Abstract
Excessive hepatic lipid accumulation is closely linked to inflammation, insulin resistance, and metabolic syndromes. We hypothesized that a combined extract containing Schisandra chinensis (SCE) could alleviate hepatic lipid accumulation. Male Sprague–Dawley rats fed a high-sucrose diet (HSD) were randomly assigned to three groups (n = 6): normal diet (ND), HSD (60% kcal from sucrose), and HSD + SCE (HSD with 2.44% SCE). Liquid chromatography–tandem mass spectrometry revealed that SCE contains chlorogenic acid (5.514 ± 0.009 mg/g) and schisandrin (0.179 ± 0.002 mg/g) as bioactive components. SCE did not alter the body weight, fat mass, lean mass, or glucose levels. Strikingly, SCE effectively reduced the plasma triglyceride (TG) and hepatic TG levels compared to the HSD group. Adiposity reduction is due to decreased activity of hepatic de novo lipogenic enzymes. These results indicated that SCE has nutraceutical potential for the prevention and treatment of hepatic steatosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01464-1.
Keywords: Chlorogenic acid, De novo lipogenesis, Hepatic steatosis, Schisandra chinensis, Schisandrin
Introduction
Hepatic steatosis, also known as fatty liver disease, is a condition in which excess fat accumulates in the liver cells. This can lead to inflammation and liver damage, and in some cases, progress to more severe forms of liver disease, such as non-alcoholic steatohepatitis (NASH) and cirrhosis (Angulo, 2002; Nature Medicine, 2017). The prevalence of fatty liver disease is increasing worldwide and is now considered a major public health concern. The global prevalence of fatty liver disease is estimated to be approximately 25%, with higher rates in some regions such as South America, the Middle East, and Asia (Lazarus et al., 2022a; b; Younossi et al., 2023). Complications of fatty liver disease include liver fibrosis, which can progress to cirrhosis, liver failure, and increased risk of liver cancer. Fatty liver disease is associated with an increased risk of cardiovascular diseases, type 2 diabetes, and metabolic syndromes (Diehl and Day, 2017; Lazarus et al., 2022a; b). Fatty liver disease is important because of its potential to progress to more severe forms of liver disease and its association with other metabolic and cardiovascular disorders (Donnelly et al., 2005).
Dietary modifications, weight loss, and regular exercise are recommended to prevent and treat non-alcoholic fatty liver disease (NAFLD). However, adherence to lifestyle changes is challenging (Vilar-Gomez et al., 2016). Although pharmacotherapeutic agents, such as metformin and thiazolidinediones, are available for NAFLD, their use is limited owing to safety concerns and side effects (Polyzos et al., 2019). The U.S. Food and Drug Administration has not approved any drugs specifically for the treatment of NAFLD; however, metformin, pioglitazone, vitamin E, and statins have been used off-label (Younossi et al., 2016). Metformin showed only marginal improvement in the hepatic tissue. Pioglitazone has been shown to reduce liver fat content and improve liver enzyme levels. However, it is associated with side effects, such as weight gain, edema, and increased risk of bone fractures (Cusi et al., 2016; Chalasani et al., 2012). To overcome these limitations, research on the development of various extracts and functional food ingredients is increasing, and there is a growing interest in natural preventive medicine and related markets (Wang et al., 2023). Many functional foods and bioactive substances have been developed for this purpose (Pathak et al., 2023).
Schisandra chinensis (SCE) is a valuable natural resource due to its pharmacological effects (Kwon and Park, 2008). It has been reported to have antioxidant, blood glucose-regulatory, and immunomodulatory effects (Jeong et al., 2009; Kim et al., 2009; Park et al., 2012a; You et al., 2023). In addition, SCE extract has been shown to reduce triglyceride (TG) and cholesterol levels in the blood of high-fat-induced obese mice (Song et al., 2013). Considering these benefits, the development of a combined extract containing Schisandra chinensis to treat hepatic steatosis has been of great interest. Identifying the bioactive compounds in SCE can offer valuable information on their mechanism of action and contribute to the standardization of herbal medicines and functional foods, thereby ensuring the consistency and effectiveness of these products. This study aimed to identify the bioactive compound in SCE and assess its effects on growth performance, body composition, and hepatic TG metabolism in rats fed a high-sucrose diet. This study explored the potential application of SCE as a nutraceutical for the treatment of hepatic steatosis.
Materials and method
Preparation of a combined extract containing Schisandra chinensis (SCE)
SCE was extracted with distilled water at approximately 98–100 ℃ for 2 h. The crude extract was filtered through microfilter paper and concentrated under reduced pressure at 50 ℃ or lower. Final sterilization was followed at 95–98 ℃ for 20 min. Chlorogenic acid and schisandrin were quantitatively analyzed using liquid chromatography as standard substances for SCE.
Sample preparation for analysis
SCE (10 g) was ground to a powder, mixed with 50 mL of 80% methanol, and homogenized at 8000 rpm (WiseTis homogenizer, HG-15D, Won-ju, Korea). The homogenized sample was sonicated for 30 min, then, filtered through a CHMLAB No. F1093-110 qualitative filter paper. The extracted sample was concentrated by a rotary evaporator (Eyela Rotary Vacuum Evaporator NN series, Eyela, Tokyo, Japan) under reduced pressure at 35 °C. For analysis, 1 µg/mL of dissolved sample in 90% methanol and centrifuged for 5 min at 14,000 g x (LaboGene 1730 R, LaboGene, Daejeon, Korea), after which the supernatant was filtered using a 13 mm diameter Nylon syringe filter with a 0.22 µm pore size (Sartorius, Darmstadt, Germany). The sample was stored at − 21 °C until analysis.
