 240 NSCLC patients. As results, mutations in 12 genes predicted longer PFS with hazard ratios (HRs) less than 1 (Fig. 1a and Fig. S1) whereas EGFR mutation predicted shorter PFS (Fig. 1a). Meanwhile, 11 gene mutations were significantly associated with DCB (Fig. 1b). Moreover, 5 gene mutations were significantly associated with better BOR (CR/PR/SD vs. PD) (Fig. 1c). Taken together, 18 unique genes among these three gene lists were identified as favorable gene alterations (Fig. 1d). The mutation spectrum of these 18 genes along with key clinical parameters including PFS in 240 NSCLC patients is shown in the Oncoprint plot in Fig. 1d. Vague associations between higher TMB and PD-L1 and longer PFS could be seen from the distribution of the individual profiles (Fig. 1d). Lastly, total TMS based on all sequenced genes in MSK-IMPACT testing and TMS18 based on 18 favorable genes were calculated and further compared with TMB. Total TMS was highly correlated with TMB (Coef = 0.98, P < 0.001, Fig. 1e) while TMS18 was also significantly correlated with TMB (Coef = 0.74, P < 0.001, Fig. 1f). The significant correlation and divergence between TMS18 and TMB were the basis for TMS18 as better biomarker for immunotherapy in NSCLC.
Fig. 1.
Mutation profiles of 18 favorable genes and characteristics of TMS18. a 13 genes were significantly associated with progression-free survival (PFS), 12 genes were favorable (Hazard ratios [HRs] < 1) except EGFR (HR > 1). b 11 genes were significantly associated with durable clinical benefit (DCB) (odds ratios [ORs] < 1). c 5 genes were significantly associated with better best overall response (BOR) (ORs < 1). a–c A total of 18 genes were identified as favorable gens. d Oncoprint plot of 18 favorable genes. e Total TMS (based on all sequenced genes) was significantly correlated with TMB. (F) TMS18 (based on 18 favorable genes) was significantly correlated with TMB
TMS18 demonstrated higher efficiency than TMB and PD-L1 in predicting response after immunotherapy
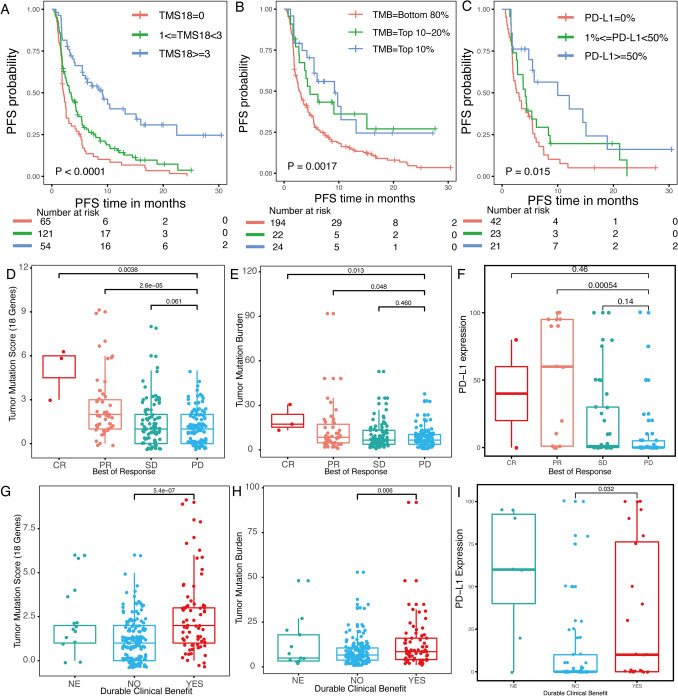
TMS18 based on 18 favorable genes was further compared with TMB and PD-L1 expression, which had been approved in NSCLC as biomarker for immunotherapy. In the survival analysis of PFS, patients were divided into Low, Moderate, and High groups as shown in Fig. 2a–c. Compared with Low group, High TMS18 (HR = 0.307, P < 0.001) demonstrated smaller HR and P values than High TMB (HR = 0.455, P = 0.004) and High PD-L1 expression (HR = 0.403, P = 0.005) (Fig. 2a–c). More importantly, intersections between Moderate and High groups were observed in the survival curves of TMB and PD-L1 expression, and distribution of the number of patients in each group in TMS18 was more reasonable, more patients were enriched in Moderate and High groups which predicted clinical benefit (Fig. 2a–c). In the evaluation of response by RECIST1.1, greater difference among different response groups was identified in TMS18 than TMB and PD-L1 expression (Fig. 2d–f). Compared with PD group, CR and PR groups had significantly higher TMS18 whereas SD group showed no significant difference. Similar trend was seen in TMB whereas only PR group had significantly higher PD-L1 expression than PD group (Fig. 2d–f). Lastly, in the relationship with DCB, the difference between DCB and non-DCB group was also the most significant in TMS18 than TMB and PD-L1 expression (Fig. 2g–i).
