Abstract
Thymocyte selection-associated high mobility group box protein (TOX) is a transcription factor implicated in the regulation of T cell exhaustion during chronic infection and cancer. While TOX is being targeted for cancer immunotherapy, limited information is available about its significance in breast cancer and other solid tumors. We performed a comprehensive analysis of TOX gene expression, its epigenetic regulation, protein localization, relation to tumor infiltrating immune cell composition, and prognostic significance in breast cancer using publicly available datasets. Our results suggest an inverse correlation between TOX expression and DNA methylation in tumor cells. However, its expression is elevated in tumor infiltrating immune cells (TIICs), which may compensates for the total TOX levels in the tumor as a whole. Furthermore, higher TOX levels in tumors are associated with T cell exhaustion signatures along with presence of active inflammatory response, including elevated levels of T cell effector cytokines. Survival analysis also confirmed that higher expression of TOX is associated with better prognosis in breast cancer. Therefore, expression of TOX may serve as a novel prognostic marker for this malignancy.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02689-3) contains supplementary material, which is available to authorized users.
Keywords: TOX, Breast cancer, Cancer immunotherapy, PD-1, Immune checkpoint proteins, T cell exhaustion
Introduction
Breast cancer is the most common cancer among females and a major health burden globally [1]. Based on the gene expression profiles, it is classified into four common subtypes, namely luminal A-like, luminal B-like, HER2-enriched (non-luminal), and basal (triple-negative breast cancer). Clinically, immunohistochemical analysis of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (ERBB2/HER2), cytokeratin 6 (CK6), and MIB1 (Ki-67) are used as surrogate marker for identifying these molecular subtypes [2]. Breast cancer microenvironment contains leucocytes of both lymphoid and myeloid lineage, which play essential roles in disease progression and influence clinical outcomes [3]. Interestingly, the immune cell composition of this malignancy varies according to the molecular features of the tumor [4].
Several studies have highlighted a complex association between tumor molecular characteristics and immune cells in the tumor microenvironment (TME). Major determinants of tumor immunogenicity include expression of antigenic peptides by tumor cells, engagement of immune cells into the tumor tissue, presentation of tumor antigens to immune cells, and abundance of immune checkpoint proteins (ICPs) in the TME [5]. Therapeutic antibodies that target immune checkpoint proteins called immune checkpoint inhibitors, have recently been introduced in the clinics for the treatment of several malignancies, including breast cancer [6]. However, the response to the immune checkpoint inhibitors displays wide variations, and only a small number of patients show substantial benefits [7]. Therefore, the identification of biomarkers, which could predict the response to these therapies and clinical outcomes, may play an essential role in the management of this malignancy.
Currently, more than 290 clinical trials for breast cancer immunotherapy are in progress, and a vast majority of them are aimed at targeting ICPs [6]. Most commonly studied ICPs includes cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1) and its ligand programmed death-ligand 1 (PD-L1). PD-1 expressed on T cells interacts with its ligand PD-L1 bearing antigen presenting cells (APCs) and tumor cells, thereby leading to T cell dysfunction or “exhaustion”. Therefore, strategies aimed at modulating PD-1 gene transcription and molecular functions have been suggested to potentiate cancer immunotherapy [8]. However, in breast cancer, a higher expression of PD-1 is associated with favorable outcomes [9, 10]. While the molecular basis of this effect is not fully understood, it has been conclusively demonstrated that tumor-infiltrating CD8+ T cells in breast cancer retain effector functions despite the expression of checkpoint proteins, including PD-1 [11, 12]. Therefore, further studies are required to determine the association of T cell exhaustion with clinical outcomes in breast cancer.
Thymocyte selection-associated HMG bOX protein (TOX) is a small family of four proteins, including TOX1 (TOX), TOX2, TOX3, and TOX4. TOX proteins belong to a large superfamily of high mobility group (HMG) box proteins and display tissues specific expression along with significant homology in their primary structure and binding motifs [13]. TOX1/TOX functions as a regulator of transcriptional programs related to T cell selection in the thymus [14]. However, recently, its role as a critical regulator of T cell exhaustion during viral infection and cancer has also been established [15–18]. Gene knockout studies unraveled its involvement in regulating the expression of T cell exhaustion markers, including PD-1 and CTLA-4 in TIICs [19]. Association of higher TOX levels with the presence of T cell exhaustion markers has also been reported in a mouse model of breast cancer [12]. These findings aroused a great deal of interest in determining the role of TOX in regulating T cell functions during various pathological conditions, including viral infection, autoimmune disorders, and cancer.
Interestingly, elevated mRNA expression of all TOX proteins has been observed in lung cancer [20], while TOX expression in tumor cells has also been reported to reduce during colon cancer progression [21]. Aberrant methylation of TOX promoter in breast cancer is documented [22], but no information was available about its effect on the expression of the TOX gene in breast cancer. Recently, higher TOX expression in breast cancer stromal cells was found to be associated with better response to neoadjuvant chemotherapy [23]. Therefore in the present study, we sought to explore the expression, protein localization, and clinical significance of TOX in breast cancer using publicly available datasets including The Cancer Genome Atlas (TCGA), Human Protein Atlas (HPA), and Gene Expression Omnibus (GEO). We focused our analysis on determining the cell-specific expression pattern of TOX in TME and regulation of TOX gene expression by genetic and epigenetic mechanisms. Further, we explored the relation of TOX expression with the level and composition of tumor infiltrating immune cells along with markers of T cell exhaustion in breast cancer. Finally, we investigated its association with patient survival in breast cancer.
Materials and methods
Gene Expression Profiling Interactive Analysis 2 (GEPIA2)
GEPIA2 (http://gepia2.cancer-pku.cn/) is a web server for analyzing the RNA sequencing data of tumor and normal tissues from The Cancer Genome Atlas (TCGA) datasets [24]. From the survival map module, we performed survival analysis of TOX family expression in TCGA breast cancer dataset. Through the correlation module of GEPIA2, we correlated mRNA levels of the TOX gene with other immunity-related genes in TCGA-BRCA tumor tissues.
Cancer Cell Line Encyclopedia (CCLE) data analysis
TOX mRNA expression and DNA methylation data obtained from RNA sequencing and multiplexed reduced representation bisulfite sequencing (mRRBS), respectively was extracted from CCLE data portal (https://portals.broadinstitute.org/ccle) and subjected to Spearman’s correlation analysis.
