Abstract
Background and objectives
Medical comorbidities (MC) are highly prevalent among patients with cancer and predict worse outcomes for traditional therapies. This association is poorly understood for checkpoint inhibitor immunotherapy (IO). We aimed to explore the relationship between common MC including cardiovascular disease (CVD), immune-related adverse events (irAEs), and overall survival (OS) among patients receiving IO for advanced cancer.
Methods
This is a retrospective cohort study of 671 patients with any cancer who received IO at our institution from 2011 to 2018. Clinical data were abstracted via chart review and query of ICD-10 codes and used to calculate modified Charlson comorbidity index (mCCI) scores. The primary outcomes were the association of individual MC with irAEs and OS using bivariate and multivariable analyses. Secondary outcomes included association of mCCI score with irAEs and OS.
Results
Among 671 patients, 62.1% had a mCCI score ≥ 1. No individual MC were associated with irAEs or OS. Increased CCI score was associated with decreased OS (p < 0.01) but not with irAEs. Grade ≥ 3 irAEs were associated with increased OS among patients without CVD (HR 0.37 [95% CI: 0.25, 0.55], p < 0.01), but not among patients with CVD.
Conclusions
No specific MC predicted risk of irAEs or OS for patients receiving IO. Increased CCI score did not predict risk of irAEs but was associated with shorter OS. This suggests IO is safe for patients with MC, but MC may limit survival benefits of IO. CVD may predict shorter OS in patients with irAEs and should be evaluated among patients receiving IO.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03371-0.
Keywords: Checkpoint inhibitor, Immunotherapy, Toxicity, Comorbidities, Cardiovascular disease
Introduction
Medical comorbidities are common among patients undergoing treatment for cancer and are becoming increasingly prevalent as cancer survivability improves with novel cancer therapeutics. While comorbidities naturally increase in prevalence with increasing age, many common conditions such as diabetes are specifically associated with increased cancer risk; in fact, most comorbidities are more common among patients with cancer than in age-matched controls [1–3]. In one study of patients with Medicare insurance, patients diagnosed with lung cancer, which is often treated with checkpoint inhibitor immunotherapy (IO), were associated with the highest prevalence of comorbidities compared to other cancer types [4]. While the prevalence of specific medical comorbidities varies among different cancer types, the current body of literature has consistently shown that these comorbidities are associated with worse survival among patients with cancer [5–7]. This effect may even exist independently of cancer treatment, since conditions such as heart disease actually carry greater mortality than cancer [8–10].
IO use in cancer treatment has become widespread in recent years as newer agents are developed and approved for use in additional cancer types. In 2020, an estimated 44% of patients with advanced cancer were candidates for these agents [11]. Research continues to show that IO is effective for improving survival in a variety of cancers [12]. Additionally, these agents have shown equal efficacy in younger and older patients [13]. IO toxicities, collectively referred to as immune-related adverse events (irAEs), are unique from adverse events caused by other anti-cancer therapeutics due to their distinct mechanism of action, which involves an upregulation of the immune response with subsequent immune-mediated tissue damage [14].
In a prior retrospective analysis of patients who received IO for multiple cancer types, patients who experienced ≥ grade 3 irAEs had significantly increased overall survival (OS) [15]. IrAEs occurred at similar rates in patients age ≥ 70 and age < 70 years. Patients age ≥ 70 had a significantly higher comorbidity burden based on modified Charlson comorbidity index (mCCI) score. In a multivariable analysis that adjusted for patient sex, ECOG performance status, BMI, race, mCCI score, cancer type, line of therapy, and duration of IO treatment, there was a significant association between patient age, presence or absence of ≥ grade 3 (high-grade) irAEs, and OS. Patients age < 70 years who experienced ≥ grade 3 irAEs experienced significant improvement in OS compared with patients age < 70 who did not experience high-grade irAEs. For patients age ≥ 70 years, there was no difference in OS based on presence or absence of ≥ grade 3 irAEs. In the multivariable analysis, increased mCCI score was significantly associated with decreased OS [15]. The association of specific medical comorbidities with irAEs and OS was not analyzed, and the association between mCCI score and OS irrespective of patient age was not described. The findings from this study suggest that the cumulative burden of medical comorbidities and/or the presence of specific medical comorbidities may impact the toxicity and survival outcomes of IO treatment and support the need for a more in-depth analysis of this issue. The results of this study also indicate that in addition to patients age ≥ 70, those with significant medical comorbidities, such as cardiovascular disease (CVD), who experience ≥ grade 3 irAEs may also have worse OS.