Fig. 2.
Comparisons among TMS18, TMB and PD-L1 expression in predicting response to ICIs in NSCLC. a Kaplan–Meier curve for patients divided into three groups with universal cutoff of TMS18. Two-sided log-rank P value was indicated for all patients. Univariate Cox regression HR of 0.307 (95% confidence interval (CI) 0.207–0.471, P < 0.001) and 0.685 (95% CI 0.500–0.940, P = 0.019) were for the TMS18 > = 3 and 1 < = TMS18 < 3 groups, respectively, compared with the TMS18 = 0 group. b Kaplan–Meier curve for patients divided into three groups by quantiles with TMB. HR of 0.455 (95% CI 0.263–0.786, P = 0.004) and 0.531 (95% CI 0.308–0.917, P = 0.023) were for the TMB = Top 10% and TMB = Top 10–20%, respectively, compared with the TMB = Bottom 80% group. c Kaplan–Meier curve for patients divided into three groups by percentages with PD-L1 expression. HR of 0.403 (95% CI 0.213–0.763, P = 0.005) and 0.708 (95% CI 0.411–1.220, P = 0.214) were for the PD-L1 > = 50% and 1% < = PD-L1 < 50%, respectively, compared with the PD-L1 = 0% group. d–f Differential analyses of TMS18, TMB and PD-L1 expression among CR/PR/SD/PD patients. g–i Differential analyses of TMS18, TMB and PD-L1 among patients with durable clinical benefit
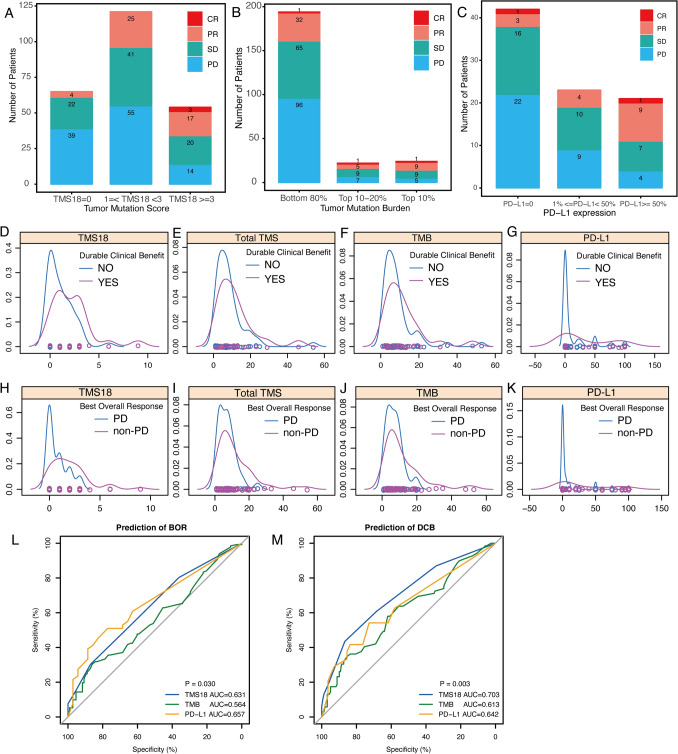
Specifically, the number of patients with different response evaluation in each group in TMS18, TMB, and PD-L1 expression is shown in Fig. 3a–c. Notably, all the three CR patients were enriched in TMS18 High group (TMS18 ≥ 3) whereas each group based on TMB had one CR patient (Fig. 3 A and B). The proportions of non-PD (CR/PR/SD) patients in High group of TMS18, TMB and PD-L1 were 74.07% (N = 54), 79.16% (N = 24) and 80.95% (N = 21), respectively (Fig. 3a–c). Although the objective response rates in High TMB and High PD-L1 groups seemed higher than that in High TMS18 group, over twofold patients were classified into the High group in TMS18, which means more patients would be predicted to benefit from immunotherapy based on TMS18. Moreover, density plots revealed that distribution of TMS18 in patients with DCB shifted right compared with that of total TMS, TMB, and PD-L1 (Fig. 3d–g). Similar character of TMS18 was also observed in comparisons between non-PD and PD patients (Fig. 3h–k). Therefore, these differences between TMS18 and TMB might improve the effect