Abstract
Hepatotoxicity is a major immune-related adverse event that may become life-threatening. The impact of adding immune checkpoint blockade (ICB) to systemic therapy on the incidence of hepatotoxicity remains unknown. We performed a systematic review and meta-analysis to compare the incidence of hepatotoxicity among patients with cancer who received therapy with and without addition of ICB. PubMed, Embase, Web of Science, and Cochrane Library were searched to select phase 3 randomized controlled trials (RCTs) evaluating the effect of adding ICB to systemic therapy, placebo, or supportive care. The odds ratio (OR) of any grade and grade 3–5 hepatitis, elevations in aspartate aminotransferase (AST), and alanine aminotransferase (ALT) was pooled for meta-analysis. 43 RCTs with 28,905 participants were analyzed. Addition of ICB increased the incidence of hepatitis (any grade: OR, 2.13, 95% confidence interval [CI] 1.52–2.97, grade 3–5: OR, 2.66, 95% CI 1.72–4.11), elevated AST (any grade: OR, 2.16, 95% CI 1.73–2.70, grade 3–5: OR, 2.72, 95% CI 1.86–3.99), and elevated ALT (any grade: OR, 2.01, 95% CI 1.59–2.54, grade 3–5: OR, 2.40, 95% CI 1.62–3.55). Subgroup analysis based on the ICB mechanism revealed no significant heterogeneity among each mechanism for hepatitis (any Grade: I2 = 11.1%, p for heterogeneity = 0.32, grade 3–5: I2 = 0%, p = 0.48). Adding ICB to systemic therapy increases the incidence of hepatotoxicity regardless of the mechanism of ICB. Hepatotoxicity is common and vigilant monitoring of liver function is required during ICB therapy for patients with cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03203-7.
Keywords: Immune checkpoint blockade, Immune checkpoint inhibitor, Hepatotoxicity, Hepatitis, Immune-related adverse event, Immuno-oncology
Introduction
Recent advances in cancer immunotherapy have resulted in a paradigm shift in oncologic treatment. Immune checkpoint blockade (ICB) has shown promise in treatment of solid tumors. ICB augments systemic antitumor immunity by blocking the inhibitory checkpoints such as cytotoxic T-lymphocyte antigen (CTLA-4) and programmed cell death 1 (PD-1) or its ligand, programmed death-ligand 1 (PD-L1), resulting in improvement in survival time in patients with many types of cancer. ICB causes immune-related adverse events (irAEs) which may result in treatment interruption, morbidity, and mortality. The commonly affected organs are the gastrointestinal tract, skin, endocrine glands, and liver [1, 2]. Incidence of hepatotoxicity has been reported to be 2–10% in patients receiving ipilimumab, nivolumab, and pembrolizumab monotherapy, and 25–30% in patients treated with nivolumab and ipilimumab combination treatment [3–6].
Severe hepatotoxicity is an irAE requiring suspension of ICB and initiation of immunosuppression with high-dose corticosteroids, mycophenolate mofetil, or azathioprine [6]. Meta-analyses have demonstrated higher incidence of hepatotoxicity with use of ICB than chemotherapy [7, 8]. However, the results of these meta-analyses were heterogeneous because PD-1 inhibitor use was associated with higher incidence of hepatotoxicity in one meta-analysis but not in another meta-analysis [9, 10]. Therefore, it is important to elucidate the accurate impact of ICB on hepatotoxicity among patients with cancer. Furthermore, recent development of the combination treatment using ICB with other systemic therapy for solid tumors requires reevaluation of the incidence of hepatotoxicity [11–13]. When more than one ICB agent is used, or ICB is given in combination with cytotoxic chemotherapy or molecular-targeted agents, incidence of irAEs including hepatotoxicity may increase. A meta-analysis evaluating the incidence of hepatotoxicity by adding PD-1 or PD-L1 inhibitors to systemic chemotherapy concluded that PD-1/PD-L1 inhibitors were associated with increased risk of hepatitis but not with elevated aspartate aminotransferase (AST) or elevated alanine aminotransferase (ALT) [8]. This was probably due to the small number of clinical trials included in analysis. Other than hepatotoxicity, another study showed association between addition of ICB to systemic therapy and the incidence of pneumonitis [14]. However, no other studies reported the add-on effect of ICB therapy on hepatotoxicity.
These previous meta-analyses compared chemotherapeutic regimens with various risks of liver toxicity to ICB therapy. Thus, oncologists require clarity regarding the incidence of hepatotoxicity when ICB is added in an anti-neoplastic regimen. We conducted a systematic review and meta-analysis to investigate the add-on effect of ICB on the incidence of hepatotoxicity. The partial result of this research was presented at the Annual Meeting of the American Society of Clinical Oncology in June 2021 [15].
Materials and methods
Data search
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Mata-Analysis (PRISMA) criteria. Database including PubMed, Embase, the Cochrane Library, and Web of Science was used to search available literature as of July 7, 2021. The search strategy is described in supplementary table S1. Only the results of phase 3 randomized clinical trials (RCTs) were pooled in this research. The protocol of this research was registered in PROSPERO (https://www.crd.york.ac.uk/prospero/) on December 17, 2020, as CRD42020221414.
Selection criteria
This is a study of the incidence and severity of hepatotoxicity (hepatitis, elevated AST, and ALT) associated with addition of ICB to a treatment regimen containing systemic chemotherapy, another ICB, or supportive care compared with that of chemotherapy, single-agent ICB, or supportive care.
