Abstract
The use of anti-programmed cell death-1 (PD-1) antibodies in treating malignancies is increasing; however, most registered clinical trials on anti-PD-1 antibodies exclude patients infected with hepatitis B virus (HBV). This retrospective study aimed to assess hepatotoxicity in cancer patients infected with HBV undergoing anti-PD1 antibody therapy and identify the associated risk factors. A total of 301 cancer patients positive for hepatitis B core antibodies (HbcAb) (negative or positive hepatitis B surface antigen [HBsAg]) who received PD-1 inhibitors were enrolled. The primary and secondary endpoints were the incidence rate of hepatotoxicity related to PD-1 inhibitor treatment, and risk factors associated with hepatic toxicity, respectively. Of the enrolled analyzed, 16.9% (n = 51) developed any grade and 4.7% (n = 14) developed grade 3–4 hepatotoxicity, respectively. Higher risk for any-grade hepatotoxicity development was associated with sero-positive HBsAg (OR = 6.30; P = 0.020), existence of liver involvement (OR = 2.10; P = 0.030), and detectable baseline HBV DNA levels (OR = 2.39; P = 0.012). Patients with prophylactic antiviral therapy decreased hazard for the incidence of grade 3–4 hepatotoxicity (OR = 0.10; P = 0.016). Our results suggested chronic (HBsAg-positive)/resolved (HBsAg-negative and HBcAb-positive) HBV-infected cancer patients are at an increased risk of hepatotoxicity following PD-1 inhibitor therapy. Cancer patients should be tested for HBsAg/HBcAb prior to the commencement of immune checkpoint inhibitor therapy. For patients with chronic/resolved HBV infection, ALT/AST and HBV DNA should be closely monitored during the whole immunotherapy period.
Keywords: PD-1; Cancer, Hepatitis B virus; Hepatotoxicity; Immunotherapy; Safety
Introduction
Anti-programmed cell death-1 (PD-1) antibody treatment is associated with promising responses across variety of cancer subtypes, including non-small-cell lung cancer [1], small-cell lung cancer [2], Hodgkin lymphoma [3, 4], and gastric cancer [5]. Approval for indications of anti-PD1 antibodies continues to be extended and the use of this agent is increasing. However, the efficacy profile of anti-PD-1 antibodies has been accompanied by a range of associated adverse events. The greater number of patients exposed to anti-PD1 antibody therapy has generated more frequent immune-related adverse events (irAEs).
The liver is one of the most common immunologically affected organs associated with anti- PD-1 immunotherapy [6, 7]. Some studies have reported that the pooled incidence rates of elevated liver transaminase were approximately 8% and 15% for anti-PD-1 antibody treatment alone [8] and PD-1 inhibitor combined with an ipilimumab regimen, respectively [9]. The incidence rate of hepatitis among patients treated with PD-1 inhibitor plus chemotherapy has been reported to be 2% [10]. Throughout most clinical trials, there has been a low incidence rate of hepatotoxicity attributed to anti-PD-1 antibody treatment.
However, most immunotherapy clinical trials have typically excluded patients with hepatitis B virus (HBV) infection. HBV infection remains the most common chronic viral infection, from which approximately 887,000 individuals die from HBV-related liver disease each year and about half of all patients are from China [11]. Among patients with malignancies who receive anti-tumor therapy, HBV infection is considered to be one risk factor for liver toxicity [12, 13]. Severe acute hepatotoxicity can occasionally progress to fulminant hepatic failure and death [14]. Due to exclusion of HBV-infected patients in most registered clinical trials, the safety of anti-PD-1 immunotherapy among this population remains unclear.
To date, there has been limited data exploring the association between HBV infection and liver toxicity induced by anti-PD-1 antibodies. This study aimed to access the prevalence of hepatotoxicity in patients with malignancies infected with HBV undergoing anti-PD-1 antibody therapy. This study also sought to identify potential risk factors for the development of immune-related hepatotoxicity.
Materials and methods
Study design and patients
Cancer patients with seropositive hepatitis B core antibodies (HBcAb) who received anti-PD-1 antibody immunotherapy at the Sun Yat-sen University Cancer Center between January 2015 and July 2018 were enrolled in this retrospective study.
