Abstract
Background
Immune-related adverse events (IrAEs) are auto-immune reactions associated with immune checkpoint inhibitor-based therapy (ICI). Steroids are currently the first-line option for irAE management; however, recent studies have raised concerns regarding their potential impairment of tumor-specific immune responses. In this study, we investigated the in vitro effects of commonly used irAE treatment drugs on the anti-tumor activity of tumor-infiltrating lymphocytes (TILs).
Methods
Impairment of anti-tumor immune responses by four drugs (antibodies: vedolizumab and tocilizumab; small molecules: mycophenolate mofetil and tacrolimus) reported to be effective in treating irAEs was tested at clinically relevant doses in vitro and compared to a standard moderate dose of corticosteroids (small molecules) or infliximab (antibodies). TIL responses against autologous tumor cell lines, in the presence or absence of irAE drugs, were determined by flow cytometry (short-term tumor-specific T-cell activation) or xCELLigence (T-cell-mediated tumor killing).
Results
None of the tested antibodies influenced T-cell activation or T-cell-mediated tumor killing. Low-dose mycophenolate and tacrolimus did not influence T-cell activation, whereas higher doses of tacrolimus (> 1 ng/ml) impaired T-cell activation comparably to dexamethasone. All tested small molecules impaired T-cell-mediated tumor killing, with high-dose tacrolimus reducing killing at levels comparable to dexamethasone-mediated inhibition. In addition, mycophenolate and tacrolimus alone also demonstrated anti-proliferative effects on tumor cells.
Conclusions
These data support clinical testing of targeted immune-regulatory strategies in the initial phase of irAE management, as a potential replacement for corticosteroids.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02760-z) contains supplementary material, which is available to authorized users.
Keywords: Immune-related adverse events, Immune checkpoint inhibitors, Immune regulatory drugs, Tumor-infiltrating lymphocytes
Introduction
Immunotherapy with checkpoint inhibitors (ICIs) has become one of the core pillars of cancer treatment [1]. ICIs block immunosuppressive axes [2], which homeostatically promote immune tolerance [3], but facilitate immune evasion in the tumor microenvironment (TME). Hence, although boosting anti-tumor immunity, these treatments may cause immunotherapy-induced auto-immune reactions or “immune-related adverse events” (irAEs). Virtually, all organs and systems can be targeted by auto-immune attacks, resulting in a range of clinical manifestations, from mild reactions to life-threatening events [4]. As the number of patients treated with ICIs is growing dramatically [5], irAEs are becoming increasingly common in clinical practice. Steroids have historically been the mainstay of irAE management [6], but in recent years, further investigation of targeted immune-regulatory strategies (TIRS) to counteract the detrimental effects of organ-specific auto-immune reactions has gained momentum. The most common TIRS for irAE management involve antibodies (i.e., infliximab, tocilizumab, and vedolizumab) or small molecule drugs (i.e., mycophenolate or tacrolimus), the use of which is increasing [6]. Corticosteroids may not represent the best choice for irAE treatment, as their inhibitory effect on T cells could hamper tumor-specific immunity [7] and promote T-cell dysfunction in the TME [8]. This study aimed to investigate whether drugs commonly used for advanced clinical management of irAEs could affect tumor-specific T-cell activation and T-cell-mediated tumor killing in vitro.
Methods
Tumor-infiltrating lymphocytes and tumor cell lines
Tumor-infiltrating lymphocytes (TILs) and autologous tumor cell lines (TCLs) were isolated and expanded as previously described [7], from stage IV melanoma biopsies, classified according to the American Joint Committee on Cancer (AJCC) 8th edition. Samples were obtained through enrollment in clinical trials at the Department of Oncology, Copenhagen University Hospital, Herlev, Denmark, and processed in the context of previously published studies [9, 10]. These trials were approved by the Ethics Committee, Capital Region of Denmark and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. Patients signed an informed consent prior to enrollment. All procedures were performed in compliance with national regulations for biomedical research. The samples used in this study were selected based on the significant killing of autologous tumors compared to control (allogeneic TILs—data not shown).