Studies were selected if they met the following criteria: (1) A published study designed as a phase 3 RCT assessing solid tumors. (2) The experimental group of the study was treated with at least one type of ICB with or without other systemic therapy and the control group was treated with systemic chemotherapy, ICB-plus-placebo or ICB monotherapy if the experimental group contained dual-ICB, best supportive care, placebo, or observation. (3) A study with more than two arms where an immune checkpoint inhibitor was included in at least one arm. (4) A trial that the impact of adding ICB can be assessed. (5) The result of the study was written in English. (6) A study revealed the results of at least grade 1–5 or grade 3–5 hepatitis, elevated AST, or elevated ALT.
Studies that did not meet inclusion criteria were excluded. If results of the same clinical trial were reported in several different articles, the article that is most updated, reporting the higher number of adverse events, or describing treatment-related adverse events (trAEs) or irAEs rather than any adverse events, was included in this meta-analysis. In such cases, consensus was reached between authors Y.F. and N.H. regarding inclusion or exclusion of an article.
Data extraction and risk of bias assessment
Data were extracted from eligible studies by two different investigators (Y.F. and N.H.). The following information was obtained from eligible studies: The name of the first author, publication year, study name, type of cancer, cancer status, the treatment setting, name of ICB added to therapy in the control group, classification of ICB, therapeutic regimens used in a control arm, the incidence of grades 1–5 and grade 3–5 hepatotoxicity (hepatitis, elevated AST, and elevated ALT), and the number of patients. Other diseases such as hepatic failure, hepatic injury, and hepatic events were not extracted. The Cochrane Risk of Bias Tool was utilized for evaluation of the risk of bias in each RCT, which was assessed by two reviewers (Y.F. and N.H.) independently [16]. The number of treated patients and the number of patients who developed grade 1–5 and grade 3–5 hepatotoxicity in each treatment arm were recorded from each RCT. We extracted the amount of each hepatic adverse event. If a study included the information of any adverse events, trAEs and irAEs were prioritized for analysis. If a study included both trAEs and irAEs, the group which contained a higher number was chosen for evaluation. When hepatitis was documented as both laboratory abnormalities and diagnoses, only hepatitis categorized as a diagnosis was extracted for analysis. If a study included more than two comparable arms, we chose only one comparable pair which could evaluate the effect of adding ICB and contained the highest number of patients.
Statistical analyses
The odds ratio (OR) for grade 1–5 and grade 3–5 hepatotoxicity was calculated. A meta-analysis for evaluating the contribution of ICB to the hepatotoxicity incidence was performed using random-effects models. Funnel plots were applied to evaluate publication bias. Significance was set for equivalence hypothesis testing using the two-tailed 0.05 level. The two-tailed 0.10 level and I2 < 50% were used to set the significance for statistical heterogeneity which was assessed by using Cochran Q statistic and I2 statistics. We used RevMan 5.4 for calculating these data [17]. We also conducted subgroup analyses based on a type of ICB (PD-1 inhibitor, PD-L1 inhibitor, and CTLA-4 inhibitor), and based on studies comparing ICB plus chemotherapy with chemotherapy alone. An exploratory analysis of the incidence of fatal AEs due to any reasons associated with addition of ICB was performed by using data extracted from articles included in this meta-analysis for hepatotoxicity.
Results
Study selection
After searching the database with title and abstract screening and duplicate removal, 310 articles were potentially eligible for analysis in this research. After detailed evaluation, 43 RCTs with 28,905 participants were included in the final analysis (Supplementary Fig. 1). Incidence of hepatitis, elevation in AST, and elevation in ALT, were analyzed in 34, 29, and 31 RCTs, respectively.
Study characteristics
Among 43 RCTs, 28 studies investigated the efficacy of ICB-containing regimens for patients with advanced cancer in the first-line setting, and 7 studies evaluated it in the second-line setting or beyond. The efficacy of ICB in the neoadjuvant and adjuvant setting was analyzed in 7 studies. No RCTs compared dual immune checkpoint inhibitors plus conventional therapy with conventional therapy alone. Atezolizumab was evaluated in 12 studies, avelumab in 3, durvalumab in 1, ipilimumab in 9, nivolumab in 5, pembrolizumab in 9, and tremelimumab in 4 studies (Table 1).
Table 1.
