Abstract
Nowadays, natural killer (NK) cell-based immunotherapy provides a practical therapeutic strategy for patients with advanced solid tumors (STs). This approach is adaptively conducted by the autologous and identical NK cells after in vitro expansion and overnight activation. However, the NK cell-based cancer immunotherapy has been faced with some fundamental and technical limitations. Moreover, the desirable outcomes of the NK cell therapy may not be achieved due to the complex tumor microenvironment by inhibition of intra-tumoral polarization and cytotoxicity of implanted NK cells. Currently, stem cells (SCs) technology provides a powerful opportunity to generate more effective and universal sources of the NK cells. Till now, several strategies have been developed to differentiate types of the pluripotent and adult SCs into the mature NK cells, with both feeder layer-dependent and/or feeder laye-free strategies. Higher cytokine production and intra-tumoral polarization capabilities as well as stronger anti-tumor properties are the main features of these SCs-derived NK cells. The present review article focuses on the principal barriers through the conventional NK cell immunotherapies for patients with advanced STs. It also provides a comprehensive resource of protocols regarding the generation of SCs-derived NK cells in an ex vivo condition.
Keywords: NK cell therapy, Stem cells technology, Advanced solid tumors, Immunotherapy, Cancer
Introduction
“Solid malignancies” is a term that refers to all types of carcinomas, sarcomas, and lymphomas, which are reported to be the most commonly diagnosed type of cancers and the leading cause of mortality, accounting for an estimated 9.6 million deaths in 2018 [1]. While enormous progress has been made in the diagnosis and treatment of these tumors, common cancer therapies have not shown satisfactory results in terms of patient survival and recurrence, particularly in cases with advanced STs [2]. Generally, the advanced STs are presented as stages III and IV of these malignancies with or without a single- and/or multi-organ metastasis [3, 4].
In many patients with advanced STs, their malignancies exhibit intrinsic resistance not only to the standard chemotherapies and radiotherapies but also to the novel immunotherapy agents such as checkpoint inhibitors (CIs) and monoclonal antibodies (mAbs) [5–7]. Numerous studies have indicated that this therapy resistance of STs may be extremely influenced by tumor’s heterogeneity and microenvironment. A mixture of heterogeneous cellular populations (malignant and non-malignant cells), extracellular matrix (ECM), and interaction of the tumor-associated components, similar to the normal tissues structure, forms the ST’s microenvironment [8, 9]. So far, many trials have been performed in efforts to develop targeted anticancer therapeutics, such as adaptive immunotherapies, through targeting the STs microenvironment [10, 11].
NK cells, which are a type of lymphocyte with a strong anticancer property, have been shown to be as an efficient cell for cancer immunotherapy in several experimental studies and clinical trials [12, 13]. The safety and efficacy of autologous and allogeneic NK cells transplantation in different types of STs cases have been clearly demonstrated in several studies (Table 1). However, in addition to the advantages of this approach, the limited number of NK cells and the variation in their anti-tumor response between different cases, as well as the high cost of their isolation and expansion, are known to be the main notable barriers for global development of the NK cell therapy in practice [12, 13].
Table 1.
Summary of some published clinical trials in solid tumors NK cell therapy according to the tumor location
| Involved patient’s tumor characteristics | Study protocol | NK cell source | NK cell activation/cell delivery method | Outcome | Ref |
|---|---|---|---|---|---|
| HER2 overexpressing recurrent breast cancer |
- N = 48 - Age: 20–70 years - Additional therapy: tumor cryoablation and herceptin |
Allogeneic, PB-isolated NK cells |
- NK cell activated with IL-2 - 12 days after culture, 8–10 × 109 harvested NK cell were divided into three parts and infused intravenously 13–15 days after purification |
- Improvement in the quality of life - Reduced levels of CTCs - Reduced CEA and CA15-3 expression - Enhanced immune function - Significant prolongation of progression-free survival |
[81] |
| Colorectal carcinoma, renal carcinoma and hepatocellular carcinoma |
- N = 5 - Age: 45–67 year - Additional therapy: subcutaneous injection of IL-2 |
Autologous, PB-isolated CD56+/CD3− NK cells |
- NK cell activated with IL-2 - 1–100 × 107 cell/kg infused intravenously |
- Infusion of the NK cells was safe even within IL-2 injection - No sign of acute graft-versus-host disease (GvHD) was observed - Serum level of α-fetoprotein in one patient with hepatocellular carcinoma decreased |
[108] |
| Gastrointestinal cancer (recurrent and metastatic) |
- N = 14 - Age: 48–78 years - Additional therapy: surgery, chemotherapy, radiation therapy |
Autologous, PB-isolated CD56+/CD3− NK cells | - NKs activated with OK432, IL-2, and modified fibronectin-CH296 (FN-CH296) induced T cells |
- NK cell therapy was very well tolerated with no severe adverse events - No clinical responses were observed |
[83] |
| Colon and lung cancer |
- N = 12 - Age: 41–69 years - Additional therapy: chemotherapy |
Autologous, PB-isolated CD56+/CD3− NK cells |
- NK cells activated with IL-2 and HSP 70-eptide - 0.1–1.5 × 109 HSP 70-activated NK cells infused intravenously in different cycle according to the patient’s response |
- No negative side effects were observed - Aggressive reaction of NK cells against Hsp70 membrane-positive colon carcinoma cells was increased in 10 of 12 patients - In some patients observed stable disease after NK cell therapy |
[12] |
| Advanced NSCLC, stages IIIb/IV |
- N = 16 - Age: 54–75 years - Additional therapy: chemotherapy |
Autologous, PB-isolated CD56+/CD3− NK cells |
- NK cell activated with IL-2 - 0.2–29 × 106 kg/dose activated NK cells infused intravenously |
- Two patients with partial response and six patients with disease stabilization were recorded - Median progression-free survival and overall survival improved - 56% 1-year survival and a 19% 2-year survival were recorded |
[109] |
| NSCLC, stages I/IV |
- N = 87 - Age: ≥ 60 < years - Additional therapy: chemotherapy, radiotherapy, immunotherapy, target therapy |
Autologous, PB-isolated CD56+/CD3− NK cells |
- NK cells activated with IL-2 and INF- γ - Totally 13.07–1.37 × 109 activated NK cell infused intravenously at days 15 and 16 of each cycle |
- 3-year overall survival rate and median overall survival time in arm 1 were significantly higher than those in arm 2 in NK cell-treated early-stage patients - NK cell therapy in advanced-stage patients could significantly improve 3-year progression-free survival and overall survival rates and also median progression-free survival and overall survival times in these cases |
[84] |
| Advanced non-small cell lung cancer, stages IIIb/IV |
- N = 19 - Age: 20–75 years - Additional therapy: docetaxel, chemotherapy |
Autologous, PB-isolated CD3−/CD16+/CD56+ NK cells |
- NK cell activated with IL-2 - 2 × 109 activated NK cells infused intravenously in 6 cores |
- Two patients achieved a PR - SD was obtained in 12 patients and PD was observed in five patients - NK cell therapy combination with docetaxel was feasible without further toxicity or complications |
[110] |
| Advanced and recurrent non-small cell lung cancer, stages IIIb/IV |
- N = 6 - Age: 55–78 years - Additional therapy: chemotherapy and radiotherapy |
Autologous, PB- isolated Vα24+/Vβ11+ and CD3−/CD56+ NK cells |
- NK cells activated with αGalCer and IL-2 - The activated Vα24 NKT cells were given in a dose escalation design at a dose level per cohort of 1 × 107 and 5 × 107 cells/m2 into steps |
- No severe adverse events were observed during this study in any patients - The number of IFN-γ-producing cells in PBMC increased after all three cases receiving the level 2 dose - After the first and second injection, an increased number of peripheral blood Vα24 NKs were observed in two of three cases |
[111] |
| Metastatic melanoma or renal cell carcinoma, stage IV |
- N = 67 - Age: > 18 years - Additional therapy: chemotherapy |
Autologous, PB- isolated CD56+/CD3− NK cell |
- NK cell activated with IL-2 - 2.5 × 1010 activated NK cell infused intravenously |
- No clinical responses were observed - No negative side effects were observed |
[112] |
| Advanced renal cell cancer, |
- N = 60 - Age: 30–80 years - Additional therapy: cryosurgery |
Autologous, PB- isolated NK cells |
- NK cell activated with IL-2 - 12 days after culture, 8–10 × 109 harvested NK cells were divided into three parts and infused intravenously 13–15 days after purification |
- Enhancing the immune system function - Improving the quality of life of the patients, - Significantly exhibiting good clinical efficacy of the patients |
[113] |
| Recurrent malignant glioma |
- N = 9 - Age: 23–70 years - Additional therapy: radiotherapy, chemotherapy, IV injection of low-dose of INF-β |
Allogeneic, PB-isolated CD3−/CD16+/CD56+ NK cell |
- NK cell activated with IL-2 - 2.3 × 109 activated NK cell injected into the tumor cavities combined with intravenous injection of 0.2–3.7 × 109 cell per one course for 10 time |
Decreased tumor volume to less than half of the pretreatment volume persisted for over 4 weeks in some patients | [104] |
| Primary glioblastoma |
- N = 33 - Median age: 57 years - Additional therapy: chemotherapy and radiotherapy |
Autologous, PB-isolated CD3−/CD16+/CD56+ NK cells |
- NK cell activated with IL-2 - 0.71–2.97 × 109 activated NK cell intraregional infused |
- Minimal toxicity effects were observed - Superior survival was observed for patients who received higher numbers of NK cell |
[114] |
| Pancreatic cancer (stages III/IV) |
- N = 87 - Median age: 55–59 years - Additional therapy: irreversible electroporation |
Allogeneic, PB-isolated CD3−/CD16+/CD56+ NK cell |
- NK cell activated with IL-2 - 12 days after culture, 8–10 × 109 harvested NK cell were divided into three parts and infused intravenously 13–15 days after purification |
- Level of CA19-9 serum marker was lower in the NK-treated group - was higher than control group after NK cell therapy - Progression-free survival in stage III and response rate in stage IV cases improved |
[82] |
| Refractory osteosarcoma, suprarenal carcinoma |
- N = 6 - Age: 11–16 years - Additional therapy: haploidentical stem cell therapy, chemotherapy, and radiotherapy |
Autologous, PB- isolated CD56+/CD3− NK cells |
- NK cell activated with IL-15 - 3–27 × 1010 activated NK cells infused intravenously |
- No toxic effects related to NK cell infusion were observed - One patient achieved very good partial remission, two patients achieved partial remission, and one patient had stable disease |
[85] |
| Patients with advance tumor |
- N = 15 - Age: 9–71 years - Additional therapy: chemotherapy |
Allogeneic, CD56+/CD3− NK-92 cell line |
- NK-92 cell line activated with IL-2 - patients were treated at three different dose levels with two infusions of NK-92 cells according to the following protocol: n = 7 at 1 × 109 cell/m2 n = 6 at 3 × 109 cell/m2 n = 6 at 1 × 1010 cell/m2 |
- No infusion-related or long-term side effects were observed - Patients with lung cancer had some anti-tumor response |
[115] |
| Recurrent ovarian and breast cancer |
- N = 20 - Age: 30–65 years - Additional therapy: lymphodepleting and chemotherapy |
Autologous, PB-isolated CD56+/CD3− NK cells |
- NK cell activated with IL-2 - 8.33 × 106–3.94 × 107 cell/kg activated NKs infused intravenously |
- just low-grade toxicities were observed in most patients - Serum IL-15 levels increased after cell therapy - T-reg was increased at day 14 compared with pre-chemotherapy |
[116] |
Within the cluster of differentiation (CD)16+/CD56+/CD3− and CD56+/CD3− phenotypes, both autologous and allogeneic NK cell sources are typically harvested from the peripheral blood (PB) of patients and umbilical cord blood (UCB), respectively (Table 1). As an unsolvable limitation, it has been shown that at best only 15% of all PB- and UCB-isolated cells display the noted NK cells phenotypes. Furthermore, due to the limited proliferative potential of the isolated NK cells, this approach yields only enough cells for a single-dose administration for the most subjects with STs [14]. Thus, other sources are required in order to provide the homogeneous NK cell population to solve this limitation for the clinical goals.