Studies examining the relationship between specific medical comorbidities and the risk of traditional (cytotoxic) cancer treatment toxicities have shown conflicting results [2], and the literature on this relationship for IO is far less robust. Pre-existing autoimmune disease is a well-established risk factor for irAEs [16], but irAEs occur in many patients without these conditions. The few studies on the association between irAEs and other medical comorbidities thus far have also yielded mixed results. In a small, retrospective analysis, Kartolo et al. identified an association between irAEs and advanced (stage 3–4) chronic kidney disease [12]. Atchley et al. found that the risk of IO-related pneumonitis, a particularly morbid toxicity, is increased in patients with chronic lung disease [17]. However, it is not known how non-pulmonary comorbidities affect this risk. Moreover, while polypharmacy, a logical reflection of comorbidity burden, has been shown to increase irAE risk, current literature on the direct association between overall comorbidity burden and irAE incidence has been inconsistent and often contradictory [12, 18–20]. Further compounding the challenges in our understanding of this topic is the historical exclusion of patients with high comorbidity burdens from prospective studies of IO agents, despite the high prevalence of comorbidities in patients who receive these agents in real-world clinical settings [13, 20]. As a result, studies on how specific medical comorbidities and overall comorbidity burden impact IO treatment outcomes in large patient cohorts have been lacking.
Among common medical comorbidities, CVD has been shown to be the most morbid and is currently the number one cause of death in the USA [10]. It is commonly comorbid with cancer, shares many of the same risk factors with cancer, and has been associated with worse cancer outcomes [21]. While cardiotoxicity has been associated with IO use, and it has been theorized that IO use may increase atherosclerotic risk, the association between comorbid CVD and IO outcomes is poorly understood [22]. Due to the gap in our knowledge of how CVD and other medical comorbidities affect IO outcomes, patients with greater comorbidity burdens are less likely to receive IO despite its established efficacy in prolonging survival of many cancers [23, 24].
As such, our primary aim in this retrospective study is to determine the association of common individual common medical comorbidities, particularly CVD, with irAEs and OS. We also aim to further describe the association of overall comorbidity burden with irAEs and OS. The results of our analyses will provide evidence about the risks and benefits of IO treatment in patients with cancer and high comorbidity burdens, particularly CVD. This would allow clinicians and patients to make informed decisions regarding treatment and guide efforts to identify, optimize, and mitigate individual patient characteristics, such as medical comorbidities, that may limit the benefit of IO.
Methods
Study design and sample
This was a single-institution, retrospective cohort study. It was approved by the institutional review board at The Ohio State University Comprehensive Cancer Center (OSUCCC). Adult patients (≥ 18y) with any cancer who received at least one dose of IO between October 6, 2011, and April 5, 2018, at the OSUCCC were included. Patients receiving IO in standard of care, clinical trial, and off-label settings were included in the study; there were no exclusion criteria. Pharmacy administration dates were used to validate receipt of IO.
Construction of variables
Patients’ demographic and clinical information (including sex, race, body mass index, cancer type and stage based on pathology and imaging reports, dates and types of treatments received, date of death or last known alive, and performance status) was abstracted by physicians from the electronic medical record and entered into a REDCap database (Vanderbilt University, v8.10.10) [25]. Detailed information regarding irAEs, including date of diagnosis, attribution, grade, and clinical interventions, was abstracted. IrAEs were graded using the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 [26]. IrAE grading was abstracted directly from provider documentation, and if the specific irAE grade was not documented in the medical record, grade was determined by abstracter evaluation of all pertinent and available data in the medical record, including provider documentation, laboratory values, and imaging reports. A subset of patient records was reviewed by multiple physician abstracters to ensure that all information regarding irAEs was abstracted consistently and comprehensively. To quantify comorbidity burden, we used the Charlson comorbidity index (CCI) as it has been well validated for and widely used in studying cancer outcomes [1, 7, 18, 19, 27]. Diagnoses listed in the CCI and documented any time prior to IO start in the patient's medical history, problem list, and/or visit diagnoses were abstracted through a query of ICD-10 codes [28]. Modified CCI (mCCI) scores were calculated by summation of points attributed to each medical comorbidity, but points attributed directly to cancer were excluded, as more specific data regarding cancer diagnoses were manually abstracted (Supplemental Table 1) [4]. Overall survival was determined based on official death status in the medical record.
Table 1.