DNA methylation analysis
MEXPRESS (https://mexpress.be/) and TCGA Wanderer (http://maplab.imppc.org/wanderer/) are web tools to analyze DNA methylation profiles and its relation to gene expression from TCGA datasets [25, 26]. These tools provide mRNA expression data of given gene and methylation values of designated CpG sites for a given gene based on “Illumina Human Methylation 450 Bead Chip” platform. MEXPRESS was utilized to assess associations of TOX expression with clinicopathological parameters in TCGA breast cancer dataset. Wanderer was used to extract data of TOX DNA methylation for manual analysis. For determining the mean methylation of TOX promoter, we selected 12 CpG sites present in the CpG island of human TOX gene sequence given in the UCSC genome browser (https://genome.ucsc.edu/), GRCh37/hg19 human genome assembly at position: chr8: 60030135–60032747. The mean methylation status of TOX was determined for 743 breast cancer and 98 normal breast tissues. Out of these, paired tumor and normal breast tissue samples were used to compare TOX gene methylation in different molecular subtypes.
cBioPortal datasets analysis
The mRNA expression of TOX in TCGA breast cancer dataset (TCGA-BRCA) sourced from NCI Genomic Data Commons (GDC, https://gdc.cancer.gov), consisting of data of 1082 patients, was downloaded from online tool cBioPortal (https://www.cbioportal.org) [27]. TOX mutation and copy number alterations data were assessed in several datasets including TCGA, METABRIC [28], INSERM [29], MBC project (https://www.mbcproject.org), BCCRC [30], Broad [31], MSKCC, and Sanger [32]. Also, correlations of TOX gene expression with immune related markers in METABRIC data were analyzed.
ABSOLUTE (tumor purity estimations)
Absolute is a scoring system for the determination of tumor purity [33]. Predetermined ABSOLUTE scores for TCGA tumor datasets were downloaded from the GDC data portal (https://gdc.cancer.gov) and used for analysis. A total of 691 breast tumor samples were used to correlate TOX gene methylation and tumor purity.
ESTIMATE (Immune infiltration scores estimations)
We extracted predetermined immune scores for TCGA breast cancer dataset using ESTIMATE, a widely accepted scoring system for the determination of overall immune infiltration in tumors [34]. Data from a total of 735 breast tumor tissues were used for correlating TOX gene methylation with the immune scores, while TOX expression was correlated with the immune scores using data from 1080 tumor tissue samples.
NCBI-GEO dataset analysis
Log transformed normalized gene expression values were downloaded as series matrix files of GSE41986 from the NCBI-GEO website [35]. TOX gene expression values were extracted and compared between 12 tumor infiltrating immune cell profiles (CD45+ immune cells) and tumor cell-specific profiles (EPCAM+ tumor cells). Non-parametric Mann–Whitney t test was applied to the mean expression values of two probe ids (A_23_P123413 and A_23_P144877) representing the TOX gene in “Agilent-014850 Whole Human Genome Microarray 4× 44 K G4112F” array platform.
Single cell sequencing data for NCBI-GEO accession number GSE75688 were extracted (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE75688) in pre-processed form. Samples were distributed into groups based on predetermined cell types for comparison of TOX mRNA expression.
The Human Protein Atlas (HPA)
The Human Protein Atlas (HPA, http://www.proteinatlas.org) is a web server consisting of immunohistochemically stained images of various tissues with normal and disease pathology [36]. Immunostained images of TOX in breast cancer tissues and normal breast tissues were analyzed for staining in tumor cells, immune cells, and normal cells. A total of 3 normal breast tissues along with 21 full size immunostained images belonging to 12 breast tumor tissues were available with pre-analyzed staining levels and patterns. These images were also reviewed by the pathologist (AC) to confirm cell-specific expression.
Tumor IMmune Estimation Resource (TIMER) analysis
Online tool TIMER (Tumor IMmune Estimation Resource; https://cistrome.shinyapps.io/timer) was used to correlate mRNA expression of TOX gene family in TCGA BRCA dataset with tumor purity and infiltration levels of six immune cell types (B cells, CD4+ T cells, CD8+ T cells, neutrophils, macrophages and dendritic cells) [37]. This tool computes immune infiltration based on a predefined signature gene matrix of the immune subsets. The gene module was used taking TOX genes as input, and TCGA breast cancer subtypes were selected as datasets.
Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts (CIBERSORT) analysis
CIBERSORT (Cell-type Identification by Estimating Relative Subsets of RNA Transcripts) is a tool to determine relative fractions of 22 different immune cells from a mixture of gene expression profiles. CIBERSORT uses an inbuilt signature matrix (LM22), which can be applied to deconvolute mRNA profile of mixed cells, including tumor tissues. Previously published CIBERSORT data for TCGA-BRCA samples were downloaded from the GDC data portal (https://gdc.cancer.gov) and used to correlate with TOX mRNA expression extracted from cBioPortal for the same patients [38, 39].
KM-Plotter survival analysis
Kaplan–Meier Plotter (http://kmplot.com) is an online database of gene expression and clinical data from multiple microarray gene expression datasets from NCBI-GEO (https://www.ncbi.nlm.nih.gov/geo). This tool provides a Kaplan–Meier survival analysis of the gene of interest in breast cancer. For the current study, we only utilized microarray gene expression profiles of breast cancer [40]. As the TOX gene is represented by two probe ids (204529_s_at and 204530_s_at) in the Affymetrix platform, the mean value of both ids was used for survival analysis.
Results
TOX mRNA expression and its genetic regulation in breast cancer
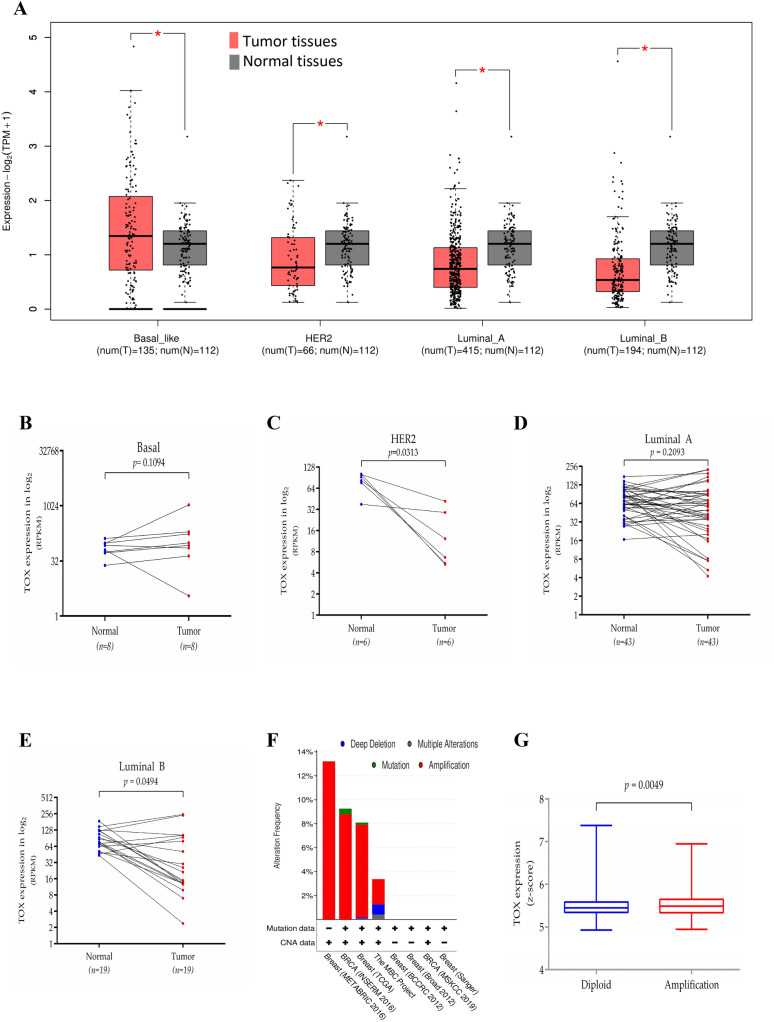
We analyzed gene expression data of breast cancer tissues and normal breast tissues from TCGA breast cancer dataset (TCGA-BRCA) using available online tool GEPIA2. As evident from Fig. 1a, significantly reduced TOX mRNA expression was observed in HER2, luminal-A, and luminal-B subtype tumors, while basal subtype tumors exhibited elevated expression of TOX compared to the normal breast tissues. Furthermore, the basal subtype expressed the highest mRNA levels as compared to the other three subtypes, where the levels of TOX expression were comparable (Supplementary Figure S1A). Confining our analysis to paired normal-tumor samples from TCGA dataset, we observed that only HER2 and luminal-B subtypes exhibited reduced expression of TOX in tumor tissues compared to their paired normal tissues (both p < 0.05, Fig. 1c, e, respectively), while no such difference was observed for basal (p = 0.109, Fig. 1b) and luminal-A (p = 0.209, Fig. 1d) subtypes. Further analysis of mutation and copy number alteration (CNA) data in several breast cancer datasets available from cBioPortal revealed less than 1% incidence of TOX mutation (Fig. 1f). Varying levels of TOX gene amplification were detected in different datasets. It was maximum (13.21%) in the METABRIC dataset [28] followed by INSERM dataset [29] and TCGA dataset, respectively; however, it was less than 4% in other datasets. The TOX mRNA levels were significantly higher in tumors with amplified TOX gene as compared to those with diploid status (Mann–Whitney t-test, p = 0.0049). However, the median mRNA expression levels were comparable (5.448 vs. 5.488) in the two groups (Fig. 1g). These results suggest that genetic alterations of TOX gene contributes minimally to TOX expression in breast cancer.
