Abstract
In recent years, the application of chimeric antigen receptor T-cell (CAR-T) therapy based on gamma delta T (γδT) cells in hepatocellular carcinoma (HCC) immunotherapy has attracted more and more attention. However, specific antigens recognized by γδT cells are rarely identified, which has become the main restriction on such therapeutic application of γδT cells. In this report, we identified a new peptide and protein antigen recognized by γδT cells in HCC using our previous established strategy. First, we investigated the diversity of the γ9/δ2 T-cell immunorepertoire by sequence analyses of the expressed complementarity-determining region 3 (CDR3) in HCC patients. Then, we constructed γ9/δ2 T-cell receptor (TCR)-transfected cell lines expressing significant HCC CDR3 sequence and identified a series of peptides capable of binding to γδT cells specifically. Next, we identified, further tested and verified the biological functions of these peptides and their matched protein by bioinformatics analysis. We identified that the new protein hepatocyte growth factor-like protein, also called as macrophage-stimulating protein (MSP), and peptide HP1, not only bound to HCC-predominant γδTCR but also effectively activated γδT cells isolated from HCC patients. Moreover, they could stimulate γδT cells in peripheral blood from HCC patients to produce cytokines, which contributed to inhibiting HCC and played an important role in mediating cytotoxicity to HCC cell lines. In conclusion, we identified MSP and HP1, which showed potential as candidates for antigens recognized by γδT cells in HCC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-020-02826-y.
Keywords: Hepatocellular carcinoma, Antigen, Γδ T cells, Macrophage stimulating protein
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies which is characterized by poor prognosis [1]. Although significant advances in treatments have increased survival time, finely targeted therapy is not yet available. In recent years, adoptive immunotherapy in which lymphocytes are genetically engineered to use chimeric antigen receptor T (CAR-T) cells has demonstrated promise in treating malignant diseases [2, 3]. The key to CAR-T technology is targeting tumor-specific antigens (TSAs). But, so far, only a few TSAs of HCC have been identified. Glypican-3 (GPC3), an oncofetal antigen overexpressed in HCC, is an effective target for cytotoxic T lymphocyte (CTL)-mediated tumor immunotherapy. However, this tumor response is rare, and most HCC patients are unlikely to benefit from the vaccination strategy targeting a single CTL epitope [4]. Accumulating evidence shows that tumor progression is regulated by cross-talk between tumor cells and the host immune microenvironment, including both innate and adaptive immunity [5]. This indicates that a type of HCC therapy that coordinates the interplay between innate and adaptive immune systems is required. The liver is commonly considered an immune organ with predominantly innate immunity [6]. Up to 35% of total liver T cells in humans carry gamma delta T-cell receptors (γδTCRs) [7]. Because γδT cells have killing effects similar to those of CTLs and are not restricted by major histocompatibility complex (MHC), they provide a strong defense against tumor formation and progression in the liver [8]. The best-known antigens for γδT cells are phosphoantigens, with research progress in γδT cells, some protein antigens recognized by γδT cells are being identified continually. The most abundantly circulating γδT cells, Vγ9Vδ2T cells, can be activated by small non-peptide phosphorylated antigens, various members of the UL16-binding protein (ULBP) and MHC class I-related molecules A/B (MICA/B) in an MHC-independent manner [9–11]. However, no liver-specific antigen or epitope of activated γδT cells has been reported.
A strategy using intact cells as probes to pan target peptides in a phage display library (PDL) was developed [12, 13]. Using HepG2 cells as a probe, the researcher obtained a target peptide, HCBP1, that might be a potential candidate for drug therapy targeted to HCC [14]. In our previous study, we established a novel strategy to screen bacillus Calmette-Guerin (BCG)-specific, γδTCR-recognized peptide and protein antigens by panning a 12-mer random-peptide using transfected cell lines as probes [15]. The strategy provides a novel means of screening mycobacterial- adjuvants or vaccine candidates. Using this strategy, we found a significant complementary determinant region 3 (CDR3) sequence in pulmonary tuberculosis (TB) patients [16], and we also identified new TB protein antigens (1-deoxy-D-xylulose 5-phosphate synthase 2, DXS2) and a peptide (EP) recognized by γδTCRs in pulmonary TB patients [17].
In this report, we again applied this strategy to identify HCC antigens recognized by γδT cells. We first investigated the diversity of the γ9/δ2 T-cell immunorepertoire by sequence analyses of the expressed CDR3 in HCC patients. Then, we constructed γ9/δ2TCR-transfected cell lines expressing the predominant HCC CDR3 sequence and identified a series of peptides capable of binding specifically to γδT cells. Next, we further tested and verified the biological functions of these peptides and their matched proteins as identified by bioinformatics analysis. We identified a new protein hepatocyte growth factor-like protein, also called as macrophage-stimulating protein (MSP), and peptide HP1, which not only bound to HCC-predominant γδTCRs but also effectively activated γδT cells isolated from HCC patients. Moreover, they could stimulate γδT cells from HCC patients to produce cytokines, which helped inhibit HCC and played an important role in mediating cytotoxicity to HCC cell lines.
Materials and methods
Subjects
Forty patients were enrolled in this study, all of them pathologically diagnosed with HCC at Renmin Hospital, Shiyan City, China in 2018–2020. To control potential confounding factors, patients (mean age, 49.7; 28 men, 12 women) who received chemotherapy or radiotherapy treatment were excluded. Twenty healthy volunteer subjects (mean age, 41; 12 men, 8 women) were recruited as a control group. Healthy volunteers did not have any changes in X-rays, hepatitis B virus (HBV) history or other underlying diseases. Exclusion criteria for the control groups were medication, pregnancy and any abnormalities in liver and renal function tests. This work received approval from the Clinical Ethics Committee of Hubei University of Medicine (2019-TH-024). All individuals gave their informed consent to participate. Basic demographic information on patients and controls is listed in Table 1.
Table 1.