Baseline patient characteristics
| Characteristic | Frequency, n (%) (n = 671) |
|
|---|---|---|
| Sex | Female | 272 (40.5) |
| Race | Non-Hispanic white | 623 (92.8) |
| Age | < 70 years | 433 (64.5) |
| ECOG PS |
0 1 ≥ 2 Unavailable |
268 (40.0) 256 (38.2) 113 (16.8) 34 (5.1) |
|
Modified CCI score |
0 1 ≥ 2 |
255 (38.0) 189 (28.2) 227 (33.8) |
| BMI category |
Underweight (BMI < 18) Normal (BMI 18–25) Overweight (BMI 25–30) Obese (BMI > 30) |
23 (3.4) 188 (28.0) 218 (32.5) 242 (36.1) |
| Cancer stage |
1–2 3 4 Unavailable |
21 (3.1) 68 (10.1) 550 (82.0) 32 (4.8) |
| Cancer type |
Melanoma NSCLC Renal cell Head and neck Bladder Hematologic Other |
266 (39.6) 147 (21.9) 60 (8.9) 33 (4.9) 26 (3.9) 29 (4.3) 110 (16.4) |
| IO agent |
Nivolumab Pembrolizumab Ipilimumab Other single agent Any 2 agents Unavailable Any IO agent(s) + chemo* |
307 (45.8) 120 (17.9) 150 (22.4) 35 (5.2) 53 (7.9) 6 (0.9) 110 (16.3) |
| IO duration |
≤ 6 weeks 6–12 weeks 12–24 weeks ≥ 24 weeks |
192 (28.6) 151 (22.5) 108 (16.1) 220 (32.8) |
| Line of therapy |
1st 2nd 3rd Unavailable |
199 (29.7) 180 (26.8) 188 (28.0) 104 (15.5) |
ECOG PS Eastern Cooperative Oncology Group performance status, CCI Charlson comorbidity index, BMI body mass index (kg/m2), IO immunotherapy
*Patients who received IO + chemotherapy are included in the totals for each specific IO agent
Outcomes
The primary outcomes were identification of association of specific medical comorbidities with irAEs and association of specific medical comorbidities with OS. Secondary outcomes included the association of comorbidity burden, as measured by mCCI score, with irAEs and OS, and association of CVD with OS based on presence or absence of high-grade (CTCAE grade 3–5) irAEs. Grade 3 or higher irAEs are of particular interest because these are defined as clinically severe and limiting activities of daily living [26].
Statistical analysis
First, baseline demographic and clinical patient characteristics were analyzed. Second, bivariate analysis and Chi-squared statistics were used to determine association of specific patient comorbidities and mCCI score with any grade and ≥ grade 3 irAEs. Third, the Kaplan–Meier method (log-rank test) was used to describe the association between specific comorbidities within the CCI, mCCI score, and OS. A multivariable analysis with Cox proportional hazards model adjusted for age, sex, Eastern Cooperative Oncology Group (ECOG) performance status [29], body mass index (BMI), race, cancer type, duration of IO treatment, and line of therapy was used to describe the effect of frequent comorbidities that were associated with decreased OS in bivariate analysis. Finally, we performed an additional analysis that was informed by the results of the bivariate analyses, which showed an association between cardiovascular comorbidities and OS. In this analysis, patients were stratified into four groups based on presence or absence of CVD (defined as congestive heart failure or previous myocardial infarction) prior to IO start and presence or absence of ≥ grade 3 irAEs. The Kaplan–Meier method (log-rank test) was used to compare OS between these groups. A multivariable analysis with Cox proportional hazards model adjusted for gender, ECOG performance status, BMI, race, cancer type, IO duration, and line of therapy was used to describe the association between CVD, ≥ grade 3 irAEs, and OS. All statistical analyses were performed using SAS, version 9.4 (Cary, NC).
Results
Baseline characteristics
In total, we identified and collected data from 671 patients (Table 1). Within this sample, 40.5% were female and 92.8% were non-Hispanic white race. The median age was 65 years (IQR 55 to 74 years), with 64.5% of patients < 70 years. All cancer types were included, with melanoma and non-small cell lung cancer (NSCLC) being the most common, present in 39.6% and 21.9% of patients, respectively.
Within our sample, 38.0% of patients had a mCCI score of 0, 28.2% had a score of 1, and 33.8% had a score of 2 or greater. The most common comorbidities present were lung disease (24%), uncomplicated diabetes (20%), and history of MI (18%) (Table 2). Some comorbidities were exceedingly rare, with HIV being present in only 2 patients, moderate or severe liver disease in 3 patients, and dementia in 3 patients.
Table 2.