List of randomized controlled trials analyzed in this meta-analysis
| First Author | Year | Study | Cancer | Cancer status | Study setting | ICI added | Control arm | Analyzed hepatotoxicity | Patients | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ICI | Control | Ref. | |||||||||
| Bajorin DF | 2021 | CheckMate 274 | Urothelial | Muscle-invasive | Adjuvant | Nivolumab | Placebo | Hepatitis, AST, ALT | 351 | 348 | [27] |
| Bellmunt J | 2021 | IMvigor 010 | Urothelial | Muscle-invasive | Adjuvant | Atezolizumab | Observation | Hepatitis, AST, ALT | 390 | 397 | [28] |
| Boyer M | 2021 | KEYNOTE 598 | NSCLC | Metastatic | 1st line | Ipilimumab | Placebo + pembrolizumab | Hepatitis, AST, ALT | 282 | 281 | [29] |
| Cortes J | 2020 | KEYNOTE 355 | Breast | Advanced | 1st line | Pembrolizumab | GEM/PTX/nab-PTX + CBDCA | ALT | 562 | 281 | [30] |
| Di Giacomo AM | 2021 | NIBIT-M2 | Melanoma | Brain metastasis | 1st line | Ipilimumab | Fotemustine | AST, ALT | 26 | 27 | [31] |
| Eggermont AMM | 2016 | EORTC 18,071 | Melanoma | Resected Stage III | Adjuvant | Ipilimumab | Placebo | AST, ALT | 471 | 474 | [32] |
| Eggermont AMM | 2018 | KEYNOTE 054 | Melanoma | Resected Stage III | Adjuvant | Pembrolizumab | Placebo | Hepatitis | 509 | 502 | [33] |
| Ferris RL | 2020 | EAGLE | Head and Neck | Recurrent or metastatic | 2nd line | Tremelimumab | Durvalumab | ALT | 247 | 240 | [34] |
| Finn RS | 2020 | KEYNOTE 240 | HCC | Advanced | 2nd line | Pembrolizumab | Placebo + BSC | Hepatitis, AST, ALT | 279 | 134 | [35] |
| Galsky MD | 2020 | IMvigor 130 | Urothelial | Metastatic | 1st line | Atezolizumab | Placebo + GEM + CDDP/CBDCA | Hepatitis | 453 | 390 | [36] |
| Govindan R | 2017 | Study 104 | NSCLC | Advanced | 1st line | Ipilimumab | Placebo + CBDCA + PTX | AST, ALT | 388 | 361 | [37] |
| Gutzmer R | 2020 | IMspire 150 | Melanoma | Advanced | 1st line | Atezolizumab | Placebo + Vemurafenib + Cobimetinib | AST, ALT | 230 | 281 | [38] |
| Hodi FS | 2010 | MDX010-20 | Melanoma | Metastatic | 2nd or later line | Ipilimumab | gp100 | Hepatitis, AST, ALT | 380 | 132 | [39] |
| Hodi FS | 2018 | CheckMate 067 | Melanoma | Advanced | 1st line | Ipilimumab | Placebo + Nivolumab | Hepatitis, AST, ALT | 313 | 313 | [40] |
| Horn L | 2018 | IMpower 133 | SCLC | Metastatic | 1st line | Atezolizumab | Placebo + CBDCA + VP-16 | Hepatitis | 198 | 196 | [41] |
| Janjigian YY | 2021 | CheckMate 649 | GEJ | Advanced | 1st line | Nivolumab | CAPOX/FOLFOX | AST, ALT | 782 | 767 | [42] |
| Jotte R | 2020 | IMpower 131 | NSCLC | Advanced | 1st line | Atezolizumab | CBDCA + nabPTX | Hepatitis | 343 | 340 | [43] |
| Kang YK | 2017 | ATTRACTION-2 | Gastric | Advanced | 3rd or later line | Nivolumab | Placebo | Hepatitis, AST, ALT | 330 | 161 | [44] |
| Kelly RJ | 2021 | CheckMate 577 | GEJ | Resected stage II-III | Adjuvant | Nivolumab | Placebo | AST | 532 | 260 | [45] |
| Kwon ED | 2014 | CA184-043 | Prostate | mCRPC | 2nd line | Ipilimumab | Placebo following radiotherapy | Hepatitis, AST, ALT | 393 | 396 | [46] |
| Lee NY | 2021 | JAVELIN Head and Neck | Head and Neck | Locally-advanced | Definitive CRT | Avelumab | Placebo + CRT | Hepatitis, AST, ALT | 348 | 344 | [47] |
| Miles D | 2021 | IMpassion 131 | Breast | Advanced | 1st line | Atezolizumab | Placebo + PTX | Hepatitis, AST, ALT | 432 | 217 | [48] |
