Abstract
Background
The aim of this study was to investigate the role of IL-17A in the cancer microenvironment and the recurrence of triple negative breast cancer (TNBC).
Methods
Using human TNBC cell lines, the role of IL17-A was investigated by knocked down of IL-17A (ΔIL-17A) and by administration of IL-17A into the culture medium. Cell proliferation assays, migration assays, as well as Western blot analysis and real-time PCR, were used to evaluate IL-17A-related signaling. Three types of 4T1 cells were implanted into BALB/c mice, namely wild type (WT), ΔIL-17A, and WT + neutralizing IL-17 antibody (WT + Ab) cells. Tumor weight, necrosis area, and the number of circulating tumor cells (CTCs) were measured. Immunohistochemistry and Western blotting were used to analyze expression of CD34, CD8, and TGF-β1 as well as anoikis resistance. The Kaplan–Meier’s method was used to correlate IL-17A expression and patient outcome, including disease-free survival (DFS) and overall survival (OS).
Results
Our results demonstrated that IL-17A was able to stimulate the migratory activity, but not the growth rate, of MDA-MB-231/468 cells. In vivo, for the ΔIL-17A group, there was an increase in necrosis area, a decrease in tumor CD34 expression and a reduction in the number of CTCs. Furthermore, in WT + Ab group, there was a decreased in tumor expression of CD34, fewer CD8 ( +) cells, and fewer CTCs, but an increase in expression of TGF-β1 expression. Both of the above were compared to the WT group. Knockdown of IL-17A also decreased anoikis resistance in human TNBC and the murine 4T1 cell lines. Kaplan–Meier analysis disclosed a negative correlation between tumor expression of IL-17A and OS in TNBC patients.
Conclusion
We conclude that IL-17A promotes migratory and angiogenic activity in tumors, enhances anoikis resistance, and modulates the immune landscape of the tumor microenvironment such changes favor cancer metastasis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02867-x.
Keywords: IL-17A, Triple negative, Breast cancer, Anoikis, Metastasis
Introduction
Breast cancer is the most common diagnosed female cancer worldwide and ranks as the fourth most important cause of death in Taiwan [1]. Triple negative breast cancer (TNBC) represents 15% to 20% of all newly diagnosed breast cancer patients and is characterized as having a large tumor size, being frequently diagnosed among younger women, and having a poor prognosis with high metastatic potential [2–4]. Recent investigations have demonstrated that TNBC seem to be composed of a number of distinct subtypes [5–7]. However, the mechanisms associated with early recurrent, which results in the poor prognosis for TNBC patients, need to be further elucidated.
IL-17, a pro-inflammatory cytokine secreted by activated T cells and it regulates the activities of MAPKs and NF-κB, which bring about increases in the expression of IL6 and cyclooxygenase-2 (COX-2) [8]. Overexpression of IL-17A is known to be highly associated with various inflammatory diseases such as multiple sclerosis, psoriasis, and rheumatoid arthritis [9]. It is generally accepted that chronic inflammation increases the risk of cancer [10], as the inflammatory response shares various molecules, and their corresponding signaling pathways, with the carcinogenic process. IL-17-related protumoral effects on cancer initiation are postulated to involve MAPK and NF-kB recruitment. By way of contrast, IL-17 cytokines also can act in an antitumor role. This duality makes IL-17 very much a “double-edged sword” [11, 12]. Notwithstanding the above, the role played by IL-17A in the survival of circulating tumor cells (CTCs), possibly an important aspect of metastasis, remains to be investigated in detail.
Evidence suggests that a poor prognosis for breast cancer might be related to the presence of CTCs [13, 14]. The survival of tumor cells in the circulation has three requirements. These are, firstly, resistance to anoikis, secondly, the ability to escape immune surveillance, and finally, being able to survive the shearing forces present in blood. Resistance to anoikis requires activation of several signaling pathways related to anchorage-independent growth and the epithelial–mesenchymal transition, including those associated with Akt, TrkB, and Src, as well as others [15]. At present, information on the role of IL-17A in the survival of CTCs has not been investigated.
Our previous findings have demonstrated that over-expression of MEGF11 in TNBC cells triggers the expression of many cytokines and chemokines, which effectively results in a cytokine cascade. In addition, positive feedback between MEGF11 and IL-17A in TNBC cells has also been demonstrated, which might explain the role of MEGF11 in TNBC recurrence [16]. Accordingly, the aim of this study was to investigate the role that IL-17A plays in the mechanisms behind TNBC recurrence, including tumor behavior, the cancer microenvironment, and the survival of CTCs.
Methods
Cell lines and reagents
The human TNBC (ER−, HER2 low) cell lines MDA-MB-468 (RRID:CVCL_0419) and MDA-MB-231 (RRID:CVCL_0062) and the mouse mammary tumor 4T1 cell line (RRID:CVCL_JG34) [17] were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and were maintained in high glucose MEM, F12 MEM (NO.12400–024,Gibco, NY), and RPMI, respectively. They were supplemented with 10% FBS, 2 mM L-glutamine, and penicillin/streptomycin and cultured at 37 °C in an atmosphere containing 5% CO2. IL-17A (InvivoGen, San Diego, CA) and anti-mouse neutralizing IL-17A antibody (InVivoMab, Bio X Cell, BE0173, Lobanon, NH) were purchased commercially.
Cell growth measurement by trypan blue dye exclusion assay and cell cycle analysis
MDA-MB-468/231 cells were cultured in low serum medium at a cell density (1 × 105 / well) in a 12-well plate, and this was followed by treatment with different doses of IL17A (0, 1, and 10 ng/mL). After 1, 2, and 3 days of treatment, the cells were washed with phosphate-buffered saline (PBS), pH 7.4, and trypsinized in TE buffer (Gibco/Invitrogen, New York). The resuspended cells were washed, and their cell numbers were counted using a hemocytometer. For cell cycle analysis, cells (2 × 105 / well) were cultured for 24 h in low serum medium (0.1% FBS), and this was followed by another 24 h culture, and then cells were harvested for cell cycle analysis by flow cytometry. Cell cycles are presented as percentages of the cell cycle fraction, namely sub G0/G1 phase, G0/G1 phase, S phase, and G2/M phase.
Cell migration assay
In vitro cell migration of MDA-MB-468/231 cells was assessed using the Transwell system (ThinCertTM cell culture inserts, 24 well, 8 μm, Greiner bio-one, Switzerland) [18]. In brief, MDA-MB-468/231 cells were cultured in the upper chamber and different doses of IL-17A (0, 1, and 10 ng/mL) were added to the lower chamber. After 4 h (MDA-MB-231) or 8 h (MDA-MB-468) of culture, the cells on the reverse side of upper chamber membrane were fixed and stained using 2% crystal violet for 10 min; this was followed by washing. Finally, the migrating cells were examined under a light microscope, photographed, and quantified.