of TMB as biomarker for immunotherapy. ROC analysis demonstrated that TMS18 significantly enhanced the prediction efficiency of TMB (Fig. 3l and m). In evaluation of BOR, the AUC of ROC of TMS18 was 0.631, which was significantly higher than 0.564 of TMB and compatible with 0.657 of PD-L1 (Fig. 3l). In evaluation of DCB, TMS18 had the highest AUC of ROC, which was 0.703, higher than 0.613 of TMB and 0.642 of PD-L1 (Fig. 3m).
Fig. 3.
Characteristics of TMs18, TMB, and PD-L1 expression as biomarker for ICIs in NSCLC. a–c Number of patients with different clinical responses among High, Moderate and Low TMS18, TMB, and PD-L1 expression. d–g Distribution of TMS18, total TMS, TMB, and PD-L1 expression between patients with or without durable clinical benefit. h–k Distribution of TMS18, total TMS, TMB, and PD-L1 expression. l ROC curves of TMS18, TMB, and PD-L1 expression in predicting best overall response (BOR: non-PD vs. PD), the AUC of TMS18 was significantly higher than that of TMB (P = 0.030). m ROC curves of TMS18, TMB, and PD-L1 expression in predicting durable clinical benefit (DCB Vs. NDB), the AUC of TMS18 was significantly higher than that of TMB (P = 0.003)
Taken together, TMS18 demonstrated higher efficiency than TMB and PD-L1 expression as biomarker for immunotherapy after immune checkpoint inhibitors.
TMS18 combined with PD-L1 expression might improve the power of patients screening with benefit
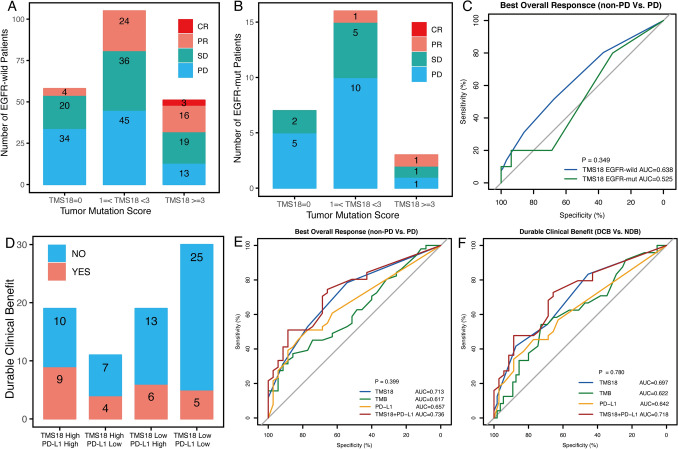
In the identification of favorable gene alterations, EGFR mutation was significantly associated with shorter PFS (Fig. 1a). Here, TMS18 was compared in EGFR-wild (N = 214) and EGFR-mut (N = 26) NSCLC patients. As results, higher response rate was identified in EGFR-wild patients with higher TMS18, which were 41.3, 57.1 and 74.5% in Low, Moderate, and High TMS18 group, respectively (Fig. 4a). However, in patients with EGFR mutation, the response rates were obviously lower than those in EGFR-wild patients, which were 28.5%, 37.5% and 66.6% (2 in 3 patients) in Low, Moderate, and High TMS18 group (Fig. 4b). Notably, only two patients with PR were found among the 26 EGFR-mut NSCLC patients (Fig. 4b). The AUC of TMS18 in EGFR-wild NSCLC patients was obviously higher than that in EGFR-mut NSCLC patients, although the difference was not statistically significant (Fig. 4c).