Bivariate analysis of baseline individual medical comorbidities and the modified Charlson comorbidity index score with overall survival
| Frequency, n (%) (n = 671) |
Median OS [95% CI] (months) |
p value | ||
|---|---|---|---|---|
|
Congestive heart failure |
Absent | 623 (92.8) | 13.9 [11.5, 16.2] | < 0.01 |
| Present | 48 (7.2) | 8.1 [4.5, 17.2] | ||
| Pulmonary disease | Absent | 510 (76.0) | 13.8 [11.2, 16.2] | 0.19 |
| Present | 161 (24.0) | 12.1 [9.1, 17.3] | ||
|
Diabetes without complications |
Absent | 537 (80.0) | 13.8 [11.0, 16.7] | 0.54 |
| Present | 134 (20.0) | 12.1 [9.3, 15.7] | ||
|
Diabetes with complications |
Absent | 649 (96.7) | 13.2 [10.8, 15.8] | 0.93 |
| Present | 22 (3.3) | 14.5 [9.3, 29.3] | ||
| Hemiparaplegia | Absent | 660 (98.3) | 13.8 [11.3, 16.0] | 0.08 |
| Present | 11 (1.6) | 8.1 [1.5, 22.5] | ||
| HIV | Absent | 669 (99.7) | 13.5 [11.2, 15.8] | 0.724 |
| Present | 2 (0.03) | NR | ||
|
Liver disease (mild) |
Absent | 607 (90.5) | 13.9 [11.4, 16.2] | 0.16 |
| Present | 64 (9.5) | 10.5 [5.8, 16.2] | ||
|
Liver disease (moderate or severe) |
Absent | 668 (99.6) | 13.6 [11.3, 16.0] | < 0.01 |
| Present | 3 (0.04) | 1.0 [0.6, 0.8] | ||
| Myocardial infarction | Absent | 550 (82.0) | 14.2 [11.4, 17.1] | < 0.01 |
| Present | 121 (18.0) | 10.1 [7.9, 14.5] | ||
| Peptic ulcer disease | Absent | 644 (96.0) | 13.6 [11.3, 15.8] | 0.56 |
| Present | 27 (4.0) | 10.1 [7.9, 14.5] | ||
|
Peripheral vascular disease |
Absent | 619 (92.3) | 13.1 [10.8, 15.7] | 0.98 |
| Present | 52 (7.7) | 16.2 [8.6, 23.9] | ||
| Renal disease | Absent | 616 (91.8) | 13.2 [10.8, 15.7] | 0.83 |
| Present | 55 (8.2) | 16.2 [8.2, 18.3] | ||
|
Cerebrovascular disease |
Absent | 601 (89.6) | 13.8 [11.5, 16.2] | 0.06 |
| Present | 70 (10.4) | 9.3 [6.9, 16.0] | ||
| Dementia | Absent | 668 (99.6) | 13.5 [11.3, 15.8] | 0.96 |
| Present | 3 (0.04) | 6.2 [4.7, NR] | ||
| Connective tissue disorder | Absent | 661 (98.5) | 13.8 [11.3, 16.2] | < 0.01 |
| Present | 10 (1.5) | 3.4 [1.0, 10.0] | ||
| mCCI score | 0 | 255 (38.0) | 17.0 [13.2, 21.3] | < 0.01 |
| 1 | 189 (28.2) | 13.1 [9.9, 17.4] | ||
| ≥ 2 | 227 (33.8) | 10 [8.2, 13.2] | ||
OS overall survival, mCCI modified Charlson comorbidity index
Toxicity
Overall, toxicities were relatively common in our sample, with 31.3% of all patients experiencing any grade irAE and 14% experiencing high-grade (≥ grade 3) irAEs. Neither any specific comorbidity nor mCCI score was significantly associated with any grade or ≥ grade 3 irAEs (p > 0.05 for all bivariate analyses). Given the lack of association between specific medical comorbidities, mCCI score, and irAEs, multivariable analysis of these relationships was not performed.