During the last decade, the exploitation of the self-renewal and differentiation potential of SCs has provided new sources for the production of homogeneous and functional NK cells. Recent studies have shown that some types of pluripotent and adult SCs are potentially capable of generating mature and functional NK cells with a powerful anti-tumor activity [15, 16]. During the long-term translational studies, researchers have succeeded in producing some universal and “off-the-shelf” NK cell sources from the SCs to treat the advanced and/or metastatic STs [16, 17]. As described in Table 2, the results of preclinical studies in animal models of human cancers have consistently shown that these next-generation NK cells, particularly NK cells derived from pluripotent SCs, can inhibit the progression of STs even in the tumors with an immune escape character. Beyond the various preclinical studies, the safety of SCs-derived NK cell therapies on patients with advanced STs is being investigated in some ongoing clinical trials (NCT03841110, IRCT20200429047241N1).
Table 2.
Summary of the main protocols for ex vivo NK cells generation represented by the published studies and the outcomes
| Stem cell type | Differentiation protocol | Generated NK cells phenotype and purity | Study phase | Cell line/tumor type | Outcome | Ref | |
|---|---|---|---|---|---|---|---|
| PSCs | Human ESC |
Step1: Feeder layer: mouse S17 BM stromal cell line Cytokines: Not used (NU) Medium: RPMI 1640 Time: 14–17 days Step2: Feeder layer: murine AFT024 cell line Cytokines: SCF (20 ng/ml), IL-3 (5 ng/ml), IL-7 (20 ng/ml), IL-15 (10 ng/ml), and Flt-3L (10 ng/ml) Medium: Ham F121DMEM 1:2 Time: 30–35 days |
Phenotype: CD56+/KIR+ Purity: not determined (ND) |
In vitro | K562 (erythroleukemia) |
- The human ESC-derived NK cells killed 70–80% of all target cells - They were remarkably able to produce IFN-γ |
[157] |
|
Step1: Feeder layer: murine M210-B4 BM stromal cell line Cytokines (ng/ml): NU Medium: RPMI 1640 Time: 2–3 days Step2: Feeder layer: murine AFT024 cell line Cytokines: SCF (20 ng/ml), IL-3 (5 ng/ml), IL-7 (20 ng/ml), and Flt-3L (10 ng/ml) Medium: Ham F12/DMEM 1:2 Time: 30–35 days |
Phenotype: CD16+/NKG2D+/KIRs+ Purity: 99% |
In vitro/ In vivo |
In vitro: K562 (erythroleukemia), MCF7 (breast cancer),NTERA2 (testicular embryonal carcinoma),PC3 (prostate cancer), and U87 (glioma) cell lines In vivo: NOD/SCID mice model of human K562 xenograft tumor |
In vitro: The human ESC-derived NK cells killed 70–100% of all target cells compared with the NK cells derived from the UCB In vivo: - All the animal models treated with human ESC-derived NK cells showed a complete clearance of the primary tumor within 2 weeks after treatment. - The human ESC-derived NK cells could significantly protect animals against the metastasis compared with the UCB-isolated NK cells-treated mice |
[134] | ||
| Human UBC-iPSCs |
Step1: Feeder layer: NU Cytokines: SCF (40 ng/ml), VEGF (20 ng/ml), and BMP-4 (20 ng/ml) Medium: RPMI 1640 Time: 11 days Step2: Feeder layer: murine AFT024 cell line Cytokines: SCF (20 ng/ml), IL-3 (5 ng/ml), IL-7 (20 ng/ml), IL-15 (10 ng/ml), and flt3-L (10 ng/ml) Medium: Ham F121DMEM 1:2 Time: 28–32 days |
Phenotype: CD16+/KIR+/NKG2A+/NKG2D+/NKp44+/NKp46+ Purity: > 97% |
In vitro/ In vivo |
In vitro: K562 (erythroleukemia) as well as MA-148 and A1847 (human ovarian carcinoma cells) In vivo: NOD/SCID/γc−/− (NSG) mice model of human MA-148 ovarian xenograft tumor |
In vitro: The human iPSCs-derived NK cells killed 40–50% of all target cells compared with the NK cells derived from PB In vivo: The human iPSCs-derived NK cells had a notable anti-ovarian cancer activity, reduced tumor mass in mice, and improved the animals overall survival |
[13] | |
| Human ESCs/iPSCs-spine EBs |
Step1 (Generation of spin EBs): Feeder layer: low-density MEFs Cytokines: SCF (40 ng/ml), VEGF (20 ng/ml), and BMP-4 (20 ng/ml) Medium: BPEL (bovine serum albumin polyvinyl alcohol essential lipids) Time: 10–20 passages Step2: Feeder layer: murine EL08-1D2 stromal cells Cytokines: 1 ml of NK cell initiating cytokines (SCF, IL-3, IL7, IL-15, and FLT-3L) Medium: RPMI 1640 Time: 11 days |
Phenotype: CD45+/CD43+/CD31+/CD73+/KIR+/CD16+/NKG2D+/NKp46+/TRAIL+ Purity: 26.2 ± 6.6% |
In vitro |
K562 (leukemia), U266, and OPM-2 (myeloma), as well as S2013 and S2VP10 (pancreatic cancer) |
- The human EBs-derived NK cells were able to kill 20–75% of all targeted cells - They had a remarkable potential to expression of INF-γ |
[154] | |
| MSPCs | Human BM-isolated CD34+ HSCs |
Feeder layer: allogeneic Irradiated stromal cells Cytokines: IL-12 (10–1000 IU/ml) and HC (10–6 M) Medium: RPMI 1640 Time: 7 days |
Phenotype: CD56+ (bright)/CD3− Purity: 75% |
In vitro | K562 (erythroleukemia) and Raji ATCC (human cell line of hematopoietic origin) | The human HSCs-derived NK cells killed 80% of all target cells | [156] |
|
Feeder layer: NU Cytokines: SCF (100 ng/ml), IL-12 (10 IU/ml), and IL-15 (100 ng/ml) Medium: RPMI 1640 Time: 21 days |
Phenotype: CD56+ (bright)/CD3− Purity: 40–85% |
In vitro | K562 (erythroleukemia) | The human HSCs-derived NKs killed 30–45% of all target cells | [178] | ||
| Human UBC-isolated CD34+ HSCs |
Feeder layer: NU Cytokines: SCF (15 ng/ml), IL-3 (4.1 ng/ml), IL-6 (0.8 ng/ml), Flt-3L (6.7 ng/ml), GM-CSF (1.3 ng/ml), and TPO (8.5 ng/ml) Medium: IMDM Time: 5 weeks |
Phenotype: CD56+ /FasLlow Purity: 54% |
In vitro | K562 (erythroleukemia) |
- The human HSCs-derived NK cells killed 27% of all target cells - They have shown a remarkable ability to IFN-γ expression |
[151] | |
|
Feeder layer: EL08.1D2 embryonic liver cell line Cytokines: SCF (20 ng/ml), IL-3 (5 ng/ml), IL-7 (20 ng/ml), IL-15 (10 ng/ml), and Flt-3L (10 ng/ml) Medium: Ham F121DMEM 1:2 Time: 3 weeks |
Phenotype: CD16+/CD94+/CD161high/NKG2Dhigh/NKp46high/KIRlow Purity: 99% |
In vitro | K562 (erythroleukemia) | The human HSCs-derived NK cells killed 48% of the all target cells | [163] | ||
|
Feeder layer: Stro-11 cells Cytokines: SCF (10 ng/ml), IL-2 (1000 IU/ml), IL-7 (10 ng/ml), and IL-15 (10 ng/ml) Medium: HAM F12 Time: 2 weeks |
Phenotype: CD16+/CD56+/CD3− Purity: 80% |
In vitro | K562 (erythroleukemia) | The human HSCs-derived NK cells killed 15% of the all target cells | [161] | ||
|
Step1: Feeder layer: NU Cytokines: SCF (25 ng/ml), IL-7 (25 ng/ml), Flt3L (25 ng/ml), and TPO (25 ng/ml) Medium: CellGro DC medium Time: 9 days Step2: Feeder layer: NU Cytokines: SCF (25 ng/ml), IL-7 (25 ng/ml), IL-15 (50 ng/ml), and Flt3L (25 ng/ml) Medium: CellGro DC medium Time: 5 Step3: Feeder layer: NU Cytokines (ng/ml): SCF (20 ng/ml), IL-7 (20 ng/ml), IL-15 (50 ng/ml), and IL-12 (0.2 ng/ml) Medium: CellGro DC medium Time: 28 days |
Phenotype: CD16+/CD56+/CD3− Purity: > 90% |
In vitro/ In vivo |
In vitro: K562 (erythroleukemia), as well as SKOV-3, IGROV1, and OVCAR-3 (human ovarian cancer cell lines) In vivo: NOD/SCID/IL2Rgnull mice model of xenograft SKOV-3 ovarian cancer |
In vitro: - The human HSCs-derived NK cells killed > 90%% of the all target cells - The generated NK cells had a remarkable ability to secretion of IFN-γ and granzyme-B against the malignant cells - The generated NK cells were effectively able to infiltrate into the ovarian cancer spheroid and kill the tumoral cell In vivo: - Progression of the ovarian tumors significantly decreased in HSCs-derived NK cells-treated animals - A notable improvement in the animal survival has been observed after NK cell therapy compared with control group |
[15] | ||
|
Step1: Feeder layer: NU Cytokines: SCF (25 ng/ml), IL-6 (50 pg/ml), IL-7 (25 ng/ml), Flt-3L (25 ng/ml), TPO (25 ng/ml), LMWH (20 µg/ml), GM-CSF (10 pg/ml), and G-CSF (250 pg/ml) Medium: GBGM Time: 9 days Step2: Feeder layer: NU cytokines: SCF (25 ng/ml), IL-6 (50 pg/ml), IL-7 (25 ng/ml), Flt3L (25 ng/ml), IL-15 (20 ng/ml), LMWH (20 µg/ml), GM-CSF (10 pg/ml), and G-CSF (250 pg/ml) Medium: GBGM Time: 5 Step3: Feeder layer: NU cytokines: SCF (25 ng/ml), IL-2 (1000 U/ml). IL-7 (25 ng/ml), and IL-15 (20 ng/ml), Medium: GBGM Time: 28 days |
Phenotype: CD16+/CD56+/CD3− Purity: > 95% |
In vitro/in vivo |
In vitro: A431 (epidermoid carcinoma), as well as COLO320, SW480, and HT-29 (colon carcinoma) cell lines In vivo: BALB/c Rag2tm1Fwa Il2rgtm1Cgn SirpaNOD mice xenograft models of SW480 and A431 colon tumor |
In vitro: - Compared with the PB-isolated NK cells, the differentiated NK cells had a remarkable cytotoxicity on the colorectal cancer cell lines. The human HSCs-derived NK cells have killed about 40–70% of all target cells In vivo: - Progression of the colorectal tumors was significantly reduced in the human HSCs-derived NK cells-treated animals compared with PB-isolated NK cells-injected groups - A notable improvement in the animal’s survival rate has been observed in the HSCs-derived NK cells-treated group compared with the PB-isolated NK cells-administrated animals |
[5] | ||
| Human UBC-isolated CD34− /line− HSCs |
Feeder layer: NU cytokines: SCF (20 ng/ml), IL-15 (50 ng/ml), IL-21 (50 ng/ml), Flt-3L (20 ng/ml), and HC (10–6 M) Medium: MyeloCultTM H5100 Time: 4 weeks |
Markers: CD16+/CD56+KIR− Purity: 92% |
In vitro | K562 (erythroleukemia) |
- The human HSCs-derived NK cells killed 59.5% of the all target cells - They also showed a remarkable ability to release IFN-γ, GM-CSF, TNF-α, and CCL3/MIP-1α in culture supernatants |
[159] | |
| Human adipose-CD34+/CD45+/KDR+ SCs (ADSC) |
Step1 (Hematopoietic induction): Feeder layer: NU Cytokines: SCF (50 ng/ml), IL-3 (20 ng/ml), IL-6 (20 ng/ml), Flt3 (50 ng/ml), GM-CSF (20 ng/ml), 2-mercaptoethanol (0.1 mM), transferrin (200 μg/ml), low-density lipoprotein (40 μg/ml), insulin (10 μg/ml), and thrombopoietin (10 ng/ml) Medium: X-VIVO-15 serum-free hematopoietic cell medium Time:7 days Step2: Feeder layer: NU cytokines (ng/ml): SCF (50 ng/ml), IL-2 (12.5 ng/ml), IL-7 (20 ng/ml), IL-15 (40 ng/ml), and Flt-3 (50 ng/ml) Medium: RPMI 1640 Time: 4 weeks |
Markers: CD56+/CD3− Purity: ND |
In vitro/In vivo |
In vitro: K562 (erythroleukemia), MCF7 (breast cancer), as well as PC3, LnCap, DuPro, C4–2, and CWR22 (prostate cancer cell lines) in vivo: Nude mice model of xenograft PC3 prostate tumor and MCF7 breast tumor |
In vitro: - The human ADSC-derived NK cells were carefully killed all of the target cancer cell lines - The human ADSC-derived NK cells were able to express granzyme-B and had a great cytokine-related anticancer activity in vivo: - Progression of the prostate and breast tumors was significantly decreased in the ADSC-NKE cells (genetically engineered ADSC-derived NK cells through transfection of E4BP4 transcription factor) received animals compared with control group just one week after the injection - Injected ADSC-NKE cells were traceable into the animal’s bone marrow and spleen during 5 weeks |
[165] | |
In this review article, we hypothesize that the application of SCs technology may be able to solve the challenges that lie ahead of conventional NK cell therapies. It may also provide a cost-benefit and more effective approach of NK cell immunotherapy for patients with advanced STs. Within a translational view, the aim of this review article is to introduce the SCs, especially induced pluripotent SCs (iPSCs), as a new and more efficient source that could produce next-generation NK cells. Based on the published studies and our experience, we also explain the current protocols, feasibility, and advantages of the ex vivo generated NK cells in the treatment of advanced STs.