Measurement of IL-17A levels
IL-17A levels in the culture medium were quantified by ELISA and the procedure followed that of a commercially available protocol (Quantikine ELISA Human IL-17A). In brief, MDA-MB-468/231 (4 × 105 / well) cells were cultured for 1 d, and this was followed by administration of different doses (0, 1, and 10 ng/mL) of IL-17A in low serum medium for 24 h. Next, the culture medium was discarded, and the cells were cultured for another 48 h. This was followed by harvesting of the cells and collection of the supernatants. Finally, the supernatants were centrifuged (300×g) for 5 min and each supernatant subjected to the IL-17A assay.
Western blotting analysis
Cultured cells were lysed in lysis buffer (10 mM Tris pH 7.4, 150 mM KCl, 150 mM KCl, 1% Triton X-100, phosphatase inhibitor and protease inhibitor cocktail (Complete Mini; Roche, Mannheim, Germany). The proteins present in each cell homogenate were quantified using the Bradford’s method [19]. In total, 30 μg of proteins were separated by 10% SDS-PAGE and then transferred to a PVDF membrane. Next, this was blocked with 5% skimmed milk and probed with each specific primary antibody, namely IL-17A (#3171, R&D Systems, Inc. Minneapolis, MN), Src (#60,521, GeneTex, Inc., Irvine, CA), phosphor-Src (#6943, Cell Signaling Technology, Beverly, MA), COX-2 (#12,282, Cell Signaling), phospho-AKT (#9271, Ser473, Cell Signaling), AKT (#9272, Cell Signaling), cdc42 (#2466, Cell Signaling), RhoA (#2117, Cell Signaling), RhoC (#3430, Cell Signaling), and anti-β-actin (#3700S, Cell Signaling). The blots were washed, and then soaked in anti-rabbit IgG HRP-linked secondary antibodies or anti-mouse IgG HRP-linked secondary antibodies (Cell Signaling Technology, Beverly, MA, USA.) After 1 h of antibody binding, the membranes were washed and developed using an ECL detection kit (Amersham Pharmacia Biotech Inc., NJ) and quantified by Multi-Gauge software analysis (Fuji Photo Film Co, Ltd, Tokyo, Japan). β-actin was used as the internal control for all experiments.
RNA extraction and reverse transcription-PCR
Total RNA were isolated by the modified single-step guanidinium thiocyanate method [20]. (TRI REAGENT, T-9424, Sigma Chem. Co., St. Louis, MO) Complementary DNA (cDNA) was prepared from the total RNA using a First Strand cDNA Synthesis Kit (Invitrogen, CA). The de novo gene expression level change for each treatment group was measured by reverse transcriptase-polymerase chain reaction (RT-PCR). Primers pairs were purchased commercially [16]. Possible contamination of any of the PCR component was excluded by performing a PCR reaction in the absence of RT product for each set of experiments. Quantification of the RNA transcripts was carried out according to a method described previously [16]. For statistical comparison, the relative expression levels of the mRNA of each specific genes were normalized against the level of GAPD expression in the same RNA extracts. All samples were analyzed in triplicate.
Lentiviral infection for short hairpin RNA (shRNA)-mediated IL-17A knockdown
Lentiviral infection for short hairpin RNA (shRNA) to silence the IL-17A gene was obtained from Academia Sinica and followed the protocol of this institute. In brief, one day after the MDA-MB-468/231 and murine 4T1 cell lines (cell density 3 × 105–1.2 × 106) were subcultured, they (30 to 40% confluence) were infected with lentivirus for 24 h with an shRNA targeting the IL-17A gene or a non-silencing control shRNA using polybrene (Hexadimethrine bromide) reagent (#H9268, Sigma). After infection, the MDA-MB-468/231 and 4T1 cells were recovered to allow further experiments to be carried out. After several passages, ∆ IL-17A cell lines were established by puromycin selection. The infection efficiency was validated by Western blot analysis (supplementary reference 1).
Measurement of anoikis resistance
Anoikis resistance was evaluated using commercially available adherent plate and non-adherent (anoikis) plates (CBA081m Cell Biolabs, San Diego, CA). Briefly, MDA-MB-231, MDA-MB-468, and murine 4T1 cells at a density of 2 × 104/well were cultured in on the adherent and non-adherent (anoikis) plates. After the cells had been cultured for 16 h (4T1 cells), 24 h, and 48 h (MDA-MB-231), mixed live (calcein-AM) and dead (ethidium homodimer-1) staining solutions (L3224, Invitrogen Detection Technologies, Eugene, OR) were added into the cultured medium for 30–45 min, and this was followed by quantification of the dead cells by fluorescent microscopy. For the flow cytometry analysis (MDA-MB-468), the protocols were the same as abovementioned ones except that the cell density was increased to 2 × 105/well.
In vivo tumor xenografts
In this study, any protocols that involved experimental mice followed the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and were approved by the Institutional Animal Committee of Taipei Veterans General Hospital (No. 2018–029). Immunodeficient NU-Foxn1nu 8-week-old mice were obtained from the National Laboratory Animal Center (Taipei, Taiwan, ROC). They were given ad libitum access to water and food and maintained in a specific pathogen-free environment under a 12 h dark–light cycle at 22–24 °C with 50% humidity. Wild type (WT) and knocked down IL-17A (∆IL-17A) MDA-MB-231 cells (1 × 107 in 0.1 mL PBS) containing a luciferase gene were injected into the back of the immunodeficient NU-Foxn1nu mice. This gave rise to a solid tumor that was noticeable at the injection site. At the 2nd, 3rd, and 5th week, the progression of the tumor was visualized using an in vivo imaging system (IVIS).
In vivo metastasis study
Three animal study groups were created, these were WT, knocked down IL-17A (ΔIL-17A), and anti-IL-17A neutralizing antibody (250 μg/100 μL, twice a week) (WT + anti-IL-17A antibody) groups. Mouse mammary 4T1 cells (5 × 106 in 0.1 mL PBS) were orthotopically injected into the fat pads (left upper and/or right lower mammary glands) of 8-week-old female BALB/c mice. The mice were sacrificed at 3 weeks later or when the tumor sizes became more than 2% of the body weight. The tumor sizes and weights were measured, and the circulating tumor cells were isolated as mentioned below.
