Abstract
Renal cell carcinoma (RCC) is known to respond to immune checkpoint blockade (ICB) therapy, whereas there has been limited analysis of T-cell responses to RCC. In this study, we utilized human carbonic anhydrase 9 (hCA9) as a model neoantigen of mouse RENCA RCC. hCA9-expressing RENCA RCC (RENCA/hCA9) cells were rejected in young mice but grew in aged mice. CD8+ T cells were the primary effector cells involved in rejection in young mice, whereas CD4+ T cells participated at the early stage. Screening of a panel of hCA9-derived peptides revealed that mouse CD8+ T cells responded to hCA9288–296 peptide. Mouse CD4+ T cells responded to lysates of RENCA/hCA9, but not RENCA cells, and showed reactivity to hCA9 276–290, which shares three amino acids with hCA9 288–296 peptide. Immunohistochemistry analysis revealed that few T cells infiltrated RENCA/hCA9 tissues in aged mice. ICB therapy of anti-PD-1/anti-CTLA-4 antibodies promoted T-cell infiltration into tumor tissues, whereas no definite antitumor effect was observed. However, additional combination with cyclophosphamide or axitinib, a vascular endothelial growth factor receptor inhibitor, induced complete regression in half of the RENCA/hCA9-bearing aged mice with increased expression of PD-L1 in tumor tissues. These results indicate that hCA9 can be a useful model neoantigen to investigate antitumor T-cell responses in mice with RCC, and that RENCA/hCA9 in aged mice can serve as a non-inflamed ‘cold’ tumor model facilitating the development of effective combined immunotherapies for RCC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02992-7.
Keywords: Renal cell carcinoma, Carbonic anhydrase 9, Immune checkpoint blockade, Combined immunotherapy, Neoantigen
Introduction
Immune checkpoint blockade (ICB) therapy is an emerging promising anti-cancer therapy [1, 2]. The main targets of this therapy are PD-1, PD-L1, and CTLA-4 [3, 4]. Several clinical studies have revealed that mutation-derived neoantigens are the major targets of T cells responsible for the therapeutic effects of ICB therapy [5]. In melanoma, neoepitopes have been primarily selected from single nucleotide variants (SNVs) [6], and SNVs and neoantigen burdens correlate with responses to ICB therapy [7, 8]. However, renal cell carcinoma (RCC) has a relatively low SNV burden but still benefits from the therapeutic effects of ICB therapy [9]. Human RCCs have been shown to have the highest proportion and number of insertion/deletion mutations [10], resulting in a large quantity of neoantigenic peptides. This makes it difficult to identify T cell-recognizing neoantigenic peptides in human RCC, although a recent study analyzed the reactivity of tumor-infiltrating T cells from RCC patients to neoepitopes derived from point and frameshift mutations [11]. Nonetheless, it remains difficult to analyze T-cell reactivity to (non-self) neoantigens, even in preclinical mouse models of RCC.
To date, several RCC-associated antigens recognized by CD8+ T cells have been reported, including SART3 [12], RAGE [13], fibroblast growth factor-5 [14], and human endogenous retrovirus type E [15]. We also reported several RCC-associated antigens and peptides that can be recognized by cytotoxic T lymphocytes (CTLs) [16–19]. Among them, carbonic anhydrase (CA)-9, which is involved in the hypoxia-related response [20], is a promising target for immunotherapy against RCC because it is highly expressed in human RCC [21]. Consequently, we have applied three human CA9 (hCA9)-derived peptides as an anti-cancer vaccine against HLA-A24 positive RCC [22]. We also established a mouse RCC RENCA cell line expressing hCA9, designated RENCA/hCA9, and reported that immunization of syngeneic mice with an hCA9 peptide could induce peptide-specific CD8+ T cells in mice [23].
In this study, we inoculated RENCA/hCA9 cells s.c. into young and aged mice and observed contrasting responses: Young mice rejected RENCA/hCA9 cells, but aged mice permitted their growth. Given that cancer frequently occurs in the elderly [24], we considered tumor-bearing aged mice to reflect the clinical situation more accurately than young mice. On this basis, we used two distinct experimental protocols: RENCA/hCA9-bearing young mice were used to analyze T-cell responses to non-self-hCA9 as a model neoantigen, while RENCA/hCA9-bearing aged mice were used to test the therapeutic efficacy of combined ICB therapies. In addition, we identified hCA9-derived peptides that can be recognized by mouse CD8+ or CD4+ T cells, which may facilitate the development of neoantigen vaccines against RCC.
Materials and methods
Mice, cell lines, and reagents
Young (6–7 weeks old) and aged (55–60 weeks old) BALB/c female mice were purchased from CLEA (Tokyo, Japan). Mice were maintained under specific pathogen-free conditions. The experiments were performed according to the ethical guidelines for animal experiments of the Shimane University Faculty of Medicine (IZ2-19). RENCA and 3T3 are RCC and fibroblast cell lines of BALB/c mouse origin, respectively. RENCA/hCA9 and 3T3/hCA9 are a RENCA cell line and a 3T3 fibroblast cell line expressing hCA9, respectively [23]. All cell lines were maintained in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum and 20 μg/mL gentamycin (Sigma-Aldrich). Anti-PD-1 mAb (clone RMP 1–14) was purchased from Bio X Cell Inc. (Lebanon, NH, USA), and anti-CTLA-4 mAb was prepared from the supernatants of hybridoma UC10-4F10-7 (American Type Culture Collection, Manassas, VA, USA). Cyclophosphamide (CP) was purchased from Shionogi Co., Ltd. (Osaka, Japan). Axitinib, a selective inhibitor of vascular endothelial growth factor (VEGF) receptor, was purchased from Selleck Co. (Tokyo, Japan).
In vivo growth of RENCA and RENCA/hCA9 cells
To examine in vivo growth, RENCA (1 × 106) or RENCA/hCA9 (3 × 106) cells were inoculated s.c. into the flanks of young and aged mice. Tumor size (mm2) was measured twice weekly.
In vivo depletion of CD4+ or CD8+ T cells
On days 5 and 9 after inoculation of RENCA/hCA9 (3 × 106) cells into young mice, anti-CD4 antibody (clone GK1.5; BioLegend, San Diego, CA, USA), anti-CD8 antibody (clone 53–6.7; BioLegend), or control rat IgG were injected i.p. at a dose of 150 μg.