NK cells development, characteristics, and trafficking
As one of the cytotoxic lymphocytes in the innate immune system, NK cells generally involve in early defenses not only against the autologous malignant cells but also against stress-induced changes in the immune system, such as infections [18]. Unlike the other effector cells of the immune system, the NK cells are able to recognize their targets even in the lack of antibodies and major histocompatibility complex (MHC), making them one of the fastest immune responders [19]. Normally, a large population of the NK cells can be detected in several organs such as spleen, liver, intestine, uterus, and also in the UCB of the fetus. These cells enter into the circulating blood early after their generation in the bone marrow (BM), lymph nodes, spleen, tonsils, and thymus. The NK cells have a half-life of approximately 7–10 days in the PB, and only 10–15% of all circulating lymphoid cells exhibit the NK cells phenotype [20, 21].
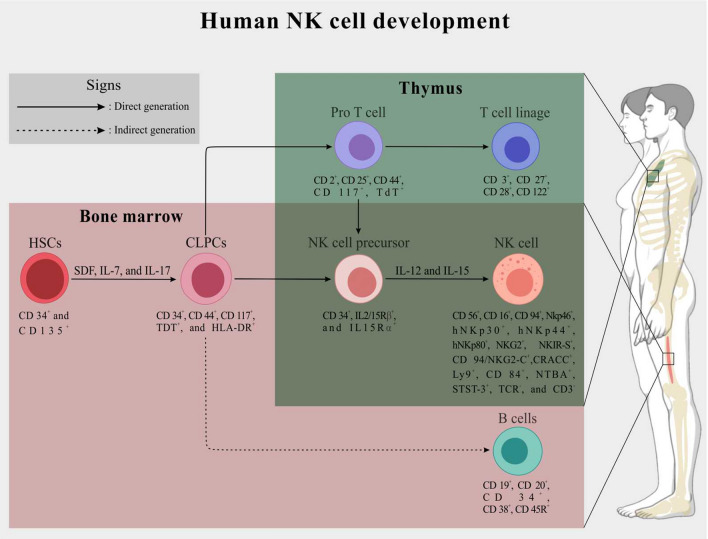
NK cell development from CD34+ hematopoietic stem cells (HSCs) is switched under the stimulation of specific growth factors and cytokines mainly stromal cell-derived factor (SDF), interleukin (IL)-7, and IL-17 [22, 23]. Early after the formation of CD34+/CD44+ common lymphoid progenitor cells (CLPCs) from the HSCs, CD34+/IL2/15Rβ+/15Rα+ NK cell progenitors and also CD19+ B lymphocytes are formed in the BM by the differentiation of CLPCs. Into the thymus, after the differentiation of CLPCs and following formation of CD2+/CD25+ pro-T cells, the NK cells progenitors are generated. Finally, under the stimulation of IL-2 and IL-15, the CD16+/CD56+/CD3− NK cells are generated by the differentiation of NK cell precursors [24, 25]. It has been found that in the aforementioned process, the NK cells development is also genetically controlled by the expression of some key transcription factors, including stem cell antigen-1 (Sca-1), stem cell growth factor receptor (SCFR or C-kit or CD117), FMS-like tyrosine kinase 3 (Flt-3), and T-cell-associated transcription factor (T-bet) [26, 27] (Fig. 1).
Fig. 1.
A schematic representation of the NK cells development and division in human BM and thymus. Briefly, in the BM stroma, human CD34+/CD135+ HSCs differentiate into CD34+/CD44+ CLPCs under the stimulation of SCF, IL-7, and IL-17. Inside the BM, CLPCs directly generate CD34+/IL2/15Rβ+/15Rα+ NK cells precursors. CD19+ B cells are also generated indirectly from the CLPCs in the BM. Unlikely, inside the thymus, the CLPCs transition to CD34+/IL2/15Rβ+/15Rα+ NK cells precursors is indirectly occurred through differentiation of the CD34+/CD44+ CLPCs to CD2+/CD25+ pro-T cells. Besides the generation of NK cells precursors, all of the CD3+ T cell lineages are produced within the differentiation of CD2+/CD25+ pro-T cells in the thymus. Finally, under the stimulation with IL-2 and IL-17, the mature CD16+/CD56+/CD3− NK cells are generated through CD34+/IL2/15Rβ+/15Rα+ NK cells precursors differentiation in both BM stroma and thymus. Below each cell, lineage-specific markers are shown. For more details, please refer to the text
The mature NK cells can be characterized by the expression of some well-known surface markers, such as low-affinity Fcgamma receptor (FcγRIII or CD16) and CD56 in human and also NK1.1 or NK1.2 in mice. As negative markers, the NK cells never express surface immunoglobulin receptors of B cells, such as T-cell antigen receptors (TCRs) and CD3 [22, 26]. In addition, human NK cells can be identified by the positive expression of other markers from the family of natural cytotoxic receptors (NCRs), including NKp46, hNKp30, hNKp44, and hNKp80, as well as natural killer group 2 (NKG2), killer cell immunoglobulin‐like receptors (KIRs), and killer activation receptors (KARs). These surface markers are also introduced as the oligomeric activation receptors (OARs) of the NK surface (Fig. 1). The human NK cell population has also been classified based on CD56 antigen expression levels. In human PB and spleen, approximately 90% of all isolated NK cells possess low levels of CD56, termed CD56dim NKs. In addition, 5–10% of NK cells express high levels of CD56 and are therefore referred to as CD56bright NK cells [28, 29]. In contrast, the common phenotype of these cells in secondary lymphoid tissues has been identified as CD56bright [30]. It has been shown that the CD56dim NK cells are more cytotoxic than the CD56high NKs, whereas the CD56bright NK cells are the primary responders to immunoregulatory cytokines [31]. Furthermore, a population of human NK cells of CD56bright/CD16− phenotype capable of expressing the CD127 (IL-7Ra) antigen has been isolated, whereas the CD56+/CD16+ NK cells are CD127− [32].
The process of NK cells stimulation and activation in the PB as well as migration to the tumor stroma is primarily mediated by the release of tumor-specific cytokines and chemokines [33]. An experimental study on mice models of lymphoma tumors (MHC class I-expressing RMA cells) demonstrated that the activation and accumulation of mice CD27high/CD3−/NK1.1+ NK cells into the malignant tissue is generally dependent on the expression of interferon-gamma (IFN-γ) [34]. Through the process of NK cells activation and trafficking, the manful roles of IL-2, IL-12, IL-15, and IL-18, as the members of tumor-derived NKs stimulators and their associated downstream targets were also understood [35, 36]. Along this pathway, mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathways, as well as GATA-binding protein 3 (GATA 3), PR domain zinc finger protein-1 (PRDM-1 or BLIMP-1), and runt-related transcription factor-3 (RUNX-3) are the major downstream targets of the NK cell stimulators [37–41]. Moreover, chemotactic migration of the human NK cells in response to the tumoral expression of chemokines, e.g., chemokine (C–C motif) ligand 2–5 (CCL 2–5), chemokine (C–X–C motif) ligand 7–8 (CXCL7-8), CXCL 9–12, and chemokine (C-X3-C motif) ligand 1 (CX3CL1 or fractalkine), has been carefully explained [42, 43]. Meanwhile, the correlation between the cytokine and the chemokine network for the NK cell matrix metalloproteinases (MMPs) expression should not be ignored. Along with the NK cell extravasation and tumoral cytotoxicity indication, expression of the specific MMPs for tumor ECM degradation is required. In this regard, the potential of human stimulated NK cells to express the MMP-1, MMP-2, MMP-9, MMP-13, MT1-MMP, MT2-MMP, MT3-MMP, and MT6-MMP has been proven [36, 44, 45]. IL-1α and β as well as IL-2, IL-6, and IL-18 beside the CCL2, CCL3, CCL8, CXCL10, and CXCL12 are the main regulatory elements in the NK cell’s MMPs expression [46–48].
NK cells-mediated cytotoxicity
In response to the malignancies, the NK cells cytotoxicity is switched on by the stimulation of their OARs, which include 1) DNAX-activating protein of 10 kD (DAP10) such as NKG2D, 2) inhibitory MHC class I-specific receptors such as NCRs, CD16, KIR-S, and NKG2C/E, and 3) immunoreceptor tyrosine-based activation motif (ITAM)-bearing molecules such as KIR-L and NKG2A [49–51]. Stimulation of the aforementioned receptors is primarily promoted by tumoral (target cell) expression of MHC class I polypeptide-related sequence (MIC)-A and B, as well as UL16-binding protein 1–3 (ULBP1-3) [52, 53]. Finally, as part of the NK cells–tumor interaction and through the activation of some vital cell death mechanisms, the targeted cells are eventually killed.