Selection of circulating mammary breast cancer 4T1 cells
After the anaesthetized, 4T1 bearing mice had been sacrificed, blood cells were obtained and centrifuged (400 g) in Ficoll-Paque PREMIUM (density: 1.084) (17–5446-02, GE Healthcare Bio-Sciences, Sweden) gradient medium. The peripheral mononuclear cells were then subjected to primary culture. After several passages, circulating 4T1 cells were selected and validated (supplementary 2) using 6-thioguanine (60 μM) (A4882, Sigma-Aldrich, MO, USA) [21], which was followed by quantification using a 2-hydroxyethyl agarose colony assay (A4018, Sigma-Aldrich, MO, USA). A colony was defined as a blue dye-stained group of cells that was ≥ 1 mm.
Subjects
After approval by the Institutional Review Board of Taipei Veterans General Hospital (2013–10-020BC), human tumor tissues from a biobank were obtained. Eighty three patients diagnosed as female breast cancer in Department of Pathology, Taipei Veterans General Hospital were enrolled from Jan. 2001 to Dec. 2010. Records including estrogen receptor (ER) status, progestin receptor (PR) status, HER2 status and clinical outcome, including overall survival (OS) and disease-free survival (DFS), were retrospectively reviewed. All data had been collected during clinical care and did not involve direct contact with the patients. In addition, written consent by the study subjects was waived by the Institutional Review Board. Every subject participated in this study (n = 83) had been followed up for ≥ 5 years. Patient outcome was subjected to a Kaplan–Meier survival analysis. ER/PR values of ≥ 1% were defined as positive, while ER/PR values of < 1% were defined as negative.
Immunohistochemical analysis
The protein expression levels of IL-17A in a tissue array (83 tumor samples) were assessed by immunohistochemical staining for IL-17A (Genetex, GTX49102). The results were analyzed by one pathologist over a short period of time (2 months). The protein expression of IL-17A (supplementary 3) was semi-quantified and expressed as (0), < 10%, (1), 11–25%, (2), 26–50%, and (3) > 50% of the tumor cells examined.
For the animal tumor tissues, the protein expression levels of CD34, CD8, and TGF-β1 were assessed by immunohistochemical staining for CD34 (#65,867, Genetex, Inc., Irvine, CA) and CD8 (#98,941, Cell Signaling, Danvers, MA), and TGF-β1 (#130,023, Genetex Inc., Irvine, CA), respectively. The CD8 ( +) cells were quantified by counting the cells within an area of 1 mm2, while the CD34 and TGF-β1 were quantified by Western blot analysis.
Statistical analysis
Results are expressed as the mean ± SEM. Differences between two groups were identified by Mann–Whitney U test or Student’s t test. Differences between groups at each time point or for each dose were identified by one-way ANOVA followed by Dunnet’s post-hoc test. Statistical comparison between two independent variables was determined by two-way ANOVA with the Bonferroni post-hoc test to correct for multiple comparisons. A p value < 0.05 is considered statistically significant compared to the vehicle or no treatment group.
DFS was defined as the time between initial breast cancer diagnosis and the date of recurrence as confirmed by pathology or an imaging study. OS was calculated from the time of initial breast cancer diagnosis to the date of death or last visit to the outpatient clinic. The Kaplan–Meier method was used to estimate the cumulative incidence of RFS and OS, and log-rank tests were then used for the various comparisons (GraphPad Prism 5).
Results
Role of IL-17A in tumor behavior
Knocked down of IL-17A does not affect the cell growth of human TNBC cell lines.
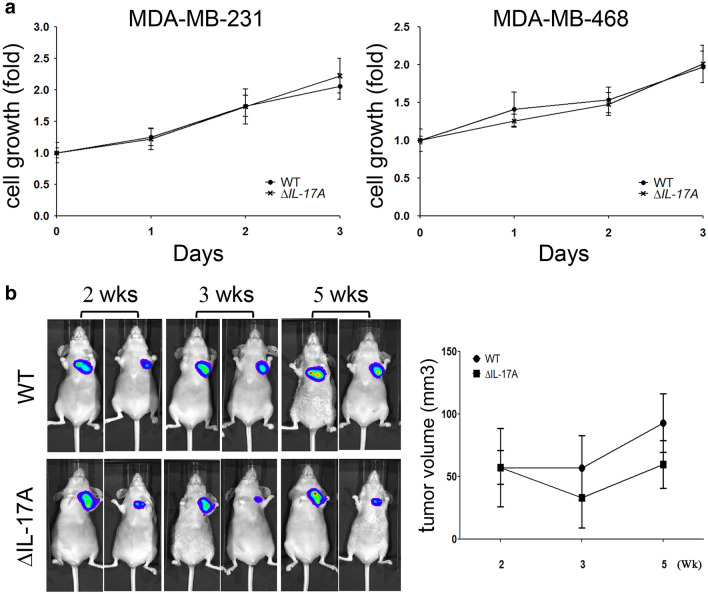
After the IL-17A gene was silenced in the TNBC MDA-MB-231 and MDA-MB-468 cell lines, there was no significantly statistical change in the growth rate of the ΔIL-17A groups compared to the WT groups and both using the cell lines (Fig. 1a) and in tumor-bearing nude mice studies (Fig. 1b).
Fig. 1.
The role of IL-17A on cell growth in MDA-MB-231 and MDA-MB-468 cell lines. After IL-17A was knocked down in MDA-MB-231 and MDA-MB-468 cells (a), cell proliferation was evaluated by trypan blue exclusion assay. Wild type (WT) and knocked down IL-17A (∆IL-17A) MDA-MB-231 cells (1 × 107 in 0.1 mL PBS) containing a luciferase gene were injected into back of immunodeficient NU-Foxn1nu mice. At the 2nd, 3rd, and 5th week, the progression of the tumors was visualized using an in vivo imaging system (IVIS) and tumor volume was quantified (b). Two-way ANOVA was used for statistical analysis for cell growth in vitro and in vivo
IL-17A does not affect cell growth but does promote cell migration of TNBC lines
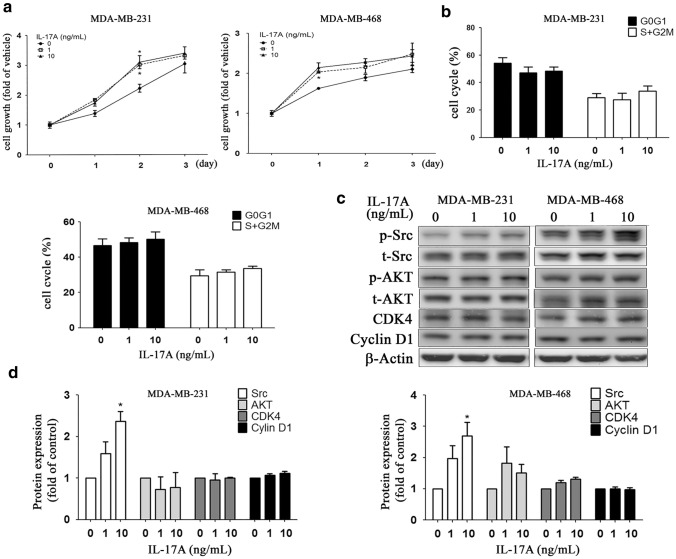
When IL-17A (0, 1, 10 ng/mL) was added to the culture medium of MDA-MB-231 and MDA-MB-468, there was an increased cell numbers in the 1-d and 2-d cultures, but not in the3-d culture (Fig. 2a, two-way ANOVA). Cell cycle analysis was able to demonstrate that there was no significant change in the various cell cycle fractions such as G0G1- or S + G2M phase (Fig. 2b, one-way ANOVA).
