Abstract
CD47 is over-expressed in Acute Myeloid Leukemia (AML) and functions as an inhibitory signal, suppressing phagocytosis by binding to signal regulatory protein α (SIRPα) on the surface of macrophages. Inhibition of CD47 restores the immune surveillance of AML cells. However, the inhibition of CD47 in AML by activated macrophages and the subsequent effects on different immune response parameters are not fully understood. Here, we demonstrate the use of a distinct co-culture method to inhibit CD47 and therefore eliminate AML cells by macrophages in vitro. Human chemically induced THP-1 macrophages were activated using different concentrations of lipopolysaccharide (LPS) and co-culturing with three AML cancer cell lines (HL-60, NB4, and THP-1), respectively, as well as normal human peripheral blood mononuclear cells (PBMC). CD47 inhibition was observed in and selective to AML but not observed in normal PBMC. Additionally, calreticulin (CRT) levels were elevated in the same cell lines simultaneously, after co-culturing with activated human macrophages, but not elevated in normal cells. We also show that the activated macrophages secreted high levels of cytokines, including IL-12p70, IL-6, and TNF-α, consistent with the elimination of AML by macrophages. Our study reveals the potential of this model for screening new drugs against AML and the possibility of using human macrophages in AML treatment in the future.
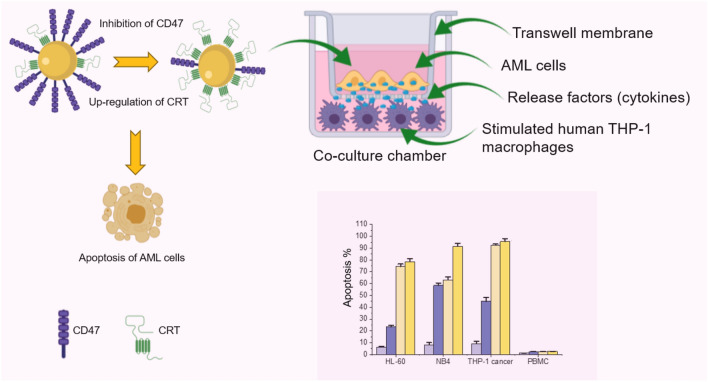
Graphic abstract
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02728-z) contains supplementary material, which is available to authorized users.
Keywords: Acute myeloid leukemia (AML), CD47, Calreticulin, Lipopolysaccharide, Stimulated-macrophage phagocytosis, Co-culture
Introduction
Acute myeloid leukemia (AML) is an aggressive malignancy with a generally poor prognosis. Chemotherapy may provide complete remission in most of AML patients (50–80%). Yet, relapse rate is high (60–90%) with approximate 5-year survival rate of 27% [1–3]. Therefore, there is a need to explore other treatment options for AML to increase the survival rate and improve overall prognosis.
Tumor immune surveillance is a way by which the immune system identifies and eliminates tumor cells based on the expression of tumor-specific antigens on their surfaces. This concept was introduced over a century ago [4]. Macrophages are the main cells involved in tumor elimination and have been shown only recently to play a vital role in regulating tumor pathogenesis [5]. In addition, macrophages mediate tumor cell elimination by production of cytokines and mainly by phagocytosis [6, 7]. AML, as is true for many types of cancers, can evade macrophage phagocytosis through the up-regulation of CD47, the “don’t eat me signal,” on their surface, whose expression is associated with a worse clinical prognosis in adult AML patients [7–9].
The phagocytic activity of macrophages can be regulated by either inhibition of the CD47 or activation of the “eat me signal,” calreticulin (CRT) [10]. CD47 is a transmembrane glycoprotein that functions as a critical inhibitory signal, suppressing phagocytosis by binding to signal regulatory protein alpha (SIRPα) on the surface of macrophages. When activated, SIRPα recruits a signal transduction cascade, resulting in inhibition of phagocytosis [11, 12]. Many studies have reported that blocking CD47 expression in many types of cancer cells, including AML, leads to phagocytic uptake of tumor cells by macrophages [13–15]. The blocking of CD47 may result in increased levels of “eat me signal” CRT, which is necessary for macrophage recognition and, therefore, triggers CRT-mediated phagocytosis [16–18].
A growing body of evidence has shown that M1 macrophages activated by lipopolysaccharides (LPS) and other pro-inflammatory cytokines (e.g., IFN-γ) kill tumor cells, inhibit angiogenesis, and improve prognosis by macrophage elimination of cancer cells [19, 20]. LPS is a bacterial, potent toxin, and it has been associated with initiation of inflammation [20, 21]. Many studies have reported the elimination of cancer cells using in vitro induced macrophage models [15, 17, 22]. However, vital immune parameters associated with this induction, specifically in AML, are still not fully elucidated and are the focus of this report.
Herein, a novel model to simultaneously inhibit CD47 and elevate CRT expression levels for elimination of AML is demonstrated using human macrophage and cancer cell co-culture. LPS at different concentrations (0–100 ng/mL) was employed. Several immune response parameters and related biomarker expressions were evaluated. The results obtained here offer a novel co-culture method useful for screening drugs against AML.
Materials and methods
Reagents
Dulbecco’s Modified Eagle’s Medium (DMEM), Iscove’s Modified Dulbecco’s Medium (IMDM), Hank’s Balanced Salt Solution (HBSS), Phosphate Buffered Saline (PBS) 1X solution (pH 7.4), Fetal Bovine Serum (FBS), penicillin–streptomycin antibiotics, and 0.5% trypsin EDTA (1X Trypsin) were purchased from Gibco (Thermo Fisher Scientific, Canada). Phorbol 12-myristate 13-acetate (PMA), lipopolysaccharide (LPS) from Escherichia coli (O127:B8), 6.5 mm Transwell permeable, 0.40 µm pore polyester membrane supports, and a Milliplex human high-sensitivity T-cell magnetic bead panel kit were products of Sigma (Canada). A PE-Annexin V Apoptosis Detection Kit and an APC-conjugated antihuman CD14 antibody were purchased from BD-Biosciences (Canada). FITC- or PE-conjugated antihuman CD11b, PE-conjugated antihuman CD47, cell staining buffer, and human TruStain FcX™ (Fc Receptor Blocking Solution) were purchased from BioLegend (USA). Alexa Fluor® 488 anti CRT antibody was obtained from Abcam (Canada).
Cell culture and THP-1 differentiation
Promyeloblast, acute promyelocytic leukemia (HL-60); monocyte, acute monocytic leukemia (THP-1); promyeloblast, acute promyelocytic leukemia with t(15;17) translocation marker (NB4); macrophage—Abelson murine leukemia virus transformed (Raw 264.7); and normal human Primary Peripheral Blood Mononuclear Cells (PBMC) were purchased from American Type Culture Collection (ATCC, USA).
THP-1, Raw 264.7, and NB4 cell lines were cultured in DMEM. PBMC were maintained in HBSS. FBS (10%) and 100 U/ml penicillin/streptomycin were added to the media of all the above cell lines. HL-60 cells were cultured in IMDM supplemented with 20% FBS and 100 U/ml penicillin/streptomycin. All cells were cultured in a 5% CO2, 95% humidified incubator at 37 °C.
THP-1 cells were differentiated into macrophage-like cells by the addition of PMA [23, 24]. Briefly, 4 − 5 × 105 cells were plated in 6-well tissue culture plate, and then, PMA (5 ng/mL) was added to the cells and incubated overnight at 37 °C. Cells were then washed twice with PBS, and fresh medium was added to the cells prior to LPS stimulation. Cell differentiation was assessed by monitoring the morphology of the cells using an optical microscope (ECHO- Revolve, USA), and quantification of the surface markers CD11b and CD14 was carried out by flow cytometry analysis.