Fig. 1.
Genetic regulation of TOX mRNA expression in breast cancer. a Expression levels of TOX among different breast cancer subtypes (red) compared to normal breast tissues (grey) in TCGA-BRCA dataset analyzed by GEPIA2. b–e Expression levels of TOX in paired tumors (red) and normal breast tissues (blue) in basal, HER2, luminal A, and luminal B subtype. f Assessment of copy number alterations and mutations of TOX gene in different datasets from cBioPortal. g Comparison of TOX gene expression in tumors diploid for TOX (blue) or with amplified TOX (red) in the METABRIC dataset (Mann–Whitney t-test, p = 0.0049). TPM, transcripts per million; RPKM, reads per kilobase million; ns, not significant
Epigenetic regulation of TOX gene in breast cancer
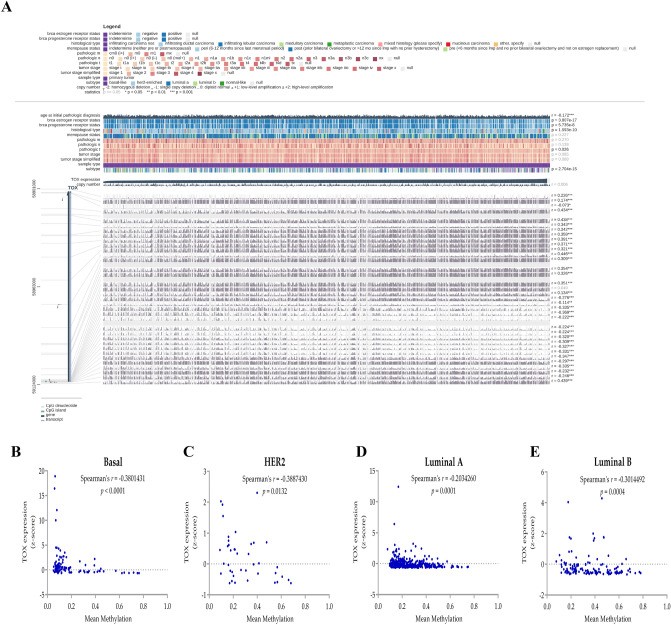
Hypermethylation of the TOX gene family has been previously reported in breast and lung cancer cell lines [22, 41]. Our analysis of 51 breast cancer cell lines from CCLE also revealed a negative correlation between TOX promoter methylation and its mRNA expression, thereby confirming the role of methylation in regulating TOX expression (Supplementary Figure S1B). To explore the association of DNA methylation and other clinicopathological features with TOX expression in breast cancer in detail, we utilized TCGA breast cancer dataset using MEXPRESS web server (Fig. 2a). Among clinicopathological features, TOX expression was negatively correlated with age at diagnosis and associated with ER status, PR status, pathologic tumor stage, tumor histology, and subtype. Correlation analysis revealed that the DNA methylation levels of the CpG sites close to the TOX transcription start site (TSS) were negatively correlated with TOX expression. Interestingly, an extended exploration of TOX gene body methylation revealed that several CpG sites within the gene body exhibited positive correlations with TOX expression (Fig. 2a). Furthermore, we observed that mean methylation levels of TOX CpG Island (represented by 12 CpG sites near TSS) were also negatively correlated in all breast cancer subtypes (Fig. 2b–e). These results suggested differential regulation of TOX expression by distinct DNA methylation patterns within the TOX genomic locus.
Fig. 2.
Epigenetic regulation of TOX expression by DNA methylation in breast cancer. a Association of TOX mRNA expression with clinicopathological features and TOX gene DNA methylation in TCGA-BRCA dataset. b Correlation of mean methylation levels of CpG sites in human TOX gene promoter with its mRNA expression in different subtypes of breast cancer
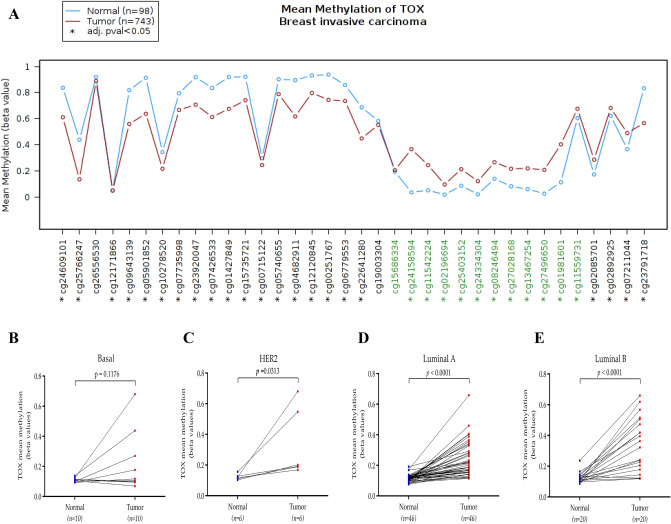
We further compared DNA methylation levels of TOX gene between normal and tumor tissues using TCGA-Wanderer and observed the extent at CpG Island to be significantly higher in tumor tissues as compared to normal tissues (Fig. 3a). We observed that methylation levels of CpG Island of the TOX gene is significantly higher in tumor tissues as compared to normal tissues. Contrary to this, the extent of DNA methylation in intragenic CpG sites was lower in tumor tissues compared to normal breast tissues. Further, comparison between paired normal and tumor tissues also revealed higher methylation of TOX promoter in HER2 (p < 0.05, Fig. 3b), luminal-A (p < 0.0001, Fig. 3c) and luminal-B (p < 0.0001, Fig. 3d) subtypes, whereas basal like subtype did not exhibit any difference (p = 0.275, Fig. 3e).