LC–MS/MS conditions
The content of chlorogenic acid and schisandrin in SCE was analyzed using a Xevo TQ-MS triple quadrupole mass spectrometer (Waters, Manchester, UK) equipped with a Waters Acquity UPLC system (Waters, Milford, MA, USA). The column was a ZORBAX Eclipse Plus C18 rapid resolution HD (2.1 × 100 mm, 1.8 Micron). A mobile phase consisting of 0.1% formic acid in acetonitrile (Thermo Fisher Scientific, cat. LS120-212) (A) and 0.1% formic acid in distilled water (Thermo Fisher Scientific, cat. LS118-212) (B) was used to inject 5 µL of the sample at a flow rate of 0.2 mL/min and analyzed by linear gradient elution. The mobile phase comprised of 5% A and 95% B from 0 to 2 min, 100% A from 6 to 12 min, and 5% A and 95% B from 15 to 18 min. The multiple reaction monitoring conditions were as follows: detection was operated in electrospray ionization source in positive ion mode, capillary voltage 3.0 kV, source temperature 150 °C, desolvation temperature 350 °C, desolvation gas flow rate 300 L/Hr. The cone voltage and collision energy were individually optimized for chlorogenic acid and schisandrin (Table 1). Precursor/product transitions (m/z) were 355 > 163 for chlorogenic acid, 433 > 415 for schisandrin. Mass data were processed using TargetLynx software (version 4.1, Waters).
Table 1.
Precursor/product transitions and parameters for multiple reaction monitoring (MRM)
| Analyte | Precursor ion (m/z) | Product ion (m/z) | Ion mode | CV* (V) | CE* (V) | RT* (min) |
|---|---|---|---|---|---|---|
| Chlorogenic acid | 355 | 163 | ESI+ | 60 | 5 | 4.50 |
| Schisandrin | 433 | 415 | ESI+ | 25 | 5 | 6.58 |
*ESI electrospray ionization, CV cone voltage, CE collision energy, RT Retention time
* ESI electrospray ionization, CV cone voltage, CE collision energy, RT Retention time
Animals and diets
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Hanyang University (HY-IACUC-2019-0182A). Eight-week-old Sprague–Dawley rats (Koatech Animal, Seoul, Korea) were purchased and housed under controlled conditions of 22 ± 1 ℃, 40–50% humidity, and a 12:12 light–dark cycle with ventilation facilities throughout the experimental period. After 1 week of adaptation, the rats were randomly assigned to three groups (n = 6): (1) normal diet (ND), (2) high-sucrose diet (HSD, 60% kcal from sucrose, negative control), and (3) HSD + SCE (HSD with 2.44% SCE). Food and water were provided ad libitum throughout the experiments. The SCE dose was calculated based on the equivalent human dose (11 g/60 kg body weight (bw)) (Table S1).
Growth performance and body composition
Growth performance, including body weight, weight gain, total energy intake, and water intake, was assessed weekly. Total energy intake (kcal) was calculated from the feed intake. Body composition analysis was conducted using dual-energy X-ray absorptiometry (DEXA; Medikors, Seongnam-si, Korea) to determine lean mass (g), fat mass (g), and bone mineral density (mg/cm2). To measure body composition, rats were anesthetized with ketamine (100 mg/kg bw; Yuhan Co., Seoul, Korea) and xylazine (10 mg/kg bw; Bayer, Leverkusen, Germany) by intraperitoneal injection 6 h fasting to measure body composition.
Fasting blood glucose and oral glucose tolerance test (OGTT)
Fasting blood glucose levels were measured every 2 weeks from the rats’ tails after 6 h of fasting using an Accu-Chek glucometer (Roche, Basel, Switzerland). After the rats were treated with SCE for 8 weeks, OGTT was performed after 6 h of fasting by oral administration of 2 g/kg bw of glucose. Blood glucose levels were measured from the tail at 0, 15, 30, 60, 120, and 240 min using a glucometer (Roche).
Plasma and hepatic TG contents
Blood was collected from rats fasted for 6 h from the retro-orbital cavity using heparin-treated tubes. Plasma was separated by centrifugation (2000× g, 15 min, 4 °C). Hepatic tissue lysates were collected using RIPA buffer. The TG levels in the plasma and liver were measured using commercially available kits (Wako, Osaka, Japan). Absorbance was measured at 600 nm using a spectrophotometer (BioTek, Winooski, VT, USA).
Immunoblotting
Total protein in the liver tissue was evaluated by lysing the tissue in NP-40 lysis buffer (Thermo Fisher Scientific, Waltham, MA, USA) containing a phosphatase and protease inhibitor cocktail (Cell Signaling, Danvers, MA, USA). A bicinchoninic acid (BCA) assay was used to determine the protein concentration in the lysates. Protein samples were mixed with 2 × Laemmli buffer (Bio-Rad, Hercules, CA, USA) and proteins were separated using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred onto polyvinylidene fluoride membranes. The membrane was blocked with 5% bovine serum albumin (GenDEPOT, Barker, TX, USA) in Tris-buffered saline (TBS) containing 0.1% Tween 20. Primary antibodies (1:1000), including acetyl-CoA carboxylase 1 (ACC1), fatty acid synthase (FAS), and stearoyl-Coenzyme A desaturase 1 (SCD1), were used for incubation overnight at 4 ℃. After that, the membrane was incubated with the HRP-conjugated secondary antibody (1:3000) for 1 h at 20 ℃. Proteins were quantified using Image Lab software (Bio-Rad).