Co-culture experiments and LPS stimulation
Suspension AML cells (HL-60, NB4, and THP-1) were co-cultured with either differentiated THP-1 macrophage or Raw 264.7 cells using 0.40 µm Transwell polyester membrane inserts into 6-well culture plate. Suspension cells (1 − 2 × 105) were plated on the membrane inserts (upper chamber), and differentiated THP-1 cells or Raw 264.7 (4 − 5 × 105) were plated in the lower chamber. All cells were allowed to grow overnight to reach confluency. Next, LPS was added to the macrophage cells (differentiated THP-1 and Raw 264.7 cells) at concentrations of 0, 5, 20, and 100 ng/mL and incubated overnight. As control experiments, suspension cells were seeded in the same way as mentioned above, but in 6-well culture plate in the absence of co-culture with no differentiated THP-1 cells or Raw 264.7, and they were treated with the same concentrations of LPS used in the co-culture experiment.
Flow cytometry and cell viability analysis
After LPS stimulation, suspension AML cells (from the upper chamber) were harvested and adherent macrophages cells (from the base of the lower chamber) were obtained by trypsin digestion (1X Trypsin for 5 min). All cells (cancer and macrophages) were then washed with cell staining buffer twice (10 mL each time) and centrifuged at 400 g for 5 min, respectively. Next, 1 × 105 cancer cells and 1 × 105 macrophages were re-suspended in 100 μL of cell staining buffer. To prevent non-specific binding, both cancer cells and macrophages were blocked with Human TruStain FcX™ (5 µL) for 15 min at room temperature. Subsequently, the cancer suspension cells were stained with both PE-conjugated antihuman CD47 and Alexa Fluor® 488 conjugated antihuman CRT antibodies simultaneously. Macrophages (differentiated THP-1 cells and Raw 264.7, respectively) were stained with both APC-conjugated antihuman CD14 and Alexa Fluor® 488 conjugated antihuman CRT at the same time.
Afterwards, stained cancer cells and THP-1 macrophages were incubated separately at room temperature for 30 min in the dark on a shaker (Boekel Scientific, USA) at 30 rpm. All cells were washed with cell staining buffer (1 mL) twice and centrifuged at 400 g for 5 min, and re-suspended in 0.5 mL of cell staining buffer containing 1% formaldehyde, and were ready for flow cytometry analysis (BD FACSCanto, USA).
Control measurements to determine the levels of CD47, CRT, and CD14 on suspension cells without co-culturing were carried out by harvesting the cells followed by staining cells with the same antibodies used in the co-culture experiment. To test macrophage surface markers, differentiated THP-1 cells were stained with both APC-conjugated antihuman CD14 and PE-conjugated antihuman CD11b antibodies following the aforementioned protocol.
To determine the percentage of CD47 down-regulation in cancer cells, the following formula was used:
where the mean of fluorescence intensity (MFI) of cancer cells at the target concentration (5, 20, or 100 ng/mL) of LPS is subtracted from the MFI of cancer cells at 0 ng/mL of LPS, and this difference in MFI is divided by the MFI of cancer cells at 0 ng/mL of LPS multiplied by 100%.
To evaluate cell viability, the Annexin V viability assay was used according to the manufacturer’s instructions. Briefly, all cells were harvested as mentioned above and washed twice with cold PBS. They were then re-suspended in 1X Binding Buffer (provided in PE-Annexin V Apoptosis Detection Kit) at a concentration of 1 × 105 cells/100 µL. Next, 5 μL of PE-Annexin V and 5 μL 7-AAD were added to sample and incubated for 15 min at room temperature in the dark on a shaker at 30 rpm. 400 μL of 1X Binding Buffer was then added to sample prior to the flow cytometry measurements.
Measuring cytokine levels
The concentrations of IFN-γ, IL-10, IL-12p70, IL-1β, IL-2, IL-6, IL-8, and TNFα in cell culture supernatants were measured using a commercially available, Milliplex, human high-sensitivity T-cell magnetic bead panel kit, following the manufacturer’s instructions. The fluorescence intensity was detected using the MAGPIX® system (Luminex, USA). The standard kit and cell samples were added in duplicate wells. Cytokine concentrations were calculated after the collection of standard curves and used as the protein level (pg/mL). The data were processed using Milliplex analyst software (version 5.1 Flex, VigeneTech, USA).
Statistical analysis
All data are presented as mean ± SEM. Experiments in this study were performed at least three times in duplicate. All statistical analysis and graphical displays were performed with OriginPro 2019 (USA). Differences between two experimental conditions were analyzed using independent two-sample t test, and differences among more than three conditions were analyzed using one-way ANOVA test. A value of P < 0.05 was considered statistically significant.
Results
THP-1 cells exhibit macrophage-like phenotype with increased expression of CD11b and CD14
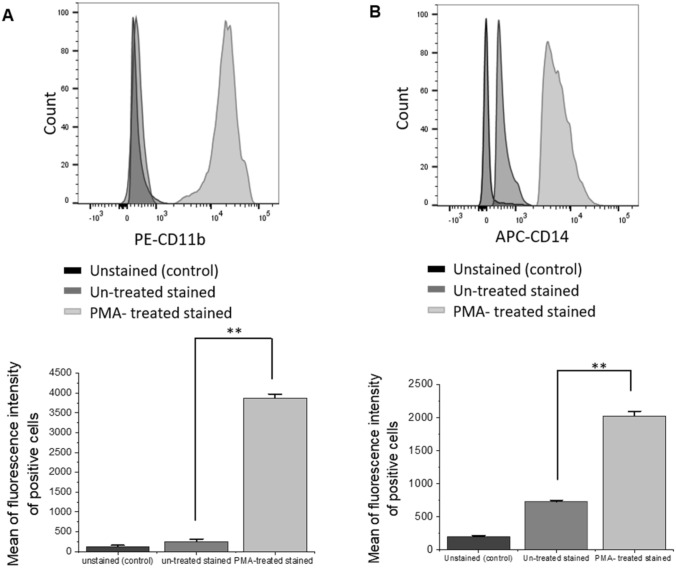
Human THP-1 cells were differentiated into macrophage-like cells (THP-1 macrophages) by incubation in the presence of PMA (5 ng/mL). Cell phenotype and morphological features, including both shape and size, were examined after the first 4 h of incubation and after overnight incubation. Cell surface expression levels of CD11b and CD14 were analyzed before and after PMA treatment by flow cytometry.
The PMA addition to THP-1 cells results in their differentiation into M1 macrophage-like cells, characterized by changes in morphology and increased cell surface expression of CD11b and CD14 [23]. In this study, the microscopy results showed changes in cell morphology, internal structure, and appearance over time (see Supporting Information Fig. S1A-S1C). Flow cytometry analysis revealed an increase in cell surface expression levels of CD11b and CD14 of PMA-treated THP-1 cells compared to non-treated (Fig. 1a, b), indicating differentiation to macrophage-like cells [24, 25]. These cells were then used as the human macrophages model to study the elimination of AML in co-culture.
Fig. 1.
