Abstract
There is an increased risk of colorectal cancer (CRC) development in patients with non-insulin-dependent type 2 diabetes. CD8+ T cells have been implicated in diabetes and are crucial for anti-tumor immunity. However, transcriptomic profiling for CD8+ T cells from CRC diabetic patients has not been explored. We performed RNA sequencing and compared transcriptomic profiles of CD8+ tumor-infiltrating T lymphocytes (CD8+ TILs) in CRC diabetic patients with CRC nondiabetic patients. We found that genes associated with ribogenesis, epigenetic regulations, oxidative phosphorylation and cell cycle arrest were upregulated in CD8+ TILs from diabetic patients, while genes associated with PI3K signaling pathway, cytokine response and response to lipids were downregulated. Among the significantly deregulated 1009 genes, 342 (186 upregulated and 156 downregulated) genes were selected based on their link to diabetes, and their associations with the presence of specific CRC pathological parameters were assessed using GDC TCGA colon database. The 186 upregulated genes were associated with the presence of colon polyps history (P = 0.0007) and lymphatic invasion (P = 0.0025). Moreover, CRC patients with high expression of the 186 genes were more likely to have poorer disease-specific survival (DSS) (Mantel–Cox log-rank P = 0.024) than those with low score. Our data provide novel insights into molecular pathways and biological functions, which could be altered in CD8+ TILs from CRC diabetic versus nondiabetic patients, and reveal candidate genes linked to diabetes, which could predict DSS and pathological parameters associated with CRC progression. However, further investigations using larger patient cohorts and functional studies are required to validate these findings.
Supplementary Information
The online version contains supplementary material available at (10.1007/s00262-021-02879-7).
Keywords: Colorectal cancer, Diabetes mellitus, CD8+ T cell transcriptome, Lymphatic invasion, Colon polyps
Introduction
Type 2 diabetes mellitus (T2DM), non-insulin dependent, is a risk factor for the development of colorectal cancer (CRC), and the incidence of both diseases increases with age [1]. Apart from this, diabetic patients with CRC usually do not perform well post tumor resection as they are more susceptible to other health complications, such as surgical site infections and myocardial ischemia, which are recognized as the main causes of high morbidity in these patients [2].
Increased serum glucose levels in CRC patients have been positively correlated with poor disease prognosis, poor overall survival (OS) and disease-free survival (DFS) [3]. CRC tumors are heterogeneous, characterized by multiple phenotypes, which are influenced by several factors including genetic, epigenetic, microbial, metabolic, environmental factors, in addition to lifestyle and diet [4]. Similarly, there are many risk factors, which may predispose individuals to develop T2DM, including genetic and epigenetic effects, lifestyle, diet, metabolic disorders, obesity and aging [5, 6]. Obesity and inflammation of adipose tissue are key factors, among others, which lead to metabolic disorders, diabetes and cardiovascular complications. Immune cells, such as CD8+ T cells, can contribute to the inflammation of adipose tissue, insulin-mediated resistance and T2DM pathogenesis/progression by increasing IFN-γ production and reducing the sensitivity to insulin [7, 8]. Patients with T2DM tend to have higher levels of CD8+ T cell-derived cytokines; however, the level of CD8+ T cells is reduced after 120 min of oral glucose tolerance test and glucose loading [9]. Moreover, obese individuals showed a higher number of CD8+ T cells releasing high levels of IFN-γ in small bowel and colon, compared to lean individuals [6]. Indeed, a positive correlation between IFN-γ levels and body mass index (BMI) has been reported in patients with T2DM [10]. In mice, high fat diet has been associated with increased body weight and epididymal fat mass, and gut inflammatory bowel disease which is characterized by increased numbers of IFN-γ-producing CD8+ T cell and CD4+ Th1 cells, and reduced number of T regulatory cells (Tregs) [6, 7].
Studies have shown that CD8+ T cells are crucial for the induction of the inflammatory cascades in adipose tissue by triggering macrophage activation and migration via the release of chemokines, such as MCP-1, MCP-3 and RANTES [7, 8]. The molecular mechanisms underlying the proliferation and activation of CD8+ T cells in obese human or mice are not fully elucidated. However, there is evidence demonstrating that CD137 (alternatively known as 4-1BB, a co-stimulatory receptor, which can be expressed by CD8+ T cells) signaling pathway could be responsible for CD8+ T cell proliferation and cytokine release [11] and may result in abnormal glucose and lipid metabolism [12]. Moreover, CD137/CD137 ligand axis could play a role in the induction of the inflammatory cascade of adipose tissue by promoting monocyte and T cell recruitment [13]. Furthermore, it has been demonstrated that type I IFN response supports CD8+ T cell accumulation in the liver of obese mice, which in turn controls gluconeogenesis and modulates hepatic insulin sensitivity [8].
The current management of CRC in diabetic patients is tumor resection and treatment with drugs such as metaformin or others to control glucose levels and blood pressure if patients were hypertensive [14]. Although the role of CD8+ T cells in the pathogenesis and progression of T2DM and their importance for anti-tumor immunity have been established [15, 16], the molecular profile and functional characteristics of CD8+ TILs from CRC diabetic patients compared to nondiabetic patients have not been investigated. In this study, we hypothesized that cytokine- and metabolism-related pathways in tumor-infiltrating CD8+ T cells are altered in diabetic CRC patients, compared with CRC patients without diabetes, which ultimately can affect CD8+ T cell anti-tumor immune responses and impact disease prognosis. Thus, our main goal was to investigate the molecular pathways altered in CD8+ TILs from CRC diabetic patients versus CRC nondiabetic patients. Then, we selected a set of genes based on their known link with diabetes and analyzed their correlation with CRC phenotypes/pathological parameters.
Consequently, we investigated the transcriptomic profiling of colorectal tumor-infiltrating CD8+ T cells in diabetic patients (n = 6), compared to nondiabetic patients (n = 6). We analyzed RNA sequencing (RNA-Seq) data in the genomic data commons (GDC) of the cancer genome atlas (TCGA) of colon cancer (COAD) patients to identify relevant genes and examine their clinical relevance and prognostic significance.
Materials and method
Study population and sample acquisition
Twelve colorectal cancer patients, who undertook surgery at Hamad Medical Corporation, Doha, Qatar, were enrolled in this study. All patients did not receive any treatment before surgery and provided written informed consent. Diabetic patients were diagnosed with type 2 diabetes before they were diagnosed with cancer. The demographic, clinical and pathological details for the study cohort are shown in Table 1. Hemoglobin A1c (HbA1c) levels (which reflect blood glucose control levels) were measured in diabetic patients at the time of operation for tumor resection and shown in Table 1. Normal HbA1c level is below 5.7%, a level of 5.7–6.4% indicates prediabetes, and a level of 6.5% or more indicates diabetes. Fasting blood sugar (FBS) levels in the preoperative setting were measured in all patients, and value of BMI was calculated (Table 1). Normal range for FBS level is 3.3–5.5 mmol/L; FBS level of 5.6–6.9 mmol/L is considered as impaired fasting glucose; and FBS level of 7.0 mmol/L or higher indicates type 2 diabetes. Normal range for BMI is 18.5–24.9 kg/m2, BMI range of 25–29.9 kg/m2 indicates overweight, while BMI of 30 kg/m2 or greater indicates obesity. Generally, the majority of diabetic patients had higher FBS levels, above the normal range, and all of them had high BMI (25.3–38 kg/m2) indicating overweight or obesity, while in the nondiabetic cohort, the BMI was normal in three patients, overweight in two patients and obese in only one patient (CRC-1).