Comorbidities and overall survival
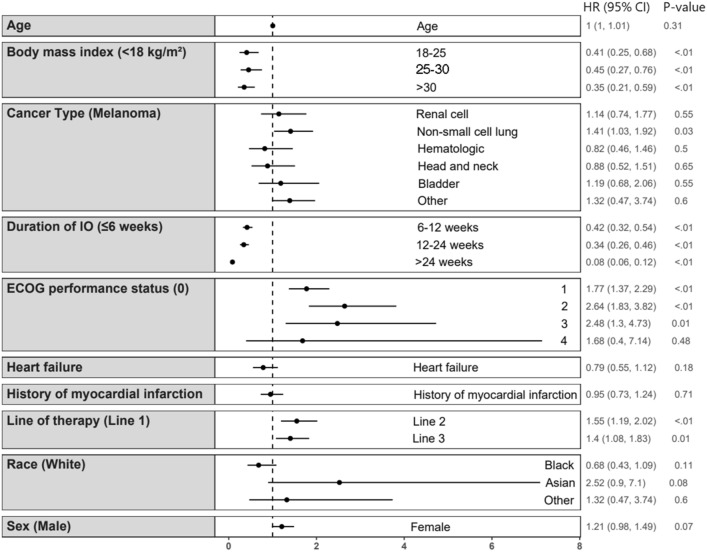
In the bivariate analysis, certain comorbidities had a significant association with OS (Table 2). Patients with congestive heart failure (CHF) had a median OS of 8.1 [95% CI: 4.5, 17.2] months, which was significantly different from a median OS of 13.9 [11.5, 16.2] months in patients without CHF (p < 0.01). A history of myocardial infarction was also associated with a reduced median OS of 10.1 [7.9, 14.5] months, compared to 14.2 [11.4, 17.1] months in patients who had never had an MI (p < 0.01) (Table 2). These associations were not significant in the multivariable analysis (Fig. 1). In addition, a significant difference in OS was seen with moderate to severe liver disease and connective tissue disorder; however, these conditions were only present in 3 (0.04%) and 10 (1.5%) of patients, respectively, and were not tested in the multivariable analysis. Increasing mCCI score was significantly associated with reduced median OS, which was 17.0 [95% CI: 13.2, 21.3] months in patients with a score of 0, 13.1 [9.9, 17.4] months in patients with a score of 1, and 10 [8.2, 13.2] months in patients with a score of 2 or higher (p < 0.01) (Table 2).
Fig. 1.
Multivariable analysis of cardiovascular comorbidities and overall survival. NOTE: Cox proportional hazards model examining the association of cardiovascular comorbidities (heart failure and history of myocardial infarction) and overall survival. Reference values for each variable are listed in parentheses. For heart failure and history of myocardial infarction, the reference value is absence of these conditions. Figure produced using ggplot2 in R: A Language and Environment for Statistical Computing
Cardiovascular disease, toxicity, and survival
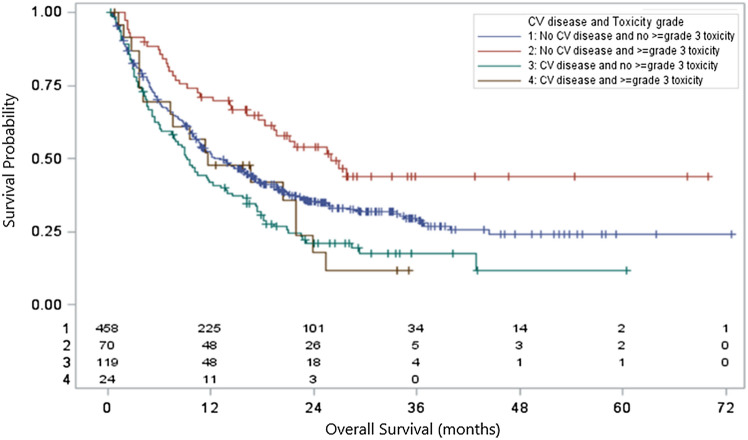
After separating patients into four groups based on the presence or absence of CVD and the presence or absence of ≥ grade 3 irAEs, there was a significant association between CVD and irAE group with OS (p < 0.0001) (Fig. 2).
Fig. 2.
Overall survival based on cardiovascular disease and ≥ grade 3 irAEs. NOTE: Overall survival for all patients stratified by presence or absence of cardiovascular disease and presence or absence of ≥ grade 3 irAEs. P < 0.0001 for the analysis of OS and CV disease/toxicity grouping by the Kaplan–Meier method (log-rank test). Figure produced using SAS, version 9.4 (Cary, NC)
Figure 2 Overall survival for all patients stratified by presence or absence of cardiovascular disease and presence or absence of ≥ grade 3 irAEs. p < 0.0001 for the analysis of OS and CV disease/toxicity grouping by the Kaplan–Meier method (log-rank test). Figure produced using SAS, version 9.4 (Cary, NC)
A multivariable analysis was performed to further describe the association between CVD, irAEs, and OS (Table 3). Among patients without CVD, the presence of ≥ grade 3 irAEs was associated with increased survival (HR 0.37 [95% CI: 0.25, 0.55], p < 0.01). This association was not seen in patients with CVD.
Table 3.