| Mittendorf EA | 2020 | IMpassion 031 | Breast | Stage II–III | Adjuvant | Atezolizumab | Placebo + nabPTX—> ADR + CPA | Hepatitis, AST, ALT | 164 | 167 | [49] |
| Moore KN | 2021 | IMagyn 050 | Ovarian | Stage III–IV | 1st line | Atezolizumab | Placebo + PTX + CBDCA + Bevacizumab | Hepatitis | 644 | 642 | [50] |
| Nishio M | 2020 | IMpower 132 | NSCLC | Stage IV | 1st line | Atezolizumab | CDDP/CBDCA + PEM | Hepatitis | 291 | 274 | [51] |
| Paz-Ares L | 2018 | KEYNOTE 407 | NSCLC | Metastatic | 1st line | Pembrolizumab | Placebo + CBDCA + PTX/nabPTX | Hepatitis | 278 | 280 | [13] |
| Paz-Ares L | 2019 | CASPIAN* | SCLC | Extended | 1st line | Durvalumab | CDDP/CBDCA + VP-16 | Hepatitis, AST**, ALT** | 265 | 266 | [52, 53] |
| Planchard D | 2020 | ARCTIC | NSCLC | Metastatic | 3rd or later line | Tremelimumab | Durvalumab | Hepatitis, AST, ALT | 173 | 117 | [54] |
| Powles T1 | 2020 | DANUBE | Urothelial | Advanced | 1st line | Tremelimumab | Durvalumab | Hepatitis, AST, ALT | 340 | 345 | [55] |
| Powles T2 | 2020 | JAVELIN Bladder 100 | Bladder | Advanced | 1st line (maintenance) | Avelumab | GEM + CDDP/CBDCA—> BSC | Hepatitis, AST, ALT | 350 | 350 | [56] |
| Powles T | 2021 | KEYNOTE 361 | Urothelial | Advanced | 1st line | Pembrolizumab | CDDP/CBDCA + GEM | Hepatitis, AST, ALT | 349 | 342 | [57] |
| Pujade-Lauraine E | 2021 | JAVELIN Ovarian 200 | Ovarian | Advanced | 2nd or later line | Avelumab | PLD | Hepatitis, AST, ALT | 182 | 177 | [58] |
| Reck M | 2016 | CA184-156 | SCLC | Extended | 1st line | Ipilimumab | CDDP/CBDCA + VP-16 | AST, ALT | 478 | 476 | [59] |
| Rizvi NA | 2020 | MYSTIC | NSCLC | Metastatic | 1st line | Tremelimumab | Durvalumab | Hepatitis | 372 | 374 | [60] |
| Robert C | 2011 | CA184-024 | Melanoma | Advanced | 1st line | Ipilimumab | Dacarbazine | Hepatitis, AST, ALT | 247 | 251 | [61] |
| Rodriguez-Abreu D | 2021 | KEYNOTE 189* | NSCLC | Metastatic | 1st line | Pembrolizumab | Placebo + CDDP/CDBCA + PEM | Hepatitis, ALT*** | 405 | 202 | [62, 63] |
| Rudin CM | 2020 | KEYNOTE 604 | SCLC | Metastatic | 1st line | Pembrolizumab | Placebo + VP-16 + CDDP/CBDCA | Hepatitis | 223 | 223 | [64] |
| Schmid P1 | 2020 | IMpassion 130 | Breast | Advanced | 1st line | Atezolizumab | Placebo + nab-PTX | Hepatitis, AST, ALT | 453 | 437 | [65] |
| Schmid P2 | 2020 | KEYNOTE 522 | Breast | Stage II–III | Neoadjuvant | Pembrolizumab | Placebo + CBDCA + PTX—> AC/EC | Hepatitis, AST, ALT | 781 | 389 | [66] |
| Shitara K | 2020 | KEYNOTE 062 | Gastric | Advanced | 1st line | Pembrolizumab | Placebo + 5-FU/Capecitabine + CDDP | Hepatitis | 250 | 244 | [67] |
| Socinski MA | 2018 | IMpower 150 | NSCLC | Metastatic | 1st line | Atezolizumab | CBDCA + PTX + Bevacizumab | Hepatitis, AST, ALT | 393 | 394 | [68] |
| Sugawara S | 2021 | TASUKI-52 | NSCLC | Advanced | 1st line | Nivolumab | Placebo + CBDCA + PTX + Bevacizumab | Hepatitis | 273 | 275 | [69] |
| West H | 2019 | IMpower 130 | NSCLC | Metastatic | 1st line | Atezolizumab | CBDCA + nabPTX | Hepatitis, AST, ALT | 473 | 232 | [70] |
*Data from two different articles were used for analysis. **Data from Goldman et al. were used for analysis. ***Data from Gandhi et al. were used for analysis
