Abstract
Chimeric antigen receptor (CAR)-modified T (CAR-T) cell therapy has been proven to be a powerful tool for the treatment of cancer, however, the limits are obvious, especially for solid tumors. Therefore, constantly optimizing the structure of CAR to improve its therapeutic effect is necessary. In this study, we generated three different third-generation CARs targeting IL13Rα2, with the same scFv, but different transmembrane domains (TMDs) from CD4, CD8 or CD28 (IL13-CD4TM-28.BB.ζ, IL13-CD8TM-28.BB.ζ and IL13-CD28TM-28.BB.ζ). CARs were transduced into primary T cells using retroviruses. The anti-GBM efficacy of CAR-T cells was monitored by flow cytometry and real-time cell analysis (RTCA) in vitro and examined in two xenograft mouse models. The differentially expressed genes related to different anti-GBM activity were screened by high throughput RNA sequencing. We observed that T cells transduced with these three CARs have similar anti-tumor activity when co-cultured with U373 cells which expressed higher IL13Rα2 but exhibited different anti-tumor activity when co-cultured with U251 cells that expressed lower IL13Rα2. All the three groups of CAR-T cells can be activated by U373 cells, but only IL13-CD28TM-28.BB.ζ CAR-T cells could be activated and expressed increased IFN-γ after co-culturing with U251 cells. IL13-CD28TM-28.BB.ζ CAR-T cells exhibited the best anti-tumor activity in xenograft mouse models which can infiltrate into the tumors. The superior anti-tumor efficacy of IL13-CD28TM-28.BB.ζ CAR-T cells was partially owing to differentially expressed extracellular assembly, extracellular matrix, cell migration and adhesion-related genes which contribute to the lower activation threshold, increased cell proliferation, and elevated migration capacity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03423-5.
Keywords: Glioblastoma, Chimeric antigen receptor-modified T cell, IL13Rα2
Introduction
Glioblastoma (GBM) is one of the most common primary malignant brain tumors worldwide that is virtually incurable with conventional therapies. Even with the best multimodal therapy, the median survival rate is only about 21 months and no substantial improvements in the overall survival rate in the past half century [1]. For most patients, the failure of treatment is largely due to the recurrence of aggressive drug-resistant malignant cells. Current knowledge suggests that the continued growth and survival of tumors is partly due to the failure to produce an effective immune response. Therefore, adoptive immunotherapy is a promising treatment [2].
Chimeric antigen receptor (CAR)-modified T cells provide a new approach to treat GBM, which can rapidly expand in vitro and express receptors that can specifically recognize antigenically distinct tumor populations by genetic engineering [3, 4]. They can migrate into brain parenchyma through chemokines to target and kill tumor cells [5, 6]. The clinical successes of CAR-T cell-mediated CD19+ hematological malignancies provide support for the further development of this therapy [7, 8]. Studies have found that CD19-CAR-T cells can reduce the incidence rate of metastatic brain leukemia [9, 10]. However, the application of CAR-T cell therapy in the treatment of brain tumors is still in the early stage.
IL-13 receptor α 2 (IL13Rα2) is a monomeric high affinity IL-13 receptor, which is expressed by stem cell like, highly differentiated malignant cells [11] and tumor infiltrating macrophages/ myeloid suppressor cells [12]. IL13Rα2 has the property of no significant expression in normal brain tissues [13, 14], and overexpression in more than 50% GBM patients, which makes it to be a potent target for CAR-T therapy against GBM [15]. Early clinical trials support the safety and tolerability of IL13Rα2 vaccine therapy [16] and IL-13-immunotoxin [17] in the treatment of GBM, especially the first-generation IL13ζ CAR-T cells and the second-generation IL13BBζ CAR-T cells for the treatment of recurrent glioblastoma, make IL13Rα2 as an attractive target for immunotherapy [18–22].
CAR-T cell therapy has been proven to be a breakthrough therapy with potent and sustained anti-tumor activity, but the occurrence of side effects and the low efficacy in solid tumors have gradually become apparent as a challenge. So tremendous efforts have been dedicated to optimizing the CAR structure. Here, we developed three different third-generation IL13Rα2-specific CARs, with the same scFv, but different transmembrane domains. We compared the anti-tumor activity of T cells transduced with these CARs in vitro and in xenograft mouse models, and partially unveiled the mechanisms contribute to better anti-tumor activity.
Materials and Methods
Cell Lines
The human GBM cell lines U251, U373 and the retrovirus packaging cell lines PG13 and Phoenix ECO were purchased from the American Tissue Culture Collection (ATCC). U251 and U373 were evaluated the expression of IL13Rα2 (APC-conjugated antihuman IL13Ra2, BD Biosciences) through flow cytometry and engineered to express the eGFP and firefly luciferase (GL) through retroviral transduction. The GBM cell lines were well cultured in DMEM media (Lonza) containing 10% fetal bovine serum (FBS, Biosera), 100 U/mL penicillin and 100 μg/mL streptomycin (EallBio Life Sciences). The retroviral producer cell lines were cultured in DMEM media containing 10% FBS without penicillin and streptomycin.
