Abstract
Background
This study aimed to assess the efficacy and safety of geptanolimab (GB226), a fully humanized, recombinant anti-programmed cell death-1 monoclonal antibody, in Chinese patients with refractory or relapsed (r/r) primary mediastinal large B-cell lymphoma (PMBCL).
Methods
This was a multicenter, open-label, single-arm phase II study (Gxplore-003), conducted at 43 hospitals in China (NCT03639181). Patients received geptanolimab intravenously at a dose of 3 mg/kg every 2 weeks until documented confirmed disease progression, intolerable toxicity, or any other cessation criteria was met. The primary endpoint was objective response rate (ORR) in the full analysis set assessed by the independent review committee (IRC) according to the Lugano Classification 2014.
Results
This study was prematurely terminated due to the slow rate of patient accrual. Between Oct 15th, 2018 and Oct 7th, 2020, 25 patients were enrolled and treated. By the data cutoff date on Dec 23rd, 2020, the IRC-assessed ORR was 68.0% (17/25; 95% confidence interval [CI] 46.5–85.1%), with the complete response rate of 24%. The disease control rate was 88% (22/25; 95%CI 68.8–97.5%). Median duration of response was not reached (NR) (95%CI, 5.62 months to NR), with 79.5% of patients having response durations of more than 12 months. Median progression-free survival was NR (95%CI, 6.83 months to NR). Treatment-related adverse events (TRAEs) were reported in 20 of 25 (80.0%) patients, and grade 3 or higher TRAEs occurred in 11 of 25 (44%) patients. No treatment-related deaths occurred. The immune-related adverse events (irAEs) of any grade were observed in 6 (24.0%) patients, and no grade 4 or grade 5 irAEs were reported.
Conclusion
Geptanolimab (GB226) demonstrated promising efficacy and a manageable safety profile in Chinese patients with r/r PMBCL.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03467-7.
Keywords: Geptanolimab (GB226), Efficacy, Safety, Primary mediastinal large B-cell lymphoma, Relapsed/refractory
Introduction
Primary mediastinal large B-cell lymphoma (PMBCL) represents a relatively rare and distinct subtype of large B-cell lymphoma, accounting for only 2–3% of all non-Hodgkin lymphomas (1). Clinically, PMBCL usually occurs in adolescents and young adults, with a female preponderance, and typically presents with a bulky, anterior mediastinal mass. Molecularly, PMBCL is truly distinct from diffuse large B-cell lymphoma, and shares substantial similarity with classic Hodgkin lymphoma (cHL) (2, 3).
Generally, initial treatment of PMBCL includes rituximab and an anthracycline-containing chemotherapy regimen, with cure rates of 85–90% across studies (4–7). Despite the favorable outcomes to frontline immunochemotherapy, 10–30% of PMBCL patients have refractory or relapsed (r/r) disease (8, 9). Clinical outcomes for patients with r/r PMBCL are dismal, with the objective response rate (ORR) to salvage chemotherapy of 25% and estimated overall survival (OS) probability at 2 years after diagnosis of r/r disease of only 15% (10). Previous retrospective studies have provided evidence that autologous stem cell transplant (ASCT) after high-dose chemotherapy may bring survival benefit for r/r PMBCL, especially for those with chemo-sensitive disease (11, 12). However, chemo-refractory relapse to second-line therapy was associated with inferior OS and progression-free survival (PFS) compared with chemo-sensitive relapse (12). Like cHL, PMBCL usually expresses CD30, albeit with heterogeneous levels (13). Unexpectedly, a phase 2 clincial trial demonstrated that brentuximab vedotin, an anti-CD30 antibody drug conjugate, yielded poor antitumor activity in r/r PMBCL, with an ORR of only 13.3% (14). Chimeric antigen receptor (CAR) T-cell therapies have demonstrated durable responses in r/r large B-cell lymphoma including PMBCL after failure of at least two lines of therapy (15–18). However, this therapeutic approach harbors a distinct toxicity profile, which limits its eligibility for many patients. Meanwhile, CAR T-cell therapy had not been approved by China National Medical Products Administration (NMPA) at the time of patient enrollment in this study. Thus, there is an unmet medical need to explore more effective therapeutic strategies or novel agents for patients with r/r PMBCL.
Genetic alterations in programmed death ligand (PD-L) locus at 9p24.1, also frequently observed in PMBCL, lead to increased expression of PD-L1/PD-L2, thus potentially rendering PMBCL sensitive to anti-programmed cell death-1 (PD-1) therapy (19, 20). The phase IB KEYNOTE-013 and phase II KEYNOTE-170 study demonstrated promising efficacy and a manageable safety profile of pembrolizumab in patients with r/r PMBCL (21, 22). Despite this, more evidence on the use of anti-PD-1 treatment for r/r PMBCL patients is warranted, especially for Chinese r/r PMBCL patients.
Geptanolimab (GB226, Genor Biopharma Co., Ltd, Shanghai, China) is a fully humanized, recombinant anti-PD-1 monoclonal antibody. The dose of 3 mg/kg every 2 weeks was determined as the recommended dose on the basis of findings from its phase 1 study in lymphoma and advanced solid tumors (data unpublished). We conducted this phase II study to evaluate the clinical efficacy and safety of geptanolimab in Chinese patients with r/r PMBCL. This study was prematurely terminated due to the slow rate of patient accrual. We herein report the final results of this study.
Methods
Study design and patients
Gxplore-003 (ClinicalTrials.gov identifier, NCT03639181) was a multicenter, open-label, single-arm phase II study of geptanolimab in r/r PMBCL conducted at 43 hospitals in China (Supplementary Table S1). Patients aged 18 years or older with a histologically confirmed diagnosis of r/r PMBCL were eligible. Relapsed disease was defined as the occurrence of a relapse after complete response (CR) or partial response (PR) with the most recent prior systemic therapy, and refractory disease was defined as the failure to achieve CR or PR with the most recent prior systemic therapy. Patients were required to experience r/r disease after ASCT or be ineligible for ASCT. Patients ineligible for ASCT had to have r/r disease after two or more prior lines of chemotherapy. Other key eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, measurable disease (defined as at least one bidimensional measurable lesion assessed by computed tomography (CT) according to the Lugano Classification 2014) (23), adequate organ functions (hematological, renal, hepatic, and thyroid function), and a life expectancy of at least 3 months.