5-FU 5-fluorouracil; AC adriamycin + cyclophosphamide; ADR adriamycin; AST aspartate aminotransferase; ALT alanine aminotransferase; BSC best supportive care; CAPOX capecitabine + oxaliplatin; CBDCA carboplatin; CDDP cisplatin; CPA cyclophosphamide; CRT chemoradiotherapy; EC epirubicin + cyclophosphamide; FOLFOX 5-fluorouracil + oxaliplatin; GEJ gastroesophageal junction; GEM gemcitabine; HCC hepatocellular carcinoma; mCRPC metastatic castration-resistant prostate cancer; nabPTX nab-paclitaxel; NSCLC non-small cell lung cancer; PEM pemetrexed; PLD pegylated liposomal doxorubicin; PTX paclitaxel; SCLC small cell lung cancer; VP-16 etoposide
Meta-analysis of hepatitis
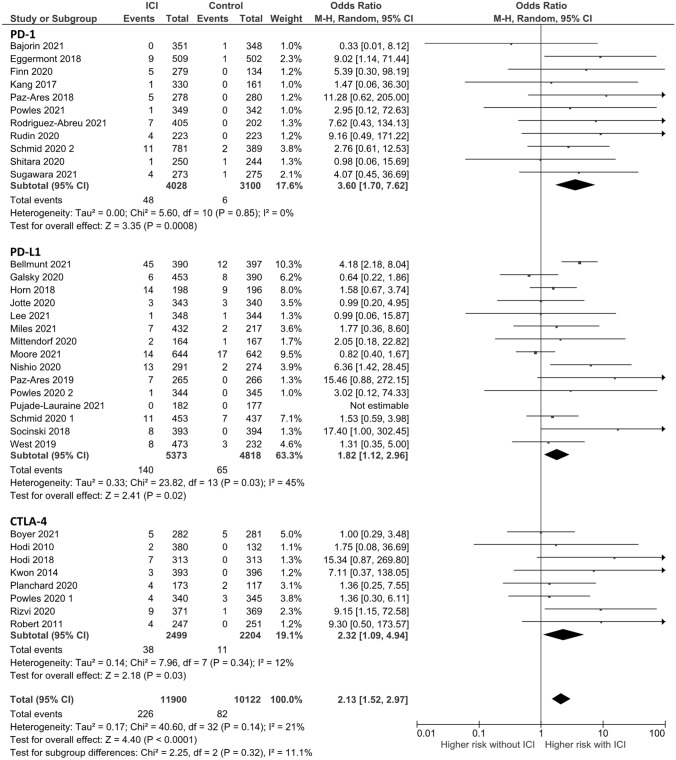
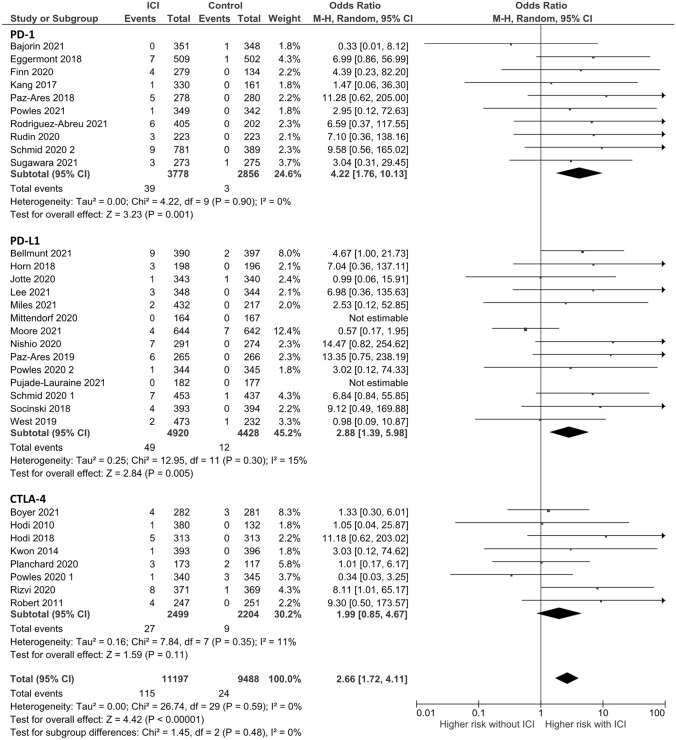
For any-grade hepatitis, 34 RCTs were analyzed with 226 events in the experimental group and 82 events in the control group. Addition of ICB to systemic therapy used in the control group was associated with an increase in the incidence of any-grade hepatitis (OR: 2.13, 95% confidence interval [CI] 1.52–2.97, p < 0.0001) (Fig. 1). For grade 3 or more hepatitis, 32 RCTs were analyzed with 115 events in the ICB group and 24 events in the control group. Addition of ICB was associated with an increase in the incidence of severe hepatitis (OR: 2.66, 95% CI 1.72–4.11, p < 0.0001) (Fig. 2).
Fig. 1.
Forest plot of any-grade hepatitis based on the mechanism of immune checkpoint blockade. ICI, immune checkpoint inhibitor; CI, confidence interval; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1; CTLA-4, cytotoxic T-lymphocyte antigen
Fig. 2.
Forest plot of grade 3–5 hepatitis based on the mechanism of immune checkpoint blockade. ICI, immune checkpoint inhibitor; CI, confidence interval; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1; CTLA-4, cytotoxic T-lymphocyte antigen
Subgroup analysis according to ICB mechanism revealed an increase in the incidence of any-grade hepatitis with addition of PD-1 inhibitors, PD-L1 inhibitors, and CTLA-4 inhibitors (OR, 3.60, 95% CI 1.70–7.62; OR 1.82, 95% CI 1.12–2.96; OR, 2.32, 95% CI 1.09–4.94, respectively). There was no significant heterogeneity among each subgroup (I2 = 11.1%, p for heterogeneity = 0.32) (Fig. 1). PD-1 inhibitors and PD-L1 inhibitors were associated with an increase in the incidence of grade 3 or more hepatitis (OR, 4.22, 95% CI 1.76–10.13; OR 2.88, 95% CI 1.39–5.98); however, CTLA-4 inhibitors were not (OR, 1.99, 95% CI 0.85–4.67). There was no significant heterogeneity among each subgroup (I2 = 0%, p for heterogeneity = 0.48) (Fig. 2).
Meta-analysis of elevations in liver enzymes
For any-grade and grade 3–5 AST elevation, 28 and 29 RCTs were analyzed with 1059 and 234 events in the experimental group, and with 476 and 59 events in the control group. For any-grade and grade 3–5 ALT elevation, 30 and 31 RCTs were assessed with 1292 and 332 events in the experimental group, and with 590 and 102 events in the control group. Addition of ICB to systemic therapy used in the control group was associated with an increase in the incidence in any-grade and grade 3–5 elevation in AST and ALT (Any-grade AST elevation: OR, 2.16, 95% CI 1.73–2.70, grade 3–5 AST elevation: OR, 2.72, 95% CI 1.86–3.99, any-grade ALT elevation: OR, 2.01, 95% CI 1.59–2.54, grade 3–5 ALT elevation: OR, 2.40, 95% CI 1.62–3.55). Subgroup analysis based on the mechanism of ICB revealed each subtype was associated with an increase in the incidence of these hepatotoxicities (Supplementary Fig. 2–5).