Fig. 2.
Effects of exogenously administration of IL-17A on cell growth in MDA-MB-231 and MDA-MB-468 cell lines. MDA-MB-468/231 cells were cultured in low serum medium with a cell density (1 × 104 / well), followed by treatment of different doses of IL17A (0-, 1-, 10 ng/mL). After 1, 2, and 3 days of treatment, cell proliferation rate was evaluated by trypan blue assay (a). For cell cycle analysis, cells (2 × 105 / well) were cultured for 24 h in low serum medium, followed by another 24 h-culture, and then cells were harvested for cell cycle analysis (b). Cell cycles were presented as percentages of cell cycle fraction, namely sub-G0/G1 phase, G0/G1 phase, S phase, and G2/M phase. Growth-related signaling proteins expression, such as Src, AKT, CDK4, and cyclinD1, were analyzed by Western blot (c) and quantified (d). Asterisk indicates a p value < 0.05 (one-way ANOVA)
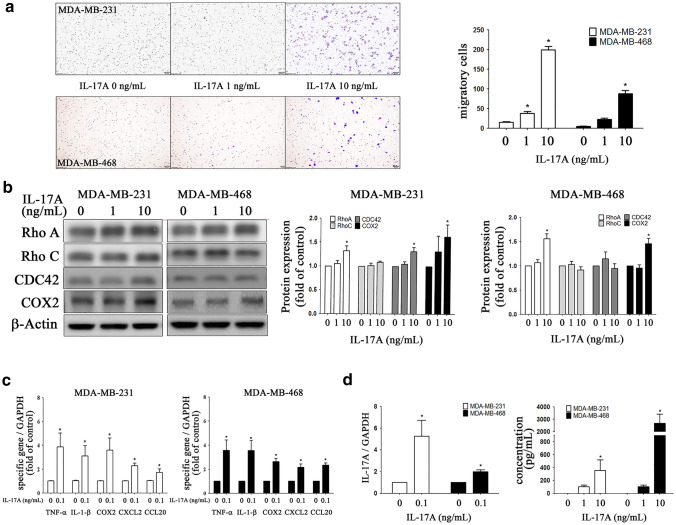
Western blot analysis (Fig. 2c) showed that there was an IL-17A dose-dependent increase in p-Src protein expression, but not in AKT, CDK4, or cyclin D1 protein expression across both TNBC lines (Fig. 2d).By way of contrast, IL-17A (0, 1, and 10 ng/mL) was able to dose-dependently increase migratory activity (Fig. 3a) via an activation in MDA-MB-231 cell line of Rho A, CDC42, and COX2, but not via activation of Rho C, while activation of Rho A and COX2, but not of Rho C and CDC42 occurred in the MDA-MB-468 line (Fig. 3b). Interestingly, our results showed that there was no significantly decreased migratory activity in ΔIL-17A TNBC cells compared to the WT type. Besides, exogenous administration of IL-17 also increased the migratory activity in ΔIL-17A TNBC lines (supplementary reference 4). In our study, we did not knockdown IL-17A receptor (IL-17AR) on TNBC lines. We attribute the fact that ΔIL-17A TNBC cells respond to IL-17A to the presence of IL-17A receptor in such cell lines. When pretreatment of anti-IL-17A neutralizing antibody (3 μg/mL) in the culture medium of wild type MDA-MB-231 or MDA-MB-468 lines, there is no significant difference on cell growth rate or migratory activity between WT and WT + Ab groups (supplementary reference 4).
Fig. 3.
Effects of exogenously administration of IL-17A on cell migration, gene expression of pro-inflammatory cytokines and chemokines in MDA-MB-231 and MDA-MB-468 cell lines. MDA-MB-468/231 cells were cultured in a transwell system as described in method followed by administration of different doses of IL17A (0-, 1-, 10 ng/mL) in the lower chamber. After 4 h (for MDA-MB-231) or 8 h (for MDA-MB-468), the migratory cells were photographed, quantified (one-way ANOVA) (a). For Western blot analysis, cells (1 × 106 / well) were cultured for 24 h in low serum medium, followed by another 24 h-culture, and then cells were harvested and probed with specific antibodies, quantified (one-way ANOVA) (b). mRNA transcripts of pro-inflammatory cytokines (TNF-α, IL-1β) and chemokines (CXCL2, CCL20) and COX2 were analyzed with real-time PCR (Mann–Whitney U test) (c). IL-17A gene expression including mRNA and protein level (d) was quantified by real-time PCR and ELISA assay (one-way ANOVA), respectively. Asterisk indicates a p value < 0.05
IL-17A upregulates gene expression of pro-inflammatory cytokines and chemokines
When IL-17A was added to the culture medium of TNBC cells, there was an increase in the mRNA transcripts of two pro-inflammatory cytokines (TNF-α, IL-1β) and two chemokines (CXCL2, CCL20), as well as of COX2; these changes occurred in both the MDA-MB-231 and MDA-MB-468 cell lines (Fig. 3c). It should also be noted that the addition of IL-17A also upregulated IL-17A gene expression at both the transcription level and the protein level (Fig. 3d). These findings suggest that IL-17A shows a degree of autoregulation that is likely to enhance the inflammatory cytokine and chemokine cascades.