Analysis of T-cell responses to hCA9-derived peptides
A panel of hCA9-derived peptides containing the H-2d binding motif was prepared based on the NetMHCpan server (http://www.cbs.dtu.dk/services/NetMHCpan). First, 21 crude peptides were purchased from Greiner (Tokyo, Japan), and four selected hCA9-derived peptides with > 90% purity were from MabProtein (Shimane, Japan). Young BALB/c mice were inoculated s.c. with RENCA/hCA9 (3 × 106) cells into the flank. On day 11, the axial and inguinal draining lymph nodes (LNs) were harvested. Following in vitro removal of CD4+ T cells using anti-CD4 mAb and sheep anti-rat IgG dynabeads (#11,035, Invitrogen, Grand Island, NY, USA), the remaining cells were cultured with the indicated peptides and IL-2 (20 U/mL) in 96-round well plates for 3 days. To identify hCA9-derivced peptides recognized by CD4+ T cells, 16 crude peptides (length, 15-mer) were purchased from Greiner, and four hCA9-derived peptides with > 90% purity were from Greiner. The spleen cells from RENCA/hCA9-immunized young mice were harvested, and CD8+ T cells were depleted in vitro using an anti-CD8 mAb and sheep anti-rat IgG Dynabeads. The remaining cells were cultured with the indicated peptides and IL-2 (20 U/mL) in 96-flat well plates for 4 days. The levels of IFN-γ in the supernatants were determined using an ELISA Development Kit (PeproTech, Rocky Hill, NJ, USA).
Cytotoxicity assays
Spleen cells from RENCA/hCA9-immunized young BALB/c mice were harvested and cultured with the indicated hCA9 peptide (10 μg/mL) and IL-2 (20 U/mL) for 4 days. Cytotoxicity was evaluated by a 6-h 51Cr-release assay.
Immunoblotting
Equal amounts of protein lysates were resolved on 10% SDS-PAGE gels, followed by transfer to polyvinylidene fluoride membranes. After blocking, the blots were incubated with anti-CA9 antibody (#ab184006; Abcam, Cambridge, UK) reactive to both human and mouse CA9, followed by peroxidase-conjugated goat anti-rabbit IgG secondary antibody (#7074; Cell Signaling Technology, Danvers, MA, USA). To detect β-actin, peroxidase-conjugated anti-β-actin antibody (#017–24,573; Wako Pure Chemicals) was used.
Assays of T-cell responses to cell lysates
Spleen cells from RENCA/hCA9-immunzed young BALB/c mice were harvested. Following in vitro removal of CD8+ T cells with anti-CD8 mAb and anti-rat IgG dynabeads, the remaining cells were cultured with lysates of the indicated cells. To prepare cell lysates, 5 × 105 cells in 1 mL were treated by freezing and thawing twice, and 100 μL of cell lysate, corresponding to 5 × 104 cells, was added into each well of 96-flat well plates. After 72 h, the levels of IFN-γ in the supernatants were determined using an ELISA Development Kit (PeproTech).
Immunohistochemistry
Immunohistochemistry was performed as described previously [25]. The sections were reacted with anti-CA9 antibody (#ab184006; Abcam), anti-CD4 antibody (rabbit monoclonal, clone D7D2Z; Cell Signaling Technology), anti-CD8 antibody (rabbit monoclonal, clone D4W2Z; Cell Signaling Technology), anti-Iba1 antibody (rabbit polyclonal; Wako Pure Chemicals), and anti-PD-L1 antibody (goat polyclonal; R&D Systems, Minneapolis, MN, USA), subsequently with horseradish peroxidase-conjugated secondary antibody (Nichirei, Tokyo, Japan). Cells were counted microscopically in four randomly selected high-power fields by two pathologists who were blinded to patient information and background data. Areas of Iba1- and PD-L1-positive staining were evaluated using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Treatment protocols
To examine the combined effects of CP and anti-PD-1/anti-CTLA-4 antibodies, young and aged BALB/c mice were inoculated s.c. into the flank with RENCA (1 × 106) or RENCA/hCA9 (3 × 106) cells. On days 10 and 14 for young and aged mice, respectively, mice received an i.p. injection of 100 mg/kg CP and were injected i.p. with an anti-PD-1 mAb and anti-CTLA-4 mAb (100 μg for young mice and 150 μg for aged mice) 1 and 3 days later. The same volumes of rat IgG and hamster IgG were injected as controls. To examine the combined effects of axitinib and anti-PD-1/anti-CTLA-4 antibodies, axitinib was administered orally at a dose of 30 mg/kg from days 10–20. As a vehicle control, the same volume of DMSO was administered.
Flow cytometry
To analyze tumor-infiltrating immune cells, the tumors were minced using glass slides and passed through gauze mesh and nylon mesh immediately before flow cytometry. The following mAbs were used: APC-conjugated anti-CD45 mAb (BioLegend), FITC-conjugated anti-Gr-1 mAb (BioLegend), PE-conjugated anti-CD11b mAb (BioLegend), FITC-conjugated anti-CD8 mAb (BioLegend), PE-conjugated anti-CD4 mAb (BioLegend), and PE/cy7-conjugated anti-Ly6C mAb (BioLegend). Analysis was performed using the FACSCalibur (Becton–Dickinson, Franklin Lakes, NJ, USA).
Statistical analysis
Data were analyzed using the unpaired two-tailed Student’s t test and the Mann–Whitney U test (between two groups) or multiple comparison Dunnett test (among more than two groups). A P value less than 0.05 was considered statistically significant.
Results
Distinct roles of CD8+ and CD4+ T cells in the rejection of RENCA/hCA9 cells in young mice
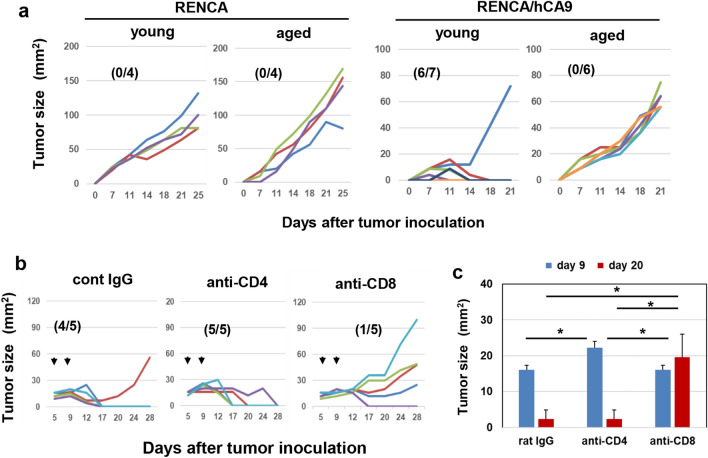
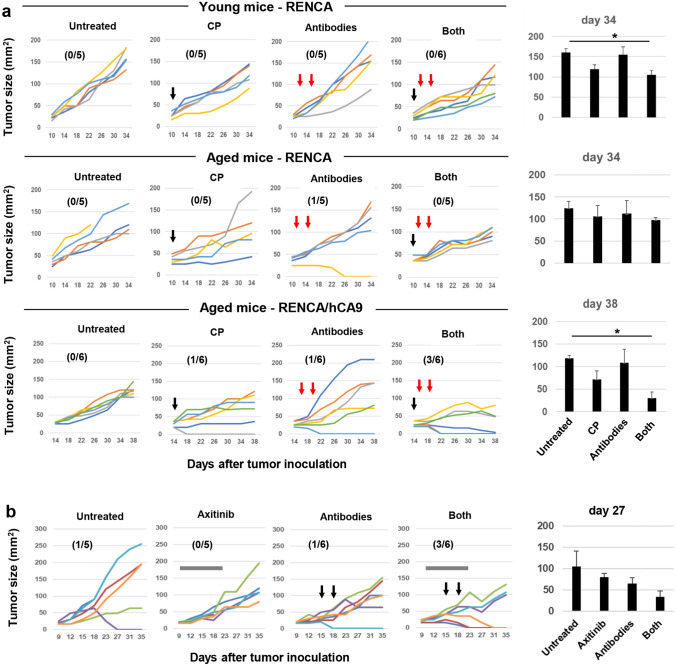
We examined the in vitro growth of RENCA and RENCA/hCA9 cells before examining in vivo growth. The growth of RENCA/hCA9 cells was significantly slower compared with the parental RENCA cells (Fig. S1). Next, we examined in vivo growth in young and aged syngeneic BALB/c mice (Fig. 1a). RENCA cells grew in both young and aged mice. Six of seven young mice rejected RENCA/hCA9 cells, whereas RENCA/hCA9 cells grew in all six aged mice. Given that hCA9 and mouse CA9 do not share 30% of their amino acids (Fig. S2), young mice must reject RENCA/hCA9 cells via recognition of hCA9-derived non-self-antigenic epitopes by T cells.
