Abstract
One of the major hurdles for the advancement of cancer immunotherapy is lack of robust, accessible experimental models. We aimed to produce an ex-vivo organ culture (EVOC) model of immunotherapy for non-small cell lung cancer (NSCLC). Freshly resected early stage tumors were collected from the operating room, fragmented to clusters < 450 µm and cultured with fetal calf serum and human autologous serum. The resulting EVOC includes cancer epithelial cells within tumor tissue clusters and immune cells. Original tissue features are reflected in the EVOCs. The response to immune checkpoint inhibitors (ICI) was assessed by IFNγ gene induction. Interestingly, IFNγ EVOC induction was numerically higher when anti-CTLA4 was added to anti-PD-L1 treatment, supporting the notion that anti-CTLA4 impacts cancer partly through tumor-resident immune cells. In parallel, immunohistochemistry (IHC) for key immune-related proteins was performed on the formalin-fixed paraffin embedded (FFPE) corresponding tumors. EVOC IFNγ induction by ICI correlated with basal non-induced IFNγ, CD8, CD4 and FOXP3 mRNA levels within EVOCs and with tumor-FFPE-IHC for CD8 and granzyme B. A weaker correlation was seen with tumor-FFPE-IHC for CD3, CD4, CD68, FOXP3 and tumor-PD-L1. Tertiary lymphoid structure density was also correlated with the ICI response. Our study provides novel data about biomarkers that correlate with ICI-induced response of early stage NSCLC. Retention of the microenvironment and minimal addition of exogenous factors suggest this model to reliably represent the original tumor. The cluster-based EVOC model we describe can provide a valuable, yet simple and widely applicable tool for the study of immunotherapy in NSCLC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-020-02828-w.
Keywords: Tumor-microenvironment, Anti-PD-L1, Anti-CTLA4, Early-stage NSCLC, IFNγ
Introduction
Lung cancer, mostly non-small cell type (NSCLC), is the leading cause of cancer-related-death worldwide, accounting for an estimated 1.6 million deaths yearly [1]. Upregulation of programmed cell death-1 ligand (PD-L1) and other immune checkpoint proteins on cancer cells enables evasion of eradication by cytotoxic T cells. Immune checkpoint-inhibitors (ICI) can re-invigorate exhausted cytotoxic T cells and allow anti-cancer immune activity. These drugs have become major tools for the treatment of melanoma, NSCLC and other cancers [2, 3]; however, in NSCLC only 20–40% of metastatic patients eventually benefit from immunotherapy treatments. Aiming to improve the efficacy of ICI, combinations of PD-L1 or PD-1 inhibitors with other immunotherapy agents such as cytotoxic T-lymphocyte-associated protein 4 (CTLA4) inhibitors or with chemotherapy or radiotherapy are being investigated [3–5]. In an effort to increase the chances of cure for early cancer patients, ICI are now considered for treatment at earlier stages of disease. Importantly, no predictive factors have been validated for the efficacy of immunotherapy in early, non-metastatic NSCLC [6].
The race to reveal the most effective treatment regimens and the optimal drug combinations for each cancer type entail huge investments in time, money, and most importantly, the trust and courage of numerous cancer patients. There is an urgent need for translational models to complement the clinical trial efforts by minimizing the range of possibilities and pointing at promising directions. In vitro models such as cell lines lack the tumor’s microenvironment (TME) and the stromal cells with their critical impact on tumor biology and drug resistance [7–9]. Many approaches have been taken along the years to solve these problems.
Patient-derived xenograft (PDX) mice models [10], one of those approaches, involve inoculation of fresh tumor sample from a cancer patient into immunodeficient mice [11]. PDX is expensive in terms of labor, time required and mice [12], and lacks a functional immune system. Engrafting these mice with hematopoietic stem cells from the same patient can overcome this obstacle, but increases substantially cost and complexity of the experiments [10, 11]. Ex-vivo organ culture (EVOC) cancer model can provide a simple and valid alternative, which includes the TME as well as the patient’s specific tumor and immune cells [13, 14]. The establishment of EVOC models requires close collaboration between thoracic surgical, pathological, medical and basic-research teams. Attempting to use core-needle tumor biopsies for this goal is problematic, as generally biopsies provide only minute amounts of tissue that are required for the routine diagnostical tests. A larger sample of tumor (namely, a surgical specimen) utilized for EVOC allows for better representation of the patient’s tumor and stromal compartments. A mechanism for fragmentation and homogenization of the tumor sample is required for EVOCs to produce a robust experimental system, allowing comparisons between replicates produced from a single tumor sample. Ex-vivo tissue slices or alternative 2–3 mm-range sized fragments [14, 15] are examples of EVOC models tested currently; however the heterogeneity between neighboring tissue fragments of this size can be substantial.
Alternative approaches for reliable models of cancer involve lab-grown organoids of cancer epithelial cells that evolve a 3D structure but lack most, if not all of the TME [13, 16]. Other examples of ex-vivo models utilize tiny tissue pieces containing tumor and TME embedded in collagen and grown in an air–liquid interface [17], or tumor spheroids retaining the immune component, grown within a collagen matrix in a complex microfluidics system [18]. Commonly, epithelial spheroids or immune cells are treated with a cocktail of growth factors and additional signaling molecules (e.g. Wnt3A, NOGGIN, EGF and more) for the preservation of cells viability and proliferation [17, 19, 20]. We aimed to establish a simple and reproducible ex-vivo organ model for lung cancer tumors that would fulfill the following criteria: (a) to include cancer cells, immune elements and other TME components; (b) to retain the original 3D structure; (c) to require minimal exogenous interventions such as addition of growth and signaling factors; (d) to allow for robust replicate testing of various experimental interventions; and (e) to have a readout of the immune activity (Supplementary Figure S1). We have utilized this model to dissect characteristics of early NSCLC specimens that correlate with enhanced immune response to ICI.