Subgroup analysis of studies comparing ICB plus chemotherapy with chemotherapy alone
The incidence of hepatitis and elevations in AST and ALT among studies that compared ICB and chemotherapy with chemotherapy alone was also analyzed. Addition of ICB to chemotherapy was associated with an increase in the incidence of any-grade hepatitis and grade 3–5 hepatitis (OR, 1.87, 95% CI 1.22–2.85; OR, 3.05, 95% CI 1.63–5.70), any-grade and grade 3–5 elevation in AST (OR, 2.03, 95% CI 1.43–2.90; OR, 2.92, 95% CI 1.47–5.82), and any-grade and grade 3–5 elevation in ALT (OR, 1.82, 95% CI 1.34–2.47; OR, 2.21, 95% CI 1.24–3.95) (Supplementary Fig. 6–11).
Subgroup analysis of trials comparing ICB with placebo or supportive care
Among 43 trials, 8 RCTs compared ICB monotherapy with placebo or supportive care In this setting, ICB monotherapy was associated with an increase in the incidence of any-grade hepatitis and grade 3–5 hepatitis (OR, 4.04, 95% CI 2.29–7.12; OR, 3.39, 95% CI 1.34–8.58), any-grade and grade 3–5 elevation in AST (OR, 2.40, 95% CI 1.79–3.22; OR, 2.97, 95% CI 1.49–6.06), and any-grade and grade 3–5 elevation in ALT (OR, 3.00, 95% CI 1.93–4.65; OR, 3.92, 95% CI 1.58–9.75).
Exploratory analysis of fatal adverse events based on the mechanism of immune checkpoint blockade
The incidence of fatal AEs of any causes by adding ICB to systemic therapy was analyzed. Information of fatal AEs was available in 43 RCTs included in the meta-analysis for hepatotoxicity. Addition of ICB to systemic therapy was associated with an increase in the incidence of fatal AEs (OR, 1.64, 95% CI 1.27–2.13). Each mechanism of ICB showed tendency to increase the incidence of fatal AEs and there was no significant heterogeneity among these subgroup (I2 = 0%, p for heterogeneity = 0.55) (Supplementary Fig. 12).
Risk of bias and publication bias assessment
The publication bias was evaluated using funnel plots shown in supplementary Fig. 13. Each funnel plot was relatively symmetrical and no obvious publication bias was observed. The summary of risk of bias for each trial is shown in supplementary Fig. 14. All studies included in this meta-analysis were RCTs and the overall risk of bias was low. Lack of blinding was seen in 16 RCTs.
Discussion
This systematic review and meta-analysis demonstrated that addition of ICB to systemic therapy such as chemotherapy or another ICB or to placebo or supportive care was associated with greater hepatotoxicity than regimens including chemotherapy alone, single-agent ICB, or supportive care. Hepatotoxicity is common during ICB-containing therapy, and therefore, vigilant monitoring of liver function tests is required while patients with advanced cancer receive ICB therapy. This is the most comprehensive analysis of the add-on effect of ICB on the incidence and severity of hepatic adverse events.
Hepatotoxicity has been variably defined in RCTs evaluating ICB but most RCTs define hepatitis as an immune-related adverse event and identify elevations in transaminase levels as a treatment-related adverse event. To comprehend the overall hepatotoxicity and eliminate potential observer bias that hepatotoxicity tends to be reported more in the ICB treatment group, this meta-analysis analyzes both hepatitis and elevated transaminase levels.
Subgroup analysis according to ICB mechanism revealed an increase in the incidence of hepatitis and transaminase elevation accompanied addition of each ICB subtype to a treatment regimen. In this analysis, CTLA-4 inhibitors were the only ICB subtype not associated with an increase in the incidence of severe hepatitis but heterogeneity was not observed among subgroups. Data regarding the difference in hepatotoxicity among each class of ICB are limited. Previous studies showed the incidence of immune-mediated hepatotoxicity is relatively low in PD-1 inhibitor use (0.7–2.1%), and intermediate in PD-L1 inhibitor use and standard-dose CTLA-4 inhibitor use (0.9–12%) [18]. Histopathologic findings in ICB-related hepatotoxicity may vary between PD-1/PD-L1 and CTLA-4 inhibitors, which could explain the different incidence of hepatotoxicity in each mechanism of ICB [19–22]. However, a relationship between differing histopathologic appearance and clinical incidence of hepatotoxicity associated with ICB subtypes has not yet been established. Our meta-analysis did not directly compare ICBs of differing mechanisms. Future research may better elucidate the risk of hepatotoxicity associated with various ICB mechanisms.
Clinical factors conferring an increased risk for ICB-related hepatotoxicity have not yet been established yet. A retrospective review of patients with autoimmune disease and melanoma treated with a CTLA-4 inhibitor demonstrated an association with irAEs, however, immune-mediated hepatitis was not observed in the study cohort [23]. Prior incidence of irAE from ICB is also associated with an increase in the risk of other irAEs, but the incidence of hepatitis in this setting remains unclear [24]. A retrospective study of patients with malignancy treated with ICB suggested prior use of ICB and female sex were associated with increased risk of grade 3–5 immune-mediated hepatitis [25]. Our study showed that the addition of ICB to systemic therapy increased the risk of hepatotoxicity, however, our data are not sufficient to address risk factors for this condition. Further research to identify clinical risk factors for hepatotoxicity is needed.
Additionally, our exploratory analysis showed addition of ICB was associated with an increased incidence of fatal toxicity. Causes of fatal toxicity in this analysis were not limited to hepatotoxicity but including any grade 5 AEs. One meta-analysis showed the incidence of fatal toxicity due to ICB use occurred early after therapy initiation [26]. Therefore, careful monitoring for AEs especially soon after initiation of ICB-containing regimens is required.