Patients with pathologically confirmed advanced cancer (not amenable to curative surgery or local treatment) were eligible for this study. At least one dose of anti-PD-1 single agent or combination therapy was prescribed to each of the participants. Patients were also required to be diagnosed as having a chronic or previous HBV infection (HBcAb-positive with either seropositive or sero-negative hepatitis B surface antigen [HBsAg]). The enrolled patients should have complete clinical and follow-up data. Adequate available clinical information, including regularly monitored liver function, was required for the recruited patients over the study period. The selected patients had no evidence of other types of viral hepatitis (e.g., hepatitis A virus [HAV], hepatitis C virus [HCV], hepatitis D virus [HDV], hepatitis E virus [HEV], or human immunodeficiency virus [HIV]) infection. Patients who displayed elevated levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBR) at the initiation of PD-1 inhibitor-involved therapy were excluded. Data regarding adverse events and laboratory abnormalities were regularly collected from medical records and reviewed by oncologists over the study period. The causality between anti-PD-1 antibody-associated treatment and hepatotoxicity was retrospectively assessed using the WHO-UMC system (Word Health Organization-Uppsala Monitoring Center) based on patient’s clinical manifestation, results of CT scans and laboratory examinations. The various causality categories included: certain/definitely; probable/likely; possible; unlikely; conditional/unclassified; and unassessable/unclassifiable [15]. All patients provided written informed consent, and the study protocol was approved by the Sun Yat-Sen University Cancer Center Institutional Review Board (Guangzhou, China).
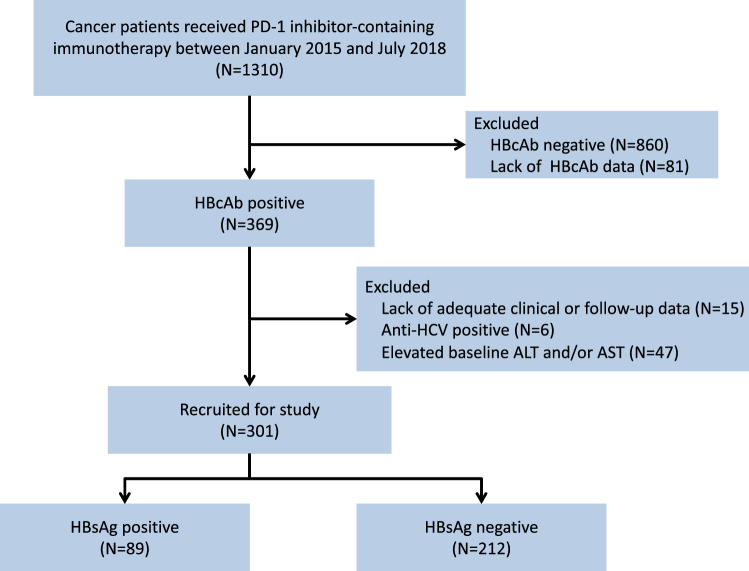
The primary objective was the total incidence rate of suspected hepatotoxicity (definitely, probably, or possibly) related to treatment with PD-1 inhibitor therapy as judged by an oncologist. Suspected hepatotoxicity was defined as an elevation in the level of aminotransferase (ALT and/or AST, whichever came first). The hepatotoxicity grade was determined using the Common Terminology Criteria for Adverse Events (CTCAE), ver. 5.0. The CTCAE defines grade 1, grade 2, grade 3, and grade 4 toxicity levels of ALT/AST as 1–3 times, 3–5 times, 5–20 times, and over 20 times the upper limit of normal, respectively. In this study, hepatotoxicity was inferred as grade 1 or higher. Severe hepatotoxicity was defined as grade 3–5 change in the level of ALT or AST. If the AST and ALT grades were discordant, the higher of the two was used for classification. The secondary objective was to identify risk factors associated with the development of hepatic toxicity in these patients (Fig. 1).
Fig. 1.
Flowchart depicting patient deposition. PD-1, programmed cell death 1; HBcAb, hepatitis B core antibody; anti-HCV, antibody to the hepatitis C virus; ALT, alanine aminotransferase; ALT, aspartate aminotransferase; HBsAg, hepatitis B surface antigen
The level of HBV DNA was assessed using a real-time viral polymerase chain reaction assay (ABI 7900; Applied Biosystems, Foster City, CA, USA). The lower limit of detection for the level of HBV DNA was 10 IU/mL.
Statistical analysis
A Chi-squared test or Fisher’s exact test was used to compare the categorical variables between patients with and without hepatotoxicity. A multivariate logistic regression analysis was used to identify predictors of hepatotoxicity. Factors with a P value < 0.2 in the univariate analysis in conjunction with strong confounding factors (e.g., age, sex, and body weight) were included in the multivariate analysis. Odds ratio (OR) and adjusted ORs were estimated by univariate and multivariate analyses, respectively. The time taken to reach hepatotoxicity was analyzed using a Kaplan–Meier survival analysis and log-rank test. Statistical significance was defined as P < 0.05 (2-tailed). Data were analyzed using Stata software (SPSS Inc., Chicago, Illinois, version 22.0, USA).
Results
Patients
A total of 348 individuals were screened. Of these, 47 patients were determined to be ineligible due to the following reasons: the pooled values were elevated for both ALT and AST (20 patients), only ALT levels were detected (9 patients), and only AST levels were detected (18 patients) at the initiation of PD-1 immunotherapy, respectively. Consequently, the data from 301 patients treated with anti-PD-1 antibodies were analyzed.
