Abstract
Background
Few real-world data are available in patients with advanced metastatic non-small cell lung cancer (NSCLC) treated with first-line immunotherapy, particularly in those with brain metastases at treatment initiation.
Methods
This was a national, retrospective, multicenter study that consecutively included all patients with PD-L1-positive (tumor proportion score ≥ 50%) advanced NSCLC who initiated first-line treatment with pembrolizumab as a single agent between May 2017 (date of availability of pembrolizumab in this indication in France) to November 22, 2019 (approval of the pembrolizumab-chemotherapy combination). Data were collected from medical records with local response assessment.
Results
The cohort included 845 patients and 176 (20.8%) had brain metastases at diagnosis. There were no significant differences in outcomes for patients with and without brain metastases: 9.2 (95% CI 5.6–15) and 8 (95% CI 6.7–9.2, p = 0.3) months for median progression-free survival (PFS) and, 29.5 (95% CI 17.2–NA) and 22 (95% CI 17.8–27.1, p = 0.3) months for median overall survival (OS), respectively. Overall response rates were 47% and 45% in patients with and without cerebral metastases. In multivariate analysis, performance status 2–4 vs. 0–1 and neutrophil-to-lymphocyte ratio ≥ 4 vs. < 4 were the main independent negative factors for OS; brain metastasis was not an independent factor for OS.
Conclusion
In this large multicenter cohort, nearly 20% of patients initiating pembrolizumab therapy for advanced NSCLC had cerebral metastases. There was no significant difference in response rates, PFS and OS between patients with and without brain metastases.
Keywords: Pembrolizumab, Non-small cell lung cancer, Advanced stage, Brain metastases, Real-world study
Introduction
Most patients (86.5%) with advanced non-small cell lung cancer (NSCLC) do not harbor oncogenic drivers, such as EGFR mutations or ALK translocation [1]. Until the recent advances in immunotherapy, treatment options in these patients at advanced stages were limited to cytotoxic chemotherapy. Among NSCLC patients, 23% to 28% have a high level of programmed death ligand 1 (PD-L1) expression defined by a tumor proportion score (TPS) ≥ 50% [2, 3]. Blocking the interaction between PD-1 expressed on effector T-cells and PD-L1 on neoplastic cells prevents tumor cells from evading anti-tumor immunity [4]. Pembrolizumab is a humanized anti-PD1 monoclonal antibody that has been evaluated in various malignancies. In the phase 1 KEYNOTE-001 and phase 3 KEYNOTE-010 studies, patients with advanced NSCLC and a TPS ≥ 50% responded more frequently to pembrolizumab than patients with lower TPS [3, 5, 6]. The results of the KEYNOTE-024 trial demonstrated the superiority of single-agent pembrolizumab over chemotherapy for the first-line treatment of advanced NSCLC characterized by a PD-L1 TPS ≥ 50% and the absence of EGFR mutation or ALK rearrangement [2].
Single-agent pembrolizumab is now a validated first-line option for the subgroup of patients with PD-L1 expression levels ≥ 50%. However, the results of the KEYNOTE-024 trial were obtained in highly selected patients. Patients were excluded if they had ECOG performance status ≥ 2, untreated brain metastases, corticosteroid treatment or a history of autoimmune disease [2]. Real-world data are important to assess the efficacy of pembrolizumab in routine care, including patients not eligible or under-represented in clinical trials [7]. The main objective of the ESCKEYP trial was to determine real-world outcomes with first-line single-agent pembrolizumab for advanced PD-L1-positive (TPS ≥ 50%) NSCLC with a focus on patients with brain metastasis at baseline, to assess the efficacy of pembrolizumab in these patients.
Materials and methods
Type of study and patients
The ESCKEYP study (GFPC 05-2018) was a national, retrospective and multicentric trial that consecutively included patients with advanced PD-L1 ≥ 50% NSCLC initiating first-line single-agent pembrolizumab.
Treatment-naive adult patients were included if they had histologically or cytologically confirmed advanced NSCLC expressing PD-L1 on more than 50% of tumor cells and if they were negative for EGFR and ALK mutations. Brain metastases at inclusion, asymptomatic, pre-treated or not, or if symptomatic, pretreated with stereotactic or pan-cerebral radiation therapy, were allowed (brain MRI was recommended). At least one measurable lesion, cerebral or extra-cerebral, must be present. Patients with autoimmune disease contraindicating immunotherapy or active infection (hepatitis B, C, HIV) and patients with organ or bone marrow transplant were excluded.
