Abstract
Background
Although disc large associated protein family (DLGAP5) has been reported to be involved in a variety of tumor pathologic processes, its expression and mechanism in gallbladder cancer (GBC) are still uncertain. Macrophages were divided into M1 and M2 macrophages. TAM is more closely defined as M2 polarized macrophages, which plays a key role in cancer progression.
Objective
To clarify the role of disc large associated protein family (DLGAP5) in gallbladder cancer (GBC) progression and investigate the mechanism.
Methods
Differential genes in 10 normal paracancer tissues and 10 GBC tissues in GSE139682 from NCBI-GEO were analyzed by R language. Bioinformation analysis and clinical sample analysis were performed to detect DLGAP5 expression in GBC and its correlation with prognosis. CCK-8, EDU, transwell, wound closure, and Immunoblot were performed to detect its effects on the function of GBC cells. GST-pulldown showed the direct interact between DLGAP5 and cAMP. Macrophage polarization assay was further conducted to detect the effects of DLGAP5 on macrophage M2 polarization. The tumor growth assays were further conducted to confirm its role in mice.
Results
Biological analysis and clinical samples confirmed that DLGAP5 was increased in GBC and strongly related to poor prognosis in patients with GBC. After overexpression of DLGAP5 in GBC cell lines, such as GBC-SD and NOZ cells, cell proliferation and migration were enhanced, and macrophages were polarized to M2. However, after DLGAP5 is knocked down, there is opposite effect. Mechanistically, DLGAP5 promotes the growth and migration of GBC-SD and NOZ cells and the M2 polarization of THP-1-derived macrophages by activating cyclic adenosine monophosphate (cAMP) pathway. In vivo, GBC-SD with DLGAP5 knockdown was subcutaneously injected into nude mice. It was found that after DLGAP5 knockdown, both tumor volume and tumor were reduced, and indicators related to proliferation and M2 polarization decreased.
Conclusion
Our study shows that DLGAP5 is significantly elevated in GBC and is strongly related to poor prognosis in patients with GBC. DLGAP5 promotes GBC proliferation, migration, and M2 polarization of macrophages through cAMP pathway, which provides a theoretical basis for the treatment of GBC and may become a promising therapeutic target.
Keywords: Gallbladder cancer, DLGAP5, Migration, Tumor-associated macrophage, cAMP
Introduction
Gallbladder carcinoma (GBC) is the most common malignant tumor of the biliary tract [1–3]. However, Patients with GBCd have a poor prognosis [2]. Therefore, it is important to clarify the underlying mechanisms and progression of gallbladder cancer. Tumor-associated macrophages are the main components of tumor microenvironment (TME), and play a key role in the cross-talk between cancer cells and TME [4–6]. Macrophages were divided into M1 and M2 macrophages. TAM is more closely defined as M2 polarized macrophages [7]. Emerging evidence suggests that M2 macrophages contribute to cancer progression and metastasis [7–9]. In addition, the presence of M2 macrophages is thought to be correlative to poor prognosis of various tumor, including breast cancer and colorectal cancer, and promote the migration and invasion of GBC cells [10].
Cyclic adenosine monophosphate (cAMP) is a second messenger molecule involved in signal transduction, such as G protein-coupled receptors. cAMP is synthesized when adenylate cyclase catalyzes the conversion of adenosine triphosphate into cAMP [11]. Elevated bile acid has been reported to increase expression levels of FGF19 and FGFR4 by activating the GPBAR1-cAMP-EGR1 pathway, FGF19 secreted by GBC cells stimulates FGFR4 and downstream ERK in an autocrine manner by using bile as a potential carrier to promote GBC progression [12]. In addition, cAMP and interleukin-4 have a synergistic effect on STAT6 expression and can act as a cofactor of macrophage reprogramming to induce cAMP to produce M2-like phenotype in macrophages that can restore TGR5-deficient inhibition [13].
The intervertebral disc large associated protein family (DLGAP), consisting of five members (DLGAP1-5), was first discovered in rats and has three key domains, including a 14-amino acid repeat domain, a dynamic protein light chain domain and a guanosine kinase-related protein homology domain [14–16]. DLGAP5 (also known as HURP or KIAA0008) is of interest because it is primarily related to many types of tumors [17–19]. DLGAP5 knockdown induces apoptosis of ovarian cancer cells [20]. High expression of DLGAP5 is closely related to poor prognosis of endometrial carcinoma patients [21]. However, the role of DLGAP5 in GBC is unclear.
The goal of this study was to explore the expression of DLGAP5 in GBC, and to illustrate the role of DLGAP5 in GBC and related mechanisms. Our study found that DLGAP5 is significantly up-regulated in GBC and is related to poor prognosis, which promotes the migration of GBC and the M2 polarization of macrophages by activating the cAMP pathway.
Methods
Bioinformatics analysis
Differential genes in 10 normal paracancer tissues and 10 GBC tissues in GSE139682 from NCBI-GEO were analyzed by R language to target DLGAP5 based on log|FC|> 2, P < 0.05. GO enrichment analysis and KEGG pathway enrichment analysis were performed for differential genes, and protein–protein interaction network was established through STRING database (https://www.string-db.org/). Then, the expression level of DLGAP5 in various tumors was analyzed by TCGA database.
Patients and clinical samples
One hundred and forty patients with GBC were recruited, and the study was approved by Ethics Committee of the Second Affiliated Ho spital of Kunming Medical University. Please refer to Table 1 for the demographic information of the patients. GBC tissue and normal paracancer tissue were collected during surgery, one sample was placed in 4% paraformaldehyde for immunohistochemical studies, and the other sample was placed in liquid nitrogen to measure gene and protein expression levels of target genes in tissue.
Table 1.
Comparison of clinicopathological profiles of GBC patients between the low and high DLGAP5 expression groups
| Variables | DLGAP5 expression level | Total (n = 140) | P value | |
|---|---|---|---|---|
| Low (n = 43) | High (n = 97) | |||
| Age | ||||
| < 60 | 12 | 34 | 46 | 0.406 |
| ≥60 | 31 | 63 | 94 | |
| Gender | ||||
| Male | 25 | 46 | 71 | 0.242 |
| female | 18 | 51 | 69 | |
| Differentiation | ||||
| High | 6 | 12 | 18 | 0.966 |
| moderate | 25 | 57 | 82 | |
| low | 12 | 28 | 40 | |
| TNM stage | ||||
| 0-II | 23 | 28 | 51 | 0.005* |
| III-IV | 20 | 69 | 89 | |
| Lymph node metastasis | ||||
| Absent | 38 | 33 | 71 | < 0.001* |
| Present | 5 | 64 | 69 | |
Statistical analyses were performed with the Chi-square test
*p < 0.05 was considered statistically significant
Cell culture and transfection
GBC cell lines GBC-SD and NOZ cells and human monocyte THP-1 were purchased from American Type Culture Collection (Manassas, VA, USA). GBC cell lines were cultured with DMEM-F12 (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) and THP-1 cells were cultured with RPMI-1640 (Gibco) containing 10% FBS. To explore the role of DLGAP5, we transfected plasmids into GBC-SD and NOZ cells with lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) to make DLGAP5 overexpressed, and realized DLGAP5 knockdown by transfection with small interfering RNA.