Generation of retroviral vectors encoding IL13Rα2-specific CAR
Three different IL13Rα2-targeted CARs were generated. IL13-CD4TM-28.BB.ζ CAR is composed of a CD4 transmembrane domain, IL13-CD8TM-28.BB.ζ CAR is composed of CD8 transmembrane domains, while IL13-CD28TM-28.BB.ζ CAR is composed of CD28 transmembrane domain. These three third-generation CARs are similar to the previously described second-generation IL13BBζ CAR and recognize IL13Rα2 through a single site (E13Y)-modified membrane-tethered IL-13 ligand and have the intracellular signaling domain of CD28 and 4-1 BB in series with CD3ζ [20]. CD28 and 4-1BB as the costimulatory sequence have been suggested to promote persistence of CAR-T cells [23].
Generation of CAR-T cells
Human peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by gradient centrifugation using Ficoll solution (GE healthcare). T cells in PBMCs were stimulated with anti-CD3/CD28 T cell Activator Dynabeads (Invitrogen). After 48 h of bead activation, T cells were transduced with retroviral supernatants by centrifugation on Retronectin (Takara)–coated plates. Transfection experiments were performed using a Calcium Phosphate Transfection Kit (Sigma) according to the instructions with minor modifications. On day 7, the T cells were subjected to flow cytometry to detect the CAR expression. The CAR positive cells were quantified by staining with FITC-labeled goat anti-mouse IgG(H + L) antibodies (Sigma) followed by flow cytometry analysis. T cells were cultured in X-VIVO-15 medium with 5% human AB serum (SIGMA), 100U/ml IL-2, 100 U/ml penicillin and 100 μg/ml streptomycin (EallBio Life Sciences). This study was approved by the institutional review committee of Beijing Shijitan Hospital and informed consent was obtained from all participants.
Carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution assay
CAR-T cells from three healthy donors were used. The cells were co-cultured with U251 cell lines, and T cell proliferation was analyzed using flow cytometry based on CFSE dye dilution. The CFSE-labeled CAR-T cells were co-cultured in triplicate at an E: T ration of 10: 1, with a density of 1× 106: 1× 105 cells/ml in a round bottom 24-well plate. Cells were well cultured in a humidified incubator at 37 °C and 5% CO2. T cell proliferation was determined by CFSE dye dilution of CD3 positive cells using BD FacsCanto II Plus instrument (BD Biosciences). The Flow cytometry data were analyzed using Flow Jo v.10 software (Tree star, Inc. Ashland, OR).
Cytotoxicity assay
Different IL13Ra2-targeted CAR-T cells were co-cultured with U373-GL or U251-GL at multiple E: T ratios in a 96-well plate. Non-transduced (NT) T cells served as controls. After 24 h, PerkinElmer photochemical imaging system was used to collect the signal, the intensity of fluorescence signal was analyzed by living imaging analysis system to evaluate the cytotoxicity of CAR-T cells. CAR-T cells were also co-cultured with U373-GL or U251-GL at 10: 1 ratio overnight to detect the target cells using flow cytometry analysis.
Real-time cell analysis (RTCA)
The xCELLigence RTCA system (Roche Applied Science, Basel, Switzerland) was used to evaluate the cytotoxicity of CAR-T cells. This system based on an electrical impedance readings of gold plate sensor electrode at the bottom of cytotoxicity plate (E-16 plate). Human GBM cells U251 and U373 were seeded in an E-plate with a density of 10,000 cells per well. After 24 h, 100,000 CAR-T cells were added in the E-16 plate to incubate with the human GBM cells and monitored every 15 min to obtain the cell index for 48 h. Every independent experiment was performed in triplicate. RTCA software was used to automatically calculate the interval slope and evaluate the change rate of cell index. To demonstrate the effect of treatments, the cell index was normalized to an equal value at the normalization time point.
Cytokine production assays
CAR-T cells were co-cultured with the human GBM cell lines at an E: T ratio of 10: 1 for 24 h. The expression of human interferon gamma (IFN-γ), tumor necrosis factor-a (TNF- α), IL4, IL-6, IL-10 and IL17A in the supernatant from co-cultured cells were assessed using commercial Cytometric Bead Array (CBA) kits (BD Biosciences) according to the procedure of the manufacturer.
For intracellular cytokine staining assay, CAR-T cells were co-cultured with target cells at a 10: 1 ratio in 24-well microplates with Golgi Stop (BD Bioscience). After 24 h, the cells were harvested and incubated with antihuman IFN-γ (BD Pharmingen, clone B27) antibodies and then measured through flow cytometry analysis.
Transcriptome sequencing
On the 12th day of culture, CAR-T cells were collected and co-cultured with U251 cells at an E: T ratio of 10: 1. Four hours later, CD4 and CD8 CAR-T cells were separated by using Dynabeads ™ CD4 positive isolation kit and Dynabeads ™ CD8 positive isolation kit (Invitrogen, Thermo Fisher Science) according to the manufacturer’s instructions. The co-cultured mixed cells, sorted CD4 CAR-T cells, CD8 CAR-T cells and U251 cells were sent to Annoroad gene technology Co., Ltd (Beijing, China) for RNA sequencing. RNA extraction, construction of a cDNA library and sequencing were strictly in accordance with transcriptome sequencing criteria.