Table 1.
Demographic, clinical, pathological and treatment details for the study cohort
| Patient ID | Diabetic | Gender | Age | A1c levels (%) | Pre-operation FBS level (mmol/L) | BMI§ | Duration of Diabetes (years) | TNM Stage | Grade | Tumor budding | Treatment details for diabetic patients |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CRC-1 | No | M | 69 | N.D | 6.1** | 34.1 | N/A | I | G2 | Low | N/A |
| CRC-2 | No | F | 60 | N.D | 5.2* | 26.5 | N/A | I | G2 | Int | N/A |
| CRC-3 | No | F | 23 | N.D | 5.0* | 21.3 | N/A | II | G2 | Low | N/A |
| CRC-4 | No | M | 43 | N.D | 6.6** | 27.0 | N/A | II | G2 | Low | N/A |
| CRC-5 | No | M | 53 | N.D | 4.7* | 23.0 | N/A | III | G2 | High | N/A |
| CRC-6 | No | F | 68 | N.D | 5.8** | 22.0 | N/A | IV | G2 | Low | N/A |
| CRC-7 | Yes | M | 76 | 6.2 | 4.2* | 25.7 | 17 | II | G2 | Low | Metformin, Aspirin, Clopidogrel |
| CRC-8 | Yes | F | 68 | 9.5 | 14.4*** | 33.8 | 19 | II | G2 | High | Metformin, Insulin Glargine |
| CRC-9 | Yes | M | 62 | 6.3 | 7*** | 26 | 17 | II | G2 | High | Metformin, Insulin Glargine |
| CRC-10 | Yes | M | 67 | 6 | 6.4** | 25.3 | 5 | IV | G2 | High | Insulin Glargine, Insulin Aspart, Bisoprolol |
| CRC-11 | Yes | M | 57 | 5.9 | 6.4** | 26.5 | 5 | IV | G2 | Low | Insulin Aspart |
| CRC-12 | Yes | M | 64 | 7.8 | 9.9*** | 38 | 15 | IV | G2 | Int | Metformin, warfarin, Clopidogrel |
CRC; colorectal cancer, N.D; not determined, Int; intermediate, A1c; hemoglobin A1c, FBS; fasting blood sugar, BMI; Body mass index, TNM; Tumor node metastasis, N/A; not applicable
*Normal range for FBS level: 3.3–5.5 mmol/L; **FBS level of 5.6 to 6.9 mmol/L is considered as impaired fasting glucose; ***FBS level of 7.0 mmol/L or higher indicates type 2 diabetes
§Normal BMI: 18.5–24.9 kg/m2, overweight: 25–29.9 kg/m2, Obesity: 30 kg/m2 or greater
Additionally, the duration of type 2 diabetes and treatment since diagnosis is indicated in Table 1. Type 2 diabetic patients were subjected to drug treatment, such as metaformin, insulin glargine and insulin aspart, to control glucose levels and blood pressure if patients were hypertensive, such as aspirin, clopidogrel, bisoprolol and warfarin (Table 1).
This study was executed under ethical approvals from Hamad Medical Corporation, Doha, Qatar (Protocol no. MRC-02–18-012) and Qatar Biomedical Research Institute, Doha, Qatar (protocol no. 2017–006).
Tissue specimens were processed and stored, as previously described [17]. Cells were dissociated from bulk tumor tissues using mechanical disaggregation, and single cell suspensions were used for cell sorting.
Fluorescence-activated cell sorting
Cell suspensions were washed with flow cytometric staining buffer (PBS with 1% FCS and 0.1% sodium azide) and stained with surface antibodies including anti-CD3-allophycocyanin-Cy7 (clone SK7, BD Pharmingen, San Jose, USA), anti-CD4-phycoerythrin (clone RPA-T4, BD Pharmingen) and anti-CD8-fluorescein isothiocyanate (clone RPA-T8; BD Pharmingen). FcR Blocking Reagent, human (Miltenyi Biotec, Bergisch Gladbach, Germany), was used for blocking Fc receptors, and 7-AAD viability dye (eBioscience, San Diego, USA) was used to gate live cells. The stained cells were kept at 4 °C for 30 min and washed twice with flow cytometric staining buffer. The cells were resuspended in Pre-Sort buffer (BD Biosciences), and BD FACSAria III SORP cell sorter on BD FACSDiva software (BD Biosciences) was used for sorting pure CD8+ T cell subpopulation (7AAD–CD3+CD4–CD8+). Applicable measures were taken to ensure minimal sorter-induced cell stress (SICS). Analyses were performed on FlowJo V10 software (FlowJo, Ashland, USA).
RNA isolation and amplification
Total RNA was isolated from sorted pure CD8+ T cells using RNA/DNA/protein purification Plus Micro Kit (Norgen Biotek Corporation, Ontario, Canada). mRNA was amplified using 5X MessageAmp™ II aRNA Amplification Kit (Invitrogen), as previously described [18]. RNA concentrations after the amplification were measured using Qubit RNA HS or Broad Range Assay Kits (Invitrogen, California, USA).
Library preparation
Amplified mRNA was used to prepare the library using Exome TruSeq Stranded mRNA Library Prep Kit (illumina, San Diego, USA), according to the manufacturer’s protocol. The quality and quantity of libraries were measured using bioanalyzer: high-sensitivity DNA kit (Agilent technologies, California, USA) and Qubit DNA HS kit (Invitrogen). The quality passed samples were further clustered using TruSeq PE Cluster Kit v3-cBot-HS (illumina) and sequenced on illumina HiSeq 4000 instrument.
Data processing and analyses of RNA-Seq
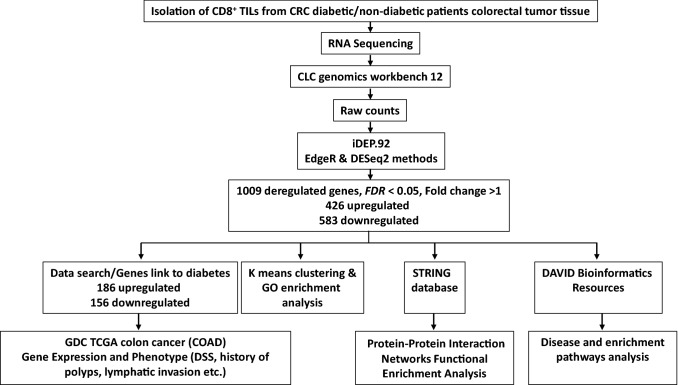
The workflow of RNA-Seq data processing and analyses is shown in Fig. 1. Differential gene expression of diabetic vs. nondiabetic patients in CD8+ TILs was performed using CLC Genomics Workbench 12 (Qiagen) using default settings. Briefly, the adaptors were trimmed out and paired end reads were aligned to hg19 human reference genome. iDEP.92 (http://bioinformatics.sdstate.edu/idep92/) was applied to identify functional clusters using the read counts and DESeq2 method and Z-scores were calculated from the TPM values, as previously described [19]. False discovery rate (FDR)-corrected differential expression tests were performed, and differentially expressed genes were identified with FDR cutoff < 0.05 (Supplementary Table 1). Using iDEP.92, k-mean enrichment analysis was performed to characterize functional clusters (Supplementary Table 2). In addition, the functional relationship among the upregulated and downregulated genes was identified by uploading them separately to STRING V11.0 tool (http://string-db.org). The Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses of DEGs were performed by the Database for Annotation, Visualization and Integrated Discovery (DAVID) tool, as previously described [17].