Cell viability and invasion
In order to detect cell proliferation, GBC-SD and NOZ cells were planted on 96-well plates, and after overexpression or knockdown of DLGAP5 with or without stimulator, cell viability was detected using cell counting kit-8 (CCK-8; Beyotime, Shanghai, China) [22]. In addition, for detecting DNA synthesis rate by staining with 5-ethynyl-20-deoxyuridine (EDU), GBC cell lines were treated and 50 μM EDU was added to cell plates, followed by DNA staining with Hoechst 33,342 for half an hour, and positive cells were observed under a microscope.
The invasion ability of GBC cell line GBC-SD and NOZ cells was detected by Transwell assay. The GBC-SD or NOZ cells (1 × 105 cells/well) were cultured in matrigel-coated transwell upper compartment and 20% FBS was added to the lower compartment. After 24 h, the cells in the lower chamber were migrating cells. In addition, The GBC-SD and NOZ cells were inoculated in 6-well plates and cultured to 30–50% density. Artificial scratches were formed with 20 μl pipette tip. The span of the scratches was observed with a microscope at 0 and 48 h after the scratches to monitor cell migration.
Flow cytometry
GBC-SD and NOZ cells with DLGAP5 knockdown were digested into single cells by 0.25% trypsin and stained with Propidium iodide (PI) and Annexin V-FITC. Apoptosis rates were measured by flow cytometry. PMA-induced THP-1 macrophages co-cultured with GBC cells were isolated and stained with anti-CD163-FITC or anti-CD206-APC, and the number of CD163 or CD206-positive cells in macrophages was analyzed by flow cytometry. The tumor tissue was dispersed into single cells, then stained with anti-CD206-APC and anti-F4/80-FITC, and F4/80+CD206+ positive cells were analyzed by flow cytometry.
All used antibodies were purchased Biolegend company (San Diego, CA, USA).
Quantitative real-time PCR and western blotting
Trizol reagent was used to extract total RNA from tissues or cells, and then the RNA was reversely transcribed into cDNA with the kit (Takara, Dalian, China) and amplified with suitable primers. The primers are as follows: DLGAP5 5’-AAGTGGGTCGTTATAGACCTGA-3’ and 5’-TGCTCGAACATCACTCTCGTTAT-3’; Arg1 5’-CCCTGGGGAACACTACATTTTG-3’ and 5’-GCCAATTCCTAGTCTGTCCACTT-3’; CD206 5’- CTACAAGGGATCGGGTTTATGGA-3’ and 5’- TTGGCATTGCCTAGTAGCGTA-3’, CD163 5’-GCGGGAGAGTGGAAGTGAAAG-3’ and 5’-GTTACAAATCACAGAGACCGCT-3’; IL-10 5’- TCAAGGCGCATGTGAACTCC-3’ and 5’- GATGTCAAACTCACTCATGGCT-3’.
Total proteins of tissues or cells were extracted, and then western blotting was performed as described above [23, 24]. The antibodies used are as follows: DLGAP5 (1:1000, Abcam, Cambridge, UK), Arg1 (1:1000, Abcam), CD206 (1:1000, Abcam), CD163 (1:1000, Abcam), and IL-10 (1:1000, Abcam).
Immunohistochemistry and immunofluorescence
For immunohistochemistry, clinical specimens or allograft tumor specimens obtained were fixed with 4% paraformaldehyde for 24 h, then embedded in paraffin and cut into 4um tissue sections. The slices were dewaxed and dehydrated, and the tissue sections were repaired by thermal repair antigen method for half an hour. Incubate with 3% hydrogen peroxide and 5% BSA for 20 min and 40 min respectively, and then incubate with antibodies. Antibodies are shown as follows: DLGAP5 (1:100, Abcam), CD206 (1:100, Abcam), Ki67 (1:100, Abcam).
For immunofluorescence, the treated cells were fixed with 4% paraformaldehyde at room temperature for 30 min, then incubated with PBS containing 0.1% Tween 20 and 5% donkey serum for 1 h [25], and then incubated with anti-E-cadherin (1:200, Abcam) and anti-vimentin (1:200, Abcam).
Macrophage polarization
The polarization of GBC cells on macrophages was detected by Transwell method. Forskolin was added or not to GBC cell lines (GBC-SD and NOZ) after overexpression or knockdown of DLGAP5, which were co-cultured with 320 ng/mL phorbol-12-myristate-13-acetate (PMA) to induce THP-1. Flow cytometry was used to detect the polarization of TPH-1 derived macrophages, and total protein and RNA of THP-1-derived macrophages were extracted for western blotting and RT-PCR.
In vivo experiments
Animal experiments have been approved by Ethics Committee of the Second Affiliated Ho spital of Kunming Medical University. Female BALB/C nude mice (4 weeks old) were purchased from Vital River Laboratory Technology (Beijing, China). GBC-SD cells (1 × 107 cells/mouse) with DLGAP5 knockdown were subcutaneously injected into the mentioned mice. Tumor growth was detected 1 week later. After 4 weeks, the mice were sacrificed and the tumors were harvested for weighing or for flow cytometry and immunohistochemistry.
Statistical analysis
All cell experiments were repeated three times and in vivo experiments five times. Data analysis was performed with Graphpad 7.0 software (GraphPad Software, LaJolla, CA, USA). Kaplan Meier was used to make survival curves. Student’s t test or the Mann–Whitney U test was used for comparison between two groups depending on distribution. Correlation analysis was performed by spearman test. P < 0.05 was considered to be statistically significant.