Fig. 3.
Epigenetic alterations of TOX in breast cancer. a Comparison of mean methylation levels of different CpG sites spanning human TOX gene between tumor and normal tissues from TCGA-BRCA dataset. 12 CpG sites within the CpG island of TOX gene used during subsequent analysis are shown in green, while other CpG sites are shown in black. *p < 0.05. b–e Mean methylation levels of CpG island of TOX gene in paired tumors (red) and normal tissue samples (blue) in basal, HER2, luminal A, and luminal B subtypes, respectively
Contribution of TIICs to tumor TOX mRNA levels in breast cancer
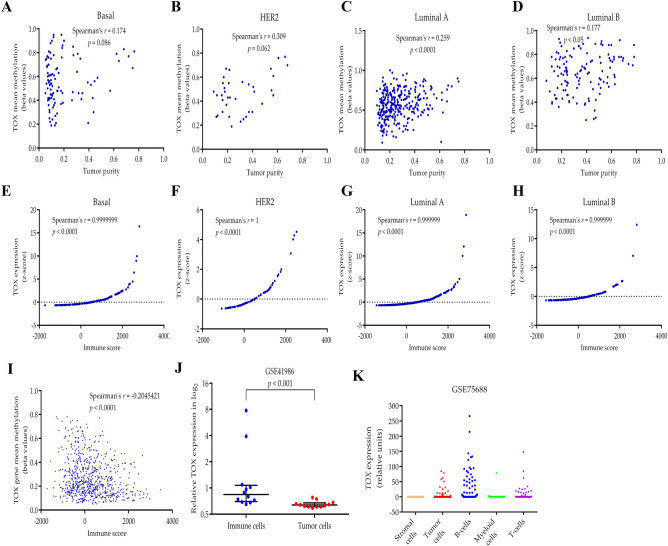
To resolve the association of altered DNA methylation and expression of TOX gene with the level of TIICs, we assessed the level of immune cell-specific mRNA in normal and tumor tissues. We utilized PTPRC (pan-immune cell marker coding for CD45 protein), CD8A (cytotoxic T cell marker) and CD4 (helper T cell marker) as surrogate markers for the level of immune cell mRNA, which revealed no difference between the normal and tumor tissues for any of these genes (Supplementary Figure S2). Hence we conclude that the number of overall immune cells, CD4+ T cells and CD8+ T cells in these tissues are comparable. Further, we correlated TOX methylation levels with the level of tumor purity (Fig. 4a–d) which revealed weak but significant positive correlation between the two in luminal-A (r = 0.259, p < 0.0001, Fig. 4c) and luminal-B subtypes (r = 0.177, p < 0.05, Fig. 4d) suggesting that the higher TOX methylation observed is also weakly associated with a higher number of tumor cells. We then assessed the relationship between TOX mRNA levels in the tumor tissue and immune score, a measure of the abundance of TIICs (Fig. 4e–h). We observed a highly significant positive correlation between the two in all breast cancer subtypes, thereby suggesting the predominant contribution of TIICs in maintaining higher mRNA levels of TOX in the tumor tissues. Also, there was a significant negative correlation between TOX methylation and immune score (Spearman’s r = − 0.2045, p < 0.0001) (Fig. 4i), which also suggest that the infiltrating immune cell may contribute DNA with hypomethylated TOX promoters. We further analyzed TOX expression in CD45+ immune cell population and EPCAM+ tumor cell population isolated from primary breast cancer patients using GSE41986 dataset (Fig. 4j). This analysis also revealed significantly higher TOX expression in TIICs compared to the tumor cells (Mann–Whitney U-test, p < 0.001). Furthermore, we utilized single cell sequencing dataset from NCBI-GEO dataset GSE75688, which consisted of mRNA expression in tumor cells, stromal cells, and multiple subsets of immune cells from cellular pool contributed by 11 breast cancer patients (Fig. 4k). We observed that the percentage of TOX expressing cells among the total number of a specific cell type analyzed was highest for B-cells (59.03%), followed by T-cells (44.44%), myeloid cells (21.05%), tumor cells (9.46%) and stromal cells (8.7%). Interestingly, the highest level of expression was observed in B-cells followed by T-cells, suggesting B-cells also contribute to TOX expression in breast cancer.
Fig. 4.
a–d Spearman’s correlation analysis of mean methylation levels of the TOX gene with tumor purity in basal, HER2, luminal A, and luminal B subtypes from TGCA-BRCA dataset. e–h Spearman’s correlation analysis of TOX mRNA expression and immune score in basal, HER2, luminal A, and luminal B subtypes. i Spearman’s correlation analysis of mean methylation levels of the TOX gene with immune score in TCGA-BRCA dataset. j Comparison of mRNA levels of TOX in isolated CD45+ immune cells (blue) and tumor cells (red) from GSE41986 dataset. Log transformed normalized gene expression values provided at the GEO profile page of GSE41986 dataset were used after antilog transformation (Mann–Whitney t-test, p < 0.001). k Single cell RNA sequencing data for TOX expression in different cellular components of breast cancer. Combined samples were contributed by 11 breast cancer patients
Protein localization of TOX in breast cancer
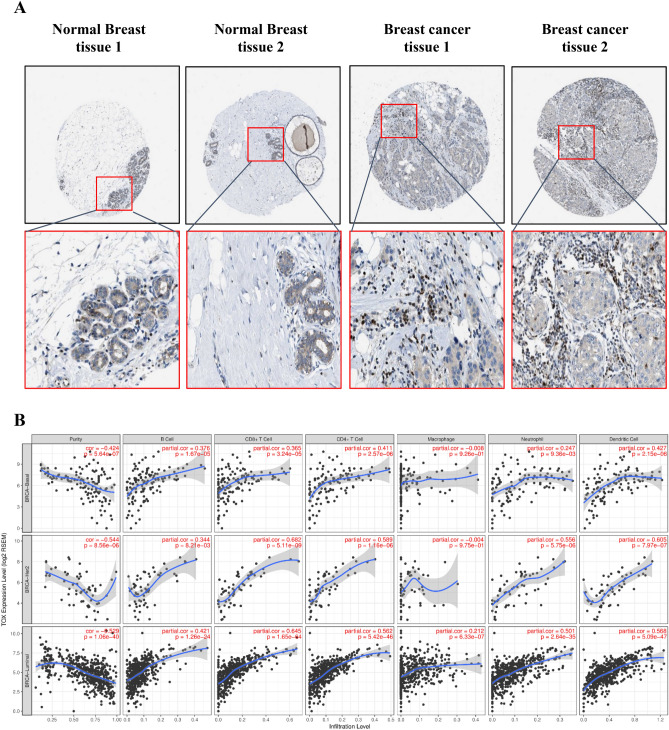
To assess localization and cellular origin of TOX protein in breast cancer and normal breast tissue, we used immunohistochemically TOX stained images available from Human Protein Atlas (https://www.proteinatlas.org/). In normal breast tissues, we observed the absence of TOX in adipocytes (3/3), moderate cytoplasmic/membranous staining in glandular cells (3/3 tissues), and weak staining in myoepithelial cells (3/3) (Fig. 3a). In tumor tissue, we observed that TOX staining was absent in tumor cells in most tissues (12/21) while in remaining tissues, there was only weak staining in the cytoplasm or cell membrane (9/21). Intriguingly, intense nuclear staining of TOX was present in tumor infiltrating immune cells (15/21) (Fig. 5a; Supplementary Table S1 and S2).