Statistical analysis
All data are presented as the mean ± standard error of the mean (SEM). All experimental results were analyzed using Prism 9 (GraphPad Software, San Diego, CA, USA) and statistical significance was evaluated using an unpaired t-test. Statistical significance was defined as p < 0.05.
Results and discussion
LC–MS/MS determined the concentration of chlorogenic acid and schisandrin in SCE
Qualitative and quantitative analyses of chlorogenic acid and schisandrin in SCE were performed using liquid chromatography-mass spectrometry. As shown in Table 2, chlorogenic acid and schisandrin concentrations ranged from 0.01 to 0.5 µg/L. The calibration equations were Y = 889.46x–19.194 (R2 = 0.999) and Y = 2313.3x–38.296 (R2 = 0.999) for chlorogenic acid and schisandrin, respectively. The limits of detection and quantification for chlorogenic acid and schisandrin were estimated to be 0.001 and 0.003 g/mL, respectively. Chlorogenic acid was quantified as 5.514 ± 0.009 mg/g and schisandrin as 0.179 ± 0.002 mg/g. The effects of extracts including chlorogenic acid and schisandrin on the SCE were investigated. Chlorogenic acid is a phenolic compound with many beneficial properties, such as antioxidative activity (Farah et al., 2008), modulation of glucose metabolism (Ong et al., 2012), and prevention of cardiovascular risk factors (Li et al., 2020). Schisandrin, a dibenzocyclooctadiene lignan, has diverse physiological activities, including anti-inflammatory (Guo et al., 2008) and hepatoprotective effects (Park and Yoon, 2015).
Table 2.
Quantification of chlorogenic acid and schisandrin from SCE by LC–MS/MS
| Analyte | Content ± SD (mg/g) | Equation (y = ax + b) | Calibration range (µg/mL) | Linearity (R2) | LODa | LOQa |
|---|---|---|---|---|---|---|
| µg/mL | ||||||
| Chlorogenic acid | 5.514 ± 0.009 | Y = 889.46x−19.194 | 0.01–0.5 | 0.999 | 0.001 | 0.003 |
| Schisandrin | 0.179 ± 0.002 | Y = 2313.3x−38.296 | 0.01–0.5 | 0.999 | 0.001 | 0.003 |
LOD Limit of detection, LOQ Limit of quantification
aLOD and LOQ were estimated as 3.3 (LOD) or 10 (LOQ) × standard deviation of the blank/slope of the calibration curve
Growth performance and body composition
Tracking physical phenotypes such as body weight, feed intake, and weight gain in experimental animals studying metabolic syndrome is a fundamental and essential research topic (Kang et al., 2021; Kim et al., 2020; Wong et al., 2016). During the 8-week intervention period, growth factors, including initial and final body weights, feed intake, and energy intake, were examined (Table 3). The initial body weight of the experimental group was 296–299 g, and no statistically significant differences were observed among the groups. The HSD group showed a significant increase in body weight (p < 0.05) and weight gain (p < 0.01) compared with the ND group. However, no significant changes in body weight or weight gain were observed following SCE administration. Energy intake was calculated by converting the amount of feed consumed into calories. Changes in feed or energy intake imply the modulation of appetite. Analysis of total energy intake in this study showed no significant changes in any of the groups, indicating that HSD increases body weight regardless of appetite regulation. These findings implied that SCE administration did not alter growth performance traits in rats with HSD-induced hepatic steatosis.
Table 3.
Growth performance and body composition in rats fed a high-sucrose diet
| ND | HSD | HSD + SCE | |
|---|---|---|---|
| Growth factor | |||
| Initial body weight (g) | 299 ± 1 | 296 ± 1 | 298 ± 3 |
| Final body weight (g) | 402 ± 5 | 424 ± 3* | 421 ± 8 |
| Weight gain (g/week) | 107 ± 2 | 126 ± 3** | 123 ± 6 |
| Total energy intake (kcal) | 4,496 ± 43 | 4,395 ± 61 | 4,466 ± 63 |
| Final water intake (g) | 206 ± 10 | 180 ± 5 | 144 ± 6 |
| Body composition | |||
| Lean mass (g) | 284 ± 6 | 300 ± 4 | 288 ± 4 |
| Fat mass (g) | 88.8 ± 2.6 | 98.7 ± 2.8* | 101 ± 4 |
| Fat in tissue (%) | 23.2 ± 0.4 | 24.6 ± 0.1* | 25.6 ± 0.7 |
| Bone mineral density (mg/cm2) | 0.22 ± 0.0 | 0.24 ± 0.0* | 0.25 ± 0.0 |
Values are expressed as mean ± standard error of the mean (n = 6)
ND normal diet, HSD high-sucrose diet, 60% kcal from sucrose; SCE, a combined extract containing Schisandra chinensis
*,**p-values compared to ND and HSD
The use of DEXA in metabolically disordered mice allows the assessment of various physical phenotypes related to body composition, including lean mass, fat mass, and bone mineral density (Jeong et al., 2022; Kim et al., 2017). It is a non-invasive and widely used method for evaluating overall body composition, fat, and lean mass distribution in experimental animals (Kishi et al., 2023; Nazarian et al., 2009). DEXA was used to trace detailed changes in body composition such as lean mass, fat mass, and bone mineral density (Table 3). HSD successfully induced adiposity (p < 0.05) without altering lean mass. However, as with growth performance, SCE did not modulate body adiposity (g or %), lean mass, or bone mineral density compared to the HSD. These findings suggest that the administration of SCE for 8 weeks did not noticeably affect the body composition of rats with HSD-induced hepatic steatosis.