Characteristics of the hepatocellular carcinoma patients and healthy controls
| Mean age Years (range) |
Male | Other complicationb | HBV positive | Treatment time > 1 year | Treatment program | |
|---|---|---|---|---|---|---|
| Patients (n = 40) | 49.7 (31–75) | 28 (70%a) | 12 (40%) | 22 (55%) | 26 (52%) | TACEc |
| Control (n = 20) | 41 (28–55) | 12 (60%) | 0 (0%) | 0 (0%) |
aThe percentage of patients with the characteristic
bOther Complication include liver cirrhosis
cThe treatment programs: TACE: Transarterial chemoembolization
Reagents and cell lines
We used a Ph.D.-12 phage display peptide library kit (New England Biolabs, US) to screen specific peptides binding to γδTCR. We obtained J.RT3-T3.5 cells from the American Type Culture Collection (ATCC, US). Peripheral blood mononuclear cells (PBMCs) were separated from peripheral blood via density gradient centrifugation with Ficoll-Hypaque (Sigma, 17144002). We sorted the γδT cells using a magnetic-activated cell sorting (MACS) system (Miltenyi, 130-050-701). PBMCs were first magnetically labeled with Biotin-Antibody Cocktail and Anti-Biotin Microbeads; then, we loaded the cell suspension onto a MACSR column, which we placed in the magnetic field of a MACS Cell Separator while retaining the magnetically labeled γδT cells within the column. Unlabeled cells were run through. After removing the column from the magnetic field, we were able to elute the magnetically retained γδT cells as the positively selected cell fraction. γδT cells was determined by flow cytometry (FCM) using FITC-αβTCRs (Biolegend, 306706) and PE-γδTCRs (Biolegend, 331209). The purity of the γδT cells was more than 90%. We stimulated the isolated γδT cells using a commercial leukocyte activation cocktail (10 μg/mL, Becton Dickinson, 550583) in the presence of RPMI 1640 medium supplemented with 12% fetal bovine serum (FBS), interleukin-2 (IL-2), penicillin, streptomycin and 5 × 10−5 M β-mercaptoethanol at 37 °C in a humidified incubator with 95% air and 5% CO2. Peptides were synthesized by Sangon Biotech Inc., Shanghai, China; the purity of these peptides was > 95% in high-performance liquid chromatography (HPLC) analysis. We labeled half the synthesized peptides with fluorescein isothiocyanate (FITC; Sangon) at their N-terminals, so the binding ability of peptide with cells can be detected by flow cytometry directly. The HepG2 cells were collected and were subjected to 5 h of sonication at room temperature in a water bath sonicator. The extracts were then spun at 13,000 g for 5 min and the supernatants were collected. Commercial MSP protein was purchased from Abnova (H00004485-P01).
Construction of transfected cells expressing γδTCRs with predominant HCC CDR3 sequence and healthy control
Using overlapping polymerase chain reactions (PCRs), we inserted the predominant HCC CDR3 sequence into the full-length γ9 and δ2 chains to substitute for the original CDR3 sequence. The detailed method of construction of expressing γδTCRs were constructed as described in our previous paper [15]. Next, we evaluated the resulting cells expressing surface γδTCRs via FCM, and we isolated positive cells via flow sorting for further experiments. Thus, we developed two artificial cell lines expressing γδTCRs, with predominant HCC CDR3 sequences and healthy-control CDR3 sequences.
Flow cytometry
To bind HP1, HP2 and HP3 to cells, we incubated the transfected cells or γδT cells with FITC-conjugated peptide and control peptide at 4 °C for 30 min. Then, we measured the percentage of γδT cells via fluorescence-activated cell sorting (FACS) using FITC-αβTCRs and PE-γδTCRs. FITC-conjugated anti-human γ9 and PE-conjugated anti-human δ2 monoclonal antibody (Biolegend) were used to detect the nature and purity of γδT induced by peptide and protein antigen. The specific binding of peptide and protein to γδ T cells were further proved by blocking with functional γδ TCR-blocking monoclonal antibody (10 ng/μL B1.1; eBioscience, 16–9959-81). FCM was performed on a MoFlo XDP flow cytometer (Beckman Coulter, US).
Cytotoxicity assay
We used the CytoTox 96 Non-radioactive Cytotoxicity Assay (Promega, G1780) to determine cytotoxic effects of γδT cells on the HCC cell line (HepG2), which is based on the colorimetric detection of the released lactate dehydrogenase enzyme. HepG2 cells were used as target cells and seeded into 96-well plates at 1 × 104 cells per well. Next, we added the γδT cells, which we used as effector cells, to each well at effector cell: target cell ratios of 1:1, 2.5:1, and 5:1. After the effector and target cells were incubated at 37 °C for 6 h, the culture supernatants were collected to detect the lactate dehydrogenase activity according to the manufacturer’s instructions.
Real-time polymerase chain reaction (RT-PCR)
We pre-coated HP1 peptide (20 μg) or HepG2 whole protein (20 μg) onto microtiter plates (Corning) and then added J.RT-T3.5 cells, HCC-specific γ9/δ2TCR-transfected cells (named as test cells) and healthy control-transfected cells (named as control cells) for 24 h. Next, we extracted total ribonucleic acid (RNA) from cells and, using Moloney murine leukemia virus reverse transcriptase (MMLV-RT), transcribed it into complementary deoxyribonucleic acid (cDNA). Using the cDNA as a template, we amplified IL-2 via RT-PCR with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for reference. Gene expression analysis was performed using Bio-Rad CFX Manager software version 3.1 (Bio-Rad Laboratories, US).
Enzyme-linked immunosorbent assay (ELISA)
Using an ELISA kit (R&D Systems, US) per manufacturer’s instructions, we determined that the γδT cells secreted T helper type 1 (Th1), Th2 and Th17 cytokines upon activation by the candidate peptide and protein. We also used ELISA kit to detect the IL-2 production level of J.RT-T3.5 cells, control cells and test cells stimulated by HepG2 whole protein.