Drugs used for testing
T cells were co-cultured with autologous tumor cells in the presence of the following drugs commonly used for treating irAEs; 40 µg/ml vedolizumab (Takeda, Tokyo, Japan) [11], 200 µg/ml tocilizumab (Roche, Basel, Switzerland) [12], 0.02 µM dexamethasone (Sigma-Aldrich/Merck KGaA, Darmstadt, Germany; equivalent to maximum free blood level after repeated administration of 25 mg prednisolone [13]), 10 µg/ml infliximab (Hospira, Hurley, UK) [14], or 200 µg/ml IgG1 k1 isotype (16-4714, eBiosciences, Thermo Fisher Scientific, Waltham, MA, USA). Unless otherwise specified, mycophenolic acid (M5255, Sigma-Aldrich/Merck KGaA, Darmstadt, Germany), the active metabolite of mycophenolate mofetil, was tested at both 3.2 µg/ml (low dose), corresponding to the target blood concentration for clinical use [15], and at 32 µg/ml (high dose). To address the high variability of target tacrolimus concentrations in the blood, a range of clinically relevant tacrolimus (F4679, Sigma-Aldrich) concentrations (1, 5, and 10 ng/ml [16]), as well as a non-clinically relevant dose of 100 ng/ml, was used to depict a dose–response effect. Doses of tacrolimus are reported as low (1 ng/ml), intermediate (5 ng/ml and 10 ng/ml), or high (100 ng/ml). Methylprednisolone is commonly used as an intravenous infusion for the treatments of irAEs, and a recent study demonstrated that distinct glucocorticoids may affect T-cell responses [17]. Hence, additional experiments were conducted with 0.4 µM methylprednisolone (Sigma-Aldrich/Merck KGaA, Darmstadt, Germany; dose equivalent to dexamethasone 0.02 µM [18]). Dimethyl sulfoxide (Capital Region of Denmark Pharmacy, DMSO) was used as a vehicle control for 20 µM methylprednisolone.
T-cell activation and T-cell-mediated killing
Tumor-specific T-cell activation was assessed via 8-h autologous TIL-TCL co-culture assays (effector/target ratio = 3:1) and subsequent detection of CD107a, CD137, TNFα, and IFNγ using multiparameter intracellular cytokine staining (ICS) as described previously [7, 19]. T-cell activation was defined as the percentage of live CD8+ or CD4+ T cells identified by the Boolean gating “CD107a+ OR CD137+ OR TNFα+ OR IFNγ+” minus control.
T-cell-mediated killing was evaluated using the xCELLigence system RTCA SP real-time cell analyzer (00380601030, ACEA Biosciences Inc. San Diego, CA, USA) and E-plate 96 plates (05232376001, ACEA Biosciences Inc.) according to the manufacturer’s instructions. The percentage of T-cell-mediated killing in the presence of the different drugs was normalized to the percentage of killing in the positive control (tumor + TILs or tumor + TILs + isotype), which was set to 100% killing (the maximum killing the TILs from each patient could perform). The effects of the different drugs on tumor cell viability were evaluated through the xCELLigence system by adding the drugs to the E-plate culture wells in the absence of T cells. Given the potential anti-proliferative or cytotoxic effects of the drugs on tumors, drug-mediated tumor killing in the absence of TILs was subtracted from overall tumor killing in the presence of TILs to remove this confounding factor.
Statistical analyses
Statistical tests were conducted using a paired Wilcoxon signed-rank test. Graphs and statistical analyses were generated using Graphpad Prism 8. Negative values deriving from the subtraction of the control values from the experimental values were converted to 0.01% for statistical analyses and generation of figures. All values are expressed as the median. Tumor-specific T-cell activation in the presence of the IgG1 k1 isotype (200 µg/ml) differed less than ± 10% from tumor-specific T-cell activation in the absence of any reagent. Therefore, tumor-specific T-cell activation in the presence of vedolizumab, tocilizumab, and infliximab was compared to the T-cell activation in the absence of any reagent.