PD-L1 expression on tumor cells was determined using immunohistochemistry (IHC) by the local pathology laboratory, based on its own routine IHC platform with 22C3 (Dako), SP263 (Ventana), 28-8 (Dako), or E1L3N (Cell Signaling Technology) antibody, all approved in France for this purpose. Considering the study design, no centralized testing was performed.
Pembrolizumab was administered intravenously at a dose of 200 mg every 3 weeks, according to its first French registration in 2015. The drug was discontinued in the event of progressive disease or unacceptable toxicity, as assessed by the investigator.
The study conformed to the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. It was approved by a national independent Ethics Committee (2019-A02073-54, on December, 11, 2019). Patients received written and oral information on the study and gave their consent to participate in the study and for the use of their medical data for research purposes.
Data collected
Data were collected from patient medical records using electronic case report forms: sociodemographic data (performance status, smoking status, occupational exposure, disease history); NSCLC characteristics (histology, stage, metastatic sites at diagnosis); PD-L1 expression, ALK and EGFR status (and other mutations or rearrangements); brain metastases at diagnosis (number, size, symptoms, treatments); other metastatic sites at diagnosis (number, locations); radiation therapy; progression (new sites or existing sites), a previous treatment in the month before pembrolizumab administration of corticosteroids (more than 10 mg/day, for more than 10 days) and a previous course of antibiotics (more than 10 days), leucocytes and lymphocytes counts.
Tumor response was assessed locally according to RECIST 1.1.
Statistical analysis
Comparisons between patient characteristics were performed using the chi-square test or Fisher’s exact test for discrete variables. The Kaplan–Meier method was used to estimate PFS and OS. OS was defined as the time from index date to the date of death from any cause or censoring, measured at the last contact date or the cut-off date. The index date was defined as the start date of the first pembrolizumab administration after diagnosis of advanced NCSLC. The cut-off date was January 18, 2021 (i.e., database extraction date). PFS was defined as the time from index date to the date of first disease progression or death from any cause. Patients without disease progression at last contact were censored at the date of last contact, or the cut-off date, if earlier. The log-rank test was used to compare survival distributions between groups.
Univariate Cox models were applied to select the most promising prognostic variables (threshold p = 0.20). A multivariate Cox model was then applied to adjust for potential confounders. The multivariable analysis was conducted using backward stepwise Cox regression modeling, with OS or PFS as the dependent variable and prognostic factors as the explanatory variables. Hazard ratios (HR) with their respective 95% CI and p-values were calculated. p-value < 0.05 was considered significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina, USA).
Results
Patient disposition and characteristics
A total of 845 patients with PD-L1 ≥ 50% advanced NSCLC treated in first line with pembrolizumab were included in 33 centers in France from May 2017 (date of availability of pembrolizumab in this indication in France) to November 22, 2019 (approval of the pembrolizumab-chemotherapy combination).
Patients had a median (range) age of 65 (59–72) years and 176 (20.8%) had brain metastases; 74 (42%) of them had radiotherapy just before or concurrently with immunotherapy. Compared to patients without brain metastasis, patients with brain metastases at diagnosis were significantly more often female (40.9% vs. 29.9%, p = 0.005), under 70 y of age (73.9% vs. 63.4%, p < 0.01), treated with corticosteroids (23.3% vs. 7.7%, p < 0.0001) and with adenocarcinomas (85.1% vs. 66.3%, p < 0.0001) (Table 1).
Table 1.