SCE did not alter fasting glucose level and OGTT
Type 2 diabetes mellitus is associated with insulin resistance, fatty liver-related obesity, and cardiovascular diseases (Lee et al., 2020). In this study, mice were fed a high-sucrose diet to investigate biomarkers associated with type 2 diabetes and glucose homeostasis. Two commonly used in vivo markers for diabetes diagnosis and monitoring are fasting glucose levels and OGTT (Roden, 2016). In rodents, elevated fasting glucose levels indicate impaired glucose regulation, which is a hallmark of diabetes (Muniyappa et al., 2008). The OGTT is a widely used diagnostic test for diabetes in rodents. It involves administering a glucose solution orally and measuring blood glucose levels at regular intervals over a set period. An elevated area under the curve (AUC) characterizes impaired glucose tolerance after administering glucose, indicating reduced insulin sensitivity or impaired insulin secretion (Andrikopoulos et al., 2008).
This study assessed fasting glucose levels at weeks 2, 4, and 8 (Fig. 1A). Throughout the intervention period, all groups maintained their blood glucose levels within the normal range (approximately 100 mg/dL), and there were no significant changes among the groups. The results of the OGTT are shown in Fig. 1B. In all groups, blood glucose levels peaked 15–30 min after glucose administration and then gradually decreased. There were no significant differences in blood glucose levels or AUC between the groups. Therefore, there is insufficient evidence that SCE improves the risk factors for type 2 diabetes, including fasting glucose levels and OGTT results.
Fig. 1.
Glucose homeostasis in rats fed a high-sucrose diet. A Fasting glucose level (mg/dL) and B Oral glucose tolerance test (OGTT, mg/dL) and the area under the curve (AUC). Data are expressed as mean ± standard error. The difference between groups was analyzed by unpaired t-test (n = 6)
SCE decreases TG in plasma and liver tissue
Plasma TG levels are crucial in the development and progression of hepatic steatosis (Go et al., 2014; Wang et al., 2015). Elevated plasma TG levels are frequently observed in individuals with NAFLD and are strongly associated with insulin resistance and dyslipidemia (Arca et al., 2020; Go et al., 2014; Heeren and Scheja, 2021; Miller et al., 2011). Increased plasma TG levels can result from the increased hepatic production of very low-density lipoproteins (VLDL) or decreased clearance of TG-rich lipoproteins (Minehira et al., 2008). Excessive accumulation of plasma TG can lead to its deposition in various tissues, including the liver (Mir et al., 2022). Hepatic TG accumulation is a key characteristic of NAFLD and closely linked to the development of liver steatosis, inflammation, and fibrosis (Diehl and Day, 2017; Samuel and Shulman, 2019; Targher et al., 2010). Excessive dietary intake of free fatty acids, along with impaired fatty acid oxidation and increased de novo lipogenesis in the liver, can contribute to the accumulation in hepatocytes (Ipsen et al., 2018; Naguib et al., 2020). Hepatic TG accumulation not only reflects the severity of liver steatosis, but also plays a critical role in the progression from simple steatosis to more advanced stages of NAFLD, such as non-alcoholic steatohepatitis (NASH) and fibrosis (Diehl and Day, 2017).
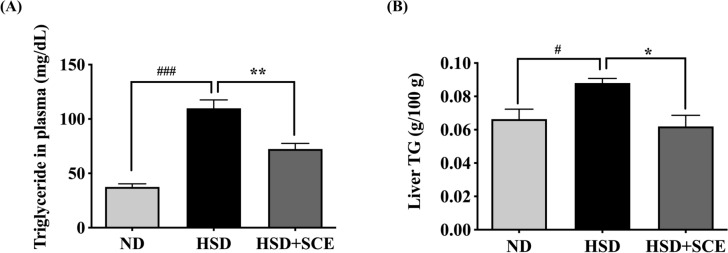
The measurement of plasma TG showed a significant increase in the HSD group (109.8 mg/dL) compared to the ND group (37.5 mg/dL) (p < 0.001) (Fig. 2A), which is consistent with previous studies that reported a significant increase in plasma neutral lipids in animals fed a high-sucrose diet compared to the control group (Kanazawa et al., 2003). In this study, the HSD + SCE group showed a significant decrease in plasma neutral lipids compared to the HSD group (72.4 mg/dL) (p < 0.01) (Fig. 2A). Previous studies have confirmed that Schisandra chinensis prevents elevated plasma TG levels. For example, the accumulation of plasma TG was reduced in Schisandra chinensis supplemented mice (p < 0.01) (Sun et al., 2017). Oral supplementation of Schisandra chinensis also resulted in a significant decrease in plasma TG levels in obese Sprague–Dawley rats (Park et al., 2012b). However, previous studies have not illustrated Schisandra chinensis’s fundamental components in reduced plasma lipid biomarkers. These findings suggest that chlorogenic acid and Schisandrin are critical for reducing plasma neutral lipid levels in the HSD + SCE group, which may improve hypertriglyceridemia and cardiovascular disease.
Fig. 2.