Results
The effects of TIRS on short-term tumor-specific T-cell activation in vitro
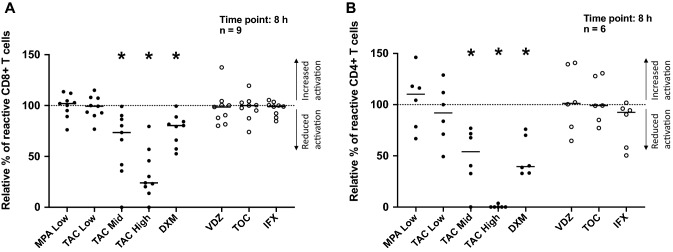
No reduction of T-cell activation was observed in the presence of the antibodies vedolizumab, tocilizumab, and infliximab (Fig. 1a, b). In contrast, although mycophenolate (both low dose and high dose, only results with low dose are shown) and low-dose tacrolimus did not impair T-cell activation (Fig. 1a, b), intermediate and high doses of tacrolimus reduced CD8+ (Fig. 1a) and CD4+ (Fig. 1b) T-cell activation to a similar or greater extent compared to dexamethasone. No difference was observed between either corticosteroid, dexamethasone, and methylprednisolone (Supplementary Fig. 1).
Fig. 1.
Effect of TIRS on T cell activation. The effects of various TIRS were evaluated with multiparameter intracellular cytokine staining after 8-h co-culture stimulation with autologous tumor cell lines, with a flow cytometry read-out. Black dots represent small molecules and empty dots represent antibodies. The background, TILs alone, was subtracted from the data and the data were normalized to tumor + TILs alone (visualized by the dotted line at 100%). The dot plots illustrate the effects of the drugs on CD8+ (a) and CD4+ (b) T cells. Tacrolimus, at 10 ng/ml and 100 ng/ml, and dexamethasone resulted in a significant reduction of CD8+ and CD4+ T-cell activation. Data are presented with median and tested for statistical significance using a Wilcoxon matched-pairs test. *p value = 0.01–0.05; MPA mycophenolate, TAC tacrolimus, DXM dexamethasone, VDZ vedolizumab, TOC tocilizumab, IFX infliximab
The effects of TIRS on T-cell-mediated tumor killing in vitro
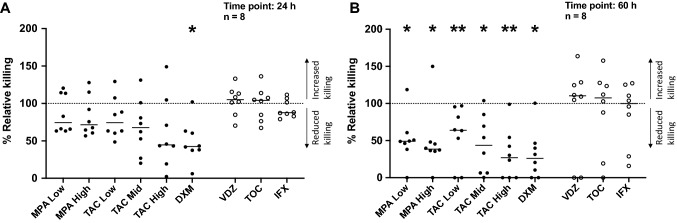
None of the tested antibodies influenced immune-mediated tumor killing (Fig. 2a, b). In contrast, all small molecules impaired tumor killing in vitro; the strongest effect being observed with high-dose tacrolimus, which was comparable to dexamethasone (Fig. 2a, b). Representative real-time tumor-survival curves are shown in Supplementary Fig. 2a and b. To gain additional insights into the effects of tacrolimus, a titration was carried out, revealing dose-dependent effects between 1 and 100 ng/ml (Supplementary Fig. 3a, b). Representative survival curves of tumors exposed to TILs and distinct doses of tacrolimus are shown in Supplementary Fig. 3c. Dexamethasone and methylprednisolone exerted similar inhibitory effects on T-cell-mediated tumor killing (Supplementary Fig. 4).
Fig. 2.