The baseline characteristics of the patients included in this study are listed in Table 1. In a total of 301 HBcAb-positive patients, HBsAg seropositivity and HBeAg seropositivity were found in 89 (29.6%) and 8 (2.7%) patients, respectively. Of the recruited patients, 71.4% (n = 215) were male and the median age was 52 years old (range: 44–84). The most common tumor types included: nasopharyngeal carcinoma (NPC, n = 84; 27.9%); non-small-cell lung cancer (NSCLC, n = 66; 21.9%); melanoma (n = 38; 12.6%); gastric cancer (n = 24; 8.0%); hepatocellular carcinoma (HCC, n = 20; 6.6%); and lymphoma (n = 21; 7.0%). There were 114 (37.8%) patients who had received more than two lines of therapy prior to receiving anti-PD-1 immunotherapy. During the immunotherapy phase, 92 (30.6%) patients received treatment with the PD-1 inhibitor alone (pembrolizumab, nivolumab, toripalimab, camrelizumab, and sintilimab), whereas 209 (69.4%) patients received combination therapy. The level of HBV DNA was detectable in 24 (8.0%) patients and undetectable in 101 (33.6%) patients. The median titer of the detectable HBV DNA was 6.57 × 102 IU/mL (range: 3.01 × 101 − 1 .69 × 105 IU/mL). There were 63 (20.9%) patients who received prophylactic antiviral therapy, including 20, 42, and 1 for the baseline HBV-DNA status of detectable, undetectable, and unknown status, respectively. Five types of antiviral drugs were used, including entecavir (n = 49), lamivudine (n = 9), tenofovir (n = 3), telbivudine (n = 1), adefovir (n = 1).
Table 1.
Baseline characteristics of the included patients (n = 301)
| No. of patients (%) | No. of any-grade hepatotoxicity (%) | Pa | No. of grade 3–4 hepatotoxicity (%) | Pa | |
|---|---|---|---|---|---|
| Total | 301 (100%) | 51 (16.9%) | 14 (4.7%) | ||
| Age (years, range 44–84) | 0.813 | 0.365 | |||
| > 52 | 143 (47.5) | 25 (17.4) | 5 (3.5) | ||
| ≤ 52 | 158 (52.5) | 26 (16.5) | 9 (5.7) | ||
| Gender | 0.409 | 0.226 | |||
| Male | 215 (71.4) | 34 (15.8) | 8 (3.7) | ||
| Female | 86 (28.6) | 17 (19.8) | 6 (7.0) | ||
| Body weight(kg) | 0.813 | 0.848 | |||
| > 60 | 143 (47.5) | 25 (17.5) | 7 (4.9) | ||
| ≤ 60 | 158 (52.5) | 26 (16.5) | 7 (4.4) | ||
| History of alcoholism | 0.900 | 0.091 | |||
| Yes | 49 (16.3) | 8 (16.3) | 0 (0.0) | ||
| No | 252 (83.7) | 43 (17.1) | 14 (5.6) | ||
| bHTN/DM/CVD | 0.858 | 0.334 | |||
| Yes | 39 (13.0) | 7 (17.9) | 3 (7.7) | ||
| No | 262 (87.0) | 44 (16.8) | 11 (4.2) | ||
| Liver cirrhosis | 0.790 | 0.030 | |||
| Yes | 21 (7.0) | 4 (19.0) | 3 (13.6) | ||
| No | 280 (93.0) | 47 (16.8) | 11 (3.9) | ||
| Hepatocellular carcinoma | 0.320 | 0.023 | |||
| Yes | 20 (6.6) | 5 (25.0) | 3 (15.0) | ||
| Noc | 281 (93.4) | 46 (16.4) | 11 (3.9) | ||
| HBsAg status | 0.757 | 0.004 | |||
| Seropositive | 89 (29.6) | 16 (18.0) | 9 (10.0) | ||
| Seronegative | 212 (70.4) | 35 (16.5) | 5 (23.6) | ||
| HBeAg status | 0.116 | 0.006 | |||
| Seropositive | 8 (2.7) | 3 (37.5) | 2 (25%) | ||
| Seronegative | 293 (97.3) | 48 (16.4) | 12 (4.1) | ||
| ECOG performance status | 0.227 | 0.665 | |||
| > 1 | 32 (10.6) | 3 (9.4) | 1 (3.1) | ||
| ≤ 1 | 269 (89.4) | 48 (17.8) | 13 (4.8) | ||
| Lines of treatment | 0.464 | 0.694 | |||
| > 2 | 114 (37.8) | 17 (14.9) | 6 (5.2) | ||
| ≤ 2 | 187 (62.1) | 34 (18.2) | 8 (4.3) | ||
| Liver involvementd | 0.022 | 0.016 | |||
| Yes | 122 (40.5) | 28 (23.0) | 10 (8.1) | ||
| No | 179 (59.5) | 23 (12.8) | 4 (2.2) | ||
| Antiviral therapye | 0.527 | 0.472 | |||
| Yes | 63 (20.9) | 9 (14.3) | 4 (6.3) | ||
| No | 238 (79.1) | 42 (17.6) | 10 (4.2) | ||
| Baseline HBV DNA level | 0.026 | 0.000 | |||
| Undetectablef | 101 (33.6) | 9 (8.9) | 4 (4.0) | ||