Plasma and liver triglyceride (TG) in rats fed a high-sucrose diet. A TG in plasma (mg/dL) and B Liver TG. Data are expressed as the mean ± standard error of the mean. #p < 0.05 compared to ND and HSD, *p < 0.05 compared to HSD and HSD + SCE
In addition to plasma triglycerides, hepatic TG is also associated with metabolic disorders such as obesity and type 2 diabetes (Seppäla-Lindroos et al., 2002). Particularly, hepatic TG increase hepatic steatosis and contribute to the pathogenesis of NAFLD (Kawano and Cohen, 2013). Therefore, we measured the hepatic neutral lipid levels (Fig. 2B). The HSD ch(p < 0.05). Animals fed a high-sucrose diet for 5 weeks showed a significant increase in hepatic neutral lipids (approximately 0.08 g/100 g tissue) compared to the control group (Huang et al., 2007), indicating that a high-sucrose diet is involved in the accumulation and inhibition of the breakdown of hepatic neutral lipids. In the current study, SCE notably decreased hepatic neutral lipids by 30% compared with the control (p < 0.05). Similarly, Schisandra chinensis berry ethanol extract significantly ameliorated lipid accumulation in HepG2 cells treated with oleic acid (p < 0.05) (Chung et al., 2017).
However, it is important to note that the specific components responsible for reducing hepatic TG levels were not identified in the previous studies. Hence, identifying chlorogenic acid and schisandrin as the active compounds in this study’s SCE for reducing hepatic TG levels is a noteworthy contribution. Only the independent effects of chlorogenic acid and schisandrin on plasma and hepatic TG levels have been demonstrated (Cho et al., 2010; Jeong et al., 2019; Sudeep et al., 2016). Schisandrin A (0.5 g/kg diet for 15 weeks) decreased the plasma and hepatic TG levels in mice fed a high-fat and high-cholesterol diet (p < 0.05) (Jeong et al., 2019). The methanol extract of Schisandra chinensis (SC extract) reduced hepatic TG levels in HFD-induced obese mice (p < 0.05). The SC extract contains 1.24 mg, which was orally administered (100 and 300 mg/kg) for 16 weeks. In addition, SC extract (10, 50, and 100 μg/mL) decreased intracellular TG levels in palmitate-treated HepG2 cells (p < 0.05) (Jang et al., 2016). Chlorogenic acid significantly reduced plasma and hepatic TG levels. Oral administration of chlorogenic acid (150 mg/kg body weight) led to a decline in plasma TG levels in high-fat-fed mice (p < 0.05) (Wang et al., 2019). Dietary supplements of chlorogenic acid (0.02 g/kg diet) in obese mice also reduced plasma TG and hepatic TG levels (p < 0.05) (Cho et al., 2010). The plasma and hepatic TG levels were consistent with those obtained in this study.
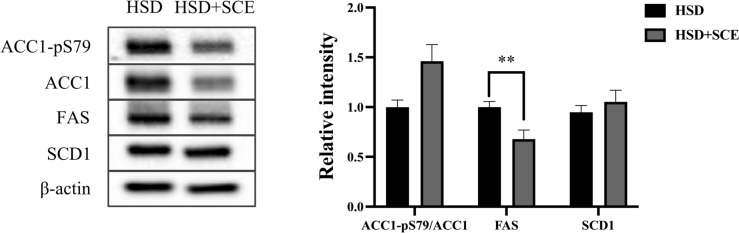
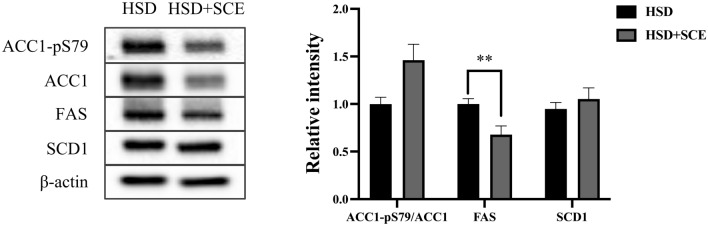
Since SCE reduces hepatic TG levels, it is important to understand the underlying mechanisms involved in hepatic steatosis. One significant pathway that contributes to hepatic steatosis is de novo lipogenesis, which involves the synthesis of lipids from non-lipid sources, particularly carbohydrates, in the liver. To investigate this, we assessed the effect of SCE on the activity of DNL-related enzymes using immunoblotting (Fig. 3). The phosphorylation of ACC1 at Ser79 inhibits lipogenic activity (Lally et al., 2019). We observed a slight increase in the ratio of phosphorylated ACC1 to total ACC1 in the HSD + SCE group compared to the HSD group, although the difference was not statistically significant (p = 0.06). FAS, a key enzyme involved in hepatic lipogenesis responsible for palmitate synthesis, showed significantly lower expression in the HSD + SCE group than in the HSD group (p < 0.05). However, the expression of SCD1, a lipogenic enzyme that generates monounsaturated fatty acids, did not differ between the HSD and HSD + SCE groups. These findings provide valuable insights into the mechanisms by which SCE may benefit hepatic steatosis, particularly by modulating lipogenic enzyme activity.
Fig. 3.