Exclusion criteria included an additional acute malignancy (other than cured in-situ cervical cancer, basal cell carcinoma or squamous cell carcinoma of the skin) in the past 5 years; confirmed central nervous system involvement; prior chemotherapy or targeted therapy within 2 weeks, or prior radical radiotherapy within 4 weeks before the first dose of geptanolimab; prior ASCT in the past 2 months or prior allogeneic stem cell transplantation in the past 5 years; systemic treatment with corticosteroids (> 10 mg daily prednisone equivalents) within 2 weeks before the first geptanolimab administration; active autoimmune disease; and prior therapy targeting checkpoint pathways or T-cell costimulation. Full eligibility and exclusion criteria are provided by the study protocol, which is available in the Supplementary Materials.
This study was conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation guidelines for Good Clinical Practice. The study protocol, amendments, and patient informed consent were approved by the independent ethics committee at each participating hospital. Written informed consent was obtained from all patients before screening.
Treatment and assessments
All patients received geptanolimab intravenously at 3 mg/kg every 2 weeks until documented confirmed disease progression, intolerable toxicity, patient withdrawal, start of other anti-tumor therapy, loss to follow-up or death, investigator decision, or the end of study (defined as a maximum treatment duration of two years for the last patient, consent withdrawal, premature termination of the study, loss to follow-up or death, whichever occurs first). Continuation of treatment with geptanolimab beyond the first occurrence of disease progression was allowed if there was evidence of clinical benefit from the treatment at the discretion of the investigator. No dose modification of geptanolimab was allowed, while dose interruption was permitted. Dose interruption lasting more than 4 weeks led to permanent discontinuation from the treatment.
At baseline, tumor assessments were performed by positron emission tomography (PET)/computed tomography (CT) and contrast-enhanced CT or magnetic resonance imaging (MRI) of neck, chest, abdomen and pelvis. Tumor response was assessed by CT or MRI every 6 weeks in the first year, and thereafter every 12 weeks until disease progression, start of new anti-tumor therapy, loss to follow-up or death. PET/CT was required to be repeated at week 13 and week 19 for response assessment, and could be performed at other time points to confirm tumor response with the consent of the sponsor. Responses were assessed by both the independent review committee (IRC) and the investigator per the Lugano Classification 2014 (23).
Safety assessments were conducted from the start of treatment until 30 and 90 days (if no new anti-tumor therapy started) after the final geptanolimab administration, or until the time that subsequent new anti-tumor therapy started (whichever occurs first). Adverse events (AEs) were graded on the basis of National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. Additionally, immune-related adverse events (irAEs) were recognized, diagnosed and managed according to the American Society of Clinical Oncology Clinical Practice Guideline for Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy (24).
Endpoints
The primary endpoint was ORR (defined as the percentage of patients with a best response of CR or PR) assessed by the IRC. Responses of CR or PR were required to be confirmed by repeat assessment 4 weeks after the response was first achieved.
Key second endpoints included duration of response (DoR), time to response (TTR), disease control rate (DCR), PFS, OS and safety. DoR was defined as the time from the first documented objective response to the date of first documented progressive disease (PD) or to death from any cause. TTR was defined as the time from first dose of geptanolimab to the first documentation of objective response. DCR was defined as the percentage of patients with a best response of CR, PR or stable disease (SD). PFS was defined as the time from the first dose to the first documented PD or to death from any cause. OS was defined as the time from the first dose to death from any cause, or last follow-up. Safety endpoints included treatment-emergent adverse event (TEAE), treatment-related adverse event (TRAE), serious adverse event (SAE), and specific laboratory abnormalities.
Statistical analysis
With the sample size of 42 patients, the study design yielded a statistical power of > 80% (one-sided 2.5% alpha) to detect that the lower limit of 95% confidence interval (CI) of ORR was > 25%, and the ORR was assumed to be 45% with the 95%CI of 29.6–61.1% for geptanolimab in r/r PMBCL. Allowing for a dropout rate of 20%, a total of 53 patients were planned to be enrolled.
Efficacy were assessed in the full analysis set (FAS), which was defined as all patients who received at least one dose of geptanolimab. Safety analyses was done in the safety set (SS), which was defined as all patients who received at least one dose of geptanolimab and had at least one safety assessment. Descriptive statistics were used to summarize baseline characteristics and safety data. The ORR and DCR were reported, and associated CIs were calculated using the Clopper-Pearson method. For time-to-event data (DoR, TTR, PFS and OS), Kaplan–Meier methods were used to estimate median values and corresponding 95%CIs. All analyses were performed using SAS statistical software, version 9.4. This study is registered with the ClinicalTrials.gov, NCT03639181.
Results
Patient characteristics
A total of 34 patients were screened between Oct 15th, 2018 and Oct 7th, 2020, and 9 patients were ineligible (Fig. 1). As a result, 25 patients were enrolled in this study and assigned to receive geptanolimab. By the data cutoff date on Dec 23rd, 2020, 15 of 25 (60%) patients remained on treatment. The reason for discontinuation included disease progression (n = 5), intolerable AEs (n = 2), patient requirement (n = 2) and death (n = 1). All 25 patients were included in FAS for efficacy and in the SS for safety analyses. As of the data cutoff date, the median duration of exposure to geptanolimab was 11.14 (range, 0.89 to 24.38) months.
Fig. 1.