Results
DLGAP5 is up-regulated in GBC
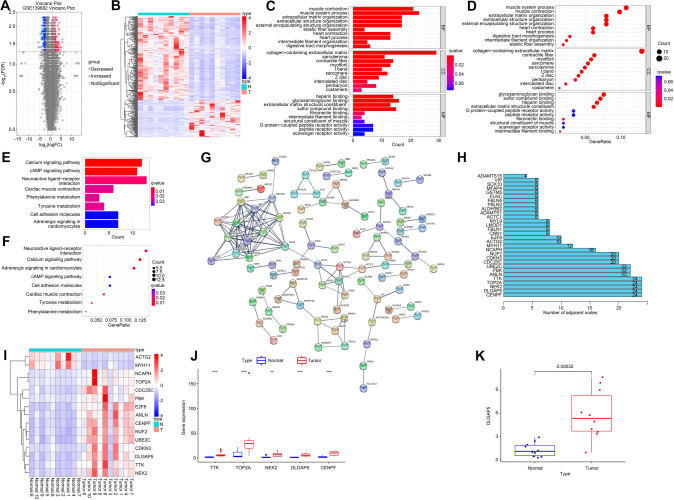
Normal and tumor tissue in GSE139682 were analyzed to gain differentially expressed genes (DEGs) based on log|FC|> 2, P < 0.05, including 100 up-regulated and 227 down-regulated genes. Volcanic map (Fig. 1A) and heat map (Fig. 1B) of RNA clustering analysis are shown. DEGs of biological processes were involved in muscle contraction and muscle system process, its cellular component participated in collagen-containing extracellular matrix, and glycosaminoglycan binding and sulfur compound binding were involved in its molecular function (Fig. 1C, D). Pathway enrichment analysis showed that the DEGs were associated with neuroactive ligand-receptor interaction, calcium signaling pathway, and cAMP signaling pathway (Fig. 1E, F). STRING database was used to analyze protein–protein interactions involving in up-regulation and down-regulation of gallbladder cancer DEGs (Fig. 1G). Protein–protein interactions analysis obtained 30 genes, all of which were nodes larger than 3 (Fig. 1H). Then, we analyzed genes with nodes no less than 10, and their expression in GBC was shown in the heat map of Fig. 1I. Subsequently, we analyzed the expression of the five genes with the most nodes, including TTK, TOP2A, NEK2, DLGAP5 and CENPF, in GBC (Fig. 1J). Studies have reported that DLGAP5 is overexpressed in many tumors, including breast cancer, liver cancer, urinary bladder cancer, meningioma and adrenocortical cancer [17, 26–28]. DLGAP5 was significantly elevated in GBC tissues (Fig. 1K). These results suggest that DLGAP5 perhaps involved in the pathological process of GBC.
Fig. 1.
Differential gene analysis in GBC expression profile. Differential genes in 10 normal paracancer tissues and 10 GBC tissues in GSE139682 from NCBI-GEO were analyzed by R language to target DLGAP5 based on log|FC|> 2, P < 0.05.It found that there were 100 up-regulated genes and 227 down-regulated genes, and then map the volcano (A) and heat maps (B). GO analysis is presented in bar charts (C) and point charts (D). KEGG analysis was presented in bar graph (E) and point graph (F) respectively. Establish PPI network (G) and obtain hub genes (H). (I) The hub genes are selected from (H) to make heat maps. (J) Genes with the largest number of adjacent nodes were selected from (H) to observe their expression levels in the two groups. (K) Expression level of DLGAP5 was analyzed in normal group and GBC tissue. **P < 0.01, ***P < 0.001 compared with normal group
The expression level of DLGAP5 is associated with poor prognosis and tumor-associated M2 macrophages
We analyzed the expression level of DLGAP5 in various tumors including cholangiocarcinoma (CHOL) through TCGA database, and found that the expression levels of DLGAP5 in CHOL were significantly elevated (Fig. 2A). There was no gallbladder cancer in the database, so we included CHOL. TCGA analysis showed that DLGAP5 was significantly overexpressed in CHOL (Fig. 2B, C). Analysis of our clinical samples showed that DLGAP5 gene levels were significantly up-regulated in GBC tissues (Fig. 2D). Consistent with gene levels, protein expression levels of DLGAP5 were also significantly increased in GBC tissues compared with normal adjacent tissues (Fig. 2E). By immunohistochemistry, we found that DLGAP5 was expressed differently in GBC tissues. In GBC tissues, 86.43% of the samples showed high expression of DLGAP5, while only 45.71% of the samples in the normal group showed high expression of DLGAP5 (Fig. 2F). In addition, we analyzed the survival rate of patients with high and low DLGAP5 in GBC and found that patients with high DLGAP5 had a lower survival rate (Fig. 2G). M2 macrophages play an important role in cancer [29–31]. Immunohistochemistry showed that the markers of M2 macrophages, CD206, were significantly up-regulated in GBC tissues, and 87.14% of samples in GBC tissues showed high expression of CD206, whereas only 23.57% of samples in the normal group showed high expression of CD206 (Fig. 2H). By correlation analysis, as shown in Fig. 2I showed that there was a positive correlation between CD206 and DLGAP5 expression levels (r = 0.683, p < 0.001). The demography and clinical data of patients with low and high expression of DLGAP5 are shown in Table 1. These results implythat DLGAP5 is overexpressed in GBC and is closely associated with poor clinical outcomes and macrophage M2 polarization.
Fig. 2.
DLGAP5 is highly expressed in GBC and associated with poor prognosis and tumor-associated M2 macrophages. (A) TCGA database showed that DLGAP5 was highly expressed in cholangiocarcinoma tissues (marked with red box). (B, C) Expression level of DLGAP5 in cholangiocarcinoma was analyzed in TCGA database. (D) Gene expression levels of DLGAP5 were detected by RT-PCR in GBC. (E) Protein expression levels of DLGAP5 were detected by western blotting in both normal group and GBC tissues. (F) Immunohistochemical representative pictures were used to demonstrate the expression of DLGAP5 in the paracancer normal group and GBC tissues. Scale bar, 200 μm. Below is an enlarged picture of them, Scale bar, 100 μm. The bar chart shows the percentage of high and low expression levels of DLGAP3 in the normal and GBC groups. (G) Survival rate in patients with high versus low DLGAP3. (H) Immunohistochemical representation of CD206 expression in the paracancer normal group and GBC tissues. Scale bar, 200 μm. Below is an enlarged picture of them, Scale bar, 100 μm. The bar chart shows the percentage of high and low expression levels of DLGAP3 in the normal and GBC group. (I) Correlation analysis of CD206 and DLGAP5 levels in GBC tissues. ***P < 0.001 compared with normal group
DLGAP5 promotes the growth and migration of GBC cells
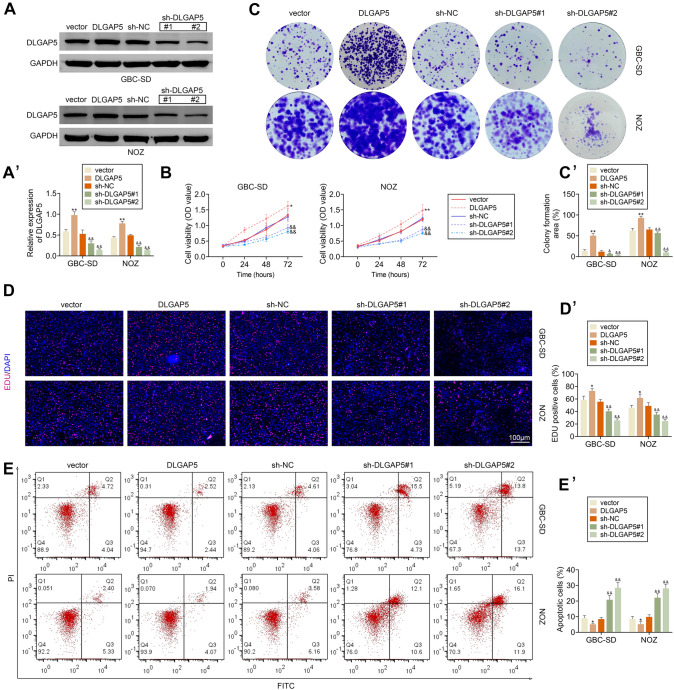
In order to explore the effect of DLGAP5 on GBC mechanism, DLGAP5 was overexpressed by plasmid and knocked down by small interfering RNA in GBC cell lines (GBC-CD and NOZ). Western blotting experiments were performed to verify the efficiency of DLGAP5 overexpression or knocking (Fig. 3A). The viability of GBC cell lines was detected by CCK8 assay, and the activity of GBC-SD and NOZ cells was enhanced after overexpression of DLGAP5, while the cell viability of GBC-SD and NOZ cells was down-regulated after knockdown of DLGAP5 (Fig. 3B). Consistent with it, we found that overexpression of DLGAP5 increased the clone number of GBC-SD and NOZ cells, while knockdown of DLGAP5 significantly descended the clone number of GBC cells (Fig. 3C). In addition, EDU experiment results also showed that GBC-SD and NOZ cells proliferation was significantly enhanced after overexpression of DLGAP5, while cell proliferation was weak after knockdown of DLGAP5 (Fig. 3D). Furthermore, we detected the effect of DLGAP5 on GBC apoptosis, and our study indicated that overexpression of DLGAP5 significantly reduced the apoptosis rate of GBC cells, while knockdown of DLGAP5 significantly increased the apoptosis rate of GBC cells (Fig. 3E). These results suggest that up-regulation of DLGAP5 promotes the growth of GBC cells.