| Detectable | 24 (8.0) | 6 (25.0) | 5 (20.8) | ||
| Unknown | 176 (58.4) | 36 (20.5) | 5 (2.8) | ||
| Treatment modality | 0.141 | 0.307 | |||
| Combined therapyg | 92 (30.6) | 20 (21.7) | 6 (6.5) | ||
| Monotherapyh | 209 (69.4) | 31 (14.8) | 8 (3.8) |
aDetermined using a χ2 test
bAbbreviations: HTN hypertension; DM diabetes mellitus; CVD cardiovascular disease
cIncluding nasopharyngeal carcinoma (n = 84), non-small-cell lung cancer (n = 66), melanoma (n = 38), gastric cancer (n = 24), colorectal cancer (n = 8), lymphoma (n = 21), urothelial carcinoma (n = 5), esophageal cancer (n = 5), gallbladder carcinoma (n = 1), spongiocytoma (n = 1), germinoma (n = 1), small-cell carcinoma of the vagina (n = 1), Breast cancer (n = 4), soft tissue sarcoma (n = 4), small-cell lung cancer (n = 4), tumor of unknown original lesion (n = 3), neuroendocrine tumor (n = 3), pancreatic cancer (n = 2), kidney cancer (n = 2), cervical cancer (n = 2), head and neck tumor (n = 2)
dMalignant lesions in the liver, including HCC and liver metastases of non-HCC tumors
eIncluding entecavir (n = 49), lamivudine (n = 9), tenofovir (n = 3), telbivudine (n = 1), adefovir (n = 1)
fHBV DNA < 10 IU/mL
gincluding PD-1 inhibitor plus chemotherapy (n = 50), targeted agent (osimertinib [n = 1], bevacizumab [n = 3], ramucirumab [n = 3], rituximab [n = 1], nimotuzumab [n = 1], sunitinib [n = 1], axitinib [n = 1], Ibrutinib [n = 1], apatinib [n = 1], cabozantinib [n = 1], lenvatinib [n = 4], vemurafenib [n = 1], pazopanib [n = 1], SHR7390 [n = 2]), chemotherapy plus targeted agent (n = 10), and ipilimumab (n = 7)
hIncluding pembrolizumab, nivolumab, toripalimab, camrelizumab, sintilimab
Abbreviations: ECOG Eastern Cooperative Oncology Group; HBV hepatitis B virus; HBeAg hepatitis B e antigen; HBsAg hepatitis B surface antigen
Development of any grade hepatotoxicity and risk factors
The proportion (number) of all subjects with suspected hepatotoxicity was associated with anti-PD-1 antibody-involved therapy was 16.9% (n = 51) (Table 1). Hepatotoxicity occurred at a median of 10.57 weeks (95%CI: 7.15–13.99 weeks) following the initiation of a PD-1 inhibitor. The median time to resolution was 4 weeks (95%CI: 3.05–4.95 weeks). Eight patients (grade 3, n = 7; grade 2, n = 1) experienced treatment delay due to hepatic AEs and successfully went back on immunotherapy after resolution. Five patients with grade 3–4 hepatotoxicity permanently discontinued PD-1 inhibitor treatment. Eight patients received steroids treatment (prednisone 0.5–2 mg/kg, tapering over 3–6 weeks) for hepatic AEs. No death was attributed to treatment-related hepatotoxicity.
Causality in these patients was considered as certain in 20/51 (37.3%), likely in 16/51 (31.3%) and possible in 15/51 (29.4%) patients. The prevalence of hepatotoxicity in combination therapy group was greater than that in monotherapy, with no statistical significance (21.7% vs. 14.8%, P = 0.141). Liver involvement and the levels of baseline HBV DNA were found to be related to hepatotoxicity occurrence (Table 1). The univariate analysis indicated that existed liver involvement existing (OR: 2.02; 95% CI: 1.10–3.71; P = 0.023) and detectable baseline levels of HBV DNA (OR: 3.41; 95% CI: 1.08–10.76; P = 0.037) were independent risk factors for the development of hepatotoxicity. The multivariate analysis demonstrated that patients with HBsAg seropositivity had a 6.30-fold increased incidence for hepatotoxicity compared with those without (95% CI: 1.33–29.80; P = 0.020). Liver involvement (P = 0.030) and baseline levels of HBV DNA (P = 0.012) also significantly increased risks for the development of hepatotoxicity (Table 2).
Table 2.