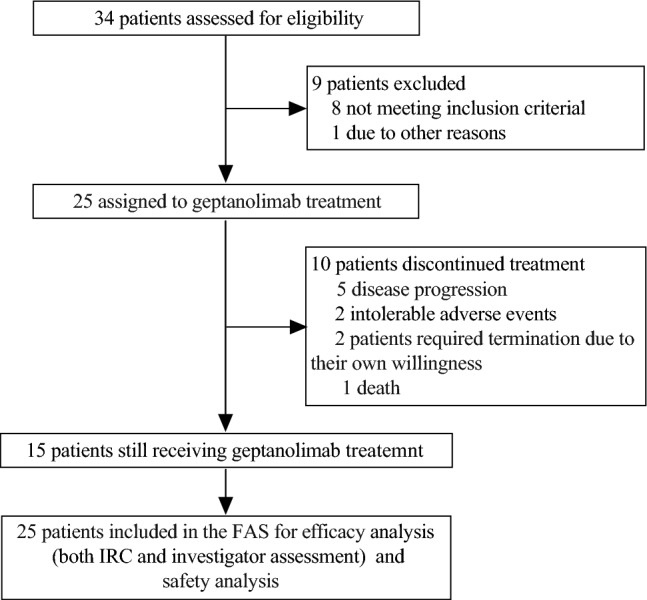
CONSORT Diagram. CONSORT, consolidated standards of reporting trials. FAS, full analysis set; IRC, independent review committee
The median age of all patients was 32 (range, 19–70) years, and 15 out of 25 (60%) patients had Ann Arbor stage III-IV disease. The median number of prior lines of therapy was 2 (range, 2–4), and 9 of 25 (36.0%) received more than 2 previous lines of therapy. Out of 25 patients, 10 (40%) received prior radiotherapy, 24 (95%) received prior rituximab, and 2 (8%) underwent prior ASCT. Overall, 13 of 25 (52.0%) patients had relapsed disease, and 12 of 25 (48%) had refractory disease. Detailed patient baseline characteristics are presented in Table 1.
Table 1.
Patient baseline characteristics in the FAS (N = 25)
| Characteristics | n (%) |
|---|---|
| Age, years | |
| Median (range) | 32 (19–70) |
| < 40 | 17 (68.0) |
| 40–60 | 6 (24.0) |
| > 60 | 2 (8.0) |
| Gender | |
| Male | 8 (32.0) |
| Female | 17 (68.0) |
| Lactate dehydrogenase | |
| < 2 × ULN | 18 (72.0) |
| ≥ 2 × ULN | 6 (24.0) |
| Missing | 1 (4.0) |
| ECOG PS | |
| 0 | 12 (48.0) |
| 1 | 13 (52.0) |
| Ann Arbor stage | |
| I | 1 (4.0) |
| II | 8 (32.0) |
| III | 2 (8.0) |
| IV | 13 (52.0) |
| Missing | 1 (4.0) |
| Disease status | |
| Relapsed* | 13 (52.0) |
| Refractory† | 12 (48.0) |
| Prior surgery | |
| Yes | 2 (8.0) |
| No | 23 (92.0) |
| Prior radiotherapy | |
| Yes | 10 (40.0) |
| No | 15 (60.0) |
| Prior rituximab | |
| Yes | 24 (96.0) |
| No | 1 (4.0) |
| Prior lines of therapy | |
| 2 | 16 (64.0) |
| > 2 | 9 (36.0) |
| Response to the most recent prior systemic therapy | |
| CR | 1 (4.0) |
| PR | 5 (20.0) |
| SD | 6 (24.0) |
| PD | 9 (36.0) |
| UE | 4 (16.0) |
| Time from completion of the last prior systemic therapy to geptanolimab treatment (months) | |
| Median (range) | 1.94 (0.59–35.32) |
| < 3 | 16 (64.0) |
| 3–6 | 5 (20.0) |
| > 6 | 4 (16.0) |
| Prior ASCT | |
| Yes | 2 (8.0) |
| No | 23 (92.0) |
| Best response to prior ASCT | |
| CR | 0 (0.0) |
| PR | 1 (4.0) |
| SD | 0 (0.0) |
| PD | 1 (4.0) |
ULN upper limit of normal; ECOG Eastern Cooperative Oncology Group; PS performance status; CR complete response; PR partial response; SD stable disease; PD progressive disease; UE unevaluable; ASCT autologous cell stem transplantation; FAS full analysis set
*Relapsed disease was defined as the occurrence of disease relapse after CR or PR with the most recent prior systemic therapy
†Refractory disease was defined as no CR or PR with the most recent prior systemic therapy
Efficacy
IRC-assessed efficacy
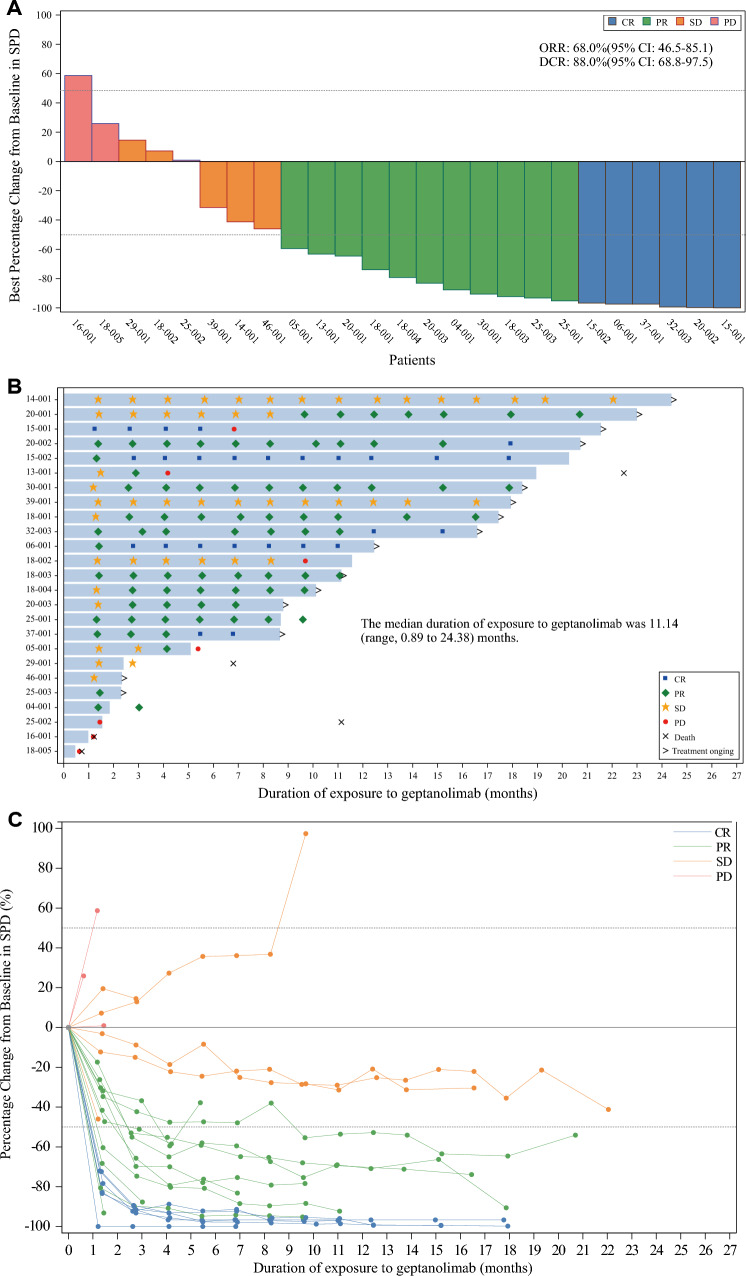
As of Dec 23rd, 2020, the median follow-up was 16.16 (range, 0.7–23.9) months. The ORR was 68.0% (17/25; 95%CI 46.5–85.1%), with the CR rate of 24% (6/25) and the PR rate of 44.0% (11/25) (Table 2). The DCR was 88.0% (22/25; 95%CI 68.8–97.5%). Of 25 patients, 20 patients (80%) had a reduction from baseline in tumor burden (Fig. 2A). Two patients changed from PR to CR after one year from the first geptanolimab administration (Fig. 2B), and the IRC-assessed percentage change of tumor size from baseline at different time points was shown in Fig. 2C. Five of six patients who achieved CR remained on treatment with geptanolimab and remained in remission at the time of data cutoff, while one patient with CR experienced subsequent disease progression.