Bioinformatics analysis
The analyzed the best matches of the germline V and J gene identified by determining alignments between the sequence results and germline sequences in the IMGT database (http://www.imgt.org). We performed homologous analysis and sequence alignment using the Basic Local Alignment Search Tool (BLAST; National Center for Biotechnology Information http://www.ncbi.nlm.nih.gov/blast) to determine matched proteins of HP1, HP2 and HP3. The first 3 matched proteins were listed. We calculated a survival analysis curve for MSP protein in HCC at the OncoLnc website (http://www.oncolnc.org/), which link TCGA survival data to mRNA, miRNA, or lncRNA expression levels. In the oncolnc website, MSP was entered to get the Cox regression results. HCC is selected, lower percentile was set on 10 and higher percentile was set on 90, the Kaplan plot for MSP in HCC was determined. We analyzed the peptides at the Heliquest website (http://heliquest.ipmc.cnrs.fr). SWISS-MODEL (http://swissmodel.expasy.org/) was performed to predict the structure of MSP using homology modeling techniques.
Statistical analysis
All data were analyzed using SPSS version 19.0 and GraphPad 5.0 software. Independent sample t tests and paired sample t tests were used to generate and statistically analyze on the data. P values < 0.05 were considered statistically significant.
Results
Sequence characteristic of γ9 and δ2 CDR3 of HCC patients
The diversity of the TCRγδ CDR3 repertoire, which reflects the proliferation of specific γδ T-cell clones, is one of the most important features of HCC patients. The CDR3 regions contained conserved “CA” at the N-terminus and conserved “FGXG” at the C-terminus. In contrast, the inner regions of CDR3 were composed of variable sequences. As shown in Tables S1 and S2, predominant γ9 and δ2 CDR3 sequences in HCC were listed. Here, a common δ2 CDR3 sequence was found in PBMC of HCC patient’s groups. Our sequence analysis also revealed that most Vδ2 T cell isolated from HCC patients and healthy controls also carried a hydrophobic amino acid residue (/leucine/ valine/ argine) at conserved position 97 (Table S2).
Construction of transfected cells expressing HCC-specific and non-specific γδTCRs
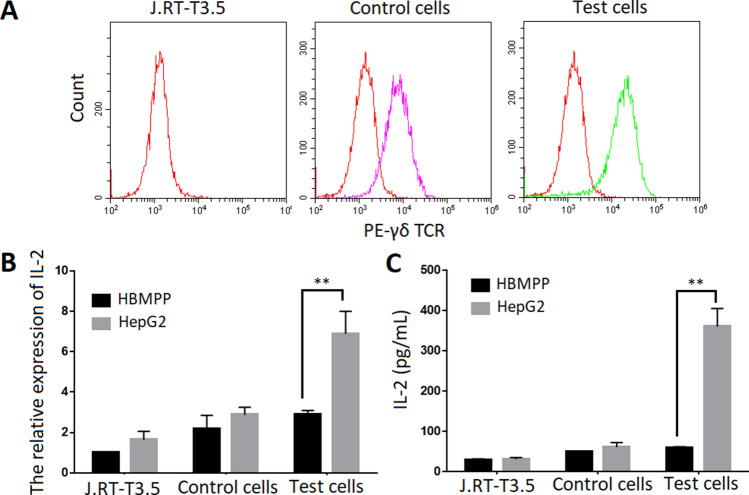
Based on predominant γ9 and δ2 CDR3 sequences in HCC listed and highlighted in Tables S1 and S2, we developed two artificial cell lines expressing HCC-specific γ9/δ2TCR-transfected cells (test cells) and healthy control-transfected cells (control cells). The full-length γ9 and δ2 chains were cloned into pREP7 and pREP9 expression vectors and co-transfected into J.RT3-T3.5 cells by electroporation. After 4 weeks of selection using G418 and hygromycin, we observed that 30% of the transfected cells expressed γδTCRs. The positive cells were further enriched by MACS and exceeded 90% (Fig. 1a). Thus, the artificial γδTCRs transfected cells were constructed successfully. They will secrete IL-2 upon stimulated by the corresponding antigens. To further check the HCC specificity of the transfected γδTCRs, we stimulated both test and control cells with protein extracts of HepG2 cells and then measured the level of secreted IL-2 by RT-PCR and ELISA. Soluble phosphoantigens, HMBPP (1 ng/mL) and protein extracts of HepG2 cells were pre-coated with 96-well plate. J.RT3-T3.5 cells, control cells and test cells were added and cultured for 24 h. As shown in Fig. 1b, c, the protein extract of HepG2 cells stimulated the test cells to produce much more IL-2 than was the case for control cells, HMBPP could not stimulate the test cells and control cells to produce IL-2, suggesting that J.RT3-T3.5-transfected cells expressing HCC-specific γδTCRs were successfully generated.
Fig. 1.
Construction of transfected cells expressing HCC-specific and non-specific γδTCRs. a Assessment of γδTCRs expressed in J.RT3-T3.5-transfected cells via flow cytometer analysis. Based on predominant γ9 and δ2 CDR3 sequences in HCC listed and highlighted in Tables S1 and S2 we developed two artificial cell lines expressing HCC-specific γ9/δ2TCR-transfected cells (test cells) and healthy control-transfected cells (control cells). The full-length γ9 and δ2 chains were cloned into pREP7 and pREP9 expression vectors and co-transfected into J.RT3-T3.5 cells by electroporation. After 4 weeks of selection using G418 and hygromycin, Cells were stained with PE-γδTCR monoclonal antibody. The positive cells were further enriched by MACS and exceeded 90%. b The protein extract of HepG2 cells stimulated the test cells to produce much more IL-2 than was the case for control cells by real-time PCR. Soluble phosphoantigens, HMBPP (1 ng/mL) and protein extracts of HepG2 cells were pre-coated with 96-well plate. J.RT3-T3.5 cells, control cells and test cells were added and cultured for 24 h. IL-2 secretion of control and test cells was measured by RT-PCR. c The protein extract of HepG2 cells stimulated the test cells to produce much more IL-2 than was the case for control cells by ELISA. Soluble phosphoantigens, HMBPP (1 ng/mL) and protein extracts of HepG2 cells were pre-coated with 96-well plate. J.RT3-T3.5 cells, control cells and test cells were added and cultured for 24 h. The IL-2 production in the supernatant of cell culture medium was measured by ELISA. **Denote P < 0.01. Data are shown as the mean of three independent experiments
Selection of three candidate peptides from a PDL using artificial γδT cells
To identify epitopes recognized by HCC-specific transfected cells, we performed γδTCR-mediated biopanning of a 12-mer random-peptide PDL. The screening method was based on our previous paper [15]. The peptides by screening are listed in Table 2. We selected 3 prominent peptides as γδTCR-recognized peptide candidates based on their high frequency of appearance, designating them as HCC-specific peptide (HP). Next, HP1, HP2 and HP3 were chemically synthesized for further analysis.