Fig. 5.
TOX localization and relation to tumor infiltrating immune cells in breast cancer. a Representative immunohistochemical staining of TOX protein in two normal breast tissues and two breast cancer tissues. Images were extracted with quantification from Human Protein Atlas. Pathological observations and quantification data for all analyzed tissue images for normal breast and tumor tissues have been given in Supplementary Tables S1 and S2, respectively. b Correlation of TOX gene expression with tumor purity and six major tumor infiltrating immune cell types in different breast cancer subtypes from TCGA-BRCA dataset
Correlation between TOX expression and immune cell composition in breast cancer
To explore the relation of TOX mRNA expression in TCGA breast cancer dataset to different immune cell populations, we examined the correlation between TOX expression and abundance of TIICs using the TIMER tool. Tumor purity normalized spearman correlation analyses revealed a positive correlation between TOX expression with B cells, CD4+ T cells, CD8+ T cells, macrophages, neutrophils, and dendritic cells in breast cancer (Fig. 5b). CD8+ T cells and CD4+ T cells exhibited higher positive correlations with TOX expression than B cells. These correlations were more pronounced in the case of luminal subtype compared to basal and HER2 subtype. We also observed a negative correlation of TOX2 with tumor purity along with positive correlation with TIICs, while TOX3 and TOX4 did not show any correlation with either tumor purity or TIICs (Supplementary Figure S3B). We further observed that mRNA levels of TOX2 exhibited positive correlation with TOX, however TOX3 and TOX4 mRNA levels displayed inverse correlation with TOX (Supplementary Figure S3A). The relative abundance of 22 different types of immune cells in TCGA-BRCA dataset was estimated by CIBERSORT tool and correlated with TOX mRNA expression. Interestingly, the strongest positive and negative correlation was found with M1 and M2 type macrophages, respectively, suggesting that TOX expression in breast cancer is associated with an overall active inflammatory response (Table 1). Also, CD4+ memory resting T cells and CD8+ T cells exhibited higher positive correlation compared to B cells, suggested their predominant contribution for TOX expression in breast cancer.
Table 1.
Correlation of TOX1 mRNA levels with relative abundance of TIICs by CIBERSORT
| Immune cell types | Spearman’s r | 95% confidence interval | p value |
|---|---|---|---|
| Macrophages M1 | 0.4655672 | 0.4169921 to 0.5114905 | < 0.0001 |
| T cells CD4 memory resting | 0.3742347 | 0.3212031 to 0.4249265 | < 0.0001 |
| T cells CD8 | 0.3701012 | 0.3168935 to 0.4209868 | < 0.0001 |
| B cells naive | 0.3668469 | 0.3135020 to 0.4178837 | < 0.0001 |
| T cells regulatory | 0.2598444 | 0.2027426 to 0.3151850 | < 0.0001 |
| T cells follicular helper | 0.2590311 | 0.2019064 to 0.3143994 | < 0.0001 |
| T cells CD4 memory activated | 0.2449999 | 0.1874915 to 0.3008345 | < 0.0001 |
| Dendritic cells resting | 0.2223254 | 0.1642492 to 0.2788655 | < 0.0001 |
| T cells gamma delta | 0.2111363 | 0.1528037 to 0.2680027 | < 0.0001 |
| NK cells activated | 0.1886026 | 0.1298010 to 0.2460823 | < 0.0001 |
| Plasma cells | 0.02994665 | − 0.03038545 to 0.09006134 | 0.3165 |
| Eosinophils | − 0.04097535 | − 0.1010031 to 0.0193497 | 0.1704 |
| Monocytes | − 0.04526551 | − 0.1052556 to 0.01505282 | 0.1299 |
| B cells memory | − 0.04849583 | − 0.1084561 to 0.01181597 | 0.1046 |
| Mast cells activated | − 0.0495772 | − 0.1095272 to 0.01073213 | 0.0971 |
| Dendritic cells activated | − 0.104156 | − 0.1634073 to − 0.04415594 | 0.0005 |
| Neutrophils | − 0.1402357 | − 0.1988323 to − 0.08064014 | < 0.0001 |
| Macrophages M0 | − 0.1479432 | − 0.2063801 to − 0.08845478 | < 0.0001 |
| T cells CD4 naive | − 0.1939834 | − 0.2513220 to − 0.1352880 | < 0.0001 |
| NK cells resting | − 0.220959 | − 0.2775398 to − 0.1628507 | < 0.0001 |
| Mast cells resting | − 0.2356439 | − 0.2917768 to − 0.1778934 | < 0.0001 |
| Macrophages M2 | − 0.4735846 | − 0.5190450 to − 0.4254525 | < 0.0001 |
Correlation between TOX expression and T cell exhaustion markers in breast cancer
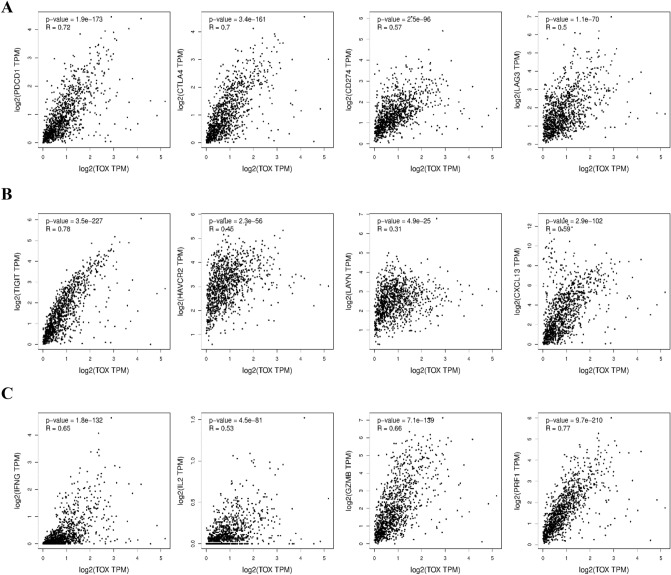
To explore the relation between TOX expression and immune response in breast cancer, we performed correlation analysis using TCGA breast cancer dataset with established ICPs using tool GEPIA2. Our results demonstrate a positive correlation of TOX mRNA levels with immune checkpoint proteins PDCD1 (PD-1), CD274 (PD-L1), CTLA4, and LAG3 (Fig. 6a) along with other established T cell exhaustion markers, including, TIGIT, HAVCR2, LAYN, and CXCL3 (Fig. 6b). Similar correlations of TOX mRNA expression with T cell exhaustion markers were also observed in the METABRIC dataset (Supplementary Table S3). It is also noteworthy that TOX mRNA exhibit a significant positive correlation with inflammatory markers such as IFNG (IFN-γ), IL2 (IL-2), GZMB (granzyme B), and PRF1 (perforin) (Fig. 4c). Similar results were also observed for breast cancer subtype specific correlation analysis for these markers in TCGA-BRCA dataset (Supplementary Table S4).