Table 2.
Efficacy of geptanolimab in the FAS
| IRC-assessed | Investigator-assessed | |
|---|---|---|
| No. of patients | 25 | 25 |
| ORR, No. (%) | 17 (68.0) | 15 (60.0) |
| 95%CI | 46.5–85.1 | 38.6–78.9 |
| DCR, No. (%) | 22 (88.0) | 22 (88.0) |
| 95%CI | 68.8–97.5 | 68.8–97.5 |
| Best objective response | ||
| CR, No. (%) | 6 (24.0) | 6 (24.0) |
| PR, No. (%) | 11 (44.0) | 9 (36.0) |
| SD, No. (%) | 5 (20.0) | 7 (28.0) |
| PD, No. (%) | 3 (12.0) | 3 (12.0) |
| Median DoR, months (95%CI) | NR (5.62 to NR) | NR (8.28 to NR) |
| Median TTR, months (95%CI) | 2.60 (1.38–2.89) | NA |
| Median PFS, months (95%CI) | NR (6.83 to NR) | NR (6.83 to NR) |
| Median OS, months (95%CI) | NR (22.4 to NR) | NA |
FAS full analysis set; IRC independent review committee; ORR objective response rate; DCR disease control rate; CI confidence interval; CR complete response; PR partial response; SD stable disease; PD progressive disease; DoR duration of response; TTR time to response; PFS progression-free survival; OS overall survival; NR not reached; NA not available
Fig. 2.
A Waterfall plot of best percentage change from baseline in SPD for the FAS assessed per the IRC (N = 25); B Duration of exposure to geptanolimab, time to response and duration of response for patients in the FAS assessed per the IRC (N = 25); C Spdier plot for IRC-assessed percentage change from baseline in SPD at different time points. In figure A and C, dotted line at − 50% represents PR at 50% reduction in SPD, and dotted line at 50% indicates PD at 50% increase in SPD as determined on the basis of Lugano Classification 2014. SPD sum of the product of the perpendicular diameters for multiple lesions; CR complete response; PR partial response; PD progressive disease; SD stable disease; IRC independent review committee; FAS full analysis set
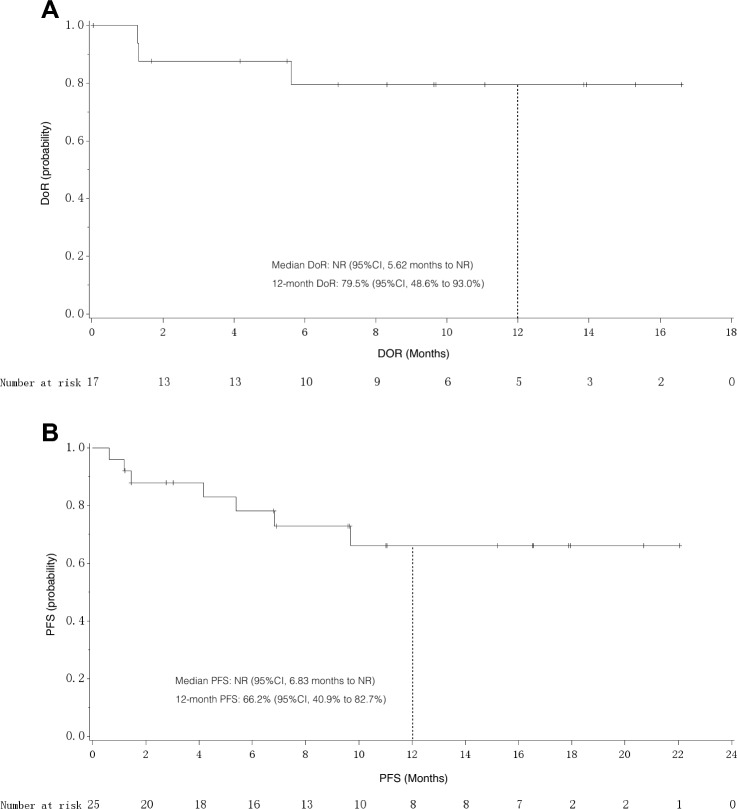
The median TTR was 2.60 (95%CI 1.38–2.89) months. Among the 17 responders, the median DoR was not reached (NR) (95%CI, 5.62 months to NR), with 79.5% of patients having response durations of > 12 months (Fig. 3A). Overall, median PFS was NR (95%CI, 6.83 months to NR), and 8 of 25 (28%) patients had a PFS event (PD, n = 7; death, n = 1). The 12-month PFS rate was 66.2% (95%CI, 40.9–82.7%) (Fig. 3B). By the data cutoff date, five out of 25 patients had died. Median OS was NR (95%CI, 22.47 months to NR), and the 12-month OS rate was 82.1% (95%CI, 58.7–93.0%).