Flow cytometric analysis of THP-1 cells exhibits increases of surface marker expression of both CD11b and CD14, after PMA treatment. The expression levels of CD11b (a) and CD14 (b) were assessed by incubating PE-conjugated antihuman CD11b and APC-conjugated antihuman CD14 with non-treated THP-1 and PMA-treated THP-1. Representative graphs of the mean of fluorescence intensity of CD11b+ and CD14+ indicate the change in CD11b (a) and CD14 (b), levels before and after PMA treatment. Un-stained THP-1 cells were used as a control. Values in the graphs are shown as means ± SEM of three trials of duplicate samples. The statistical significance was determined by independent two-sample t test (OriginPro 2019). ** P < 0.01
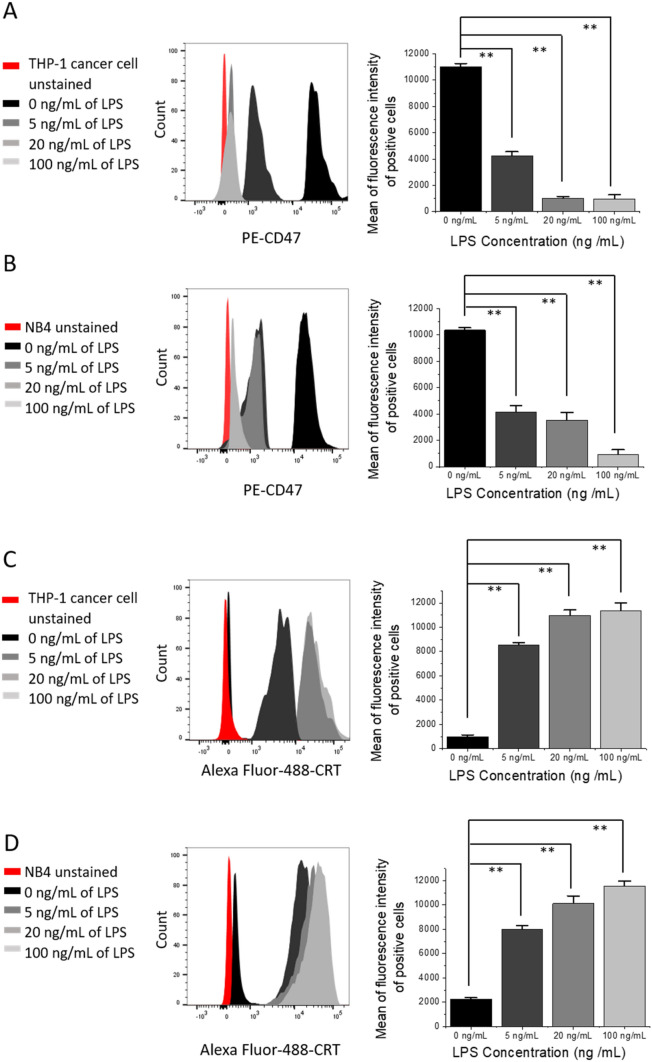
Simultaneous down-regulation of CD47 and increased CRT expression on HL-60 leukemia cell in co-culture
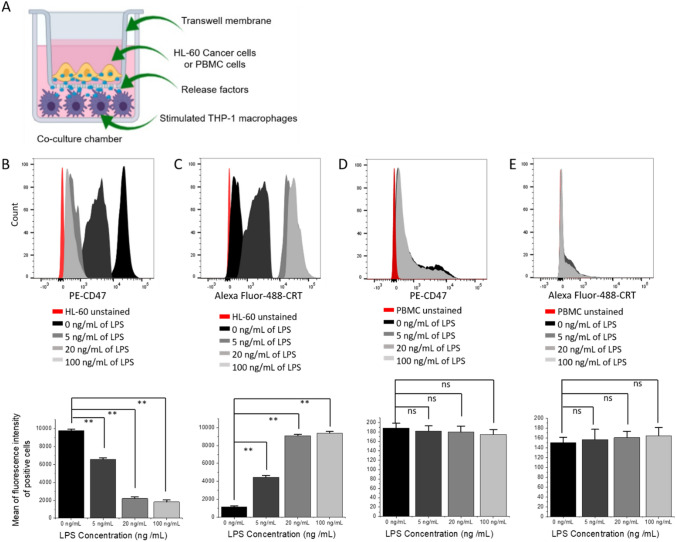
In this study, our hypothesis stated that the down-regulation of CD47 expression in leukemia cells (e.g., via LPS activation of macrophages) could increase their elimination by macrophages in co-culture. To test this hypothesis, human HL-60 cells were first co-cultured with THP-1 macrophages in paired chambers (Fig. 2a). LPS at 0, 5, 20, and 100 ng/mL was added to the bottom chambers containing THP-1 macrophages cells and incubated for 16–18 h (overnight). All cells were then harvested and the levels of CD47 and CRT on HL-60 after co-culture were assessed using flow cytometry. As described earlier, PMA was removed by adding fresh medium to culture (after washing with PBS) prior to the addition of LPS.
Fig. 2.
Levels of CD47 and CRT in HL-60 and PBMC after co-culturing with stimulated THP-1 macrophages. a Scheme of the THP-1 macrophages co-culturing with HL60 or PBMC in a co-culture chamber. Flow cytometric analysis of CD47 b and CRT c in HL-60 cells; CD47 d and CRT e in PBMC cells after co-culturing with THP-1 macrophages. The expression levels of CD47 and CRT were assessed by incubating PE-conjugated anti human CD47 and Alexa Fluor® 488 conjugated antihuman CRT antibodies on cancer (HL-60) and normal cells (PBMC). Representative graphs of CD47+ and CRT+ cells show the mean of fluorescence intensity of positive counts of cancer or normal cells at 0, 5, 20, and 100 ng/mL of LPS used to activate THP-1 macrophages 24 h to flow cytometry measurements. Values in the graphs are shown as means ± SEM of three trials of duplicate samples. The statistical significance between the different concentration of LPS with and without co-culture was determined by one-way ANOVA (OriginPro 2019). ** P < 0.01, ns non-significant, P > 0.05
M1 macrophage-like cells activated by LPS were previously shown to have the ability to eliminate tumor cells [26]. In our study, as shown in Fig. 2b, the levels of CD47 on HL-60 decreased with increasing amounts (ng/mL) of LPS used to stimulate THP-1 macrophages (Fig. 2b). Surprisingly, the levels of CRT on HL-60 also increased with increasing doses of LPS (Fig. 2c). The stimulation of THP-1 macrophages resulted in the down-regulation of the “don’t eat me signal” CD47. Interestingly, it also increased the levels of “eat me signal” CRT on the surface of HL-60 cells. CD47 levels were highest at 0 ng /mL of LPS and conversely CRT levels were the lowest at 0 ng/mL of LPS (Fig. 2b, c). This pattern was seen at all LPS concentrations used to stimulate THP-1 macrophages co-cultured with HL-60 cells (Fig. 2b, c). CD47 level showed 81% down-regulation at 100 ng/mL of LPS. Down-regulations of 32–77% were seen at 5–20 ng/mL of LPS, respectively (Fig. 2b). The correlated side scatter plots of CD47 and CRT expression levels can also be found in Fig. S2A-S2E.
The pattern of CD47 down-regulation and CRT up-regulation was only seen in co-culture. When HL-60 cells were cultured alone and treated with the same LPS concentrations used when co-cultured with THP-1 macrophages, CD47 and CRT levels did not show any significant changes compared to 0 ng/mL (P > 0.05). However, the change in the levels of both proteins was significant after co-culture, when compared to the levels in the absence of co-culture (P < 0.01 at 5, 20, and 100 ng/mL) (Fig. S2F, and S2G), indicating the necessity of the co-culture model in order for this inhibition to take place.
Second, we examine whether the down-regulation of CD47 occurs in normal blood cells. PBMC normal blood cells [24] were co-cultured with differentiated THP-1 macrophages under the same conditions as HL-60 and used as the control (Fig. 2b). PBMC exhibited similarly low CD47 expression levels at all LPS concentrations used, including that of 0 ng/mL (Fig. 2d). Similar results were observed with CRT levels (Fig. 2e). As is the case with most normal cells, PBMC did not express high levels of CD47 compared to HL-60, meaning that PBMC threshold levels of CD47 and CRT were low (Fig. S2H) and, therefore, no down-regulation of CD47 (Fig. S2I) or elevation of CRT (Fig. S2J) was seen in PBMC normal cells at any concentration of LPS used when co-cultured with THP-1 macrophages after LPS stimulation and when cultured alone (without co-culture, P > 0.05) (Fig. 2d, e, Fig. S2K and S2L). These results suggested that the activation of macrophage-like cells and subsequent inhibition of CD47 and increase of CRT levels could be selective for only cancer cells, and could be applied gradually at different concentrations of the material used for activation. Moreover, the inhibition of CD47 and the induction of CRT levels can only be obtained in co-culture, indicating the potential of the model to guide the development of therapies for leukemia.