Fig. 1.
Flowchart summarizes the workflow of the RNA-Seq data processing and analyses. Online tools and database used in each step are included
GDC TCGA analysis
Out of 1009 differentially expressed genes with FDR cutoff < 0.05 (Supplementary Table 1), 342 genes were selected based on their link with diabetes, obtained from data search of PubMed, OMIM and http://amp.pharm.mssm.edu/Harmonizome which includes GAD Gene-Disease Associations, DISEASES Experimental Gene-Disease Associations, dbGAP Gene-Trait Associations, DISEASES Curated Gene-Disease Association, DISEASES Text-mining Gene-Disease Association, HPO Gene-Disease Associations, CTD Gene-Disease Associations, GWASdb SNP-Disease Associations, GWASdb SNP-Phenotype Associations and HuGE Navigator Gene-Phenotype Associations [20] (Supplementary Table 3). The selected genes (186 upregulated and 156 downregulated) were evaluated in the GDC TCGA colon cancer dataset accessed via the UCSC Xena platform (http://xena.ucsc.edu/) and tested for the association with specific CRC risk factor or pathological parameters, including the presence of polyps history, lymphatic invasion, venous invasion and perineural invasion.
The average RNA-Seq expression of the 186-upregulated and for the 156-downregulated genes were calculated from the GDC colon RNA-Seq patients’ dataset, and Kaplan-curves for disease-specific survival (DSS) were compared between patients with high (n = 229, top 50%) and low (n = 229, bottom 50%) scores (Supplementary Table 4). Furthermore, the distribution of patients with high and low scores across several risk factors or CRC-associated phenotypes (BMI, gender, age, history of polyps, lymphatic invasion, venous invasion, perineural invasion, synchronous colon cancer and tumor residue) were determined and Chi-squared test was used to reveal the association between the high and low score and each of the phenotypes.
Results
Differentially expressed genes in colorectal CD8+ TILs from diabetic and nondiabetic patients form distinct clusters
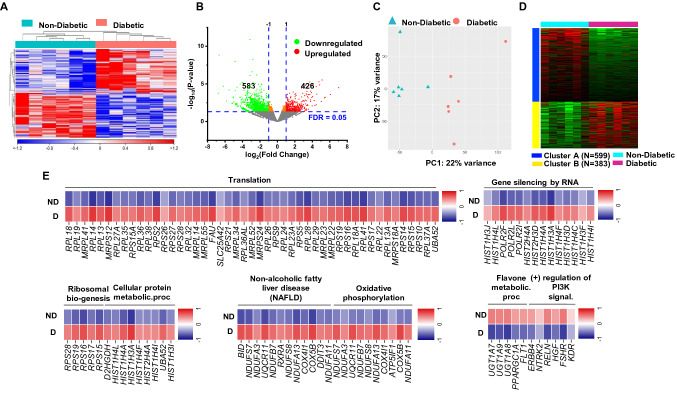
CD8+ T cells play a pathogenic role in T2DM patients, and they are usually activated and hyperproliferative [15]; however, CD8+ T cell-mediated anti-tumor immune response is crucial for tumor eradication and regression [16]. Therefore, we sought to investigate the potential alterations of the molecular signature and functional characteristics of colorectal CD8+ TILs from diabetic and nondiabetic CRC patients. We compared the transcriptomic profiling of tumor-infiltrating CD8+ T cells in CRC diabetic patients (n = 6) and CRC nondiabetic patients (n = 6). Hierarchical clustering shows distinct clusters of upregulated and downregulated genes comparing CRC diabetic and nondiabetic patients (Fig. 2a). Differentially expressed genes (DEGs) between diabetic and nondiabetic patients with FDR < 0.05 showed 426 upregulated and 583 downregulated genes (Fig. 2b). Principal component analysis (PCA) based on DEGs showed distinct clusters (Fig. 2c), illustrating the key differences in gene expression in CD8+ TILs from CRC patients with or without diabetes. PCA confirmed the close relativeness of the biological replicates within each group; PC1 shows 22% variance, while PC2 shows 17% (Fig. 2c).
Fig. 2.
Differentially expressed genes in CD8+ TILs in CRC diabetic versus nondiabetic patients. Hierarchical clustering for RNA-Seq data was performed on samples from sorted CD8+ TILs, isolated from tumor tissues of 12 CRC patients, comparing the transcriptomic profile of 6 patients with diabetes and 6 patients without diabetes a. Volcano plot of genes that were significantly upregulated (in red) or downregulated (in green), or remained unchanged (in grey) b. Principle component analysis (PCA) of differentially expressed genes, with FDR < 0.05, between CRC patients with or without diabetes c. K-mean clustering analysis was performed using iDEP.92 to show the clusters of differentially expressed genes in CD8+ TILs from CRC diabetic and nondiabetic patients d. Representative heatmap of Z-score for significantly downregulated or upregulated genes, from selected pathways, in CRC patients with diabetes vs. without diabetes e
Up/downregulated pathways in colorectal CD8+ TILs from diabetic patients, compared to nondiabetic patients
K-Means clustering and GO (biological process) enrichment analyses were performed using iDEP.92 for 1009 DEGs (FDR < 0.05) and showed that two different clusters (A and B) were mainly enriched in the deregulated genes. Genes in cluster A were downregulated, while genes in cluster B were upregulated in CRC diabetic patients, compared to CRC nondiabetic patients (Fig. 2d, Supplementary Table 2). Next, we performed functional annotation analysis on DEGs with FDR < 0.05, using DAVID web-based tool. We found that several genes associated with ribosome biogenesis, translation and protein synthesis, gene silencing by epigenetic regulations, metabolic regulation and oxidative phosphorylation were upregulated in CD8+ TILs from CRC patients with diabetes (Fig. 2e). These data indicate the potential involvement of ribosomal biogenesis, epigenetic/metabolic alterations and oxidative phosphorylation pathways in altering functional properties of CD8+ TILs in CRC diabetic patients [21]. In contrast, genes related to PI3K signaling and flavone metabolic pathways were downregulated in CD8+ TILs from patients with diabetes (Fig. 2e). This could be associated with the overexpression of inhibitory immune checkpoints, such as PD-1, which can in turn negatively influence PI3K/Akt/mTOR signaling pathway leading to attenuated aerobic glycolysis in activated T cells and impaired effector functions [22], and potentially induce metabolic reprograming and immunosuppressive functions [23].