Along with the cytotoxicity of NKs, the MAPK/ERK signaling pathway is the first initiated downstream target that gets triggered immediately after OARs stimulation. Activation of the MAPK/ERK cascade plays a direct role in the expression of some important cytokine genes such as INF-γ, tumor necrotic factor-alpha (TNF-α), and also granulocyte–macrophage colony-stimulating factor (GM-CSF) in NK cells [54, 55]. During this cascade, the INF-γ directly stimulates the INFs receptor (INFsR) of NK cell and it is followed by the activation of JAK/STAT signaling pathway, which ends with the expression of TNF-related apoptosis-inducing ligand (TRAIL) on the surface of NK cells [56]. Moreover, the INF–INFsR interaction on tumor cells promotes the expression of the first apoptosis signal receptor (FAS or CD95) [57]. A study by Saric et al. (2002) [58] has indicated that INF–INFsR interaction plays a key role in the expression of antigenic peptides, particularly the MHC class I molecules, on the NK’s target cells in the context of endoplasmic reticulum aminopeptidase-1 (ARTS-1) expression. On the other hand, activation of ERK1/2, as a member of MAPK/ERK mediators, leads to the formation and then exocytosis of some important cytotoxic granules from the NK cell’s cytoplasm to the membrane, localization, and accumulation of the FASL (CD95L) on the NK cell’s surface, and finally release of granzymes (kinds of serine proteases) into the microenvironment of STs [54, 55]. As the result of this cascade, tumoral cell apoptosis is directly induced by the TRAIL–TRAIL receptor (TRAILR), FAS–FASL interaction, and also activation of caspase-3-related cascade through the granzymes penetration [59]. However, part of the evidence suggests the existence of a close correlation between the NK cells cytotoxicity and the tumor microenvironment (TME). A deep knowledge of the nature of the TME and its impact on the anti-tumor activities of NK cells can definitely support the development of more effective immunotherapy approaches.
NK cells and TME
Histologically, STs are introduced as “complex rogue organs” with the characteristics of a heterogeneous population of malignant (transformed) and non-malignant (tumor-associated normal and infiltrating) cells in a tumor-specific ECM. Interactions between the tumor cells through a paracrine, autocrine, and juxtacrine complex network form a dynamic state referred to as TME [60, 61]. Based on the various observations, the heterogeneous structure of tumors and the TME play a mediatory role in the anticancer activity of NK cells.
According to the histopathological observations, two main populations of tumor cells, including the transformed cells and tumor-associated normal cells, can be traced in the STs stroma. Undifferentiated malignant cells, differentiated malignant cells, tumor-associated fibroblasts (TAFs), mesenchymal SCs (MSCs), adipocytes, pericytes, vascular endothelial cells (VECs), and also lymphatic endothelial cells (LECs) are the main detectable cells in most tumors. In addition, myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), T regulatory (T-reg) cells, T lymphocytes, B lymphocytes, and also NK cells are defined as the intra-tumor infiltrative immune cells [60–64]. All of the above cells directly influence tumor progression as well as the anti-tumor activities of NK cells through the expression of various paracrine/autocrine factors.
Several studies have shown a direct correlation between the poor prognosis in survival of patients with STs and high tumoral recruitment of immunosuppressive leukocytes including TAMs, MDSCs, and T-reg cells [65–67]. The accumulation of these immunosuppressive cells and then their cytokine activity can directly hinder the NK cell’s responses against the malignant cells. In TME, it has been shown that TAMs, through expression of some anti-inflammatory factors such as IL-10 and also indoleamine-2, 3-dioxygenase (IDO), directly lead to a decrease in the secretion of INF-γ and eventually to the degranulation of NK cells. Through the expression of epidermal growth factor (EGF), basic fibroblast growth factor (b-FGF), transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), CXCL8, CCL17, CCL18, and CCL22 into the TME, TAMs actively promote tumor progression via proliferation of VECs, remodeling of the ECM, and also recruitment of T family cells [10, 68]. In addition to TAMs, tumor-infiltrated MDSCs play a critical role in the suppressing the anti-tumor activity of NK cells. Through the secretion of TGF-β and IL-10, arginase-1, and inducible nitric oxide synthase (iNOS), MDSCs ultimately suppress the NK’s cytotoxicity via inhibition of IFN-γ and NKG2D [69–71]. T-reg cells, as the important immunosuppressive cells, can also inhibit the NK cell’s activity through secretion of different types of the anti-inflammatory cytokine, such as IL-10, suppressing the NK’s IFN-γ and NKG2D expression [72, 73]. Like T-regs, neutrophils can directly inhibit the NK cells function through the secretion of arginase-1 [74].
The TAFs not only induce tumor progression but also exhibit some known suppressive effects on the NK cell functions. It appears that TAFs are able to effectively inhibit NK activity via downregulation of NKp44, NKp30, and DNAX Accessory Molecule-1 (DNAM-1 or CD226). Moreover, these tumor-associated cells directly stimulate malignant cell proliferation and invasion as well as tumor angiogenesis and ECM remodeling in the context of VEGF, TGF-β, insulin-like growth factor (IGF), and hepatocyte growth factor (HGF) secession [75, 76]. On the other hand, the tumor-infiltrated MSCs are known as another inhibitor of the NK’s activity. In addition to inhibiting cytokine-induced NK cell proliferation, MSCs can potentially suppress the anti-tumor activity of NKs and also cytokine production by downregulation of the NK surface activating receptors such as NKp30, NKp44, and NKG2D. In this regard, IDO and prostaglandin E2 (PGE2) have been shown to be key players in the MSCs-induced NK cell inhibition [77].
Beyond the TME-associated cells, tumoral expression of programmed death-ligand 1 (PD-L1) (a member of immune checkpoints) plays a crucial role in tumor immune escaping [78]. Nowadays, scientists consider the PD-L1/PD-1 (PD-L1 receptor) axis as one of the possible suppressors of the NK cell’s activities. It has been indicated that the NK’s PD-1 stimulation by the tumor PD-L1 could effectively inhibit the activator signals of NK cells [78, 79]. This overview of the TME complex network has clearly shown the destructive effects of the STs for achieving an efficient NK cell therapy. Application of some innovative approaches, such as adaptive NK cell therapy and concomitant administration of TME targeting agendas like immune CIs, may improve the clinical outcome of conventional NK cell therapies.
STs NK cells therapy in practice
The safety and efficacy of NK cell-based immunotherapy on patients with various stages of STs have been studied based on many clinical trials (Table 1). In general, a complete cycle of the NK cell therapy would consist of 1) isolation of the patient’s peripheral blood mononuclear cells (PBMCs) and/or UCB mononuclear cells (UCBMCs), 2) sorting out the NK cells from the PBMCs and/or UCBMCs, 3) in vitro expansion and overnight activation of the isolated NK cells, and 4) transfer of the activated NK cells to the patient. In this context, both human PB- and UCB-derived CD56+/CD3− and CD16+/CD56+/CD3− NK cells have been used as the main functional NK cell sub-types in clinical practices (Table 1). Currently, UCB-derived NK cells are widely applied for clinical aims of cancer immunotherapy. As like as the PB-isolated NK cells, the NKs isolated from UCB have a beneficial cytotoxicity potential and also more capacity to cytokine production [80]. Different protocols have been developed for the UCB-derived NKs isolation and banking. The UCB-derived NK cells consist of a population of UCBMCs with CD56+/CD16+/CD3− phenotype, and they can be efficiently harvested by cell sorting technology used for therapeutic objectives [80]. Besides autologous transfection of NK cells, using types of off-the-shelf UCB-derived NK cells are now one of the common strategies for the using of immune cells to treatment of patients with cancers (NCT01619761, NCT02280525, and NCT01729091).
Till now, different types of the STs, including breast cancer [81], pancreatic cancer [82], advanced and metastatic gastrointestinal (GI) cancer [83], non-small cell lung carcinoma (NSCLC) [84], and sarcoma tumors [85] have been targeted in trials to prove the NK cell-based immunotherapy benefits and outcomes. According to the aforementioned studies, it has been clearly shown that the success of a NK cell therapy on the patients with STs is largely affected by some elements including the stage of disease, the tumor grade and location, the NK cells source, the NK cell’s activating method, the method of NK cell’s administration, and finally the number of administrated NK cells.
Throughout the decades, it has been demonstrated that systemic administration of some cytokines, such as INFs within the stimulation of endogenic and circulating NK cells, can efficiently improve the NK’s intra-tumoral infiltration, cytotoxicity, and finally therapy responses in patients with advanced STs [86, 87]. Alongside the INFs, as the most commonly used NK cell stimulator, the IL-2 high-dose injection, can also effectively reduce the malignant tumor’s progression via polarization of the NK cells toward the tumor stroma and can then induce an anti-tumoral response. Additionally, IL-15 and IL-18 have also shown a proper anti-tumor efficacy in some preclinical examinations through activation of the endogenous NK cells. These observations were the initial efforts for developing an effective cancer immune cell-based immunotherapy. However, toxicities which resulted from cytokine application in patients with STs were the main notable issue [88–92].
Nowadays, adaptive transfer of the NK cells within the in vitro activation of them by cytokines and some peptides has a crucial role to improve the NK cell therapy efficiency. Aside from the heat shock protein 70 (HSP 70)-peptide and a-galactosylceramide (a-GalCer), IL-1α, IL-2, IL-15, and IFN-γ have been applied as the well-established factors in the adaptive NK cell therapy. It should be noted that the clinical outcomes and benefits of the adaptive NK cell therapy have been practically examined on different types of STs through several clinical trials (Table 1).
In a clinical trial of 48 patients with human epidermal growth factor receptor-2-positive (HER-2+) recurrent breast cancer, Liang et al. (2017) recognized that intravenous infusion of the IL-2-activated allogeneic PB-derived NK cell resulted in an improvement in the patient’s quality of life. They have also observed a significant reduction in the number of circulative tumor cells (CTCs), decreasing in the level of carcinoembryonic antigen (CEA) and cancer antigen 15–3 (CA15-3) tumor markers, as well as improvement in the patient’s immune system function following the NK cell immunotherapy [81]. Similarly, Lin et al. (2017) in a clinical examination of 71 patients with stage III/IV pancreatic cancer have clearly shown that intravenous infusion of the allogeneic PB-derived CD16+/CD56+/CD3− NK cells activated by IL-2 could dramatically improve the patient’s overall survival and disease control rate. Furthermore, they have discovered that the patients who had undergone the NK cell immunotherapy had a higher median progression-free survival in stage III, better response rate in stage IV, and lower serum level of CA19-9 tumor marker [82]. Likewise, the effects of the IL-2-adaptive NK cells on the other types of STs patient’s survival, therapeutic response, and quality of life, like recurrent malignant glioma, primary glioblastoma, advanced GI cancer, advanced NSCLC, advanced renal cell cancer, metastatic melanoma, and recurrent ovarian cancer have been evaluated (Table 1).
Beside the IL-12, IL-15-activated NK cells have had satisfactory safety and efficacy results for patients with STs. In this regard, through intravenous infusion of the PB-derived CD56+/CD3− NK cells activated by IL-15 on patients with refractory osteosarcoma and suprarenal carcinoma simultaneously, Pérez-Martínez et al. (2015) have observed no toxicity related to immunotherapy. They also recognized a partial tumor remission in three patients and a stable disease condition in one case [85]. Furthermore, IL-2 co-adapted NK cells with IL-1α, IL-15, HSP-70, and α-GalCer in cases with GI cancer and NSCLC have also displayed a significant efficacy on patient’s therapeutic responses, as well as enhancement in their immune function and survival (Table 1). More recently, Multhoff et al. (2020) have evaluated the therapeutic outcomes of autologous NK cells activated with Hsp70 peptide /IL-2 on 16 patients with NSCLC [93]. The results have proven that their intervention was completely safe and tolerated for the patients. They have also shown a favorable improvement of the NSCLC patient’s progressive-free survival flowing a years after the immune cell therapy [93].