Fig. 3.
A Kaplan–Meier estimates of the DoR among the 17 patients who had achieved a response assessed by the IRC; B Kaplan–Meier estimates of PFS assessed by the IRC. DoR duration of response; PFS progression-free survival; CI confidence interval; NR not reached; IRC independent review committee
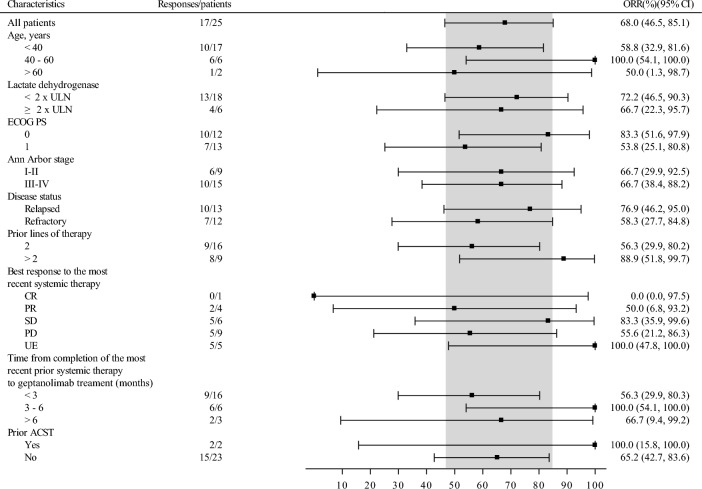
Subgroup analyses showed similar ORR and DCR stratified by age, ECOG PS, number of previous lines of systemic therapy, disease status, disease stage, history of ASCT, lactate dehydrogenase level, and best response to the most recent systemic therapy (Fig. 4 and Supplementary Table S2).
Fig. 4.
Forest plot for subgroup analyses on ORR assessed by the IRC according to the Lugano Classification 2014 in the FAS. ORR objective response rate; IRC independent review committee; CI confidence interval; ULN upper limit of normal; ECOG Eastern Cooperative Oncology Group; PS performance status; CR complete response; PR partial response; SD stable disease; PD progressive disease; UE unevaluable; ASCT autologous cell stem transplantation
Investigator-assessed efficacy
The ORR assessed by investigator was 60.0% (15/25; 95%CI 38.6–78.87%), with 6 (24%) patients achieving CR. Consistent with that assessed by the IRC, the investigator-assessed DCR was 88.0% (22/25; 95%CI 68.8–97.5%). As of the data cutoff date, the median DoR was NR (95%CI, 8.28 months to NR), and median PFS was also NR (95%CI, 6.83 months to NR).
Safety
Among 25 patients in the SS, 23 (92.0%) patients experienced at least one TEAE, and serious TEAEs occurred in 8 (32.0%) patients. TRAEs were reported in 20 (80.0%) patients. Serious TRAEs were observed in 6 (24%) patients, including abnormal liver function (n = 2, 8%), infectious pneumonia (n = 1, 4%), bronchitis (n = 1, 4%), interstitial lung disease (n = 1, 4%) and immune mediated lung disease (n = 1, 4%). Dose interruption occurred in 9 of 25 (36%) patients. In 3 of 25 (12%) patients, geptanolimab was permanently discontinued due to TEAEs. One patient died as a result of TEAEs (sudden cardiac death), which was judged not related to geptanolimab treatment by investigator. The overview of AEs is shown in Supplementary Table S3.
According to investigator, the most common laboratory TRAEs (incidence ≥ 10%) included white blood cell count decreased (n = 13, 52.0%), neutrophil count decreased (n = 11, 44.0%), lymphocyte count decreased (n = 7, 28%), thyroid stimulating hormone elevated (n = 6, 24%), alanine aminotransferase elevated (n = 4, 16%), aspartate aminotransferase elevated (n = 4, 16%), alkaline phosphatase elevated (n = 4, 16%), γ-glutamyltransferase elevated (n = 3, 12%) and lactate dehydrogenase elevated (n = 3, 12%). The most common non-laboratory TRAEs (incidence ≥ 10%) were weight gain (n = 4, 16%), urinary tract infection (n = 3, 12%), upper respiratory tract infection (n = 3, 12%), hypertriglyceridemia (n = 3, 12%), abnormal liver function (n = 3, 12%), and anemia (n = 3, 12%). Grade 3 or higher TRAEs occurred in 11 of 25 (44%) patients. The most common laboratory TRAEs of ≥ grade 3 included white blood cell count decreased (5/25, 20.0%), neutrophil count decreased (4/25, 16.0%), and lymphocyte count decreased (4/25, 16.0%). The detailed TRAEs are summarized in Table 3.
Table 3.