Characteristics of patients with and without brain metastases at initiation of first-line pembrolizumab
| All population (n = 845) | With brain metastases (n = 176) | Without brain metastases (n = 669) | p-value | |
|---|---|---|---|---|
| Female gender, n (%) | 272 (32.2) | 72 (40.9) | 200 (29.9) | 0.005 |
| Age ≥ 70 y, n (%) | 290 (34.3) | 46 (26.1) | 244 (36.5) | 0.01 |
| Smoking status, n (%) | n = 818 | n = 170 | n = 648 | |
| Current/former smoker | 764 (93.4) | 160 (94.1) | 604 (93.2) | 0.67 |
| No smoker | 54 (6.6) | 10 (5.9) | 44 (6.8) | |
| Weight loss, n (%) | n = 722 | n = 150 | n = 572 | |
| < 5% | 461 (63.9) | 102 (68.0) | 359 (62.8) | 0.23 |
| ≥ 5% | 261 (36.1) | 48 (32.0) | 213 (37.2) | |
| BMI kg/m, n (%) | n = 805 | n = 169 | n = 636 | |
| < 18 | 51 (6.3) | 15 (8.9) | 36 (5.7) | 0.26 |
| 18–30 | 692 (85.9) | 143 (84.6) | 549 (86.3) | |
| ≥ 30 | 62 (7.7) | 11 (6.5) | 51 (8.0) | |
| ECOG performance status, n (%) | n = 781 | n = 169 | n = 612 | |
| 0–1 | 610 (78.1) | 133 (78.7) | 477 (77.9) | 0.83 |
| 2–3 | 171 (20.2) | 36 (21.3) | 135 (22.1) | |
| Corticosteroid treatment, n (%) | n = 845 | n = 176 | n = 669 | |
| Yes | 92 (10.9) | 41 (23.3) | 51 (7.7) | < 0.0001 |
| Antibiotic treatmenta n (%) | n = 826 | n = 173 | n = 653 | |
| Yes | 142 (17.2) | 17 (9.8) | 125 (19.1) | 0.004 |
| Histology, n (%) | n = 838 | n = 174 | n = 664 | |
| Adenocarcinoma | 588 (70.2) | 148 (85.1) | 440 (66.3) | < 0.0001 |
| Other | 250 (29.8) | 26 (14.9) | 224 (33.7) | |
| Metastases, n (%) | n = 845 | n = 176 | n = 669 | |
| Bone | 549 (65.0) | 122 (69.3) | 427 (63.8) | 0.17 |
| Liver | 296 (35.0) | 54 (30.7) | 242 (36.2) | 0.37 |
| PD-L1-positive tumor, n (%) | n = 774 | n = 161 | n = 613 | |
| TPS > 75% | 413 (53.4) | 86 (53.4) | 327 (53.3) | 0.98 |
| Genetics, n (%) | n = 845 | n = 176 | n = 669 | |
| KRAS | 234 (27.7) | 58 (33.0) | 176 (26.3) | 0.07 |
| BRAF | 29 (3.4) | 5 (2.8) | 24 (3.6) | 0.62 |
| Biological parameters, n (%) | ||||
| Albumin | n = 460 | n = 100 | n = 360 | |
| ≤ 30 g/L | 107 (23.3) | 20 (20) | 87 (24.2) | 0.38 |
| C-reactive protein | n = 352 | n = 77 | n = 275 | |
| > 5 mg/L | 304 (86.4) | 60 (77.9) | 244 (88.7) | 0.01 |
| White blood cells | n = 678 | n = 145 | n = 533 | |
| > 10,000/mm3 | 288 (42.5) | 63 (43.4) | 225 (42.2) | 0.78 |
| Neutrophil-to-lymphocyte ratio | n = 642 | n = 139 | n = 503 | |
| ≥ 4 | 388 (60.4) | 76 (54.7) | 312 (62.0) | 0.11 |
TPS tumor proportion score
aWithin 3 months before pembrolizumab
Tumor response rates
For the overall cohort (n = 791 evaluable patients), the best response was complete response for 37 (4.7%) patients, partial response for 334 (42.2%), stable disease for 189 (23.9%) and progressive disease for 231 (29.2%). The overall response rate was 45% (95% CI 42–49). The mean duration of response was 11.7 (95% CI 10.8–12.6) months.
The best systemic response according to the presence or absence of cerebral metastases is described in Table 2; there was no significant difference between the groups for the response rate. Regarding brain response in the 74 patients with brain metastases who had brain radiotherapy at diagnosis or at initiation of pembrolizumab, 48.6% (36/74), 24.3% (18/74) and 27% (20/74) had objective response, stable disease and progression of central nervous system (CNS) disease, respectively; in the 76 patients with brain metastases who did not have cerebral radiotherapy (n = 76), these rates were 35.5% (27/76), 15.8% (12/76) and 48.7% (37/76), respectively.