Materials and methods
EVOC preparation
Tissue was collected directly from the operating room in RPMI medium (Biological Industries) supplemented with 8% fetal calf serum (FCS; Gibco) at 4 ℃. Five ml of blood were collected from each patient at the beginning of the operation, and the patient’s serum was separated by centrifugation at 2000 g for 20 min. The tissue was washed with cold PBS and cut to fragments of ~ 1.5 mm diameter in ice-cold RPMI supplemented with 8% FCS, followed by dissociation by GentleMACS (Miltenyi Biotec). The released cells and clusters were collected, filtered with a 450 µm mesh, and centrifuged for 5 min at 200 g in 4 ℃ (producing a pellet of the ‘clusters fraction’). The supernatant containing mostly single cells was centrifuged again for 10 min at 400 g (producing the ‘single-cells fraction’). The clusters and single-cell fractions were washed, joined, and resuspended in RPMI supplemented with 8% FCS, 2% autologous human serum, 1% transferrin–insulin–selenium mix (Gibco), 1% glutamine, 1% pen/strep, 0.5% HEPES, 0.6% non-essential amino acids, amphotericin 2.5 µg/ml, and gentamycin 50 µg/ml (all from Biological Industries). The resuspended EVOC was divided into 500 µl/well in 24-well plates and cultured at 37 ℃, 5% CO2.
Drugs and treatments
Drugs were added to the cultured EVOC within 15 h of EVOC initiation and incubated for additional 4 days. Durvalumab (anti-PDL1; AstraZeneca) and ipilimumab (anti-CTLA4, Bristol Myers Squibb) were used at a final concentration of 35 and 50 µg/ml, respectively [18, 21, 22]. Recombinant IL-2 (Novartis) was used at a final concentration of 1.1 µg/ml.
Real-time qRT-PCR
Total RNA from EVOCs, collected at 4 days post-treatment, was isolated using PureLink RNA Mini Kit (Invitrogen) according to the manufacturer’s protocol. RNA was reverse-transcribed using qScript cDNA synthesis kit (Quantabio). Quantitative real-time PCR (StepOnePlus instrument, Applied Biosystems, California, USA) was performed either using gene-specific primers or TaqMan probes (Applied Biosystems; details in Supplementary Table S4) using SYBR-FAST Green PCR Master Mix or a TaqMan master mix (Applied Biosystems). Gene induction level was calculated as relative fold-change normalized to GAPDH housekeeping gene in the same sample and to a reference control sample (non-treated sample; 2−∆∆Ct). Non-induced relative gene expression levels determined using the delta Ct (∆Ct)) method (Ct [target gene] − Ct [GAPDH]).
FFPE (Bio-agar) of EVOC culture
EVOCs were collected to Eppendorf tubes and centrifuged at 400 g for 5 min, the pellet was washed with cold PBS and fixed with 1:1 96% ethanol: 4% paraformaldehyde for 5 min. The fixed pellet was centrifuged at 1000 g for 5 min and resuspended in 5–20 µl melted Bio-Agar gel (Bio-Optica). The gel containing the EVOC was flattened to a thin disc, cooled at ~ 0 ℃, coated on both sides with additional melted Bio-Agar gel, cooled again, followed by routine FFPE processing.
Tissues fixation, processing, and embedding
Tissues were processed according to the standard operating procedures of the Pathology department. Briefly, samples were fixed in 4% paraformaldehyde and processed on automated tissue processing machine (Tissue-Tek VIP, Sakura). Dehydration of the tissue was done by serial immersion in increasing concentrations of alcohol (70–100%) and removal of the dehydrant with Xylene. The tissue was embedded in paraffin, sectioned at 3.5 µm, and mounted on microscope slides. The slides were placed in an oven for 1 h at 60 ℃ or overnight at 37 ℃ before immunohistochemical staining.
IHC staining
H&E stains were performed on an automated ST5020 device (Leica Biosystems, Germany) according to the manufacturer's instructions. IHC stains were performed on a Benchmark XT staining module (Ventana Medical Systems Inc.; USA) using iVIEW DAB Detection Kit (catalog #: 760-091, Ventana Medical Systems Inc.) or Ultra VIEW Universal DAB Detection Kit (catalog #: 760–500, Ventana Medical Systems Inc.; USA). Antibody details are described in supplementary Table S5. Following immunostaining, sections were counterstained with hematoxylin (Ventana Medical Systems Inc.), rinsed in distilled water, and dehydrated manually in graded ethanols. Finally, the sections were cleared in xylene and mounted with Entellan (Surgipath Medical Industries Inc.) on glass slides.
IHC results scoring
IHC results were quantified using Olympus BX50 microscope (Olympus Corporation, Japan) by an expert thoracic pathologist via identification of positively stained cells. For most immunostains, the percentage of the area including positively stained cells was evaluated relative to the total tumor area (including tumor cells and tumor stroma). KI67 immunostain was quantified as the percent of positively stained tumor cells out of the total tumor cells. In each case, an average was calculated for ten randomly selected fields per tumor. PD-L1 tumor cell staining evaluation was done according to the standard scoring procedure [23]. PD-L1 IHC staining on immune cell was scored as described in Bellmunt et al. [24]. Care was taken not to include areas of necrosis in the evaluated areas. Intra-observer reproducibility was tested by repeat scoring for 50 randomly selected stained slides, resulting in a Pearson correlation r value of 0.959.