In vivo metastasis
The role of IL-17A in the tumor behavior
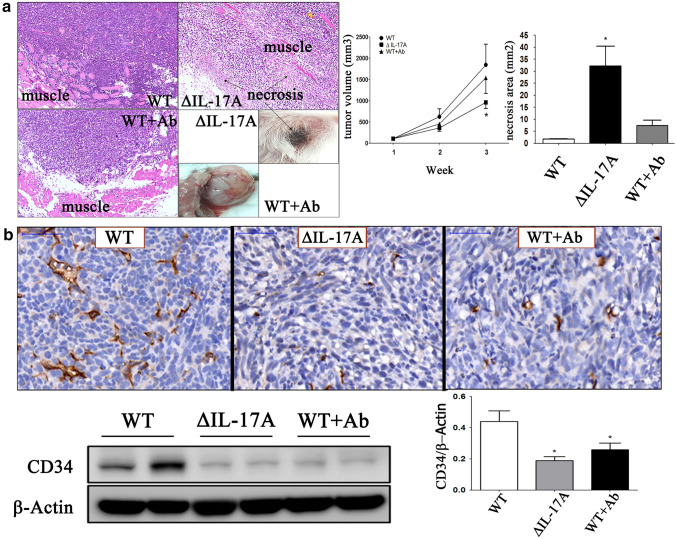
Using the murine mammary 4T1 cell line as an in vivo metastatic model, three animal study groups were created, namely the WT group, the knocked down IL-17A (∆IL-17A) group, and WT + anti-IL-17A neutralizing antibody (WT + Ab) group. The roles of IL-17A in tumors (the ∆IL-17A group) and surrounding tumor microenvironment (the WT + Ab group) were compared to the WT group. The results showed that there was a significant decrease in tumor volume and tumor weight (supplementary reference 5) and an increase area of tumor necrosis (Fig. 4a) in the ∆IL-17A group compared to the WT group. To investigate the role of IL-17A on angiogenesis in vivo, CD34 expression was measured by immunohistochemistry and Western blotting. It was found that there was a significant decrease in CD34 expression (Fig. 4b) in the ∆IL-17A and WT + Ab groups compared to the WT group.
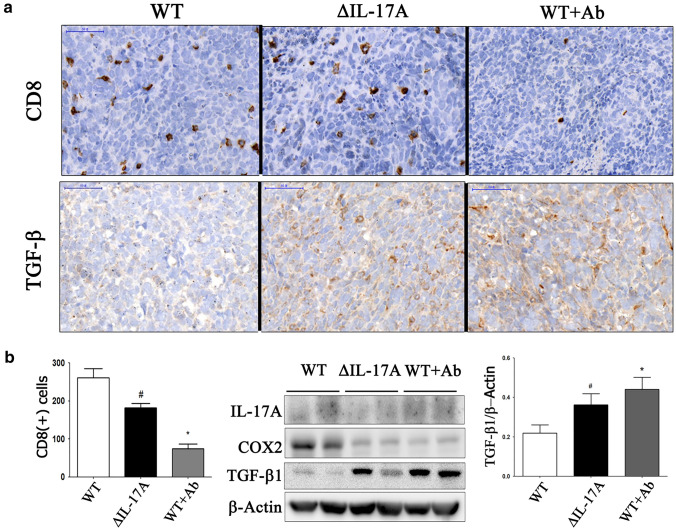
Fig. 4.
The role of IL-17A on in vivo implanted 4T1 cells in BALB/c mice. Three groups of 4T1–bearing BALB/c mice were designed, namely wild type (WT), ΔIL-17A, and WT + neutralizing IL-17 antibody (WT + Ab). After tumor cells were implanted. Neutralizing IL-17A antibody (250 μg/100μL) was injected, twice a week, intraperitoneally in WT + Ab group. After 3 weeks of tumor cells implantation, animals were sacrificed under adequate anesthetized. Tumor volume curve and necrosis area were photographed and measured (a). Immunohistochemistry and Western blot for CD34 expression were quantified (b). Bar indicated 50 μm. Asterisk indicates a p value < 0.05, Mann–Whitney U test
Role of IL-17A on tumor microenvironment
When dissecting the tumors away from the mice chest wall, it was surprising to find that the WT + Ab group tumors, which were no smaller in size than those of the WT group, were more easily freed from the adjacent chest wall due to either reduced adhesion or reduced vascularity (Fig. 4a). To investigate the role of IL-17A on the immune landscape in vivo, CD8 and TGF-β1 expression levels were measured by immunohistochemistry, while at the same time the expression level of TGF-β1 was also measured by Western blotting. The results for the ∆IL-17A and WT + Ab groups compared to the WT group showed that there was a significant decrease in CD8 ( +) T cells (Fig. 5a), and that this was accompanied by a decreased expression of COX2 and an increased expression of TGF-β1 expression (Fig. 5b).
Fig. 5.