Table 2.
Response rates of patients with and without brain metastases to first-line single-agent pembrolizumab
| With brain metastases | Without brain metastases | |||
|---|---|---|---|---|
| N = 176 | % (95% CI)a | N = 669 | % (95% CI)a | |
| Best response | ||||
| Objective response rate (ORR)b | N = 80 | 47 (39–55) | N = 291 | 45 (41–49) |
| Stable disease | N = 36 | 20 (14–26) | N = 153 | 23 (20–26) |
| Progressive disease | N = 55 | 31 (24–38) | N = 206 | 31 (27–34) |
| Not evaluable | N = 5 | – | N = 19 | – |
NA not applicable
aExact method for binomial confidence interval
bComplete response plus partial response rates
Survival
The median duration of follow-up was 25.8 (95% CI 24.8–26.7) months for the entire cohort.
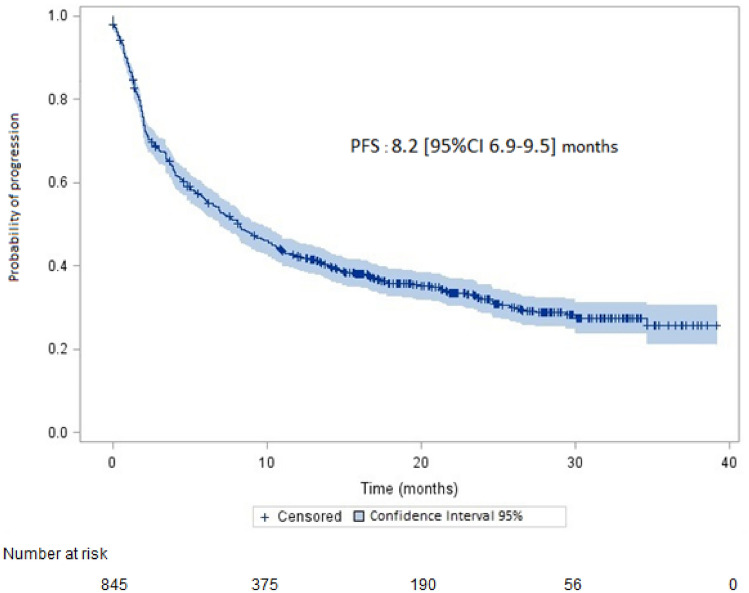
Median PFS was 8.2 (95% CI 6.9–9.5) months (Fig. 1); the median time to pembrolizumab discontinuation was 8 (95% CI 7–9) months. Median PFS was 8 (95% CI 6.7–9.2) months in patients without brain metastases and 9.2 (95% CI 5.6–15) months in patients with brain metastases (p = 0.33).
Fig. 1.
Progression-free survival (PFS) (95% confidence interval in light blue on the survival curve)
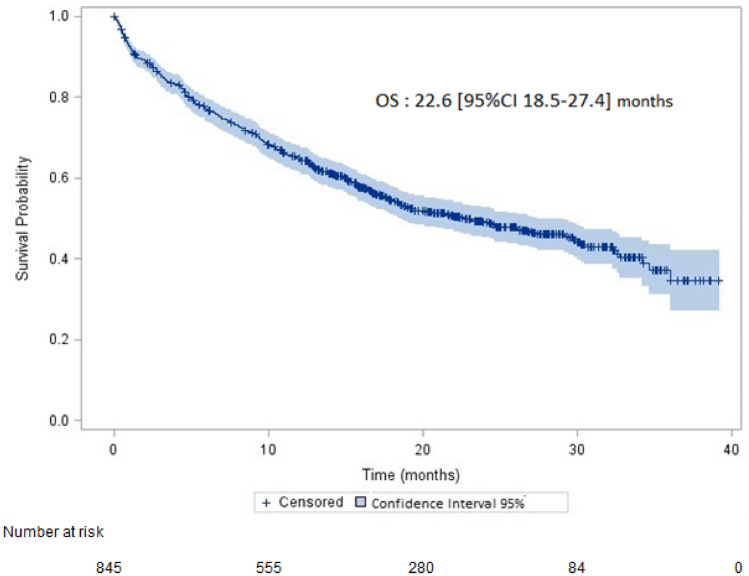
Median OS was 22.6 (95% CI 18.5–27.4) months (Fig. 2). Survival rates at 6, 12 and 18-month were 76.8%, 64.8% and 54.3%, respectively. Median OS was 22 (95% CI 17.8–27.1) in patients without brain metastases and 29.5 (95% CI 17.2–not reached) months in patients with brain metastases (p = 0.37).