TLS quantification
Mature tertiary lymphoid structures (TLS) were defined as lymphocyte aggregates with CD21 or CD23 network-like positive staining (Supplementary Figure S3). Slides were scanned with Ultra Fast Scanner 1.8 (Philips, Holland) and whole-section area for each slide was measured using Image Management System 3.3.1 Philips IntelliSite Pathology Solution software. TLS were counted independently by two readers and average TLS counts were divided by section area to produce the TLS density for each tumor.
Statistics
Comparisons between parameters were done by two-tailed Student’s t test, Mann–Whitney test, or Fisher’s exact test, as appropriate. Pearson correlation was tested to examine correlation between different parameters. R software version 3.5.3 (2019-03-11) was used for Box plot generation and statistics. For heatmap generation, IHC quantification was normalized to z score, and unsupervised complete linkage clustering was performed using correlation coefficients, depicting Manhattan distance metric and average linkage. P values lower than 0.05 were highlighted as significant. 95% confidence interval was calculated by the Wilson procedure.
Results
Establishing ex-vivo organ culture from resected primary lung tumors
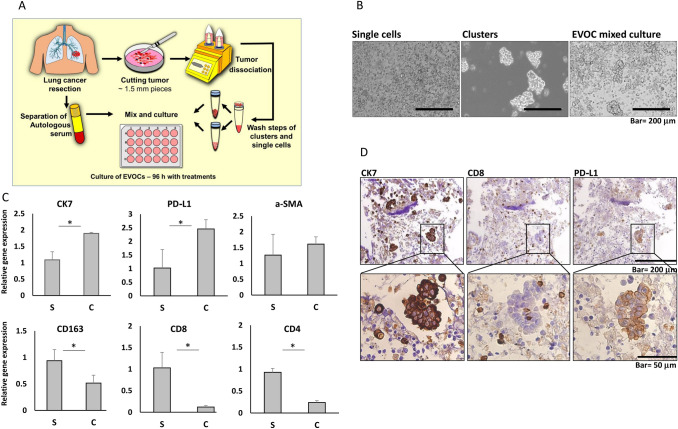
Aiming to establish a relevant model for the study of lung cancer that will be appropriate to test tumors' response to immunotherapy, we took advantage of early stage NSCLC surgically resected specimens where the amount of available tissue exceeds the requirements for routine pathologic assessment. Fragments of resected tumors were collected directly from the operating room and processed within half an hour (see Fig. 1a and Methods for details). The protocol we have developed produces clusters of tumor cells and various types of single cells released from the tumor tissue, thus partly preserving TME 3D structure (Fig. 1b). We found the clusters to contain more tumor epithelial cells (see Fig. 1c; CK7 as a marker for adenocarcinoma cells). The single-cell fraction contained more immune cells, based on the expression profile of CD4 (T-helper and T-regulatory lymphocytes), CD8 (cytotoxic T cells), and CD163 (tissue macrophages). Fibroblasts were present similarly in both fractions (see α-SMA expression; Fig. 1c). We thus combined the clusters and the single cells to compose the final EVOC containing tumor cells with their TME as well as resident immune cells (Fig. 1b, d). The small size of the produced clusters (less than 450 μm) facilitated uniform mixtures and reduced variability among replicates. Immunohistochemistry (IHC) on EVOCs reaffirmed the presence of tumor cells mostly within clusters, while cytotoxic T cells are detected both in the single-cell fraction as well as within the epithelial clusters (Fig. 1d). PD-L1 expression could be detected mainly on epithelial cell clusters in the EVOC. We have thus produced an EVOC model including the major components of the TME.
Fig. 1.
EVOC preparation and component analysis. a Tumor tissue is collected directly from the operating room, rapidly dissected with surgical razor blades, followed by gentleMACS dissociation. Released cells and clusters are washed and cultured in medium supplemented with autologous serum and fetal calf serum. Drugs are added to the culture medium and culture is incubated for four days (see materials and methods for details). b Bright field microscopic images of: single cells fraction (< 30 μm; left panel), clusters fraction (> 30 μm; < 450 μm, middle panel) and mixed clusters and single cells (as used in all experiments; right panel). c Gene expression in single-cells fraction (S) and clusters fraction (C) determined by real-time PCR (normalized to GAPDH; single cells fraction was used as the reference sample): CK7 (representing adenocarcinoma epithelial cells), PD-L1, α-SMA (fibroblasts), CD163 (macrophages), CD8 (cytotoxic T cells), CD4 (helper T cells). *p < 0.05, student’s t-test. d IHC of a representative EVOC; upper panel—microscopic images of 10 × magnification, lower panels—enlargement of the cluster area. IHC of CK7 (left panel), CD8 (middle panel) and PD-L1 (right panel)
Correlation of tumor morphology and EVOC composition
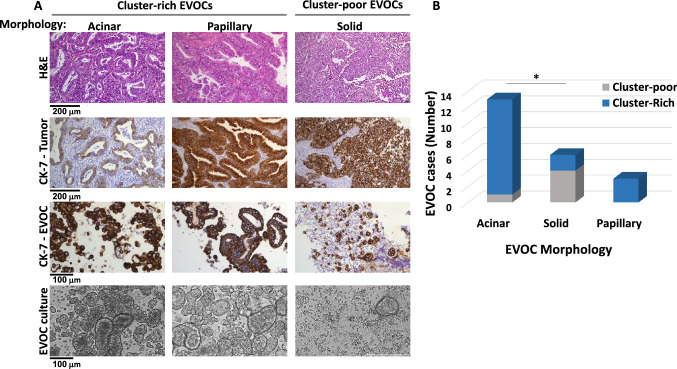
In the process of establishing the EVOC protocol, we have encountered significant heterogeneity of the produced EVOCs, some rich in clusters, while others were composed mostly of single cells (Fig. 2 and supplementary Table S1). EVOCs were designated as cluster-rich (defined arbitrarily as ≥ 10 clusters/1.85 mm2), or as cluster poor (< 10 clusters/1.85mm2). This separation was based on a bimodal distribution, with cluster-poor EVOCs having no more than 6 clusters/1.85 mm2. In parallel, FFPE sections of the resected tumors were stained for hematoxylin and eosin (H&E) and markers of lung cancer subtypes, as done for the routine pathologic analysis of lung cancer specimens. A significant correlation was found between the cluster density of the established EVOCs and the morphological subtype of the corresponding tumors. EVOCs produced from adenocarcinoma tumors of acinar or papillary subtypes were mostly cluster-rich, while those produced from solid-morphology adenocarcinoma tumors were mostly composed of single cells (acinar vs. solid morphology, Fisher’s exact test p = 0.022; Fig. 2 and supplementary Table S1). The solid NSCLC adenocarcinoma subtype is considered to be less differentiated, more aggressive and to have a worse prognosis relatively to other morphologies [25, 26]. It can be speculated that these characteristics are reflected in the formation of cluster-poor EVOCs, probably indicative of weaker cell–cell contacts between tumor cells and the TME and potentially corresponding with a higher tendency to metastasize. Therefore, the EVOC setup seems to retain some biological features of the resected tumors.