Table 2.
The amino acid sequences of 12 peptides and multiple sequence alignment
| Name | Sequence | Frequency |
|---|---|---|
| HP1 | TSRHQSWSPQDL | 13/40 |
| HP2 | YQLLTPSETFSY | 9/40 |
| HP3 | TPLYKELDLPLQ | 8/40 |
| HP4 | ATEVAKSESCLH | 2/40 |
| HP5 | YKILPHGQWRRS | 2/40 |
| HP6 | TDAHPESDVDRD | 2/40 |
| HP7 | KHPVYPFDPSRP | 2/40 |
| HP8 | AQTPVSYSPTTF | 2/40 |
BLAST analysis
We performed a protein BLAST search to identify related proteins containing the sequences for HP1, HP2 and HP3. The results are listed in Table 3 according to the E value score. Among theses, the most matched protein from Homo sapiens with peptide HP1 is Hepatocyte growth factor-like protein.
Table 3.
Blast analysis of candidate peptide (HP1, HP2 and HP3)
| No | Reference | Protein | Species | E value | Matching part |
|---|---|---|---|---|---|
| HP1 | XP_011532034.1 | Hepatocyte growth factor-like protein isoform X1 | Homo sapiens | 34.1 | -TSRHQSW-PQ- |
| HP1 | MCB09754.1 | Immunoglobulin heavy chain junction region | Homo sapiens | 25.7 | -RHQ-WSP-D- |
| HP1 | NP_001315537.1 | Pentraxin-4 isoform 1 precursor | Homo sapiens | 25.2 | -R-Q-WSPQ- |
| HP2 | AHA56119.1 | Immunoglobulin heavy chain variable region | Homo sapiens | 25.7 | -LLTPSET-S- |
| HP2 | NP_001093634.1 | Phosphatase and actin regulator 2 isoform 1 | Homo sapiens | 24.8 | -QLLTP-SE-FS- |
| HP2 | AAF61928.1 | Protocadherin fat 2 | Homo sapiens | 23.1 | Y-LL-P-E-FS |
| HP3 | XP_024309834.1 | farnesyl pyrophosphate synthase isoform X1 | Homo sapiens | 26.1 | -LY-ELDLP-LQ- |
| HP3 | 2RAH_A | Chain A, human Fdps synthase in complex with novel inhibitor | Homo sapiens | 26.1 | -LY-ELDLP-LQ- |
| HP3 | 3CP6_A | Chain A, farnesyl pyrophosphate synthetase | Homo sapiens | 26.1 | -LY-ELDLP-LQ- |
Confirmation of the peptide’s capability to bind to HCC-specific transfected cells
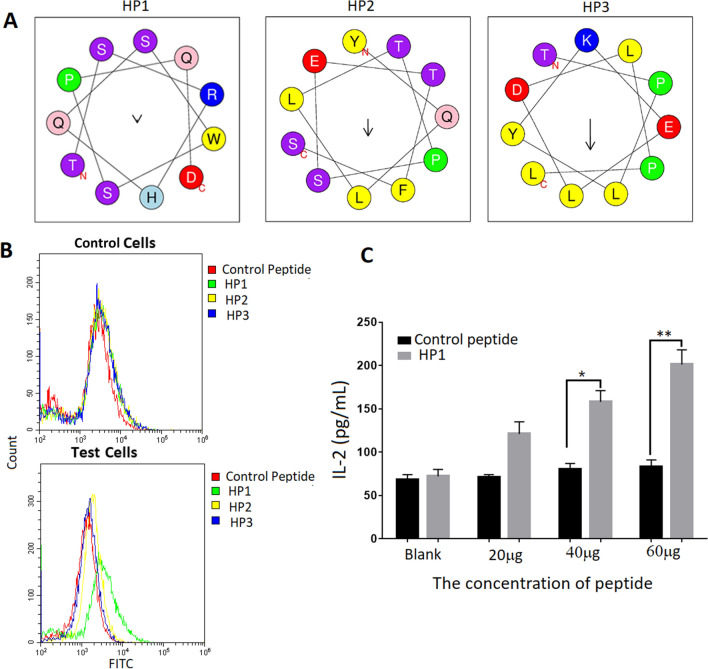
Using the Heliquest website, we predicted the spiral structure of HP1, HP2 and HP3; this structure is presented in Fig. 2a. We performed binding assays to determine whether the synthesized peptides could specifically bind to test cells. After we co-cultured the peptides (10 μg) with such cells, FACS results showed that HP1 was better able to bind to test cells than that of control cells (Green line represents HP1; Fig. 2b). Next, we confirmed that HP1 could also stimulate test cells to secrete IL-2 in a dose-dependent manner (Fig. 2c), while control cells have no this ability. Collectively, these data suggested that HP1 could specifically bind to HCC-transfected cells.
Fig. 2.