The role of IL-17A on CD8 and TGF-β1 4T1 cells-implanted BALB/c mice. Three groups of 4T1–bearing BALB/c mice were designed, namely wild type (WT), ΔIL-17A, and WT + neutralizing IL-17 antibody (WT + Ab). After tumor cells were implanted. Neutralizing IL-17A antibody (250 μg/100μL) was injected, twice a week, intraperitoneally in WT + Ab group. After 3 weeks of tumor cells implantation, animals were sacrificed under adequate anesthetized. CD8 and TGF-β1 expressions were performed by immunohistochemistry (a) or/and Western blot (b). Bar indicated 50 μm. *, p value < 0.05, Mann–Whitney U test, compared to the WT group; #, p < 0.05, Student t test, compared to the WT group
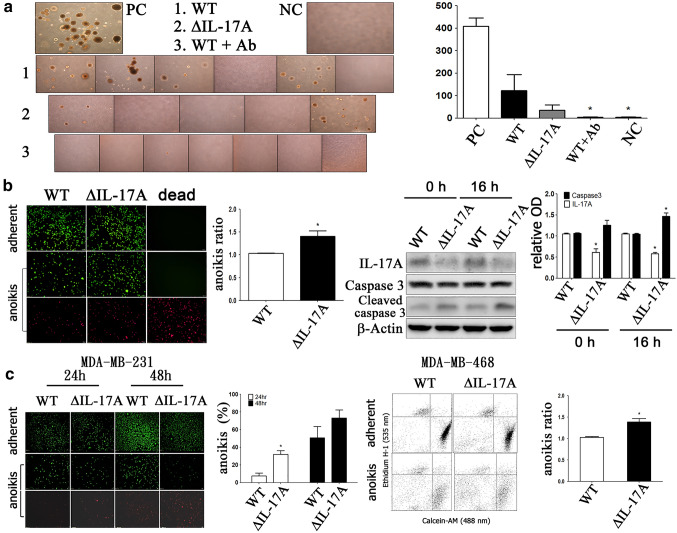
Role of IL-17A in the anoikis resistance of circulating tumor cells (CTCs)
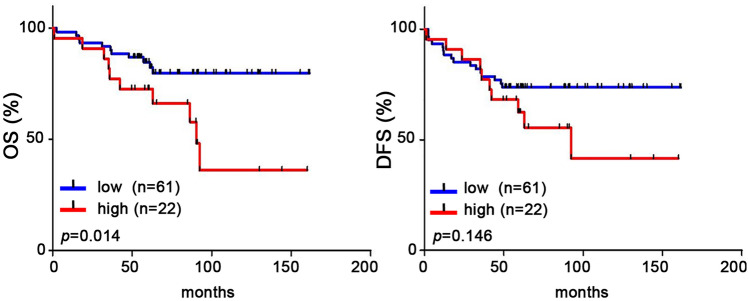
The CTCs obtained from the blood of sacrificed mice were isolated, subcultured, and counted by colony assay. It was found that there was a significant decrease in the number of CTC colonies from both the ∆IL-17A group and WT + Ab group compared to the WT group (Fig. 6a). Using the anoikis plate assay system as an in vitro model, the results showed that there was a decreased anoikis resistance in ∆IL-17A 4T1 group compared to the WT 4T1 group due to caspase 3 activation (Fig. 6b). Similarly, there was also a decrease in anoikis resistance (Fig. 6c) in the ∆IL-17A MDA-MB-231/468 group compared to the WT MDA-MB-231/468 group. These findings suggest that IL-17A plays an important role in maintaining CTC survival in the presence of anoikis. Clinically, the expression of IL-17A was found to correlate negatively with the OS of studied patients (Fig. 7a), and this was accompanied by a negative trend in relation to the DFS of the studied patients (Fig. 7b).
Fig. 6.
The role of IL-17A on circulating tumor cells (CTCs) and anoikis resistance in 4T1 cells-implanted BALB/c mice. Three groups of 4T1–bearing BALB/c mice were designed, namely wild type (WT), ΔIL-17A, and WT + neutralizing IL-17 antibody (WT + Ab). After tumor cells were implanted. Neutralizing IL-17A antibody (250 μg/100μL) was injected, twice a week, intraperitoneally in WT + Ab group. After 3 weeks after tumor cells implantation, animals were sacrificed under adequate anesthetized. CTCs were isolated, subcultured, selected with 6-thioquanine (60 μM), and ready for quantification with a 2-hydroxyethyl agarose colony assay (a). For anoikis assay, WT and ΔIL-17A 4T1 cells (b) or MDA-MB-231/MDA-MB-468 cells (c) were seeded onto adherent and anoikis (non-adherent) plate for 24 h, followed by stained with calcein-AM and ethidium homodimer-1 (live and dead stain) solutions and quantified by fluorescence microscopy (MDA-MB-231), flow cytometry (MDA-MB-468) or Western blot analysis as described in Methods. Relative optic density (O.D.) indicated IL-17A/β-actin or caspase (cleaved/total) ratio. Asterisk indicated a p value < 0.05, compared to the WT group at the same time point (Mann–Whitney U test)
Fig. 7.
Clinicopathologic correlation between IL-17A expression and patients’ prognosis. IL-17A protein expression by immunohistochemistry was correlated with patient survival, including overall survival (OS, a) and disease-free survival (DFS, b). The protein expression of IL-17A was semi-quantified and expressed as (0), < 10%, (1), 11–25%, (2), 26–50%, and (3) > 50% of tumor cells. The MEGF11 expression level was defined as low (≤ 60%, n = 61) and high (> 60%, n = 22). Asterisk indicated a p value < 0.05 by Kaplan–Meier survival analysis
Discussion
Using paired (non-recurrent and recurrent) resected tumor tissues obtained from breast cancer patients, we were able to identify a novel gene, MEGF11. This gene is highly associated with tumor recurrence among TNBC patients. In addition, overexpression of the MEGF11 gene is able to upregulate IL-17A gene expression in TNBC MDA-MB-231 and MDA-MB-468 cells. Although IL-17A is well known to be highly associated with inflammatory diseases, including rheumatoid arthritis and psoriasis [9], the role of IL-17A in TNBC recurrence needed further elucidation. In this study, we have demonstrated that IL-17A promotes the migratory activity of the tumors and changes the tumor’s immune landscape toward a tumor microenvironment that favors cancer metastasis.
The experiments involving knockdown of IL-17A were designed to investigate the role of IL-17A in tumor behavior. On the other hand, the WT + anti-IL17A antibody group was designed to investigate tumor microenvironment. Tumor necrosis, a form of cell death, is commonly associated with hypoxia, rapidly growth, and aggressive forms of breast cancer [22]. There is evidence that angiogenesis is essential for tumor growth and metastasis [23] and it is known that IL-17A promotes tumor angiogenesis via stimulation of endothelial fatty acid β-oxidation [24]. Using CD34 as a marker for hematopoietic stem cells [25], our results showing a significant increase in the area affected by necrosis and a decrease in CD34 expression by ΔIL-17A group compared to the WT group. This suggests that IL-17A enhances angiogenesis and hence reduces ischemic necrosis when there is rapid tumor progression.
On the other hand, when the tumors were dissected away from the mice chest wall, the tumors from the WT + Ab group were easily detached due to reduced adhesion and lower vascularity (Fig. 5a). The decrease in CD34 expression level supports the hypothesis that this is due to decreased angiogenesis in this group. Recent investigations have suggested that the immune system plays a dual role in tumor progression; specifically, it can act to either inhibit or promote tumor expansion. For example, TGF-β1, which is produced during chronic inflammation, is known to actively promote tumor growth and metastasis [10, 26]. In addition, the TGF-β signaling pathway is also known to play a key role in the early development of Treg cells in the thymus [27]. These cells are involved in maintaining the homeostasis of peripheral CD4 + T cells via increasing IL-7Rα expression (28), as well as controlling CD8+ T-cell homeostasis [29]. It should be noted that there are several lines of evidence indicating that TGF-β has the opposite effect on Th17 cells; specifically, there is an effect on the immunoregulatory/pathogenic roles of Th17 cells in a clinical setting [30, 31].