Fig. 2.
Tumor morphological features and EVOC cluster formation. Adenocarcinoma FFPE specimens (of standard pathologic processing) with a single dominant adenocarcinoma subtype and their corresponding EVOCs were analyzed (n = 21). EVOCs analyzed were either cluster-rich (≥ 10 clusters/1.85 mm2) or cluster-poor (< 10 clusters/1.85 mm2). a Two upper panels: microscopic-images of representative FFPE adenocarcinoma specimens with a dominant subtype of acinar, papillary or solid. IHC of H&E and cytokeratin 7 epithelial cell marker (CK7-tumor); Two bottom panels—microscopic images of FFPE Bio-Agar embedded cell block of morphologically similar EVOCs stained with epithelial cells marker (CK7-EVOC) and light microscopy images of the EVOCs (EVOC culture). b Analysis of the number of cluster-rich and cluster-poor EVOCs, within each adenocarcinoma subtype group (acinar, solid or papillary). Correlation between the acinar and solid predominant morphology subtypes of resected tumors and the corresponding cases of cluster-poor or cluster-rich EVOCs; *p = 0.022, Fisher's exact test
Immune activation in EVOCs treated by checkpoint inhibitors
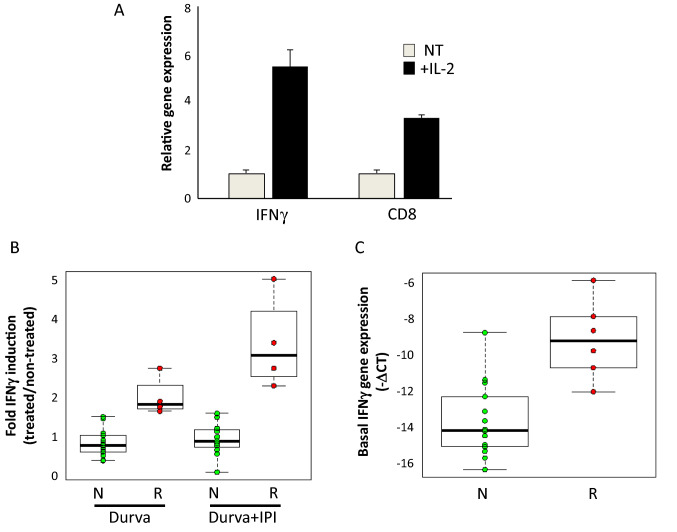
We next examined whether the immune cells in our EVOC model are responsive to activating signals. IFNγ expression and activity are well correlated with benefit from immune checkpoint blockade in NSCLC and in other cancers [27–29]. We thus studied treatment-induced IFNγ gene expression as a readout for the immune activation of the EVOCs. EVOCs treated with interleukin-2 (IL-2) for 96 h demonstrated elevated IFNγ and CD8 gene expression (conceivably indicative of cytotoxic T-cell activation; Fig. 3a). Next, IFNγ induction was evaluated in a cohort of 20 EVOCs (see supplementary Table S2 for clinical details) treated with ICIs, durvalumab (anti-PD-L1; AstraZeneca) alone or in combination with ipilimumab (anti-CTLA4; Bristol Myers Squibb). In 6 out of 20 EVOCs (30%; 95% confidence interval 14.5–51.9%) the immunotherapy-induced IFNγ elevation (either by durvalumab alone or in combination with ipilimumab) was statistically significant (by Student’s t test comparing three repeats of treated versus non-treated) as well as greater than an arbitrary threshold of 1.9-fold induction. These EVOCs were designated as “responders”; while the rest were termed “non-responders” (Fig. 3b). The induction of IFNγ in the responders group ranged between 1.9- and 5.2-fold with the highest induction seen with a combination of durvalumab and ipilimumab (Fig. 3b). The average IFNγ fold induction with durvalumab treatment was 2.2, while the combined durvalumab and ipilimumab treatment-induced IFNγ 3.5-fold. This enhancement of the IFNγ induction by the addition of ipilimumab did not reach statistical significance; however, it suggests that anti-CTLA4 may be active also within the TME.
Fig. 3.