Fig. 3.
DLGAP5 promotes cell growth of GBC. DLGAP5 was knocked down in GBC-SD and NOZ cells. (A) Protein expression levels of DLGAP5 were detected by western blotting. The expression level of DLGAP5 in each group was analyzed according to the gray value of the bands. (B) CCK8 assay was detected the cell viability of GBC and NOZ cells. (C) After overexpression or knockdown of DLGAP5, cell clonogenesis was counted. (D) EDU detected cell proliferation in each group. (E) Apoptosis rate was detected by flow cytometry. *P < 0.05, **P < 0.01 compared with vector group; &P < 0.05, &&P < 0.01 compared with sh-NC group
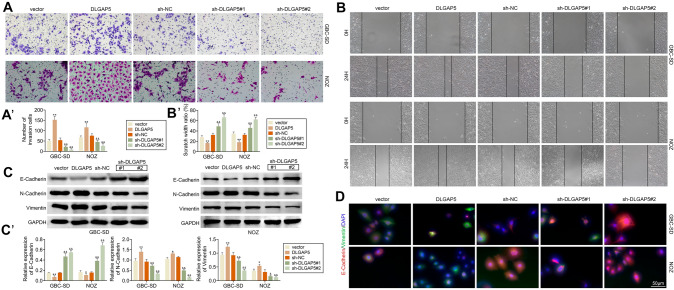
Further, we studied the effect of DLGAP5 on GBC cell migration. Transwell assay showed that the invasion ability of GBC-SD and NOZ cells was significantly enhanced after DLGAP5 overexpression, while the invasion ability of GBC cells was significantly weakened after DLGAP5 knockdown (Fig. 4A). Consistent with the results of invasion experiment, through scratch experiment, it was found that the scratch width of GBC-SD and NOZ cells was significantly reduced after overexpression of DLGAP5, while the scratch width of GBC cells was increased after knock down of DLGAP5 (Fig. 4B). In addition, we examined the protein levels of E-cadherin, N-cadherin, and vimentin, and we found that overexpression of DLGAP5 decreased E-cadherin and elevated N-cadherin and vimentin levels. However, the opposite effect appears after DLGAP5 is knocked down (Fig. 4C). Immunofluorescence showed that the expression levels of E-cadherin and vimentin in each group were consistent with the above results. This suggests that up-regulated DLGAP5 can promote GBC cells invasion and migration.
Fig. 4.
DLGAP5 promotes the migration of GBC cells. In GBC-SD and NOZ cells, DLGAP5 was knocked down and cell invasion was detected by transwell (A) and cell migration was detected by scratch assay (B). (C) Western blotting was used to detect the expression levels of E-cadherin, N-cadherin and vimentin in GBC-SD and NOZ cells. (D) Immunofluorescence representative pictures of E-cadherin and vimentin were shown. Scale bar, 50 μm. **P < 0.01 compared with vector group; &P < 0.05, &&P < 0.01 compared with sh-NC group
DLGAP5 promotes the M2 polarization of macrophage in GBC
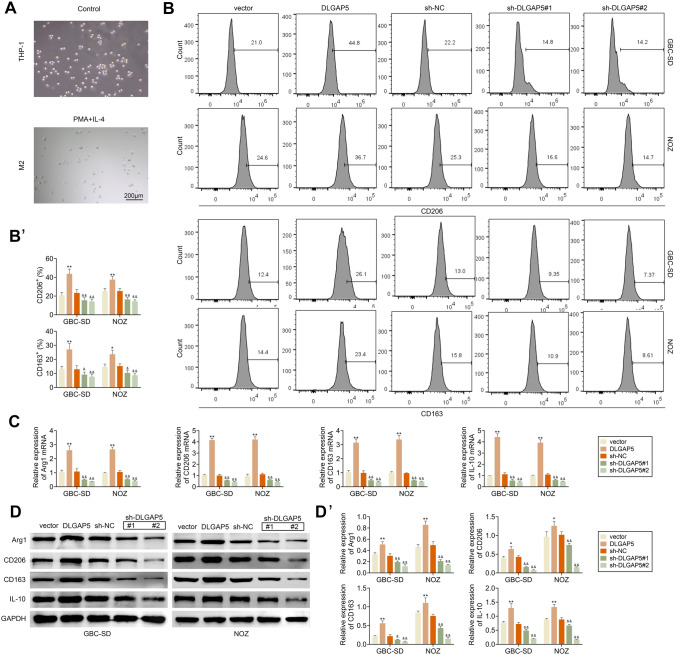
M2 polarization of macrophages plays an important role in tumor [31–33]. We explored whether DLGAP5 affects the polarization of tumor-associated macrophages. Human monocyte THP-1 can polarize into M2 macrophages under the stimulation of PMA and IL-4, and the morphology of the cells under the microscope is shown in Fig. 5A. GBC-SD and NOZ cells overexpressed or knocked down DLGAP5 were co-cultured with PMA-induced THP-1. Flow cytometry was used to detect CD163 and CD206 positive cells of THP-1-derived macrophages, which were markers of M2 macrophages. It indicated that CD163 and CD206 were up-regulated in THP-1-derived macrophages after overexpression of DLGAP5, while the expression levels of CD163 and CD206 were decreased after knockdown of DLGAP5 (Fig. 5B). In addition, after overexpression of DLGAP5 in GBC-SD and NOZ cells, the relative mRNA expression levels of Arg1, CD206, CD163 and IL-10 in THP-1-derived macrophages were up-regulated by RT-PCR assay, while this result was reversed after knockdown of DLGAP5 (Fig. 5C). Consistent with gene expression levels, western blotting results showed that after overexpression of DLGAP5 in GBC-SD and NOZ cells, protein expression levels of Arg1, CD206, CD163 and IL-10 in THP-1-derived macrophages were up-regulated (Fig. 5D).