Generally, there is a straight correlation between the patient’s stage of disease and their response to the NK cell immunotherapy. Based on the studies, it has been shown that the chance of a successful NK cell therapy in the subjects with early stage of STs is higher than the patients with the advanced disease. Through phase II clinical trials on stages I–IV patients with lung cancer, Li et al. (2012) have shown that the rate of 3-year overall survival in cases with early-stage lung cancer (stages I–IIIa) was significantly higher than that in patients with advanced lung tumors (stages IIIb–IV) following the NK cell therapy. However, they have announced a notable improvement in the 3-year overall survival and progression-free survival in advanced-stage patients as a result of NK cell therapy [83]. Beside the stage and ST’s character, the number of administrated NK cells seems to be another important issue that affects the outcome [94].
Interestingly, advantageous outcomes have resulted from combining NK cell therapy with other common approaches including tumor cryoablation and herceptin therapy in HER-2 overexpress recurrent breast cancer [81], irreversible electroporation in pancreatic cancer [82], docetaxel-based chemotherapy in advanced NSCLC [95], and immunoglobulin (Ig) G1 administration in GI cancer [96].
In order to achieve the most efficient and cost-effective NK cell therapy, researchers have tried to generate the new unlimited and universal source of NK cells. In this context, NK-92 cell line, or “cytotoxic natural killer cell line-92,” has been described as the first unlimited and off-the-shelf NK cell source [97, 98]. The NK-92 cell line, similar to the natural NK cells, can effectively promote the Fas–FasL cell death-associated cascade with producing various types of inflammatory cytokines, such as TNF-α and IFN-γ, against the target tumoral cells [98–100]. Intravenous infusion of different dosages of NK-92 cells (1 × 109–1 × 1010 cell/m2) displayed acceptable effectiveness and safety in patients with various cancers (Table 1). So far, a few clinical observations have been registered in the “U.S. National Library of Medicine” (clinicaltrials.gov) in order to investigate the efficacy of NK-92 cell therapy on different types of STs (NCT03656705 and NCT03383978). However, in order to prevent the risk of NK/T-cell lymphoma formation, the NK-92 cells should be irradiated before the infusion [101].
Immunologically, it seems that reduction or lack of MHC-I expression on the tumoral cells surfaces can be a primary factor for the NK cell’s response against malignant cells [102, 103]. Different observations showed that some tissues and organs (named immune-privileged sites) physiologically present the lower level of MHC-I and may possibly be undesirable targets for the NK cell’s anti-tumor activities. Central nervous system, anterior chamber of the eye, seminiferous tubules in the testis, hair follicles, and also placenta are known as the main immune-privileged sites in human body [104].
Similar to the other cancer medications, NK cell immunotherapies may result in some complications and immunological symptoms [105]. According to the European Society for Medical Oncology (ESMO) last guideline for the management of toxicities from immunotherapy, different prevalent complications, including immune-related skin toxicity, immune-related endocrinopathies, immune-related hepatotoxicity, immune-related gastrointestinal toxicities, and immune-related pneumonitis toxicities, may commonly be observed after the immunotherapies [106]. However, the NK cell therapy has been considered as one of the safest immunotherapy plans compared to the other approaches such as chimeric antigen receptor (CAR)-T cells and checkpoint inhibitors [107]. Unfortunately, global application of NK cell therapy in its current form has been faced with many challenges. To achieve a cost-effective and efficient NK cell therapy for patients with advanced STs, developing the next-generation sources of NK cells with a universal or personalized character is an inevitable issue.
SCs sources and technology in modern medicine
SCs-advanced technology provides many autologous and commercially available therapeutic platforms for modern medicine. The feasibility and performance of SCs-based therapeutic approaches for the treatment of various diseases have already been investigated. SCs are introduced as a population of cells with the capacity for self-renewing and a potential to differentiate into different specialized cell types [117]. Through the mammalians’ lifetime, these cells play a crucial role in organ development, regeneration, and hemostasis [117–119]. In modern medicine, several types of human SCs have been isolated and characterized depending on their clinical application. According to the origin and differentiation potential of SCs, they are generally classified into three types, including totipotent SCs (TSCs), pluripotent SCs (PSCs), and multipotent stem/progenitor cells (MSPCs) [117–120]. Several studies have shown that only a limited number of them are able to differentiate onto hematopoietic lineages, such as NK cells (Table 2). In the following, the best known human SCs sources are briefly reviewed.
TSCs
Totipotency is a term used to introduce a single cell with the ability to differentiate into all animal cell lineages, including intra-embryonic and also extra-embryonic tissues such as the placenta. In humans, the zygote, which is the first cell to form following fertilization, is known as the first totipotent stem cell [121]. The totipotent state of mammalian’s blastomeres at the 2–8-cell stage has also been confirmed [122]. After fertilization, the totipotent character of the zygote is mainly regulated by the phenomenon of zygotic gene activation (ZGA) and in the context of epigenetic reprogramming of parental genomes [123, 124]. In this regard, zinc finger and scan domain containing-4 (ZSCAN-4) [125], double homeobox (Dux) [126, 127], and the endogenous retrovirus MERVL [128] genes recognized as the key mediators of ZGA in the mammalian’s embryo development. It has also been demonstrated that negative elongation factor-A (NELF-A), the regulator of RNA polymerase II pausing, is the key factor controlling ZGA in TSCs [129]. Although several experimental studies have carefully shown the hematogenic potential of TSCs, the clinical applications of TSCs are severely limited due to some technical, ethical, and legal issues [130].
PSCs
In the field of developmental biology, pluripotency is known as a property of a cell that is capable of differentiating into all three germ layers: endoderm, mesoderm, and ectoderm. Unlike TSCs, the PSCs cannot create extra-embryonic tissues [131]. Generally, PSCs are classified into two embryonic SCs (ESCs) and iPSCs types. As the first discovered PSCs, ESCs are directly harvested from the inner cell mass (ICM) of the blastocyst [131, 132]. Several studies have clearly demonstrated that the in vitro expanded human ESCs are able to differentiate into all types of the hematopoietic lineages, such as NK cells [133, 134]. These cells are characterized by positive expression of CD133 and CD326 markers. Moreover, the pluripotent property of ESCs is controlled by the expression of several nuclear transcription factors, including binding transcription factor-4 (Oct-4), sex-determining region Y-box-2 (Sox-2), and homeobox protein NANOG [118, 135].
Similar to ESCs, human iPSCs have shown a great potential to differentiate between all mature hematopoietic lineages [13]. As a manipulated stem cell, the iPSCs are generated from cultured animal somatic cells through transfection of some pluripotency transcription factor genes such as Oct-4, Sox-2, kruppel-like factor-4 (KLF-4), and myelocytomatosis virus oncogenic cellular homolog (c-MYC) [118, 136]. They are also characterized by the expression of stage-specific embryonic antigen-3/4 (SSEA-3/4), tumor resistance antigen1-60 (TRA1-60), TRA1-81, and TRA2-49/6E markers [137]. The iPSCs can be manipulated from different types of human somatic cells such as fibroblasts [138], PBMCs [139], circulating T cells [140], urine-derived exfoliated renal epithelial cells[141], as well as some other MSPCs [142–144].
MSPCs (adult SCs)
In general, multipotency refers to a population of cells with the ability to differentiate into specialized cell lineages, based on their niche and gene activity [145]. Within various differentiation potentials, MSPCs can be isolated during all of the human fetal, neonatal, and post-neonatal life span [117]. HSCs, MSCs, neural SCs, and cardiac SCs are some of the well-known MSPCs in practice [146–149]. Of all the adult SCs described, only a limited type of them, including HSCs, have shown hematopoiesis potential. Frequently, human HSCs can be isolated from different ontogenic niches such as BM, PB, and fetus UCB [150–152]. Human HSCs are characterized by the expression of CD34 marker. Furthermore, they show different phenotypes in the expression of CD38, CD45, CD90, CD105, CD133, and c-kit markers [118, 153]. The potential of UCB- and BM-isolated CD34+ HSCs to produce a functional NK cells has been well described in several studies (Table 2).
Approved protocols for ex vivo NK cell generation
After the discovery of the NK cell’s anti-tumor efficacy, an increasing trend has been observed in the application of these cells in cancer studies. However, several challenges still exist when it comes to development of cancer NK cell therapies. Currently, SCs have realized a highly potent source concerning the generation of mature NK cells. In this context, PSCs and HSCs are recognized as the main stem cell populations for the ex vivo generation of NK cells. Unlimited sources of SCs, besides their high self-renewal and hematopoiesis potential, are believed to be an effective method in clinical-scale production of next-generation NK cells. In this chapter, we outline the major SCs sources and the established protocols for ex vivo NK cell generation.
In recent years, human H1, H9, and R1 ESCs lines as well as iPSCs derived from human fibroblast, UCBMCs, and PBMCs [16, 154, 155] have been used as the most suitable stem cell types for ex vivo NK cell generation, in addition to human CD34+ HSCs isolated from BM and UBC [13, 156]. In this way, a variety of the NK cell differentiating cytokines are used to stimulate the cultured SCs, e.g., IL-2, IL-3, IL-6, IL-7, IL-12, IL-15, IL-21, SCF, Flt-3 ligand (Flt-3L), VEGF, bone morphogens protein (BMP), GM-CSF, thrombopoietin (TPO), and haptocorrin (HC) [13, 16, 154–156] (Table 2).
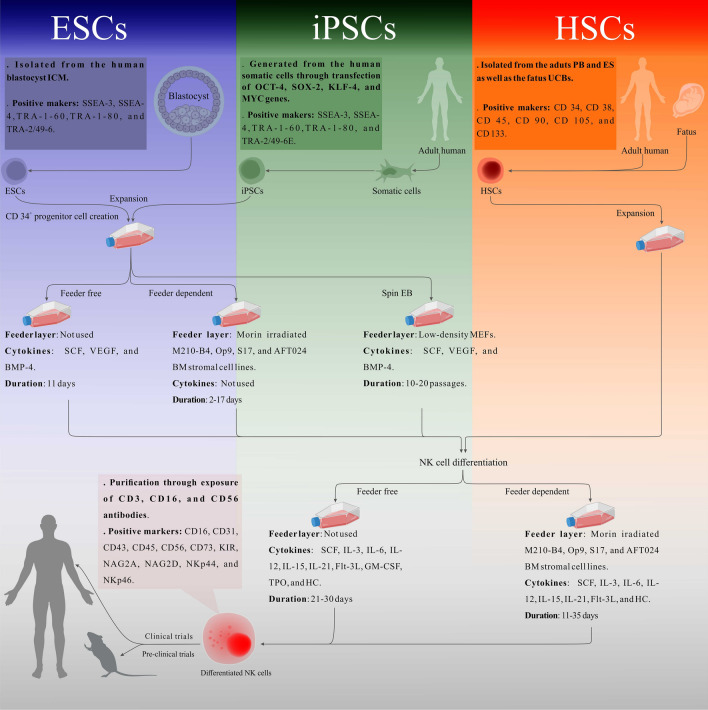
Today, human PSCs are the most common type of SCs for the production of universal NK cells. Processes leading to the production of NK cells from human PSCs generally consist of two main steps: (1) generation of CD34+ progenitor cells (precursor cells) by differentiation of PSCs and (2) generation of NK cells by differentiation of CD34+ progenitors. Along this path, there are two feeder‐dependent and feeder‐free protocols [16, 154, 155]. In the feeder‐dependent protocols, irradiated mice BM stromal cell lines such as M210‐B4, OP9, S17, and also AFT024 perform a crucial role in the inductions of PSCs and their ability to differentiate into CD34+ progenitors in step1 and in the enhancement of NK cell formation through cytokine production in step 2 [13, 16, 134, 157] (Fig. 2 ).