EVOC responsiveness to immune signals. a EVOCs were treated (+ IL2) or not (NT) with IL-2 for 4 days. Representative experiment showing average and standard deviation of IFNγ and CD8 gene expression (qRT-PCR; each condition was tested in triplicate). b EVOCs were treated for four days with either durvalumab (Durva; n = 17) or combination of durvalumab and ipilimumab (Durva + Ipi; n = 15; different numbers due to limited material, thus not all EVOC experiments included both treatment types). Immunotherapy-induced IFNγ fold-induction was calculated as the ratio of treated to non-treated IFNγ expression (measured as in A) for each EVOC/treatment. Each point represents one EVOC/treatment. IFNγ fold-induction is shown for “responders” (R), and “non-responders” (N). Centre line is the median, the box limits are the lower and upper quartiles, and the whiskers extend to the most extreme values within 1.5 × the interquartile range. The differences between responders and non-responders group in each treatment group are statistically significant at p < 0.0014 (Mann–Whitney test). c Basal (non-induced) IFNγ expression levels determined in non-treated EVOCs, four days after EVOC initiation, in non-responder EVOCs (N) and responders (R; represented as -∆CT; normalized to GAPDH). Box plot representation as in B. The differences between the groups are statistically significant (p < 0.003, Mann–Whitney test)
Interestingly, we found that basal, non-induced IFNγ levels (−∆CT; measured 4 days after EVOC initiation) were on average 1.5-fold higher in the responders vs. the non-responders (Mann–Whitney p < 0.003; Fig. 3c). Thus, the non-induced immune activation level of EVOCs, determined by IFNγ expression level, may be a predictive factor for response to ICI. In addition, basal mRNA expression levels of CD8, CD4, and FOXP3 in non-treated-EVOCs were also found to be statistically higher in the responders' group versus the non-responders (Supplementary Figure S2) stressing the importance of the non-induced immune status in the EVOC model.
Immunotherapy-responsive EVOCs correlate with ‘hot’ tumors
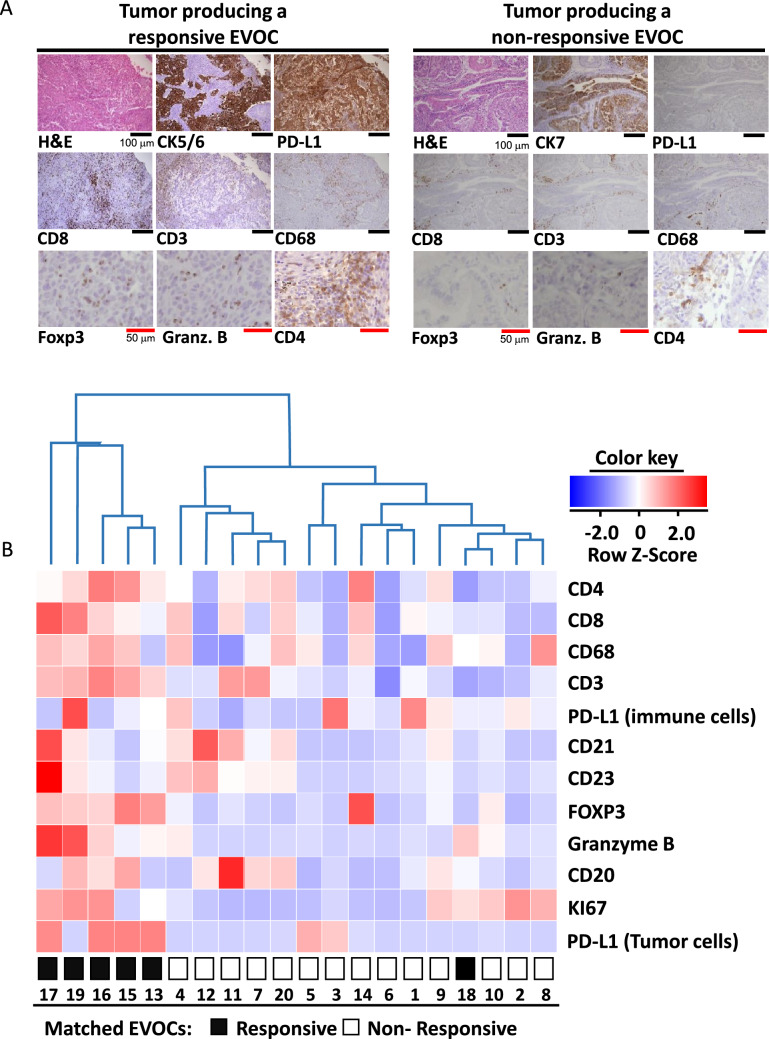
To characterize the immune status of the tumors from which the EVOCs originated, we conducted IHC analysis of several key proteins in the tumors. IHC was performed to assess the major immune cell types based on CD3 (representing T cells), CD8 (for cytotoxic T cells), CD4 (mostly representing T-helper cells, in some cases also regulatory T cells [T-regs]), CD68 (for macrophages), CD21 and CD23 (for follicular dendritic cells), CD20 (for B cells), and FOXP3 (for T-regs, in some cases expressed also by effector T cells). In addition, Granzyme B was evaluated as a measure of activation level of cytotoxic T cells and PD-L1 expression on immune cells and on tumor cells was interrogated. Representative IHC images of tumors corresponding to immunotherapy-responsive and non-responsive EVOCs are shown in Fig. 4a. Regarding the general pattern of immune cell infiltration, no clear cases of T-cell exclusion were seen. Interestingly, we noted two cases where CD20-positive cells were relatively concentrated in the tumor–stroma interphase (data not shown). Of these two cases, one was a clear responder. We next performed detailed quantitative analysis of the various IHC results. Unsupervised hierarchical clustering of the IHC results separated the tumors into five ‘hot’ and 15 ‘cold’ tumors (right and left, respectively, of the first fork in the dendrogram, Fig. 4b). All of the EVOCs originating from ‘hot’ tumors were responders as defined by the in vitro IFNγ induction (five of five), and almost all of the EVOCs stemming from ‘cold’ tumors were non-responders (14 of 15). Fisher’s test for the correlation between the tumor-IHC-based separation and the response status of the corresponding EVOCs demonstrated a significance at p < 0.001. We further investigated the correlation between average IFNγ induction in each of the EVOC experiments and protein expression in the matched FFPE specimens (Table 1). CD8 and granzyme B protein expression were highly correlated with EVOC response to either durvalumab or durvalumab and ipilimumab combination regimens. A significant but weaker correlation was found for FOXP3, CD3, CD4, CD68, and tumor-PD-L1 with the response to durvalumab treatment only.