Elimination of HL-60 by activated THP-1 macrophages in co-culture and cells viability
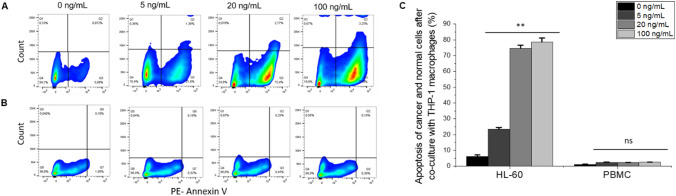
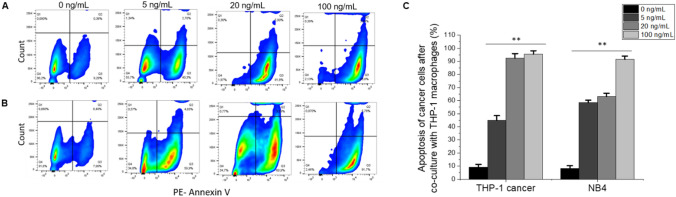
In the co-culture, LPS was used to activate THP-1 macrophages and incubated overnight with HL-60 leukemia cells. Therefore, it was necessary to study the effect of LPS on the viability of HL-60 and PBMC before co-culture. Moreover, to further test our hypothesis and to investigate the elimination of cancer cells by macrophages, the apoptosis rate of HL-60 and PBMC (as the control) after co-culture with macrophages was determined. PE-Annexin V Apoptosis-based detection followed by flow cytometric analysis was performed.
It was shown that the viabilities of HL-60 and PBMC remain above 90% at all LPS concentrations over 16 − 18 h of incubation (Fig. S3A) when these two cell lines were cultured alone and treated with LPS. However, after co-culture with THP-1 macrophages, an increase of apoptosis was observed as the LPS concentration was increased (Fig. 3a, c). The apoptosis of HL-60 cells increased from 22% at 5 ng/mL of LPS to 77% at 100 ng/mL of LPS (Fig. 3a, c). However, PBMC did not show any significant increase of apoptosis at any concentrations of LPS compared to 0 ng/mL of LPS (Fig. 3b, c). The results above indicate that the elimination of cancer cells by macrophages as a result of LPS activation is selective to cancer cells and that the co-culture model is necessary for this cancer cell elimination to take place.
Fig. 3.
Elimination of HL-60 by LPS-activated THP-1 macrophages in co-culture. Side scatter plots showing apoptosis rate (PE-Annexin V) of cancer and normal cells after co-culturing with activated THP-1 macrophages at 0, 5, 20, and 100 ng/mL of LPS, respectively. Side scatter plots of HL-60 (a) and PBMC (b). All plots showed apoptosis at 0, 5, 20, and 100 ng/mL of LPS. c A graph shows the percentage (%) of apoptosis of cancer and normal cells after co-culturing with THP-1 macrophages. Values in the graph are shown as means ± SEM of three trials of duplicate samples. The statistical significance was determined by one-way ANOVA (OriginPro 2019) ** P < 0.01, ns non-significant, P > 0.05. The side scatter plots shown here are representative and show one of three trials
Down-regulation of CD47 and elimination by THP-1 macrophages extend to other leukemia cell lines in co-culture
To investigate whether the inhibitory effect of CD47 extends to other types of leukemia cell lines, NB4 and THP-1 cancer cell lines were used. Cancer cells were co-cultured in the upper chamber with the THP-1 macrophages in the lower chamber, as mentioned previously (Fig. 2a). LPS was used to activate THP-1 macrophages at the same concentrations as mentioned earlier, with cells incubated overnight. After that, cells were harvested, and levels of CD47 and CRT on THP-1 and NB4 cancer cells were evaluated, and the apoptosis rates were determined using flow cytometry.
As found with HL-60, the levels of CD47 expressed in both THP-1 and NB4 cancer cells decreased with increasing amounts of LPS used, and the converse was observed for CRT levels (Fig. 4). However, the percentage of CD47 down-regulation was the highest when THP-1 cancer cells were co-cultured with THP-1 macrophages, in comparison with HL-60 and NB4 cells (Figs. 2b, 4a, b). The percentage of CD47 down-regulation in THP-1 cancer cells was around 90% when 20–100 ng/mL of LPS were used to activate macrophage-like cells (Fig. 4a). Moreover, the levels of CRT were the highest in THP-1 cancer cells and NB4 at 20–100 ng/mL of LPS compared to HL-60 after co-culture (Figs. 2c, 4c, d).
Fig. 4.
Down-regulation of CD47 and increased levels of CRT on THP-1 and NB4 cancer cells after co-culturing with stimulated THP-1 macrophages. Flow cytometric analysis of CD47 on THP-1 cancer cells (a) and NB4 (b); analysis of CRT on THP-1 cancer cells (c) and NB4 (d), after co-culturing with THP-1 macrophages. The expression levels of CD47 and CRT were assessed by incubating PE-conjugated antihuman CD47 and Alexa Fluor® 488 conjugated antihuman CRT with cancer cells for 30 min at room temperature. Representative graphs indicate the mean of fluorescence intensity of positive counts of cancer cells at 0, 5, 20, and 100 ng/mL of LPS used to activate THP-1 macrophages 24 h prior to flow cytometry analysis. Values in the graphs are shown as means ± SEM of three trials of duplicate samples each. The statistical significance between the different concentration of LPS with and without co-culture was determined by one-way ANOVA (OriginPro 2019). ** P < 0.01
When NB4 and THP-1 cancer cell lines were cultured alone (without THP-1 macrophages) and treated with the same LPS concentrations used in the co-culture experiment, CD47 and CRT levels on both cells did not show any significant changes compared to the levels at 0 ng/mL of LPS (P > 0.05, Fig. S4A-S4D). However, the changes in the levels of the same proteins obtained after co-culture were significant when compared to the levels without co-culture (P < 0.01, Fig. 4 and Fig. S4). This result showed a similar trend as was seen when HL-60 cells were cultured alone and treated with LPS (Fig. S2F and S2G). Most importantly, the control (without co-culture) experiments prove that the stimulation of THP-1 macrophages with LPS did indeed induce the down-regulation of CD47 and the up-regulation of CRT on the surface of leukemia cells, leading to their elimination, as proven by the apoptosis results seen in Figs. 3 and 5.
Fig. 5.
Elimination of cancer cells by LPS-activated THP-1 macrophages extends to other cell lines in co-culture. Side scatter plots showing apoptosis rate (PE-Annexin V) of cancer cells a THP-1 and b NB4, after co-culturing with activated THP-1 macrophages at 0, 5, 20, and 100 ng/mL of LPS respectively. All plots showed apoptosis at 0, 5, 20, and 100 ng/mL of LPS. c A graph showing the percentage (%) of apoptosis of THP-1 and NB4 after co-culturing with THP-1 macrophages. Values in the graph are shown as means ± SEM of three trials of duplicate samples. The statistical significance was determined by one-way ANOVA (OriginPro 2019) ** P < 0.01, ns: non-significant, P > 0.05. Side scatter plots shown here is one representative out of three trials
In our results, all of the cell lines mentioned above were responsive to the co-cultured macrophages, and furthermore, inhibition of CD47 and increased CRT expression levels were observed in all cancer cells, suggesting that the macrophage elimination of leukemia cells only happens when co-culturing together. To support this conclusion, apoptosis rates were determined in all of the cell lines mentioned above after co-culture with activated THP-1 macrophages. Apoptosis of THP-1 and NB4 cancer cells was increased with increasing amounts of LPS used to activate macrophages in a dose-dependent manner (Fig. 5). The highest levels of apoptosis (over 95%) were seen in THP-1 cancer cells after co-culture with THP-1 macrophages at 100 ng/mL of LPS compared to 0 ng/mL (Fig. 5a, c). The NB4 apoptosis percentage was very close to that of THP-1 cancer cells (91%) and slightly higher than HL-60 at the same LPS concentration, but both values were significantly higher than what was observed at 0 ng/mL LPS (Fig. 5b, c). It is worthwhile to note that at 5 ng/mL of LPS, over 50% of apoptosis was observed in THP-1 and NB4 cancer cells (Fig. 5a–c), suggesting that THP-1 macrophage co-culture is a sensitive in vitro model for testing targeted drugs to eliminate AML cells.