TRAEs in the SS
| TRAEs | Geptanolimab (N = 25) | |||
|---|---|---|---|---|
| All grade, n (%) | Grade 1, n (%) | Grade 2, n (%) | ≥ Grade 3, n (%) | |
| Any | 20 (80.0) | 5 (20.0) | 4 (16.0) | 11 (44.0) |
| White blood cell count decreased | 13 (52.0) | 3 (12.0) | 2 (20.0) | 5 (20.0) |
| Neutrophil count decreased | 11 (44.0) | 3 (12.0) | 4 (16.0) | 4 (16.0) |
| Lymphocyte count decreased | 7 (28.0) | 0 | 3 (12.0) | 4 (16.0) |
| Thyroid stimulating hormone elevated | 6 (24.0) | 6 (24.0) | 0 | 0 |
| Alanine aminotransferase elevated | 4 (16.0) | 4 (16.0) | 0 | 0 |
| Weight gain | 4 (16.0) | 2 (8.0) | 2 (8.0) | |
| Aspartate aminotransferase elevated | 4 (16.0) | 4 (16.0) | 0 | 0 |
| Alkaline phosphatase elevated | 4 (16.0) | 4 (16.0) | 0 | 0 |
| γ-glutamyltransferase elevated | 3 (12.0) | 1 (4.0) | 2 (8.0) | 0 |
| Lactate dehydrogenase elevated | 3 (12.0) | 2 (8.0) | 1 (4.0) | 0 |
| Urinary tract infection | 3 (12.0) | 3 (12.0) | 0 | |
| Upper respiratory tract infection | 3 (12.0) | 1 (4.0) | 2 (8.0) | 0 |
| Infectious pneumonia | 2 (8.0) | 0 | 1 (4.0) | 1 (4.0) |
| Bronchitis | 1 (4.0) | 0 | 1 (4.0) | |
| Hypertriglyceridemia | 3 (12.0) | 2 (8.0) | 1 (4.0) | |
| Abnormal liver function | 3 (12.0) | 1 (4.0) | 1 (4.0) | 1 (4.0) |
| Autoimmune hepatitis | 1 (4.0) | 0 | 0 | 1 (4.0) |
| Pneumonitis | 1 (4.0) | 0 | 0 | 1 (4.0) |
| Interstitial lung disease | 1 (4.0) | 0 | 0 | 1 (4.0) |
| Hypotension | 2 (8.0) | 1 (4.0) | 0 | 1 (4.0) |
| Anemia | 3 (12.0) | 1 (4.0) | 0 | 2 (8.0) |
| Proteinuria | 2 (8.0) | 1 (4.0) | 0 | 1 (4.0) |
SS safety set; TRAEs treatment-related adverse events
The irAEs of any grade were observed in six (24.0%) patients, with two (8.0%) patients having grade 1, one (4%) having grade 2 and three (12%) having grade 3 (Supplementary Table S4). The grade 3 irAEs included interstitial lung disease (n = 1, 4%), immune mediated lung disease (n = 1, 4%) and autoimmune hepatitis (n = 1, 4%). No patient occurred grade 4 or grade 5 irAEs.
Discussion
In this multicenter, phase II study, geptanolimab (GB226), a novel anti-PD-1 antibody, showed promising efficacy in Chinese patients with r/r PMBCL. The ORR assessed by the IRC was 68.0% (17/25; 95%CI 46.5–85.1%), with the CR rate of 24.0% (6/25), and the IRC-assessed DCR was 88.0% (22/25; 95%CI 68.8–97.5%). The ORR and DCR assessed by the investigator were comparable to those assessed by the IRC. Moreover, the responses were durable, and median DoR was NR as of the data cutoff date. The estimated 12-month PFS rate by the IRC assessment was 66.2%, and the 12-month OS rate was 82.1%. Geptanolimab was well tolerated in this study, with a low proportion of patients experiencing AEs leading to treatment discontinuation. To the best of our knowledge, this study was the first multicenter, phase II study to assess the clinical efficacy and safety of an anti-PD-1 monoclonal antibody in Chinese patients with r/r PMBCL.
Due to the rarity of PMBCL, there is a paucity of prospective and in particular, randomized studies, resulting in no optimal therapeutic approach to r/r PMBCL. Like other aggressive B-cell lymphomas, salvage therapy followed by consolidative ASCT is considered as the current standard of care for chemo-sensitive patients (25). Since conventional salvage immunochemotherapy exhibits unsatisfactory results, recent efforts have focused on elucidating new therapeutic targets and exploring novel therapies in patients with PMBCL. CAR T-cell therapy has emerged as a new promising therapeutic strategy for lymphoma including PMBCL in recent years (26, 27). In the ZUMA-1 and TRANSCEND NHL001 studies, axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel, respectively, were studied in r/r aggressive large B-cell lymphomas after failure of two or more lines of systemic therapy, and both demonstrated promising efficacy and manageable safety (15–17). These results led to US Food and Drug Administration (FDA) approval of axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel on Oct 18th, 2017 and Feb 5th, 2021, respectively, for the treatment of adult patients with r/r large B-cell lymphoma including PMBCL after two or more lines of systemic therapy. However, no CAR T-cell products were available until June 23rd, 2021 on which axicabtagene ciloleucel (axi-cel) was approved by China NMPA with the same indication as approved by US FDA.
PMBCL shares substantial clinical and biological features with cHL, and is considered as an inflamed lymphoma subtype, harboring genetic alterations that facilitate immune escape (28). Chromosomal rearrangements involving PD-L locus (9p24.1) occur prominently in PMBCL, resulting in enhanced PD-L1 and PD-L2 expression (19, 20). Other common chromosomal alterations in PMBCL include translocation involving the class II transactivator CIITA (29, 30). These aberrations have been established as genetic mechanisms of immune escape in PMBCL. Additionally, like cHL, PMBCL also exhibits microsatellite instability, and an apolipoprotein B mRNA editing catalytic polypeptide-like (APOBEC) signatures, which were associated with increased mutational load and neo-antigen generation, and might provide additional mechanisms of sensitivity to anti-PD-1 therapy in PMBCL (31–33).
Previous studies have demonstrated that treatment with pembrolizumab yielded favorable clinical outcomes in r/r PMBCL. In the phase 1b KEYNOTE-013 study, pembrolizumab showed promising anti-tumor activity among 21 patients with r/r PMBCL, with an ORR of 48% and CR rate of 33% (21, 22). The phase 2 KEYNOTE-170 study of pembrolizumab enrolling 53 patients with r/r PMBCL reported an ORR of 45% and a CR rate of 13% (21, 22). The estimated 12-month PFS was 47% and 38% in KEYNOTE-013 and KEYNOTE-170, respectively, and 12-month OS was 65% in the KEYNOTE-013 and 58% in KEYNOTE-170. As a result, the US FDA approved pembrolizumab for the treatment of patients with r/r PMBCL who have received two or more prior lines of therapy on June 13, 2018. In our study, geptanolimab demonstrated an IRC ORR of 60.0%, with a CR rate of 24%; the estimated 12-month PFS was 66.2%, and the 12-month OS rate was 82.1%. The results reported with geptanolimab herein seemed to be numerically more favorable compared with that for pembrolizumab.
Notably, consistent with that observed in cHL, the benefit from anti-PD-1 monoclonal antibody was durable (34, 35) The median DoR remained NR after a median follow-up time of 29.1 months in KEYNOTE-013 and 12.5 months in KEYNOTE-170 study(21). No patient who achieved CR experienced relapse during follow-up in both studies of pembrolizumab. In our study, the median DoR was also NR with a median follow-up of 16.16 months, and 79.5% of patients had response durations of > 12 months. Five of six patients with CR remained in remission by the time of data cutoff. Besides, consistent benefit could be obtained across all subgroups. Taken together, though only small numbers of patients were enrolled in our study, geptanolimab demonstrated promising clinical efficacy in Chinese patients with r/r PMBCL.