Effect of TIRS on T-cell-mediated tumor killing. The effect of various TIRS on T-cell-mediated tumor killing was measured via real-time tumor-killing assays on the xCELLigence platform and evaluated after a 24 h and b 60 h of co-culture of tumor, TILs, and drug. Small molecules were normalized to the control “tumor + TILs” and are represented as black dots, whereas all antibodies were normalized to the control “tumor + TILs + isotype” and are represented as empty dots. Controls are presented as a dotted line at 100%. a A significant reduction in T-cell-mediated killing was observed in the presence of dexamethasone after 24 h of co-culture. A trend of T-cell-mediated killing reduction is also evident with increasing doses of tacrolimus. b A significant reduction in T-cell-mediated killing was observed in the presence of mycophenolate, tacrolimus, and dexamethasone after 60 h of co-culture. Data are presented with median and tested for statistical significance using a Wilcoxon matched-pair test. p values: *0.01–0.05, **0.001–0.01; MPA mycophenolate, TAC tacrolimus, DXM dexamethasone, VDZ vedolizumab, TOC tocilizumab, IFX infliximab
Tumor-cell viability is affected by TIRS in vitro
To assess the direct effect of TIRS on tumor growth, we measured tumor cell growth in the presence of the different drugs without T cells. Neither the antibodies nor dexamethasone inhibited tumor growth (Supplementary Fig. 5a, b). In contrast, both mycophenolate and tacrolimus (despite heterogeneous responses) impaired tumor growth (Supplementary Fig. 5a, b), and a peak reduction of 50% was observed for mycophenolate at 60 h, regardless of dose level (Supplementary Fig. 5b). Representative real-time tumor-survival curves, including the tacrolimus titration, are shown in Supplementary Figs. 3d, 5c and d.
Discussion
The clinical efficacy of multiple TIRS for irAE management is being investigated in numerous clinical trials [6]; however, very little is known regarding how strategies counteracting irAEs affect immune responses to cancer. Increasing evidence indicates that the occurrence of irAEs may be a sign of ICI activity [20, 21], suggesting prolonged tumor control even after treatment discontinuation [22] and thus highlighting the importance of administering a treatment that does not hamper T-cell function in the TME. To address these issues, we used a robust model reproducing TIL–tumor interactions to screen a number of drugs commonly used for irAE treatment for their effects on both T cells and tumor cells. A moderate dose of corticosteroids (corresponding to an oral dose of 25 mg prednisolone) and a clinically relevant dose of infliximab, which we have previously shown not to affect the anti-tumor activity of TILs [7], were used as a benchmark to evaluate the effects of additional TIRS.
All the antibodies used (vedolizumab, tocilizumab, and infliximab) did not significantly affect T-cell activation and T-cell-mediated tumor killing. In addition, recent preclinical data showed that TNF blockade may even contribute to strengthening anti-tumor immune responses [23], and a real-world study suggested that baseline corticosteroids may impair clinical outcomes after PD-(L)1 blockade [24]. These observations indicate that such antibodies may be a better therapeutic choice than corticosteroids. In contrast, another real-world study described a possible detrimental effect of TNF blockade on clinical outcomes following immunotherapy [25]. Prospective studies are needed to draw firm conclusions about the impact of corticosteroids and TNF blockade on clinical outcomes following immunotherapy. Steroid-sparing strategies using early infliximab/vedolizumab [26] or tocilizumab (EudraCT no. 2018-002595-41) are already being investigated in the clinical setting.
Small immune-regulatory molecules currently represent a valid alternative to targeted antibodies in treating steroid-refractory irAEs. Previously, these drugs were primarily employed in the management of ICI-induced hepatitis [6]. The administration of mycophenolate has historically been the TIRS of choice in steroid-refractory ir-hepatitis [6], and tacrolimus has also been used with success in similar settings [27]. Our results showed that tacrolimus, especially when used at doses above 10 ng/ml, may impair anti-tumor T-cell activity in short-term assays. However, its net impairment on T-cell-mediated tumor killing after prolonged cancer-immune interplay and at clinically relevant doses (1–10 ng/ml) was similar to that of mycophenolate. Based on these results, we recommend clinical trials of tacrolimus as a treatment for irAEs to maintain a circulating level of < 5 ng/ml to reduce the risks of an involuntary inhibition of the anti-cancer mechanisms. Remarkably, the effect of 100 ng/ml tacrolimus, a concentration almost ten times higher than clinical target levels when used to prevent transplant rejection [16], was comparable to the inhibition exerted by a concentration of dexamethasone equivalent to 25 mg oral prednisolone. Based on our previous results, higher doses of steroids, typically used for managing CTCAE grade 3–4 irAEs, may result in even greater inhibition of tumor-specific T-cell activation [7]. As long as the efficacy of the small immune-regulatory molecules for treating steroid-refractory irAEs has already been proven multiple times in the clinic [4], our results support and represent a rationale to study the early use of these drugs as a valid alternative to steroids.