Fig. 2.
Overall survival (OS) (95% confidence interval in light blue on the survival curve)
Patients with white blood cell count ≤ 10,000/mL at pembrolizumab initiation had higher OS (29.8 vs. 15.8 months; p < 0.0001) and PFS (10.3 vs. 5.9 months; p = 0.0027) that those with > 10,000/mL white blood cell count. A neutrophil-to-lymphocyte ratio < 4 was significantly associated to a higher OS (32.7 vs. 14.3 months; p < 0.0001) and PFS (15.7 vs. 5 months; p < 0.0001) in comparison with a neutrophil-to-lymphocyte ratio ≥ 4.
In the multivariate analysis, neutrophil-to-lymphocyte ratio ≥ 4 vs. < 4 was an independent predictive factor for shorter PFS (HR 1.64, 95% CI 1.30–2.07; p < 0.0001) (Table 3). Performance status 2–4 vs. 0–1 (1.68, 95% CI 1.26–2.22) and neutrophil-to-lymphocyte ratio ≥ 4 vs. < 4 (1.67, 95% CI 1.25–2.22) were independent predictive factors for shorter OS. The presence of brain metastases at diagnosis was not an independent factor for OS.
Table 3.
Independent predictive factors of progression-free survival and overall survival in multivariate analysis (Cox proportional hazards model)
| Factors | Test vs. reference | HRa | 95% CI | p-value |
|---|---|---|---|---|
| Progression-free survivalb | ||||
| Neutrophil-to-lymphocyte ratio | ≥ 4 vs. < 4 | 1.64 | 1.30–2.07 | < 0.0001 |
| Smoker | No vs. current/former | 1.35 | 0.90–2.00 | 0.15 |
| Performance status | 2–4 vs. 0–1 | 1.24 | 0.96–1.60 | 0.10 |
| Liver metastases | Yes vs. no | 1.24 | 0.94–1.64 | 0.13 |
| Bone metastases | Yes vs. no | 1.16 | 0.93–1.44 | 0.20 |
| Histology | No vs. adenocarcinoma | 1.13 | 0.89–1.45 | 0.32 |
| Brain metastases | Yes vs. no | 1.08 | 0.83–1.40 | 0.58 |
| Age, years | ≥ 70 vs. < 70 | 1.05 | 0.84–1.32 | 0.66 |
| Leukocytes | > 10,000 vs. ≤ 10,000 | 0.97 | 0.78–1.22 | 0.80 |
| Gender | Female vs. male | 0.90 | 0.71–1.14 | 0.38 |
| PD-L1 > 75% | Yes vs. no | 0.89 | 0.72–1.10 | 0.27 |
| Corticosteroid treatment | No vs. yes | 0.83 | 0.59–1.16 | 0.27 |
| Antibiotic treatment | No vs. yes | 0.80 | 0.62–1.03 | 0.08 |
| Overall survivalc | ||||
| Performance status | 2–4 vs. 0–1 | 1.68 | 1.26–2.22 | 0.0004 |
| Neutrophil-to-lymphocyte ratio | ≥ 4 vs. < 4 | 1.67 | 1.25–2.22 | 0.0005 |
| Smoker | No vs. current/former | 1.65 | 1.06–2.57 | 0.02 |
| Histology | No vs. adenocarcinoma | 1.4 | 1.05–1.86 | 0.02 |
| Bone metastases | Yes vs. no | 1.32 | 1.03–1.71 | 0.03 |
| Age, years | ≥ 70 vs. < 70 | 1.31 | 1.02–1.70 | 0.04 |
| Brain metastases | Yes vs. no | 1.17 | 0.87–1.59 | 0.29 |
| Leukocytes | > 10,000 vs. ≤ 10,000 | 1.13 | 0.86–1.48 | 0.38 |
| Liver metastases | Yes vs. no | 1.11 | 0.81–1.53 | 0.51 |
| Corticosteroid treatment | No vs. yes | 0.91 | 0.6–1.3 | 0.65 |
| PD-L1 > 75% | Yes vs. no | 0.85 | 0.67–1.09 | 0.20 |
| Gender | Female vs. male | 0.81 | 0.62–1.09 | 0.15 |
| Antibiotic treatment | No vs. yes | 0.75 | 0.56–0.99 | 0.04 |
aHazard ratio (HR) above 1 indicates poorer survival