Multivariable analysis of OS based on CVD and ≥ grade 3 irAEs
| Covariate | Median OS [95% CI] (months) |
HR [95% CI] | p value | |
|---|---|---|---|---|
| CVD | ≥ Grade 3 irAEs | |||
| Absent | Absent | 13.1 [10.8, 15.8] | Reference | |
| Present | 26.0 [18.3, NR] | 0.37 [0.25, 0.55] | < 0.0001 | |
| Present | Absent | 9.3 [6.2, 12.3] | 1.02 [0.80, 1.31] | 0.86 |
| Present | 11.6 [4.1, 21.9] | 1.02 [0.61, 1.69] | 0.94 | |
Cox proportional hazards model examining the association of CVD and overall survival. The model was adjusted for CVD (history of heart failure or MI) status and ≥ grade 3 irAE group, gender, ECOG performance status, BMI, race, cancer type, IO duration, and line of therapy. OS for each group shown in the table was calculated using the Kaplan–Meier method
CVD cardiovascular disease, irAEs immune-related adverse events, OS overall survival
Discussion
Implications
In this study, we aimed to determine the association of common medical comorbidities and overall comorbidity burden, as characterized by CCI score, with toxicity and survival among patients receiving IO for advanced cancer. First, our results showed no association between any specific comorbidity, or overall comorbidity burden, and risk of irAEs including severe toxicities. This is an important finding because patients with cancer commonly have significant medical comorbidities that lead to exclusion from cancer treatment clinical trials. Patients with these conditions are also less likely to receive newer therapeutics in practice despite their well-documented survival benefits among patients with advanced cancer [13, 21]. As a result, the safety and efficacy of IO use in patients with significant medical comorbidities has not been fully established, which further hinders the widespread use of these potentially life-extending cancer therapeutics. This is especially problematic when considering that patients with advanced-stage cancer are at an inherently increased risk of having medical comorbidities due to shared risk factors (i.e. increased age) between cancer and comorbidities. [3, 30]. Although more studies are needed to validate our findings and guide clinical practice, the results here suggest these agents may be safely used in patients with cancer and significant comorbidity burden.
Second, we did not find an association between any specific comorbidity and decreased OS after controlling for patient characteristics. However, the bivariate analysis performed in our study showed that increasing comorbidity burden was associated with decreased OS. This finding is consistent with the results of a previous retrospective multivariable analysis of patients receiving IO for multiple types of advanced cancer, where the association of mCCI score > 0 with decreased OS was maintained in a model that included age and ≥ grade 3 irAEs, sex, ECOG performance status, BMI, race, cancer type, line of therapy, and duration of IO treatment [15]. This suggests that the impact on survival is inherent to the medical comorbidities themselves, which carry significant mortality risk even in the absence of cancer [10]. In fact, the association between comorbidity burden and decreased OS was also reported in existing studies of non-IO cancer treatments and has even been established independent of treatment type [6, 8]. As such, we do not believe it reflects a safety concern of using IO agents in patients with significant comorbidity burden.
Furthermore, we found that the presence of any high-grade irAEs predicted increased OS in patients without CVD, but no association with survival was present among patients with CVD who experienced high-grade irAEs. The association between the development of irAEs and improved IO treatment outcomes has been reported in other studies, with some postulating that irAEs signify a sufficiently high IO clinical activity for tumor response [31]. CVD, a highly morbid condition that causes more deaths than cancer annually in the USA, likely impairs survival to such a degree that it negates the increased benefits of IO therapy that are often seen in patients with irAEs, resulting in no difference in survival despite potentially greater IO activity [10]. Additionally, chronic immune and inflammatory processes have been shown to underlie the pathogenesis of atherosclerosis, the major cause of CVD [32]. As such, it is plausible that these inflammatory processes could undermine the immune upregulation that is necessary for IO efficacy. Further research is necessary to improve our understanding of how CVD affects IO response and its risk-versus-benefit profile in cancer treatment. Overall, our findings support the importance of assessing cardiovascular risk factors and disease status among patients receiving IO.
Finally, while a cost-effectiveness analysis of IO use in patients with comorbidities is beyond the scope of our present study, this is another important area of focus for future investigations. Existing cost-effectiveness studies of IO drugs are largely derived from randomized controlled trials that excluded patients with high comorbidity burden and therefore may not be representative of patients treated outside of clinical trials [33]. Given that overall comorbidity burden can significantly impact OS, it stands to reason that immunotherapy drugs, which are relatively novel and expensive, may lose their cost-effectiveness in patients with these conditions who do not derive the same survival benefit as healthier patients. In fact, a microsimulation model by Criss et al. found to this to be the case for the treatment of non-small cell lung cancer with pembrolizumab [33]. Follow-up studies across all cancer types are needed to comprehensively evaluate the effects of comorbidities on the cost effectiveness of IO cancer treatments.
