Abstract
Background
Immunotherapy has determined unprecedented long-term responses in several hematological and solid tumors. In the MOUSEION-03 study, we conducted a meta-analysis to determine the possibility of achieving complete remissions (CR) with immunotherapy or immuno-oncology combinations in cancer patients.
Methods
The primary endpoint was to assess the incidence of CR in cancer patients receiving immune checkpoint inhibitors (ICIs) alone or in combination with other agents versus control treatments. The pooled odds ratio (OR) and 95% confidence interval (CI) for CR rate were extracted.
Results
A total of 12,130 potentially relevant trials were identified; 5 phase II and 80 phase III randomized studies (37 monotherapies and 48 combinations) and 49,425 cancer patients were included. The most frequent types of malignancies were non-small cell lung cancer (n = 14,249; 29%), urothelial cancer (n = 6536; 13%), renal cell carcinoma (n = 5743; 12%), and melanoma (n = 2904; 6%). In patients treated with immunotherapy (as monotherapy or in combination with other anticancer agents), the pooled OR was 1.67 (1.52–1.84). The highest OR was registered by immune-based combinations with two ICIs (3.56, 95% CI 1.28–9.90).
Conclusions
To the best of the authors’ knowledge, no comprehensive meta-analysis on the use of ICIs and ICI-based combinations in solid tumors to systematically investigate the probability to achieve CR has been published so far. Although CR is not a common event in several cancer patients receiving immunotherapy, the MOUSEION-03 suggests that the use of ICIs may significantly increase the chance of achieving CR in comparison with control treatments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03349-4.
Keywords: Pembrolizumab, Cancer, Complete response, Immuno-oncology combinations, Immunotherapy, Meta-analysis
Introduction
Immunotherapy has revolutionized the treatment scenario for hematological and solid tumors and has reported unprecedented clinical benefits in several settings. Multiple recent reports have supported the long-term benefit of immunotherapy, with even the possibility—in selected cases—to cure cancer patients [1, 2]. The current armamentarium of available immunotherapies for cancer patients encompasses several types of anticancer agents, including immune checkpoint inhibitors (ICIs) targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed death 1 (PD-1) or its ligand [3, 4]; in addition, beyond immunomodulatory antibodies, several other agents and immune-based treatments have been assessed and are currently under evaluation, including adoptive cell transfer (ACT), oncolytic virus therapy, and vaccines. More recently, the possibility of combining immunotherapy with other systemic chemotherapies, antiangiogenic agents, or targeted therapies as well as other ICIs has emerged as a novel standard of care in a variety of tumors, including hepatocellular carcinoma, renal cell carcinoma (RCC), and non-small cell lung cancer (NSCLC) [5–7].
According to RECIST 1.1 criteria [8], complete remission (CR) is defined as the disappearance of all target lesions in response to therapy, and achieving CR and maintaining it for more than 5 years is the “sine qua non” for considering a patient as potentially cured. Nowadays, the short median duration of the follow-up of cancer patients treated by immunotherapy or immuno-oncology combinations in clinical trials hardly allows estimating the rate of subjects who will maintain lifelong CR. At this regard, assessing the possibility to obtain CR results fundamental to enter in the second phase of the immunotherapy era, in which clinicians will finally not be afraid to tell a patient: “You have been definitively cured.” Therefore, it is of pivotal importance to assess the probability of achieving CR with immunotherapy, and whether the use of ICIs would increase CR in cancer patients.
To the best of our knowledge, the MOUSEION-03 study is the first study aimed to systematically investigate the possibility of achieving CR in patients affected by solid tumors treated with immunotherapy or immuno-oncology combinations through a large up-to-date study-level meta-analysis of available randomized trials.
Materials and methods
Selection of studies and data extraction
Study selection was carried out according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [9]. To identify relevant clinical trials, four authors (MS, FM, AR, and VM) reviewed citations from PubMed, MEDLINE, Embase, and Scopus from January 1966 to February 2022. The search was performed by combining the words “cancer” or “solid tumor” with the following words: “atezolizumab,” “avelumab,” “camrelizumab,” “cemiplimab,” “CTLA-4,” “cytotoxic T-lymphocyte-associated protein-4,” “durvalumab,” “immune checkpoint inhibitor,” “ipilimumab,” “nivolumab,” “PD-1,” “PD-L1,” “pembrolizumab,” “programmed cell death receptor-1,” “tislelizumab,” and “tremelimumab.” The search was limited to human studies and randomized clinical trials published in English that met the following criteria: (1) prospective randomized phase III trials of patients with solid tumors; (2) random assignment of participants to treatment with immunotherapy or control (active therapy) and (3) available data on outcome in males and females. When multiple publications of the same clinical trial were encountered, only the most recent or most complete reporting of that trial was included. Studies including ≥ 3 treatment arms were divided to compare each experimental arm with the control arm. Disagreements about trials were discussed and resolved by all investigators.
The primary objective of this study was to assess the possibility, expressed as OR, of achieving CR in patients treated by immunotherapy alone or combined with other immuno-, chemo- or targeted therapies. Phase I, phase II, and randomized phase III trials including immunotherapy in both experimental and control arms were excluded, as well as studies with placebo as control arm. The meta-analysis was conducted according to PRISMA guidelines (Supplementary Material).
Statistical design
All statistical analyses were performed using RStudio.
Odds Ratios (ORs) were used to analyze dichotomous variables, including CR rate in cancer patients treated with immunotherapy versus control arms. Forest plots were used to assess ORs. Statistical heterogeneity between the included trials was examined using the chi-square test and the I2 statistic; substantial heterogeneity was present when the I2 value was greater than 50% or there was a low p value (< 0.10) in the chi-square test. When no heterogeneity was noted, the fixed-effects model was used, while the random-effects model was applied in the presence of significant heterogeneity.
Results
Search results
A total of 12,130 potentially relevant studies investigating immunotherapy or immuno-oncology combinations in cancer patients were identified; 10,086 studies were excluded for at least one of the following reasons: observational and in vitro studies, review articles, meta-analyses, case reports, editorials, letters, or commentaries. Subsequently, among the 2044 selected clinical trials, 1959 studies were immediately excluded for at least one of the following reasons: phase I or phase II non-randomized studies, both control and treatment groups who received immunotherapy, non-active therapy as control arm or insufficient data on CR (Fig. 1). At the end of this review process, 77 papers [10 − 86] were considered to be of adequate quality and relevance for this analysis. Nine of them [15, 19, 29, 30, 37, 45, 63, 71, 81] were divided into 2 distinct studies for each one due to presence of 2 experimental arms and 1 control arm in each one, for a total of 85 randomized controlled trials (Fig. 1). The baseline characteristics of each trial are summarized in Table 1.