bFactors (p-values) of progression-free survival in univariate analysis: gender (p = 0.3), age ≥ 75 y (p = 0.03), smoker (p = 0.14), weight loss ≥ 5% (p = 0.12), performance status (p = 0.004), body mass index (p = 0.8), corticosteroid treatment (p = 0.09), antibiotic treatment (p = 0.0004), histology (p = 0.5), brain metastases (p = 0.3), bone metastases (p = 0.002), liver metastases (p = 0.0007), PD-L1 > 75% (p = 0.46), white blood cells > 10,000 (p < 0.003), neutrophil-to-lymphocyte ratio ≥ 4 (p < 0.0001)
cFactors (p-values) of overall survival in univariate analysis: gender (p = 0.12), age ≥ 75 y (p = 0.0002), smoker (p = 0.056), weight loss ≥ 5% (p = 0.014), performance status (p < 0.0001), body mass index (p = 0.7), corticosteroid treatment (p = 0.051), antibiotic treatment (p = 0.002), histology (p = 0.004), brain metastases (p = 0.3), bone metastases (p < 0.0001), liver metastases (p = 0.008), PD-L1 > 75% (p = 0.51), white blood cells > 10,000 (p < 0.0001), neutrophil-to-lymphocyte ratio ≥ 4 (p < 0.0001)
Discussion
In this large real-world cohort of patients with PD-L1 ≥ 50% advanced NSCLC, including 20.8% of patients with brain metastases at diagnosis and 20.2% of PS ≥ 2, treated as first-line therapy with single-agent pembrolizumab, median PFS (primary endpoint) was 8.2 (95% CI 6.9–9.5) months and median OS was 22.6 (95% CI 18.5–27.4) months. These outcomes were less favorable compared to the results of the pivotal studies, but patients were less selected in our study. In the KEYNOTE-024 trial, median PFS was 10.3 months (95% CI 6.7–not reached) in the single-agent pembrolizumab arm [2]; median OS was 26.3 (95% CI,
18.3 to 40.4) months in an updated analysis [8]. In this trial, only patient with PS 0 or 1 can be included and patients with not-treated brain metastasis or with > 10 mg of corticosteroids were excluded. As a consequence, patients with brain metastases (20.8% treated or untreated vs. 11.7% treated), corticosteroid treatment (10.9% vs 0%) and performance status ≥ 2 (20.2% vs. < 1%) were more frequent in our study than in the KEYNOTE-024 trial, respectively.
An Italian analysis including advanced NSCLC, PD-L1 ≥ 50%, also in real-world management, reported comparable rates of treated PS ≥ 2 patients (15.2%) and patients with brain metastasis (18.3%). The outcomes associated to the pembrolizumab treatment in the Italian cohort were close to our results with PFS and OS of 7.9 (95% CI 6.9–9.5) months and 17.2 (95% CI 15.3–22.3) months, respectively [9]. The ORR, 46.9% in our cohort, 44.5% in the Italian cohort and 44.8% in KEYNOTE-024 study, suggested a comparable efficacy of single-agent pembrolizumab in real-word management compared to the phase III pivotal study. Differences in OS were most likely related to more unfavorable prognostic conditions in real-world setting. In multivariate analysis, PS ≥ 2 was an independent factor for poorer OS in our cohort. This was also reported in other analyses [9–11].