The effect of LPS on THP-1 and NB4 cancer cells, as well as THP-1 macrophages when cultured alone was also addressed here. The results show that cancer cells and macrophages remain viable (over 90%) at all concentrations of LPS used (Fig. S3B), indicating that the apoptosis seen here is a result of the elimination of cancer cells by activated macrophages in a co-culture setting.
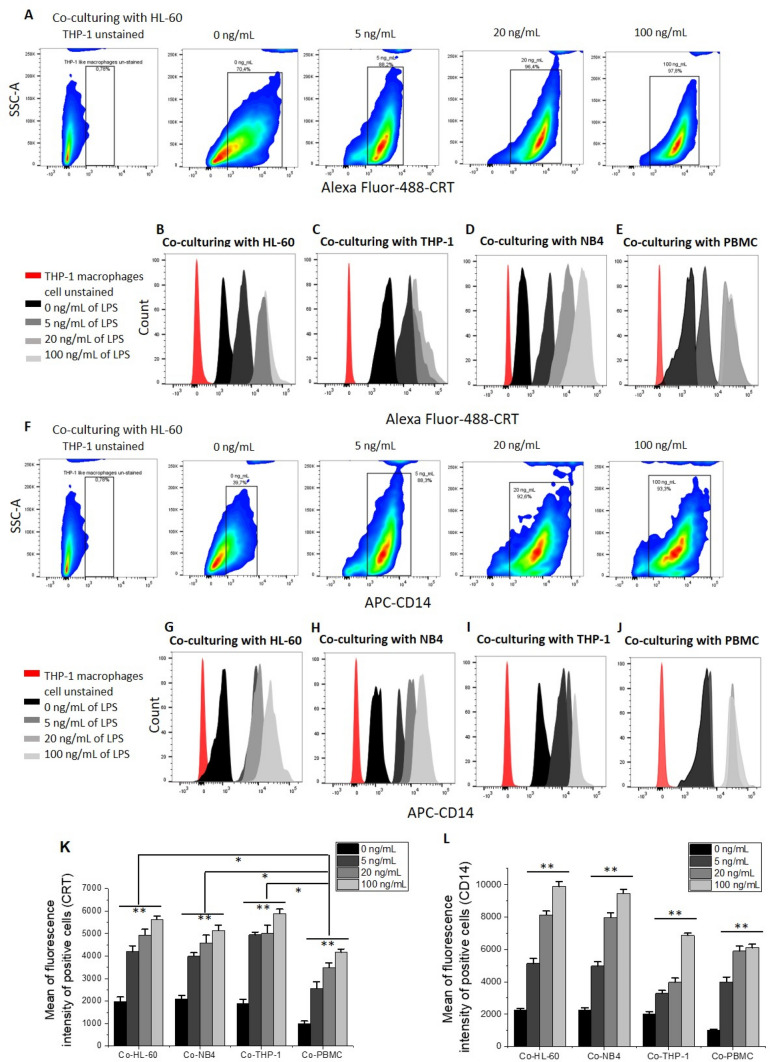
Increased levels of CRT and CD14 on LPS-activated THP-1 macrophages in co-culture
So far, we have shown that LPS activation induced an inhibition of the “do not eat me signal” CD47 and an increase of the “eat me signal” CRT levels on leukemia cells in co-culture, as well as increased cancer cell elimination by THP-1 macrophages (Figs. 2 and 5). To further the study, we investigated the levels of CRT and CD14 on LPS-activated macrophages and their link to phagocytosis. We noticed that the alteration of the expression of biomarkers on the cancer cells only occurs in co-culture with macrophages (Fig. 6). This could be the reason behind their elimination via CRT-mediated phagocytosis [16]. When co-cultured with HL-60 and THP-1 cancer cells, THP-1 macrophages showed the highest levels of CRT (Fig. 6a–c and k). Levels of CRT were similar when THP-1 macrophages were co-cultured with NB4 and lower when co-cultured with PBMC. (Fig. 6d, e and k). It should be noted here that the levels of CRT on THP-1 macrophages (when co-cultured with cancer cells) were significantly higher (P < 0.05) than the levels of CRT obtained after co-culture with PBMC normal cells (Fig. 6k), indicating higher levels of CRT alteration occurred on THP-1 macrophages in co-culture, and, therefore, ultimately resulted in phagocytosis and elimination of cancer cells by co-culture with macrophages.
Fig. 6.
Increased levels of CRT and CD14 on THP-1 macrophages after co-culturing with leukemic cells. Representative examples of side scatter plots show an increase of CRT (a) and CD14 (f) levels on THP-1 macrophages when activated with 0, 5, 20, and 100 ng/mL of LPS in co-culture with HL-60. Flow cytometric histograms showing CRT (b–e) and CD14 (g–j) levels on THP-1 macrophages when activated with 0, 5, 20, and 100 ng/mL of LPS and co-cultured with HL-60 (b, g); THP-1 cancer cells (c, i); NB4 (d, h); and PBMC normal cells (e, j). The expression levels of CRT and CD14 were assessed by incubating Alexa Fluor® 488 conjugated antihuman CRT and APC-conjugated antihuman CD14 with THP-1 macrophages after co-culturing with each cell line mentioned above for 30 min at room temperature. Then, cells were washed and re-suspended with 0.5 mL of cell staining buffer containing 1% formaldehyde and was ready for flow cytometry analysis. Graphs representing CRT (k) and CD14 (l) levels on THP-1 macrophages after co-culturing with HL-60, NB4, THP-1 cancer cells, and PBMC normal cells. Values are shown as means ± SEM of three trials of duplicate samples each. The statistical significance of the effect of different LPS concentrations in each group was determined by one-way ANOVA (OriginPro 2019), and the statistical significance between each cancer cell line and the normal cell line PBMC was determined using independent two-sample t test (OriginPro 2019). * P < 0.05, ** P < 0.01
When THP-1 macrophages were cultured alone and stimulated with different concentrations of LPS, no significant increase of CRT level was observed at 5 and 20 ng/mL of LPS treatment (P > 0.05), and an increase of CRT level was found at 100 ng/mL LPS treatment, compared to the level at 0 ng/mL (P < 0.05, Fig. S5A). However, CRT levels on THP-1 macrophages were significant increased at all LPS concentrations used in co-culture with cancer cells (P < 0.05, Fig. 6k and Fig. S5A). This indicates that in order for the cancer cell elimination to take place, THP-1 macrophages and cancer cells have to be in co-culture.