Fig. 2.
A schematic representation of the standard protocols for the NK cells ex vivo generation. Human PSCs including ESCs and iPSCs as well as HSCs are presented as the main SCs sources for NK cells production. As the first step, blastocyst-isolated ESCs and also somatic cells-generated iPSCs are differentiated into CD34+ HSCs through three different methods: feeder-free, feeder dependent, and also spin EB. In a feeder-free method, HSCs are generated only through stimulation of the expanded PSCs with SCF, VEGF, and BMP-4 after 11 days. Without the cytokines application, HSCs are differentiated by the co-culture of the PSCs with murine-irradiated M210-B4, O19, S17, and AFT024 stroma cell lines in a 17–20 period of days. In spin EB method, through the application of low-density mouse embryonic fibroblasts (MEFs) and under the SCF, VEGF, and BMP-4 stimulation, human ESCs- and iPSCs-derived spin EBs are differentiated into the HSCs after 10–20 passages. In the next step, the functional NK cells are generated from both the PSCs-derived HSCs and PB-, BM-, and UCB-isolated HSCs through one of the feeder-free and/or feeder-dependent conditions. In a feeder-free system, under the stimulation with various cytokines and growth factors including SCF, IL-3, IL-6, IL-12, IL-15, IL-21, Flt-3L-GM-CSF, TPO, and HC, the human HSCs are differentiated into the NK cells during a 21–30 period of days. In addition to the cytokines and growth factors, mature NK cells are generated through co-culture of human HSCs with murine-irradiated M210-B4, Sro-11, and AFT024 stroma cell lines in 11–35 days. The generated NK cells can be isolated under the exposure of CD3, CD16, and CD56 antibodies
In two series studies published in 2005 and 2009, Woll et al. succeeded in generating CD34+ progenitor cells without the application of cytokines and growth factors. They co-cultured the human ESCs with the murine-irradiated M210-B4 as well as S17 BM stromal cell lines within the 2–3- and the 14–17-day period, respectively [134, 157]. In the above studies, the researchers produced functional NK cells via co-culture of generated CD34+ cells with AFT024 cell line and under the stimulation by SCF, IL-3, IL-7, IL-15, and Flt-3L within 30–50 days. With about 99% purity, their produced ESC-derived NK cells were able to express a variety of CD16, NKG2D, and KIRs markers as well as secretion of IFN-γ. Moreover, in vitro assessments on the human K562, MCF7, U87, PC3, and NTERA2 cancer cells showed an excellent anticancer activity of ESCs-derived NK cells. Using a three-step feeder-dependent protocol, Tabatabaei et al. (2007) have also managed to produce NK cells from human ESCs. They first cultured human R1 ESCs in a differentiation medium to generate CD34+ ESCs. The isolated CD34+ ESCs were then seeded on the OP9 stromal cells and cultured in medium enriched with IL-6, IL-7, SCF, and Flt-3L for 7 days to generate CD34+ progenitor cells. Subsequently, during the co-culturing of differentiated CD34+ progenitor cells with mice OP9 stromal cells in OP9 media (αMEM supplemented with FBS) containing IL-2 and IL-15, they were able to generate a mature and functional population of NK cells in a period of only 7–14 days. The produced NK cells had an excellent ability to express CD94, NKG2A/C/E, and secretion of granzyme-B [155].
To produce the NK cells in a clinical scale, researchers have provided a great opportunity through the application of human PSCs. As a three-dimensional structure of PSCs, spin embryo bodies (EBs) have shown a profitable outcome to generate NK cells in both feeder‐dependent and feeder-free systems [16, 154]. In an experimental study, Knorr et al. (2013) presented a “feeder-free system that can be used to generate large numbers of cytotoxic NK cells for clinical translation.” In the above study, mature and functional NK cells have been obtained from human ESCs- and iPSCs-derived spin EBs in both feeder‐dependent and feeder‐free systems. Two separate systems, based on the medium enriched with NK cell initiating cytokines alone and also irradiated EL08‐1D2 stromal cells employed to differentiate HSCs into the NK cells. Results of flow cytometry and enzyme-linked immunosorbent assay (ELISA) have shown that the spin EBs-derived NK cells in both systems were able to express a similar level of NKs effector molecules including KIR and CD16, as well as secretion of INF-γ. Without a significant difference between the stromal-dependent and the feeder-free systems, all of the human spin EBs-derived NK cells had an equivalent cytotoxicity on the malignant target cells [154]. There are also other studies which demonstrated that PSCs-derived NK cells have a similar genotype and phenotype in both feeder-free and feeder-dependent protocols [134, 158].
Similar to PSCs, the feeder-dependent and feeder-free systems have been applied to produce mature NK cells from BM- and UCB-isolated HSCs [156, 159]. In this regard, human and rodent EL08-1D2, AFT024, and MS-5 stromal cell lines have been utilized as the most commonly used feeder layers [160–162]. Grzywacs et al. (2006) through co-culture of the human HSCs isolated from UCB on EL08-1D2 stromal cells and under the stimulation of SCF, IL-3, IL-7, IL-15, and Flt-3L managed to successfully generate functional NK cells in a period of about 28–35 days. Their ex vivo generated NK cells were able to express CD16, NKG2A/D, NKp44, CD161, and NKp30 markers and also had a strong cytotoxic effect on the cancer target cells [163]. A similar study using human UBC-isolated CD34+ HSCs yielded an attractive result to produce functional NK cells. During the human UCB-isolated HSCs co-culturing on the MSCs layer and under the stimulation with SCF, IL-2, IL-7, and IL-15, Frias et al. (2008) have discovered that their ex vivo generated NK cells were able to express a CD16 marker and also to kill the malignant cells [161]. Further studies have also demonstrated the ability of human HSCs to generate the functional NK cells with anti-tumor activity under the feeder-dependent systems (Table 2).
Some studies have successfully evaluated the NK cell differentiation potential of human HSCs in a feeder-free system. Bonanno et al. (2009), through applying a feeder-free system and via stimulation of the human UCB-isolated CD34−/Lin− cells, a rare population of HSCs, with SCF, IL-15, IL-21, Flt-3L, and HC potentially could generate functional NK cells with the ability to kill their target cancer cells [159]. Likewise, Perez et al. (2006) showed that functional NK cells with the ability to express CD16, NKG2D, and NKp46, as well as anticancer activities, could be generated upon stimulation of human CD34+ HSCs by IL-15, IL-21, Flt-3L, and HC [164]. In addition to HSCs, human CD34+/CD45+/KDR+ adipose-derived SCs (ADSCs) presented a great capacity to form the NK-like cells through feeder-free and two-step protocols. Using a culture medium enriched with SCF, IL-3, IL-6, Flt-3L, GM-CSF, 2-mercaptoethanol, transferrin, low-density lipoprotein, insulin, and thrombopoietin, Hongxiu et al. (2014) were able to differentiate ADSCs into hematopoietic progenitor cells in 7 days. They were also able to generate a functional population of NK cells with the CD56+/CD3− phenotype by differentiating the produced HSCs under the stimulation of SCF, IL-2, IL-7, IL-15, and Flt-3L [165].
Beyond the above-mentioned studies, Spanholtz et al. (2011) developed a high-performance method to generate clinical-grade functional NK cells by establishment of a closed-system culture process. They could practically produce the HSCs-derived NK cells in a large-scale bioreactor using human serum-, IL-7-, IL-12-, IL-15-, and SCF-enriched medium for adoptive immunotherapy subjects. This feeder-free method achieved a purity of > 90% for CD16+/CD56+/CD3− NK cells within a period of 5–6 weeks [166] (Table 2).
SCs-derived NK cells in the serve of cancer immunotherapy
Compared to human PB- and UCB-isolated NK cells, experimental studies have discovered enhanced intra-tumoral polarization, cytokine secretion, and ultimately cytotoxic potential of SCs-derived NK cells. Furthermore, animal studies on human highly-metastatic and immunotherapy-resistant STs have demonstrated increased anti-tumor efficacy resulting from SCs-derived NK cell immunotherapy (Table 2). Interestingly, the safety and remarkable therapeutic benefits of ex vivo generated NK cells were demonstrated in a recent clinical trial on advanced and metastatic STs. To explain the feasibility and advantages of the SCs-derived NK cell-based immunotherapy, we have specifically reviewed some of the impressive published and currently ongoing studies in the field of cancer SCs-derived NK cell therapy.
Some of the remarkable observations have already reported complicated dysfunction and poor intra-tumoral infiltration of the implanted autologous and/or allogeneic NK cells in patients with advanced STs [5, 15]. Adoptive transfer of in vitro expanded NK cells [167, 168] and also concomitant therapy with mAbs or immune CIs have been practically presented to resolve the above challenge in the way of STs NK cell therapies [5, 168]. However, the poor therapeutic response of patients with advanced STs to conventional NK cell immunotherapies is still one of the challenges in modern oncology [168]. Veluchamy et al. (2017) investigated the utility and therapeutic potential of human HSCs-derived NK cells compared to an allogeneic NK cell source, termed A-PBNK, on the animal models of metastatic and immunotherapy-resistant colorectal cancer. They also evaluated both HSCs-derived NK cells and A-PBNK anti-tumoral activities in combination with cetuximab, an epidermal growth factor receptor (EGFR) inhibitor mAbs. Compared with A-PBNK monotherapy and even A-PBNK concomitant treatment with cetuximab, HSCs-derived NK cells alone had more cytotoxic capacity against two human metastatic and cetuximab-resistant colorectal cancer cell lines: the EGFR+ RASmut SW-480 and EGFR+++ RASwt A-431 cells. Interestingly, their experimental observations on the human colorectal cancer mice models (SW-480 and A-431 cells-associated tumors) found a remarkable reduction in the rate of tumoral cell loading and organ metastasis resulting from the infusion of human HSCs-derived NK cell compared to the control, A-PBNK alone, and cetuximab co-treated groups. They have also reported a significant increase in the animal survival after the human HSCs-derived NK cell therapy [5]. However, no significant therapeutic differences were observed between the HSCs-derived NK cell monotherapy and concurrent treatment with cetuximab. It seems that the low levels of some NK’s specific markers, such as CD16, in the ex vivo generated NK cells could be one of the involved effectors for the failure of efficacy of HSCs-derived NK cells and cetuximab combination [5, 169].
High intra-tumoral infiltration capacity of the SCs-derived NK cells has also been observed in some studies. Hoogstad et al. (2017) have demonstrated that their generated NK cells from human UBC-isolated HSCs could potentially infiltrate into the 3D spheroids of ovarian cancer and kill the malignant cells accordingly. In a mice model of human ovarian cancer peritoneal carcinomatosis, they have observed efficient anti-tumor and anti-metastatic responses through the intraperitoneal injection of human HSCs-derived NK cells. Ultimately, the generated NK cells dramatically prolonged the overall survival of the mice compared with the control group [15].