Fig. 5.
DLGAP5 promotes the M2 polarization of macrophage. (A) THP-1 cells were treated with 320 ng/mL PhorBOL-12-myriSTATE-13-acetate (PMA) for 1 day and then were cultured with 20 ng/mL IL-4 and 20 ng/mL IL-13 for 48 h to induce M2 polarization of macrophages. Cell morphology was observed under a microscope. (B–D) After overexpressing DLGAP5 or knocking down DLGAP5 in GBC-SD and NOZ cells, they were co-cultured with PMA-induced THP-1 cells and THP-1 cells were used to detect indicators associated to M2 macrophage. (B) The expression levels of CD206 and CD163 were determined by flow cytometry. (C) Arg1, CD206, CD163 and IL-10 gene expression levels were detected by RT-PCR. (D) The protein levels of Arg1, CD206, CD163 and IL-10 were detected by western blotting. **P < 0.01 compared with vector group; &P < 0.05, &&P < 0.01 compared with sh-NC group
DLGAP5 promotes the progression of GBC by regulating cAMP pathway
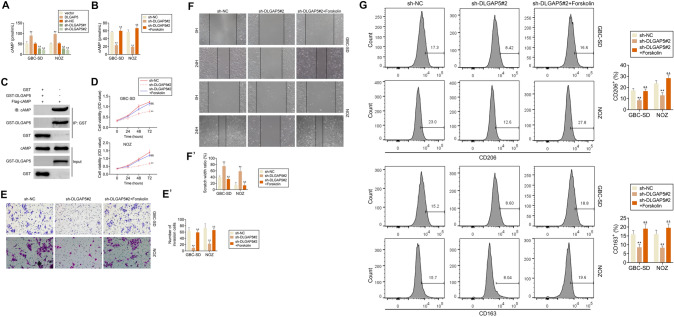
DLGAP5 promotes the growth, invasion and migration and the M2 polarization of macrophages in GBC cells. First, we investigated whether DLGAP5 could regulate cAMP, and our results indicated that cAMP level was up-regulated in GBC-SD and NOZ cells after overexpression of DLGAP5, while cAMP expression was down-regulated after DLGAP5 knockdown (Fig. 6A). To explore whether DLGAP5 is involved in the progression of GBC through the cAMP pathway, forskolin was added, which is cAMP agonist, after DLGAP5 knockdown of GBC-SD and NOZ cells. After DLGAP5 was knocked down, the level of cAMP was significantly down-regulated, while after forskolin was added, the level of cAMP was recovered (Fig. 6B). The direct interact between DLGAP5 and cAMP had been further confirmed by the GST-pulldown assay (Fig. 6C). Subsequently, the activity of GBC cells was detected by CCK8 assay, and it was found that after DLGAP5 was knocked down, the cell activity was significantly decreased, while after forskolin was given, the cell activity was rescued (Fig. 6D). We further study the effect of cAMP pathway on GBC cell proliferation and migration. By transwell experiment, the invasion ability of GBC cells was reduced after DLGAP5 was knocked down, while the invasion ability of GBC cells was recovered after forskolin was added (Fig. 6E). The results of scratch test indicated that the migration ability of GBC-SD and NOZ cells was weakened after DLGAP5 was knocked down, while the migration ability of GBC-SD and NOZ cells was rescued after forskolin was given (Fig. 6F). After DLGAP5 knockdown, GBC cells were co-cultured with PMA-induced THP-1 cells. Flow cytometry results showed that THP-1-derived macrophages had low expression levels of CD163 and CD206. CD163 and CD206 levels of THP-1-derived macrophages were restored when forskolin was added (Fig. 6G). These results elucidate that DLGAP5 is involved in the pathogenesis of GBC through the cAMP pathway.
Fig. 6.
DLGAP5 promotes GBC progression by regulating cAMP pathway. (A) Overexpression or knockdown of DLGAP5 in GBC-SD and NOZ cells. The expression levels of cAMP were detected in each group. (B–F) After DLGAP5 was knocked down in GBC-SD and NOZ cells, forskolin was added or not, and cAMP expression was detected (B). (C). GST-pulldown confirmed the direct interact between DLGAP5 and cAMP. The cell activity was detected by CCK8 assay (D), cell migration was detected by transwell (E) and scratch (F) assay. (G) After DLGAP5 was knocked down in GBC-SD and NOZ cells treated with or without forskolin, and then co-cultured with PMA-induced THP-1 cells. Flow cytometry was used to detect the expression levels of CD206 and CD163 in THP-1 cells. **P < 0.01 compared with sh-NC group; &P < 0.05, &&P < 0.01 compared with sh-DLGAP5 group
Knockdown of DLGAP5 inhibits tumor progression in vivo
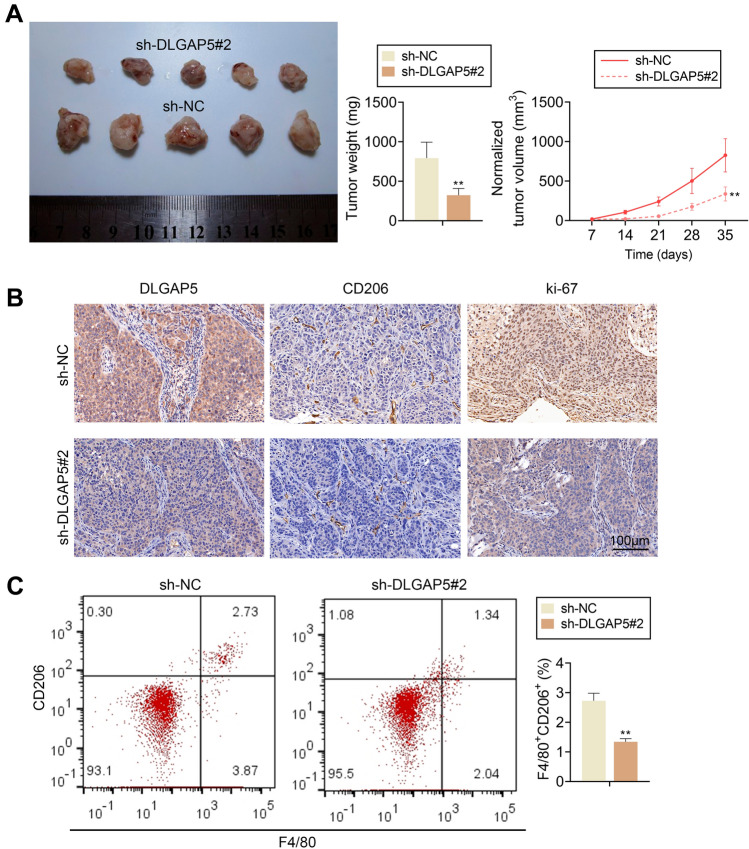
GBC-SD cells with DLGAP5 knockdown were subcutaneously xenografted into BALB/C nude mice. Results showed that among tumors formed by GBC-SD cells with DLGAP5 knockdown, the sh-DLGAP5 #2 group showed decrease in tumor weight and volume (Fig. 7A). The expression levels of DLGAP5, Ki67 and CD206 in tumor tissues were detected by immunohistochemistry, and the results showed that the expression levels of DLGAP5, Ki67 and CD206 were down-regulated in the sh-DLGAP5 #2 group (Fig. 7B). Moreover, F4/80+CD206+ cells in tumor tissue were significantly decreased in the sh-DLGAP5 #2 group (Fig. 7C). These data suggest that DLGAP5 knockdown can inhibit tumor progression in vivo.