Fig. 6.
Spearman’s correlation analysis of TOX gene expression with expression of immunity related markers in breast cancer. a Correlation of TOX with immune checkpoint proteins, including PDCD1 (PD-1), CD274 (PD-L1), CTLA4 and LAG3. b Correlation of TOX with T cell exhaustion markers, including TIGIT, HAVCR2, LAYN, and CXCL3. c Correlation of TOX with markers of T cell effector functions, including IFN-γ, IL-2, granzyme B, and perforin. All correlations were determined by using GEPIA2 for analysis of TCGA-BRCA dataset of tumor tissues
Prognostic significance of TOX expression in breast cancer
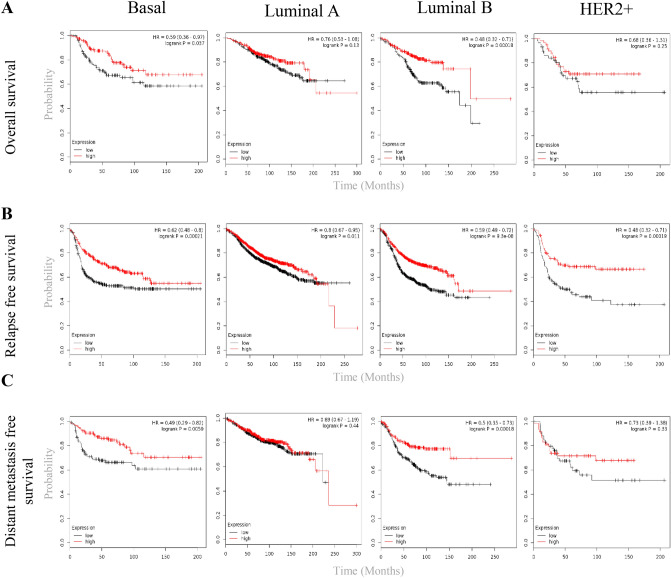
To assess the prognostic importance of TOX mRNA expression in breast cancer, we utilized microarray based gene expression datasets of breast cancer from NCBI-GEO using tool KM-Plotter. We observed a strong association between high TOX mRNA levels and favorable overall survival (Fig. 7a), relapse-free survival (Fig. 7b), and distant metastasis free survival (Fig. 7c) in breast cancer. The number of patients and survival estimates have been summarized in Supplementary Table S5. We further analyzed TCGA breast cancer dataset using the tool GEPIA2 to assess the prognostic significance of the expression of TOX family genes. Similar to GEO datasets, different breast cancer subtypes in TCGA-BRCA dataset also revealed the relation of TOX mRNA overexpression with better overall survival (OS) and relapse-free survival (RFS) while other TOX family members did not show significant association with prognosis (Supplementary Figure S4A, S4B and Table S6). We also performed a survival analysis for cytotoxic T cell marker CD8A in both the datasets (Supplementary Tables S5 and S6). We observed that the survival significance of TOX and CD8A was comparable, with TOX exhibiting better prognostic associations, most notably in the luminal-B subtype. In basal subtype, TOX was better associated with DMFS (Supplementary Table S5), while CD8A was better associated with OS and RFS.
Fig. 7.
Prognostic significance of TOX expression in breast cancer subtypes. Kaplan–Meier survival plots for TOX mRNA expression in different subtypes of breast cancer, including overall survival (a), relapse free survival (b) and distant metastasis free survival (c). All survival analysis were done using GEO datasets in KM-Plotter tool. Mean value of TOX expression from two TOX probes (204529_s_at and 204530_s_at) was used with median TOX expression as group cut-off for survival analysis. All numeric values have been summarized in Supplementary Table S5
Discussion
Immune checkpoint inhibitors-based therapies have been successfully used to treat certain cancers, while their efficacy in the management of other malignancies remains suboptimal. Role of TOX in the regulation of T cell exhaustion during chronic infection and cancer has been extensively documented [15–17, 42]. In the TME, T cell exhaustion is mediated by multiple immune checkpoint proteins such as PD-1, LAG-3, CD224, CD160, T cell immunoglobulin mucin-3 (Tim-3), and CTLA-4 [5]. Most of these checkpoint proteins are transcriptionally upregulated by TOX protein in CD8+ T cells. In hepatocellular carcinoma, downregulation of TOX expression blocks PD-1-mediated CD8+ T cell exhaustion leading to enhancement of sensitivity to anti PD-1 therapy [19].
The main objective of the present study was to evaluate the expression and prognostic significance of TOX in breast cancer. Surprisingly, our analysis revealed a downregulation of TOX mRNA expression in breast tumor tissues compared to tumor adjacent normal tissues. Also events of genetic alterations in TOX gene were observed to be low among breast cancer patients. Since no systematic study has been carried to dissect the cellular origin of TOX in breast tumor tissues, our analysis for the first time reveals the TOX mRNA expression in breast tumor tissues is majorly contributed by T cells. These observations were further corroborated by the absence or weak immunostaining pattern for TOX in tumor cells along with limited expression observed in single cell RNA sequencing data analysis. We further validated immune cell enriched expression of TOX in TCGA-BRCA dataset by relating it with the degree of tumor purity and immune cell infiltration.
Hypermethylation of TOX promoter has previously been reported in breast cancer [22, 41]. Our promoter methylation analysis also revealed significant hypermethylation of the TOX promoter as compared to normal breast tissue in agreement with earlier reports. We also observed that the TOX gene exhibit distinct DNA methylation patterns. In the TOX promoter region, DNA methylation levels were negatively correlated with TOX expression and breast tumors exhibited higher DNA methylation levels compared to adjacent normal breast tissues. On the contrary, gene body methylation was positively correlated with TOX expression and reduced in tumor tissues compared to normal breast tissues. This suggests that distinct DNA methylation patterns at different sites in the TOX gene may regulate TOX gene expression. Also, these results suggest epigenetic regulation as a potential mechanism for the regulation of immune cell activities in breast cancer.
Our immunohistochemical analysis revealed dominant cytoplasmic TOX staining in glandular cells of normal tissue while in breast tumor tissue, we observed a prominent nuclear localization in infiltrating immune cells along with low cytoplasmic staining in both tumor and immune cells. Cytoplasmic localization of TOX has also been reported in the colon and hepatocellular carcinoma [19, 21]. Interestingly, TOX has been demonstrated to physically interact with PD-1 in the endosomal compartment of exhausted CD8+ T cells of hepatocellular carcinoma, thereby preventing its degradation [19]. While our analysis confirms TOX localization in TIICs, the localization and potential molecular functions of cytoplasmic TOX in breast cancer cells are not clear and require detailed exploration. However, it has been previously reported that TOX knockdown does not alter the proliferation or migration of breast cancer cells [22].