Similar to the HSCs-derived NK cells, human iPSCs-derived NK cells have presented remarkable tumor infiltration and anticancer activities on the NOD/SCID/γc−/− (NSG) mice models of human ovarian cancer. In this regard, Hermanson et al. (2015) have observed a notable anti-tumor response and also improvement in the animals' survival through a single-dose intraperitoneal administration of the iPSC‐derived NK cells activated by artificial antigen-presenting cells (aAPCs) compared with the human PB‐isolated NK cells activated with two separate aAPCs and IL-2 factors. A similar result was obtained by multiple-dose injections of the aAPCs-activated iPSC‐derived NK cells as opposed to the single-dose injection. Notably, a higher intra-tumoral trafficking of the iPSC‐derived NK cells has been observed compared to the PB‐isolated NK cells [13].
As a result of an experimental study, researchers managed to establish potentially functional NK cells from human CD34+/CD45+/KDR+ ADSCs, termed ADSCs-NK cells. Outcomes of the in vitro evaluations on the human PC-3, LnCap, DuPro, C4-2, and CWR-22 prostate cancer and also MCF-7 breast cancer cell lines have demonstrated a strong cytotoxicity of ADSCs-NK cells compared with human NK leukemia (hNKL) cells. Interestingly, through transfection of E4-binding protein-4 (E4BP-4) transcription factor gene, they successfully could produce ADSC-NKE cells as a gene-modified ADSCs-NK type. Their results showed a notable higher cytotoxicity and cytokine activity of the ADSC-NKEs in comparison with not only the hNKL cells but also ADSC-NK cells. Unlike the ADSC-NK cells, the ADSC-NKE cells could express most of the NK’s specific markers and also have more ability to express lysosomal-associated membrane protein-1 (LAMP-1 or CD107). However, both NK cell types mentioned above were similar in the expression of CD56 and CD32 antigens. Finally, their in vivo evaluations on the nude mice models of human PC3 prostate and MCF7 breast tumor have confirmed a significant decrease in tumor progression in the treated animals with ADSC-NKE as early as one week after treatment [165].
The phenotypic and functional differences between the ex vivo generated NK cells from the human PSCs and also MASCs have been further investigated by Woll et al. (2009). They showed that the NK cells derived from ESCs were able to express higher levels of NK-specific markers such as CD16, NKG2D, and KIRs than the NK cells differentiated from the human UCB-isolated progenitor cells. Their in vitro anti-tumor evaluations showed a significant increase in cytotoxicity of the produced ESCs-derived NK cells on human K562 erythroleukemia, MCF7 breast cancer, PC3 prostate cancer, U87 glioma, and NTERA2 testicular embryonal carcinoma cell lines compared to the HSCs-derived NK cells. In the K562 tumor-bearing mice, a complete clearance of primary tumors was observed in all treated mice (all 13 mice) as early as 2 weeks after intravenous infusion of the ESCs-derived NK cells. While tumor progression was significantly inhibited in animals treated with the HSCs-derived cells, only of 13 mice showed complete tumor clearance. Furthermore, the human ESCs-derived NK cells exposed a more significant ability to inhibit the tumor-associated metastasis to various organs of the animals, including the liver, lung, spleen, and kidneys, compared with the HSCs-derived NK cells [134].
The results of this experience enabled us to large-scale production of clinical-grade NK cells using the SCs technology [16, 166]. Within a long-term translational study, Bjordahl et al. (2018) were able to successfully produce a universal and “off-the-shelf” NK cell source, named FT500, from the human iPSCs for the STs immunotherapy targets. The FT500 cells are unable to express PD-1 (receptor of PD-L1). Hence, it seems that the PD-L1 of the tumor cell has no interfering effect on the FT500 intra-tumoral migration and cytotoxicity. Within the FT500 monotherapy, both in vitro and in vivo studies have confirmed a powerful inhibition of the progression and metastasis of immune escaping STs. In the peritoneal ovarian cancer mice models, intraperitoneal injection of the FT500 cells additionally caused in a recruit of circulating T lymphocytes into the peritoneal cavity, which resulted in the secretion of soluble factors and then the expression of some chemokine [17]. Ultimately, their results represent a set of fascinating evidence for the potential benefit of FT500 monotherapy and in combination with immune CIs in subjects with advanced STs. Now, a phase I non-randomized clinical trial is launched to study on more than 18 different types of advanced STs in efforts to evaluate the dose-limiting toxicities, the maximum tolerated dose, and ultimately the efficacy of FT500 monotherapy and in combination with immune CIs (NCT03841110).
Currently, promising approaches of cancer immunotherapy are being developed through the generation of CAR-engineered immune cells. As an artificial receptor, CARs consist of four principal parts, including extracellular antigen-binding domain, transmembrane regions, and intracellular co-stimulatory, and finally main activating domains. When CARs are stimulated by an antigen-expressing target cell, the crucial activating signaling pathways of immune cells are triggered [170]. Currently, CARs approach case is used to produce autologous and allogeneic CAR-NK cells [171]. Accessibility to more flexible candidates of tumor antigens, higher targeting potential, and enhanced anti-tumor responses are some of the main advantages of CAR-NK cell therapies. Interestingly, application of the allogeneic NK cell source, including NK-92, NKT, and gamma delta (γδ) T cells, has led to the generation of universal products of CAR-NK cells [172]. Several clinical trials on patients with advanced cancers are investigating the safety and feasibility of allogeneic CAR-NK cell products (NCT02944162, NCT03774654, and NCT02656147). Along the way, CD33-CAR (NCT02944162), CD7-CAR (NCT02742727), CD19-CAR (NCT02892695), CD19-CAR + IL-15 (NCT03774654), NKG2DL-targeting CAR (NCT04107142), and CD19-CAR (NCT02656147) are some of the applied genetically modified receptors in clinical trials of CAR-NK cell immunotherapy. Currently, ex vivo generated NK cells are proposed as one of the best options for the production of CAR-NK cells in an off-the-shelf platform. In the previously published studies, novel allogeneic CAR-NK cells were generated by iPSC-derived NK cells. Strong cytokine activity and anti-tumor response have been also reported following the preclinical evaluation of iPSC-derived CAR-NK cells [173].
Together with all the reviewed benefits, the allogeneic sources of NK cells may be unintentionally rejected via the reaction of the recipient T and NK cells against the HLA-A of the implanted NK cells [174–176]. Based on a recently published study, it has been demonstrated that the application of autologous iPSCs for the cancer immunotherapy aims, besides the safety and the histocompatibility, would be able to improve the immunotherapy efficacy through the inhibition of STs progression and recurrent [177]. Thus, it appears that the administration of NK cells derived from the patient’s owns iPSCs, in addition to providing an unlimited source of autologous NK cells with potent anti-tumor activities, would be able to solve most of the challenges ahead of advanced and/or metastatic STs immunotherapy. Within a personalized setting, we have currently initiated an international multicenter, non-randomized phase I clinical trial to evaluate the safety and the efficacy of autologous iPSCs-derived NK cell therapy in patients with advanced breast cancer (IRCT20200429047241N1). Here, the authors believe that the application of SCs-derived NK cell immunotherapy approaches, particularly in the context of personalized medicine, could be the next revolutionary step in the modern oncology.
Conclusion
Conventional NK cell-based immunotherapy on patients with advanced STs faces many challenges. Along this path, outcomes of the current preclinical and clinical studies have revealed a variety of benefits resulting from the SCs-derived NK cells immunotherapy. Through a longstanding international collaboration, we have highlighted the most appropriate and achievable solutions for the challenges ahead of STs NK cell therapy in this paper. To defend our hypotheses, the benefits of ex vivo generated NK cells in the treatment of advanced STs, we have reviewed high-quality published articles in the field of advanced STs NK cell immunotherapy, SCs biology, and ex vivo NK cell generation, within a multidisciplinary framework. As a comprehensive resource, this article facilitates the access of researchers to current protocols of NK cell production from the human SCs and helps them to develop their scientific goals.
Acknowledgements
The figures were created by using of “BioRender.com” and CorelDRAW Graphics Suite X8 (version 2018).
Authors’ contributions
HK, SK, HM, KN, and JH designed the study; HK, SK, EE, and FH wrote the manuscript; SK, HK, and EE were involved in graphical art works and tables; MV, EE, HM, FH, KN, JH, TS, WS, and SVG critically revised the work; KN and HM were involved in supervision.
Funding
This research was funded by International Center for Personalized Medicine (ICPM), Düsseldorf, Germany (grant number: ICPM-2019–001).
Declaration
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hamid Khodayari and Saeed Khodayari have contributed equally.
Contributor Information
Habibollah Mahmoodzadeh, Email: hmahmoodzadeh@tums.ac.ir.