Fig. 1.
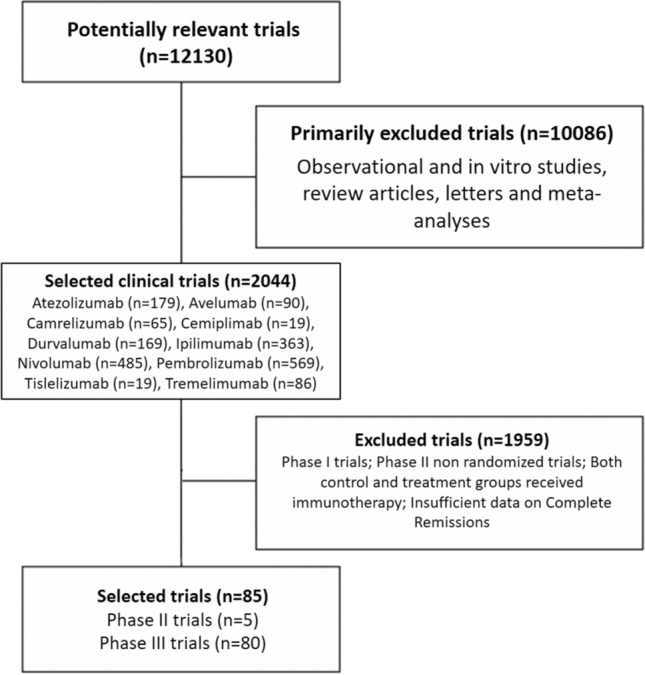
Selection of randomized controlled trials (RCTs) included in the meta-analysis according to PRISMA statement
Table 1.
Baseline characteristics of randomized trials included in the meta-analysis
| Author | Year | REF | Phase | Malignancy | Treatment arm | Control arm | N events (Treatment arm) | N of patients (Treatment arm) | N events (Control arm) | N of patients (Control arm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Rittmeyer et al. | 2017 | 10 | 3 | NSCLC | Atezolizumab | Chemotherapy | 6 | 425 | 1 | 425 |
| Schmid et al. | 2018 | 11 | 3 | TNBC | Atezolizumab + nab-paclitaxel | Nab-paclitaxel | 32 | 445 | 7 | 445 |
| Socinski et al. | 2018 | 12 | 3 | Non-squamous NSCLC | Atezolizumab + bevacizumab + chemotherapy | Bevacizumab + chemotherapy | 13 | 353 | 4 | 331 |
| Powles et al. | 2018 | 13 | 3 | UC | Atezolizumab | Chemotherapy | 16 | 467 | 16 | 464 |
| Horn et al. | 2018 | 14 | 3 | SCLC | Atezolizumab + chemotherapy | Chemotherapy | 5 | 201 | 2 | 202 |
| Eng et al. (A) | 2019 | 15A | 3 | CRC | Atezolizumab + cobimetinib | Regorafenib | 0 | 183 | 0 | 90 |
| Eng et al. (B) | 2019 | 15B | 3 | CRC | Atezolizumab | Regorafenib | 0 | 90 | 0 | 90 |
| West et al. | 2019 | 16 | 3 | Non-squamous NSCLC | Atezolizumab plus chemotherapy | Chemotherapy | 11 | 447 | 3 | 226 |
| Rini et al. | 2019 | 17 | 3 | RCC | Atezolizumab + bevacizumab | Sunitinib | 24 | 454 | 10 | 461 |
| Finn et al. | 2020 | 18 | 3 | HCC | Atezolizumab + bevacizumab | Sorafenib | 18 | 326 | 0 | 159 |
| Galsky et al. (A) | 2020 | 19A | 3 | UC | Atezolizumab + chemotherapy | Chemotherapy | 56 | 447 | 27 | 397 |
| Galsky et al. (B) | 2020 | 19B | 3 | UC | Atezolizumab | Chemotherapy | 22 | 359 | 27 | 397 |
| Gutzmer et al. | 2020 | 20 | 3 | Melanoma | Atezolizumab + vemurafenib + cobimetinib | Vemurafenib + cobimetinib | 40 | 256 | 44 | 258 |
| Mittendorf et al. | 2020 | 21 | 3 | TNBC | Atezolizumab + chemotherapy | Chemotherapy | 95 | 165 | 69 | 168 |
| Jotte et al. | 2020 | 22 | 3 | Squamous NSCLC | Atezolizumab + carboplatin + nab-paclitaxel | Carboplatin + nab-paclitaxel | 8 | 342 | 5 | 339 |
| Bang et al. | 2018 | 23 | 3 | GC/GEJC | Avelumab | Chemotherapy | 1 | 185 | 1 | 186 |
| Barlesi et al. | 2018 | 24 | 3 | NSCLC | Avelumab | Docetaxel | 5 | 396 | 2 | 396 |
| Choueiri et al. | 2020 | 25 | 3 | RCC | Avelumab + axitinib | Sunitinib | 17 | 442 | 9 | 444 |
| Powles et al. | 2020 | 26 | 3 | UC | Avelumab | BSC | 6 | 350 | 0 | 350 |
| Moehler et al. | 2021 | 27 | 3 | GC/GEJC | Avelumab | Chemotherapy | 8 | 249 | 5 | 250 |
| Lee et al. | 2021 | 28 | 3 | HNT | Avelumab + chemoradiotherapy | Chemoradiotherapy | 167 | 350 | 177 | 347 |
| Pujade-Lauraine et al. | 2021 | 29A | 3 | Ovarian cancer | Avelumab | PLD | 0 | 188 | 0 | 190 |
| Pujade-Lauraine et al. | 2021 | 29B | 3 | Ovarian cancer | Avelumab ± PLD | PLD | 2 | 188 | 0 | 190 |
| Monk et al. | 2021 | 30A | 3 | Ovarian cancer | Chemotherapy—> manteinance avelumab | Chemotherapy—> observation | 17 | 328 | 30 | 334 |
| Monk et al. | 2021 | 30B | 3 | Ovarian cancer | Chemotherapy + avelumab—> manteinance avelumab | Chemotherapy—> observation | 20 | 329 | 30 | 334 |
| Huang Jing et al. | 2020 | 31 | 3 | Oesophageal cancer | Camrelizumab | Chemotherapy | 1 | 228 | 1 | 220 |
| Zhu et al. | 2020 | 32 | 3 | NSCLC | Camrelizumab + chemotherapy | Chemotherapy | 8 | 205 | 5 | 207 |
| Luo et al. | 2021 | 33 | 3 | Oesophageal cancer | Camrelizumab + chemotherapy | Chemotherapy | 7 | 298 | 5 | 298 |
| Yang et al. | 2021 | 34 | 3 | Nasopharingeal carcinoma | Camrelizumab + chemotherapy | Chemotherapy | 7 | 134 | 4 | 129 |
| Sezer et al. | 2021 | 35 | 3 | NSCLC | Cemiplimab | Chemotherapy | 6 | 283 | 3 | 280 |
| Paz-Ares et al. | 2019 | 36 | 3 | SCLC | Durvalumab + carboplatin + etoposide | Carboplatin + etoposide | 6 | 268 | 2 | 269 |
| Powles et al. (A) | 2020 | 37A | 3 | UC | Durvalumab + tremelimumab | Chemotherapy | 27 | 342 | 22 | 344 |