Generally, geptanolimab was well tolerated with manageable AEs. The safety profile in the current study is consistent with that reported in the previous reports on geptanolimab (36, 37), with no new safety signal detected. However, the overall incidence of AEs appeared to be higher in the current study, probably due to the small sample size. In a phase 2 study on geptanolimab in r/r peripheral T cell lymphoma (PTCL), hematologic disorders were also the most common TRAEs (white blood cell count decreased [20/102, 19.6%], and anemia [13/102, 12.7%]) (37). In contrast, in a phase II study of geptanolimab in alveolar soft part sarcoma (ASPS), rash (7/37, 18.9%) and increased blood thyroid stimulating hormone (7/37, 18.9%) were the most common TRAEs, with hematologic disorders being less frequent (white blood cell count decreased [5/37, 13.5%], and anemia [5/37, 13.5%]) (36). Although increased blood thyroid stimulating hormone (6/25, 24%) was also common in the current study, rash was not observed. Additionally, the spectrum of TRAEs with geptanolimab was similar but somewhat not identical to that observed with other anti-PD-1 antibodies in PMBCL (21, 22, 38). The most common TRAEs of any grade and grade 3 or higher in our study were white blood cell count decreased, neutrophil count decreased, and lymphocyte count decreased. Gastrointestinal disorders, such as decreased appetite, diarrhea and nausea, which were frequently observed with pembrolizumab, occurred at a low rate in our study (21). Moreover, infusion-related reactions, including pyrexia, which were frequently found in other anti-PD-1 monoclonal antibodies for patients with r/r cHL, were less common in our study (34, 39, 40).
Several limitations of this study need to be acknowledged. The major limitation of this study is that the sample size is small, since this study was prematurely terminated due to slow patient accrual. Given that the number of patients enrolled was less than originally planned, the statistical analysis of this study remained underpowered. Other limitations included that data on DoR, PFS and OS were immature as of the data cutoff date. Despite these limitations, the efficacy and safety of geptanolimab for Chinease patients with r/r PMBCL in this study are promising. Since current treatment options for r/r PMBCL are limited, and it is difficult to conduct randomized studies specifically in this rare patient population, the results of this study contribute to supporting the use of PD-1 monoclonal antibodies for the treatment of r/r PMBCL.
Geptanolimab has demonstrated promising efficacy and manageable safety profiles for lymphoma and advanced solid tumors in its phase 1 study (Gxplore-001, NCT03374007) (data unpublished), as well as for r/r PTCL (Gxplore-002, NCT03502629) and unresectable, recurrent, or metastatic ASPS (Gxplore-005, NCT03623581) in two phase II studies (36, 37). Further phase II studies are underway to evaluate the use of geptanolimab in solid tumors, including cervical cancer (NCT03808857), non-small cell lung cancer (NCT03976856) and colorectal cancer (NCT03977090). In the future, more clinical trials will be conducted to investigate the efficacy and safety of geptanolimab as monotherapy or in combination with other agents.
In conclusion, this study demonstrated that geptanolimab showed promising efficacy and a manageable safety profile in Chinese patients with r/r PMBCL. Further investigations are needed to confirm the clinical benefit of geptanolimab in the management of patients with r/r PMBCL.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank for all the patients and their families, all the investigators and study teams that contributed to this study. The authors would also like to thank Dr. Haizhu Chen (National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, China) for providing medical writing assistance with this article, funded by Genor Biopharma Co., Ltd., Shanghai, China.
Authors' contributions
YKS was the leading principal investigator, and contributed to the conceptualization and design of this study, study supervision, data acquisition and interpretation, manuscript writing and revision. YKS, JC, HZ, XHZ, LQZ, JNC, YHG, CJ, XLL, HL, ZGP, LPX, HLZ, WHZ, HYZ, LYZ and FZ contributed to patient recruitment and data acquisition. GG and WDH contributed to data collection, data analysis and data interpretation. All authors had full access to the data in this study, reviewed and approved the final version for submission.
Funding
This study was funded by Genor Biopharma Co., Ltd., Shanghai, China and partly supported by China National Major Project for New Drug Innovation (2017ZX09304015).
Data availability
Data generated and analyzed in this study are on file with Genor Biopharma Co., Ltd., Shanghai, China and are not publicly available according to the company.
Declarations
Conflict of interest
Genny Guo and Wenduo He are employees of Genor Biopharma Co., Ltd., Shanghai, China. All other authors declare no competing interests.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation guidelines for Good Clinical Practice. The study protocol, amendments, and patient informed consent were approved by the independent ethics committee at each participating hospital.
Consent to participate
Written informed consent was obtained from all patients before screening.