Fig. 4.
Correlation between key immune proteins’ expression in tumors and immunotherapy response in the matched EVOCs. a Representative microscopic images of IHC conducted for the indicated proteins on FFPE specimens of the tumors from which the EVOCs were produced. CK7 or CK5/6 were stained to identify epithelial cancer cells. Left—tumor producing a responsive EVOC, right—tumor producing a non- responsive EVOC. Black scale bars represent 100 μm, red scale bars—50 μm. b Heat map of 12 proteins expression in immune-responsive and non-responsive EVOCs with hierarchical clustering. Rows correspond to IHC quantification of individual proteins, columns correspond to EVOC samples. Besides the proteins depicted in A, IHC results for CD21 and CD23, identifying follicular dendritic cells, and KI67, indicative of cell proliferation rate, were also included. IHC of PD-L1, quantified separately for immune and tumor cells was incorporated. At the bottom: immunotherapy response of the corresponding EVOCs, coded as indicated. H&E: Hematoxylin and eosin. Granz.B: granzyme B
Table 1.
Correlation between expression of key proteins in FFPE specimens analyzed by IHC, and the IFNγ-induction in the corresponding EVOCs (for each treatment, IFNγ mRNA expression in the treated EVOC is compared to non-treated EVOC)
| Durvalumab | Durvalumab+Ipilimumab | |||
|---|---|---|---|---|
| Marker | r | p | r | p |
| Granzyme B | 0.831 | < 0.001 | 0.853 | < 0.001 |
| CD8 | 0.811 | < 0.001 | 0.783 | < 0.001 |
| FOXP3 | 0.651 | 0.006 | 0.464 | 0.051 |
| PD-L1 (Tumor cells) | 0.626 | 0.009 | 0.373 | 0.171 |
| CD68 | 0.537 | 0.032 | 0.383 | 0.159 |
| CD4 | 0.533 | 0.034 | 0.312 | 0.257 |
| CD3 | 0.513 | 0.042 | 0.508 | 0.053 |
| KI67 | 0.412 | 0.113 | 0.113 | 0.051 |
| CD20 | 0.125 | 0.644 | 0.090 | 0.749 |
| PD-L1 (immune cells) | − 0.412 | 0.089 | 0.155 | 0.582 |
Results are shown for durvalumab treatments (n = 17), and for combined durvalumab with ipilimumab treatments (n = 15). r; Pearson correlation coefficient. P; p-value of this correlation. Significant results are highlighted in bold
TLS are ectopic lymphoid structures, developing outside lymphoid organs at sites of prolonged inflammation or cancer [30], whose baseline prevalence in cancer correlates with better outcome and with response to immunotherapy [30, 31]. We therefore evaluated TLS abundance in the FFPE specimens as a potential predictor of immune response in the EVOC model (see Supplementary Figure S3 for representative TLSs). Indeed, a positive correlation of moderate strength was found between the TLS density and the IFNγ induction levels in the corresponding EVOCs (Supplementary Table S3). No difference was found in the strength of this correlation when evaluated separately in the durvalumab treatment results or in the durvalumab-ipilimumab combination treatment results. In addition, we found a moderate positive correlation between TLS density and the density of CD8-positive cells, as well as between TLS density and the density of granzyme B-positive cells (Supplementary Table S3). TLS in tumors require further studies as potentially important mediators of the response to ICIs.
Discussion
We have developed a novel and simple protocol for ex-vivo modeling of cancer, fulfilling several pre-defined required characteristics. Specifically, the partial disintegration protocol we have utilized retains the epithelial cancer cells within their native TME and incorporates the tissue-resident immune cells. Unlike apparently similar protocols [14], the procedure we used involves random mixing of cell clusters and single cells from all parts of the tumor sample, aimed at ensuring their representation in all of the replicates of an EVOC experiment. We believe that, in this manner, we avoid the problem of heterogeneity between replicates that may exist in models using larger fragments. Importantly, our model is based on fresh tumor samples, without the addition of specific growth factors or signaling molecules, therefore remaining as close as possible to the actual tumor. Preliminary use of this model allowed us to identify a set of genes and proteins whose levels correlate with the response of early NSCLC EVOCs to checkpoint inhibitors. Basal CD8 and Granzyme B quantified by IHC staining were found to be the biomarkers most highly correlated with response to immunotherapy in the EVOC model. Interestingly, addition of anti-CTLA-4 to anti-PD-L1 demonstrated a trend for stronger activation of local immune response. Since our model completely lacks the tumor-draining lymph nodes, these results suggest that immune activation by anti-CTLA-4 may not require the involvement of lymphoid organs as commonly accepted [32]. Therefore, our observations contribute to the ongoing debate regarding the site of ICI-facilitated T-cell priming, occurring in the TME or within tumor-draining lymph nodes [6].