Risk factors for any-grade and grade 3–4 hepatotoxicity development
| Any-grade hepatotoxicity | Grade 3–4 hepatotoxicity | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| Covariate | OR | P | OR | P | OR | P | OR | P |
| (95%CI) | (95%CI) | (95%CI) | (95%CI) | |||||
| Age (years) | 0.834 | 0.370 | ||||||
| > 52 | 1.07 | 0.60 | ||||||
| (0.58–1.95) | (0.20–1.83) | |||||||
| ≤ 52 | 1 | 1 | ||||||
| Gender | 0.400 | 0.233 | ||||||
| Male | 0.76 | 0.52 | ||||||
| (0.40–1.45) | (0.17–1.53) | |||||||
| Female | 1 | 1 | ||||||
| Body weight(kg) | 0.813 | 0.848 | ||||||
| > 60 | 1.08 | 1.11 | ||||||
| (0.59–1.97) | (0.38–3.25) | |||||||
| ≤ 60 | 1 | 1 | ||||||
| History of alcoholism | 0.900 | 0.997 | ||||||
| Yes | 0.95 | 0.00 | ||||||
| (0.42–2.17) | (0.00–NA) | |||||||
| No | 1 | 1 | ||||||
| HTN/DM/CVD | 0.858 | 0.341 | ||||||
| Yes | 1.08 | 1.90 | ||||||
| (0.45–2.61) | (0.51–7.14) | |||||||
| No | 1 | 1 | ||||||
| Liver cirrhosis | 0.790 | 0.492 | 0.043 | 0.682 | ||||
| Yes | 1.17 | 0.47 | 4.08 | 1.88 | ||||
| (0.37–3.62) | (0.05–4.13) | (1.04–15.93) | (0.09–38.43) | |||||
| No | 1 | 1 | 1 | 1 | ||||
| Hepatocellular carcinoma | 0.325 | 0.437 | 0.036 | 0.892 | ||||
| Yes | 1.70 | 2.30 | 4.33 | 1.23 | ||||
| (0.59–4.92) | (0.28–18.83) | (1.10–17.00) | (0.06–25.438) | |||||
| No | 1 | 1 | 1 | 1 | ||||
| HBsAg status | 0.757 | 0.020 | 0.007 | 0.123 | ||||
| Sero-positive | 1.11 | 6.30 | 4.66 | 5.80 | ||||
| (0.58–2.13) | (1.33–29.80) | (1.51–14.32) | (0.62–54.20) | |||||
| Sero-negative | 1 | 1 | 1 | 1 | ||||
| HBeAg status | 0.134 | 0.096 | 0.018 | 0.080 | ||||
| Sero-positive | 3.06 | 4.15 | 7.81 | 6.83 | ||||
| (0.71–13.25) | (0.78–22.18) | (1.42–42.79) | (0.80–58.66) | |||||
| Sero-negative | 1 | 1 | 1 | 1 | ||||
| ECOG performance status | 0.237 | 0.667 | ||||||
| > 1 | 0.48 | 0.64 | ||||||
| (0.14–1.63) | (0.08–5.02) | |||||||
| ≤ 1 | 1 | 1 | ||||||
| Lines of treatment | 0.464 | 0.694 | ||||||
| > 2 | 0.79 | 1.24 | ||||||
| (0.42–1.49) | (0.42–3.68) | |||||||
| ≤ 2 | 1 | 1 | ||||||
| Liver involvement | 0.023 | 0.030 | 0.024 | 0.162 | ||||
| Yes | 2.02 | 2.10 | 3.91 | 2.64 | ||||
| (1.10–3.71) | (1.08–4.10) | (1.20–12.76) | (0.68–10.29) | |||||
| No | 1 | 1 | 1 | 1 | ||||
| Antiviral therapy | 0.528 | 0.475 | 0.016 | |||||
| Yes | 0.78 | 1.55 | 0.10 | |||||
| (0.36–1.70) | (0.47–5.10) | (0.014–0.65) | ||||||
| No | 1 | 1 | 1 | |||||
| Baseline HBV DNA level | 0.032 | 0.012 | 0.003 | 0.080 | ||||
| Detectablea | 3.41 | 0.037 | 2.39 | 0.181 | 6.38 | 0.010 | 7.49 | 0.025 |
| (1.08–10.76) | (0.67–8.53) | (1.57–25.98) | (1.28–43.72) | |||||
| Unknown | 2.63 | 0.015 | 8.09 | 0.005 | 0.71 | 0.615 | 1.60 | 0.668 |
| (1.21–5.71) | (1.87–34.93) | (0.19–2.70) | (0.19–13.61) | |||||
| Undetectable | 1 | 1 | 1 | 1 | ||||
| Treatment modality | 0.143 | 0.241 | 0.312 | 0.515 | ||||
| Combined therapy | 1.60 | 1.49 | 1.75 | 1.51 | ||||
| (0.85–2.98) | (0.77–2.88) | (0.59–5.20) | (0.44–5.25) | |||||
| Monotherapy | 1 | 1 | 1 | 1 | ||||
aHBV DNA < 10 IU/mL
Abbreviations: OR odd ratio; CI confidence interval; HTN hypertension; DM diabetes mellitus; CVD cardiovascular disease; ECOG Eastern Cooperative Oncology Group; HBV hepatitis B virus; HBeAg hepatitis B e antigen; HBsAg hepatitis B surface antigen
Development of severe hepatotoxicity (grade 3/4) and risk factors
The incidence of grade 3–4 hepatotoxicity related to PD-1 inhibitor-involved therapy was estimated at 14/301 (4.7%). The existence of liver cirrhosis, diagnosis of primary hepatocellular carcinoma, seropositive HBsAg, seropositive HBeAg, existence of liver involvement, and detectable baseline HBV DNA levels were significantly associated with a higher incidence of severe hepatotoxicity (Table 1).