CD14 is known to be involved in the binding of LPS and the subsequent generation of pro-inflammatory responses [27, 28]. In our results, LPS-activated THP-1 macrophages, indeed, showed high levels of CD14 when co-culturing with HL-60 and NB4 (Fig. f–h and l). Lower levels were seen on THP-1 macrophages after co-culturing with PBMC normal cells (Fig. 6j, l). All cancer cells mentioned above were cultured alone and treated with 0, 5, 20, and 100 ng/ mL of LPS, after which CD14 was measured on their surfaces by flow cytometry. The levels of CD14 on HL60 and NB4 cancer cells were not significantly increased after 5 ng/mL of LPS treatment (Fig. S5B-S5C). For THP-1 cancer and PBMC normal cells, the increase of CD14 at all concentrations was insignificant (P > 0.05) when compared to the level at 0 ng/mL of LPS (Fig. S5D-S5F). At 20 and 100 ng /mL of LPS treatment, the increase in CD14 levels on HL60 and NB4 was significant (P < 0.05) (Fig. S5B, S5C and S5F); however, CD14 levels on all cell line cells were significantly higher (P < 0.05) when co-cultured with THP-1 macrophages (when compared to the levels of cultured alone, Figs. 6 and S5). Similarly, the levels of CD14 on THP-1 macrophages when cultured alone increased with increasing the concentration of LPS, but these increased levels were much lower than the increased CD14 levels on the THP-1 macrophages after co-culture with cancel cells (Figs. 6l and S5G). These pieces of evidence indicate that CRT-mediated macrophage elimination of cancer cells likely took place in co-culture.
Increased levels of M1 macrophage polarization cytokines after LPS activation of THP-1 macrophages
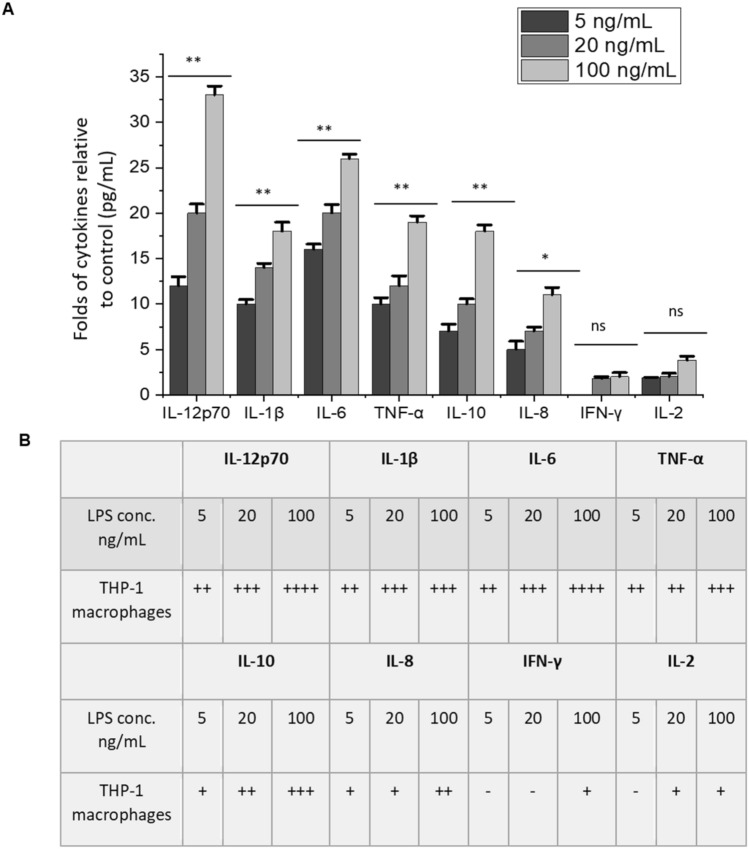
It has been reported that various CD47 inhibition methods (including the use of LPS to activate macrophages) result in increased production of certain cytokines and stimulate phagocytosis [22, 29]. In our study, LPS was used to stimulate THP-1 macrophages at various concentrations. As a result, CD47 was down-regulated in all leukemic cell lines used. We also found that LPS stimulation resulted in significant productions of IL-12p70, IL-6, TNF-α, IL-1β, IL-10, and IL-8, compared to the control in a dose-dependent manner (Fig. 7). IL-12p70 was 30-fold higher than the control (0 ng/mL of LPS) when THP-1 macrophages were activated at 100 ng/mL of LPS (Fig. 7a). IL-6 and TNF-α followed by an increase of over 20-fold at the same concentration of LPS as the control (Fig. 7). All the cytokines mentioned above are secreted from type M1 macrophages as a result of mainly LPS stimulation [30]. M1 macrophages have a role in the elimination and phagocytosis of microbes [30]. The presence of these cytokines in high levels in the supernatant of the media in co-culture indicates that THP-1 macrophages are M1 type, and their main role was to eliminate cancer cells. These results are another piece of evidence that support our hypothesis that upon LPS stimulation of macrophages in co-culture, CD47 and CRT levels change to mark cancer cells for elimination by M1 macrophages. This was shown earlier by the alteration of CD47 and CRT levels, as well as the elimination (apoptosis) of cancer cells.
Fig. 7.
Increased levels of M1 macrophage polarization markers after LPS activation of THP-1 macrophages. a Levels of produced cytokines (pg/mL) relative to the control of THP-1 macrophages (at 0 ng/mL of LPS) after activation of LPS at 0, 5, 20, and 100 ng/mL. Values in the graph were shown as means ± SEM of three trials of duplicate samples. The statistical significance was determined by one-way ANOVA (OriginPro 2019).* P < 0.05, ** P < 0.01, ns: non-significant, P > 0.05. b The fold-change of cytokine levels in THP-1 macrophages relative to the control. The amount of cytokines secreted by macrophages (pg/mL) is presented by folds, which was relative to the amount secreted without activation of LPS (control, 0 ng/mL). A cut-off of twofold was determined to be the threshold. Symbols: “−“: < 2, “ + ”: 2–9, “ + + ”: 10–17, “ + + + ”: 18–25, “ + + + + ”: 26–33 folds
IL-2 and IFN-γ are known as markers of M2 macrophages, and they are mainly secreted as a result of certain cytokines stimulation. They also have a role in tissue repairing and wound healing [30, 31]. These cytokines in low levels in the supernatant of the media in co-culture support the assertion that THP-1 macrophages are type M1 not M2 (Fig. 7). The data shown here indicate the need for co-culture to test the elimination of cancer cells.
Investigating CD47 down-regulation and cancer cells elimination in co-culture using murine macrophage model Raw 264.7
To support our findings and determine whether the changes of CD47 and CRT levels observed in our co-culture model with human THP-1 macrophages can be detected in a murine model, cancer cells (HL-60, NB4, and THP-1), as well as normal cells (PBMC) were each co-cultured that were activated with the same concentrations of LPS as the THP-1 macrophages (Fig. 2a). CD47, CRT, and CD14 levels on cancer cells without co-culture with Raw 264.7 were also tested. The results showed that CD47 down-regulation went from 41 to 64% at 5–20 ng/mL of LPS, respectively; and CRT levels increased at the same LPS concentrations in HL-60 cells (Supporting Information Fig. S6A and S6B). HL-60 and NB4 cells showed similar trends of decreased CD47 and increased CRT levels (Fig. S6C and S6D). However, at 100 ng/mL, both cells showed a significant reduction in CD47 down-regulation and increase of CRT levels compared to the same concentration of LPS (100 ng/mL) when both cell lines were co-cultured with THP-1 macrophages (Fig. S6C-S6D). This trend was not seen in THP-1 cancer cells when co-cultured with Raw 264.7. CD47 down-regulation and CRT up-regulation were seen as LPS concentrations increased (Fig. S6E and S6F). The inhibition of CD47 went from 59% at 5 ng/mL of LPS to 95% at 100 ng/mL of LPS (Fig. S6E and S6F). When PBMC normal cells were co-cultured with Raw 264.7, no significant increase in CD47 down-regulation or CRT levels was observed in any of the LPS concentrations used to activate Raw 264.7 (Fig. S6G and S6H). CD47 and CRT levels remain unaltered in all of the cancer and normal cells when cultured alone and treated with the same concentrations of LPS (0, 5, 20, and 100 ng/mL) used in co-culture (Fig. S2 and Fig. S4).