The differentially expressed genes between two CAR-T cells were subjected to gene ontology (GO) analysis using an online bioinformatics tool: Database for Annotation, Visualization, and Integrated Discovery (DAVID) Bioinformatics Resources 6.8. Data visualization and analysis were processed by custom R studio scripts following the packages (ggplot2 and Treemap). Fisher’s exact test was used for the gene-enrichment analysis.
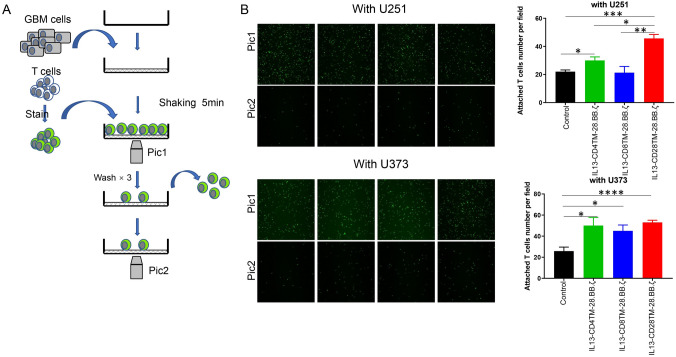
Cell binding assay
CAR-T cells were first labeled with CFSE, and then co-cultured with U373 and U251 cells. After 5 min, unconjugated CAR-T cells were washed with PBS. The CAR-T cells adhered to the tumor were displayed and counted by fluorescence microscope.
Xenograft mouse model with glioma cells intracerebral implantation
Six- to eight-week-old NOD-SCID mice were purchased from Charles River Laboratories. Mice were anesthetized with a ketamine/xylazine cocktail solution and secured in a stereotaxic head frame. A 1 cm midline scalp incision was made, and 2×105 U373-eGFP-luc or U251-eGFP.Luc cells in 5 μL PBS were injected into the left striatum (coordinates: 2.5 mm lateral and 0.5 mm posterior to the bregma) through a burr hole in the skull using a 10 μL BD syringe to deliver tumor cells to a 3.5-mm intraparenchymal depth. The burr hole in the skull was sealed with bone wax, and the incision was closed using medical adhesive glue (COMPONT). Five days after tumor cell injection, 2×106 or 3×106 CAR-T cells were injected through the tail vein. Tumor development was monitored using the IVIS in vivo imaging system (IVIS, Xenogen, Alameda, CA, USA). All experiments with mice were approved by the Beijing Shijitan Hospital Institutional Review Board.
Immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) sections of tumor specimens were deparaffinized and then incubated with rabbit anti-CD3 or rabbit anti-IL13Rα2 primary antibody (Cell Signaling Technology) at 4 °C overnight. After incubation with HRP- conjugated goat anti-rabbit secondary antibody, immunostaining was visualized with DAB chromogen (Sigma-Aldrich) and Mayer’s hematoxylin following the manufacturer’s instructions. The images were obtained using a microscopy (Nikon).
Statistical analysis
Data analysis was performed with GraphPad Prism 7 software (GraphPad Software, San Diego, CA). Data are presented as mean ± SEM using One-way ANOVA to evaluate the difference. Overall survivals of mice with GBM xenografts were measured using the Kaplan-Meier method, with Cox proportional hazard regression analysis for group comparison. Statistically significant was established when p < 0.05.
Results
IL13Rα2 is overexpressed in glioma cell lines
It has been reported that IL13Rα2 is highly expressed in GBM patients (more than 50%), but not in normal brain tissues, indicating that IL13Rα2 is a candidate target for CAR-T therapy. To evaluate the treatment potential of using IL13Rα2 specific CAR-T cells against glioblastoma, two glioma cell lines U373 and U251 were subjected to flow cytometry to examine the IL13Rα2 level on the cell surface. As shown in Fig.S1A, the expression of IL13Ra2 was 36.1% on the surface of U251 cells and 90.9% on the surface of U373 cells.
Development of the third-generation IL13 Rα2 CAR-T cells
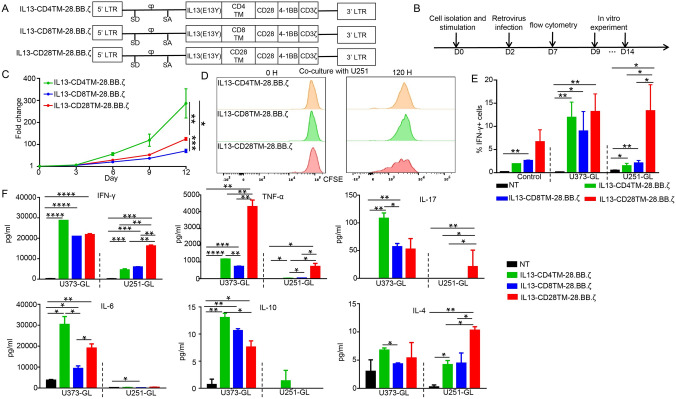
CAR-T cell therapy has been developed to treat multiple kinds of cancers, but the research for optimizing CAR structures was never stopped. To understand the impacts of transmembrane domains on CAR’s function, we generated three IL13Rα2 specific CARs containing different transmembrane domains derived from CD4, CD8 or CD28 (Fig. 1A). Primary T cells were stimulated and then isolated from healthy donor peripheral blood using anti-CD3 and anti-CD28 beads. The T cells were infected with one of the retroviruses and then subjected to flow cytometry analysis 5 days after infection to examine the efficiency. As shown in Fig. S1B, CARs were successfully transduced into T cells. Cells were collected for experiments among the 9th-14th day. The detailed process is shown in Fig. 1B. All these CAR-T cells exhibited good viability and proliferation capacity, with IL13-CD4TM-28.BB.ζ CAR-T cells having the best expansion (Fig. 1C).