Fig. 7.
Knockdown DLGAP5 inhibits tumor progression in vivo. (A) After DLGAP5 knockdown in GBC-SD cells, both tumor size and tumor volume were down-regulated. (B) Representative images showing the expression levels of DLGAP5, CD206 and Ki-67 positive cells. (C) M2 macrophage marker F4/80+CD206+ cells in xenograft tumor tissue were detected by flow cytometry. **P < 0.01 compared with sh-NC group
Discussion
Our study found that DLGAP5 levels are increased in GBC, and DLGAP5 expression levels are closely related to the prognosis of GBC patients. DLGAP5 promotes the growth and migration of GBC cells and the M2 polarization of macrophages. Mechanistically, DLGAP5 promotes the pathological progression of GBC through cAMP pathway.
The differential expression genes between GBC tissues and the normal group using a database were analyzed. Through analyzing protein–protein interaction, 30 genes with more nodes were obtained. Then, 5 genes with the most nodes were selected from the 30 genes for analysis. Previous studies have found that DLGAP5 is upregulated in many tumors, which is closely related to tumor migration and proliferation [34–36]. Our study found that DLGAP5 was overexpressed in patient with GBC. Furthermore, the expression level of DLGAP5 in various tumors was analyzed through the database. Since there was no data of GBC tissues in the database, we analyzed the data of similar CHOL tissues. DLGAP5 was found to be significantly overexpressed in CHOL. Combined with clinical samples, by detecting the expression level of DLGAP5 in GBC tissues, it found that DLGAP5 was significantly increased in GBC tissues. GBC patients with high DLGAP5 have a poor prognosis. In addition, GBC tissue significantly overexpressed CD206, M2 macrophage markers. There was a significant positive correlation between the expression level of DLGAP5 and CD206, suggesting that DLGAP5 may be related to the polarization of tumor-associated macrophages. All these suggest that DLGAP5 may be involved in the underlying pathological mechanism of GBC. Cell proliferation and migration play an important role in tumors. Studies have shown that DLGAP5 promotes the growth and migration of tumor cells [36, 37]. Toexplored whether DLGAP5 also affects the proliferation and migration of GBC cells, the role of DLGAP5 on the proliferation and migration of GBC was elucidated by overexpression of DLGAP5 or si-RNA knockdown of DLGAP5. Interestingly, GBC-SD and NOZ cells overexpressing DLGAP5 revealed more clonal formation, more cell activity, and lower cell apoptosis rate. However, GBC-SD and NOZ cells in DLGAP5 knockdown showed opposite results. Transwell assay, scratch assay and western blotting showed that cell invasion ability was enhanced in GBC-SD and NOZ cells overexpressing DLGAP5, and the expression of N-cadherin and vimentin was up-regulated, which are epithelialmesenchymal transition-related indicators. After DLGAP5 knockdown, the migration ability of GBC-SD and NOZ cells decreased, and the expression of N-cadherin and vimentin was down-regulated. It implies that DLGAP5 promotes the proliferation and migration of GBC cells.
Polarization of macrophages plays an important role in tumor [38, 39]. According to the data in Fig. 2, we found that DLGAP5 was correlated with M2 macrophages. We hypothesized that DLGAP5 promoted M2 polarization of tumor-associated macrophages. GBC-SD and NOZ cells that were overexpressed or knocked down by DLGAP5 were co-cultured with PMA-induced THP-1 cells. CD163 and CD206 were highly expressed in THP-1 macrophages co-cultured with dLGAP5-overexpressing GBC cells, which are markers of M2 macrophages. Moreover, our study results showed that the expression levels of Arg1, IL-10, CD163 and CD206 related to M2 macrophages were up-regulated after overexpression of DLGAP5. However, in the co-culture of GBC cells with DLGAP5 knockdown and PMA-induced THP-1 cells, M2-related indicators in THP-1-derived macrophages were down-regulated. It suggests that DLGAP5 promotes the M2 polarization of macrophages in GBC. cAMP pathway plays an important role in proliferation, migration and M2 polarization of macrophages in various tumors [40–43]. We explored whether DLGAP5 plays a role in GBC proliferation and macrophage polarization through the cAMP pathway. We found that overexpression of DLGAP5 significantly increased the cAMP levels, while knockdown of DLGAP5 remarkably decreased the expression level of cAMP. After DLGAP5 knockdown, GBC-SD and NOZ cells showed decreased cell activity, decreased migration ability, and decreased polarization of macrophages toward M2. However, these phenomena were reversed after the administration of forskolin, an agonist of cAMP. It suggests that DLGAP5 is involved in the growth, invasion, migration of GBC cells and the M2 polarization of macrophages through the cAMP pathway. The effect of DLGAP5 on GBC was verified in vivo. The sh-DLGAP5 #2 group showed down-regulation of tumor weight and volume, and decreased expression of CD206. All these suggest that knockdown DLGAP5 can inhibit tumor progression in vivo.
Macrophage polarization regulation mainly plays a role through cell signaling mediators. As an important nuclear transcription factor, cAMP response element-binding protein (CREB) promotes M2 polarization of macrophages by binding to cAMP. In addition, cAMP itself has an important influence on the regulation of metabolism, and the change of its level also affects the M2 polarization process by affecting cell metabolism.
DLGAP5 is an expression product of a cell cycle regulatory gene. The expression level of DLGAP5 increases in the G2/M phase and decreases sharply in the early to middle stages of G1. The expression level of DLGAP5 changes periodically throughout the cell cycle. In addition, DLGAP5 could mediate cell cycle in cancer cells. However, We found that DLGBP5 affects tumor progression through TAM, but do not rule out the possibility of simultaneous regulation through the cell cycle. Given the complex effects of DLGBP5 on gallbladder cancer and its impact on macrophage-related phenotypes, we suggest that DLGBP5 appears to have a greater influence on the progression of gallbladder cancer through TAM.
Patients with different expression of DLGAP5 had different stages by TNM, which was confirmed by our clinical data. However, if accumulation of expression is just stage-related, or this is independent factor of poor prognosis, is still unclear, in this study. Therefore we need further function assays to confirm it. In addation, while therapeutic potential of DLGAP5 is promising, further investigations are needed to clarify the prognostic role of this marker.