Fig. 1.
Distinct roles of CD8+ and CD4+ T cells in the rejection of RENCA/hCA9 cells in young mice. a Young and aged mice were inoculated s.c. with RENCA (1 × 106) or RENCA/hCA9 (3 × 106) cells into the flank and tumor sizes (mm2) were measured. Numbers in parentheses indicate rejected mice/total mice. b Five and nine days after s.c. inoculation of RENCA/hCA9 (3 × 106) cells into young mice, anti-CD4 antibody, anti-CD8 antibody, or control rat IgG were injected i.p. at a dose of 150 μg. c Tumor sizes (mm2) were evaluated statistically. Data represent the means ± SEM of five mice. *P < 0.05
Next, we determined the effector cells responsible for the rejection of RENCA/hCA9 cells in young mice. In vivo depletion of CD8+ T cells allowed RENCA/hCA9 cells to grow in four of five young mice, whereas depletion of CD4+ T cells appeared to transiently permit the growth of RENCA/hCA9 cells on day 9 after tumor inoculation (Fig. 1b). Tumor sizes in CD4+ T-cell-depleted mice on day 9 after tumor inoculation were statistically larger than those in control or CD8+ T-cell-depleted mice (Fig. 1c). These results indicate that CD8+ T cells were the main effectors of the rejection of RENCA/hCA9 cells in young mice, and that CD4+ T cells participated at the early stage.
Identification of a hCA9-derived peptide recognized by CD8+ T cells from RENCA/hCA9-bearing young mice
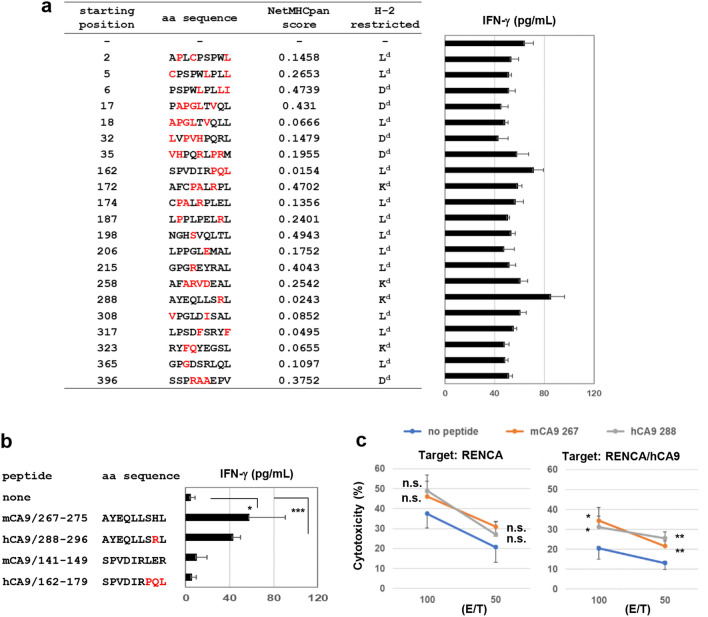
Next, we aimed to identify the peptides recognized by RENCA/hCA9-reactive CD8+ T cells. Based on the H-2d binding motif, 21 peptides were prepared (Fig. 2a). Following in vitro depletion of CD4+ T cells, draining LNs from RENCA/hCA9-bearing young mice were cultured with the indicated peptide individually, and the responses were estimated based on IFN-γ production. The response to the hCA9288–296 peptide (AYEQLLSRL) was higher than any others. Intriguingly, this peptide was one of the peptides used in the anti-cancer vaccine for patients with HLA-A24+ RCC [22]. A similar result was observed when highly purified peptides were used (Fig. 2b). Interestingly, we also observed a response to mouse CA9267–275 peptide (AYEQLLSHL), of which the second amino acid from the C-terminus differs from that of the hCA9288–296 peptide. This result suggests that RENCA/hCA9-reactive CD8+ T cells may respond to both the hCA9288–296 peptide and the mouse CA9267–275 peptide.
Fig. 2.
Identification of an hCA9-derived peptide recognized by CD8+ T cells from RENCA/hCA9-bearing young mice. a Twenty-one peptides with H-2d binding ability were prepared. From draining LN cells of RENCA/hCA9-bearing young mice, CD4+ T cells were depleted in vitro, and the remaining cells were cultured with the indicated peptide and IL-2 (20 U/mL) in 96-well plates. After 3 days, the levels of IFN-γ in the supernatant were measured by ELISA. The amino acids of hCA9 shown in red letters differ from those of mouse CA9. Data represent the means ± SD of 3 wells. b Similar experiments were done using highly purified four peptides. *P < 0.05. ***P < 0.005. c Spleen cells from RENCA/hCA9-immunized young mice were cultured with or without the indicated CA9 peptide (10 μg/mL) in the presence of IL-2 (20 U/mL) for 4 days. Thereafter, the cultured cells were examined for cytotoxicity against RENCA and RENCA/hCA9 cells. Data represent the means ± SD of 3 wells. *P < 0.05, **P < 0.01, n.s., not significant
Cytotoxicity against RENCA and RENCA/hCA9 cells following in vitro stimulation with the hCA9288–296 peptide or the mouse CA9267–275 peptide
Next, we examined the cytotoxicity of T cells, which were stimulated with the hCA9288–296 or mouse CA9267–275 peptide, against RENCA and RENCA/hCA9 cells. The spleen cells from RENCA/hCA9-immunized young mice were stimulated in vitro with the indicated peptide and examined for cytotoxicity against RENCA and RENCA/hCA9 cells (Fig. 2c). When RENCA cells were the target, the spleen cells stimulated with either peptide showed higher levels of cytotoxicity against RENCA cells compared with no peptide stimulation. However, this difference in cytotoxicity was not significant. On the other hand, spleen cells stimulated with either peptide showed significantly higher levels of cytotoxicity against RENCA/hCA9 cells compared with no peptide stimulation, suggesting that both peptides had the potential to induce CTLs reactive to RENCA/hCA9 cells. In addition, spleen cells cultured without peptide showed higher cytotoxicity against RENCA cells than RENCA/hCA9 cells (Fig. S3a). This result suggests that RENCA/hCA9 cells were more resistant to immune cell-mediated cytotoxicity than parental RENCA cells. In support of this, RENCA/hCA9 cells were more resistant to poly(I:C)-augmented NK activity than RENCA cells (Fig. S3b and c).