Fig. 1.
Engineering and Characterization of CAR-T Cells. A Schematic diagram of three different cDNA open reading frames of chimeric antigens targeting IL13Rα2. B Schematic of the manufacturing process. C The expansion of CAR-T cells without the stimulation of target cells. Cells were counted once every 3 days for a total of 12 days. D The expansion of CAR-T cells with the stimulation of target cells. E The percentage of IFN-γ positive cells. F The release of cytokines in the co-culture process. GL: eGFP and luciferase
T cells transduced with IL13-CD28TM-28.BB.ζ CAR exhibit higher sensitivity for IL13Rα2 in vitro
In order to further compare the anti-tumor activity of these three different CAR-T cells, we firstly evaluate the cell growth and cytokine release of these CAR-T cells after co-culturing with tumor cells. As shown in Fig. 1D, IL13-CD28TM-28.BB.ζ CAR-T cells have the best expansion. Meanwhile, the proportion of IFN-γ positive cells was all increased after co-culturing with U373 cells, but only IL13-CD28TM-28.BB.ζ CAR-T cells had the significantly increased IFN-γ expression after co-culturing with U251 cells (Fig. 1E). Similar phenomenon was observed in the cytokines level of the medium after 24 h co-culture. The level of IFN-γ, TNF-α, IL-17 and IL-4 was increased in the medium after co-culturing with U373 cells, but only IL13-CD28TM-28.BB.ζ CAR-T cells had the significantly increased IFN-γ, TNF-α, IL-17 and IL-4 levels after co-culturing with U251 cells. These results suggested that IL13-CD28TM-28.BB.ζ CAR-T cells had a lower “activation threshold”.
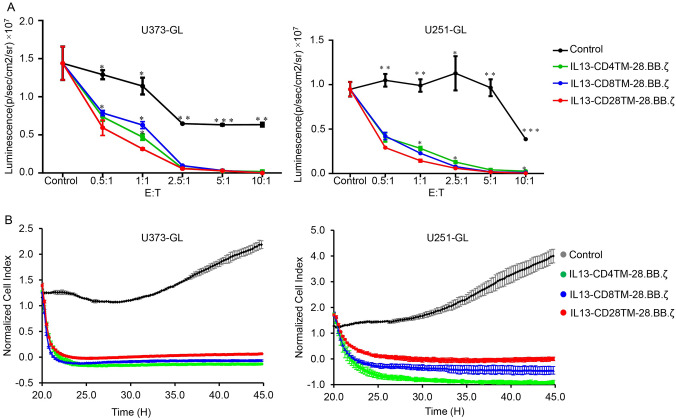
To further evaluate the cell killing capacity, CAR-T cells were co-cultured with U251-GL or U373-GL cells at the effector to target (E: T) ratio of 1: 1, 5: 1 and 10: 1 for 24 h, and the cell killing capacity was determined by measuring the luminescence signal. As shown in Fig. 2A, all these three groups of CAR-T cells significantly repressed the luminescence signal when compared with NT group, and IL13-CD28TM-28.BB.ζ CAR-T cells had the strongest repression effect, especially at low E: T ratios (0.5: 1 and 1: 1). The results from real-time cell growth monitoring (RTCA) system and flow cytometry indicated that all these three groups of CAR-T cells had significantly elevated anti-tumor activity at 10: 1 E: T ratio, but there was no significant difference within these three CAR-T groups (Fig. 2B and Fig. S2).
Fig. 2.