In many types of tumors (breast, bladder, prostate, head and neck, glioma, melanoma, and non-Hodgkin lymphoma), macrophage polarization often suggests a poor prognosis, which can be explained by the pro-tumor function of macrophages. M2 macrophages are abundant in malignant gliomas and predict poor prognosis, which could serve as markers for the early diagnosis of GBC. We here also indicated the co-relation of DLGAP5 expression level and poor GBC prognosis, suggesting that DLGAP5 promotes M2 polarization of macrophages and therefore promotes GBC progression [40, 41]. In this study, we focused on the effect of this protein on the polarization of macrophages, because the literature reports that this phenomenon has a more significant effect on GBC. The experimental results further confirm our hypothesis. In addition, we expect to further analyze the effects of other types of immune cells on the tumor immune microenvironment and the progression and prognosis of GBC in future studies. We plan to further verify the synergistic effect of relevant immune cells on GBC by means of monocytomics and immunofluorescence.
Our study shows that DLGAP5 is significantly elevated in GBC and is strongly associated with poor prognosis in patients with GBC. DLGAP5 promotes proliferation, migration, and M2 polarization of macrophages through cAMP pathway in GBC. DLGAP5 provides a theoretical basis for the treatment of GBC and may become a promising therapeutic target.
Author contribution
All authors contributed to the study conception and design. Material preparation and the experiments were performed by Jie Huang. Data collection and analysis were performed by Mengyao Zheng, and Yan Li. The first draft of the manuscript was written by Dingwei Xu and Daguang Tian and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Yun nan Fundamental Research Projects (Grant No.202101AT070239) and Medical Reserve Talents project of Yunnan Provincial Health Commsion (Grant No. H-2018065).
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interests
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the standards upheld by the Ethics Committee of the Second Affiliated Ho spital of Kunming Medical University and with those of the 1964 Helsinki Declaration and its later amendments for ethical research involving human subjects. All animal experiments were approved by the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University for the use of animals and conducted in accordance with the National Institutes of Health Laboratory Animal Care and Use Guidelines.
Informed consent
Written informed consent was obtained from a legally authorized representative(s) in the Second Affiliated Hospital of Kunming Medical University for anonymized patient information to be published in this article.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hickman L, Contreras C. Gallbladder cancer: diagnosis, surgical management, and adjuvant therapies. Surg Clin North Am. 2019;99:337–355. doi: 10.1016/j.suc.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Recio-Boiles A, Kashyap S, Babiker HM (2022) Gallbladder Cancer. In: StatPearls. Treasure Island (FL), [PubMed]
- 3.Zaidi MY, Maithel SK. Updates on gall bladder cancer management. Curr Oncol Rep. 2018;20:21. doi: 10.1007/s11912-018-0664-3. [DOI] [PubMed] [Google Scholar]
- 4.Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Liu Q, Dou R, Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18:64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40:310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Tian T, Zhang J. Tumor-associated macrophages (TAMs) in colorectal cancer (CRC): from mechanism to therapy and prognosis. Int J Mol Sci. 2021;22:22168470. doi: 10.3390/ijms22168470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, Liu B, Su L, Qiu Z. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic cancer metastasis. Cancer Res. 2018;78:4586–4598. doi: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- 8.Guan Y, Du Y, Wang G, Gou H, Xue Y, Xu J, Li E, Chan DW, Wu D, Xu P, Ni P, Xu D, Hu Y. Overexpression of PLXDC2 in Stromal cell-associated M2 Macrophages is related to EMT and the progression of gastric cancer. Front Cell Dev Biol. 2021;9:673295. doi: 10.3389/fcell.2021.673295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thanee M, Loilome W, Techasen A, Namwat N, Boonmars T, Pairojkul C, Yongvanit P. Quantitative changes in tumor-associated M2 macrophages characterize cholangiocarcinoma and their association with metastasis. Asian Pac J Cancer Prev. 2015;16:3043–3050. doi: 10.7314/apjcp.2015.16.7.3043. [DOI] [PubMed] [Google Scholar]
- 10.Zhao S, Liu Y, He L, Li Y, Lin K, Kang Q, Liu L, Zou H. Gallbladder cancer cell-derived exosome-mediated transfer of leptin promotes cell invasion and migration by modulating STAT3-mediated M2 macrophage polarization. Anal Cell Pathol Amst. 2022;2022:9994906. doi: 10.1155/2022/9994906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amunjela JN, Tucker SJ. Dysregulation of POPDC1 promotes breast cancer cell migration and proliferation. Biosci Rep. 2017;37:1039. doi: 10.1042/BSR20171039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T, Liu H, Liu Z, Li K, Qin R, Wang Y, Liu J, Li Z, Gao Q, Pan C, Yang F, Zhao W, Zhang Z, Xu Y. FGF19 and FGFR4 promotes the progression of gallbladder carcinoma in an autocrine pathway dependent on GPBAR1-cAMP-EGR1 axis. Oncogene. 2021;40:4941–4953. doi: 10.1038/s41388-021-01850-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhao L, Zhang H, Liu X, Xue S, Chen D, Zou J, Jiang H. TGR5 deficiency activates antitumor immunity in non-small cell lung cancer via restraining M2 macrophage polarization. Acta Pharm Sin B. 2022;12:787–800. doi: 10.1016/j.apsb.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabio G, Arthur JS, Kuma Y, Peggie M, Carr J, Murray-Tait V, Centeno F, Goedert M, Morrice NA, Cuenda A. p38gamma regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 2005;24:1134–1145. doi: 10.1038/sj.emboj.7600578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naisbitt S, Valtschanoff J, Allison DW, Sala C, Kim E, Craig AM, Weinberg RJ, Sheng M. Interaction of the postsynaptic density-95/guanylate kinase domain-associated protein complex with a light chain of myosin-V and dynein. J Neurosci. 2000;20:4524–4534. doi: 10.1523/JNEUROSCI.20-12-04524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong J, Yang H, Eom SH, Chun C, Im YJ. Structure of the GH1 domain of guanylate kinase-associated protein from Rattus norvegicus. Biochem Biophys Res Commun. 2014;452:130–135. doi: 10.1016/j.bbrc.2014.08.073. [DOI] [PubMed] [Google Scholar]
- 17.de Reynies A, Assie G, Rickman DS, Tissier F, Groussin L, Rene-Corail F, Dousset B, Bertagna X, Clauser E, Bertherat J. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol. 2009;27:1108–1115. doi: 10.1200/JCO.2008.18.5678. [DOI] [PubMed] [Google Scholar]