Recently, Seo et al. [17] demonstrated the cooperation of TOX and TOX2 with transcription factor NR4A to facilitate T cell exhaustion. Our study revealed a negative correlation between TOX/TOX2 levels and tumor purity. However, their expression exhibit a positive association with each other. In contrast, TOX3 and TOX4 showed positive correlations to tumor purity, which can be attributed to their predominant expression and functions in tumor cells, which has been previously reported [43].
CIBERSORT analysis revealed a positive correlation between TOX mRNA expression and inflammatory TIIC types, being most robust with M1 type macrophages. In agreement with this observation, a strong negative correlation between TOX mRNA and anti-inflammatory cells, such as M2 type macrophages, further strengthened the association between TOX and inflammation. While several studies have focused on the expression and functions of TOX specifically in tumor infiltrating T cells, it is noteworthy that TOX is also expressed by other immune cells and may perform essential functions in those cells as well [44]. Single cell RNA sequencing data analysis revealed lesser TOX expression in cells of myeloid lineage therefore, the correlations observed with macrophages can be attributed to the development of overall inflammatory TME. Also, both single cell analysis and CIBERSORT correlation revealed that TOX expression in breast cancer is contributed by T-cells, and also up to some extent, by B-cells. This also concludes that the immune cell population in the adjacent normal tissues may express higher TOX mRNA than TIICs present in the tumor tissues, which further requires detailed exploration.
TOX being a transcription factor regulates the expression of multiple ICPs, including PD-1 and CTLA-4 [17]. TOX is also known to stabilize PD-1 in CD8+ T cells during T cell exhaustion [19]. Therefore, elevated expression of TOX may also be responsible for increasing levels of ICP expression in breast cancer. In line with these reports, we observed a strong positive correlation of TOX mRNA with the expression of various ICPs, including PD-1 and CTLA-4. Although PD-1 is known to mediate CD8+ T cell exhaustion, we observed a positive correlation between mRNA levels of TOX/PD-1 and T cell effector cytokines such as IFN-γ and IL-2 along with proteases including granzyme B and perforin in breast cancer. These observations are in agreement with a recent study by Egelston et al. [11], which conclusively demonstrated that PD-1 expressing CD8+ T cells retain effector functions in breast cancer.
Survival analysis suggested a strong association of higher TOX mRNA levels with better overall and disease-free survival as well as metastasis-free survival in different subtypes of breast cancer. However, other TOX family members did not display any such correlation with patient survival. Since we also observed a positive correlation between TOX and inflammatory macrophages, our results are in agreement with Jeong et al. [45], who reported a strong association of M1 macrophages with a favorable outcome while M2 macrophages with poor survival in breast cancer.
Thus, our findings suggest that TOX may serve as a novel clinical prognostic marker for breast cancer. Recently, Wang et al. [19] have shown that the TOX levels in peripheral lymphocytes positively correlates with its expression in the tumor infiltrating CD8+ T cells, suggesting its prognostic implications. While our survival analysis is based on mRNA expression data from bulk tumors, further investigation on differential expression of TOX mRNA and protein levels in various tumor infiltrating immune cells in breast cancer may add to its prognostic utility. Therefore, a thorough understanding of cell-specific TOX expression and its overall effect on therapeutic and survival outcomes in breast cancer is highly warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- APCs
Antigen presenting cells
- CNA
Copy number alteration
- CK6
Cytokeratin 6
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- ER
Estrogen receptor
- GEO
Gene Expression Omnibus
- HMG
High mobility group
- HER2
Human epidermal growth factor receptor 2
- HPA
Human Protein Atlas
- ICPs
Immune checkpoint proteins
- PR
Progesterone receptor
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death-ligand 1
- TCGA
The Cancer Genome Atlas
- TOX
Thymocyte selection-associated high mobility group box protein
- TIICs
Tumor infiltrating immune cells
- TME
Tumor microenvironment
Authors’ contributions
MA conceptualized the study. SSC supervised the study and arranged funding. MA, SK, JS and AC performed data curation, interpretation and statistical analysis. SK performed the validation of all results. MA wrote the original manuscript. SK, AC and SSC edited the manuscript. All the authors have approved the manuscript.
Funding
This study was supported by grants from Indian Council of Medical Research (ICMR, India), Grant number 2019-2914 to SSC.
Availability of data and materials
All data sources are freely available to access and have been mentioned in “Materials and methods” section.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salgado R, Loi S. Tumour infiltrating lymphocytes in breast cancer: increasing clinical relevance. Lancet Oncol. 2018;19:3–5. doi: 10.1016/S1470-2045(17)30905-1. [DOI] [PubMed] [Google Scholar]
- 4.Glajcar A, Szpor J, Hodorowicz-Zaniewska D, Tyrak KE, Okoń K. The composition of T cell infiltrates varies in primary invasive breast cancer of different molecular subtypes as well as according to tumor size and nodal status. Virchows Arch. 2019;475:13–23. doi: 10.1007/s00428-019-02568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams S, Gatti-Mays ME, Kalinsky K, Korde LA, Sharon E, Amiri-Kordestani L, Bear H, McArthur HL, Frank E, Perlmutter J, Page DB, Vincent B, Hayes JF, et al. Current landscape of immunotherapy in breast cancer: a review. JAMA Oncol. 2019;5:1205. doi: 10.1001/jamaoncol.2018.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedognetti D, Ceccarelli M, Galluzzi L, Lu R, Palucka K, Samayoa J, Spranger S, Warren S, Wong K-K, Ziv E, Chowell D, Coussens LM, De Carvalho DD, et al. Toward a comprehensive view of cancer immune responsiveness: a synopsis from the SITC workshop. J ImmunoTher Cancer. 2019;7:131. doi: 10.1186/s40425-019-0602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Aranda M, Redondo M. Targeting protein kinases to enhance the response to anti-PD-1/PD-L1 immunotherapy. IJMS. 2019;20:2296. doi: 10.3390/ijms20092296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhercik M, Sanders AJ, Owen S, Davies EL, Sharma AK, Jiang WG, Mokbel K. Clinical significance of PD1 and PDL1 in human breast cancer. Anticancer Res. 2017;37:4249–4254. doi: 10.21873/anticanres.11817. [DOI] [PubMed] [Google Scholar]