In the univariate analysis, liver cirrhosis (OR: 4.08; 95%CI: 1.04–15.93; P = 0.043), primary hepatocellular carcinoma (OR: 4.33; 95%CI: 1.10–17.00; P = 0.036), liver involvement (OR: 3.91; 95%CI: 1.20–12.76, P = 0.024), seropositive HBsAg (OR: 4.66; 95%CI: 1.51–14.32; P = 0.007), seropositive HBeAg (OR: 7.81; 95%CI: 1.42–42.79, P = 0.018), and detectable baseline HBV DNA (OR, 6.38; 95%CI: 1.57–25.98, P = 0.010) levels were independent risk factors for grade 3–4 hepatotoxicity development. None of the significant aforementioned risk factors for grade 3–4 hepatotoxicity were identified in the multivariate analysis. The administration of antiviral therapy was found to be a significant beneficial factor by a 90% reduction in the risk for the incidence of grade 3–4 hepatotoxicity (P = 0.016) (Table 2).
Discussion
This study reports a large cohort and systematic analysis emphasizing liver toxicity in cancer patients with chronic or resolved HBV infection who were treated with anti-PD-1 immunotherapy. Our findings showed that the rate of any grade and grade 3–4 hepatotoxicity in this population were 16.9% and 4.7%, respectively. Significant risk factors for the development of hepatotoxicity included the existence of liver involvement, HBsAg seropositivity, and detectable baseline HBV DNA levels. Our data indicate that HBV infection may not be a contraindication to treatment with an PD-1 inhibitors regimen. Moreover, these findings may provide clinically relevant information for this patient population while receiving PD-1 inhibitor treatment by enhancing the awareness of its hepatic profiles.
Anecdotally, hepatic profiles are infrequent but common immune-related adverse events. Hepatotoxicity occurs in 5–10% (of which 1–2% is grade 3) of patients during therapy with PD-1 inhibitor at the approved doses as single agents [16]. Available data reported from large-scale phase III clinical trials of the administration of a single anti-PD-1 antibody showed that the pooled incidence of grade 1–4/3–4 hepatic adverse events was reported to be 10.7%/3.8% and 7%/1% by checkmate-227 [9] and keynote-042 [17], respectively. Most participants included in these trials were negative for HBV infection. Currently, our results indicate 16.9%/4.7% for 1–4/3–4 hepatotoxicity in HBV-infected cancer patients undergoing anti-PD-1 immumotherapy, which is higher than previously reported. To our knowledge, HBV infection is not cytopathic and does not trigger immunomediated necroinflammatory liver damage. In patients with chronic HBV infection (CHB), an inadequate HBV-specific T cell response can trigger substantial non-antigen-specific cellular infiltration, amplifying the level of liver damage through bystander T cells [18, 19]. Even among patients resolved HBV (RHB), most individuals may have detectable HBV DNA in the liver, and some also have detectable HBV DNA in the serum [20]. Potential liver damage by HBV may be the primary and most rational explanation for the higher incidence rate of liver toxicity compared with that in most registered clinical trials excluding viral hepatitis patients. Furthermore, HBV reactivation is a another potential complication of anti-PD-1/PD-L1 therapy in HBV-infected patients with cancers, which could occur in both CHB and RHB populations and result in hepatitis [21]. Our previous work reported a 5.3% incidence rate of HBV reactivation among 114 CHB cancer patients who developed HBV reactivation with abnormal liver function [22]. In addition, immune checkpoint inhibitor (ICI) combination therapy has been reported to induce a greater number of hepatic profiles than a single agent [23]. In our study, 30.6% patients received combination therapy while 69.4% received monotherapy. Either all grade or severe grade hepatotoxicity, the incidence rate caused by the former was higher than that by the latter (grade 1–5: 21.7% vs. 14.8%; Grade ≥ 3:6.5% vs. 3.8%), although the outcomes were of no significant differences.