Despite the high frequency of brain metastases in NSCLC patients [12], few studies evaluated first-line immunotherapy in patients with untreated brain metastases at baseline. Indeed, in the phase III studies that evaluated immune checkpoint inhibitors (ICI), patients must have been irradiated and brain metastases must be stable; patients with untreated brain metastases were excluded [2, 6, 13–18]. In a retrospective study, Gauvain et al. included 43 NSCLC patients with brain metastases at diagnosis (treated in 34 patients and active in 16 patients) who received nivolumab [19]. Intracerebral and extracerebral ORR were equal to 9% (95% CI 3–23) and 11% (95% CI 4–26), respectively. A recent meta-analysis of 12 studies (566 patients), mostly retrospective, has evaluated the potential benefit of ICI in NSCLC patients with brain metastases [20]. ICI treatment was associated to an intracerebral ORR of 16.4% (95% CI 9.8–24) and intracerebral disease control rate (DCR) equal to 45%. There was no significant difference among patients with brain metastases previously treated or not treated with radiation therapy before ICI administration. In the 9 studies that evaluated extracranial response, ORR was 16.4% (95% CI 10.0–23.9). However, almost all of these studies assessed the efficacy of ICIs in second line and above in a patient population not selected on PD-L1 status, making it difficult to compare with first-line results in patients with PD-L1 ≥ 50%.
In a phase 2 prospective study, Goldberg et al. evaluated the intracerebral efficacy of pembrolizumab in patients with asymptomatic brain who did not require corticosteroids [21]. Brain MRI was performed at 4 weeks and brain response was evaluated by modified RECIST in which the sum of diameters of up to 5 brain metastases were allowed and lesions were considered measurable if they were at least twice the MRI section thickness. Patients should have a PS < 2 and no neurological symptoms. Of the 37 PD-L1 positive patients, 11 (29.7%, 95% CI 15.9–47.0%) had a confirmed brain metastasis response (7 partial responses and 4 complete responses). An additional 4 patients had stable disease in the brain (2 unconfirmed), 16 had progressive disease and 6 were unevaluable due to rapid systemic progression precluding adequate imaging of the CNS. Eleven of the 37 patients (29.7%, 95% CI 15.9–47.0%) had a systemic response. Among the 27 patients evaluable for both CNS and systemic response, 6 had discordant outcomes. Of these cases, the brain was the site of progression in 3 patients (11.1%) with response in the body, whereas the other 3 (11.1%) had the reverse scenario. Median duration of systemic response was 6.9 (IQR 3.7–22.4) months. More recently, a pooled retrospective data for advanced or metastatic PD-L1 ≥ 1% NSCLC patients with previously treated or untreated brain metastasis included in clinical trials evaluated outcomes to determine whether baseline brain metastases were associated with the efficacy of pembrolizumab versus chemotherapy [22]. All studies included patients with previously treated stable brain metastases. A total of 3170 patients were included, 293 (9.2%) with and 2877 (90.8%) without brain metastases at baseline. In patients with PD-L1 ≥ 50%, pembrolizumab improved OS versus chemotherapy with an HR of 0.67 (95% CI 0.44‒1.02).
This study allowed to include a large number of patients managed consecutively at a time when only pembrolizumab as monotherapy was available. Therefore, selection bias was reduced. Nevertheless, this study has some limitations. Thus, response rates were assessed locally and there was no centralized evaluation. Even though MRI was recommended at inclusion, not all patients had the same type of imaging. Moreover, when cerebral radiotherapy was performed, the modalities and the delays from the start of pembrolizumab treatment were not homogeneous. Similarly, it was recommended that an assessment be performed every 6 to 8 weeks, but some assessments may have been delayed, which may have resulted in some bias in the calculation of PFS. Finally, there were no adjustments for multiple comparisons and there might be heterogeneity in the group with brain metastases (treated or untreated).
In conclusion, in this large multicenter cohort, nearly 20% of patients initiating pembrolizumab therapy for advanced NSCLC had brain metastases. There were no significant differences in response rates, PFS and OS between patients with and without brain metastases.
Authors' contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RD, LG, CC and CD. The first draft of the manuscript was written by RD, CC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for conducting this study.