The set of proteins and genes we have identified as correlated with immune activation by ICIs includes proteins that are known to be involved in tumor-immune rejection, although not studied so far as biomarkers of response to ICI of early stage tumors. PD-L1 tumor expression is the only biomarker utilized today for prediction of response to ICI in advanced NSCLC, and has not yet been validated in early NSCLC. Density of CD8, Granzyme B, and CD68-positive cells were shown to correlate with the density of PD-L1-positive cells in NSCLC [33]. CD8-positive T-cell density in resected NSCLC specimens was demonstrated to be a robust prognostic marker, regardless of treatments administered [34]. Baseline mRNA IFNγ levels were tested as part of an ‘effector T-cell gene signature’ (T-eff) composed of PD-L1, CXCL9, and IFNγ. In a study of advanced NSCLC patients, comparing atezolizumab (anti-PD-L1 antibody) versus chemotherapy, both high T-eff patients as well as low T-eff patients benefited from ICI [35]. Although the biomarkers for ICI response we highlight are mostly not unexpected, demonstration of their potential value in the context of early resectable cancer is novel.
One of the proteins we highlight is FOXP3, recognized as a marker of T-reg cells, thus might be expected to negatively correlate with immune response. However, the role of T-reg cells is not always immune-inhibitory; in a model of viral infection, T-regs were required for effective immune response in peripheral tissues, while an inhibitory effect was seen in the draining lymph nodes [36]. Positive correlation between T-reg infiltration assessed by FOXP3 IHC and response to anti-CTLA-4 in melanoma patients has been reported [37, 38]. Additional consideration is the transient and lower level but identifiable expression of FOXP3 in effector T cells [39] that might be alternative explanation for the positive correlation we found between FOXP3 and immune activation. T-regs density might simply correlate with a general state of immune cell infiltration as an expected self-regulatory mechanism. Nevertheless, the strong correlation we found between FOXP3-expressing cells and ICI activity supports the need for further studies of the role of T-regs in early versus advanced tumors.
Considering the current interest in neo-adjuvant immunotherapy for early resectable NSCLC [6, 40] and considering the potential difference in the immune system between localized and metastatic tumors [6], our results might be most relevant in the context of neo-adjuvant clinical studies. Interestingly, most of the biomarkers we identify correlate with response to durvalumab and not with the response to combination durvalumab and ipilimumab. The combination of anti-PD-1 with anti-CTLA-4 has recently been proven to be of benefit for advanced NSCLC, but at the price of heightened toxicity [41]. Both anti-PD-(L)1 alone or in combination with anti-CTLA-4 are being tested currently also in the early, neo-adjuvant setting, stressing the need for the identification of biomarkers specific for a single ICI versus an ICI combination regimen.
Our goal of using minimal exogenous interventions implied a short lifespan of the EVOC (up to 5 days; data not shown), since we did not use factors known to prolong in vitro cell survival, immune cell activation, and growth [17, 42]. This time window did not allow us to demonstrate robust tumor cell eradication by immunotherapy or to study T-cell expansion and clonal evolution. Instead, we focused on IFNγ induction, known to occur as part of the activation of cytotoxic T cells and to be strongly correlated with tumor rejection [27]. Another drawback of our study is the fact that due to the limiting amounts of tissue, we did not test the impact of anti-CTLA-4 alone in the EVOC model. However, considering the lack of clinical efficacy of such treatment as a single checkpoint inhibitor in lung cancer [43], such experiments seemed unlikely to be informative and were not carried out. The interpretation of our results requires caution due to the relatively small number of samples included, and due to the lack of statistically significant differences between the immune response of the EVOC to durvalumab versus durvalumab and ipilimumab. Nevertheless, the EVOC model we suggest provides a valuable tool for the investigation of immune responses in situ. Retaining the TME and avoiding the use of specific signaling molecules provide a model that is highly representative of the original, naïve tumor. The lack of requirement for complex media components or microfluidic systems may facilitate its dissemination as a useful and widely applicable research tool of cancer immunotherapy. Our novel EVOC model provides a set of potentially predictive biomarkers for response to neo-adjuvant ICI in early NSCLC, that requires further validation.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- EVOC
Ex-vivo organ culture
- FCS
Fetal calf serum
- FFPE
Formalin-fixed paraffin embedded
- H&E
Hematoxylin and eosin
- ICI
Immune checkpoint inhibitors
- IHC
Immunohistochemistry
- NSCLC
Non-small cell lung cancer
- PDX
Patient-derived xenograft
- PD-L1
Programmed cell death-1 ligand
- TLS
Tertiary lymphoid structures
- TME
Tumor microenvironment
- T-regs
Regulatory T cells
Author contributions
IK, EBD and JB designed the experiments; OZ, EO, IDM, GOHS, ALBN, IB and AO: acquisition of data and performance of experiments. IK, EBD and TG: data analysis. IK, EBD and JB wrote the manuscript. All authors read the final version and approved the manuscript.
Funding
This study was partly supported by a research grants from AstraZeneca, [NCR-15-11358]; Ministry of health, Israel [#3-14487]; and The Israel Cancer Association through ICA-USA Board of Directors [#20190081].
Compliance with ethical standards
Conflict of interest
A.O. reports research funding for the institution from Boehringer Ingelheim, Roche, Sanofi-Aventis, Xcovery, AstraZeneca, BMS and MSD, advisory fees from Boehringer Ingelheim, MSD, Takeda and AstraZeneca, and honoraria from Takeda, Roche and Boehringer Ingelheim. J.B reports research funding for the institution from Roche, MSD, BMS, AstraZeneca, Pfizer, Abbvie, and consulting fees from Roche, MSD, BMS, AstraZeneca, Pfizer, Takeda, Novartis, Boehringer Ingelheim, VBL and Bayer. All reported above are unrelated to the submitted work; All other authors declare no potential conflict of interests.