There are conflicting roles for IL-17A in carcinogenesis. Overexpression of IL-17A in tumor cells could suppress tumor growth through enhanced antitumor activity or promote it through enhanced angiogenesis. The tumor-inhibitory activity of IL-17A observed in immunocompetent mice is postulated to through the generation of cytotoxic T lymphocytes (CTLs). Thus, in IL-17 knockdown and anti-IL-17 antibody-injected mice, the tumor growth, being not affected by IL-17A-mediated CTL cytotoxicity, persists. While tumor cells secrete TGF-β and TGF-β positively regulate the tumor growth, it is expected TGF-beta, as a tumor promoting factor plays the important role on tumor growth in the IL-17 knockdown and anti-IL-17 group. Actually, our results disclosed that there was no statistical significance in tumor volume and weight between wild type (WT) group and WT + Ab group. In contrast, there was a significant decrease in tumor volume and tumor weight (supplementary reference 5) and an increase area of tumor necrosis in the ∆IL-17A group compared to the WT group. The markedly increased necrotic area (Fig. 4a) lead us to speculate the possibility of rapid tumor growth without adequate blood supply or neoangiogenesis. Our results that there was a significant decrease in CD34 expression (Fig. 4b) in the ∆IL-17A and WT + Ab groups compared to the WT group support such speculation. On the other hand, TGF-β secreted by tumor cells is known to suppress the immune surveillance response. Recent evidence suggests that IL-17 and TGF-β synergistically protects 4T1 cells from apoptosis and IL-17 is confirmed as an important pro-survival factor for 4T1 cells. Besides, TGF-β promotes immune subversion by enhancing IL-6 expression and by synergizing with IL-17 to enhance tumor cell survival. When IL-17A is knocked down or blocked by anti-IL-17A neutralizing antibody, TGF-β (without IL-17A) positively regulates tumor growth and its secretion by tumor cells and suppresses immune surveillance, including CD8 ( +) cytotoxic T cells. Our results show that there is a decrease in CD8 ( +) cells and an increase in TGF-β expression when the WT + Ab (anti-IL-17A) group is assessed and this has led us to speculate that TGF-β plays an immunosuppressive role that can lead to the immune escape of implanted 4T1 tumors, which is in agreement with a previous investigation [32].
The presence of circulating tumor cells (CTCs) is well known to be a mechanism whereby tumor metastasis or cancer recurrence can occur [33]. Several hypotheses concerning the evasion of immune surveillance by CTCs have been proposed, such as adhesion to platelets, adhesion to or myeloid-derived suppressor cells [34, 35], and evasion mediated by PD-L1 [36]. Our results showing that there is a significantly lower number of CTC colonies obtained from the ΔIL-17A and WT + anti-IL17A Ab groups compared to the WT group. This demonstrates that there is a negative correlation between IL-17A expression and the number of CTCs in the blood, which is consistent with previous findings on colon cancer [37].
There is consensus that persistent chronic inflammation increases the risk of cancer [10] and that necrosis has pro-inflammatory and tumor promoting potential [38]. The use of nonsteroidal anti-inflammatory drugs (NSAIDs) is well known to decrease the incidence and mortality of many cancers [39]. The IL-17E/IL-17E receptor axis possibly underlies TNBC resistance to EGFR inhibitors and this suggests that inhibiting IL-17E or its receptor, in combination with EGFR inhibitor administration, may improve TNBC management [40]. Recent studies have suggested that IL-17A inhibitors might play an important role in the immune therapies used to treat psoriasis and ankylosing spondylitis patients [41].
Conclusion
Our findings demonstrate that IL-17A stimulates the migratory activity of TNBC cells and modulates the immune landscape of the tumor microenvironment such that it favors cancer metastasis. A blockade of IL-17A might provide a co-treatment option when trying to prevent tumor metastasis or tumor recurrence in TNBC patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are in debt to Chou, SS, Wang, YL, and Hsu, TH for their technique supports. This work was supported by the biobank from the Division of Experimental Surgery, Department of Surgery, Taipei Veterans General Hospital. We thank the Taiwan Animal Consortium (MOST 107-2319-B-001-002)--Taiwan Mouse Clinic, which is funded by the Ministry of Science and Technology (MOST) of Taiwan, for technical support in the IVIS animal experiment. We also thanks Ralph Kirby for English edition of this manuscript. Funding was provided by Ministry of Health and Welfare (Grant No. MOHW108-TDU-B-212-112015) and Health Promotion Administration, Ministry of Health and Welfare (Grant No. MOHW109-TDU-B-212-010001).
Author contributions
JHC formed the idea. LMT and JHC contributed equally in this manuscript. LMT supervised the experiments. CYL, LMT, YFT, CCH, and YSL provided clinical samples and data. CPH performed the experiments. CYH read the pathology slices.
Funding
This work was supported by grants from the Ministry of Health and Welfare (Center of Excellence for Cancer Research at Taipei Veterans General Hospital phase II, MOHW108-TDU-B-212–112,015, phase III, MOHW109-TDU-B-212–010,001).
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Compliance with ethical standards
Conflict of interest
We declare that we have no conflicts of interest, including financial and non-financial interests such as the following items.
Unpaid membership in a government or non-governmental organization.
Unpaid membership in an advocacy or lobbying organization.
Unpaid advisory position in a commercial organization.
Writing or consulting for an educational company.
Acting as an expert witness.
Ethics approval and consent to participate
Study protocols involving experimental mice followed ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and were approved by the Institutional Animal Committee of and Taipei Veterans General Hospital (No. 2018–029). The human study for tumor tissue utilization from the biobank was approved by the Institutional Review Board of Taipei Veterans General Hospital (# 2013–10-020BC).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ling-Ming Tseng, Jen-Hwey Chiu contributed equally in this manuscript.