Disease functional gene annotation in colorectal CD8+ TILs from diabetic, compared to nondiabetic patients
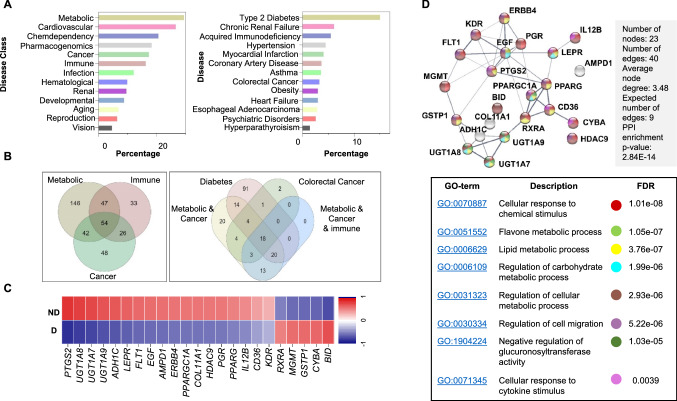
Based on the GAD_DISEASE_CLASS category, we found that 29% of DEGs were significantly enriched in the term “Metabolic” followed by “Cardiovascular” while 17% and 16% of DEGs were enriched within “Cancer” or “immune” terms, respectively (Fig. 3a). In GAD disease, 18% of DEGs were enriched within “Type 2 diabetes,” while smaller proportions of DEGs were related to chronic renal failure, hypertension, myocardial dysfunction, CRC and heart failure (Fig. 3a).
Fig. 3.
Functional gene enrichment analysis of differentially expressed genes in CD8+ TILs in CRC diabetic versus nondiabetic patients. Differentially expressed genes (up/downregulated) between CRC diabetic and nondiabetic patients were imported into DAVID platform for functional annotations. Bar plots for gene annotations based on GAD disease class and disease terms a. Venn diagrams for the significantly upregulated and downregulated genes in CD8+ TILs from CRC diabetic patients shared within different disease classes and terms b. Heatmap of Z-score for 23 down/upregulated genes, shared within the “Colorectal cancer,” and “Diabetes” disease terms, in CRC patients with diabetes c. Protein–protein interaction (PPI) network analysis of the 23 genes shared within “Colorectal cancer” and “Diabetes” disease terms, along with the Gene Ontology terms for each gene d
Next, we looked at the overlap between genes among different disease classes and disease terms. There were 54 DEGs shared genes between “Immune,” “Metabolic” and “Cancer,” and 23 DEGs were common between “Colorectal Cancer” and “Diabetes” (Fig. 3b). A heatmap showing the differential expression of these 23 genes is depicted in Fig. 3c and their functional annotation pathways in Fig. 3d. Results from disease functional annotation confirm the high specificity of the comparative transcriptomic profile for diabetes and its related pathways including metabolism- and immune-related pathways.
Protein–protein interaction network analysis for differentially expressed genes between CRC diabetic and nondiabetic patients
Using STRING web-based tool, we performed protein–protein interaction (PPI) and enrichment network analysis for some of the upregulated genes in CRC diabetic patients (Supplementary Fig. 1). Based on Gene Ontology annotations, some of these upregulated genes were related to amide biosynthesis, translation, ribosome biogenesis, endoplasmic reticulum (ER) stress, unfolding protein response, cell cycle arrest, oxidative phosphorylation, mitochondria ATP synthesis, response to oxidative stress and negative regulation of gene expression (Supplementary Fig. 1). STRING database identified 135 nodes and 691 edges with PPI enrichment P value < 1.0E–16, average clustering coefficient of 0.516 and average node degree of 10.2 (Supplementary Fig. 1).
PPI and enrichment network analysis were also performed for some of the downregulated genes in CRC diabetic patients (Supplementary Fig. 2). Based on Gene Ontology annotations, some of these downregulated genes were related to cellular response to cytokines, sodium ion transport channels (channels involved in glucose level control), response to lipid and cytokine signaling (Supplementary Fig. 2). STRING database identified 86 nodes and 104 edges with PPI enrichment P value < 1.0E-16, average clustering coefficient of 0.502 and average node degree of 2.42 (Supplementary Fig. 2).
Collectively, these data rationalize that increased expression of genes associated with fatty acid metabolism, oxidative stress, protein synthesis, protein misfolding and increased ATP production in CRC CD8+ TILs from diabetic patients could suppress CD8+ T cell effector functions [21]. Additionally, CRC CD8+ TILs from diabetic patients exhibit reduced ability to respond to cytokine signaling, lipids and glucose, which could in turn affect their effector function within the TME and favor tumorigenesis and immunosuppression [21].
TCGA analysis for RNA-Seq data predicts the correlation of diabetes to CRC-associated clinical phenotypes and the contribution to poor disease-specific survival
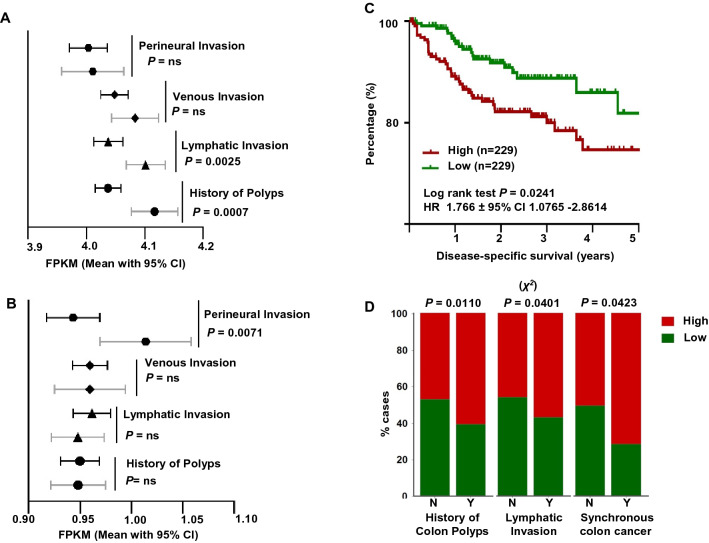
There are different pathological parameters which have been linked either to increase the risk of CRC development, such as history of adenomatous polyposis, or disease metastasis and poor prognosis, such as lymphatic, perineural and venous invasion [24, 25]. In order to understand the contribution of DEGs obtained from comparing the transcriptomes in diabetic and nondiabetic CRC patients to the risk of colon cancer and its prognosis, we examined the DEGs with FDR cutoff < 0.05 and selected 186 upregulated and 156 downregulated genes based on their link to diabetes. The expressions of the selected genes were evaluated in the GDC TCGA colon cancer databases for the patient’s phenotypes of history of polyps, and lymphatic, venous and perineural invasion.
Similar expression patterns were found when we compared the expression of the upregulated genes in patients with positive history of polyps or who exhibited lymphatic invasion with Welch's t test P value of 0.0007 and 0.003, respectively. Such significant correlations were not observed in patients with venous or perineural invasion (Fig. 4a). On the other hand, there was no significant similarity in the expression pattern of the downregulated genes for history of polyps, lymphatic invasion and venous invasion (Fig. 4b). Only a significant negative correlation between the expression pattern of downregulated genes and perineural invasion was observed (P value of 0.007, Fig. 4b).