hCA9-recognizing CD4+ T cells induced in RENCA/CA9-cured young mice
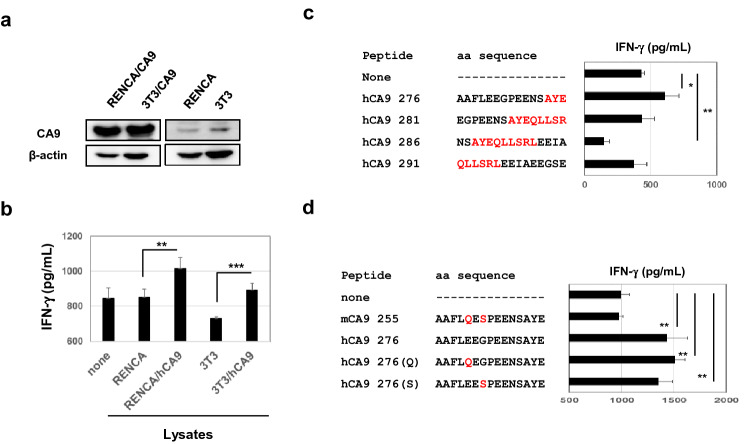
Next, we examined the protein expression of CA9 in RENCA and RENCA/hCA9 cells, as well as 3T3 and 3T3/hCA9, using an antibody that responds to both mouse and human CA9 proteins. This antibody detected high levels of CA9 expression in RENCA/hCA9 and 3T3/CA9 cells, whereas very low levels of CA9 were detected in the parental cell lines RENCA and 3T3 (Fig. 3a). Based on these results, we then determined whether CA9-recognizing CD4+ T cells were induced in RENCA/hCA9-cured young mice. Spleen cells from RENCA/CA9-cured young mice were examined for reactivity against lysates of RENCA, RENCA/hCA9, 3T3, or 3T3/hCA9 cells. The results clearly showed that the spleen cells responded to lysates of RENCA/hCA9 and 3T3/hCA9 cells, but not to those of their parental cell lines (Fig. 3b), indicating that hCA9-recognizing CD4+ T cells were induced in RENCA/hCA9-cured young mice.
Fig. 3.
Identification of an hCA9-derived peptide recognized by mouse CD4+ T cells. a Expression of CA9 in the indicated cell lines was examined using an antibody that recognizes both mouse and human CA9 proteins. β-Actin was used as a control. b To examine the reactivity of CD4+ T cells, spleen cells from RENCA-hCA9-immunized young mice were depleted of CD8+ T cells in vitro and were cultured with the indicated lysates. Data represent the means ± SD of 3 wells. **P < 0.01, ***P < 0.005. c, d Similarly, CD8+ T-cell-depleted spleen cells from RENCA/hCA9-immunized young mice were cultured with the indicated peptide and IL-2 (20 U/mL) in 96-well plates. After 4 days, the level of IFN-γ in the supernatant was measured by ELISA. Data are means ± SD of 3 wells. *P < 0.05. **P < 0.01
Next, we aimed to identify the peptides recognized by RENCA/hCA9-reactive CD4+ T cells. We screened 16 hCA9-derived 15-mer peptides: seven peptides from the N-terminus, five from the C-terminus, and four that encompass the hCA9288–296 peptide. As a result, the hCA9 276–290 peptide was recognized by CD4+ T cells (Fig. 3c, Fig. S4). In experiments with highly purified peptides, CD4+ T cells showed no response to the mCA9 255–279 peptide, whereas substitution of glutamic acid (E) to glutamine (Q) at position 280, or of glycine (G) to serine (S) at position 282, maintained the ability to stimulate CD4+ T cells (Fig. 3d).
RENCA/hCA9 as a non-inflamed ‘cold’ tumor in aged mice
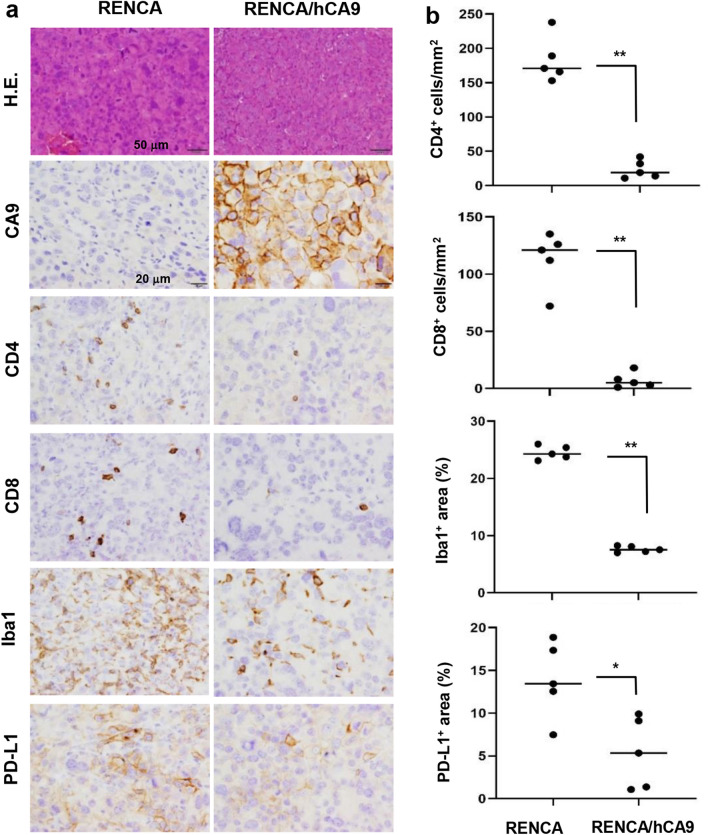
Next, we examined the expression of CA9 and the infiltration of immune cells of RENCA and RENCA/hCA9 tissues in aged mice. RENCA/hCA9 tissues were strongly positive for CA9, while RENCA tissues appeared to be negative (Fig. 4a). In addition, the infiltration of CD4+ T cells, CD8+ T cells, and Iba-1+ macrophages was significantly hampered in RENCA/hCA9 tissues compared with those of RENCA (Fig. 4a, b). Also, the expression of PD-L1 was lower in RENCA/hCA9 tissues. These results indicate that RENCA/hCA9 tumors in aged mice could be regarded as non-inflamed ‘cold’ tumors. Because of the remarkable necrosis seen in RENCA/hCA9 tissues in young mice, evaluation of infiltrating immune cells was difficult (Fig. S5). However, expression of PD-L1 around the margin of the necrotic area appeared to be enhanced.