Confirmation of the peptide’s capability to bind to HCC-specific transfected cells. a The spiral structure of three peptides were predicted by the Heliquest website http://heliquest.ipmc.cnrs.fr/?tdsourcetag=s_pcqq_aiomsg. Enter the website, click the button of analysis, enter the sequence of HP1, HP2 and HP3 in the blank. Helix type is 3–10, and click the button of process. The predicted spiral structure of the three peptides was presented. b HP1 was able to bind to test cells by FACS analysis. Three peptide and control peptide conjugated with FITC (10 μg) were co-cultured with test cells and control cells, respectively. The results showed that HP1 was better able to bind to test cells than that of the control cells (Green line represents HP1). Results are representative of three independent experiments. c HP1 could also stimulate test cells to secrete IL-2 in a dose-dependent manner. Different dosages of HP1 and control peptide were co-cultured with test cells and control cells for 24 h. Production of IL-2 in the supernatant of cell culture medium was detected by ELISA. Data are shown as the mean of three independent experiments. *Denote P < 0.05. **Denote P < 0.01
HP1 could bind to and activate γδT cells from HCC patients in vitro
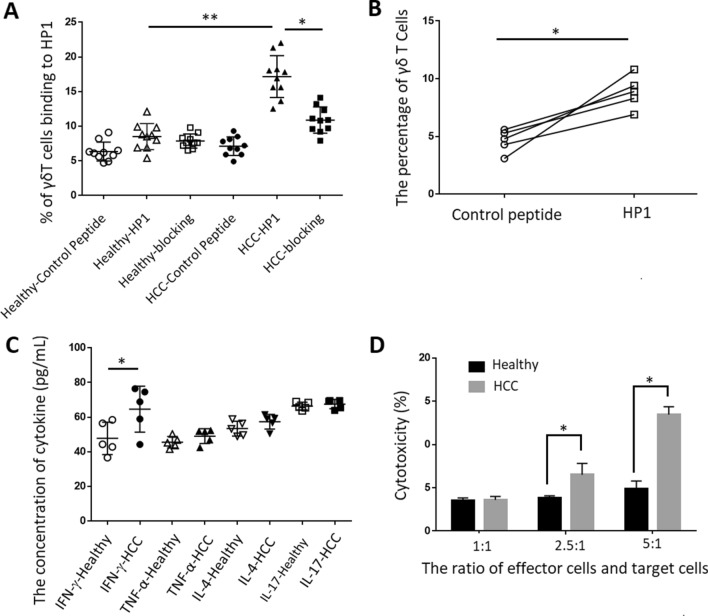
To investigate whether natural γδT cells could recognize and become activated by the HP1 peptide, we detected HP1′s ability to bind to γδT cells isolated from HCC patients and healthy controls, as well as its ability to induce natural γδT cells proliferation. After γδT cells from 10 HCC patients and 10 healthy subjects were sorted by MACS, control peptide and HP1 peptide were co-cultured with these γδT cells under the condition with or without functional blocking antibody for 30 min. FACS results showed that γδT cells from HCC patients with HP1 had greater binding ability than such γδT cells from healthy controls (P < 0.01; Fig. 3a). Meanwhile, the binding of HP1 with γδT cells from HCC patients could be blocked by functional blocking antibody. It means that HP1 could specifically bind to γδT cells from HCC patients in vitro. Moreover, HP1 was shown to be abler than the control peptide to induce proliferation of γδT cells in PBMCs from the same HCC patients (P < 0.05; Fig. 3b). 74.16 percent of these γδT cells belong to γ9/δ2T cells (Figure. S1A). Furthermore, to check the immune function of HP1-activated γδT cells, we detected cytokine production and cytotoxicity effect. The results showed that production of HP1-induced interferon gamma (IFN-γ) in γδT cells from HCC patients was higher than in γδT cells from healthy controls (P < 0.05; Fig. 3c). Levels of other cytokines such as tumor necrosis factor-alpha (TNF-α), IL-4 and IL-17 did not differ between γδT cells from HCC patients and those from healthy controls. In addition, we found that HP1-activated γδT cells could kill HepG2 cells effectively (P < 0.01; Fig. 3d).
Fig. 3.
HP1 could bind to and activate γδT cells from HCC patients in vitro. a HP1 bound to more γδT cells from HCC patients than γδT cells from healthy controls. γδT cells in PBMCs from 10 HCC patients and 10 healthy controls were obtained by MACS. HP1 and control peptide conjugated with FITC were co-cultured with cells under the condition with or without functional blocking antibody (10 ng/μL) for 30 min at 4 °C. The binding of HP1 and control peptide to γδT cells was measured by FACS. b Immobilized HP1 could induce proliferation of γδT cells from HCC patients. Immobilized HP1 and control peptide were pre-coated onto microtiter plates, on which γδT cells from 5 HCC patients were incubated for 2 weeks. The percentage of γδT cells was measured by FACS. c HP1-activated T cells could secrete Th1-based cytokines. Immobilized HP1 was pre-coated on microtiter plates, on which γδT cells from 5 HCC patients and 5 healthy controls were added. Supernatants were collected after 48 h and measured for IFN-γ, TNF-α, IL-4 and IL-17 secretion by ELISA. d HP1-induced γδT cells from HCC patients have the cytotoxicity of to HepG2 by CytoTox 96 non-radioactive cytotoxicity assay. After γδT cells from HCC patients inoculated by HP1 were sorted and cultured, cytotoxic activities of γδT cells against HepG2 in vitro was evaluated. Data are shown as the mean of three independent experiments. *Denote P < 0.05. **Denote P < 0.01
Effector function of MSP-induced γδT cells from HCC patients
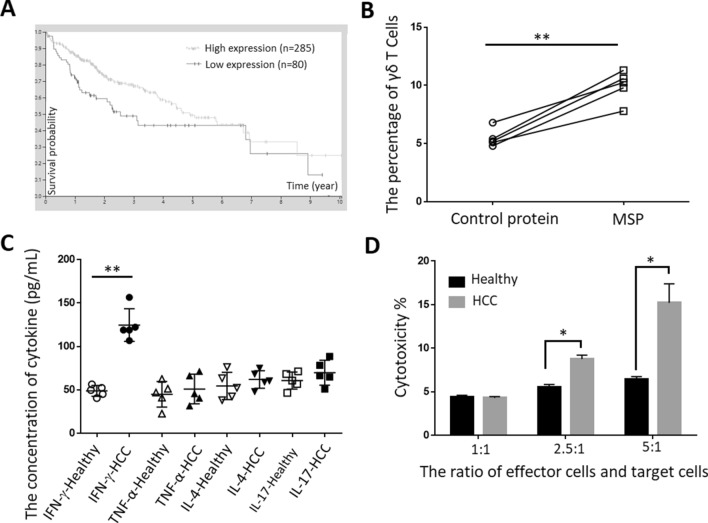
Using the OncoLnc website to demonstrate the relationship between MSP and survival probability for HCC patients, we found that patients with higher MSP expression had longer survival probabilities (Fig. 4a). Then, to investigate whether MSP could activate natural γδT cells, we detected the percentage of γδT cells induced by MSP. The results showed that MSP was better able to induce proliferation of γδT cells in PBMCs from the same HCC patients than in PBMCs from control protein (P < 0.01; Fig. 4b). 42.35 percent of these γδT cells belong to γ9/δ2T cells (Figure. S1B). To check the immune function of MSP-activated γδT cells, we detected cytokine production and cytotoxicity effect, and we found that production of IFN-γ (P < 0.01) in MSP-induced γδT cells from HCC patients was higher than in such cells from healthy controls (Fig. 4c). We found that functional blocking antibody for γδTCR caused a reduction in IFN-γ production in MSP-stimulated γδT cells (Figure S2A). It was suggested that the activation of γδ T cells by MSP is depend on γδ TCR. Furthermore, we observed that MSP protein-activated γδT cells could kill HepG2 cell lines (P < 0.05; Fig. 4d). Collectively, these results suggested that γδT cells activated by MSP might act against HCC by secreting cytokines and/or by exerting an CTL-like effect.