When looking at the viability of Raw 264.7 before co-culturing, it was shown that the cells remain over 90% viable at all concentrations except at 100 ng/mL of LPS, at which point the cells showed only a 51% viability (Fig. S3C). This may explain the unexpected results of the decreased CD47 down-regulation and CRT levels at 100 ng/mL of LPS in HL-60 and NB4 after co-culturing with Raw 264.7 (Fig. S6C-S6D).
When investigating the apoptosis rate in all cancer cells mentioned above, we found similar CD47 and CRT level changes. In HL-60 and NB4 cells, the apoptosis rate increased as the LPS concentration increased compared to the control (0 ng/mL), except at 100 ng/mL, it drops from 58% at 20 ng/mL to 35% at 100 ng/mL for HL-60 (Fig. S7A and S7E), and from 57% at 20 ng/mL to 29% at 100 ng/mL for NB4 (Fig. S7B and S7E). As the CD47 down-regulation and CRT levels were the highest in THP-1 cancer cells at 100 ng/mL of LPS, the apoptosis rate was also the highest with around 60% of THP-1 cancer cells being killed (Fig. S7C and S7E). This is likely due to THP-1 cancer cells possibly being more sensitive to elimination by Raw 264.7 than HL-60 or NB4. PBMC normal cells did not show any significant increase of the apoptosis rate at all LPS concentrations used for activation when compared to the control (0 ng/mL) (Fig. S7D and S7E).
CRT and CD14 levels obtained on Raw 264.7 after co-culturing with leukemic cells were comparable to those obtained from THP-1 macrophages, except at 100 ng/mL LPS. The levels dropped significantly after co-culturing with HL-60 and NB4 (Fig. S8A, S8B, S8E, S8F, S8I, and S8J). However, at the same concentration of LPS, CRT and CD14 levels were the highest after co-culturing with THP-1 cancer cells (Fig. S8C, S8G, S8I, and S8J). In co-culture with PBMC normal cells, Raw 264.7 showed increased CRT and CD14 levels at all LPS concentrations used (Fig. S8D, S8H, S8I, and S8J). When cultured alone, all cancer and normal cells showed an increase of CD14 levels (Fig. S5). However, the levels of CD14 measured after co-culturing with Raw 264.7 were significantly higher (Fig. S8).
To support the elimination results, cytokines were measured in the supernatant of Raw 264.7 after activation of the different concentrations of LPS. The data showed a similar trend as that for THP-1 macrophages, with IL-12p70 recording the highest levels compared to the control, followed by IL-6 and TNF-α at the same concentration of LPS (Fig. S9A and S9B). Moreover, the productions of M2 macrophages markers IFN-γ and IL-2 were twofold higher than the control at all concentrations of LPS used to stimulate Raw 264.7 cells (Fig. S9A and S9B).
Data obtained using the murine Raw 264.7 as a model to eliminate leukemia cancer cells showed selectivity to only cancer cells in the set examined and were comparable to the data obtained from using human macrophages as an elimination model (Figs. 2 and 7), except at one concentration of LPS where Raw 264.7 cells were more in-tolerant.
Discussion
Using LPS -activated human THP-1 macrophages in a co-culture model to eliminate AML cells, we have defined a distinct method to simultaneously inhibit the “don’t eat me signal” CD47 and up-regulate the “eat me signal” CRT on the surface of AML cells to flag them for elimination by macrophages in vitro. There is limited literature on restoring immune surveillance in AML cells in vitro using this method [32–34]. However, previously published studies to restore immune surveillance in many tumors in vitro were found to either focus on CD47 inhibition or activation of CRT [16, 17]. Nevertheless, in our findings, the activation of macrophages by LPS led to both actions taking place at the same time. The investigation of these two signals in AML cells in vitro may help in understanding the high relapse rate of AML and establish new ways to diagnosis and treat the diseases.
Many studies reported that using monoclonal antibodies directed against CD47 leads to inhibition of CD47 levels on AML cells [8, 35, 36]. In addition, other studies reported that the level of CD47 on these cells determines the probability that they were engulfed by macrophages [17, 18]. However, the inhibition of CD47 using a model like the one described here in AML cells has not been previously demonstrated. In our study, we showed that CD47 inhibition as well as CRT up-regulation in AML cells were correlated with the amount of LPS (ng/mL) used to activate THP-1 macrophages, and this was comparable to the apoptosis rate of AML cells in co-culture chamber. For instance, HL-60, which was first used here in a co-culture model with macrophages to study expression levels of CD47 and CRT to restore immune surveillance showed an 81% inhibition of CD47 at 100 ng/mL of LPS. This was correlated with their apoptosis rate, which was around 80%. Slightly higher inhibition of CD47 and apoptosis rate were seen in both NB4 and THP-1 cancer cells at the same LPS concentration in the co-culture chamber. These results are consistent with those in the literature when monoclonal antibodies against CD47 were used as the inhibition method [35, 36]. In addition to CD47 inhibition, CRT levels on all AML cells were increased as the inhibition of CD47 increased, suggesting the increase of AML cell uptake by CRT-mediated phagocytosis, which was reported elsewhere [17]. Our data revealed that using a small amount (as low as 5 ng/mL) of LPS can trigger over 45% of AML uptake by macrophages. In order for our model to have a potential use in the diagnosis and treatment of AML, it has to be selective for only cancer cells. Our data confirmed that the inhibition of CD47 and the up-regulation of CRT in co-culture was only selective to AML cells. Normal human blood cells (PBMC) did not show any significant change in their CD47 nor CRT expression levels before and after co-culture with LPS-activated macrophages.
It has been reported that when activated, macrophages increase the expression levels of CRT and therefore, increase the uptake of cancer cells [37, 38]. Our results showed that CRT levels on THP-1 macrophages increased as LPS concentration increased indicating the uptake of AML cells by these macrophages in co-culture. Moreover, as an LPS receptor and an element of inflammatory response, CD14 showed increased expression levels similarly to CRT and was correlated to LSP concentrations. This further indicates the uptake of AML cells by macrophages in co-culture. It has been reported that the inhibition of CD47 induces a broad antitumor immune response, including the shift toward an inflammatory phenotype macrophages (M1), and increased pro-inflammatory cytokine production [39–41]. Our results are consistent with these findings, as we observed an increased production of many M1 cytokines including IL 12p70, IL-6, and TNF-α in the supernatant after co-cultured with AML cancer cells.
Consistent with human cell model data, the murine cell results showed comparable signatures when Raw 264.7 was co-cultured with AML cancer cells and PBMC normal cells. Elimination of AML cells by Raw 264.7 was dependent on the concentration of LPS used, except for at 100 ng/mL in the case of HL-60 and NB4 cancer cells. Future work to further investigate this could explain the results obtained here.
In conclusion, our findings demonstrate the successful use of human macrophage model to inhibit CD47 and up-regulate CRT in AML cancer cells to increase their elimination in co-culture. Moreover, our data showed that this model is selective to AML cells, and does not affect several other types of normal blood cells, implicating the use of this model in drug screening targeted against AML.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Mr. Henk van Faassen for his assistance on the flow cytometry measurements.
Abbreviations
- AML
Acute myeloid leukemia
- CRT
Calreticulin
- DMEM
Dulbecco’s Modified Eagle’s Medium
- FBS
Fetal bovine serum
- HBSS
Hank’s balanced salt solution
- LPS
Lipopolysaccharide
- MFI
Mean of fluorescence intensity
- PBMC
Primary peripheral blood mononuclear cells
- SIRPα
Signal regulatory protein alpha
Authors’ contributions
Conception and design: S.Z and G.W. Development of methodology: E.H and S.Z. Acquisition of data: E.H. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, and computational analysis): E.H and S.Z. Writing, review, and/or revision of the manuscript: E.H, S.Z, G.W, and C.W. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): E.H and S.Z. Study supervision: S.Z.