Fig. 4.
Prognostic value of candidate genes in CD8+ TILs. Bar graphs showing the mean with 95% CI to the average RNA-Seq expression for the 186 upregulated a and for the 156 downregulated genes b calculated from GDC TCGA colon cancer database for patients with the absence (black line) or presence (grey line) of history of polyps (n = 432), lymphatic invasion (n = 462), perineural invasion (n = 182) or venous invasion (n = 445). Welch's t test was used to calculate the significant probability and are indicated near each bar. Kaplan–Meier curves for disease-specific survival were compared between patients with high (top 50%) or low (bottom 50%) score c. High and low scores were determined based on the average RNA expression of the upregulated genes, and patients were divided into two groups based on the score; the number (n) of patients in each group and the log-rank P value from Mantel–Cox test and HR ± 95% CI are indicated. Column graphs showing the distribution of CRC patients with high and low score across the different CRC clinical parameters d. The association between high and low scores, presence or absence of history of polyps (n = 387), lymphatic invasion (n = 416) or synchronous colon cancer (n = 409) were evaluated by Chi-squared (χ2) test (n.s.: not significant). (MedCalculator v12.7, https://www.medcalc.org/) was used to create the figures and calculate the Chi-squared (χ2)
Next, we calculated the average RNA-Seq expression for the upregulated and for the downregulated genes of 458 patients from GDC TCGA colon RNA-Seq database and established two scores of high and low (n = 229 each) for each sets of genes (Supplementary Tables 4). We then performed Kaplan–Meier analysis and found that CRC diabetic patients with a high score of gene expression comprising upregulated genes were more likely to have a shorter DSS (log-rank test P = 0.024, Fig. 4c). However, there was no significant difference in DSS between CRC diabetic patients with a high score or low score of gene expression comprising downregulated genes (log-rank test P = 0.6202, data not shown).
The distribution of patients with high and low scores of the upregulated genes across other CRC pathological parameters was assessed. CRC patients with high score were more likely to have history of polyps (P = 0.010, χ2 test), lymphatic invasion (P = 0.040, χ2 test) and synchronous colon cancer (P = 0.041, χ2 test) (Fig. 4d). There was no difference in the distribution of patients with high and low score and the presence or absence of perineural invasion and venous invasion (Supplementary Fig. 3A). However, patients with high score tend to have more residual tumor after therapy, compared to patients with low score (P = 0.088, χ2 test, Supplementary Fig. 3A). The distribution of patients with high and low score across different risk factors (BMI, age and gender) which could be related to diabetes, obesity and cancer was also assessed. The distribution of patients with high and low scores did not show any significant differences with BMI, gender or age (Supplementary Fig. 3b).
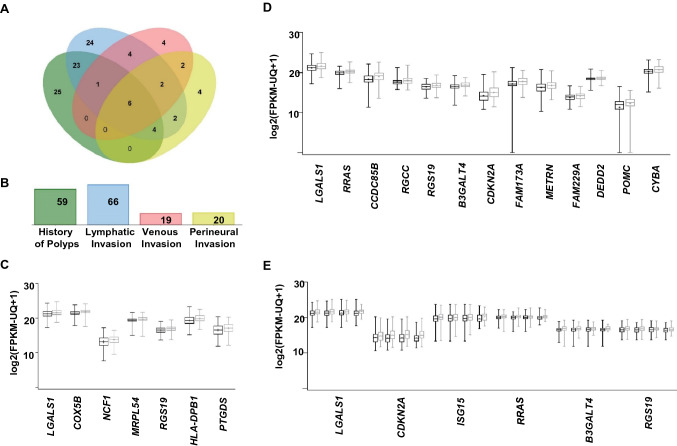
We further analyzed the overlaps between the upregulated genes which have similar expression pattern and P value of < 0.05 in CRC tumors with history of polyps, lymphatic invasion, venous invasion or perineural invasion (Fig. 5a and Supplementary Table 5). Out of the significantly upregulated genes, lymphatic invasion and history of polyps shared 23 genes, which account for 35% and 39% of the total number of significantly upregulated genes in each clinical parameter, respectively (Fig. 5a). The number of upregulated genes associated with each pathological parameter is shown in Fig. 5b. The most significant upregulated genes (n = 7, LGALS1, COX5B, NCF1, MRPL54, RGS19, HLA-DPB1 and PTGDS) in patients with history of polyps (Welch's t test P value < 0.001) are shown in Fig. 5c. While the most significant upregulated genes (n = 13, LGALS1, RRAS, CCDC85B, RGCC, RGS19, B3GALT4, CDKN2A, FAM173A, METRN, FAM229A, DEDD2, POMC and CYBA) in patients with lymphatic invasion (Welch's t test P value < 0.0001) are shown in Fig. 5d. Among these genes, there were 6 genes (LGALS1, RRAS, RGS19, B3GALT4, CDKN2A and ISG15), showing a significant association with the four parameters (Welch's t test P value < 0.05, Fig. 5e). Overall, these findings implicated the potential of the identified gene signature to predict CRC progression.
Fig. 5.
Gene overlaps among different clinical parameters associated with colorectal cancer. Venn diagram showing the overlaps between genes from the selected significantly upregulated genes (Welch's t test P < 0.05) in TCGA colon cancer patients with clinical parameters; history of polyps, lymphatic invasion, venous invasion or perineural invasion a. The number of shared genes among CRC clinical parameters is shown in the Venn diagram. Bar plots showing the number of the significantly upregulated genes associated with each CRC clinical parameter b. Box and whiskers plots showing the expression of most significant upregulated genes associated with history of polyps (n = 7, Welch's t test P value < 0.001) c and lymphatic invasion (n = 13, Welch's t test P value < 0.0001) d. Box and whiskers plot showing the expression of the overlapped genes (n = 6), which were significantly upregulated in GDC TCGA colon cancer patients with history of polyps, lymphatic invasion, perineural invasion or venous invasion e. The log2 (FPKM-UQ + 1) dataset was accessed via the UCSC Xena platform (http://xena.ucsc.edu/). Gray color indicates “presence of the phenotype,” and black color indicates the absence of the phenotype c–e
Discussion
The contribution of CD8+ T cells to the pathogenesis and progression of T2DM and to anti-tumor immunity has been established [15, 16]. However, the transcriptomic profile and functional characteristics of CD8+ TILs from CRC diabetic patients compared to nondiabetic patients have not been yet explored. Therefore, we compared the transcriptomic profile of colorectal CD8+ TILs in diabetic patients to nondiabetic patients, aiming to obtain insights into the altered biological mechanisms within colorectal CD8+ TILs in relation to diabetes.