Importantly, both tacrolimus and mycophenolate appeared to impair tumor growth directly, demonstrating some intrinsic anti-cancer effects. However, the durability of clinical responses to immunotherapy is based on continuous immune surveillance despite treatment interruption, as is the case during irAEs. Therefore, the role of these small molecules in tumor control may not be clinically meaningful, given their limited administration duration. Moreover, Engl et al. demonstrated that mycophenolate may reduce the adhesion of tumor cells, which could confound the results of our xCELLigence analysis, as it relies on cell adhesion to estimate viability [28].
In conclusion, our data support a paradigm change in the clinical management of irAE towards steroid-sparing strategies. Despite its limitations, our model offers a specific focus on T cells, the primary drivers of responses, indicating that all TIRS (antibodies, small molecules at certain doses) can maintain high levels of tumor killing, in contrast to corticosteroids. Clinical trials testing the early initiation of TIRS in the context of steroid-sparing strategies are highly warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank all the patients who donated the samples used and the funding sources who generously supported this research (Danish Cancer Society R184-A11806, Sundhedsstyrelsen “Empowering Cancer Immunotherapy in Denmark”). Dr. Morten Hansen and Dr. Michael Douglas Crowther are acknowledged for technical assistance with the flow cytometry setup. Kasper Mølgaard Jensen is acknowledged for assistance in performing mycoplasma testing.
Abbreviations
- ICI
Immune checkpoint inhibitors
- IrAE
Immune-related adverse events
- TILs
Tumor-infiltrating lymphocytes
- TIRS
Targeted immune-regulatory strategies
- TME
Tumor microenvironment
Compliance with ethical standards
Conflict of interest
Marco Donia has received honoraria for lectures from Roche and Novartis (past 2 years); Inge Marie Svane has received honoraria for consultancies and lectures from Novartis, Roche, Merck, and Bristol-Myers Squibb; a restricted research grant from Novartis; and financial support for attending symposia from Bristol-Myers Squibb, Merck, Novartis, Pfizer, and Roche. All other authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mario Presti and Marie Christine Wulff Westergaard have contributed equally to this work.
References
- 1.Kelly PN. Special section the cancer immunotherapy revolution. Science. 2018;359:1344–1345. doi: 10.1126/science.359.6382.1344. [DOI] [PubMed] [Google Scholar]
- 2.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 3.Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 5.IQVIA (2019) Global oncology trends 2019. https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncology-trends-2019. Accessed 28 Oct 2020
- 6.Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Prim. 2020;6:38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draghi A, Borch TH, Radic HD, et al. Differential effects of corticosteroids and anti-TNF on tumor-specific immune responses: implications for the management of irAEs. Int J Cancer. 2019;145:1408–1413. doi: 10.1002/ijc.32080. [DOI] [PubMed] [Google Scholar]
- 8.Acharya N, Madi A, Zhang H, et al. Endogenous glucocorticoid signaling regulates CD8+ T cell differentiation and development of dysfunction in the tumor microenvironment. Immunity. 2020;53:658–671.e6. doi: 10.1016/j.immuni.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen R, Donia M, Ellebaek E, et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated il2 regimen. Clin Cancer Res. 2016;22:3734–3745. doi: 10.1158/1078-0432.CCR-15-1879. [DOI] [PubMed] [Google Scholar]
- 10.Andersen R, Borch TH, Draghi A, et al. T cells isolated from patients with checkpoint inhibitor-resistant melanoma are functional and can mediate tumor regression. Ann Oncol. 2018;29:1575–1581. doi: 10.1093/annonc/mdy139. [DOI] [PubMed] [Google Scholar]