Consent to publish
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alaggio R, Amador C, Anagnostopoulos I et al. (2022) The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia, 36:1720–1748. Doi: 10.1038/s41375-022-01620-2 [DOI] [PMC free article] [PubMed]
- 2.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102:3871–3879. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 4.Giulino-Roth L, O'Donohue T, Chen Z, et al. Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R. Br J Haematol. 2017;179:739–747. doi: 10.1111/bjh.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayden AR, Tonseth P, Lee DG, et al. Outcome of primary mediastinal large B-cell lymphoma using R-CHOP: impact of a PET-adapted approach. Blood. 2020;136:2803–2811. doi: 10.1182/blood.2019004296. [DOI] [PubMed] [Google Scholar]
- 6.Martelli M, Ceriani L, Zucca E, et al. [18F]fluorodeoxyglucose positron emission tomography predicts survival after chemoimmunotherapy for primary mediastinal large B-cell lymphoma: results of the International Extranodal Lymphoma Study Group IELSG-26 Study. J Clin Oncol. 2014;32:1769–1775. doi: 10.1200/JCO.2013.51.7524. [DOI] [PubMed] [Google Scholar]
- 7.Melani C, Advani R, Roschewski M, et al. End-of-treatment and serial PET imaging in primary mediastinal B-cell lymphoma following dose-adjusted EPOCH-R: a paradigm shift in clinical decision making. Haematologica. 2018;103:1337–1344. doi: 10.3324/haematol.2018.192492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rieger M, Osterborg A, Pettengell R, et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol. 2011;22:664–670. doi: 10.1093/annonc/mdq418. [DOI] [PubMed] [Google Scholar]
- 9.Savage KJ, Al-Rajhi N, Voss N, Paltiel C, Klasa R, Gascoyne RD, Connors JM. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol. 2006;17:123–130. doi: 10.1093/annonc/mdj030. [DOI] [PubMed] [Google Scholar]
- 10.Kuruvilla J, Pintilie M, Tsang R, Nagy T, Keating A, Crump M. Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma. 2008;49:1329–1336. doi: 10.1080/10428190802108870. [DOI] [PubMed] [Google Scholar]
- 11.Aoki T, Shimada K, Suzuki R, et al. High-dose chemotherapy followed by autologous stem cell transplantation for relapsed/refractory primary mediastinal large B-cell lymphoma. Blood Cancer J. 2015;5:e372. doi: 10.1038/bcj.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H, Xu-Monette ZY, Xiao L, et al. Prognostic factors, therapeutic approaches, and distinct immunobiologic features in patients with primary mediastinal large B-cell lymphoma on long-term follow-up. Blood Cancer J. 2020;10:49. doi: 10.1038/s41408-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steidl C, Gascoyne RD. The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood. 2011;118:2659–2669. doi: 10.1182/blood-2011-05-326538. [DOI] [PubMed] [Google Scholar]
- 14.Zinzani PL, Pellegrini C, Chiappella A, Di Rocco A, Salvi F, Cabras MG, Argnani L, Stefoni V. Brentuximab vedotin in relapsed primary mediastinal large B-cell lymphoma: results from a phase 2 clinical trial. Blood. 2017;129:2328–2330. doi: 10.1182/blood-2017-01-764258. [DOI] [PubMed] [Google Scholar]
- 15.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 18.Savage KJ. Primary mediastinal large B-cell lymphoma. Blood. 2022;140:955–970. doi: 10.1182/blood.2020008376. [DOI] [PubMed] [Google Scholar]
- 19.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123:2062–2065. doi: 10.1182/blood-2013-10-535443. [DOI] [PubMed] [Google Scholar]
- 21.Armand P, Rodig S, Melnichenko V, et al. Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma. J Clin Oncol. 2019;37:3291–3299. doi: 10.1200/JCO.19.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130:267–270. doi: 10.1182/blood-2016-12-758383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer JR, Lacchetti C, Thompson JA. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J oncol Pract. 2018;14:247–249. doi: 10.1200/jop.18.00005. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Chen H, Qin Y, et al. Clinical characteristics and treatment outcomes of Chinese diffuse large B-cell lymphoma patients in the era of rituximab (2005–2018)☆. Cancer Pathog Ther. 2023;1:3–11. doi: 10.1016/j.cpt.2022.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H, Zhou Y, Han X, Shi Y. The changing landscape of anti-lymphoma drug clinical trials in mainland China in the past 15 years (2005–2020): a systematic review. Lancet Reg Health West Pac. 2021;8:100097. doi: 10.1016/j.lanwpc.2021.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Y. Current status and progress of lymphoma management in China. Int J Hematol. 2018;107:405–412. doi: 10.1007/s12185-018-2404-8. [DOI] [PubMed] [Google Scholar]
- 28.Kline J, Godfrey J, Ansell SM. The immune landscape and response to immune checkpoint blockade therapy in lymphoma. Blood. 2020;135:523–533. doi: 10.1182/blood.2019000847. [DOI] [PubMed] [Google Scholar]
- 29.Mottok A, Woolcock B, Chan FC, et al. Genomic alterations in CIITA are frequent in primary mediastinal large B cell lymphoma and are associated with diminished MHC class II expression. Cell Rep. 2015;13:1418–1431. doi: 10.1016/j.celrep.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Steidl C, Shah SP, Woolcock BW, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471:377–381. doi: 10.1038/nature09754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapuy B, Stewart C, Dunford AJ, et al. Genomic analyses of PMBL reveal new drivers and mechanisms of sensitivity to PD-1 blockade. Blood. 2019;134:2369–2382. doi: 10.1182/blood.2019002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Jia M, He Z, Liu XS. APOBEC3B and APOBEC mutational signature as potential predictive markers for immunotherapy response in non-small cell lung cancer. Oncogene. 2018;37:3924–3936. doi: 10.1038/s41388-018-0245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Su H, Song Y, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019;6:e12–e19. doi: 10.1016/S2352-3026(18)30192-3. [DOI] [PubMed] [Google Scholar]
- 35.Su H, Song Y, Jiang W, et al. Sintilimab for relapsed/refractory classical Hodgkin's lymphoma: long-term follow-up on the multicenter, single-arm phase II ORIENT-1 study. J Clin Oncol. 2020;38:8034. doi: 10.1200/JCO.2020.38.15_suppl.8034. [DOI] [Google Scholar]
- 36.Shi Y, Cai Q, Jiang Y, et al. Activity and safety of geptanolimab (GB226) for patients with unresectable, recurrent, or metastatic alveolar soft part sarcoma: a phase II. Single-arm Study Clin Cancer Res. 2020;26:6445–6452. doi: 10.1158/1078-0432.CCR-20-2819. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Wu J, Wang Z, et al. Efficacy and safety of geptanolimab (GB226) for relapsed or refractory peripheral T cell lymphoma: an open-label phase 2 study (Gxplore-002) J Hematol Oncol. 2021;14:12. doi: 10.1186/s13045-021-01033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zinzani PL, Santoro A, Gritti G, et al. Nivolumab combined with brentuximab vedotin for relapsed/refractory primary mediastinal large B-Cell lymphoma: efficacy and safety from the phase II CheckMate 436 study. J Clin Oncol. 2019;37:3081–3089. doi: 10.1200/JCO.19.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35:2125–2132. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–1294. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated and analyzed in this study are on file with Genor Biopharma Co., Ltd., Shanghai, China and are not publicly available according to the company.