There are many risk factors which may predispose individuals to develop CRC and T2DM, including aging, diet, metabolic disorders, and genetic and epigenetic factors [4, 5]. Metabolic stress, possibly induced by inflammation, oxidative stress, glucolipotoxicity or endoplasmic reticulum (ER) stress, is one of the key determinant factors which leads to the loss of β-cell function and T2DM development [26]. Here, we found that genes enriched within ribosome biogenesis, translation and protein synthesis were upregulated in CD8+ TILs from CRC patients with diabetes. Increased ribosomal biogenesis in CD8+ TILs could be an indication of TCR-mediated cell activation; weakly stimulated T cells possess lower capacity of protein synthesis [21]. Oxidative phosphorylation and altered metabolism are key factors, which can favor tumorigenesis and impact immune cell functions; targeting oxidative phosphorylation has been pointed to as one of the potential therapeutic strategies which could be utilized to improve current cancer therapies [27]. In parallel with this, genes enriched within oxidative phosphorylation and metabolic pathways were also upregulated in CD8+ TILs from diabetic patients, implying the potential proliferative capacity of these cells and their capability of exerting effector function [28]. However, this does not rule out the possibility that activated antigen-specific CD8+ TILs could have impaired effector T cell function as a result of increased glucose consumption by tumor cells causing nutrient deprivation [29]. In diabetes, high levels of glucose can increase the capacity of mRNA translation via increased ribosomal biogenesis, resulting in increased protein synthesis and ER stress, and ultimately cause renal hypertrophy [30]. Moreover, increased ribosomal biogenesis plays a role in cancer pathogenesis by inducing tumor formation and cell proliferation via supporting the progression of cell cycle and has implicated in the neoplastic formation of chronically inflamed tissues [31].
The finding that ribosomal biogenesis-related genes were highly expressed in CD8+ TILs from CRC diabetic patients may not only indicate cell activation and activated TCR-mediated signaling [21], but may also indicate T cell exhaustion in response to persistent antigen stimulation resulting in the overexpression of inhibitory immune checkpoints, such as PD-1, CTLA-4 and TIM-3. Activation of PD-1-mediated signaling pathways can negatively influence PI3K/Akt/mTOR signaling pathway resulting in the attenuation of aerobic glycolysis in activated T cells and thereby the inhibition of their effector functions [22]. Here, we found that genes enriched within PI3K signaling pathway were downregulated in CD8+ TILs from patients with diabetes, possibly suggesting a potential for metabolic reprograming causing a switch from aerobic glycolysis to lipolysis and fatty acid metabolism, which ultimately would influence their effector functions [23]. The suppressive milieu within the tumor microenvironment (TME) can also contribute to carcinogenesis and tumor progression [32, 33]. In support of this, we found that genes related to immune responses, including cytokine response, were downregulated in CD8+ TILs from diabetic patients, compared to nondiabetic patients.
History of polyps is one of the predisposing risk factor to CRC development [24], and lymphatic invasion is one of the risk factors for CRC metastasis, especially in patients with early disease stages [25]. Synchronous CRC refers to the detection of more than one primary CRC in a single patient, and its presence could arise because of genetic and environmental factors, and predispose patients to poorer cancer-specific survival, compared to solitary CRC [34]. Additionally, familial adenomatous polyposis, serrated polyps/hyperplastic polyposis and inflammatory bowel diseases are predisposing factors, which increase the risk of synchronous colorectal carcinoma [34]. In this study, we analyzed RNA-Seq data in the GDC of TCGA of COAD patients and identified a set of upregulated genes with a clinical relevance and prognostic significance in CRC patients. In light of the findings indicating the relationship between the history of polyps, lymphatic invasion and synchronous CRC, this study found that CRC diabetic patients with a high score of gene expression were more likely to have a shorter DSS, history of polyps, evidence of lymphatic invasion and synchronous colon cancer. Furthermore, these patients tended to have slightly more residual tumor after therapy, compared to patients with low scores.
Several genes among the most significantly upregulated genes in patients with lymphatic invasion were also upregulated in CRC patients with venous and perineural invasion. Our results are in line with other findings indicating the importance of these genes in T2DM and progression of CRC. One of these genes was LGALS1, encodes galectin-1 (an adipokine), which has been associated with T2DM and CRC progression and metastasis [35, 36]. Moreover, both secretion of galectin-1 (Gal-1) from tumor cells and endogenous expression of this gene in CD8+ T cells can negatively influence CD8+ T cell function and anti-tumor immune responses [37]. Here, we found that LGALS1 was upregulated in CD8+ TILs from CRC diabetic patients by more than twofold log change (FDR 1.06E-06), compared to that of CRC nondiabetic patients. Furthermore, significantly upregulated level of this gene in CRC diabetic patients was associated with CRC-related pathological parameters, highlighting the overlapping roles of this gene in T2DM, CRC and immunity.
The second gene is B3GALT4 which encodes beta1,3-galactosyltransferase-4, an enzyme which is related to metabolic pathways, insulin resistance and control of lipid concentration, and proposed to be a biomarker for gynecological carcinomas [38, 39]. High expression of B3GALT4 in patients with CRC was found to be associated with poor overall survival, and B3GALT4 was suggested along with B7-H3, the transmembrane glycoprotein that has role in immunosuppression, as novel prognostic biomarkers for CRC [40].
It is interesting that CDKN2A was among the top upregulated genes associated with the presence of history of polyps and lympho-vascular invasion. Although the physiological role of p16Ink4a (protein encoded by CDKN2A gene) is known to be a tumor suppressor protein, and its downregulation has been detected in various tumors [41], overexpression of CDKN2A has been implicated in the prognosis and invasive of several cancer types, including colorectal adenocarcinomas [42]. Additionally, CDKN2A is a risk factor for T2DM and has been implicated in the modulation of T cell phenotype/function in T2DM and cancer development [43, 44]. It has been reported that levels of CDKN2A mRNA and p16Ink4a protein levels were significantly lower in patients with T2DM and T2DM/coronary heart disease, compared to controls [43]. Moreover, both patient groups exhibited high levels of activated CD3+CD69+ T cells and low levels of Tregs in the circulation; however, the in vivo administration of p16Ink4a mimetic drug in mice increased the levels of Tregs [43]. The upregulation of CDKN2A mRNA in CD8+ T cells is associated with senescence and disrupted T cell differentiation [45].
Overall, data from this study provide novel insights into the molecular pathways and biological functions, which could be altered in CD8+ TILs from CRC diabetic vs. nondiabetic patients, and reveal candidate genes linked to diabetes, which could predict phenotypes associated with CRC progression and DSS. Additionally, this study demonstrates the importance of assessing the transcriptome CD8+ T cell profile in the TME among CRC diabetic patients and provides a path to conduct further analysis. However, further investigations in a larger number of samples and functional studies are warranted to validate these findings. It is also important to note that our patient cohort in both diabetic and nondiabetic groups had different disease stages; this could have some impact on the differential expression/pathway analyses. Therefore, it would be useful to validate our findings in diabetic and nondiabetic CRC patients with the same disease stages. For future studies, differential expression analysis could be performed to examine the transcriptomic profiling of CD8+ T cells from normal colon and compare it to CD8+ TILs in CRC tissue. This should provide more information on the impact of the TME on altering the molecular profile of CD8+ T cells. Moreover, comparative analysis for the transcriptomes of circulating CD8+ T cells in diabetic and nondiabetic patients could also be performed to examine the alterations, which could be induced by high glucose levels and the inflammation status in diabetic patients.