- 10.Yeong J, Lim JCT, Lee B, Li H, Ong CCH, Thike AA, Yeap WH, Yang Y, Lim AYH, Tay TKY, Liu J, Wong S-C, Chen J, et al. Prognostic value of CD8+ PD-1+ immune infiltrates and PDCD1 gene expression in triple negative breast cancer. J ImmunoTher Cancer. 2019;7:34. doi: 10.1186/s40425-019-0499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egelston CA, Avalos C, Tu TY, Simons DL, Jimenez G, Jung JY, Melstrom L, Margolin K, Yim JH, Kruper L, Mortimer J, Lee PP. Human breast tumor-infiltrating CD8+ T cells retain polyfunctionality despite PD-1 expression. Nat Commun. 2018 doi: 10.1038/s41467-018-06653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doedens AL, Rubinstein MP, Gross ET, Best JA, Craig DH, Baker MK, Cole DJ, Bui JD, Goldrath AW. Molecular programming of tumor-infiltrating CD8+ T cells and IL15 resistance. Cancer Immunol Res. 2016;4:799–811. doi: 10.1158/2326-6066.CIR-15-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Flaherty E, Kaye J. TOX defines a conserved subfamily of HMG-box proteins. BMC Genomics. 2003;4:13. doi: 10.1186/1471-2164-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aliahmad P, Kaye J. Development of all CD4 T lineages requires nuclear factor TOX. J Exp Med. 2008;205:245–256. doi: 10.1084/jem.20071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, Attanasio J, Yan P, George SM, et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, Trivedi P, Menocal L, Appleby H, Camara S, Zamarin D, Walther T, Snyder A, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571:270–274. doi: 10.1038/s41586-019-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo H, Chen J, González-Avalos E, Samaniego-Castruita D, Das A, Wang YH, López-Moyado IF, Georges RO, Zhang W, Onodera A, Wu C-J, Lu L-F, Hogan PG, et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. PNAS. 2019;116:12410–12415. doi: 10.1073/pnas.1905675116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K, Park S, Kim GM, Park SM, Park SY, Kim DH, Park YM, Koh YW, Kim HR, Ha S-J, Lee I. Single-cell transcriptome analysis revealed a role of the transcription factor TOX in promoting CD8+ T-cell exhaustion in cancer. Cancer Biol. 2019 doi: 10.1101/641316. [DOI] [Google Scholar]
- 19.Wang X, He Q, Shen H, Xia A, Tian W, Yu W, Sun B. TOX promotes the exhaustion of antitumor CD8+ T cells by preventing PD1 degradation in hepatocellular carcinoma. J Hepatol. 2019 doi: 10.1016/j.jhep.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Zeng D, Lin H, Cui J, Liang W. TOX3 is a favorable prognostic indicator and potential immunomodulatory factor in lung adenocarcinoma. Oncol Lett. 2019;18:4144–4152. doi: 10.3892/ol.2019.10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen T, Li Q, Zhang X, Long R, Wu Y, Wu J, Fu X. TOX expression decreases with progression of colorectal cancers and is associated with CD4 T-cell density and Fusobacterium nucleatum infection. Hum Pathol. 2018;79:93–101. doi: 10.1016/j.humpath.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Tessema M, Yingling CM, Grimes MJ, Thomas CL, Liu Y, Leng S, Joste N, Belinsky SA. Differential epigenetic regulation of TOX subfamily high mobility group box genes in lung and breast cancers. PLoS ONE. 2012 doi: 10.1371/journal.pone.0034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katayama MLH, Vieira RAdC, Andrade VP, Roela RA, Lima LGCA, Kerr LM, Campos APd, Pereira CAdB, Serio PAdMP, Encinas G, Maistro S, Petroni MdAL, Brentani MM, et al. Stromal cell signature associated with response to neoadjuvant chemotherapy in locally advanced breast cancer. Cells. 2019 doi: 10.3390/cells8121566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556–W560. doi: 10.1093/nar/gkz430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Díez-Villanueva A, Mallona I, Peinado MA. Wanderer, an interactive viewer to explore DNA methylation and gene expression data in human cancer. Epigenet Chromatin. 2015 doi: 10.1186/s13072-015-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch A, Jeschke J, Van Criekinge W, van Engeland M, De Meyer T. MEXPRESS update 2019. Nucleic Acids Res. 2019;47:W561–W565. doi: 10.1093/nar/gkz445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira B, Chin S-F, Rueda OM, Vollan H-KM, Provenzano E, Bardwell HA, Pugh M, Jones L, Russell R, Sammut S-J, Tsui DWY, Liu B, Dawson S-J, et al. The somatic mutation profiles of 2433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7:11479. doi: 10.1038/ncomms11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefebvre C, Bachelot T, Filleron T, Pedrero M, Campone M, Soria J-C, Massard C, Lévy C, Arnedos M, Lacroix-Triki M, Garrabey J, Boursin Y, Deloger M, et al. Mutational profile of metastatic breast cancers: a retrospective analysis. PLoS Med. 2016;13:e1002201. doi: 10.1371/journal.pmed.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, Bashashati A, Prentice LM, Khattra J, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L, Cortes ML, Fernandez-Lopez JC, Peng S, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–409. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, Nik-Zainal S, Martin S, Varela I, Bignell GR, Yates LR, Papaemmanuil E, Beare D, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, Laird PW, Onofrio RC, Winckler W, Weir BA, Beroukhim R, Pellman D, Levine DA, et al. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. 2012;30:413–421. doi: 10.1038/nbt.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, Treviño V, Shen H, Laird PW, Levine DA, Carter SL, Getz G, Stemke-Hale K, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuckerman NS, Yu HX, Simons DL, Bhattacharya N, Carcamo-Cavazos V, Yan N, Dirbas FM, Johnson DL, Schwartz EJ, Lee PP. Altered local and systemic immune profiles underlie lymph node metastasis in breast cancer patients. Int J Cancer. 2013;132:2537–2547. doi: 10.1002/ijc.27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 37.Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77:e108–e110. doi: 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang T-H, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, Ziv E, Culhane AC, Paull EO, et al. The immune landscape of cancer. Immunity. 2018;48(812–830):e14. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–259. doi: 10.1007/978-1-4939-7493-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 41.Chung W, Kwabi-Addo B, Ittmann M, Jelinek J, Shen L, Yu Y, Issa J-PJ. Identification of novel tumor markers in prostate, colon and breast cancer by unbiased methylation profiling. PLoS ONE. 2008 doi: 10.1371/journal.pone.0002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, Utzschneider DT, von Hoesslin M, Cullen JG, Fan Y, Eisenberg V, Wohlleber D, Steiger K, et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. 2019;571:265–269. doi: 10.1038/s41586-019-1326-9. [DOI] [PubMed] [Google Scholar]
- 43.Seksenyan A, Kadavallore A, Walts AE, de la Torre B, Berel D, Strom SP, Aliahmad P, Funari VA, Kaye J. TOX3 is expressed in mammary ER+ epithelial cells and regulates ER target genes in luminal breast cancer. BMC Cancer. 2015;15:22. doi: 10.1186/s12885-015-1018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aliahmad P, Seksenyan A, Kaye J. The many roles of TOX in the immune system. Curr Opin Immunol. 2012;24:173–177. doi: 10.1016/j.coi.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeong H, Hwang I, Kang SH, Shin HC, Kwon SY. Tumor-associated macrophages as potential prognostic biomarkers of invasive breast cancer. J Breast Cancer. 2019;22:38–51. doi: 10.4048/jbc.2019.22.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data sources are freely available to access and have been mentioned in “Materials and methods” section.