Our study has several limitations. First, though this meta-analysis includes more than 40 RCTs, a number of malignancies including colorectal cancer, renal cell carcinoma, and hematologic malignancies were not analyzed in this research. This is because trials of these cancer types contained a different agent in the experimental and control group and did not meet inclusion criteria in this meta-analysis. Caution should be exercised when applying the results of the study to treatment of these malignancy types. Second, the unclear definition of hepatitis may lead to under or overestimation of the incidence of hepatitis in each trial. The majority of RCTs in this meta-analysis reported hepatitis under the category of irAE or AE of special interest, suggesting potential observer bias regarding the incidence of hepatitis. However, more than half of the clinical trials in this meta-analysis are double-blind placebo-controlled trials, mitigating the potential for bias. Furthermore, the incidence of hepatitis is consistent with that of transaminase elevation, which was categorized as treatment-related adverse effect. Third, though our meta-analysis suggests that addition of ICB to an anti-neoplastic regimen may increase the incidence of hepatotoxicity, a network meta-analysis would be necessary to compare the impact of each mechanism of ICB on this adverse effect.
Conclusion
The addition of ICB to a systemic treatment regimen was associated with an increase in the incidence of hepatitis, severe hepatitis, and elevation in transaminase levels among patients with solid tumors regardless of the mechanism of ICB. Hepatotoxicity is common during ICB therapy, and therefore, clinicians should maintain vigilance for hepatotoxicity while patients with advanced cancer are treated with an ICB-containing therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- CI
Confidence interval
- CTLA-4
Cytotoxic T-lymphocyte antigen
- ICB
Immune checkpoint blockade
- irAE
Immune-related adverse event
- OR
Odds ratio
- PD-1
Programmed cell death 1
- PD-L1
Programmed death-ligand 1
- PRISMA
Preferred reporting items for systematic reviews and mata-analysis
- RCT
Randomized clinical trial
- trAE
Treatment-related adverse event
Author contributions
YF had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: YF, NH, HN. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: YF, NH, MH. Critical revision and final approval of the manuscript: All authors. Supervision: NH, MH, MDG.
Funding
This research was not supported by a specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information.
Declarations
Conflict of interest
None of the authors have a conflict of interest to report for the submitted work. M.D.G. reports stock from Rappta Therapeutics; a consulting/advisory role for BioMotiv, Janssen, Dendreon, Merck, GlaxoSmithKline, Lilly, Astellas Pharma, Genentech, Bristol-Myers Squibb, Novartis, Pfizer, EMD Serono, AstraZeneca, Seattle Genetics, Incyte, Aileron Therapeutics, Dracen, Inovio Pharmaceuticals, NuMab, Dragonfly Therapeutics, Basilea, Urogen, Infinity Pharmaceuticals, and Gilead; and institutional research funding from Janssen Oncology, Dendreon, Novartis, Bristol-Myers Squibb, Merck, AstraZeneca, and Genentech/Roche.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 2.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/jco.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim RA, Berman DM, Depril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29:8583. doi: 10.1200/jco.2011.29.15_suppl.8583. [DOI] [Google Scholar]
- 4.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/s0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziemer M, Koukoulioti E, Beyer S, Simon JC, Berg T. Managing immune checkpoint-inhibitor-induced severe autoimmune-like hepatitis by liver-directed topical steroids. J Hepatol. 2017;66:657–659. doi: 10.1016/j.jhep.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/jco.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin LL, Lin GF, Yang F, Chen XQ. A systematic review and meta-analysis of immune-mediated liver dysfunction in non-small cell lung cancer. Int Immunopharmacol. 2020;83:106537. doi: 10.1016/j.intimp.2020.106537. [DOI] [PubMed] [Google Scholar]
- 8.Guo X, Li W, Hu J, Zhu EC, Su Q. Hepatotoxicity in patients with solid tumors treated with PD-1/PD-L1 inhibitors alone, PD-1/PD-L1 inhibitors plus chemotherapy, or chemotherapy alone: systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76:1345–1354. doi: 10.1007/s00228-020-02903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Ran Y, Wang K, Zhu Y, Li J. Incidence and risk of hepatic toxicities with PD-1 inhibitors in cancer patients: a meta-analysis. Drug Des Devel Ther. 2016;10:3153–3161. doi: 10.2147/dddt.S115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Lie P, Guo M, He J. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis of published data. Int J Cancer. 2017;141:1018–1028. doi: 10.1002/ijc.30678. [DOI] [PubMed] [Google Scholar]
- 11.Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 12.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 13.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara Y, Horita N, Namkoong H, Galsky MD. The effect of adding immune checkpoint inhibitors on the risk of pneumonitis for solid tumours: a meta-analysis of phase III randomised controlled trials. Eur J Cancer. 2021;150:168–178. doi: 10.1016/j.ejca.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara Y, Horita N, Namkoong H, Miyashita H, Harrington M, Galsky MD. Incidence of hepatitis associated with addition of immune checkpoint blockade to conventional solid tumor therapy: a meta-analysis of phase 3 randomized clinical trials. J Clin Oncol. 2021;39:2645. doi: 10.1200/JCO.2021.39.15_suppl.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Review Manager (RevMan) [Computer program]. Version 5.4 TCC (2020)
- 18.Peeraphatdit TB, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR. Hepatotoxicity from immune checkpoint inhibitors: a systematic review and management recommendation. Hepatology. 2020;72:315–329. doi: 10.1002/hep.31227. [DOI] [PubMed] [Google Scholar]
- 19.De Martin E, Michot JM, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68:1181–1190. doi: 10.1016/j.jhep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Johncilla M, Misdraji J, Pratt DS, Agoston AT, Lauwers GY, Srivastava A, Doyle LA. Ipilimumab-associated Hepatitis: clinicopathologic characterization in a series of 11 cases. Am J Surg Pathol. 2015;39:1075–1084. doi: 10.1097/pas.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 21.Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol. 2018;31:965–973. doi: 10.1038/s41379-018-0013-y. [DOI] [PubMed] [Google Scholar]