- 11.Pouillon L, Vermeire S, Bossuyt P. Vedolizumab trough level monitoring in inflammatory bowel disease: a state-of-the-art overview. BMC Med. 2019;17(1):89. doi: 10.1186/s12916-019-1323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Georgy A, Rowell L. Pharmacokinetics and pharmacodynamics of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, following single-dose administration by subcutaneous and intravenous routes in healthy subjects. Int J Clin Pharmacol Ther. 2013;51:443–455. doi: 10.5414/CP201819. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Winkler J, Sabarinath SN, Derendorf H. Assessment of the impact of dosing time on the pharmacokinetics/pharmacodynamics of prednisolone. AAPS J. 2008;10:331–341. doi: 10.1208/s12248-008-9038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlsen A, Omdal R, Leitao KØ, et al. Subtherapeutic concentrations of infliximab and adalimumab are associated with increased disease activity in Crohn’s disease. Ther Adv Gastroenterol. 2018 doi: 10.1177/1756284818759930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koehl GE, Wagner F, Stoeltzing O, et al. Mycophenolate mofetil inhibits tumor growth and angiogenesis in vitro but has variable antitumor effects in vivo, possibly related to bioavailability. Transplantation. 2007;83:607–614. doi: 10.1097/01.tp.0000253756.69243.65. [DOI] [PubMed] [Google Scholar]
- 16.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623–653. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 17.Okoye IS, Xu L, Walker J, Elahi S. The glucocorticoids prednisone and dexamethasone differentially modulate T cell function in response to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade. Cancer Immunol Immunother. 2020;69:1423–1436. doi: 10.1007/s00262-020-02555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mager DE, Lin SX, Blum RA, et al. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43:1216–1227. doi: 10.1177/0091270003258651. [DOI] [PubMed] [Google Scholar]
- 19.Combined detection of CD137 and type 1 functions improves identification and characterization of the activated T lymphocyte repertoire - Annals of Oncology. https://www.annalsofoncology.org/article/S0923-7534(20)34468-9/abstract. Accessed 4 May 2020
- 20.Xing P, Zhang F, Wang G, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer. 2019;7:341. doi: 10.1186/s40425-019-0779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519–527. doi: 10.1001/jamaoncol.2019.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schadendorf D, Wolchok JD, Stephen Hodi F, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35:3807–3814. doi: 10.1200/JCO.2017.73.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Ruiz E, Minute L, Otano I, et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature. 2019;569:428–432. doi: 10.1038/s41586-019-1162-y. [DOI] [PubMed] [Google Scholar]
- 24.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non–small-cell lung cancer. J Clin Oncol. 2018;36:2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 25.Verheijden RJ, May AM, Blank CU, et al. Association of anti-TNF with decreased survival in steroid refractory ipilimumab and anti-PD1-treated patients in the Dutch melanoma treatment registry. Clin Cancer Res. 2020;26:2268–2274. doi: 10.1158/1078-0432.CCR-19-3322. [DOI] [PubMed] [Google Scholar]
- 26.Abu-Sbeih H, Ali FS, Wang X, et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2019;7:93. doi: 10.1186/s40425-019-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beardslee T, Draper A, Kudchadkar R. Tacrolimus for the treatment of immune-related adverse effects refractory to systemic steroids and anti-tumor necrosis factor a therapy. J Oncol Pharm Pract. 2019;25:1275–1281. doi: 10.1177/1078155218793709. [DOI] [PubMed] [Google Scholar]
- 28.Engl T, Makarević J, Relja B, et al. Mycophenolate mofetil modulates adhesion receptors of the beta1 integrin family on tumor cells: impact on tumor recurrence and malignancy. BMC Cancer. 2005;5:4. doi: 10.1186/1471-2407-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.