Funding
This work was financially supported by the National Research Council of Canada.
Data availability
The datasets are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no competing interests.
Ethical Approval and consent to participate
No human or animal sample was involved in the study. The research is carried out in compliance with the biosafety and biosecurity protocols at the National Research Council Canada.
Consent to publication
A submission approval is granted by the National Research Council Canada, with consent emails from all authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu M, Li C, Zhu X. FLT3 inhibitors in acute myeloid leukemia. J Hematol Oncol. 2018;11:133. doi: 10.1186/s13045-018-0675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talati C, Sweet K. Recently approved therapies in acute myeloid leukemia: a complex treatment landscape. Leuk Res. 2018;73:58–66. doi: 10.1016/j.leukres.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 3.National cancer institute: SEER cancer stat facts: acute myeloid leukemia. https://seer.cancer.gov/statfacts/html/amyl.html. Accessed 6 Apr 2020
- 4.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31:212–219. doi: 10.1016/j.it.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munn DH, Cheung NKV. Phagocytosis of tumor cells by human monocytes cultured in recombinant macrophage colony-stimulating factor. J Exp Med. 1990;172:231–237. doi: 10.1084/jem.172.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao MP, Majeti R, Weissman IL. Programmed cell removal: a new obstacle in the road to developing cancer. Nat Rev Cancer. 2012;12:58–67. doi: 10.1038/nrc3171. [DOI] [PubMed] [Google Scholar]
- 8.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaiswal S, Jamieson CHM, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrova PS, Viller NN, Wong M, Pang X, Lin GHY, Dodge K, et al. TTI-621 (SIRPαFc): a CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res. 2017;23:1068–1079. doi: 10.1158/1078-0432.CCR-16-1700. [DOI] [PubMed] [Google Scholar]
- 11.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 12.Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis-Mortari A, Oldenborg PA, Iizuka K, et al. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J Exp Med. 2001;194:541–549. doi: 10.1084/jem.194.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Huang Q, Xiao W, Zhao Y, Pi J, Xu H, et al. Advances in anti-tumor treatments targeting the CD47/SIRPα axis. Front Immunol. 2020;11:18. doi: 10.3389/fimmu.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao MP, Weissman IL, Majeti R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24:225–232. doi: 10.1016/j.coi.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng M, Chen JY, Weissman-Tsukamoto R, Volkmer JP, Ho PY, McKenna KM, et al. Macrophages eat cancer cells using their own calreticulin as a guide: roles of TLR and Btk. Proc Natl Acad Sci USA. 2015;112:2145–2150. doi: 10.1073/pnas.1424907112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng M, Marjon KD, Zhu F, Weissman-Tsukamoto R, Levett A, Sullivan K, et al. Programmed cell removal by calreticulin in tissue homeostasis and cancer. Nat Commun. 2018;9:3194. doi: 10.1038/s41467-018-05211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krysko DV, Ravichandran KS, Vandenabeele P. Macrophages regulate the clearance of living cells by calreticulin. Nat Commun. 2018;9:4464. doi: 10.1038/s41467-018-06807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446. doi: 10.1615/CritRevImmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Carter CD, Miller MS, Bochsler PN. CD14 and tissue factor expression by bacterial lipopolysaccharide-stimulated bovine alveolar macrophages in vitro. Infect Immun. 1995;63:51–56. doi: 10.1128/IAI.63.1.51-56.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, Shao R, Huang H, Wang X, Rong Z, Lin Y. Engineering macrophages to phagocytose cancer cells by blocking the CD47/SIRPɑ axis. Cancer Med. 2019;8:4245–4253. doi: 10.1002/cam4.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starr T, Bauler TJ, Malik-Kale P, Steele-Mortimer O. The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS ONE. 2018;13:e0193601. doi: 10.1371/journal.pone.0193601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daigneault M, Preston JA, Marriott HM, Whyte MKB, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE. 2010;5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohro T, Tanaka T, Murakami T, Wada Y, Aburatani H, Hamakubo T, et al. A comparison of differences in the gene expression profiles of phorbol 12-myristate 13-acetate differentiated THP-1 cells and human monocyte-derived macrophage. J Atheroscler Thromb. 2004;11:88–97. doi: 10.5551/jat.11.88. [DOI] [PubMed] [Google Scholar]
- 26.Anfray C, Ummarino A, Andón FT, Allavena P. Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells. 2020;9:46. doi: 10.3390/cells9010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pugin J, Heumann D, Tomasz A, Kravchenko VV, Akamatsu Y, Nishijima M, et al. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 28.He Z, Riva M, Björk P, Swärd K, Mörgelin M, Leanderson T, et al. CD14 is a co-receptor for TLR4 in the S100A9-induced pro-inflammatory response in monocytes. PLoS ONE. 2016;11:e0156377. doi: 10.1371/journal.pone.0156377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiskopf K, Ring A, Garcia CK, et al. CD47-blocking therapies stimulate macrophage cytokine secretion and are effective in a model of peritoneal carcinomatosis. J Immunother Cancer. 2015;3(Suppl 2):P248. doi: 10.1186/2051-1426-3-S2-P248. [DOI] [Google Scholar]
- 30.Yao Y, Xu XH, Jin L. Macrophage polarization in physiological and pathological pregnancy. Front Immunol. 2019;10:792. doi: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoeksema MA, Scicluna BP, Boshuizen MCS, van der Velden S, Neele AE, Van den Bossche J, et al. IFN-γ priming of macrophages represses a part of the inflammatory program and attenuates neutrophil recruitment. J Immunol. 2015;194:3909–3916. doi: 10.4049/jimmunol.1402077. [DOI] [PubMed] [Google Scholar]
- 32.Kauder SE, Kuo TC, Harrabi O, Chen A, Sangalang E, Doyle L, et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS ONE. 2018;13:e0201832. doi: 10.1371/journal.pone.0201832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manna PP, Frazier WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64:1026–1036. doi: 10.1158/0008-5472.CAN-03-1708. [DOI] [PubMed] [Google Scholar]
- 35.Majeti R. Monoclonal antibody therapy directed against human acute myeloid leukemia stem cells. Oncogene. 2011;30:1009–1019. doi: 10.1038/onc.2010.511. [DOI] [PubMed] [Google Scholar]
- 36.Pietsch EC, Dong J, Cardoso R, Zhang X, Chin D, Hawkins R, et al. Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J. 2017;7:e536. doi: 10.1038/bcj.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han A, Li C, Zahed T, Wong M, Smith I, Hoedel K, et al. Calreticulin is a critical cell survival factor in malignant neoplasms. PLoS Biol. 2019;17:e3000402. doi: 10.1371/journal.pbio.3000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osman R, Tacnet-Delorme P, Kleman JP, Millet A, Frachet P. Calreticulin release at an early stage of death modulates the clearance by macrophages of apoptotic cells. Front Immunol. 2017;8:1034. doi: 10.3389/fimmu.2017.01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu M, O’Connor RS, Trefely S, Graham K, Snyder NW, Beatty GL. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47−mediated ‘don’t-eat-me’ signal. Nat Immunol. 2019;20:265–275. doi: 10.1038/s41590-018-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He W, Kapate N, Shields CW, Mitragotri S. Drug delivery to macrophages: a review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv Drug Deliv Rev. 2020 doi: 10.1016/j.addr.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, Hutter G, Kahn SA, Azad TD, Gholamin S, Xu CY, et al. Anti-CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 polarized macrophages and promotes M1 polarized macrophages in vivo. PLoS ONE. 2016;11:e0153550. doi: 10.1371/journal.pone.0153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.