Study limitations
Our study had limitations that should be considered. Given the retrospective and single-institution nature of the study, we were unable to establish causality. Also, our sample was fairly homogeneous with respect to many patient characteristics, particularly race and cancer stage. As such, we cannot comment on the generalizability of our findings to cohorts with different characteristics. Along these same lines, the majority of our patients received single-agent IO in the second-line or later setting; with recent advances, IO is now often used as first-line treatment and in combination with chemotherapy or as a doublet (for example, nivolumab plus ipilimumab), these changes may limit application of our findings to current practice. Second, some of the comorbidities analyzed in this study were poorly represented. For example, severe liver disease, HIV, and dementia were found in fewer than 1% of patients within our cohort. Consequently, we cannot make confident conclusions about any associations between these comorbidities and our study outcomes. However, for the most common comorbidities in our data set, we found their prevalence in our cohort to be comparable to those described for the general population of cancer patients in the USA [4]. Finally, the presence or absence of specific comorbidities was determined via computerized analysis of ICD codes linked to patient encounters and documented medical history. As a result, provider and institutional biases in diagnosing and documenting these conditions, as well as variable patient access to primary or specialty care at our institution, could result in a skewed representation of their true prevalence. We also did not take into account if and how well comorbid conditions were managed at the time of IO treatment, so we cannot comment on the effects of disease severity on the outcomes described.
Conclusion
In conclusion, our study found no association between specific medical comorbidities or overall comorbidity burden and increased risk for irAEs. We did observe an association between increased comorbidity burden, but not individual comorbidities, and decreased OS. Patients without CVD who experienced ≥ grade 3 irAEs had improved OS, but this benefit was not seen in patients with CVD. Taken together, these findings suggest that IO agents are safe to use in patients with medical comorbidities, although the benefits to survival conferred by IO therapy may be negated by the morbidity of these medical conditions, particularly CVD. Future efforts should be directed toward studying whether and how severity and management of medical comorbidities affects IO treatment outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge the contributions of Megan Reynolds and Kamila Jaroniec in the preparation of this manuscript.
Author contributions
A.C.J. and C.J.P. developed the study concept and methodology. A.C.J. and M.Y. wrote the main manuscript text. L.W. performed the statistical analysis. M.Y. prepared Tables 1, 2, and 3 and Supplemental Table 1. R.H. and D.S. prepared Fig. 1. L.W. prepared Fig. 2. M.G. assisted with project administration. A.C.J, S.H.P., M.L., M.H., K.L.K., J.T.B., and D.H.O. curated patient data. All authors contributed to revision and preparation of the final manuscript text. All authors read and approved the final manuscript.
Funding
Research support provided by the REDCap project and The Ohio State University Center for Clinical and Translational Science grant support (National Center for Advancing Translational Sciences, Grant UL1TR002733). This work was also supported by The National Institute of Aging (C.J.P., 1K76AG074923-01; R03AG064374) and The Ohio State University Comprehensive Cancer Center and the National Institutes of Health (P30 CA016058).
Declarations
Conflict of interest
Gregory Otterson reports speaker honoraria from OncLive and NCCN. He reports institution-directed research funding from: Roche/Genentech, AstraZeneca, BMS, Merck, AbbVie, and Elevation Oncology. Dwight Owen reports institution-directed research funding from BMS, Merck, Palobiofarma, Genentech, Pfizer, and Onc.AI.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Land LH, Dalton SO, Jensen M-B, Ewertz M. Impact of comorbidity on mortality: a cohort study of 62,591 danish women diagnosed with early breast cancer, 1990–2008. Breast Cancer Res Treat. 2011;131(3):1013–1020. doi: 10.1007/s10549-011-1819-1. [DOI] [PubMed] [Google Scholar]
- 2.Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment: cancer and comorbidity. CA A Cancer J Clin. 2016;66(4):337–350. doi: 10.3322/caac.21342. [DOI] [PubMed] [Google Scholar]
- 3.Sogaard M, Thomsen RW, Bossen HH, Sørensen T, Nørgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013 doi: 10.2147/CLEP.S47150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards BK, et al. Annual report to the nation on the status of cancer, 1975‐2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2013;120(9):1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho H, et al. Assessing non–cancer-related health status of US cancer patients: other-cause survival and comorbidity prevalence. Am J Epidemiol. 2013;178(3):339–349. doi: 10.1093/aje/kws580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monirul Islam KM, et al. Comorbidity and survival in lung cancer patients. Cancer Epidemiol Biomark Prev. 2015;24(7):1079–1085. doi: 10.1158/1055-9965.epi-15-0036. [DOI] [PubMed] [Google Scholar]