Fig. 4.
Effector function of MSP-induced γδT cells from HCC patients. a The relationship of MSP with the survival probability of HCC patients, as shown by the OncoLnc website. In the oncolnc website, MSP was entered to get the Cox regression results. HCC is selected, lower percentile was set on 10 and higher percentile was set on 90, the Kaplan plot for MSP in HCC was determined. b Immobilized MSP could induce proliferation of γδT cells from HCC patients. Immobilized MSP and control protein were pre-coated onto microtiter plates, on which γδT cells from 5 HCC patients were incubated for 2 weeks. The percentage of γδT cells was measured by FACS. c MSP-activated T cells could secrete Th1-based cytokines. Immobilized MSP was pre-coated on microtiter plates, on which γδT cells from 5 HCC patients and 5 healthy controls were added. Supernatants were collected after 48 h and measured for IFN-γ, TNF-α, IL-4 and IL-17 secretion. d MSP-induced γδT cells from HCC patients have the cytotoxicity of to HepG2 by CytoTox 96 Non-radioactive Cytotoxicity Assay. After γδT cells from HCC patients inoculated by MSP were sorted and cultured, cytotoxic activities of γδT cells against HepG2 in vitro was evaluated. Data are shown as the mean of three independent experiments. *Denote P < 0.05. **Denote P < 0.01
Discussion
Selection of target antigens is a key determinant of the specificity and effectiveness of the CAR-T immunotherapy method [18]. In recent years, the application of CAR-T therapy based on γδT cells in tumor immunotherapy has attracted more and more attention. However, tumor antigens recognized by γδT cells are rarely identified, which has become the main restriction on this application of γδT cells. In this study, we used previously established methods to identify one candidate protein and one candidate peptide recognized by γδT cells [15]. J.RT-T3.5 cells do not express any TCR. When the full-length γ9 and δ2 sequences are transfected into the cells, γδTCR is expressed on their surface. The cells secrete IL-2 after being stimulated by corresponding antigens. This method has also been used to identify mycobacterium TB protein antigens recognized by γδT cells [17]. In present study, we first investigated the diversity of the γ9/δ2 T-cell immunorepertoire by sequence analyses of the expressed CDR3 in HCC patients. The difference of δ2 TCR chain was most pronounced presumably because of the increased potential for diverse sequences due to the additional D gene segment rearrangements and nucleotide additions/substitution [19]. Here, a common δ2 CDR3 sequence was found in PBMC of HCC patient’s groups. Our sequence analysis revealed that most Vδ2 T cell isolated from HCC patients and healthy controls also carried a hydrophobic amino acid residue (/leucine/valine/argine) at conserved position 97 (Table S2). The results suggested that the hydrophobic amino acid residue is not a prerequisite for γδ T cell reactive to HCC patients.
Then, we constructed γ9/δ2 TCR-transfected cells expressing the predominant HCC CDR3 sequence, identified a group of peptides capable of binding specifically to γδT cells and candidate peptide-matched proteins by bioinformatics analysis. Thus, we obtained a candidate HCC protein and peptides recognized by γδT cells. They were able to bind to γδTCRs with the HCC CDR3 sequence on the transfected cells and stimulate the expansion of γδT cells from HCC patients.
New antigen recognized by γδT cells that we identified is macrophage-stimulating protein (MSP), also known as hepatocyte growth factor-like protein. It is a kind of glycoprotein synthesized in the liver, and it has immunoregulatory activity [20]. MSP may bind to its specific Ron-receptor tyrosine kinase to stimulate macrophages to regulate macrophage motility and its own phagocytic activity and to induce the production of oxygen-free radicals and cytokines; this in turn induces activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and regulates cell differentiation, migration and matrix invasion [21]. Additionally, MSP could reduce palmitic acid (PA)-induced lipid accumulation and lipogenesis in the HepG2 cell line [22]. Satoru et al. demonstrated that plasma MSP levels are associated with prostate cancer progression [23]. Our bioinformatics analysis showed that HCC patients with higher expression of MSP had longer survival probability (Fig. 4a), suggesting the potential significance MSP in the diagnosis and progression of HCC.
MSP consists of six domains: an N-terminal domain homologous to plasminogen preactivation peptide; four copies of the kringle domain; and a C-terminal serine proteinase homology domain [24]. SWISS-MODEL (http://swissmodel.expasy.org/) were performed to predict the structure of MSP using homology modeling techniques. The result was presented in Figure S2B. However, due to the diversity of γδTCR, we cannot predict the binding of γδTCR and MSP by molecular docking.
γδT cells recognize a wide variety of antigens, such as lipids, proteins, and phosphoantigens (P-Ag). The crystallographic structure of γδTCRs shows that CDR3δ might be in direct contact with peptides and MHC-like protein and serve as a key determinant of specificity of antigen recognition [25]. He et al. demonstrated that recognition of γδT cells by human mutS homolog 2 (hMSH2) depends on γδTCRs and another activating receptor, NKG2D [26]. Other activating receptors, such as toll-like receptors (TLRs), also may provide costimulatory signals for γδT cell activation by different non-MHC protein ligands [27]. The mechanism by which γδT cells recognize MSP protein also needs further study.