| Powles et al. (B) | 2020 | 37B | 3 | UC | Durvalumab | chemotherapy | 27 | 346 | 22 | 344 |
| Robert et al. | 2011 | 38 | 3 | Melanoma | Ipilimumab + dacarbazine | dacarbazine | 4 | 250 | 2 | 252 |
| Reck et al. | 2016 | 39 | 3 | SCLC | Ipilimumab + platinum + etoposide | Platinum + etoposide | 1 | 478 | 0 | 476 |
| Govindan R et al | 2017 | 40 | 3 | Squamous NSCLC | Ipilimumab + carboplatin + paclitaxel | Carboplatin + paclitaxel | 1 | 388 | 2 | 361 |
| Robert et al. | 2015 | 41 | 3 | Melanoma | Nivolumab | Dacarbazine | 16 | 210 | 2 | 208 |
| Weber et al. | 2015 | 42 | 3 | Melanoma | Nivolumab | Chemotherapy | 4 | 120 | 0 | 47 |
| Borghaei et al. | 2015 | 43 | 3 | Non-squamous NSCLC | Nivolumab | Docetaxel | 4 | 292 | 1 | 290 |
| Brahmer et al. | 2015 | 44 | 3 | Squamous NSCLC | Nivolumab | Docetaxel | 1 | 135 | 0 | 137 |
| Motzer et al. | 2015 | 45 | 3 | RCC | Nivolumab | Everolimus | 4 | 410 | 2 | 411 |
| Motzer et al. | 2018 | 46 | 3 | RCC | Nivolumab + ipilimumab | Sunitinib | 40 | 425 | 5 | 422 |
| Gillison | 2018 | 47 | 3 | HNT | Nivolumab | Chemotherapy | 6 | 240 | 1 | 121 |
| Hellmann MD et al. | 2019 | 48 | 3 | NSCLC | Nivolumab + ipilimumab | Chemotherapy | 33 | 576 | 10 | 570 |
| Kato et al. | 2019 | 49 | 3 | Oesophageal cancer | Nivolumab | Chemotherapy | 1 | 171 | 2 | 158 |
| Wu et al. | 2019 | 50 | 3 | NSCLC | Nivolumab | Docetaxel | 1 | 338 | 0 | 166 |
| Reardon et al. | 2020 | 51 | 3 | Glioblastoma | Nivolumab | Bevacizumab | 2 | 184 | 4 | 185 |
| Tsujikawa et al. | 2020 | 52 | 2 | Pancreatic | Cyclophosphamide/Nivo/GVAX + Nivo/CRS-207 | Cyclophosphamide/GVAX + CRS-207 | 0 | 51 | 0 | 42 |
| Hamanishi et al. | 2021 | 53 | 3 | Ovarian cancer | Nivolumab | Chemotherapy | 2 | 119 | 2 | 114 |
| Baas et al. | 2021 | 54 | 3 | MPM | Nivolumab + ipilimumab | Chemotherapy | 5 | 303 | 0 | 302 |
| Reck et al. | 2021 | 55 | 3 | NSCLC | Nivolumab + ipilimumab + chemotherapy | Chemotherapy | 12 | 361 | 4 | 358 |
| Sugawara et al. | 2021 | 56 | 3 | NSCLC | Nivolumab + CHT/Beva | Placebo + CHT/Beva | 14 | 275 | 8 | 275 |
| Spigel et. al. | 2021 | 57 | 3 | SCLC | Nivolumab | chemotherapy (topotecan or amrubicin) | 1 | 284 | 1 | 285 |
| Janjigian et al. | 2021 | 58 | 3 | Gastric/G-O junction | Nivolumab + chemotherapy | Chemotherapy | 59 | 603 | 39 | 608 |
| Choueiri et al. | 2021 | 59 | 3 | RCC | Nivolumab + cabozantinib | Sunitinib | 26 | 323 | 15 | 328 |
| Ribas et al. | 2015 | 60 | 2 | Melanoma | Pembrolizumab | Chemotherapy | 9 | 357 | 0 | 171 |
| Herbst et al. | 2016 | 61 | 2/3 | NSCLC | Pembrolizumab | Docetaxel | 0 | 682 | 0 | 309 |
| Paz-Ares et al. | 2018 | 62 | 3 | Squamous NSCLC | Pembrolizumab + chemotherapy | Chemotherapy | 4 | 278 | 6 | 281 |
| Gandhi et al. | 2018 | 63 | 3 | NSCLC | Pembrolizumab + chemotherapy | Chemotherapy | 2 | 410 | 1 | 206 |
| Burtness et al. (A) | 2019 | 64A | 3 | HNT | Pembrolizumab | Cetuximab + chemotherapy | 6 | 300 | 3 | 287 |
| Burtness et al. (B) | 2019 | 64B | 3 | HNT | Pembrolizumab + chemotherapy | Cetuximab + chemotherapy | 9 | 278 | 1 | 266 |
| Fradet et al. | 2019 | 65 | 3 | UC | Pembrolizumab | Chemotherapy | 25 | 270 | 8 | 272 |
| Cohen et al. | 2019 | 66 | 3 | HNT | Pembrolizumab | Chemotherapy | 1 | 246 | 0 | 234 |
| Mok et al. | 2019 | 67 | 3 | NSCLC | Pembrolizumab | Chemotherapy | 3 | 637 | 3 | 637 |
| Rini et al. | 2019 | 68 | 3 | RCC | Pembrolizumab + axitinib | Sunitinib | 25 | 432 | 8 | 429 |
| Powles et al. | 2020 | 69 | 3 | ccRCC | Pembrolizumab + axitinib | Sunitinib | 38 | 429 | 13 | 425 |
| Paz-Ares et al. | 2020 | 70 | 3 | Squamous NSCLC | Pembrolizumab + chemotherapy | Placebo + chemotherapy | 6 | 278 | 9 | 281 |
| Schmid et al. | 2020 | 71 | 3 | TNBC | Pembrolizumab + chemotherapy | Chemotherapy | 260 | 401 | 103 | 201 |
| Shitara et al. | 2020 | 72A | 3 | GC/GEJC | Pembrolizumab | Chemotherapy | 9 | 256 | 14 | 250 |
| Shitara et al. | 2020 | 72B | 3 | GC/GEJC | Pembrolizumab + chemotherapy | Chemotherapy | 24 | 257 | 14 | 250 |