Supplementary Information
Acknowledgements
We would like to thank the patients for donating their samples. This work was supported by a start-up Grant [VR04] for Professor Eyad Elkord from Qatar Biomedical research Institute, Qatar Foundation.
Abbreviations
- CD8+ TILs
CD8+ Tumor-infiltrating lymphocytes
- CRC
Colorectal cancer
- DAVID
Database for annotation, visualization and integrated discovery
- DEGs
Differentially-expressed genes
- DSS
Disease-specific survival
- GDC
Genomic data commons
- iDEP
Integrated differential expression and pathway analysis
- RNA-Seq
RNA Sequencing
- T2DM
Type 2 diabetes mellitus
- TCGA
The cancer genome atlas
Author contributions
RS contributed to data curation, methodology, formal analysis, investigation and writing the original draft; VN contributed to data curation, methodology and formal analysis; KM and MAN contributed to sample acquisition and investigation; EE contributed to conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, validation, investigation, visualization, methodology, project administration, writing-review and editing; and RS contributed to conceptualization, data curation, formal analysis, validation, visualization, methodology, writing-review and editing.
Data availability and material
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This study was executed under ethical approvals from Hamad Medical Corporation, Doha, Qatar (Protocol no. MRC-02-18-012) and Qatar Biomedical Research Institute, Doha, Qatar (Protocol no. 2017-006).
Footnotes
The original online version of this article was revised: The gene names on Figure 3C in the proofs were missing.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/12/2021
A Correction to this paper has been published: 10.1007/s00262-021-02903-w
Contributor Information
Eyad Elkord, Email: e.elkord@salford.ac.uk.
Ranad Shaheen, Email: ranad_shaheen@hotmail.com.
References
- 1.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, 3rd, Fuchs CS. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21(3):433–440. doi: 10.1200/jco.2003.07.125. [DOI] [PubMed] [Google Scholar]
- 2.Sehgal R, Berg A, Figueroa R, Poritz LS, McKenna KJ, Stewart DB, Koltun WA. Risk factors for surgical site infections after colorectal resection in diabetic patients. J Am Coll Surg. 2011;212(1):29–34. doi: 10.1016/j.jamcollsurg.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Yang IP, Tsai HL, Huang CW, Lu CY, Miao ZF, Chang SF, Juo SH, Wang JY. High blood sugar levels significantly impact the prognosis of colorectal cancer patients through down-regulation of microRNA-16 by targeting Myb and VEGFR2. Oncotarget. 2016;7(14):18837–18850. doi: 10.18632/oncotarget.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogino S, Fuchs CS, Giovannucci E. How many molecular subtypes? Implications of the unique tumor principle in personalized medicine. Exp Rev Mol Diagn. 2012;12(6):621–628. doi: 10.1586/erm.12.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Candia P, Prattichizzo F, Garavelli S, De Rosa V, Galgani M, Di Rella F, Spagnuolo MI, Colamatteo A, Fusco C, Micillo T, Bruzzaniti S, Ceriello A, Puca AA, Matarese G. Type 2 diabetes: how much of an autoimmune disease? Front Endocrinol. 2019 doi: 10.3389/fendo.2019.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, Lei H, Luk CT, Shi SY, Surendra A, Copeland JK, Ahn J, Prescott D, Rasmussen BA, Chng MH, Engleman EG, Girardin SE, Lam TK, Croitoru K, Dunn S, Philpott DJ, Guttman DS, Woo M, Winer S, Winer DA. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21(4):527–542. doi: 10.1016/j.cmet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 8.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Blüher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28(7):1304–1310. doi: 10.1161/atvbaha.108.165100. [DOI] [PubMed] [Google Scholar]
- 9.Miya A, Nakamura A, Miyoshi H, Takano Y, Sunagoya K, Hayasaka K, Shimizu C, Terauchi Y, Atsumi T. Impact of glucose loading on variations in CD4(+) and CD8(+) T Cells in Japanese participants with or without Type 2 diabetes. Front Endocrinol (Lausanne) 2018;9:81. doi: 10.3389/fendo.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francisco CO, Catai AM, Moura-Tonello SC, Arruda LC, Lopes SL, Benze BG, Del Vale AM, Malmegrim KC, Leal AM. Cytokine profile and lymphocyte subsets in type 2 diabetes. Brazilian J Med Biol Res. 2016;49(4):e5062. doi: 10.1590/1414-431x20155062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu TH, Kim CS, Kang JH, Nam-Goong IS, Nam CW, Kim ES, Kim YI, Choi JI, Kawada T, Goto T, Park T, Yoon Park JH, Choi MS. Yu R (2014) Levels of 4–1BB transcripts and soluble 4–1BB protein are elevated in the adipose tissue of human obese subjects and are associated with inflammatory and metabolic parameters. Int J Obes. 2005;38(8):1075–1082. doi: 10.1038/ijo.2013.222. [DOI] [PubMed] [Google Scholar]
- 12.Kim CS, Kim JG, Lee BJ, Choi MS, Choi HS, Kawada T, Lee KU, Yu R. Deficiency for costimulatory receptor 4–1BB protects against obesity-induced inflammation and metabolic disorders. Diabetes. 2011;60(12):3159–3168. doi: 10.2337/db10-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu TH, Kim CS, Goto T, Kawada T, Kim BS, Yu R. 4–1BB/4-1BBL interaction promotes obesity-induced adipose inflammation by triggering bidirectional inflammatory signaling in adipocytes/macrophages. Mediators Inflamm. 2012;2012:972629. doi: 10.1155/2012/972629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci. 2015;112(6):1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar NP, Sridhar R, Nair D, Banurekha VV, Nutman TB, Babu S. Type 2 diabetes mellitus is associated with altered CD8(+) T and natural killer cell function in pulmonary tuberculosis. Immunology. 2015;144(4):677–686. doi: 10.1111/imm.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostroumov D, Fekete-Drimusz N, Saborowski M, Kuhnel F, Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci. 2018;75(4):689–713. doi: 10.1007/s00018-017-2686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasidharan Nair V, Saleh R, Toor SM, Taha RZ, Ahmed AA, Kurer MA, Murshed K, Alajez NM, Abu Nada M, Elkord E. Transcriptomic profiling disclosed the role of DNA methylation and histone modifications in tumor-infiltrating myeloid-derived suppressor cell subsets in colorectal cancer. Clin Epigenet. 2020;12(1):1–15. doi: 10.1186/s13148-020-0808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saleh R, Toor SM, Taha RZ, Al-Ali D, Sasidharan Nair V. Elkord E (2020) DNA methylation in the promoters of PD-L1, MMP9, ARG1, galectin-9, TIM-3, VISTA and TGF-beta genes in HLA-DR(-) myeloid cells, compared with HLA-DR(+) antigen-presenting cells. Epigenetics. 2020 doi: 10.1080/15592294.2020.1767373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malone BM, Tan F, Bridges SM, Peng Z. Comparison of four ChIP-Seq analytical algorithms using rice endosperm H3K27 trimethylation profiling data. PLoS ONE. 2011;6(9):e25260. doi: 10.1371/journal.pone.0025260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouillard AD, Gundersen GW, Fernandez NF, Wang Z, Monteiro CD, McDermott MG, Ma'ayan A. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Datab: J Biol Datab Cur. 2016 doi: 10.1093/database/baw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan TCJ, Knight J, Sbarrato T, Dudek K, Willis AE, Zamoyska R. Suboptimal T-cell receptor signaling compromises protein translation, ribosome biogenesis, and proliferation of mouse CD8 T cells. Proc Natl Acad Sci. 2017;114(30):E6117–E6126. doi: 10.1073/pnas.1700939114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, Li L, Boussiotis VA. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanitis E, Dangaj D, Irving M, Coukos G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann Oncol. 2017 doi: 10.1093/annonc/mdx238. [DOI] [PubMed] [Google Scholar]