We further evaluated the function of these γδT cells activated by candidate protein and peptides. They performed their major immunological effector functions in response to HCC mainly via secreting cytokines and performing cytotoxic effect. Based on two mechanisms, we detected the roles played by MSP protein and HP1 peptide-activated γδT cells against HCC.
Cytokines secreted by γδT cells, like those secreted by αβT cells, include Th1-, Th2- and Th17-type cytokines. γδT cells are thus divided into IFN-γ+γδT cells, IL-4+γδT cells and IL-17+γδT cells [28]. HCC is a typical inflammation-related cancer; various cytokines play important roles in its tumorigenesis and development. IFN-γ and TNF-α are Th1-type, IL-4 and IL-10 are Th2-type and IL-17 is a Th17-type cytokine, respectively. As a key cytokine in the control of HCC, IFN-γ can activate effector cells and cytotoxic T cells, which can kill or inhibit HCC [29, 30]. TNF-α is an important inflammatory factor and has been confirmed to promote tumor growth and poor prognosis in HCC [31, 32]. IL-4 is produced by Th2 cells, mast cells, basophils and natural-killer (NK) cells. Like other cytokines, it can have a variable effect on a number of different target cells, including B cells, T cells, monocytes, endothelial cells and hepatocytes [33, 34]. IL-4 plays important roles in regulating antibody production, in the development of effector T-cell responses, in hematopoiesis and in inflammation. In the inflammatory state, IL-4 can inhibit proinflammatory cytokines and chemokine production in monocytes, and it enhances the expression of vascular cell adhesion molecule 1 (VCAM-1) in endothelial cells. IL-10 is a pleiotropic cytokine produced by immune cells and has been shown to inhibit various immune reactions (35). In HCC, IL-17+ T cells have been found in increased numbers within tumors and correlate with poor survival rates and increased postoperative recurrence, indicating that Th17 cells and IL-17 may promote tumor progression in HCC [36]. In our study, we first detected cytokines secreted by γδT cells that had been activated by MSP protein and HP1. As shown in Fig. 3c, the production of IFN-γ in HP1-induced γδT cells from HCC patients was higher than in such cells from healthy controls; so was the production of IFN-γ in MSP-induced γδT cells from HCC patients (Fig. 4c). Levels of other cytokines, such as TNF-α, IL-4 and IL-17, did not differ between γδT cells activated by MSP protein and HP1 from HCC patients and those from healthy controls. Thus, MSP protein cells and HP1 peptide stimulated Vγ9Vδ2 T showed potential as Th1 biased immune reaction for HCC immunotherapy.
We then determined the cytotoxicity of γδT cells activated by MSP protein and HP1 to the HCC cell line HepG2. γδT cells expanded by MSP protein and HP1 had cytotoxic effects on HepG2 cells (Figs. 3d, 4d). However, we verified this function of γδT in only 10 HCC patients in the current study. We intend to expand the sample size in subsequent research.
In conclusion, our findings add to the understanding of the mechanism by which γδTCRs recognize protein antigens. We identified a peptide (HP1) and a protein (MSP) as new candidate HCC antigens recognized by γδT cells. They could be potential candidate peptides or antigenic components to develop new targets for immunotherapy, as well as biomarkers of HCC. Further studies are in progress to confirm the bio-function of MSP protein and the HP1 peptide in γδT cell-based anti-HCC therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Conceived and designed the experiments: XX, BD. Performed the experiments: MZ, YG, FQ. Analyzed the data: XX BD. Contributed reagents/materials/analysis tools: GL FL. Wrote the paper: XX BD.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81772649,31370890). Innovative Research Program for Graduated of Hubei University of Medicine (YC2019012, YC2020008).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This work received approval from the Clinical Ethics Committee of Hubei University of Medicine (2019-TH-024).
Informed consent
All individuals gave their informed consent to participate.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xueyan Xi, Yang Guo and Min Zhu contributed equally to this report.
Contributor Information
Xueyan Xi, Email: xixueyan2001@126.com.
Boyu Du, Email: du.boyu@hotmail.com.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Z, Jiang X, Chen S, et al. Anti-GPC3-CAR T cells suppress the growth of tumor cells in patient-derived xenografts of hepatocellular carcinoma. Front Immunol. 2017;7:690. doi: 10.3389/fimmu.2016.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busato D, Mossenta M, Baboci L, Di Cintio F, Toffoli G, Dal Bo M. Novel immunotherapeutic approaches for hepatocellular carcinoma treatment. Expert Rev Clin Pharmacol. 2019;12:453–470. doi: 10.1080/17512433.2019.1598859. [DOI] [PubMed] [Google Scholar]
- 4.Sawada Y, Yoshikawa T, Nobuoka D, et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18:3686–3696. doi: 10.1158/1078-0432.CCR-11-3044. [DOI] [PubMed] [Google Scholar]
- 5.Yarchoan M, Xing D, Luan L, et al. Characterization of the immune microenvironment in hepatocellular carcinoma. Clin Cancer Res. 2017;23:7333–7339. doi: 10.1158/1078-0432.CCR-17-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 7.Norris S, Collins C, Doherty DG, et al. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol. 1998;28:84–90. doi: 10.1016/S0168-8278(98)80206-7. [DOI] [PubMed] [Google Scholar]
- 8.Kasper HU, Ligum D, Cucus J, Stippel DL, Dienes HP, Drebber U. Liver distribution of gammadelta-T-cells in patients with chronic hepatitis of different etiology. APMIS. 2009;117:779–785. doi: 10.1111/j.1600-0463.2009.02540.x. [DOI] [PubMed] [Google Scholar]
- 9.Holtmeier W, Kabelitz D. Gammadelta T cells link innate and adaptive immune responses. Chem Immunol Allergy. 2005;86:151–183. doi: 10.1159/000086659. [DOI] [PubMed] [Google Scholar]