| André et al. | 2020 | 73 | 3 | CCR | Pembrolizumab | Chemotherapy | 17 | 153 | 6 | 154 |
| Rudin et al. | 2020 | 74 | 3 | SCLC | Pembrolizumab + chemotherapy | Placebo + chemotherapy | 4 | 228 | 2 | 225 |
| Ferrucci et al. | 2020 | 75 | 2 | Melanoma | Pembrolizumab + dabrafenib + trametinib | Placebo + dabrafenib + trametinib | 12 | 60 | 9 | 60 |
| Nishiyama et al. | 2020 | 76 | 3 | UC | Pembrolizumab | Chemotherapy | 3 | 30 | 1 | 22 |
| Tolaney et al. | 2020 | 77 | 2 | Breast cancer | Pembrolizumab + eribulina | Eribulina | 0 | 44 | 0 | 44 |
| Motzer et al. | 2021 | 78 | 3 | RCC | Pembrolizumab + lenvatinib | Everolimus + lenvatinib / sunitinib | 57 | 355 | 35 (E + L) / 15 (S) | 357 (E + L) / 357 (S) |
| Rodriguéz-Abreu et al. | 2021 | 79 | 3 | Non-squamous NSCLC | Pembrolizumab + pemetrexed + platinum | Platinum + pemetrexed + placebo | 5 | 410 | 1 | 206 |
| Awad et al. | 2021 | 80 | 2 | Non-Squamous NSCLC | Pembrolizumab + chemotherapy | Chemotherapy | 5 | 60 | 2 | 63 |
| Colombo et al. | 2021 | 81 | 3 | Cervical cancer | Pembrolizumab + chemotharapy + bevacizumab | Placabo + chemotherapy + bevacizumab | 2 | 307 | 1 | 309 |
| Powles et al. | 2021 | 82A | 3 | UC | A) Pembrolizumab + chemotherapy B) pembrolizumab | Chemotherapy | 3 | 347 | 1 | 342 |
| Powles et al. | 2021 | 82B | 3 | UC | A) Pembrolizumab + chemotherapy B) pembrolizumab | Chemotherapy | 2 | 304 | 1 | 342 |
| Sun et al. | 2021 | 83 | 3 | Oesophageal cancer | Pembrolizumab + chemotherapy | Placebo + chemotherapy | 0 | 370 | 1 | 370 |
| Winer et al. | 2021 | 84 | 3 | TNBC | Pembrolizumab | Chemotherapy | 11 | 312 | 5 | 310 |
| Lu et al. | 2021 | 85 | 3 | NSCLC | Tislelizumab + chemotherapy | Chemotherapy | 6 | 223 | 1 | 111 |
| Ribas et al. | 2013 | 86 | 3 | Melanoma | Tremelimumab | Chemotherapy | 11 | 328 | 8 | 327 |
Population characteristics
A total of 49,425 patients were available for this meta-analysis, with 25,647 that were included in the experimental arms and 23,778 in the control arms; 14,249 (29%) of them presented a diagnosis of NSCLC [10, 12, 16, 22, 24, 32, 35, 40, 43, 44, 48, 50, 55, 56, 61–63, 67, 70, 79, 80, 85], 6536 (13%) urothelial cancer (UC) [13,19A,19B,26,37A,37B,65,76,82A,82B], 5743 (12%) RCC [17, 25, 45, 46, 59, 68, 69], 2904 (6%) melanoma [20, 38, 41, 42, 75, 86], and 2669 (5%) head and neck tumors (HNT) [28,34,64A,64B,66] (Table 1).
In the 37 trials exploring single immunotherapies, 11,100 patients were included in the experimental arms and 10,105 in the control arms; 4 trials had atezolizumab as experimental drug [10,13,15B,19B], 7 avelumab [23,24,26,27,29A,30A,30B], 1 camrelizumab [31], 1 cemiplimab [35], 1 durvalumab [37B], 11 nivolumab [41─45,47,49,50,51,53,57], 11 pembrolizumab [60,61,64A,65─67,72A,73,76,82B,84], and 1 tremelimumab [86] (Table 1).
In the 48 studies investigating immuno-oncology combinations, 14,547 patients were included in the experimental arms and 13,673 in the control arms; 30 trials explored the combination of chemo–immunotherapy [11,14,16,19A,21,22,28,29B,32–34,36,38–40,52,58,62,63,64B,70,71,72B,74,77,79,80,82A,83,85], 4 immuno–immunotherapy [37A,46,48,54], 1 immuno–immuno–chemotherapy [55], 3 immuno-targeted therapy [15A,20,75], 7 immuno-antiangiogenetic drugs [17, 18, 25, 59, 68, 69, 78], and 3 immuno-targeted therapy–chemotherapy [12, 56, 81]. Eleven of these combinations included atezolizumab [11,12,14,15A,16–18,19A,20–22], 4 avelumab [25,28,29B,30B], 3 camrelizumab [32–34], 2 durvalumab [36,37A], 3 ipilimumab [38–40, 46, 48], 4 both ipilimumab and nivolumab [46, 48, 54, 55], 4 nivolumab [52, 56, 58, 59], 18 pembrolizumab [62–64,68-72B,74,75,77–83], and 1 tislelizumab [85] (Table 1).
Among the 25,647 patients included in the experimental arms with immunotherapy or immuno-combinations, 1474 CRs were reported. On the other hand, in the 23,778 patients treated in the control arms, we registered 855 CRs. The baseline characteristics of each trial are summarized in Table 1.