Comparison of Anti-tumor Effects of the three Different CAR-T Cells In Vitro. A The killing ability of CAR-T cells was measured by examining the luciferase activity. B The killing ability of CAR-T cells was detected by RTCA system. GL: eGFP and luciferase
IL13-CD28TM-28.BB.ζ CAR-T showed a better anti-tumor efficacy in intracranial mouse model
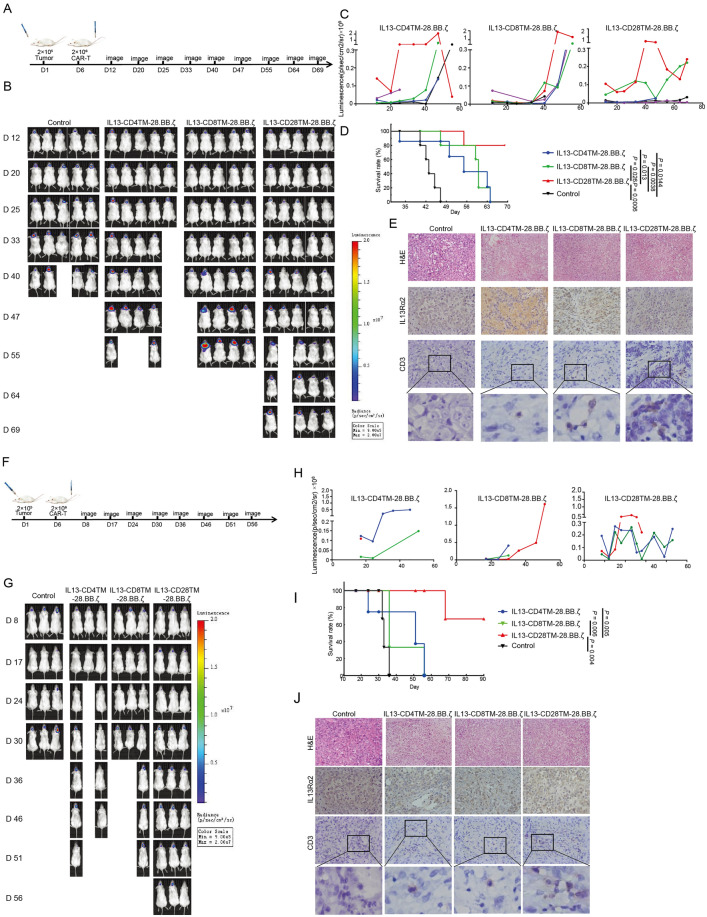
To evaluate the anti-tumor effects of these three groups of CAR-T cells, Xenograft mouse model with glioma cells intracranial implantation was successfully constructed.
Six days after tumor cell implantation, CAR-T cells were injected into mice through tail vein and the tumor growth was monitored through detecting the fluorescence signal. As shown in Fig. 3A–D, IL13-CD28TM-28.BB.ζ CAR-T cells treated U373-GL injected mice had the longest survival which reached to more than 90 days. Meanwhile, CAR-T cells could be found infiltrated into tumor tissues, but the control T cells could not. Similar results were observed in U251-GL generated xenograft mouse model. As shown in Fig. 3 E–H, mice treated by IL13-CD28TM-28.BB.ζ CAR-T cells had the highest survival rate, and CAR-T cells could infiltrate into U251 cells generated tumors, while control T cells could not.
Fig. 3.
IL13-CD28TM-28.BB.ζ CAR-T Cells Mediate Superior Anti-Tumor Efficacy in vivo. A Schematic diagram of the construction and treatment process of U373-GL NSG mouse model. B Representative mice from each group showing relative U373-GL tumor burden over time using Xenogen Living Image. C Each linear line represents the luminescence value for each mouse from U373-GL NSG mice model over time. D Kaplan–Meier survival analysis demonstrating improved survival for U373-GL mice model treated with CAR-T cells. p value was calculated by using the log rank (Mantel–Cox) test. E Immunohistochemistry was used to determine the IL13Rα2 level in tumors and the T cells infiltration. F Schematic diagram of the construction and treatment process of U251-GL NSG mouse model. G Representative mice from each group showing relative U251-GL tumor burden over time using Xenogen Living Image. H Each linear line represents the luminescence value for each mouse from U251-GL NSG mice model over time. I Kaplan–Meier survival analysis demonstrating improved survival for U251-GL mice model treated with CAR-T cells. p value was calculated by using the log rank (Mantel–Cox) test. J Immunohistochemistry was used to determine the IL13Rα2 level in tumors and the T cells infiltration
Altered genes expression profile contributed to enhanced anti-tumor efficacy of IL13-CD28TM-28.BB.ζ CAR-T cells
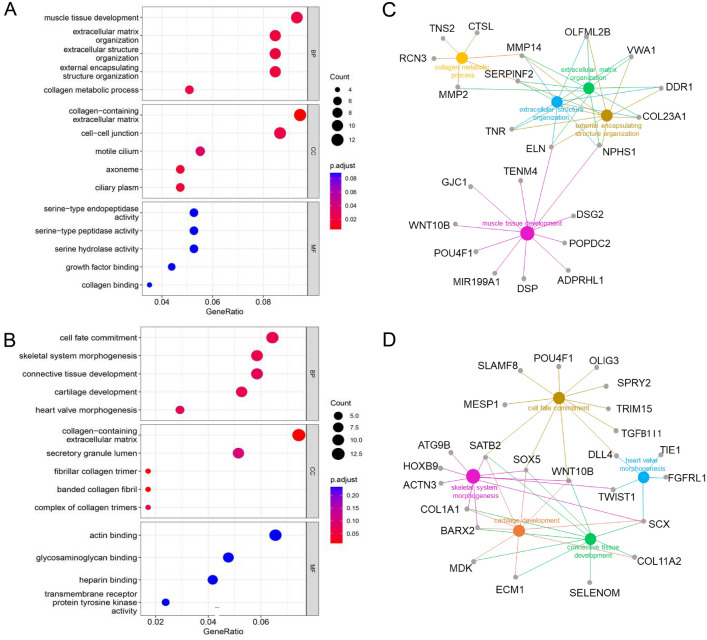
To explore the mechanism through which IL13-CD28TM-28.BB.ζ CAR-T cells exhibit the best anti-tumor efficacy in vivo, the CAR-T cells after 4 h co-culture with U251 cells were firstly separated into CD4+ T cells and CD8+ T cells, and then subjected to high throughput sequencing to examine the differentially expressed genes. As shown in Fig. S3, 413 genes were upregulated in IL13-CD28TM-28.BB.ζ CAR transfected CD4+ cells, and 509 genes were upregulated in IL13-CD28TM-28.BB.ζ CAR transfected CD8+ cells compared with the cells that were transfected with CARs using CD8TM or CD4TM. The results of GO analysis indicated that most of the upregulated genes in IL13-CD28TM-28.BB.ζ CAR-T cells were localized in the extracellular region and functionally enriched in extracellular matrix assembly and disassociation (Fig. 4A and B). Meanwhile, the GO enrichment plot indicated that MMP14, SERPINF2, ELN and NPHS1 are the hub genes that are involved in the four key GO terms in CD4 + cells (Fig. 4C). While, six upregulated genes (COLA1, SCX, BARX2, SATB2, SOX5 and WNT10B) are involved in at least three GO terms in CD8 + cells and function as hub genes (Fig. 4D).