Fig. 4.
RENCA/hCA9 cells induce non-inflamed tumors in aged mice. a RENCA (1 × 106) or RENCA-hCA9 (3 × 106) cells were inoculated s.c. into the flank of aged mice. On day 20, the tumor masses were removed and examined for the expression of CA9, CD4, CD8, Iba-1, and PD-L1. Scale bar of hematoxylin and eosin staining, 50 μm; scale bar of immunohistochemistry, 20 μm. b Cell density and positive staining were evaluated, and the Mann–Whitney U test was performed. *P < 0.05 **P < 0.01
ICB therapy combined with CP or axitinib against RENCA and RENCA/hCA9
Next, we examined the therapeutic efficacy of ICB therapy combined with CP. First, we s.c. inoculated RENCA into young and aged mice. Monotherapy with CP or ICB antibodies showed no significant antitumor effect on RENCA in young or aged mice, whereas in combination, they significantly suppressed tumor growth to two-thirds that of the untreated group, on day 34 in RENCA-bearing young mice (Fig. 5a, upper). This effect was weaker in RENCA-bearing aged mice (Fig. 5a, middle). In RENCA/hCA9-bearing aged mice, monotherapy with CP or ICB antibodies cured only one of six mice of RENCA/hCA9 tumors, but in combination, they cured three of six (Fig. 5a, lower). The tumor size on day 38 was one-fourth that of the untreated group. Two months after tumor regression, the three cured mice were re-challenged with RENCA/hCA9 cells, which were rejected by all of the mice (Fig. S6).
Fig. 5.
Combined ICB therapy against RENCA/hCA9-bearing aged mice. a (upper and middle) 10 days after s.c. inoculation of RENCA (1 × 106) cells into young and aged mice, CP (100 mg/kg) was i.p. injected. On days 11 and 14, some mice were injected i.p. with anti-PD-1/anti-CTLA-4 antibodies (100 mg for young mice and 150 μg for aged mice). (lower) Fourteen days after s.c. inoculation of RENCA/hCA9 (3 × 106) cells into aged mice, CP (100 mg/kg) was i.p. injected. On days 15 and 18, some mice were injected i.p. with anti-PD-1/anti-CTLA-4 antibodies (150 μg). Numbers in parentheses indicate rejected mice/total mice. The right histograms are the means ± SEM. *P < 0.05 (multiple comparison Dunnett’s test). b Ten days after s.c. inoculation of RENCA/hCA9 (3 × 106) cells into aged mice, daily oral administration of axitinib (30 mg/kg) was performed until day 20. On days 15 and 18, some mice were injected i.p. with anti-PD-1/anti-CTLA-4 antibodies (150 μg). The right histograms are the means ± SEM
Given that the combination of ICB therapy with axitinib, a selective VEGF receptor inhibitor, has been reported to be effective against metastatic RCC patients [26, 27], we tested if this effect could be replicated in our RENCA/hCA9 mouse model. Axitinib alone showed no definite effect on the growth of RENCA/hCA9, and ICB therapy induced tumor regression in only one of six mice (Fig. 5b). Although combination of them cured three of six mice and the tumor size on day 27 was one-third that of the untreated group, no statistical significance in tumor size was observed among the four groups.
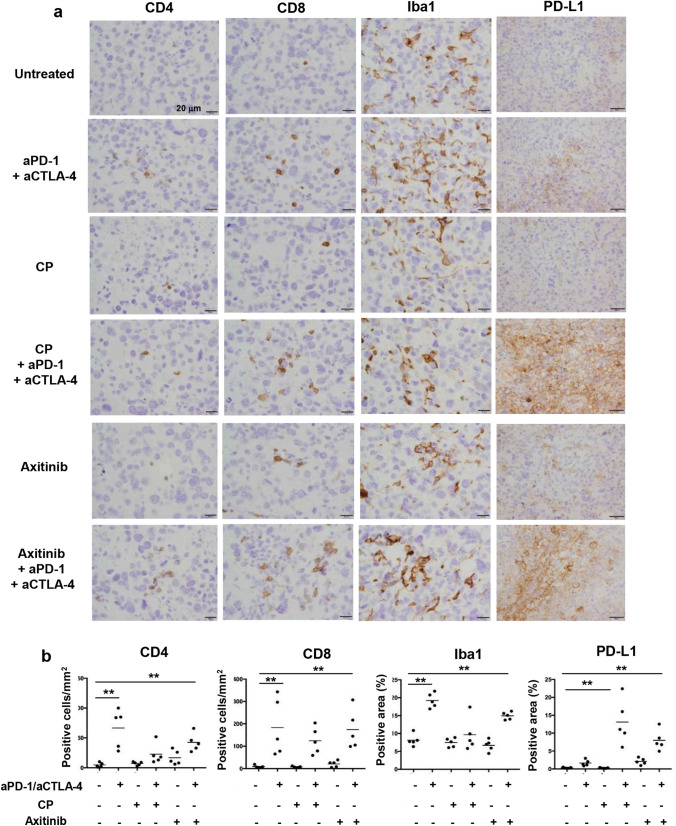
Enhanced expression of PD-L1 in RENCA/hCA9 after ICB therapy with CP or axitinib
Next, we examined the infiltration of immune cells and expression of PD-L1 in RENCA/hCA9 tissues in aged mice after ICB therapy combined with CP or axitinib by immunohistochemistry (Fig. 6a, b). Although ICB therapy alone showed no definite antitumor effect (Fig. 5), the infiltration of CD4+ or CD8+ T cells, and Iba-1+ macrophages, was significantly promoted by the ICB antibodies. Monotherapy with CP or axitinib did not affect the infiltration of these cells. Although monotherapy with ICB, CP, or axitinib exerted no effect on PD-L1 expression in RENCA/hCA9 tissues, its expression was significantly enhanced when ICB therapy was combined with CP or axitinib.
Fig. 6.