Immunotherapy versus control
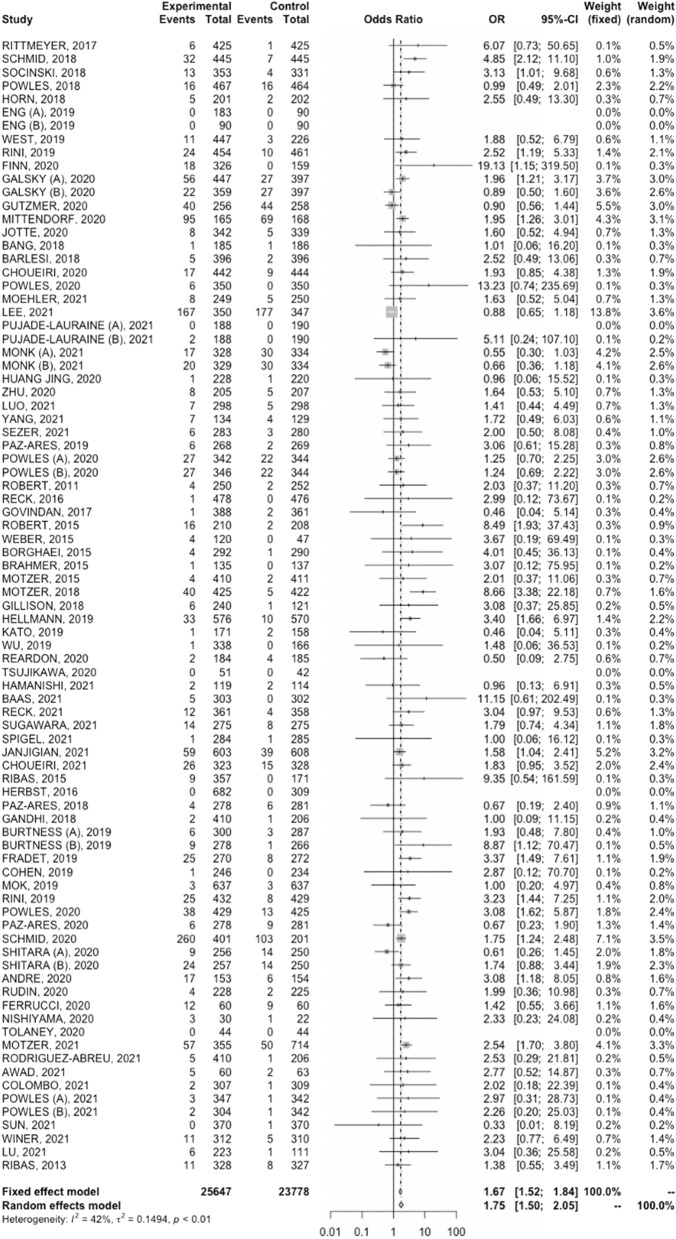
Higher CR rate was reported in cancer patients treated with immunotherapy compared with control treatments (OR, 1.67; 95% CI, 1.52–1.84, Fig. 2). The analysis reported low heterogeneity (I2 of 42%), and a fixed-effect model was used.
Fig. 2.
Forest plot of comparison between immunotherapy alone and immuno-combinations versus control arms in cancer patients; the outcome (Effect Size) was odds ratio (OR) of complete response (CR) rate. Abbreviations: CI: confidence interval; OR: odds ratio
Similarly, CR rate was higher in patients receiving chemo–immunotherapy versus control treatments, with a OR of 1.60 (95% CI, 1.39–1.84, Supplimentary Fig. 2). A fixed-effect model was used due to low heterogeneity (I2 = 21%).
As for the 4 studies investigating immuno–immuno combinations (Supplimentary Fig. 3A), we observed the highest OR (3.56, 95% CI 1.28–9.90). In this case, a random-effects model was used due to high heterogeneity (I2 = 79%). On the other hand, the OR was 2.84 (95% CI 2.20–3.56) in the 7 studies comparing the combination of immunotherapy with antiangiogenetic agents versus controls (Supplimentary Fig. 3B); a fixed-effect model was used due to low heterogeneity (I2 = 0%).
Complete response rate according to primary tumor
We specifically focused our analyses on available data in terms of CR rate according to primary tumor (NSCLC, RCC, UC, and melanoma).
NSCLC
For 14,249 NSCLC patients treated with immunotherapy (ICI monotherapy or in combination with other anticancer agents) (n = 7794) versus control treatment (n = 6455), the pooled OR of CR rate was 2.0 (95% CI, 1.5–2.65, Supplimentary Fig. 4A). The analysis showed low heterogeneity (I2 of 0), and thus, a fixed-effects model was used.
RCC
According to our analysis, for 5743 RCC patients receiving immune-based combinations (n = 2838) versus control treatment (n = 2905), CR rate was higher in the immunotherapy arm (OR, 2.4; 95% CI, 1.55–3.72, Spplimentary Fig. 4B). The analysis presented substantial heterogeneity (I2 = 59%), and a random-effects model was used.
UC
For 6536 UC patients receiving ICIs (n = 3262) versus control treatment (n = 3274), the pooled OR for CR rate was 1.51 (95% CI, 1.19–1.91). The analysis was conducted according to fixed-effect model, due to low heterogeneity observed (I2 = 30%).
Melanoma
Higher CR rate was reported in melanoma patients treated with immunotherapy (n = 1581) compared with control (n = 1323) treatments (OR, 1.45; 95% CI, 1.03–2.03). A fixed-effect model was used due to heterogeneity lower than 50% (I2 of 48%).
Discussion
The introduction of ICIs and ICI-based combination therapies into clinical practice has been an undoubted breakthrough in the treatment of cancer patients [87–89]. In particular, ICIs have been suggested to induce durable and robust anticancer responses in an important proportion of patients, and even CR, in selected cases [90–92]. Of note, although several case reports have been published in recent years, the likelihood of achieving a CR in cancer patients treated with immunotherapy has not been systematically determined [93–95].
Achieving the cure is the Holy Grail of anticancer treatment for metastatic disease, and CR is independently associated with improved survival in several solid tumors. Therefore, it is fundamental to assess the probability of achieving CR with ICIs, and whether the use of immunotherapy would increase CRs in cancer patients. At the same time, several questions regarding modern immunotherapy remain unanswered. Among these, ICIs present a specific set of treatment-related toxicities, which are commonly called as immune-related adverse events (irAEs) and are due to the erroneous activation of the immune system against self-antigens [96, 97]. Several organ systems may be affected by irAEs, with the incidence and severity of irAEs depending on multiple factors, including the type of ICI, the tumor type, and the disease setting, and some studies have also associated irAEs with anticancer response, and even CR [98]. On the other side, patients achieving CR could stop therapy without compromising clinical outcomes and, in this way, by minimizing toxicities and treatment-related costs. However, few clinical trials have specifically addressed this crucial point, and available data are limited on potential predictors of CR and patient disposition after ICIs discontinuation following CR; the mechanisms underlying durable CR certainly require further investigation. Another fundamental issue in current and future cancer immunotherapy is the identification of reliable biomarkers of response or resistance. In fact, despite ICIs seem to have found their role in several tumors in monotherapy or as part of combinatorial strategies, the lack of validated biomarkers of response represents an important issue since only a proportion of cancer patients benefit from immunotherapy [99–101]. Based on these premises, a greater understanding of the role of potential biomarkers including programmed death ligand 1 (PD-L1) expression, tumor mutational burden (TMB), microsatellite instability (MSI) status, gut microbiota, concomitant medications, and several others, and their association with CR, is of great importance [102–104]. In addition, clinical trials on cancer immunotherapy frequently differ in terms of drugs, patients, designs, and inconsistent clinical outcomes, including CR rate.