Relative intensity of hepatic lipogenic enzymes in rats fed a high-sucrose diet. Immunoblotting analysis for hepatic lipogenic enzymes acetyl-CoA carboxylase 1 (ACC1), fatty acid synthase (FAS), and stearoyl-Coenzyme A desaturase 1 (SCD1). Data are expressed as the mean ± standard error of the mean. **p < 0.01 compared to HSD and HSD + SCE
This study aimed to investigate the effects of extracts including chlorogenic acid and Schisandrin on plasma TG, hepatic TG, and lipogenic enzymes. Although we observed improvements in these parameters, the effects were relatively weaker than those reported in previous studies. These differences could be attributed to variations in intervention protocols, including differences in treatment duration and dosage regimens. Nevertheless, present findings support the role of chlorogenic acid and Schisandrin as bioactive compounds that prevent lipid accumulation. The combination of these compounds in this trial presents a promising nutraceutical approach for reducing adiposity.
Supplementary Information
Below is the link to the electronic supplementary material.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haneul Lee, Eun Young Kang, and Joowon Lee have contributed equally to this work.
Change history
12/26/2023
A Correction to this paper has been published: 10.1007/s10068-023-01480-1
Contributor Information
Cao Lei, Email: caolei0630@gmail.com.
Gwang-woong Go, Email: gwgo1015@hanyang.ac.kr.
References
- Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. American Journal of Physiology-Endocrinology and Metabolism. 2008;295:1323–1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. New England Journal of Medicine. 2002;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Arca M, Veronesi C, D’Erasno L, Borghi C, Colivicchi F, Ferrari GMD, Desideri G, Pontremoli R, Temporelli PL, Perrone V, Esposti LD. Association of hypertriglyceridemia with all-cause mortality and atherosclerotic cardiovascular events in a low-risk Italian population: the TG-REAL retrospective cohort analysis. Journal of American Heart Association. 2020;9(19):e015801. doi: 10.1161/JAHA.119.015801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, Lee MK. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food and Chemical Toxicology. 2010;48(3):937–943. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Chung MY, Shin EJ, Choi HK, Kim SH, Sung MJ, Park JH, Hwang JT. Schisandra chinensis berry extract protects against steatosis by inhibiting histone acetylation in oleic acid-treated HepG2 cells and in the livers of diet-induced obese mice. Nutrition Research. 2017;46:1–10. doi: 10.1016/j.nutres.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Annals of Internal Medicine. 2016;165(5):305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. New England Journal of Medicine. 2017;377(21):2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah A, Monteiro M, Donangelo CM, Lafay S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. Journal of Nutrition. 2008;138(12):2309–2315. doi: 10.3945/jn.108.095554. [DOI] [PubMed] [Google Scholar]
- Go GW, Srivastava R, Hernandez-Ono A, Gang G, Smith SB, Booth CJ, Ginsberg HN, Mani A. The combined hyperlipidemia caused by impaired Wnt-LRP6 signaling is reversed by Wnt3a rescue. Cell Metabolism. 2014;19(2):209–220. doi: 10.1016/j.cmet.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo LY, Hung TM, Bae KH, Shin EM, Zhou HY, Hong YN, Kang SS, Kim HP, Kim YS. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. European Journal of Pharmacology. 2008;591(1–3):293–299. doi: 10.1016/j.ejphar.2008.06.074. [DOI] [PubMed] [Google Scholar]
- Heeren J, Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Molecular Metabolism. 2021;50:101238. doi: 10.1016/j.molmet.2021.101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Dedousis N, O’Doherty RM. Hepatic steatosis and plasma dyslipidemia induced by a high-sucrose diet are corrected by an acute leptin infusion. Journal of Applied Physiology. 2007;102(6):2260–2265. doi: 10.1152/japplphysiol.01449.2006. [DOI] [PubMed] [Google Scholar]
- Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cellular and Molecular Life Sciences. 2018;75(18):3313–3327. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MK, Nam JS, Kim JH, Yun YR, Han CW, Kim BJ, Jeong HS, Ha KT, Jung MH. Schisandra chinensis extract ameliorates nonalcoholic fatty liver via inhibition of endoplasmic reticulum stress. Journal of Ethnopharmacology. 2016;185:96–104. doi: 10.1016/j.jep.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Jeong EJ, Lee WJ, Kim KY. Effects of Schizandra chinensis Extract on the Growth of Intestinal Bacteria Related with Obesity. Korean Journal of Food Science and Technology. 2009;41(6):673–680. doi: 10.9721/KJFST.2012.44.6.673. [DOI] [Google Scholar]
- Jeong MJ, Kim SR, Jung UJ. Schizandrin A supplementation improves nonalcoholic fatty liver disease in mice fed a high-fat and high-cholesterol diet. Nutrition Research. 2019;64:64–71. doi: 10.1016/j.nutres.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Jeong EW, Park GR, Kim J, Baek Y, Go GW, Lee HG. Whey Proteins-Fortified Milk with Adjusted Casein to Whey Proteins Ratio Improved Muscle Strength and Endurance Exercise Capacity without Lean Mass Accretion in Rats. Foods. 2022;11(4):574. doi: 10.3390/foods11040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa M, Xue CY, Kageyama H, Suzuki E, Ito R, Namba Y, Osaka T, Kimura S, Inoue S. Effects of a high-sucrose diet on body weight, plasma triglycerides, and stress tolerance. Nutrition Reviews. 2003;61:S27–33. doi: 10.1301/nr.2003.may.S27-S33. [DOI] [PubMed] [Google Scholar]