Fig. 4.
Relationship between TMS18 and EGFR mutation or PD-L1 expression in predicting response to ICIs in NSCLC. a Number of patients with different clinical responses in High, Moderate and Low TMS18 groups in EGFR-wild NSCLC patients. b Number of patients with different clinical responses in High, Moderate and Low TMS18 groups in EGFR-mut NSCLC patients. c ROC curves of TMS18 in EGFR-wild and EGFR-mut NSCLC patients. d Number of patients with durable clinical benefit in groups divided by TMS18 (stratified into 0 or > = 1 groups as Low vs. High) combined with PD-L1 expression (stratified into 0% or > = 1% groups as Low vs. High). e ROC curves of TMS18, TMB, PD-L1, and TMS18 + PD-L1 in predicting best overall response (BOR: non-PD vs. PD). f ROC curves of TMS18, TMB, PD-L1, and TMS18 + PD-L1 in predicting durable clinical benefit (DCB vs. NDB)
Previous reports demonstrated that TMB combined with PD-L1 expression may enhance the efficiency of predicting clinical benefit from immunotherapy [4]. Here, 47.3% patients had DCB among the 19 patients with both High TMS18 and High PD-L1, whereas only 16.6% patients had DCB in patients with Low TMS18 and Low PD-L1 (Fig. 4d). Furthermore, ROC curves demonstrated that TMS18 combined with PD-L1 expression improved the accuracy. In identification of BOR, the AUC of TMS18 plus PD-L1 was 0.736, while the AUC of TMS18 was 0.718, both were higher than those of PD-L1 and TMB (Fig. 4e). Similar findings were also identified in evaluation of DCB (Fig. 4f). TMS18 plus PD-L1 expression had the highest AUC compared with the individual biomarker (Fig. 4e, f). However, the differences were not statistically significant, which might due to small cohort with missing data in some patients.
TMS18 outperformed TMB in an independent validation cohort.
In order to validate these findings, TMS18 was compared with TMB in an independent validation cohort consisting 34 NSCLC patients reported by Rizvi NA [19]. TMS18, TMB by MSK-IMPACT and TMB by WES were calculated using WES data from Rizvi cohort. As results, in the survival analysis based on previous grouping criteria, Higher TMS18 indicated significantly longer PFS in NSCLC (Fig. 5a), whereas there was no significant association between higher TMB by IMPACT or WES method and longer PFS (Fig. 5b, c). Meanwhile, if NSCLC patients were divided into two groups rather than three groups, patients with TMS18 > = 2 showed significantly longer PFS than patients with TMS18 < = 1 (Fig. 5d), High TMB groups by both IMPACT and WES method indicated significantly longer PFS than Low TMB groups (Fig. 5e, f). Notably, TMS18 outperformed TMB by IMPACT with lower P value while TMB by WES demonstrated the highest efficiency in predict PFS (Fig. 5d–f). The underlying reasons for these differences might due to small cohort and TMB by WES were more discrete than TMS18 since most patients had TMS18 of 1 in the Rizvi cohort. Similarly, in prediction of BOR, The AUC of TMS18 was 0.74, which was significantly higher than 0.72 of TMB by IMPACT and was significantly lower than 0.84 of TMB by WES (Fig. S2A–D). Similar results were also found in prediction of DCB, but the differences were not statistically significant (Fig. S2E–H). Lastly, all 3 patients had PR in the TMS18 > = 3 group while PD patient was found in high TMB (Top 10%) groups (Fig. 5g–i). These results in the Rizvi cohort were largely consistent with our previous findings even with small cohort. Therefore, TMS18 outperformed TMB in predicting response to immunotherapy in NSCLC.