To the best of the authors’ knowledge, our study represents the first study-level meta-analysis in the literature to systematically assess the probability to achieve CR in cancer patients receiving ICIs. In MOUSEION-03, 49,425 patients from 85 clinical trials were included in the analysis. Higher CR rate was reported in cancer patients treated with immunotherapy compared with control treatments as well as in those receiving chemo–immunotherapy versus control treatments. Of note, the highest CR rate was observed with the dual checkpoint blockade through the combination of two ICIs (OR 3.56, 95% CI 1.28–9.90).
The effectiveness of anticancer treatment depends on a plethora of elements, including the indication for which a specific agent is used, as well as the biology of the disease itself, the setting and line of treatment. According to our results, ICI-based treatment was associated with a higher likelihood of achieving CR in patients affected by the most common malignancies included in the current study. In NSCLC patients OR was 2.0 (95% CI, 1.5–2.65), in RCC 2.4 (95% CI, 1.55–3.72), in UC 1.51 (95% CI, 1.19–1.91), and in melanoma 1.45 (95% CI, 1.03–2.03). Our results are consistent with the observations which have been previously reported by several authors, including those highlighted by Li and colleagues, who assessed the chance of obtaining CR in 4803 NSCLC patients enrolled in 9 randomized controlled trials [105]. Compared to systemic chemotherapy, ICI-based treatment was associated with higher possibility of archiving CR in this study (RR 2.89, 95% CI: 1.44–5.81, P = 0.003) [105].
Our analysis presents some strengths, including the quality of statistical analysis and the selection of the most updated results of clinical trials including a large sample size (49,425 cancer patients treated with ICIs—immunotherapy = 25,647; control = 23,778). However, some limitations should be acknowledged. First, this is a study-level meta-analysis based on pooled data, and thus, the potential presence of confounding factors and single-patient variables (e.g., patient age, comorbidities, concomitant medications, etc.) was not included. In addition, despite random-effects modeling was performed to address heterogeneity among clinical trials, some analyses were associated to substantial heterogeneities. This issue is particularly relevant for analyses regarding RCC, UC, and melanoma (59%, 30%, and 48%, respectively), and I2 was used to test heterogeneity. Of note, all statistical tests to assess heterogeneity are weak—including I2 —and the clinical implications of this issue are considerable and should be examined on a case-by-case basis; in particular, the perception of statistical heterogeneity as well as homogeneity is often involved in influencing clinicians in important decision. In fact, at the same time, lack of evidence of heterogeneity is not evidence of homogeneity and putting too much trust in homogeneity of effects itself may give a false sense of reassuring the “one fits all.” Thus, our results must be interpreted cautiously. Lastly, most of the included studies were industry funded, and thus, were prone to reporting bias; all the immunotherapeutic agents present different and not superimposable features, and thus, this element may have introduced some bias. In addition, not all studies included central response assessment.
Conclusions
In recent years, immunotherapy has shown outstanding efficacy in pan-tumors, and an impressive number of clinical trials of ICIs has been published. In the MOUSEION-03, we conducted an updated and comprehensive meta-analysis enrolling a large sample size with the aim of assessing CR rate in cancer patients receiving immunotherapy versus control treatment. We obtained data from 85 identified international trials and almost 50.000 patients were included in our analysis, which showed that immunotherapy may confer therapeutic advantages in several settings and varying on multiple factors, including primary tumor site as well as the use of ICIs as monotherapy or as part of combinatorial strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Fernando Sabino Marques Monteiro has received research support from Janssen, Merck Sharp and Dhome and honoraria from Janssen, Ipsen, Bristol Myers Squibb and Merck Sharp and Dhome. All unrelated to the present paper. Andrey Soares has received honoraria: Janssen, Pfizer, Bayer, Novartis, AstraZeneca, Astellas Pharma, Pierre Fabre, Merck Serono, Sanofi,Roche, Ipsen, Zodiac. Consulting or Advisory Role: Astellas Pharma, Janssen, Roche, Bayer, Lilly, AstraZeneca, Novartis, MSD, Bristol Myers Squibb, Zodiac, Amgem, Ipsen, United, Zodiac. Research Funding: Bristol Myers Squibb (Inst), Astellas (Inst), AstraZeneca (Inst). Travel, Accommodations, Expenses: AstraZeneca,Pfizer, AstellasPharma, Bristol Myers Squibb, Bayer, Roche, Janssen, Merck Serono, Sanofi, Ipsen, Zodiac. Enrique Grande has received honoraria for speaker engagements, advisory roles or funding of continuous medical education from Adacap, AMGEN, Angelini, Astellas, Astra Zeneca, Bayer, Blueprint, Bristol Myers Squibb, Caris Life Sciences, Celgene, Clovis-Oncology, Eisai, Eusa Pharma, Genetracer, Guardant Health, HRA-Pharma, IPSEN, ITM-Radiopharma, Janssen, Lexicon, Lilly, Merck KGaA, MSD, Nanostring Technologies, Natera, Novartis, ONCODNA (Biosequence), Palex, Pharmamar, Pierre Fabre, Pfizer, Roche, Sanofi-Genzyme, Servier, Taiho, and Thermo Fisher Scientific. EG has received research grants from Pfizer, Astra Zeneca, Astellas, and Lexicon Pharmaceuticals. Francesco Massari reports personal fees from Astellas, BMS, Janssen, Ipsen, MSD, and Pfizer outside the submitted work.
Author contributions
M.S, A.R. and F.M. wrote the main manuscript text. All authors reviewed the manuscript
Funding
None to declare.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Matteo Santoni and Alessandro Rizzo have equally contributed to this work.