Availability of data and material
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
L. Greillier reports grants, personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, Roche, Sanofi Aventis, Bristol-Myers Squibb, Merck Sharp & Dohme, Lilly, Novartis, Pfizer, Takeda, Bayer and Amgen, outside the submitted work. C. Chouaid reports grants, personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, GSK, Roche, Sanofi Aventis, Bristol-Myers Squibb, Merck Sharp & Dohme, Lilly, Novartis, Pfizer, Takeda, Bayer and Amgen, outside the submitted work. C. Decroisette reports personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, Roche, Sanofi Aventis, Bristol-Myers Squibb, Merck Sharp & Dohme, Lilly, Novartis, Pfizer, Takeda, and Amgen, outside the submitted work. M. Pérol reports personal fees and non-financial support from Roche, Eli Lilly, Pfizer, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Novartis, AstraZeneca, Takeda, Gritstone, Sanofi, GlaxoSmithKline, Amgen, Chugai, Illumina, Daïchi-Sankyo and Abbvie outside the submitted work. R. Descourt reports personal fees and non-financial support from AstraZeneca, Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, Takeda, and Chugai, outside the submitted work. C. Ricordel, J.B. Auliac, L. Falchero, R. Gervais, R. Veillon, S. Vieillot, F. Guisier, M. Marcq, G. Justeau, L. Bigay-Game, M. Bernardi, P. Fournel, H. Doubre, J. Pinsolle and K. Amrane report no conflict of interest.
Ethical approval
The study conformed to the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. It was approved by a national independent Ethics Committee (2019-A02073-54, on December, 11, 2019).
Consent to participate
Patients received written and oral information on the study and gave their consent to participate in the study and for the use of their medical data for research purposes.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Institut National du Cancer (2016) Accès aux tests moléculaires EGFR, RAS et BRAF. Résultats d’une enquête dans 5 régions françaises
- 2.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 3.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 4.Kwok G, Yau TC, Chiu JW, Tse E, Kwong YL. Pembrolizumab (Keytruda) Hum Vaccin Immunother. 2016;12:2777–2789. doi: 10.1080/21645515.2016.1199310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee M, Turner DC, Felip E, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol. 2016;27:1291–1298. doi: 10.1093/annonc/mdw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 7.Pasello G, Pavan A, Attili I, Bortolami A, Bonanno L, Menis J, Conte P, Guarneri V. Real world data in the era of Immune Checkpoint Inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer Treat Rev. 2020;87:102031. doi: 10.1016/j.ctrv.2020.102031. [DOI] [PubMed] [Google Scholar]
- 8.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score >/= 50. J Clin Oncol. 2021;39:2339–2349. doi: 10.1200/JCO.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortellini A, Tiseo M, Banna GL, et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of >/= 50. Cancer Immunol Immunother. 2020;69:2209–2221. doi: 10.1007/s00262-020-02613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gounant V, Duruisseaux M, Soussi G et al (2021) Does very poor performance status systematically preclude single agent anti-PD-1 immunotherapy? A multicenter study of 35 consecutive patients. Cancers 13. 10.3390/cancers13051040 [DOI] [PMC free article] [PubMed]
- 11.Roborel de Climens F, Chouaid C, Poulet C, Leroy V, Stoven L, Cortot AB, Dhalluin X, Gauvain C. Salvage immunotherapy with pembrolizumab in patients hospitalized for life-threatening complications of NSCLC. JTO Clin Res Rep. 2021;2:100147. doi: 10.1016/j.jtocrr.2021.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Guo H, Xu H, Yu H, Chen Y, Zhao G (2021) Research progress and challenges in the treatment of central nervous system metastasis of non-small cell lung cancer. Cells 10. 10.3390/cells10102620 [DOI] [PMC free article] [PubMed]
- 13.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govindan R, Szczesna A, Ahn MJ, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35:3449–3457. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 19.Gauvain C, Vauleon E, Chouaid C, Le Rhun E, Jabot L, Scherpereel A, Vinas F, Cortot AB, Monnet I. Intracerebral efficacy and tolerance of nivolumab in non-small-cell lung cancer patients with brain metastases. Lung Cancer. 2018;116:62–66. doi: 10.1016/j.lungcan.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Loiola T, de Alencar V, Guedes Camandaroba MP, Pirolli R, Fogassa CAZ, Cordeiro de Lima VC. Immunotherapy as single treatment for patients with NSCLC With brain metastases: a systematic review and meta-analysis-the META-L-BRAIN study. J Thorac Oncol. 2021;16:1379–1391. doi: 10.1016/j.jtho.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–983. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansfield AS, Herbst RS, de Castro G Jr et al (2021) Outcomes with pembrolizumab monotherapy in patients with programmed death-ligand 1-positive NSCLC with brain metastases: pooled analysis of KEYNOTE-001, 010, 024, and 042. JTO Clin Res Rep 2: 100205. 10.1016/j.jtocrr.2021.100205 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.