Infiltration of immune cells and PD-L1 expression in RENCA/hCA9 tissues after ICB therapy with CP or axitinib. a RENCA/hCA9 (3 × 106) cells were inoculated s.c. into the flank of aged mice. These mice were treated by the protocols described in Fig. 5. On day 20, the tumor masses were removed and examined for the expression of CD4, CD8, Iba-1, and PD-L1. Scale, 20 μm. b Cell density and positive staining area were evaluated by multiple comparison Dunnett’s test. **P < 0.01
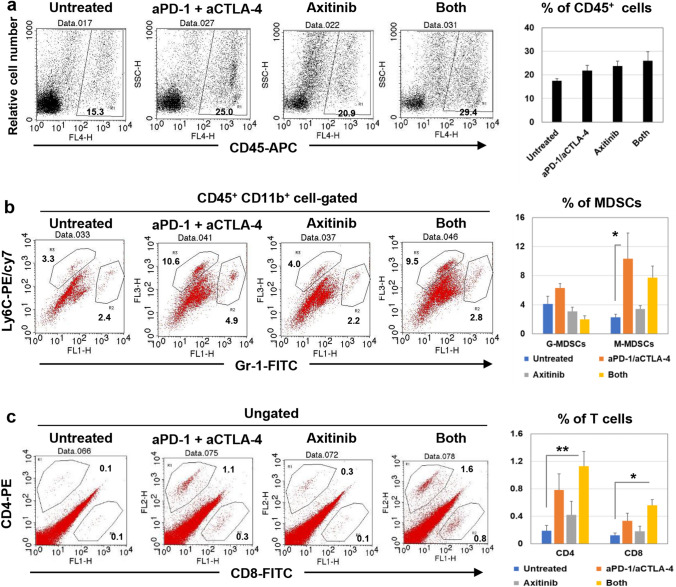
MDSCs and T cells in RENCA/hCA9 tissues in treated aged mice
Myeloid-derived suppressor cells (MDSCs), which exert immunosuppressive effects in cancer-bearing hosts, are divided into two subsets: CD11b+ Gr-1+ Ly6Clow granulocytic MDSCs (G-MDSCs) and CD11b+ Gr-1− Ly6C+ monocytic MDSCs (M-MDSCs) [28]. In addition, MDSCs express the VEGF receptor [29], which is a target of axitinib. Therefore, we next examined the effects of axitinib and/or ICB therapy on MDSCs in the RENCA/hCA9 tissues of aged mice. Either or both of axitinib and ICB therapy increased the proportions of CD45+ immune cells in tumor sites, but not significantly (Fig. 7a). Axitinib alone had no obvious effect on the proportion of MDSCs, but ICB therapy alone significantly increased the proportion of M-MDSC in tumor sites (Fig. 7b). Tumor-infiltrating T cells were also examined. Either ICB therapy or axitinib increased the proportions of CD4+ or CD8+ T cells, but not significantly (Fig. 7c). However, the combination of them significantly increased their proportions.
Fig. 7.
Flow cytometry analysis of immune cells in the RENCA/hCA9 tissues of treated aged mice. Aged mice were s.c. inoculated with RENCA/hCA9 (3 × 106) cells and treated with the same protocol shown in Fig. 5b. On day 19, RENCA/hCA9 tissues were harvested and analyzed by flow cytometry. a The proportion of CD45+ cells is shown. b The proportions of Gr-1+ Ly6Clow G-MDSCs and Gr-1− Ly6C+ M-MDSCs among CD45+ CD11b+ cells are shown. c The proportions of CD4+ and CD8+ T cells are shown. The data are mean ± SEM of four mice. Percentages are also shown. * P < 0.05, ** P < 0.01 (Dunnett’s test for multiple comparisons)
Discussion
Although RCC benefits from ICB therapy [9], only one study has shown T-cell reactivity to neoantigens of human RCC [11], and to the best of our knowledge, there has been no such report in preclinical models. In this study, we used RENCA/hCA9 cells as a model of neoantigen-expressing RCC in syngeneic mice. To date, several reports have demonstrated the growth of hCA9-expressing RENCA cells in syngeneic young mice [30–34]. However, our RENCA/hCA9 cells were rejected in young mice (Fig. 1a), probably because of their high expression of hCA9 (Fig. 3a). In this regard, we measured CA9 expression in RENCA/hCA9 cells and two human RCC lines, which were treated with or without desferrioxamine (DFO), an iron chelator that mimics hypoxia (Fig. S7). RENCA/hCA9 cells expressed more CA9 than the human RCC lines, irrespective of DFO treatment, suggesting that the expression level of CA9 in RENCA/hCA9 cells was relatively high. On the other hand, although head-to-head comparison of hCA9-expressing RENCA cells was not feasible, the observed different immunogenicity may be specific for their transgenic line and number of cells injected. One of the most likely explanation might be that we used high cell doses but that other investigators used substantial lower cell numbers to initiate tumor growth in vivo. The use of high cell numbers may have led to substantial cell necrosis, probably inducing or enhancing the anti-hCA9 response.
Young and aged mice showed distinct T-cell responses to RENCA/hCA9. In this regard, we suppose that this difference was due to impaired T-cell responses in aged compared with young mice. Indeed, we previously compared the immunological status of young and aged BALB/c mice [35]. The serum levels of the proinflammatory cytokines IL-6 and TNF-α were higher in aged than young BALB/c mice, and the efficacy of anti-cancer vaccination using inactivated cancer cells was low in aged mice. In addition, the proportion of B220+ B cells was higher in aged than young mice, while that of CD8+ T cells was lower. The proportions of CD4+ T and Treg cells did not differ. Three types of myeloid cells (G-MDSCs, M-MDSCs, and monocytes) tended to increase in the spleens of aged mice. In this study, we newly examined the proportions of naïve and effector/memory T cells; as expected, the proportion of CD44− CD62L+ (naïve) T cells decreased, but that of CD44+ (effector/memory) T cells increased, in aged mice (Fig. S8). These findings indicate that older mice may be less able to respond to unencountered antigens than young mice.
Young, but not aged, mice rejected RENCA/hCA9 (Fig. 1a). We suppose that rejection or acceptance of cancer cells could be determined by a balance between the immunogenicity of cancer cells and the immunological status of hosts. hCA9-expressing RENCA is highly immunogenic; younger mice exhibited potent T-cell responses to hCA9, which induced severe cell death/necrosis in RENCA/hCA9 tissues (Fig. S5). On the other hand, as we explain above, the immunological status (especially T-cell potency) of aged mice is impaired, which must be associated with reduced induction of anti-hCA9 T cells. Thus, T cells did not infiltrate the tumor sites, and RENCA/hCA9 in aged mice served as a non-inflamed ‘cold’ tumor.
The hCA9288–296 peptide (AYEQLLSRL) was recognized by mouse CD8+ T cells. This peptide is identical to one that is used in anti-cancer vaccine therapy for patients with HLA-A24+ RCC [22]. This coincidence could have arisen because HLA-A24 and H-2Kd molecules share a binding motif [36]. Unexpectedly, the mouse CA9267–275 peptide (AYEQLLSHL) was also recognized by mouse CD8+ T cells. This peptide differs from the hCA9288–296 peptide in the second amino acid from the C-terminus. The amino acid at this position might not influence recognition by hCA9-recognizing CD8+ T cells. Alternatively, the levels of cytotoxicity against RENCA/hCA9 following in vitro stimulation with the hCA9288–296 peptide or the mouse CA9267–275 peptide were significant but low (Fig. 2c, Fig. S3a). We suggest two possibilities for this. First, given the similar reactivity to the hCA9288–296 and mouse CA9267–275 peptides (Fig. 2b), CTLs with high avidity to the hCA9288–296 peptide might be deleted by negative selection in the thymus of mice. Second, the expression of hCA9 rendered RENCA cells more resistant to immune cell-mediated cytotoxicity. Actually, RENCA/hCA9 cells were more resistant to poly (I:C)-augmented NK activity compared with RENCA cells (Fig. S3c).