References
- 1.Borcoman E, Kanjanapan Y, Champiat S, et al. Novel patterns of response under immunotherapy. AnnOncol. 2019;30:385–396. doi: 10.1093/annonc/mdz003. [DOI] [PubMed] [Google Scholar]
- 2.Faury S, Foucaud J. Health-related quality of life in cancer patients treated with immune checkpoint inhibitors: a systematic review on reporting of methods in randomized controlled trials. PLoS ONE. 2020;15:e0227344. doi: 10.1371/journal.pone.0227344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11:3801. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Most RG, Robinson BW, Lake RA. Combining immunotherapy with chemotherapy to treat cancer. Discov Med. 2005;5(27):265–270. [PubMed] [Google Scholar]
- 6.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol. 2015;26(9):1813–1823. doi: 10.1093/annonc/mdv209. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1. 1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Rittmeyer A, Barlesi F, Waterkamp D, et al; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentrerandomised controlled trial. Lancet. 2017 389:255–265. [DOI] [PMC free article] [PubMed]
- 11.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 12.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 13.Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigr211): a multicenter, open-label, phase 3 randomized controlled trial. Lancet. 2018;391:748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 14.Horn L, Mansfield AS, Szczęsna A, et al; IMpower133 Study Group. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–9. [DOI] [PubMed]
- 15.Eng C, Won Kim T, Bendell J, Argiles G, Tebbutt NC, Di Bartolomeo M, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicenter, open-label, phase 3, randomized, controlled trial. Lancet Oncol. 2019;20:849–861. doi: 10.1016/S1470-2045(19)30027-0. [DOI] [PubMed] [Google Scholar]
- 16.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 17.Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, Bracarda S, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicenter, open-label, phase 3, randomized controlled trial. Lancet. 2019;393:2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 18.Finn RS, Qin S, Ikeda M, et al; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–1905. [DOI] [PubMed]
- 19.Galsky MD, Arija JÁA, Bamias A, et al; IMvigor130 Study Group. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1547–1557. [DOI] [PubMed]
- 20.Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (Imspire150): primary analysis of the randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835–1844. doi: 10.1016/S0140-6736(20)30934-X. [DOI] [PubMed] [Google Scholar]
- 21.Mittendorf EA, Zhang H, Barrios CH, Saji S, Hae Jung K, Hegg R, et al. Neoadjuvant Atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (Impassion031): a randomized, double-blind, phase 3 trial. Lancet. 2020;396:1090–1100. doi: 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 22.Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in combination with carboplatin and Nab-Paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J ThoracOncol. 2020;15:1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052–2060. doi: 10.1093/annonc/mdy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19:1468–1479. doi: 10.1016/S1470-2045(18)30673-9. [DOI] [PubMed] [Google Scholar]
- 25.Choueiri TK, Motzer RJ, Rini BI, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31:1030–1039. doi: 10.1016/j.annonc.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 27.Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu MH, Muntean AS, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol. 2021;39(9):966–977. doi: 10.1200/JCO.20.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22(4):450–462. doi: 10.1016/S1470-2045(20)30737-3. [DOI] [PubMed] [Google Scholar]
- 29.Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 2021;22(7):1034–1046. doi: 10.1016/S1470-2045(21)00216-3. [DOI] [PubMed] [Google Scholar]
- 30.Monk BJ, Colombo N, Oza AM, Fujiwara K, Birrer MJ, Randall L, et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(9):1275–1289. doi: 10.1016/S1470-2045(21)00342-9. [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9(3):305–314. doi: 10.1016/S2213-2600(20)30365-9. [DOI] [PubMed] [Google Scholar]
- 33.Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of Camrelizumab vs Placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell Carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916–925. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2021;22(8):1162–1174. doi: 10.1016/S1470-2045(21)00302-8. [DOI] [PubMed] [Google Scholar]
- 35.Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592–604. doi: 10.1016/S0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- 36.Paz-Ares L, Dvorkin M, Chen Y, et al; CASPIAN investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. [DOI] [PubMed]
- 37.Powles T, van der Heijden MS, Castellano D, et al; DANUBE study investigators. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21:1574–1588. [DOI] [PubMed]
- 38.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 39.Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of Ipilimumab plus etoposide and platinum versus Placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34:3740–3748. doi: 10.1200/JCO.2016.67.6601. [DOI] [PubMed] [Google Scholar]
- 40.Govindan R, Szczesna A, Ahn MJ, et al. Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J ClinOncol. 2017;35:3449–3457. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 41.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 42.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 43.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motzer RJ, Escudier B, McDermott DF, et al; CheckMate 025 Investigators. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015; 373:1803–13. [DOI] [PMC free article] [PubMed]
- 46.Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate 214 Investigators. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018; 378:1277–1290. [DOI] [PMC free article] [PubMed]
- 47.Gillison ML, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. CheckMate 141: 1-Year update and subgroup analysis of Nivolumab as first-line therapy in patients with recurrent/Metastatic head and neck cancer. Oncologist. 2018;23(9):1079–1082. doi: 10.1634/theoncologist.2017-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus Ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 49.Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 50.Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14(5):867–875. doi: 10.1016/j.jtho.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of Nivolumab vs Bevacizumab in patients with recurrent Glioblastoma: the checkMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsujikawa T, Crocenzi T, Durham JN, Sugar EA, Wu AA, Onners B, et al. Evaluation of Cyclophosphamide/GVAX pancreas followed by Listeria-Mesothelin (CRS-207) with or without Nivolumab in patients with pancreatic cancer. Clin Cancer Res. 2020;26(14):3578–3588. doi: 10.1158/1078-0432.CCR-19-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamanishi J, Takeshima N, Katsumata N, Ushijima K, Kimura T, Takeuchi S, et al. Nivolumab Versus Gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: open-label, randomized trial in Japan (NINJA) J Clin Oncol. 2021;39(33):3671–3681. doi: 10.1200/JCO.21.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397(10272):375–386. doi: 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 55.Reck M, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open. 2021;6(5):100273. doi: 10.1016/j.esmoop.2021.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2021;32(9):1137–1147. doi: 10.1016/j.annonc.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331☆. Ann Oncol. 2021;32(5):631–641. doi: 10.1016/j.annonc.2021.01.071. [DOI] [PubMed] [Google Scholar]