- 10.Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. Gammadelta T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology. 2014;3:e27572. doi: 10.4161/onci.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 12.Brown KC. New approaches for cell-specific targeting: identification of cell-selective peptides from combinatorial libraries. Curr Opin Chem Biol. 2000;4:16–21. doi: 10.1016/S1367-5931(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 13.Alderson MR, Bement T, Day CH, et al. Expression cloning of an immunodominant family of Mycobacterium tuberculosis antigens using human CD4(+) T cells. J Exp Med. 2000;191:551–560. doi: 10.1084/jem.191.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Zhang Y, Wang J, et al. Screening and identification of a targeting peptide to hepatocarcinoma from a phage display peptide library. Mol Med. 2007;13:246–254. doi: 10.2119/2006-00115.Zhang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xi X, Zhang X, Wang B, et al. A novel strategy to screen Bacillus Calmette-Guerin protein antigen recognized by gammadelta TCR. PLoS ONE. 2011;6:e18809. doi: 10.1371/journal.pone.0018809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xi X, Han X, Li L, Zhao Z. Gammadelta T cells response to Mycobacterium tuberculosis in pulmonary tuberculosis patients using preponderant complementary determinant region 3 sequence. Indian J Med Res. 2011;134:356–361. [PMC free article] [PubMed] [Google Scholar]
- 17.Xi X, Han X, Li L, Zhao Z. Identification of a new tuberculosis antigen recognized by gammadelta T cell receptor. Clin Vaccine Immunol. 2013;20:530–539. doi: 10.1128/CVI.00584-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Chang-Yong E, Gong ZW, et al. Chimeric antigen receptor-engineered T-cell therapy for liver cancer. Hepatobiliary Pancreat Dis Int. 2018;17:301–309. doi: 10.1016/j.hbpd.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Allison TJ, Winter CC, Fournié JJ, Bonneville M, Garboczi DN. Structure of a human gammadelta T-cell antigen receptor. Nature. 2001;411:820–824. doi: 10.1038/35081115. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Chanda D, van Gorp PJ, et al. Macrophage Stimulating Protein Enhances Hepatic Inflammation in a NASH Model. PLoS ONE. 2016;11:e0163843. doi: 10.1371/journal.pone.0163843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T, Chen X, Zhang W, Xiang X, Leng C, Jia Q. Roles of macrophage stimulating protein and tyrosine kinase receptor RON in smoke-induced airway inflammation of rats. Int J Clin Exp Pathol. 2015;8:8797–8808. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu M, Paddock GV. Expression of the hepatocyte growth factor-like protein gene in human hepatocellular carcinoma and interleukin-6-induced increased expression in hepatoma cells. Biochim Biophys Acta. 1999;1449:63–72. doi: 10.1016/S0167-4889(98)00171-2. [DOI] [PubMed] [Google Scholar]
- 23.Sugie S, Mukai S, Yamasaki K, Kamibeppu T, Tsukino H, Kamoto T. Plasma macrophage-stimulating protein and hepatocyte growth factor levels are associated with prostate cancer progression. Hum Cell. 2016;29:22–29. doi: 10.1007/s13577-015-0123-5. [DOI] [PubMed] [Google Scholar]
- 24.Carafoli F, Chirgadze DY, Blundell TL, Cheradi E. Crystal structure of the beta-chanin of human hepatocyty fcator-like/macrophage stimulating protein. FEBS J. 2005;272:5799–5807. doi: 10.1111/j.1742-4658.2005.04968.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, He X, Wang Z, et al. Identification of human T cell receptor gammadelta-recognized epitopes/proteins via CDR3delta peptide-based immunobiochemical strategy. J Biol Chem. 2008;283:12528–12537. doi: 10.1074/jbc.M708067200. [DOI] [PubMed] [Google Scholar]
- 26.Dai Y, Chen H, Mo C, Cui L, He W. Ectopically expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human gammadelta T cells to induce innate anti-tumor/virus immunity. J Biol Chem. 2012;287:16812–16819. doi: 10.1074/jbc.M111.327650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serrano R, Wesch D, Kabelitz D. Activation of human γδ t cells: modulation by toll-like receptor 8 ligands and role of monocytes. Cells. 2020;9:713. doi: 10.3390/cells9030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J, Luo X, Chen D, Fang H, Xie H. Proportion and characteristics of γδT cells in different tissues and organs of C57BL/6 mice. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013;29(449–52):457. [PubMed] [Google Scholar]
- 29.Li P, Du Q, Cao Z, et al. Interferon-γ induces autophagy with growth inhibition and cell death in human hepatocellular carcinoma (HCC) cells through interferon-regulatory factor-1 (IRF-1) Cancer Lett. 2012;314:213–222. doi: 10.1016/j.canlet.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou J, Zhuang M, Yu X, et al. MYC inhibition increases PD-L1 expression induced by IFN-gamma in hepatocellular carcinoma cells. Mol Immunol. 2018;101:203–209. doi: 10.1016/j.molimm.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Zhang GP, Yue X, Li SQ. Cathepsin C interacts with TNF-alpha/p38 MAPK signaling pathway to promote proliferation and metastasis in hepatocellular carcinoma. Cancer Res Treat. 2020;52:10–23. doi: 10.4143/crt.2019.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jing Y, Sun K, Liu W, et al. Tumor necrosis factor-alpha promotes hepatocellular carcinogenesis through the activation of hepatic progenitor cells. Cancer Lett. 2018;434:22–32. doi: 10.1016/j.canlet.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Wu Z, Peng Q, et al. Role of IL-4 gene polymorphisms in HBV-related hepatocellular carcinoma in a Chinese population. PLoS ONE. 2014;9:e110061. doi: 10.1371/journal.pone.0110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao Y, Li J, Lu Z, et al. Proteomic analysis of the interleukin-4 (IL-4) response in hepatitis B virus-positive human hepatocelluar carcinoma cell line HepG2.2.15. Electrophoresis. 2011;32:2004–2012. doi: 10.1002/elps.201100147. [DOI] [PubMed] [Google Scholar]
- 35.Xue H, Lin F, Tan H, Zhu ZQ, Zhang ZY, Zhao L. Overrepresentation of IL-10-expressing B cells suppresses cytotoxic CD4+ T cell activity in HBV-induced hepatocellular carcinoma. PLoS ONE. 2016;11:e0154815. doi: 10.1371/journal.pone.0154815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu FM, Li QL, Gao Q, et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer. 2011;10:150. doi: 10.1186/1476-4598-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.