- Kang EY, Kim HK, Jung JY, Kim JH, Woo TK, Choi JI, Kim JH, Ahn C, Lee HG, Go GW. Combined Extract of Leonurus japonicus Houtt, Eclipta prostrata L., and Pueraria lobata Ohwi Improved Hot Flashes and Depression in an Ovariectomized Rat Model of Menopause. Foods. 2021;10(1):180. doi: 10.3390/foods10010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. Journal of Gastroenterology. 2013;48(4):434–441. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SI, Sim KH, Ju SY, Han YS. A Study of Antioxidative and Hypoglycemic Activities of Omija (Schizandra chinensis Baillon) Extract under Variable Extract Conditions. The Korean Journal of Food and Nutrition. 2009;22(1):41–47. [Google Scholar]
- Kim H, Choe JH, Choi JH, et al. Medium-Chain Enriched Diacylglycerol (MCE-DAG) Oil Decreases Body Fat Mass in Mice by Increasing Lipolysis and Thermogenesis in Adipose Tissue. Lipids. 2017;52(8):665–673. doi: 10.1007/s11745-017-4277-7. [DOI] [PubMed] [Google Scholar]
- Kim HK, Jeong J, Kang EY, Go GW. Red Pepper (Capsicum annuum L.) Seed Extract Improves Glycemic Control by Inhibiting Hepatic Gluconeogenesis via Phosphorylation of FOXO1 and AMPK in Obese Diabetic db/db Mice. Nutrients. 2020;12(9):2546. doi: 10.3390/nu12092546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi K, Goto M, Tsuru Y, Hori M. Noninvasive monitoring of muscle atrophy and bone metabolic disorders using dual-energy X-ray absorptiometry in diabetic mice. Experimental Animals. 2023;72(1):68–76. doi: 10.1538/expanim.22-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HJ, Park CS. Biological Activities of Extracts from Omija(Schizandra chinensis Baillon) Korean Journal of Food Preservation. 2008;15(4):587–592. [Google Scholar]
- Lally JSV, Ghoshal S, DePeralta DK, Moaven O, Wei L, Masia R, Erstad DJ, Fujiwara N, Leong V, Houde VP, Anagnostopoulos AE, Wang A, Broadfield LA, Ford RJ, Foster RA, Bates J, Sun H, Wang T, Liu H, Ray AS, Saha AK, Greenwood J, Bhat S, Harriman G, Miao W, Rocnik JL, Westlin WF, Muti P, Tsakiridis T, Harwood HJ, Jr, Kapeller R, Hoshida Y, Tanabe KK, Steinberg GR, Fuchs BC. Inhibition of Acetyl-CoA Carboxylase by Phosphorylation or the Inhibitor ND-654 Suppresses Lipogenesis and Hepatocellular Carcinoma. Cell Metabolism. 2019;29(1):174–182.e5. doi: 10.1016/j.cmet.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, Cortez-Pinto H, Crespo J, Cusi K, Dirac MA, Francque S, George J, Hagström H, Huang TT, Ismail MH, Kautz A, Sarin SK, Loomba R, Miller V, Newsome PN, Ninburg M, Ocama P, Ratziu V, Rinella M, Romero D, Romero-Gómez M, Schattenberg JM, Tsochatzis EA, Valenti L, Wong VW, Yilmaz Y, Younossi ZM, Zelber-Sagi S. NAFLD Consensus Consortium. Advancing the global public health agenda for NAFLD: a consensus statement. Nature Reviews Gastroenterology & Hepatology. 2022;19(1):60–78. doi: 10.1038/s41575-021-00523-4. [DOI] [PubMed] [Google Scholar]
- Lazarus JV, Mark HE, Villota-Rivas M, Palayew A, Carrieri P, Colombo M, Ekstedt M, Esmat G, George J, Marchesini G, Novak K, Ocama P, Ratziu V, Razavi H, Romero-Gómez M, Silva M, Spearman CW, Tacke F, Tsochatzis EA, Yilmaz Y, Younossi ZM, Wong VW, Zelber-Sagi S, Cortez-Pinto H, Anstee QM. NAFLD policy review collaborators. The global NAFLD policy review and preparedness index: Are countries ready to address this silent public health challenge. Journal of Hepatology. 2022;76(4):771–780. doi: 10.1016/j.jhep.2021.10.025. [DOI] [PubMed] [Google Scholar]
- Lee BW, Lee YH, Park CY, Rhee EJ, Lee WY, Kim NH, Choi KM, Park KG, Choi YK, Cha BS, Lee DH. Non-Alcoholic fatty liver disease in patients with type 2 diabetes mellitus: a position statement of the fatty liver research group of the korean diabetes association. Diabetes and Metabolism Journal. 2020;44(3):382–401. doi: 10.4093/dmj.2020.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Su C, Chen X, Wang Q, Jiao W, Luo H, Tang J, Wang W, Li S, Guo S. Chlorogenic Acids in Cardiovascular Disease: A Review of Dietary Consumption, Pharmacology, and Pharmacokinetics. Journal of Agricultural and Food Chemistry. 2020;68(24):6464–6484. doi: 10.1021/acs.jafc.0c01554. [DOI] [PubMed] [Google Scholar]
- Miller M, Stone NJ, Ballantyne C, Bittner C, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- Minehira K, Young SG, Villanueva CJ, Yetukuri L, Oresic M, Hellerstein MK, Farese RV, Jr, Horton JD, Preitner F, Thorens B, Tappy L. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. Journal of Lipid Research. 2008;49(9):2038–2044. doi: 10.1194/jlr.M800248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir BA, Majeed T, Chauhan A. Nonalcoholic Fatty Liver Disease. New England Journal of Medicine. 2022;386(3):295. doi: 10.1056/NEJMc2118255. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. American Journal of Physiology-Endocrinology and Metabolism. 2008;294:15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- Naguib G, Morris N, Yang S, Fryzek N, Haynes-Williams V, Huang WA, Norman-Wheeler J, Rotman Y. Dietary fatty acid oxidation is decreased in non-alcoholic fatty liver disease: A palmitate breath test study. Liver International. 2020;40(3):590–597. doi: 10.1111/liv.14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nature Medicine. Cutting out the liver fat. 23(12):1385 (2017) [DOI] [PubMed]