In the immunoblot assays, RENCA/hCA9 cells were highly positive for CA9, whereas both parental RENCA cells were slightly positive for CA9 (Fig. 3a). This suggests that RENCA cells expressed mouse CA9 only at a very low level. On the other hand, the same antibody clearly detected the expression of CA9 in RENCA/hCA9, but not RENCA, tissues (Fig. 4a), probably because the antibody could not detect the low expression of mouse CA9 in immunohistochemical samples. Importantly, CD4+ T cells from RENCA/hCA9-cured young mice responded to lysates of RENCA/hCA9 and 3T3/hCA9 cells, but not to those of the parental cells RENCA and 3T3 (Fig. 3b), indicating that hCA9-recognizing CD4+ T cells were generated in RENCA/hCA9-cured young mice. Because CD4+ T cells are essential for maturation of dendritic cells and cross-priming of tumor-specific CD8+ T cells [37], hCA9-recognizing CD4+ T cells might increase the antigen-presenting ability of dendritic cells and thereby promote induction of hCA9-recognizing CD8+ T cells. Indeed, CD4+ T-cell neo-epitope vaccination can reshape the tumor microenvironment and induce CD8+ T cells against another antigen in mice, indicating the induction of antigen-spreading [38]. In addition, both neoantigen-recognizing CD8+ T cells and CD4+ T cells can exert a greater antitumor effect in vivo compared with neoantigen-recognizing CD8+ T cells alone [39]. To this end, we tried to identify hCA9-derived peptides recognized by mouse CD4+ T cells and found that the hCA9 276–290 peptide was recognized by mouse CD4+ T cells (Fig. 3c). Interestingly, the C-terminus of the hCA9276–290 peptide shares three amino acids with the hCA9 288–296 peptide. The hCA9 276–296 peptide contains epitopes for both CD8+ and CD4+ T cells. We plan to apply the hCA9 276–296 peptide as a neoantigen peptide vaccine in our RENCA/hCA9-bearing aged mice model.
The infiltration of CD4+ and CD8+ T cells, and Iba1+ macrophages was significantly suppressed in RENCA/hCA9 tissues compared with RENCA tissues in aged mice, and the expression of PD-L1 in RENCA/hCA9 tissues was low compared with RENCA tissues (Fig. 4). The difference could be explained by adaptive immune resistance [40]. Therefore, RENCA/hCA9 can be considered as a non-inflamed ‘cold’ tumor. Clinically, ‘cold’ tumors have been obstacles in improving the therapeutic efficacy of ICB therapy [41, 42]. To this end, we examined the therapeutic efficacy of combined therapy with anti-PD-1/anti-CTLA-4 antibodies with CP or axitinib, because we observed combined effects of CP and ICB therapy in several mouse models [43] and because the combination of ICB therapy with axitinib was effective for the treatment of patients with RCC [26, 27]. As a result, the combined ICB therapy with CP or axitinib induced a complete cure in half of the RENCA/hCA9-bearing aged mice (Fig. 5a, b). In terms of the mechanisms involved, given that the presence of antitumor T cells in tumor sites is essential for the therapeutic efficacy of ICB therapy [44], we supposed that CP or axitinib promoted the infiltration of antitumor T cells into tumor sites. That is, CP mitigated Treg-mediated immunosuppression and induced immunogenic cancer cell death [45, 46], while axitinib targeted the VEGF receptor and thereby promoted infiltration of immune cells into tumor sites [47]. Unexpectedly, the infiltration of immune cells was promoted only by ICB therapy (Fig. 6), whereas no definite antitumor effect was observed in RENA/hCA9-bearing aged mice (Fig. 5). However, additional combination with CP or axitinib caused tumor regression in half of the mice with the enhanced expression of PD-L1 in RENCA/hCA9 tissues (Fig. 6). Given that this enhanced expression of PD-L1 could reflect adaptive immune resistance, tumor-infiltrating T cells in responder mice after combined ICB therapy with CP or axitinib may acquire the potential to produce cytokines, such as IFN-γ. We plan to characterize these T cells in the next study.
Because axitinib targets VEGF receptor [47] and MDSCs express this receptor [29], we examined the effects of axitinib on MDSCs in the RENCA/hCA9 tissues of aged mice. Axitinib showed no definite effect on the proportion of MDSCs in tumor sites of aged mice. However, the proportions of CD4+ or CD8+ T cells in tumor sites increased significantly when axitinib was combined with ICB antibodies (Fig. 7c). These results confirm the in vivo antitumor effect induced by this combination (Fig. 5b). Given that axitinib can induce differentiation of M-MDSCs toward antigen-presenting phenotype [47], differentiated M-MDSCs might promote antitumor T-cell responses. Further studies are required to elucidate the underlying mechanism.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported in part by JSPS KAKENHI Grants (No. 17K07217 to M Harada, No. 17K11301 to K Yoshikawa, and No. 19H03794 for H Uemura), Suzuken Memorial Foundation to Y Iida, and SHIMANE “SUIGAN” Project to M Harada.