Ethics approval
All patients provided written informed consent for the use of blood samples and tumor specimens for research. This study was approved by the Sheba Medical Center ethics committee (approvals #2019-SMC and #8990-11-SMC).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Iris Kamer and Elizabeta Bab-Dinitz equal contribution.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7–H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18:31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 5.Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer. JAMA Oncol. 2019;5:1276. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367:eaax0182. doi: 10.1126/science.aax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bar J, Moskovits N, Oren M. Involvement of stromal p53 in tumor-stroma interactions. Semin Cell Dev Biol. 2010;21:47–54. doi: 10.1016/j.semcdb.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 10.Aparicio S, Hidalgo M, Kung AL. Examining the utility of patient-derived xenograft mouse models. Nat Rev Cancer. 2015;15:311–316. doi: 10.1038/nrc3944. [DOI] [PubMed] [Google Scholar]
- 11.Lai Y, Wei X, Lin S, et al. Current status and perspectives of patient-derived xenograft models in cancer research. J Hematol Oncol. 2017;10:106. doi: 10.1186/s13045-017-0470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behrens D, Rolff J, Hoffmann J. Predictive in vivo models for oncology. Handb Exp Pharmacol. 2016;232:203–221. doi: 10.1007/164_2015_29. [DOI] [PubMed] [Google Scholar]
- 13.Endo H, Okami J, Okuyama H, et al. Spheroid culture of primary lung cancer cells with neuregulin 1/HER3 pathway activation. J Thorac Oncol. 2013;8:131–139. doi: 10.1097/JTO.0b013e3182779ccf. [DOI] [PubMed] [Google Scholar]
- 14.Majumder B, Baraneedharan U, Thiyagarajan S, et al. Predicting clinical response to anticancer drugs using an ex vivo platform that captures tumour heterogeneity. Nat Commun. 2015;6:6169–6183. doi: 10.1038/ncomms7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karekla E, Liao W-J, Sharp B, Pugh J, Reid H, Quesne JL, Moore D, Pritchard C, MacFarlane M, Pringle JH. Ex vivo explant cultures of non-small cell lung carcinoma enable evaluation of primary tumor responses to anticancer therapy. Cancer Res. 2017;77(8):2029–2039. doi: 10.1158/0008-5472.CAN-16-1121. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, Mun H, Sung CO, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019;10:3991–4005. doi: 10.1038/s41467-019-11867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neal JT, Li X, Zhu J, et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175:1972–1988.e16. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins RW, Aref AR, Lizotte PH, et al. Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov. 2018;8:196–215. doi: 10.1158/2159-8290.CD-17-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dijkstra KK, Cattaneo CM, Weeber F, et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174:1586–1598.e12. doi: 10.1016/j.cell.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019 doi: 10.15252/embj.2018100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic Anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3:1052–1062. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 22.Centanni M, Moes DJAR, Trocóniz IF, et al. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet. 2019;58:835–857. doi: 10.1007/s40262-019-00748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuman T, London M, Kania-Almog J, et al. A harmonization study for the use of 22C3 PD-L1 immunohistochemical staining on ventana’s platform. J Thorac Oncol. 2016;11:1863–1868. doi: 10.1016/j.jtho.2016.08.146. [DOI] [PubMed] [Google Scholar]
- 24.Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015;26:812–817. doi: 10.1093/annonc/mdv009. [DOI] [PubMed] [Google Scholar]
- 25.Ujiie H, Kadota K, Chaft JE, et al. Solid predominant histologic subtype in resected stage I lung adenocarcinoma is an independent predictor of early, extrathoracic, multisite recurrence and of poor postrecurrence survival. J Clin Oncol. 2015;33:2877–2884. doi: 10.1200/JCO.2015.60.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung JJ, Yeh YC, Wu YC, et al. Prognostic factors in completely resected node-negative lung adenocarcinoma of 3 cm or smaller. J Thorac Oncol. 2017;12:1824–1833. doi: 10.1016/j.jtho.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Ayers M, Lunceford J, Nebozhyn M, et al. IFNγ-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 29.Blons H, Garinet S, Laurent-Puig P, Oudart JB. Molecular markers and prediction of response to immunotherapy in non-small cell lung cancer, an update. J Thorac Dis. 2019;11:S25–S36. doi: 10.21037/jtd.2018.12.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 31.Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 33.Silva MA, Ryall KA, Wilm C, et al. PD-L1 immunostaining scoring for non-small cell lung cancer based on immunosurveillance parameters. PLoS ONE. 2018;13:e0196464. doi: 10.1371/journal.pone.0196464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnem T, Hald SM, Paulsen E-E, et al. Stromal CD8 + T-cell density—A promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res. 2015;21:2635–2643. doi: 10.1158/1078-0432.CCR-14-1905. [DOI] [PubMed] [Google Scholar]
- 35.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 36.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribas A, Comin-Anduix B, Economou JS, et al. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res. 2009;15:390–399. doi: 10.1158/1078-0432.CCR-08-0783. [DOI] [PubMed] [Google Scholar]
- 38.Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 40.Rhodin KE, Rucker AJ, Ready NE, et al. The immunotherapeutic landscape in non–small cell lung cancer and its surgical horizons. J Thorac Cardiovasc Surg. 2020;159:1616–1623. doi: 10.1016/j.jtcvs.2019.08.138. [DOI] [PubMed] [Google Scholar]
- 41.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 42.Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 43.Govindan R, Szczesna A, Ahn MJ, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non–small-cell lung cancer. J Clin Oncol. 2017;35:3449–3457. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.