- Nazarian A, Cory E, Müller R, Snyder BD. Shortcomings of DXA to assess changes in bone tissue density and microstructure induced by metabolic bone diseases in rat models. Osteoporos International. 2009;20(1):123–132. doi: 10.1007/s00198-008-0632-0. [DOI] [PubMed] [Google Scholar]
- Ong KW, Hsu A, Tan BKH. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE. 2012;7(3):e32718. doi: 10.1371/journal.pone.0032718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Yoon J. Schizandrin inhibits fibrosis and epithelial-mesenchymal transition in transforming growth factor-β1-stimulated AML12 cells. International Immunopharmacology. 2015;25(2):276–284. doi: 10.1016/j.intimp.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Park HJ, Cho JY, Kim MK, Koh PO, Cho KW, Kim CH, Lee KS, Chung BY, Kim GS, Cho JH. Anti-obesity effect of Schisandra chinensis in 3T3-L1 cells and high fat diet-induced obese rats. Food Chemistry. 2012;134(1):227–234. doi: 10.1016/j.foodchem.2012.02.101. [DOI] [Google Scholar]
- Park SY, Hwang HY, Seo EA, Kwon KB, Ryu DG. Inhibition effects of Galla chinenisis extract on adipocyte differentiation in OP9 cells. Journal of Physiology & Pathology in Korean Medicine. 2012;26(4):455–461. [Google Scholar]
- Pathak MP, Pathak K, Saikia R, Gogoi U, Patowary P, Chattopadhyay P, Das A. Therapeutic potential of bioactive phytoconstituents found in fruits in the treatment of non-alcoholic fatty liver disease: A comprehensive review. Heliyon. 2023;9(4):e15347. doi: 10.1016/j.heliyon.2023.e15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Roden M. Diabetes mellitus – definition, klassifikation und diagnose. Wien Klin Wochenschr. 2016;128:S37–40. doi: 10.1007/s00508-015-0931-3. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Nonalcoholic Fatty Liver Disease, Insulin Resistance, and Ceramides. New England Journal of Medicine. 2019;381(19):1866–1869. doi: 10.1056/NEJMcibr1910023. [DOI] [PubMed] [Google Scholar]
- Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, Halavaara J, Yki-Järvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. Journal of Clinical Endocrinology and Metabolism. 2002;87(7):3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- Song YO, Lee SJ, Park HJ, Jang SH, Chung BY, Song YM, Kim GS, Cho JH. Hepatoprotective effect of Schisandra chinensis on high-fat diet-induced fatty liver in rats. Korean Journal of Veterinary Service. 2013;36(1):45–52. doi: 10.7853/kjvs.2013.36.1.45. [DOI] [Google Scholar]
- Sudeep HV, Venkatakrishna K, Patel D, Shyamprasad K. Biomechanism of chlorogenic acid complex mediated plasma free fatty acid metabolism in rat liver. BMC Complementary and Alternative Medicine. 2016;16(1):274. doi: 10.1186/s12906-016-1258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JH, Liu X, Cong LX, Li H, Zhang CY, Chen JG, Wang CM. Metabolomics study of the therapeutic mechanism of Schisandra Chinensis lignans in diet-induced hyperlipidemia mice. Lipids in Health and Disease. 2017;16(1):145. doi: 10.1186/s12944-017-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. New England Journal of Medicine. 2010;363(14):1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- Vilar-Gomez E, Yasells-Garcia A, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Villa-Jimenez O, Friedman SL, Diago M, Romero-Gomez M. Development and validation of a noninvasive prediction model for nonalcoholic steatohepatitis resolution after lifestyle intervention. Hepatology. 2016;63(6):1875–1887. doi: 10.1002/hep.28484. [DOI] [PubMed] [Google Scholar]
- Wang S, Song K, Srivastava R, Dong C, Go GW, Li N, Iwakiri Y, Mani A. Nonalcoholic fatty liver disease induced by noncanonical Wnt and its rescue by Wnt3a. The FASEB Journal. 2015;29(8):3436–3445. doi: 10.1096/fj.15-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Lam KL, Hu J, Ge S, Zhou A, Zheng B, Zeng S, Lin S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Science and Nutrition. 2019;7(2):579–588. doi: 10.1002/fsn3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, Zhang PH, Yan HH. Functional foods and dietary supplements in the management of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Frontiers in Nutrition. 2023;10:1014010. doi: 10.3389/fnut.2023.1014010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SK, Chin KY, Suhaimi FH, Fairus A, Ima-Nirwana S. Animal models of metabolic syndrome: a review. Nutrition & Metabolism. 2016;13:65. doi: 10.1186/s12986-016-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You YL, Lee JY, Choi HS. Schisandra chinensis-derived gomisin C suppreses lipid accumulation by JAK2-STAT signaling in adipocyte. Food Science and Biotechnology. 2023;32:1225–1233. doi: 10.1007/s10068-023-01263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335–1347. doi: 10.1097/HEP.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.