- 24.Bonnington SN, Rutter MD. Surveillance of colonic polyps: Are we getting it right? World J Gastroenterol. 2016;22(6):1925–1934. doi: 10.3748/wjg.v22.i6.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan H, Dong Q, Zheng B, Hu X, Xu JB, Tu S. Lymphovascular invasion is a high risk factor for stage I/II colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(28):46565–46579. doi: 10.18632/oncotarget.15425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, Powers AC, Rhodes CJ, Sussel L, Weir GC. beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. J Clin Endocrinol Metab. 2014;99(6):1983–1992. doi: 10.1210/jc.2014-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res : An Offl J Am Assoc Cancer Res. 2018;24(11):2482–2490. doi: 10.1158/1078-0432.ccr-17-3070. [DOI] [PubMed] [Google Scholar]
- 28.Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, Tonc E, Schreiber RD, Pearce EJ, Pearce EL. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariappan MM, D'Silva K, Lee MJ, Sataranatarajan K, Barnes JL, Choudhury GG, Kasinath BS. Ribosomal biogenesis induction by high glucose requires activation of upstream binding factor in kidney glomerular epithelial cells. Am J Physiol Renal Physiol. 2011;300(1):F219–230. doi: 10.1152/ajprenal.00207.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penzo M, Montanaro L, Treré D, Derenzini M. The ribosome biogenesis-cancer connection. Cells. 2019 doi: 10.3390/cells8010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saleh R, Elkord E. Acquired resistance to cancer immunotherapy: Role of tumor-mediated immunosuppression. Semin Cancer Biol. 2020;65:13–27. doi: 10.1016/j.semcancer.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Saleh R, Elkord E. FoxP3(+) T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020;490:174–185. doi: 10.1016/j.canlet.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 34.He W, Zheng C, Wang Y, Dan J, Zhu M, Wei M, Wang J, Wang Z. Prognosis of synchronous colorectal carcinoma compared to solitary colorectal carcinoma: a matched pair analysis. Eur J Gastroenterol Hepatol. 2019;31(12):1489–1495. doi: 10.1097/MEG.0000000000001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fryk E, Strindberg L, Lundqvist A, Sandstedt M, Bergfeldt L, Mattsson Hultén L, Bergström G, Jansson P-A. Galectin-1 is inversely associated with type 2 diabetes independently of obesity—a SCAPIS pilot study. Metabolism Open. 2019;4:100017. doi: 10.1016/j.metop.2019.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanjuán X, Fernández PL, Castells A, Castronovo V, van den Brule F, Liu FT, Cardesa A, Campo E. Differential expression of galectin 3 and galectin 1 in colorectal cancer progression. Gastroenterology. 1997;113(6):1906–1915. doi: 10.1016/s0016-5085(97)70010-6. [DOI] [PubMed] [Google Scholar]
- 37.Banh A, Zhang J, Cao H, Bouley DM, Kwok S, Kong C, Giaccia AJ, Koong AC, Le QT. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Can Res. 2011;71(13):4423–4431. doi: 10.1158/0008-5472.can-10-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seko A, Kataoka F, Aoki D, Sakamoto M, Nakamura T, Hatae M, Yonezawa S, Yamashita K. Beta 1,3-galactosyltransferases-4/5 are novel tumor markers for gynecological cancers. Tumour Biol : J Int Soc Oncodevelop Biol Med. 2009;30(1):43–50. doi: 10.1159/000203129. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki N, Itakura Y, Toyoda M. Ganglioside GM1 contributes to the state of insulin resistance in senescent human arterial endothelial cells. J Biol Chem. 2015;290(42):25475–25486. doi: 10.1074/jbc.M115.684274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T, Wang F, Wu JY, Qiu ZC, Wang Y, Liu F, Ge XS, Qi XW, Mao Y, Hua D. Clinical correlation of B7–H3 and B3GALT4 with the prognosis of colorectal cancer. World J Gastroenterol. 2018;24(31):3538–3546. doi: 10.3748/wjg.v24.i31.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romagosa C, Simonetti S, López-Vicente L, Mazo A, Lleonart ME, Castellvi J, Ramon y Cajal S. p16(Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene. 2011;30(18):2087–2097. doi: 10.1038/onc.2010.614. [DOI] [PubMed] [Google Scholar]
- 42.Jung A, Schrauder M, Oswald U, Knoll C, Sellberg P, Palmqvist R, Niedobitek G, Brabletz T, Kirchner T. The invasion front of human colorectal adenocarcinomas shows co-localization of nuclear beta-catenin, cyclin D1, and p16INK4A and is a region of low proliferation. Am J Pathol. 2001;159(5):1613–1617. doi: 10.1016/s0002-9440(10)63007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.VinuÉ Á, MartÍnez-HervÁs S, Herrero-Cervera A, SÁnchez-GarcÍa V, AndrÉs-Blasco I, Piqueras L, Sanz MJ, Real JT, Ascaso JF, Burks DJ, GonzÁlez-Navarro H, Changes in CDKN2A/2B expression associate with T-cell phenotype modulation in atherosclerosis and type 2 diabetes mellitus. Transl Res. 2019;203:31–48. doi: 10.1016/j.trsl.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Brenner E, Schörg BF, Ahmetlić F, Wieder T, Hilke FJ, Simon N, Schroeder C, Demidov G, Riedel T, Fehrenbacher B, Schaller M, Forschner A, Eigentler T, Niessner H, Sinnberg T, Böhm KS, Hömberg N, Braumüller H, Dauch D, Zwirner S, Zender L, Sonanini D, Geishauser A, Bauer J, Eichner M, Jarick KJ, Beilhack A, Biskup S, Döcker D, Schadendorf D, Quintanilla-Martinez L, Pichler BJ, Kneilling M, Mocikat R, Röcken M. Cancer immune control needs senescence induction by interferon-dependent cell cycle regulator pathways in tumours. Nat Commun. 2020;11(1):1335. doi: 10.1038/s41467-020-14987-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grange M, Giordano M, Mas A, Roncagalli R, Firaguay G, Nunes JA, Ghysdael J, Schmitt-Verhulst AM, Auphan-Anezin N. Control of CD8 T cell proliferation and terminal differentiation by active STAT5 and CDKN2A/CDKN2B. Immunology. 2015;145(4):543–557. doi: 10.1111/imm.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on request.