- 7.Etienne A, et al. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer. 2007;109(7):1376–1383. doi: 10.1002/cncr.22537. [DOI] [PubMed] [Google Scholar]
- 8.Gross CP, McAvay GJ, Krumholz HM, David Paltiel A, Bhasin D, Tinetti ME. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Annal Intern Med. 2006;145(9):646. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]
- 9.Lee L, Cheung WY, Atkinson E, Krzyzanowska MK. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol. 2011;29(1):106–117. doi: 10.1200/JCO.2010.31.3049. [DOI] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 11.von Itzstein MS, Gonugunta AS, Mayo HG, Minna JD, Gerber DE. Immunotherapy use in patients with lung cancer and comorbidities. Cancer J. 2020;26(6):525–536. doi: 10.1097/PPO.0000000000000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kartolo A, et al. Predictors of Immunotherapy-Induced Immune-Related Adverse Events. Curr Oncol. 2018;25(5):403–410. doi: 10.3747/co.25.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yildiz B. Immunotherapy in Geriatric Patients with Advanced Cancer. Eurasian J Med Oncol. 2020 doi: 10.14744/ejmo.2020.44446. [DOI] [Google Scholar]
- 14.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johns AC, et al. Checkpoint inhibitor immunotherapy toxicity and overall survival among older adults with advanced cancer. J Geriatr Oncol. 2021;12(5):813–819. doi: 10.1016/j.jgo.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F, Thompson JA, Obeid M. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 17.Atchley WT, et al. Immune checkpoint inhibitor-related pneumonitis in lung cancer. Chest. 2021;160(2):731–742. doi: 10.1016/j.chest.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakozaki T, Hosomi Y, Shimizu A, Kitadai R, Mirokuji K, Okuma Y. Polypharmacy as a prognostic factor in older patients with advanced non-small-cell lung cancer treated with anti-PD-1/PD-L1 antibody-based immunotherapy. J Cancer Res Clin Oncol. 2020;146(10):2659–2668. doi: 10.1007/s00432-020-03252-4. [DOI] [PubMed] [Google Scholar]
- 19.Zeng X, Zhu S, Cheng X, Wang Z, Xingxing S, Zeng D, Long H, Zhu B. Effect of comorbidity on outcomes of patients with advanced non-small cell lung cancer undergoing anti-PD1 immunotherapy. Med Sci Monit. 2020 doi: 10.12659/MSM.922576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanz BA, et al. Safety and efficacy of anti-PD-1 in patients with baseline cardiac, renal, or hepatic dysfunction. J Immunother Can. 2016 doi: 10.1186/s40425-016-0166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson CB, Davis MK, Law A, Sulpher J. Shared risk factors for cardiovascular disease and cancer: implications for preventive health and clinical care in oncology patients. Can J Cardiol. 2016;32(7):900–907. doi: 10.1016/j.cjca.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Lutgens E, Seijkens TTP. Cancer patients receiving immune checkpoint inhibitor therapy are at an increased risk for atherosclerotic cardiovascular disease. J Immunother Cancer. 2020;8(1):e000300. doi: 10.1136/jitc-2019-000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman S. Caleb, Satish M, Walters RW. Comorbidity burden on receipt of adjuvant immunotherapy and survival in patients with stage iii melanoma: an analysis of the national cancer database. Int J Dermatol. 2020;59(11):1381–1390. doi: 10.1111/ijd.15019. [DOI] [PubMed] [Google Scholar]
- 24.Zhang D, Tailor TD, Kim C, Atkins MB, Braithwaite D, Akinyemiju T. Immunotherapy utilization among patients with metastatic NSCLC: impact of comorbidities. J Immunother. 2021;44(5):198–203. doi: 10.1097/CJI.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris Paul A, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support.". J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Cancer Institute Cancer Therapy Evaluation Program (2022) Common terminology criteria for adverse events (CTCAE) v4.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40. 14 June 2010. Accessed 28 September 2022.
- 27.Charlson ME, Pompei P, Ales KL, Ronald MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288–1294. doi: 10.1016/j.jclinepi.2004.03.01. [DOI] [PubMed] [Google Scholar]
- 29.Oken MM, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–656. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Jørgensen TL, et al. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106(7):1353–1360. doi: 10.1038/bjc.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Indini Alice, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-pd1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol. 2018;145(2):511–521. doi: 10.1007/s00432-018-2819-x. [DOI] [PubMed] [Google Scholar]
- 32.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38(6):1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Criss SD, et al. Cost-effectiveness of pembrolizumab for advanced non-small cell lung cancer patients with varying comorbidity burden. PLoS ONE. 2020;15(1):e0228288. doi: 10.1371/journal.pone.0228288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.