References
- 1.Nik-Zainal S, Davies H, Staaf J et al Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Doi: D - NLM: EMS68344 EDAT- 2016/05/03 06:00 MHDA- 2016/06/29 06:00 CRDT- 2016/05/03 06:00 PHST- 2015/06/29 00:00 [received] PHST- 2016/03/17 00:00 [accepted] PHST- 2016/05/03 06:00 [entrez] PHST- 2016/05/03 06:00 [pubmed] PHST- 2016/06/29 06:00 [medline] AID - nature17676 [pii] AID - 10.1038/nature17676 [doi] PST - ppublish
- 2.Podo F, Buydens LMC, Degani H, et al. Triple-negative breast cancer: Present challenges and new perspectives. Mol Oncol. 2010;4:209–229. doi: 10.1016/j.molonc.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P. Biology and management of patients with triple-negative breast cancer. Oncologist. 2016;21:1050–1062. doi: 10.1634/theoncologist.2016-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thike AA, Cheok PY, Jara-Lazaro AR, Tan B, Tan P, Tan PH. Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Modern Pathol. 2010;23:123–133. doi: 10.1038/modpathol.2009.145. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann BD, Pietenpol JA, Tan AR (2015) Triple-negative breast cancer: molecular subtypes and new targets for therapy. American Society of Clinical Oncology Educational Book. E31-e9. 10.14694/edbook_AM.2015.35.e31 [DOI] [PubMed]
- 6.Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015;121:8–16. doi: 10.1002/cncr.28914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng L-M, Chiu J-H, Liu C-Y, Tsai Y-F, Wang Y-L, Yang C-W, Shyr Y-M. A comparison of the molecular subtypes of triple-negative breast cancer among non-Asian and Taiwanese women. Breast Cancer Res Treat. 2017;163:241–254. doi: 10.1007/s10549-017-4195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widera D, Martínez Aguilar R, Cottrell GS, Toll-like receptor 4 and protease-activated receptor 2 in physiology and pathophysiology of the nervous system: more than just receptor cooperation? Neural Regen Res. 2019;14:1196–1201. doi: 10.4103/1673-5374.251290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung MK, Kwak J-E, Shin E-C. IL-17A-producing Foxp3+ regulatory T cells and human diseases. Immune Netw. 2017;17:276–286. doi: 10.4110/in.2017.17.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabre JAS, Giustinniani J, Garbar C, Merrouche Y, Antonicelli F, Bensussan A. The interleukin-17 family of cytokines in breast cancer. Int J Mol Sci. 2018 doi: 10.3390/ijms19123880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welte T, Zhang XH. Interleukin-17 could promote breast cancer progression at several stages of the disease. Mediators Inflamm. 2015;2015:804347. doi: 10.1155/2015/804347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oeckinghaus A, Ghosh S. The NF-kappab family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Lv Y, Niu Y, Su H, Feng A (2017) Role of circulating tumor cell (CTC) monitoring in evaluating prognosis of triple-negative breast cancer patients in China. Med Sci Monit 23:3071–9. 10.12659/msm.902637 [DOI] [PMC free article] [PubMed]
- 15.Paoli P, Giannoni E, Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1833:3481–98. Https://doi.org/10.1016/j.bbamcr.2013.06.026 [DOI] [PubMed]
- 16.Chiu J-H, Tseng L-M, Huang T-T, Liu C-Y, Wang J-Y, Huang C-P, Tsai Y-F, Hsu C-Y. MEGF11 is related to tumour recurrence in triple negative breast cancer via chemokine upregulation. Sci Rep. 2020;10:8060. doi: 10.1038/s41598-020-64950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 Breast tumor model. Curr Protoc Immunol. 2000;39:20.2.1–20.2.16. doi: 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 18.Chiu JH, Chen FP, Tsai YF, Lin MT, Tseng LM, Shyr YM. Effects of Chinese medicinal herbs on expression of brain-derived Neurotrophic factor (BDNF) and its interaction with human breast cancer MDA-MB-231 cells and endothelial huvecs. BMC Complement Altern Med. 2017;17:401. doi: 10.1186/s12906-017-1909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: twenty-something years on. Nat Protoc. 2006;1:581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 21.Huang S, Houghton PJ. Targeting mtor signaling for cancer therapy. Curr Opin Pharmacol. 2003;3:371–377. doi: 10.1016/S1471-4892(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 22.Tata A, Woolman M, Ventura M, et al. Rapid detection of necrosis in breast cancer with desorption electrospray ionization mass spectrometry. Sci Rep. 2016;6:35374. doi: 10.1038/srep35374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folkman J. Tumor angiogenesis: therapeutic implications. New Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 24.Wang R, Lou X, Feng G, et al. IL-17A-stimulated endothelial fatty acid β-oxidation promotes tumor angiogenesis. Life Sci. 2019;229:46–56. doi: 10.1016/j.lfs.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 25.Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32:1380–1389. doi: 10.1002/stem.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun S-C. The noncanonical NF-κb pathway. Immunol Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–653. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouyang W, Oh Soyoung A, Ma Q, bivonamichael R, Zhu J, liming O, TGF-β cytokine signaling promotes CD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor α expression. Immunity. 2013;39:335–346. doi: 10.1016/j.immuni.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan MH, Chiou YS, Tsai ML, Ho CT. Anti-inflammatory activity of traditional Chinese medicinal herbs. J Tradit Complement Med. 2011;1:8–24. doi: 10.1016/s2225-4110(16)30052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gagliani N, Vesely MCA, Iseppon A, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma M, Kaveri SV, Bayry J. Th17 cells, pathogenic or not? TGF-β3 imposes the embargo. Cell Mol Immunol. 2013;10:101–102. doi: 10.1038/cmi.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam JS, Terabe M, Kang MJ, et al. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915–3923. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Lou X-L, Sun J, Gong S-Q, Yu X-F, Gong R, Deng H. Interaction between circulating cancer cells and platelets: clinical implication. Chin J Cancer Res. 2015;27:450–460. doi: 10.3978/j.issn.1000-9604.2015.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Q, Liao Q, Zhao Y. Myeloid-derived suppressor cells (MDSC) facilitate distant metastasis of malignancies by shielding circulating tumor cells (CTC) from immune surveillance. Med Hypotheses. 2016;87:34–39. doi: 10.1016/j.mehy.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Mazel M, Jacot W, Pantel K, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol. 2015;9:1773–1782. doi: 10.1016/j.molonc.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tseng JY, Yang CY, Liang SC, et al. Interleukin-17A modulates circulating tumor cells in tumor draining vein of colorectal cancers and affects metastases. Clin Cancer Res. 2014;20:2885–2897. doi: 10.1158/1078-0432.CCR-13-2162. [DOI] [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Yan L, Anderson GM, dewitte M, Nakada MT, Therapeutic potential of cytokine and chemokine antagonists in cancer therapy. Eur J Cancer. 2006;42:793–802. doi: 10.1016/j.ejca.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Merrouche Y, Fabre J, Cure H et al. (2016) IL-17E synergizes with EGF and confers in vitro resistance to EGFR-targeted therapies in TNBC cells. Oncotarget 7:53350–61. 10.18632/oncotarget.10804 [DOI] [PMC free article] [PubMed]
- 41.Reis J, Vender R, Torres T. Bimekizumab: the first dual inhibitor of interleukin (IL)-17A and IL-17F for the treatment of psoriatic disease and ankylosing spondylitis. BioDrugs. 2019;33:391–399. doi: 10.1007/s40259-019-00361-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).