- 22.Simonelli M, Di Tommaso L, Baretti M, Santoro A. Pathological characterization of nivolumab-related liver injury in a patient with glioblastoma. Immunotherapy. 2016;8:1363–1369. doi: 10.2217/imt-2016-0057. [DOI] [PubMed] [Google Scholar]
- 23.Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016;2:234–240. doi: 10.1001/jamaoncol.2015.4368. [DOI] [PubMed] [Google Scholar]
- 24.Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28:368–376. doi: 10.1093/annonc/mdw443. [DOI] [PubMed] [Google Scholar]
- 25.Kitagataya T, Suda G, Nagashima K, et al. Prevalence, clinical course, and predictive factors of immune checkpoint inhibitor monotherapy-associated hepatitis in Japan. J Gastroenterol Hepatol. 2020;35:1782–1788. doi: 10.1111/jgh.15041. [DOI] [PubMed] [Google Scholar]
- 26.Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajorin DF, Witjes JA, Gschwend JE, et al. adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384:2102–2114. doi: 10.1056/NEJMoa2034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellmunt J, Hussain M, Gschwend JE, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:525–537. doi: 10.1016/s1470-2045(21)00004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyer M, Şendur MAN, Rodríguez-Abreu D, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%: randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol. 2021;39:2327–2338. doi: 10.1200/jco.20.03579. [DOI] [PubMed] [Google Scholar]
- 30.Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/s0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 31.Di Giacomo AM, Chiarion-Sileni V, Del Vecchio M, et al. Primary analysis and 4-year follow-up of the phase III NIBIT-M2 trial in melanoma patients with brain metastases. Clin Cancer Res. 2021;27:4737–4745. doi: 10.1158/1078-0432.Ccr-21-1046. [DOI] [PubMed] [Google Scholar]
- 32.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 34.Ferris RL, Haddad R, Even C, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31:942–950. doi: 10.1016/j.annonc.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind Phase III Trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/jco.19.01307. [DOI] [PubMed] [Google Scholar]
- 36.Galsky MD, Arija JÁA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1547–1557. doi: 10.1016/s0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 37.Govindan R, Szczesna A, Ahn MJ, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35:3449–3457. doi: 10.1200/jco.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 38.Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835–1844. doi: 10.1016/s0140-6736(20)30934-x. [DOI] [PubMed] [Google Scholar]
- 39.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492. doi: 10.1016/s1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 41.Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 42.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/s0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15:1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 44.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/s0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 45.Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384:1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 46.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/s1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee NY, Ferris RL, Psyrri A, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22:450–462. doi: 10.1016/s1470-2045(20)30737-3. [DOI] [PubMed] [Google Scholar]
- 48.Miles D, Gligorov J, André F, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32:994–1004. doi: 10.1016/j.annonc.2021.05.801. [DOI] [PubMed] [Google Scholar]
- 49.Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090–1100. doi: 10.1016/s0140-6736(20)31953-x. [DOI] [PubMed] [Google Scholar]
- 50.Moore KN, Bookman M, Sehouli J, et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39) J Clin Oncol. 2021;39:1842–1855. doi: 10.1200/jco.21.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishio M, Barlesi F, West H, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16:653–664. doi: 10.1016/j.jtho.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 52.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/s0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 53.Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22:51–65. doi: 10.1016/s1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 54.Planchard D, Reinmuth N, Orlov S, et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol. 2020;31:609–618. doi: 10.1016/j.annonc.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21:1574–1588. doi: 10.1016/s1470-2045(20)30541-6. [DOI] [PubMed] [Google Scholar]
- 56.Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 57.Powles T, Csőszi T, Özgüroğlu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:931–945. doi: 10.1016/s1470-2045(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 58.Pujade-Lauraine E, Fujiwara K, Ledermann JA, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 2021;22:1034–1046. doi: 10.1016/s1470-2045(21)00216-3. [DOI] [PubMed] [Google Scholar]
- 59.Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34:3740–3748. doi: 10.1200/jco.2016.67.6601. [DOI] [PubMed] [Google Scholar]
- 60.Rizvi NA, Cho BC, Reinmuth N, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020;6:661–674. doi: 10.1001/jamaoncol.2020.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 62.Rodríguez-Abreu D, Powell SF, Hochmair MJ, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32:881–895. doi: 10.1016/j.annonc.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 64.Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38:2369–2379. doi: 10.1200/jco.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/s1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 66.Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 67.Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571–1580. doi: 10.1001/jamaoncol.2020.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 69.Sugawara S, Lee JS, Kang JH, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2021;32:1137–1147. doi: 10.1016/j.annonc.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 70.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/s1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information.