Fig. 5.
Validations results in the Rizvi cohort with 34 NSCLC patients. All patients received whole-exome sequencing (WES), TMS18 and TMB by MSK-IMPACT were calculated based on the gene panels using the WES data, accordingly. a–c Survival analyses of progression-free survival (PFS) with TMS18, TMB by IMPACT and TMB by WES based on previous grouping criteria. Higher TMS18 indicated significantly longer PFS in NSCLC (a), whereas there was no significant association between higher TMB by IMPACT or WES method and longer PFS (b–c). d–f Survival analyses of PFS with TMS18, TMB by IMPACT and TMB by WES divided into two groups as indicated. TMS18 outperformed TMB by IMPACT with lower P value while TMB by WES demonstrated the highest efficiency in predict PFS. g–i Number of patients with different clinical responses in High, Moderate and Low groups of TMS18, TMB by WES and TMB by IMPACT
Discussion
In this study, we developed novel TMS as predictive biomarker for NSCLC patients treated with ICIs. TMS was defined as number of genes with nonsynonymous somatic mutations among certain candidate genes and was compared with TMB. Firstly, in the training MSK-IMPACT cohort, 18 genes were characterized as favorable genes by significant associations with longer PFS or better responses. TMS18 was then calculated accordingly. Secondly, TMS18 demonstrated higher efficiency than TMB in predicting PFS and clinical responses in the 240 NSCLC patients. Moreover, TMS18 combined with PD-L1 expression achieved even higher prediction accuracy in NSCLC patients. Lastly, these findings were further validated in Rizvi cohort. These results highlighted that novel TMS18 might be better biomarker than TMB for NSCLC patients receiving ICIs treatment.
Currently, TMB is usually measured by targeted sequencing with gene panels [7]. MSK-IMPACT testing and F1CDx offer a reliable identification of true somatic mutations [1]. The gene lists between these two panels were different and germline DNA was not sequenced in F1CDx but filtering germline status was carried out using public and private variant databases [1, 28]. Therefore, TMB may differ across platforms and gene panels. Furthermore, different thresholds for high TMB were reported recently with no consensus widely accepted worldwide [1, 28]. There was no universal cutoff value for high TMB. More importantly, the predict efficiency of TMB as biomarker for immunotherapy was not satisfactory. With more insights into the relationship between mutations and response to ICIs in cancer, TMB non-selectively included adverse or unnecessary mutations in the calculation which attenuated its power in predicting response to ICI. Therefore, compared with TMB, the key concept of TMS18 was to only keep favorable gene mutations to achieve better effect. More importantly, we did not define genes associated with poor response with negative values in TMS and excluded only these genes in the analysis, since our concept was to make TMS easy to calculate and thus achieve a universal cutoff value. The simple scoring algorithm of TMS18 rather than complicated models such as nomograms or normalization like TMB was adopted to overcome differences across platforms and future changes of candidate gene list. Accordingly, this approach might dramatically reduce the number of genes in NGS panels which was more cost-effective than TMB. Therefore, easy calculation and universal cutoff value of TMS18 will not be affected across platforms and is more feasible in clinic.
In identifying favorable genes, the results were largely consistent with previous reports. For example, EGFR mutation predicted worse PFS in NSCLC (Fig. 1a). It is worth noting that PIK3CA, APC, and KRAS which were reported to be favorable factors in response to ICIs, were statistically significant not in the 18 favorable genes. The reason for low mutation rate genes PIK3CA and APC might be due to small cohort while for frequently mutated KRAS, KRAS encompasses a complex and heterogeneous set of mutations and concurrent KRAS and TP53 mutation could be a predictive factor for response to immunotherapy [29] (Fig. 1). In our analysis, we only chose genes with mutation rate greater or equal to 2% (5/240) to generate at least 5 patients with mutations to perform survival analysis. Therefore, the number of candidate genes might be expanded with larger cohort by including genes with lower mutation rates. Simple scoring system of TMS18 would not be affected with these changes since all favorable genes were regarded as independent factors. As proof-of-concept study, the specific gene list and mechanistic study of these genes were needed in further investigations.