Declarations
Conflicts of interest
The authors have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian S, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19(19):5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 4.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turajlic S, Litchfield K, Xu H, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 2017;18(8):1009–1021. doi: 10.1016/S1470-2045(17)30516-8. [DOI] [PubMed] [Google Scholar]
- 11.Hansen UK, Ramskov S, Bjerregaard AM, et al. Tumor-infiltrating T cells from clear cell renal cell carcinoma patients recognize neoepitopes derived from point and frameshift mutations. Front Immunol. 2020;11:373. doi: 10.3389/fimmu.2020.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawagoe N, Shintaku I, Yutani S, Etoh H, Matsuoka K, Noda S, Itoh K. Expression of the SART3 tumor rejection antigen in renal cell carcinoma. J Urol. 2000;164(6):2090–2095. doi: 10.1016/S0022-5347(05)66975-3. [DOI] [PubMed] [Google Scholar]
- 13.Flad T, Spengler B, Kalbacher H, et al. Direct identification of major histocompatibility complex class I-bound tumor-associated peptide antigens of a renal carcinoma cell line by a novel mass spectrometric method. Cancer Res. 1998;58(24):5803–5811. [PubMed] [Google Scholar]
- 14.Hanada K, Yewdell JW, Yang JC. Immune recognition of a human renal cancer antigen through post-translational protein splicing. Nature. 2004;427(6971):252–256. doi: 10.1038/nature02240. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi Y, Harashima N, Kajigaya S, et al. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J Clin Invest. 2008;118(3):1099–1109. doi: 10.1172/JCI34409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minami T, Minami T, Shimizu N, Yamamoto Y, De Velasco M, Nozawa M, Yoshimura K, Harashima N, Harada M, Uemura H. Identification of erythropoietin receptor-derived peptides having the potential to induce cancer-reactive cytotoxic T lymphocytes from HLA-A24+ patients with renal cell carcinoma. Int Immunopharm. 2014;20(1):59–65. doi: 10.1016/j.intimp.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Minami T, Minami T, Shimizu N, Yamamoto Y, De Velasco M, Nozawa M, Yoshimura K, Harashima N, Harada M, Uemura H. Identification of programmed death ligand 1-derived peptides capable of inducing cancer-reactive cytotoxic T lymphocytes from HLA-A24+ patients with renal cell carcinoma. J Immunother. 2015;38(7):285–291. doi: 10.1097/CJI.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 18.Minami T, Matsumura N, Sugimoto K, Shimizau N, De Velsasco Nozawa M, Yoshimura K, Harashima N, Harada M, Uemura H. Hypoxia-inducing factor (HIF)-1a-derived peptide capable of inducing cancer-reactive cytotoxic T lymphocytes from HLA-A24+ patients with renal cell carcinoma. Int Immunopharm. 2017;44:197–202. doi: 10.1016/j.intimp.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Komohara Y, Harada M, Ishihara Y, Suekane S, Nogichi M, Yamada A, Itoh K. HLA-G as a target molecule in specific immunotherapy against renal cell carcinoma. Oncol Rep. 2007;18(6):1463–1468. [PubMed] [Google Scholar]
- 20.Pantuck AJ, Zeng G, Belldegrun AS, et al. Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res. 2003;9(13):4641–4652. [PubMed] [Google Scholar]
- 21.Uemura H, Okajima E, Debruyne FM, Oosterwijk E. Internal image anti-idiotype antibodies related to renal-cell carcinoma-associated antigen G250. Int J Cancer. 1994;56(4):609–614. doi: 10.1002/ijc.2910560424. [DOI] [PubMed] [Google Scholar]
- 22.Uemura H, Fujimoto K, Tanaka M, Yoshikawa M, Hirao Y, Uejima S, Yoshikawa K, Itoh K. A phase I trial of vaccination of CA9-derived peptides for HLA-A24-positive patients with cytokine-refractory metastatic renal cell carcinoma. Clin Cancer Res. 2006;12(6):1768–1775. doi: 10.1158/1078-0432.CCR-05-2253. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu K, Uemura H, Yoshikawa M, Yoshida K, Hirao Y, Iwashima K, Saga S, Yoshikawa K. Induction of antigen specific cellular immunity by vaccination with peptides from MN/CA IX in renal cell carcinoma. Oncol Rep. 2003;10(5):1307–1311. [PubMed] [Google Scholar]
- 24.Lustgarten J. Cancer, aging and immunotherapy: lessons learned from animal models. Cancer Immunol Immunother. 2009;58(12):1979–1989. doi: 10.1007/s00262-009-0677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawa T, Ohnishi K, Kosaki Y, Saito Y, Horlad H, Fujiwara Y, Takeya M, Komohara Y. Optimum immunohistochemical procedures for analysis of macrophages in human and mouse formalin fixed paraffin-embedded tissue samples. J Clin Exp Hematop. 2017;57(1):31–36. doi: 10.3960/jslrt.17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 28.Youn JI, Nagaraj S, Collazo M, Gabrilovich Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;3(9):978. doi: 10.3389/fimmu.2018.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai D, Shan H, Wang G, et al. Combining DNA vaccine and AIM2 in H1 nanoparticles exert anti-renal carcinoma effects via enhancing tumor-specific multi-functional CD8+ T-cell responses. Mol Cancer Ther. 2019;18(2):323–334. doi: 10.1158/1535-7163.MCT-18-0832. [DOI] [PubMed] [Google Scholar]
- 31.Shvarts O1, Janzen N, Lam JS, Leppert JT, Caliliw R, Figlin RA, Belldegrun AS, Zeng G. RENCA/carbonic anhydrase-IX: a murine model of a carbonic-IX-expressing renal cell carcinoma. Urology. 2006;68(5):1132–1138. doi: 10.1016/j.urology.2006.08.1073. [DOI] [PubMed] [Google Scholar]
- 32.Kim BR, Yang EK, Kim DY, Kim SH, Moon DC, Lee JH, Kim HJ, Lee JC. Generation of anti-tumour immune response using dendritic cells pulsed with carbonic anhydrase IX-Acinetobacter baumannil outer membrane protein A fusion proteins against renal cell carcinoma. Clin Exp Immunol. 2012;167(1):73–83. doi: 10.1111/j.1365-2249.2011.04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbert N, Haferkamp A, Schmitz-Winnenthal HF, Zöller M. Concomitant tumor and autoantigen vaccination supports renal cell carcinoma rejection. J Immunol. 2010;185(2):902–916. doi: 10.4049/jimmunol.0902683. [DOI] [PubMed] [Google Scholar]
- 34.Birkhäuser FD, Koya RC, Neufeld C, et al. Dendritic cell-based immunotherapy in prevention and treatment of renal cell carcinoma: efficacy, safety, and activity of Ad-GM·CAIX in immunocompetent mouse models. J Immunother. 2013;36(2):102–111. doi: 10.1097/CJI.0b013e31827bec97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishikawa S, Matsui Y, Wachi S, Yamaguchi H, Harashima N, Harada M. Age-associated impairment of antitumor immunity in carcinoma-bearing mice and restoration by oral administration of Lentinula edodes mycelia extract. Cancer Immunol Immunotherapy. 2016;65(8):961–972. doi: 10.1007/s00262-016-1857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rammensee HG. Chemistry of peptides associated with MHC class I and class II molecules. Curr Opin Immunol. 1995;7(1):85–96. doi: 10.1016/0952-7915(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 37.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2(11):1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 38.Kreiter S, Vormehr, van de Romer N, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520(7780):692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alspach E, Lussier DM, Miceli AP, et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574:696–701. doi: 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonaventura P, Shekarian T, Alcazer V, et al. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 43.Iida Y, Harashima N, Motoshima T, et al. Contrasting effects of cyclophosphamide on anti-CTLA-4 blockade therapy in two tumor mouse models. Cancer Sci. 2017;108(10):1974–1984. doi: 10.1111/cas.13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 45.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 46.Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol. 2011;33(4):369–368. doi: 10.1007/s00281-011-0245-0. [DOI] [PubMed] [Google Scholar]
- 47.Four SD, Maenhout SK, Pierre KD, Renmans D, Niclou SP, Thielemans K, Neyns B, Aerts JL. Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic MDSCs in an intracranial mouse melanoma model. Oncoimmunol. 2015;22(4):e998107. doi: 10.1080/2162402X.2014.998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.