So far, there has been a lack of data regarding the risk factors of liver toxicity in cancer patients with HBV infection receiving immunotherapy. The current finding suggests that when receiving anti-PD-1 therapy, patients with positive HBsAg were at a much higher risk of developing hepatotoxicity than those with negative HBsAg and positive HBcAb by 4.66 times (P = 0.020). The persistent presence of HBsAg establishes the diagnosis of CHB, which, as part of its natural course, may lead to cirrhosis, liver failure, and/or HCC. Negative HBsAg and positive HBcAb indicates a previous exposure to HBV infection (resolved HBV); the majority of this population recovered from acute HBV infection earlier in life and anti-HBs titers have waned to undetectable levels. In RHB patients, the risk of liver dysfunction, cirrhosis or HCC due to HBV is minimal [24]. It has been wildly observed that CHB patients are in a high risk of HBV reactivation when receiving anticancer therapy [24]. For RHB patients, covalently closed circular DNA remains and is capable of replicating in the liver of individuals with this serologic profile. This population is also potentially at risk for HBV reactivation but the incidence is much lower [24]. Detectable baseline HBV DNA was also reported to be a risk factor for the incidence of hepatotoxicity in our study. The level of HBV DNA is a direct measurement of the viral load, which demonstrates the replication activity of the virus and has been recognized as a risk factor for HBV reactivation in previous studies [25]. Worthy of note, in a recent retrospective study evaluating the safety of ICIs in cancer patients with HBV infection, the authors demonstrated that hepatitis flare (alanine aminotransferase > 2 times of the upper limit of normal) occurred in 39.3% HBsAg-positive and 30.4% HBsAg-negative patients, and CHB or RHB had no impact on the emergence of hepatic flare [26]. However, the primary endpoint, criteria of patient enrollment and the type of ICIs performed in this study are quite different from ours. The natural bias of retrospective study could also give rise to heterogeneous conclusions. Nevertheless, both studies delivered clinically relevant and nonoverlapping information to the area of ICI application in HBV-involved cancer patients. Hepatotoxicity related to PD-1 inhibitors in this population deserves greater vigilance and further study, especially in patients with positive HBsAg.
Our result suggested liver involvement as another potential risk factor for the development of hepatotoxicity while receiving ICIs. In accordance with the finding above, subgroup analysis from CheckMate 017 and 057 demonstrated that rates of treatment-related hepatic AEs were slightly higher in nivolumab-treated NSCLC patients with liver metastases than in the overall study population (all hepatic events: 10% vs. 6%; grade 3–4 hepatic events: 2% vs. 1%) [27]. Liver involvements of malignancy could directly reduce the volume of functional healthy liver or induce intrahepatic and extrahepatic biliary obstruction [28]. Humoral and immunological factors associated with cancer may also increase cholestasis and inflammatory damage in the liver [28]. Assessment of liver function is a fundamental part of work-up for patients with liver involvements before initiation of anti-PD-1 immunotherapy.
Another relevant finding is that antiviral prophylaxis substantially reduced the risk of developing severe hepatic profiles, suggesting that some of hepatic profiles in our study are HBV-related hepatotoxicity. Moreove, we previously declared that a lack of antiviral prophylaxis was a risk factor for HBV-related hepatitis (OR: 13.44; P = 0.019) among HBsAg-positive cancer patients receiving ICIs [22]. According to these data and the current guidance [24], we suggest all HBsAg-positive patients receive effective antiviral drugs before and during anti-PD-1 treatment. For patients with resolved HBV infection, considering the low risk of HBV reactivation, treatment can begin on prophylaxis, or ALT, HBV DNA, and HBsAg can be carefully monitored with the intent for on-demand anti-HBV therapy [24]. Entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide, are the recommended drugs.
One limitation of this study is that most of the recruited patients only provided test results for HBV DNA levels at the initiation of immunotherapy, as few patients were monitored for HBV DNA levels throughout the treatment period, particularly patients who had previously been infected with HBV. This lack of monitoring regarding the changes in HBV DNA levels may result in an impairment in the timely discovery of viral reactivation and initiation of relevant treatment. Another limitation was the relatively small sample size included in the present study. We found that several virological factors were of significance in the univariate analysis, which were determined to be false-negative factors in the multivariate analysis, which may have led to the concealment of critical risk factors. In addition, causality between anti-PD-1 antibody treatment and hepatotoxicity was mainly assessed retrospectively based on available clinical and laboratory data, which may lead to some bias.
In summary, the findings of the present study indicate that anti-PD-1 therapy has acceptable safety in patients with chronic or resolved HBV infection and cancers. Although the development of adverse events in the liver is more frequent in this population compared to HBV-negative patients, the hepatic adverse events induced by PD-1 inhibitors therapy are considered manageable. Thus, cancer patients should be tested for HBsAg/HBcAb prior to the commencement of immune checkpoint inhibitor therapy. For patients with chronic or resolved HBV infection, physicians should closely monitor the levels of ALT/AST and HBV DNA, as well as consider the proper use of preemptive antiviral drugs throughout the entire immunotherapy period.
Acknowledgements
We sincerely appreciate all the patients and their families who were included in this retrospective study.
Author contributions
SH and LZ conceived and designed the study. ZL, XZ and YZ collected, analyzed and interpreted the data. All authors were involved in the drafting, review, and approval of the report and the decision to submit for publication.