Fig. 4.
Transcriptional analysis of CAR-T cells with different transmembrane domain. A The differentially expressed genes in CD4+ IL13-CD4TM-28.BB.ζ, IL13-CD8TM-28.BB.ζ and IL13-CD28TM-28.BB.ζ CAR-T cells were subjected to gene ontology (GO) analysis using an online bioinformatics tool: DAVID Bioinformatics Resources 6.8. B The differentially expressed genes in CD8+ IL13-CD4TM-28.BB.ζ, IL13-CD8TM-28.BB.ζ and IL13-CD28TM-28.BB.ζ CAR-T cells were subjected to gene ontology (GO) analysis using an online bioinformatics tool: DAVID Bioinformatics Resources 6.8. C–D Protein–protein interaction analysis of up-regulation expressed genes in CD4+ C and CD8+ D IL13-CD828BBζ CAR-T cells. BP, Biological Processes; CC, Cellular Components; MF, Molecular Functions
High affinity binding of IL13-CD28TM-28.BB.ζ CAR to IL13Rα2 triggers differentially expressed genes
Recently, researchers reported that replacing a CD8-hinge and transmembrane domain with a CD28-hinge and TMD, lowers the threshold for CAR activation to CD19 [24]. So, we hypothesized that CAR-T cells with the CD28 TMD have stronger affinity to IL13Rα2 when compared with CAR-T cells with the TMD from CD4 or CD8 and can easily be activated by tumor cells expressing lower IL13Rα2 density like U251 cells. So, we used a simple cell binding assay to examine the capacity of CAR-T cells recognize tumor cells in vitro (Fig. 5A). As shown in Fig. 5B, more IL13-CD28TM-28.BB.ζ CAR-T cells bound with U251 cells after 5 min incubation, but the numbers of these three groups of CAR-T cells bound with U373 cells were similar.
Fig. 5.
IL13-CD28TM-28.BB.ζ CAR-T cells increased affinity to IL13Rα2. A A schematic diagram of cell binding assay. B Cell binding assay. Results were analyzed by One-way ANNOVA. p < 0.05 was considered as significant. *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
T cell therapy was first applied in treatment of hematologic B cell malignancies and exhibited effective and encouraging results [25, 26]. However, CAR-T cell treatment for solid tumors has shown limited anti-tumor activity and is still in the experimental stage. Meanwhile, the occurrence of side effects and the low efficacy in solid tumors have gradually become apparent as a challenge. So tremendous efforts have been dedicated to optimize the CAR structure. The transmembrane domains of CD8 and CD28 were firstly chosen for CAR design because they are relative inert compared to the CD3ζ-derived transmembrane domain [27]. Subsequently, Dongrui Wang et al. constructed IL13Rα2-CAR using CD4 transmembrane and identified that CD4+ CAR-T cells are key player for long-term anti-tumor response for effective CAR-T therapy [28]. In this study, we compared the anti-tumor efficacy of CARs using one of these three commonly used transmembrane domains incorporate to the same scFv in solid tumor for the first time. We found IL13Rα2 CAR-T cell containing CD28 transmembrane domain have the best anti-GBM activity in vivo, which provide a good choice to optimize the CAR structure for GBM immunotherapy.