In the validation of these findings, the results were largely consistent in the Rizvi cohort with 34 NSCLC patients. Patients in Rizvi cohort only received pembrolizumab treatment while 34 patients in MSK-IMPACT cohort received PD(L)1 and CTLA-4 combined treatment (Table 1). Meanwhile, the response evaluation of these two cohorts were evaluated by RECIST1.1, which about 10% PD patients might be evaluated as pseudoprogressions by irRECIST/iRECIST [30]. Lastly, WES data were required since the specific genes within different gene panels varied a lot. For example, ARID1B, MGA, and TET1 were not in F1CDx. Therefore, if the 18 favorable genes were incorporated into the big commercial gene panels, TMS18 may be used independently or as complement to the currently used gene panels specific for NSCLC. Since big gene panels may provide other druggable targets for patients, inclusion of these 18 favorable genes is recommended in future gene panels.
The core concept and the methods of this study are similar to our previous report of TMS55 [25, 26]. However, there are several main differences between these two studies. Firstly, the previous study was to uncover biomarker for the overall survival after ICIs in pan-cancer while this study is to uncover biomarker for the response to ICIs only in NSCLC. Generally, this study was more precise and specific compared with the previous study which may be affected by heterogeneous patients and many factors related to overall survival. As results, the candidate genes were further narrowed down to 18 genes in NSCLC from 55 genes in pan-cancer.
Taken together, we developed a more feasible and robust algorithm of TMS compared with TMB as biomarker for cancer patients treated with ICIs. TMS18, consisting of 18 favorable genes, still needs further investigations in prospective larger cohort. Novel TMS may provide a universal way to promote the application of mutation-based biomarkers for cancer patients after immunotherapy.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary information 1 (DOCX 2834 kb)
Author contributions
J.H., Y.L., and W.P.T. designed the study. Y.L. and Z.H.C. performed the analysis. Y.L. and N.S. wrote the manuscript. All authors reviewed the manuscript and approved the final version.
Funding
This work is supported by National Natural Science Foundation of China under Grant 81902369.
Compliance with ethical standards
Conflicts of interest
The authors declare that there is no conflict of interest.
Availability of data and material
The data and material are available as described in the methods and within this publication and its supplementary material.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nan Sun, Email: sunnan@vip.126.com.
Jie He, Email: prof.jiehe@gmail.com.
References
- 1.Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30(1):44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu H, Chen Z, Ballman KV, Watson MA, Govindan R, Lanc I, Beer DG, Bueno R, Chirieac LR, Chui MH, Chen G, Franklin WA, Gandara DR, Genova C, Brovsky KA, Joshi MM, Merrick DT, Richards WG, Rivard CJ, Harpole DH, Tsao MS, van Bokhoven A, Shepherd FA, Hirsch FR. Correlation of PD-L1 Expression with Tumor Mutation Burden and Gene Signatures for Prognosis in Early-Stage Squamous Cell Lung Carcinoma. J Thorac Oncol. 2019;14(1):25–36. doi: 10.1016/j.jtho.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, Investigators K. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N, Hollmann T, Schalper KA, Gainor JF, Shen R, Ni A, Arbour KC, Merghoub T, Wolchok J, Snyder A, Chaft JE, Kris MG, Rudin CM, Socci ND, Berger MF, Taylor BS, Zehir A, Solit DB, Arcila ME, Ladanyi M, Riely GJ, Schultz N, Hellmann MD. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol. 2018;36(7):633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 6.Jiang T, Shi J, Dong Z, Hou L, Zhao C, Li X, Mao B, Zhu W, Guo X, Zhang H, He J, Chen X, Su C, Ren S, Wu C, Zhou C. Genomic landscape and its correlations with tumor mutational burden, PD-L1 expression, and immune cells infiltration in Chinese lung squamous cell carcinoma. J Hematol Oncol. 2019;12(1):75. doi: 10.1186/s13045-019-0762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ, Omuro A, Kaley TJ, Kendall SM, Motzer RJ, Hakimi AA, Voss MH, Russo P, Rosenberg J, Iyer G, Bochner BH, Bajorin DF, Al-Ahmadie HA, Chaft JE, Rudin CM, Riely GJ, Baxi S, Ho AL, Wong RJ, Pfister DG, Wolchok JD, Barker CA, Gutin PH, Brennan CW, Tabar V, Mellinghoff IK, DeAngelis LM, Ariyan CE, Lee N, Tap WD, Gounder MM, D'Angelo SP, Saltz L, Stadler ZK, Scher HI, Baselga J, Razavi P, Klebanoff CA, Yaeger R, Segal NH, Ku GY, DeMatteo RP, Ladanyi M, Rizvi NA, Berger MF, Riaz N, Solit DB, Chan TA, Morris LGT. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goto Y. Tumor Mutation Burden: Is It Ready for the Clinic? J Clin Oncol:JCO2018793398. 2018 doi: 10.1200/JCO.2018.79.3398. [DOI] [PubMed] [Google Scholar]
- 9.Soo RA, Lim SM, Syn NL, Teng R, Soong R, Mok TSK, Cho BC. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: Current controversies and future directions. Lung Cancer. 2018;115:12–20. doi: 10.1016/j.lungcan.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco SE, Gay L, Ali SM, Elvin JA, Singal G, Ross JS, Fabrizio D, Szabo PM, Chang H, Sasson A, Srinivasan S, Kirov S, Szustakowski J, Vitazka P, Edwards R, Bufill JA, Sharma N, Ou SI, Peled N, Spigel DR, Rizvi H, Aguilar EJ, Carter BW, Erasmus J, Halpenny DF, Plodkowski AJ, Long NM, Nishino M, Denning WL, Galan-Cobo A, Hamdi H, Hirz T, Tong P, Wang J, Rodriguez-Canales J, Villalobos PA, Parra ER, Kalhor N, Sholl LM, Sauter JL, Jungbluth AA, Mino-Kenudson M, Azimi R, Elamin YY, Zhang J, Leonardi GC, Jiang F, Wong KK, Lee JJ, Papadimitrakopoulou VA, Wistuba II, Miller VA, Frampton GM, Wolchok JD, Shaw AT, Janne PA, Stephens PJ, Rudin CM, Geese WJ, Albacker LA, Heymach JV. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018;8(7):822–835. doi: 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, Corral Jaime J, Gray JE, Powderly J, Chouaid C, Bidoli P, Wheatley-Price P, Park K, Soo RA, Huang Y, Wadsworth C, Dennis PA, Rizvi NA, Investigators A. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, Yang JC. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol. 2017;12(2):403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Scheel AH, Ansen S, Schultheis AM, Scheffler M, Fischer RN, Michels S, Hellmich M, George J, Zander T, Brockmann M, Stoelben E, Groen H, Timens W, Perner S, von Bergwelt-Baildon M, Buttner R, Wolf J. PD-L1 expression in non-small cell lung cancer: Correlations with genetic alterations. Oncoimmunology. 2016;5(5):e1131379. doi: 10.1080/2162402X.2015.1131379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarchoan M, Johnson BA, 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17(4):209–222. doi: 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med. 2017;377(25):2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, Utikal J, Hassel JC, Weide B, Kaehler KC, Loquai C, Mohr P, Gutzmer R, Dummer R, Gabriel S, Wu CJ, Schadendorf D, Garraway LA. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, Ready N, Hiltermann TJN, Nair S, Juergens R, Peters S, Minenza E, Wrangle JM, Rodriguez-Abreu D, Borghaei H, Blumenschein GR, Jr, Villaruz LC, Havel L, Krejci J, Corral Jaime J, Chang H, Geese WJ, Bhagavatheeswaran P, Chen AC, Socinski MA, CheckMate I. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, Borghaei H, Ramalingam SS, Brahmer J, Reck M, O'Byrne KJ, Geese WJ, Green G, Chang H, Szustakowski J, Bhagavatheeswaran P, Healey D, Fu Y, Nathan F, Paz-Ares L. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, Rizvi NA, Hirsch FR, Selvaggi G, Szustakowski JD, Sasson A, Golhar R, Vitazka P, Chang H, Geese WJ, Antonia SJ. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell. 2018;33(5):853–861. doi: 10.1016/j.ccell.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, Dawson NA, der vanHeijden MS, Dreicer R, Srinivas S, Retz MM, Joseph RW, Drakaki A, Vaishampayan UN, Sridhar SS, Quinn DI, Duran I, Shaffer DR, Eigl BJ, Grivas PD, Yu EY, Li S, Kadel EE, Boyd Z, Bourgon R, Hegde PS, Mariathasan S, Thastrom A, Abidoye OO, Fine GD, Bajorin DF, Group IMS Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy MJ, Crown J. Biomarkers for Predicting Response to Immunotherapy with Immune Checkpoint Inhibitors in Cancer Patients. Clin Chem. 2019;65(10):1228–1238. doi: 10.1373/clinchem.2019.303644. [DOI] [PubMed] [Google Scholar]
- 24.Nandakumar V, Mills JR. The Now and Beyond of Tumor Mutational Burden as a Predictor of Response to Immune Checkpoint Inhibitors. Clin Chem. 2019;65(2):357. doi: 10.1373/clinchem.2018.295097. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Chen Z, Wu L, Tao W. Novel tumor mutation score versus tumor mutation burden in predicting survival after immunotherapy in pan-cancer patients from the MSK-IMPACT cohort. Annals of Translational Medicine. 2020;8(7):446. doi: 10.21037/atm.2020.03.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Chen Z, Wu L, Tao W. Novel tumour mutation score versus tumour mutation burden in predicting survival after immunotherapy in pan-cancer from MSK-IMPACT cohort. Ann Oncol. 2019 doi: 10.1093/annonc/mdz438.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, Hellmann MD, Barron DA, Schram AM, Hameed M, Dogan S, Ross DS, Hechtman JF, DeLair DF, Yao J, Mandelker DL, Cheng DT, Chandramohan R, Mohanty AS, Ptashkin RN, Jayakumaran G, Prasad M, Syed MH, Rema AB, Liu ZY, Nafa K, Borsu L, Sadowska J, Casanova J, Bacares R, Kiecka IJ, Razumova A, Son JB, Stewart L, Baldi T, Mullaney KA, Al-Ahmadie H, Vakiani E, Abeshouse AA, Penson AV, Jonsson P, Camacho N, Chang MT, Won HH, Gross BE, Kundra R, Heins ZJ, Chen HW, Phillips S, Zhang H, Wang J, Ochoa A, Wills J, Eubank M, Thomas SB, Gardos SM, Reales DN, Galle J, Durany R, Cambria R, Abida W, Cercek A, Feldman DR, Gounder MM, Hakimi AA, Harding JJ, Iyer G, Janjigian YY, Jordan EJ, Kelly CM, Lowery MA, Morris LGT, Omuro AM, Raj N, Razavi P, Shoushtari AN, Shukla N, Soumerai TE, Varghese AM, Yaeger R, Coleman J, Bochner B, Riely GJ, Saltz LB, Scher HI, Sabbatini PJ, Robson ME, Klimstra DS, Taylor BS, Baselga J, Schultz N, Hyman DM, Arcila ME, Solit DB, Ladanyi M, Berger MF. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Truesdell J, Miller VA, Fabrizio D. Approach to evaluating tumor mutational burden in routine clinical practice. Transl Lung Cancer Res. 2018;7(6):678–681. doi: 10.21037/tlcr.2018.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torralvo J, Friedlaender A, Achard V, Addeo A. The Activity of Immune Checkpoint Inhibition in KRAS Mutated Non-small Cell Lung Cancer: A Single Centre Experience. Cancer Genomics Proteomics. 2019;16(6):577–582. doi: 10.21873/cgp.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, Balleyguier C, Planchard D, Gazzah A, Soria JC, Marabelle A, Besse B, Caramella C. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. doi: 10.1016/j.ejca.2017.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information 1 (DOCX 2834 kb)