- 58.Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 62.Paz-Ares L, Luft A, Vicente D, et al; KEYNOTE-407 Investigators. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040–2051. [DOI] [PubMed]
- 63.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al; KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed]
- 64.Burtness B, Harrington KJ, Greil R, et al; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–1928. [DOI] [PubMed]
- 65.Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019;30(6):970–976. doi: 10.1093/annonc/mdz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen EEW, Soulières D, Le Tourneau C, et al; KEYNOTE-040 investigators. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156–167. [DOI] [PubMed]
- 67.Mok TSK, Wu YL, Kudaba I, et al; KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819–1830. [DOI] [PubMed]
- 68.Rini BI, Plimack ER, Stus V, et al; KEYNOTE-426 Investigators. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1116–1127. [DOI] [PubMed]
- 69.Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–1573. doi: 10.1016/S1470-2045(20)30436-8. [DOI] [PubMed] [Google Scholar]
- 70.Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-Specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15(10):1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 71.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 72.Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–1580. doi: 10.1001/jamaoncol.2020.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383(23):2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 74.Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–2379. doi: 10.1200/JCO.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferrucci PF, Di Giacomo AM, Del Vecchio M, Atkinson V, Schmidt H, Schachter J, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8(2):e001806. doi: 10.1136/jitc-2020-001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nishiyama H, Yamamoto Y, Sassa N, Nishimura K, Fujimoto K, Fukasawa S, et al. Pembrolizumab versus chemotherapy in recurrent, advanced urothelial cancer in Japanese patients: a subgroup analysis of the phase 3 KEYNOTE-045 trial. Int J Clin Oncol. 2020;25(1):165–174. doi: 10.1007/s10147-019-01545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tolaney SM, Barroso-Sousa R, Keenan T, Li T, Trippa L, Vaz-Luis I, et al. Effect of eribulin with or without pembrolizumab on progression-free survival for patients with hormone receptor-positive, ERBB2-negative metastatic breast cancer: a randomized clinical trial. JAMA Oncol. 2020;6(10):1598–1605. doi: 10.1001/jamaoncol.2020.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 79.Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32(7):881–895. doi: 10.1016/j.annonc.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Awad MM, Gadgeel SM, Borghaei H, Patnaik A, Yang JC, Powell SF, et al. Long-term overall survival from KEYNOTE-021 Cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous NSCLC. J Thorac Oncol. 2021;16(1):162–168. doi: 10.1016/j.jtho.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 81.Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385(20):1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- 82.Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SY, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931–945. doi: 10.1016/S1470-2045(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 83.Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021 ;398(10302):759–771. [DOI] [PubMed]
- 84.Winer EP, Lipatov O, Im SA, Goncalves A, Muñoz-Couselo E, Lee KS, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(4):499–511. doi: 10.1016/S1470-2045(20)30754-3. [DOI] [PubMed] [Google Scholar]
- 85.Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, et al. Tislelizumab Plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): a randomized phase 3 trial. J Thorac Oncol. 2021;16(9):1512–1522. doi: 10.1016/j.jtho.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Powles T, Walker J, Andrew Williams J, Bellmunt J. The evolving role of PD-L1 testing in patients with metastatic urothelial carcinoma. Cancer Treat Rev. 2020;82:101925. doi: 10.1016/j.ctrv.2019.101925. [DOI] [PubMed] [Google Scholar]
- 88.Massari F, Rizzo A, Mollica V, Rosellini M, Marchetti A, Ardizzoni A, Santoni M. Immune-based combinations for the treatment of metastatic renal cell carcinoma: a meta-analysis of randomised clinical trials. Eur J Cancer. 2021;154:120–127. doi: 10.1016/j.ejca.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 89.de Miguel M, Calvo E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell. 2020;38:326–333. doi: 10.1016/j.ccell.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 90.Dizman, N.; Arslan, Z.E.; Feng, M.; Pal, S.K. Sequencing Therapies for Metastatic Renal Cell Carcinoma. Urol. Clin. North Am.2020, 47, :305–318. [DOI] [PubMed]
- 91.Gao X, McDermott DF. Ipilimumab in combination with nivolumab for the treatment of renal cell carcinoma. Exp Opin Biol Ther. 2018;18:947–957. doi: 10.1080/14712598.2018.1513485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bergerot P, Lamb P, Wang E, Pal SK. Cabozantinib in Combination with Immunotherapy for Advanced Renal Cell Carcinoma and Urothelial Carcinoma: Rationale and Clinical Evidence. Mol Cancer Ther. 2019;18:2185–2193. doi: 10.1158/1535-7163.MCT-18-1399. [DOI] [PubMed] [Google Scholar]
- 94.Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72:307–319. doi: 10.1016/j.jhep.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 95.Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med. 2017;377:1409–1412. doi: 10.1056/NEJMp1709968. [DOI] [PubMed] [Google Scholar]
- 96.Rizzo A, Santoni M, Mollica V, Logullo F, Rosellini M, Marchetti A, Faloppi L, Battelli N, Massari F. Peripheral neuropathy and headache in cancer patients treated with immunotherapy and immuno-oncology combinations: the MOUSEION-02 study. Exp Opin Drug Metab Toxicol. 2021;17(12):1455–1466. doi: 10.1080/17425255.2021.2029405. [DOI] [PubMed] [Google Scholar]
- 97.Herzyk DJ, Haggerty HG. Cancer immunotherapy: factors important for the evaluation of safety in nonclinical studies. AAPS J. 2018;20(2):28. doi: 10.1208/s12248-017-0184-3. [DOI] [PubMed] [Google Scholar]
- 98.Hu D, Zhang W, Tang J, Zhou Z, Liu X, Shen Y. Improving safety of cancer immunotherapy via delivery technology. Biomaterials. 2021;265:120407. doi: 10.1016/j.biomaterials.2020.120407. [DOI] [PubMed] [Google Scholar]
- 99.Massari F, Mollica V, Rizzo A, Cosmai L, Rizzo M, Porta C. Safety evaluation of immune-based combinations in patients with advanced renal cell carcinoma: a systematic review and meta-analysis. Exp Opin Drug Saf. 2020;19:1329–1338. doi: 10.1080/14740338.2020.1811226. [DOI] [PubMed] [Google Scholar]
- 100.Sheng IY, Rini BI. Immunotherapy for renal cell carcinoma. Exp Opin Biol Ther. 2019;19:897–905. doi: 10.1080/14712598.2019.1628946. [DOI] [PubMed] [Google Scholar]
- 101.Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, Zhu B, Wang S, Zhuo M, Sun J, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 2019;5:696–702. doi: 10.1001/jamaoncol.2018.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mollica V, Santoni M, Matrana MR, Basso U, De Giorgi U, Rizzo A, Maruzzo M, Marchetti A, Rosellini M, Bleve S, Maslov D, Tawagi K, Philon E, Blake Z, Massari F. Concomitant proton pump inhibitors and outcome of patients treated with nivolumab alone or plus ipilimumab for advanced renal cell carcinoma. Target Oncol. 2022;17(1):61–68. doi: 10.1007/s11523-021-00861-y. [DOI] [PubMed] [Google Scholar]
- 105.Li J, He Q, Yu X, Khan K, Weng X, Guan M. Complete response associated with immune checkpoint inhibitors in advanced non-small-cell lung cancer: a meta-analysis of nine randomized controlled trials. Cancer Manag Res. 2019;18(11):1623–1629. doi: 10.2147/CMAR.S188551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.