Several groups have compared the anti-tumor efficacy of T cells transduced with different CARs, and recently, the function of TMD and HD (hinge domain) are partially unveiled [24, 29, 30]. MC Ramello et al. compared a PSCA-specific second-generation CAR, containing the CD28 TMD and costimulatory domains linked to CD3ζ, and a third-generation CAR of the same antigen specificity containing the CD8 transmembrane sequence linked to both the CD28 and 4-1BB costimulatory domains and CD3ζ [29]. They found that the second-generation CAR with CD28 TMD has superior anti-tumor capacity than the third-generation CAR. Subsequently, Muller YD et al. analyzed the roles of TMD (CD8 or CD28) in Anti-CD19-CAR [30]. They found that CARs containing a CD28-TMD, but not a CD8-TMD, could form heterodimers with the endogenous CD28 in human T cells which could modulate CAR-T cell activities in vitro. Our findings confirmed their findings in CAR-T cells when treating solid tumor in vivo. We found that IL13Rα2-targeted CAR-T cells with CD28 TMD has significantly increased cell proliferation and migration, indicating that the heterodimer induced by CD28 TMD made T cells more easily to be activated.
Some studies have shown that CAR-T cells still maintain tumor killing activity even when antigen density on the tumor cells is very low [31–33]. However, Dr. Mackall recently reported that the CAR-T cells cytotoxicity evidence in vitro is not sufficient for in vivo activity evaluation [24]. The antigen density on tumor cell surface must be above the thresholds to sustain effective antigen-antibody interaction, and then trigger the cytokine production and proliferation of CAR-T cells. They also reported that replacing a CD8-hinge and transmembrane domain with a CD28-hinge and transmembrane domain (TMD) lowers the threshold for CAR activation to CD19. In this study, T cells transduced with IL13-CD4TM-28.BB.ζ or IL13-CD8TM-28.BB.ζ CAR had significantly increased intracellular IFN-γ level after being co-cultured with U373 cells but no increased intracellular IFN-γ level after being co-cultured with U251 cells. That may be caused by the relatively low IL13Rα2 level on the U251 cells, and CAR-T cells with CD4 or CD8 TMD needed a higher threshold for activation. Meanwhile, IL13-CD28TM-28.BB.ζ CAR-T cells expressed similar IFN-γ before co-cultured with GBM cells and exhibited similar anti-tumor activity in vitro as T cells transduced with IL13-CD4TM-28.BB.ζ or IL13-CD8TM-28.BB.ζ CAR. However, IL13-CD28TM-28.BB.ζ CAR-T cells had the best anti-tumor activity in vivo, that may be caused by CARs incorporating a CD28-transmembrane domain induced a more stable and efficient immunologic interaction and reduced the threshold for activation.
To confirm our hypothesis above, we developed a cell adhesion detection assay to examine the tumor cells recognition capacity. We observed similar cell interactions between these three groups of CAR-T cells with U373 cells, but different interactions with U251 cells indicating that TMDs alter the CAR-T cells tumor cell binding capacity, which exhibited when co-cultured with tumor cells expressed less antigen.
The following transcriptome sequencing data supported our hypothesis above. The differentially expressed genes in IL13-CD28TM-28.BB.ζ CAR-T cells are enriched in the GO terms including extracellular space, extracellular matrix organization, cell fate commitment and downstream T cell activation-related pathways. The increased cell proliferation, migration, activation of tyrosine kinase and transcriptional activator activity may be induced by stronger interaction between IL13-CD28TM-28.BB.ζ CAR-T cells and tumor cells, and also may contribute to the better anti-tumor efficacy in vivo.
In conclusion, T cells transduced with IL13Rα2-CAR-T using CD28 transmembrane domain had the best anti-GBM activity in vivo owing to lower activation threshold and altered transcriptome.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Authors’ contributions
AG designed experiments, collected and analyzed data, processed the pictures and wrote manuscripts; YB participated in flow cytometry analysis; FW participated in cytokine detection; CZ participated in preparation of CAR-T cells; CX participated in mouse experiments; ZA and YZ participated in cell culture; SZ participated in statistical analysis; YH and XZ supervised the project and designed the study. All authors read and approved the final manuscript.
Funding
Beijing Municipal Science and Technology Commission, Brain Science Research Fund (Z16110000021636).
Availability of data and materials
Data available within the article or its supplementary materials.
Declarations
Conflict of interest
No competing interest.
Ethical approval and consent to participate
This research was approved by the Beijing Shijitan Hospital Institutional Review Board.
Consent for publication
Written informed consent was obtained from all the participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aiqin Gu, Yue Bai and Can Zhang have contributed equally to this work.
Contributor Information
Yi Hu, Email: huyi3795@bjsjth.cn.
Xiaosong Zhong, Email: zhongxiaosong7113@bjsjth.cn.