- 18.Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, Ji XN, Liu H, Xia JL, Wu ZQ, Fan J, Ma ZC, Zhou XD, Lin ZY, Liu KD. A decade's studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PubMed] [Google Scholar]
- 19.Tsou AP, Yang CW, Huang CY, Yu RC, Lee YC, Chang CW, Chen BR, Chung YF, Fann MJ, Chi CW, Chiu JH, Chou CK. Identification of a novel cell cycle regulated gene, HURP, overexpressed in human hepatocellular carcinoma. Oncogene. 2003;22:298–307. doi: 10.1038/sj.onc.1206129. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Liu Y, Tang S, Qin X, Li L, Zhou J, Zhang J, Liu B. Knockdown of DLGAP5 suppresses cell proliferation, induces G2/M phase arrest and apoptosis in ovarian cancer. Exp Ther Med. 2021;22:1245. doi: 10.3892/etm.2021.10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng R, Shi Z, Li W, Yu J, Wang Y, Zhou Q. Identification and prognostic value of DLGAP5 in endometrial cancer. PeerJ. 2020;8:e10433. doi: 10.7717/peerj.10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Y, Li Q, Zhou W, Gu Y, Jiang Y. Coniferyl aldehyde alleviates LPS-induced WI-38 cell apoptosis and inflammation injury via JAK2-STAT1 pathway in acute pneumonia. Allergol Immunopathol Madr. 2021;49:72–77. doi: 10.15586/aei.v49i5.464. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Bao X, Zhang J, Hu Q, Wei B. Devazepide suppresses cell proliferation and migration, and induces apoptosis in bladder carcinoma. Transl Androl Urol. 2021;10:2113–2121. doi: 10.21037/tau-21-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato K, Tatsunami R, Wakame K. Epalrestat suppresses inflammatory response in lipopolysaccharide-stimulated RAW264.7 cells. Allergol Immunopathol Madr. 2021;49:1–8. doi: 10.15586/aei.v49i5.102. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Wang L, Hu H, Dong P. MiR-224 ameliorates inflammation and symptoms in mouse model of allergic rhinitis by targeting CDK9. Allergol Immunopathol Madr. 2021;49:80–88. doi: 10.15586/aei.v49i6.451. [DOI] [PubMed] [Google Scholar]
- 26.Bassal S, Nomura N, Venter D, Brand K, McKay MJ, van der Spek PJ. Characterization of a novel human cell-cycle-regulated homologue of Drosophila dlg1. Genomics. 2001;77:5–7. doi: 10.1006/geno.2001.6570. [DOI] [PubMed] [Google Scholar]
- 27.Gomez CR, Kosari F, Munz JM, Schreiber CA, Knutson GJ, Ida CM, El Khattouti A, Karnes RJ, Cheville JC, Vasmatzis G, Vuk-Pavlovic S. Prognostic value of discs large homolog 7 transcript levels in prostate cancer. PLoS One. 2013;8:e82833. doi: 10.1371/journal.pone.0082833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuart JE, Lusis EA, Scheck AC, Coons SW, Lal A, Perry A, Gutmann DH. Identification of gene markers associated with aggressive meningioma by filtering across multiple sets of gene expression arrays. J Neuropathol Exp Neurol. 2011;70:1–12. doi: 10.1097/NEN.0b013e3182018f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, Zhang Z, Cai S, Xu Y, Li X, He X, Zhong X, Li G, Chen Z, Li D. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13:156. doi: 10.1186/s13045-020-00991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Zhang S, Wang Q, Zhang X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol Oncol. 2017;10:36. doi: 10.1186/s13045-017-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lan J, Sun L, Xu F, Liu L, Hu F, Song D, Hou Z, Wu W, Luo X, Wang J, Yuan X, Hu J, Wang G. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79:146–158. doi: 10.1158/0008-5472.CAN-18-0014. [DOI] [PubMed] [Google Scholar]
- 32.Weng YS, Tseng HY, Chen YA, Shen PC, Al Haq AT, Chen LM, Tung YC, Hsu HL. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol Cancer. 2019;18:42. doi: 10.1186/s12943-019-0988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharifi L, Nowroozi MR, Amini E, Arami MK, Ayati M, Mohsenzadegan M. A review on the role of M2 macrophages in bladder cancer; pathophysiology and targeting. Int Immunopharmacol. 2019;76:105880. doi: 10.1016/j.intimp.2019.105880. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Pan Y, Fu H, Zhang J. Nucleolar and spindle associated protein 1 (NUSAP1) inhibits cell proliferation and enhances susceptibility to epirubicin in invasive breast cancer cells by regulating cyclin D kinase (CDK1) and DLGAP5 expression. Med Sci Monit. 2018;24:8553–8564. doi: 10.12659/MSM.910364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Y, Li F, Yan J, Guo X, Wang F, Shi H, Du J, Zhang H, Gao Y, Li D, Yao Y, Hu W, Han J, Zhang M, Ding R, Wang X, Huang C, Zhang J. Pan-cancer analysis and experiments with cell lines reveal that the slightly elevated expression of DLGAP5 is involved in clear cell renal cell carcinoma progression. Life Sci. 2021;287:120056. doi: 10.1016/j.lfs.2021.120056. [DOI] [PubMed] [Google Scholar]
- 36.Yu DC, Chen XY, Zhou HY, Yu DQ, Yu XL, Hu YC, Zhang RH, Zhang XB, Zhang K, Lin MQ, Gao XD, Guo TW. TRIP13 knockdown inhibits the proliferation, migration, invasion, and promotes apoptosis by suppressing PI3K/AKT signaling pathway in U2OS cells. Mol Biol Rep. 2022;49:3055–3064. doi: 10.1007/s11033-022-07133-6. [DOI] [PubMed] [Google Scholar]
- 37.Liao W, Liu W, Yuan Q, Liu X, Ou Y, He S, Yuan S, Qin L, Chen Q, Nong K, Mei M, Huang J. Silencing of DLGAP5 by siRNA significantly inhibits the proliferation and invasion of hepatocellular carcinoma cells. PLoS One. 2013;8:e80789. doi: 10.1371/journal.pone.0080789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020;11:583084. doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL, Gong WY, Dong JC, Liu BJ. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J Exp Clin Cancer Res. 2018;37:207. doi: 10.1186/s13046-018-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polumuri S, Perkins DJ, Vogel SN. cAMP levels regulate macrophage alternative activation marker expression. Innate Immun. 2021;27:133–142. doi: 10.1177/1753425920975082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang K, Yao G, Hu L, Yan Y, Liu J, Shi J, Chang Y, Zhang Y, Liang D, Shen D, Zhang G, Meng S, Piao H. MOB2 suppresses GBM cell migration and invasion via regulation of FAK/Akt and cAMP/PKA signaling. Cell Death Dis. 2020;11:230. doi: 10.1038/s41419-020-2381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Safi W, Kraus A, Grampp S, Schodel J, Buchholz B. Macrophage migration inhibitory factor is regulated by HIF-1alpha and cAMP and promotes renal cyst cell proliferation in a macrophage-independent manner. J Mol Med Berl. 2020;98:1547–1559. doi: 10.1007/s00109-020-01964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi H, Sun X, Kong A, Ma H, Xie Y, Cheng D, Wong CKC, Zhou Y, Gu J. Cadmium induces epithelial-mesenchymal transition and migration of renal cancer cells by increasing PGE2 through a cAMP/PKA-COX2 dependent mechanism. Ecotoxicol Environ Saf. 2021;207:111480. doi: 10.1016/j.ecoenv.2020.111480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.