Funding
This study was funded by grants 8217102281, 81972898, and 81872499 from the National Natural Science Funds of China; 16zxyc04 from the Outstanding Young Talents Program of Sun Yat-sen University Cancer Center; 2019A1515011090 from the Natural Science Foundation of Guangdong Province. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declarations
Conflict of interest
The authors declare no conflict of interest that pertain to this work.
Data availability
Data will be provided upon request for reasonable academic studies by the corresponding author.
Footnotes
Zuan Lin, Xuanye Zhang and Yixin Zhou have contributed equally.
The original online version of this article was revised: following article note “Zuan Lin, Xuanye Zhang and Yixin Zhou have contributed equally” is missing.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/6/2021
A Correction to this paper has been published: 10.1007/s00262-021-03112-1
Contributor Information
Shaodong Hong, Email: hongshd@sysucc.org.cn.
Li Zhang, Email: Zhangli6@mail.sysu.edu.cn.
References
- 1.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ready N, Farago AF, de Braud F, et al. Third-line nivolumab monotherapy in recurrent SCLC: checkmate 032. J Thorac Oncol. 2019;14:237–244. doi: 10.1016/j.jtho.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribrag V, Avigan DE, Green DJ, et al. Phase 1b trial of pembrolizumab monotherapy for relapsed/refractory multiple myeloma: KEYNOTE-013. Br J Haematol. 2019;186:e41–e44. doi: 10.1111/bjh.15888. [DOI] [PubMed] [Google Scholar]
- 4.Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134:1144–1153. doi: 10.1182/blood.2019000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehra R, Seiwert TY, Gupta S, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018;119:153–159. doi: 10.1038/s41416-018-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 7.Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (Checkmate 017 and Checkmate 057) J Clin Oncol. 2017;35:3924–3933. doi: 10.1200/JCO.2017.74.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khodadoust MS, Rook AH, Porcu P, et al. Pembrolizumab in relapsed and refractory mycosis fungoides and sezary syndrome: a multicenter phase II Study. J Clin Oncol. 2020;38:20–28. doi: 10.1200/JCO.19.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 10.Gadgeel S, Rodriguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38:1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Yan L, Shi Y, Lv D, Shang J, Bai L, Tang H. Hepatitis B virus infection: overview. Adv Exp Med Biol. 2020;1179:1–16. doi: 10.1007/978-981-13-9151-4_1. [DOI] [PubMed] [Google Scholar]
- 12.Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559–574. doi: 10.1093/annonc/mdv623. [DOI] [PubMed] [Google Scholar]
- 13.Jennings JJ, Mandaliya R, Nakshabandi A, Lewis JH. Hepatotoxicity induced by immune checkpoint inhibitors: a comprehensive review including current and alternative management strategies. Expert Opin Drug Metab Toxicol. 2019;15:231–244. doi: 10.1080/17425255.2019.1574744. [DOI] [PubMed] [Google Scholar]
- 14.Varma A, Biritxinaga L, Saliba RM, et al. Impact of hepatitis B core antibody seropositivity on the outcome of autologous hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2017;23:581–587. doi: 10.1016/j.bbmt.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–2232. [PubMed] [Google Scholar]
- 16.Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K, Committee EG. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018 doi: 10.1093/annonc/mdy162. [DOI] [PubMed] [Google Scholar]
- 17.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 18.Pallett LJ, Gill US, Quaglia A, et al. Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells. Nat Med. 2015;21:591–600. doi: 10.1038/nm.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 20.Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 21.Tapia Rico G, Chan MM, Loo KF. The safety and efficacy of immune checkpoint inhibitors in patients with advanced cancers and pre-existing chronic viral infections (Hepatitis B/C, HIV): a review of the available evidence. Cancer Treat Rev. 2020;86:102011. doi: 10.1016/j.ctrv.2020.102011. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Zhou Y, Chen C, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer. 2019;7:322. doi: 10.1186/s40425-019-0808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv JW, Li JY, Luo LN, Wang ZX, Chen YP. Comparative safety and efficacy of anti-PD-1 monotherapy, chemotherapy alone, and their combination therapy in advanced nasopharyngeal carcinoma: findings from recent advances in landmark trials. J Immunother Cancer. 2019;7:159. doi: 10.1186/s40425-019-0636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297–1309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong GL, Wong VW, Hui VW, et al. Hepatitis flare during immunotherapy in patients with current or past hepatitis B virus infection. Am J Gastroenterol. 2021;116:1274–1283. doi: 10.14309/ajg.0000000000001142. [DOI] [PubMed] [Google Scholar]
- 27.Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29:959–965. doi: 10.1093/annonc/mdy041. [DOI] [PubMed] [Google Scholar]
- 28.Field KM, Dow C, Michael M. Part I: liver function in oncology: biochemistry and beyond. Lancet Oncol. 2008;9:1092–1101. doi: 10.1016/S1470-2045(08)70279-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be provided upon request for reasonable academic studies by the corresponding author.