References
- 1.Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide versus maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA, J Am Med Assoc. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bielamowicz K, Khawja S, Ahmed N. Adoptive cell therapies for glioblastoma. Front Oncol. 2013;3:275. doi: 10.3389/fonc.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed N, Salsman VS, Kew Y, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16:474–485. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampson JH, Choi BD, Sanchez-Perez L, et al. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20:972–984. doi: 10.1158/1078-0432.CCR-13-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong JJ, Rosenberg SA, Dudley ME, et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16:4892–4898. doi: 10.1158/1078-0432.CCR-10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaghoubi SS, Jensen MC, Satyamurthy N, et al. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6:53–58. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadelain M, Brentjens R, Riviere I, Park J. CD19 CAR therapy for acute lymphoblastic Leukemia. Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Annu Meet. 2015;35:e360–363. doi: 10.14694/EdBook_AM.2015.35.e360. [DOI] [PubMed] [Google Scholar]
- 8.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–2635. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in Leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramson JS, McGree B, Noyes S, et al. Anti-CD19 CAR T cells in CNS diffuse large-B-Cell lymphoma. N Engl J Med. 2017;377:783–784. doi: 10.1056/NEJMc1704610. [DOI] [PubMed] [Google Scholar]
- 11.Brown CE, Starr R, Aguilar B, et al. Stem-like tumor-initiating cells isolated from IL13Ralpha2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18:2199–2209. doi: 10.1158/1078-0432.CCR-11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fichtner-Feigl S, Terabe M, Kitani A, et al. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Can Res. 2008;68:3467–3475. doi: 10.1158/0008-5472.CAN-07-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK. Expression of interleukin-13 receptor alpha2 in glioblastoma multiforme: implications for targeted therapies. Can Res. 2007;67:7983–7986. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- 14.Joshi BH, Puri RA, Leland P, et al. Identification of interleukin-13 receptor alpha2 chain overexpression in situ in high-grade diffusely infiltrative pediatric brainstem glioma. Neuro Oncol. 2008;10:265–274. doi: 10.1215/15228517-2007-066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng J, Zhang J, Yang YZ, et al. IL13RA2 is overexpressed in malignant gliomas and related to clinical outcome of patients. Am J Transl Res. 2020;12:4702–4714. [PMC free article] [PubMed] [Google Scholar]
- 16.Iwami K, Shimato S, Ohno M, et al. Peptide-pulsed dendritic cell vaccination targeting interleukin-13 receptor alpha2 chain in recurrent malignant glioma patients with HLA-A*24/A*02 allele. Cytotherapy. 2012;14:733–742. doi: 10.3109/14653249.2012.666633. [DOI] [PubMed] [Google Scholar]
- 17.Kunwar S, Prados MD, Chang SM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25:837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 18.Brown CE, Badie B, Barish ME, et al. Bioactivity and safety of il13ralpha2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21:4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown CE, Aguilar B, Starr R, et al. Optimization of IL13Ralpha2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther J Am Soc Gene Therapy. 2018;26:31–44. doi: 10.1016/j.ymthe.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K, Gwak HS, Han N, et al. Chimeric antigen receptor T cells with modified interleukin-13 preferentially recognize IL13Ralpha2 and suppress malignant glioma: a preclinical study. Front Immunol. 2021;12:715000. doi: 10.3389/fimmu.2021.715000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin Y, Rodriguez JL, Li N, et al. Locally secreted BiTEs complement CAR T cells by enhancing killing of antigen heterogeneous solid tumors. Mol Ther. 2022;30:2537–2553. doi: 10.1016/j.ymthe.2022.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4–1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majzner RG, Rietberg SP, Sotillo E, et al. Tuning the antigen density requirement for CAR T-cell activity. Cancer Discov. 2020;10:702–723. doi: 10.1158/2159-8290.CD-19-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridgeman JS, Hawkins RE, Bagley S, Blaylock M, Holland M, Gilham DE. The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J Immunol. 2010;184:6938–6949. doi: 10.4049/jimmunol.0901766. [DOI] [PubMed] [Google Scholar]
- 28.Wang D, Aguilar B, Starr R, Alizadeh D, Brito A, Sarkissian A, Ostberg JR, Forman SJ, Brown CE. Glioblastoma-targeted CD4+ CAR T cells mediate superior antitumor activity. JCI Insight. 2018 doi: 10.1172/jci.insight.99048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramello MC, Benzaid I, Kuenzi BM, et al. An immunoproteomic approach to characterize the CAR interactome and signalosome. Sci Signal. 2019;12:568. doi: 10.1126/scisignal.aap9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller YD, Nguyen DP, Ferreira LMR, et al. The CD28-transmembrane domain mediates chimeric antigen receptor heterodimerization with CD28. Front Immunol. 2021;12:639818. doi: 10.3389/fimmu.2021.639818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe K, Terakura S, Martens AC, et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. J Immunol. 2015;194:911–920. doi: 10.4049/jimmunol.1402346. [DOI] [PubMed] [Google Scholar]
- 32.Stone JD, Aggen DH, Schietinger A, Schreiber H, Kranz DM. A sensitivity scale for targeting T cells with chimeric antigen receptors (CARs) and bispecific T-cell Engagers (BiTEs) Oncoimmunology. 2012;1:863–873. doi: 10.4161/onci.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nerreter T, Letschert S, Gotz R, et al. Super-resolution microscopy reveals ultra-low CD19 expression on myeloma cells that triggers elimination by CD19 CAR-T. Nat Commun. 2019;10:3137. doi: 10.1038/s41467-019-10948-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available within the article or its supplementary materials.