Karim Nayernia, Email: nayernia@p7medicine.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Shaked Y. The pro-tumorigenic host response to cancer therapies. Nat Rev Cancer. 2019;19:667–685. doi: 10.1038/s41568-019-0209-6. [DOI] [PubMed] [Google Scholar]
- 3.Gavhane Y, Shete A, Bhagat A, Shinde V, Bhong K, Khairnar G, Yadav A. Solid tumors: facts, challenges and solutions. Int J Pharm Sci Res. 2011;2:1–12. [Google Scholar]
- 4.Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36:1611. doi: 10.1200/JCO.2017.76.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veluchamy JP, Lopez-Lastra S, Spanholtz J, et al. In vivo efficacy of umbilical cord blood stem cell-derived NK cells in the treatment of metastatic colorectal cancer. Front Immunol. 2017;8:87. doi: 10.3389/fimmu.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Restifo NP, Smyth MJ, Snyder A. Acquired resistance to immunotherapy and future challenges. Nat Rev Cancer. 2016;16:121. doi: 10.1038/nrc.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohsenikia M, Farhangi B, Alizadeh AM, et al. Therapeutic effects of dendrosomal solanine on a metastatic breast tumor. Life Sci. 2016;148:260–267. doi: 10.1016/j.lfs.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Dupuis F, Lamant L, Gerard E, et al. Clinical, histological and molecular predictors of metastatic melanoma responses to anti-PD-1 immunotherapy. Br J Cancer. 2018;119:193–199. doi: 10.1038/s41416-018-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somasundaram R, Zhang G, Fukunaga-Kalabis M, et al. Tumor-associated B-cells induce tumor heterogeneity and therapy resistance. Nat Commun. 2017;8:1–16. doi: 10.1038/s41467-017-00452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiri S, Alizadeh AM, Baradaran B, Farhanghi B, Shanehbandi D, Khodayari S, Khodayari H, Tavassoli A. Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pac J Cancer Prev. 2015;16:3917–3922. doi: 10.7314/apjcp.2015.16.9.3917. [DOI] [PubMed] [Google Scholar]
- 11.Farhanji B, Latifpour M, Alizadeh AM, Khodayari H, Khodayari S, Khaniki M, Ghasempour S. Tumor suppression effects of myoepithelial cells on mice breast cancer. Eur J Pharmacol. 2015;765:171–178. doi: 10.1016/j.ejphar.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Krause SW, Gastpar R, Andreesen R, Gross C, Ullrich H, Thonigs G, Pfister K, Multhoff G. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase I trial. Clin Cancer Res. 2004;10:3699–3707. doi: 10.1158/1078-0432.CCR-03-0683. [DOI] [PubMed] [Google Scholar]
- 13.Hermanson DL, Bendzick L, Pribyl L, McCullar V, Vogel RI, Miller JS, Geller MA, Kaufman DS. Induced pluripotent stem cell-derived natural killer cells for treatment of ovarian cancer. St Cells. 2016;34:93–101. doi: 10.1002/stem.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koepsell SA, Miller JS, McKenna DH., Jr Natural killer cells: a review of manufacturing and clinical utility. Transfusion. 2013;53:404–410. doi: 10.1111/j.1537-2995.2012.03724.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoogstad-van Evert JS, Cany J, van den Brand D, et al. Umbilical cord blood CD34+ progenitor-derived NK cells efficiently kill ovarian cancer spheroids and intraperitoneal tumors in NOD/SCID/IL2Rgnull mice. Oncoimmunology. 2017;6:e1320630. doi: 10.1080/2162402X.2017.1320630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng J, Tang SY, Toh LL, Wang S. Generation of “off-the-shelf” natural killer cells from peripheral blood cell-derived induced pluripotent stem cells. St Cell Rep. 2017;9:1796–1812. doi: 10.1016/j.stemcr.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjordahl R, Mahmood S, Gaidarova S, et al. FT500, an off-the-shelf NK cell cancer immunotherapy derived from a master pluripotent cell line, enhances T-cell activation and recruitment to overcome checkpoint blockade resistance. AACR. 2018 doi: 10.1158/1538-7445.AM2018-3576. [DOI] [Google Scholar]
- 18.O’Brien KL, Finlay DK. Immunometabolism and natural killer cell responses. Nat Rev Immunol. 2019;19:282–290. doi: 10.1038/s41577-019-0139-2. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Tian Z. NK cell education via nonclassical MHC and non-MHC ligands. Cell Mol Immunol. 2017;14:321–330. doi: 10.1038/cmi.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lysakova-Devine T, O'Farrelly C. Tissue-specific NK cell populations and their origin. J Leukoc Biol. 2014;96:981–990. doi: 10.1189/jlb.1RU0514-241R. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Wallace DL, De Lara CM, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121:258–265. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walzer T, Bléry M, Chaix J, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Sun R, Hao X, Lian Z-X, Wei H, Tian Z. IL-17 constrains natural killer cell activity by restraining IL-15–driven cell maturation via SOCS3. Proc Natl Acad Sci. 2019;116:17409–17418. doi: 10.1073/pnas.1904125116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 2010;11:585. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- 25.Cichocki F, Grzywacz B, Miller JS. Human NK cell development: one road or many? Front Immunol. 2019;10:2078. doi: 10.3389/fimmu.2019.02078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Male V, Brady HJ. Transcriptional control of NK cell differentiation and function. Transcr Control Lineage Differ Immune Cells. 2014 doi: 10.1007/82_2014_376. [DOI] [PubMed] [Google Scholar]
- 27.Male V, Nisoli I, Kostrzewski T, Allan DS, Carlyle JR, Lord GM, Wack A, Brady HJ. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J Exp Med. 2014;211:635–642. doi: 10.1084/jem.20132398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jonge K, Ebering A, Nassiri S, Maby-El Hajjami H, Ouertatani-Sakouhi H, Baumgaertner P, Speiser DE. Circulating CD56 bright NK cells inversely correlate with survival of melanoma patients. Sci Rep. 2019;9:1–10. doi: 10.1038/s41598-019-40933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crome SQ, Lang PA, Lang KS, Ohashi PS. Natural killer cells regulate diverse T cell responses. Trends Immunol. 2013;34:342–349. doi: 10.1016/j.it.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Ferlazzo G, Thomas D, Lin S-L, Goodman K, Morandi B, Muller WA, Moretta A, Münz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 31.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 32.Vosshenrich CA, García-Ojeda ME, Samson-Villéger SI, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 33.Grégoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-γ and CXCR3 ligands. Can Res. 2008;68:8437–8445. doi: 10.1158/0008-5472.CAN-08-1440. [DOI] [PubMed] [Google Scholar]
- 35.Hydes T, Noll A, Salinas-Riester G, et al. IL-12 and IL-15 induce the expression of CXCR6 and CD49a on peripheral natural killer cells. Immun Inflamm Dis. 2018;6:34–46. doi: 10.1002/iid3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishida Y, Migita K, Izumi Y, et al. The role of IL-18 in the modulation of matrix metalloproteinases and migration of human natural killer (NK) cells. FEBS Lett. 2004;569:156–160. doi: 10.1016/j.febslet.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 37.Li C, Ge B, Nicotra M, Stern JN, Kopcow HD, Chen X, Strominger JL. JNK MAP kinase activation is required for MTOC and granule polarization in NKG2D-mediated NK cell cytotoxicity. Proc Natl Acad Sci. 2008;105:3017–3022. doi: 10.1073/pnas.0712310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moriggl R, Sexl V, Piekorz R, Topham D, Ihle JN. Stat5 activation is uniquely associated with cytokine signaling in peripheral T cells. Immunity. 1999;11:225–230. doi: 10.1016/s1074-7613(00)80097-7. [DOI] [PubMed] [Google Scholar]
- 39.Samson SI, Richard O, Tavian M, et al. GATA-3 promotes maturation, IFN-γ production, and liver-specific homing of NK cells. Immunity. 2003;19:701–711. doi: 10.1016/s1074-7613(03)00294-2. [DOI] [PubMed] [Google Scholar]
- 40.Kallies A, Carotta S, Huntington ND, Bernard NJ, Tarlinton DM, Smyth MJ, Nutt SL. A role for Blimp1 in the transcriptional network controlling natural killer cell maturation. Blood. 2011;117:1869–1879. doi: 10.1182/blood-2010-08-303123. [DOI] [PubMed] [Google Scholar]
- 41.Ohno S-i, Sato T, Kohu K, Takeda K, Okumura K, Satake M, Habu S. Runx proteins are involved in regulation of CD122, Ly49 family and IFN-γ expression during NK cell differentiation. Int Immunol. 2008;20:71–79. doi: 10.1093/intimm/dxm120. [DOI] [PubMed] [Google Scholar]
- 42.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 43.Inngjerdingen M, Damaj B, Maghazachi AA. Expression and regulation of chemokine receptors in human natural killer cells. Blood. 2001;97:367–375. doi: 10.1182/blood.v97.2.367. [DOI] [PubMed] [Google Scholar]
- 44.Edsparr K, Speetjens FM, Mulder-Stapel A, Goldfarb RH, Basse PH, Lennernäs B, Kuppen PJ, Albertsson P. Effects of IL-2 on MMP expression in freshly isolated human NK cells and the IL-2-independent NK cell line YT. J Immunother. 2010;33:475. doi: 10.1097/CJI.0b013e3181d372a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taub DD, Sayers TJ, Carter C, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
- 46.Albertsson P, Kim M, Jonges L, Kitson R, Kuppen P, Johansson B, Nannmark U, Goldfarb R. Matrix metalloproteinases of human NK cells. Vivo (Athens, Greece) 2000;14:269–276. [PubMed] [Google Scholar]
- 47.Goda S, Inoue H, Umehara H, et al. Matrix metalloproteinase-1 produced by human CXCL12-stimulated natural killer cells. Am J Pathol. 2006;169:445–458. doi: 10.2353/ajpath.2006.050676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnatty RN, Taub DD, Reeder SP, Turcovski-Corrales SM, Cottam DW, Stephenson TJ, Rees RC. Cytokine and chemokine regulation of proMMP-9 and TIMP-1 production by human peripheral blood lymphocytes. J Immunol. 1997;158:2327–2333. [PubMed] [Google Scholar]
- 49.Quatrini L, Molfetta R, Zitti B, et al. Ubiquitin-dependent endocytosis of NKG2D-DAP10 receptor complexes activates signaling and functions in human NK cells. Sci Signal. 2015;8:ra108–ra108. doi: 10.1126/scisignal.aab2724. [DOI] [PubMed] [Google Scholar]
- 50.Sivori S, Vacca P, Del Zotto G, Munari E, Mingari MC, Moretta L. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol. 2019;16:430–441. doi: 10.1038/s41423-019-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luu TT, Nguyen N-A, Ganesan S, Meinke S, Kadri N, Alici E, Höglund P. A brief IL-15 pulse results in JAK3-dependent phosphorylation of ITAM-associated signaling molecules and a long-lasting priming imprint in mouse NK cells. SSRN Electron J. 2019 doi: 10.2139/ssrn.3351831. [DOI] [Google Scholar]
- 52.Raneros AB, Puras AM, Rodriguez RM, et al. Increasing TIMP3 expression by hypomethylating agents diminishes soluble MICA, MICB and ULBP2 shedding in acute myeloid leukemia, facilitating NK cell-mediated immune recognition. Oncotarget. 2017;8:31959. doi: 10.18632/oncotarget.16657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.López-Cobo S, Romera-Cárdenas G, García-Cuesta EM, Reyburn HT, Valés-Gómez M. Transfer of the human NKG 2D ligands UL 16 binding proteins (ULBP) 1–3 is related to lytic granule release and leads to ligand retransfer and killing of ULBP-recipient natural killer cells. Immunology. 2015;146:70–80. doi: 10.1111/imm.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang L, Shen M, Xu LJ, Yang X, Tsai Y, Keng PC, Chen Y, Lee SO. Enhancing NK cell-mediated cytotoxicity to cisplatin-resistant lung cancer cells via MEK/Erk signaling inhibition. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-017-08483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 56.Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, Yagita H, Okumura K. Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) contributes to interferon γ–dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001;193:661–670. doi: 10.1084/jem.193.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wigginton JM, Gruys E, Geiselhart L, et al. IFN-γ and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J Clin Investig. 2001;108:51–62. doi: 10.1172/JCI10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saric T, Chang S-C, Hattori A, York IA, Markant S, Rock KL, Tsujimoto M, Goldberg AL. An IFN-γ–induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I–presented peptides. Nat Immunol. 2002;3:1169–1176. doi: 10.1038/ni859. [DOI] [PubMed] [Google Scholar]
- 59.Koya T, Yanagisawa R, Higuchi Y, Sano K, Shimodaira S. Interferon-α-inducible dendritic cells matured with OK-432 exhibit TRAIL and fas ligand pathway-mediated killer activity. Sci Rep. 2017;7:1–11. doi: 10.1038/srep42145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125(23):5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 61.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 62.Garris CS, Luke JJ. Dendritic cells, the T-cell-inflamed tumor microenvironment, and immunotherapy treatment response. Clin Cancer Res. 2020;26:3901–3907. doi: 10.1158/1078-0432.CCR-19-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khodayari H, Chamani R, Khodayari S, Alizadeh AM. A glance into cancer stem cells. J Kerman Univ Med Sci. 2016;23:515–542. [Google Scholar]
- 65.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H. CD4 (+) CD25 high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–1071. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Porembka MR, Mitchem JB, Belt BA, Hsieh C-S, Lee H-M, Herndon J, Gillanders WE, Linehan DC, Goedegebuure P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61:1373–1385. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murray S, Lundqvist A. Targeting the tumor microenvironment to improve natural killer cell-based immunotherapies: on being in the right place at the right time, with resilience. Hum Vaccin Immunother. 2016;12:607–611. doi: 10.1080/21645515.2015.1096458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ren J, Zeng W, Tian F, Zhang S, Wu F, Qin X, Zhang Y, Lin Y. Myeloid-derived suppressor cells depletion may cause pregnancy loss via upregulating the cytotoxicity of decidual natural killer cells. Am J Reprod Immunol. 2019;81:e13099. doi: 10.1111/aji.13099. [DOI] [PubMed] [Google Scholar]
- 70.Hoechst B, Voigtlaender T, Ormandy L, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-β1. J Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 72.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+ CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 73.Littwitz-Salomon E, Malyshkina A, Schimmer S, Dittmer U. The cytotoxic activity of natural killer cells is suppressed by IL-10+ regulatory T cells during acute retroviral infection. Front Immunol. 2018;9:1947. doi: 10.3389/fimmu.2018.01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costantini C, Cassatella MA. The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. J Leukoc Biol. 2011;89:221–233. doi: 10.1189/jlb.0510250. [DOI] [PubMed] [Google Scholar]
- 75.Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318:154–161. doi: 10.1016/j.canlet.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 76.Li T, Yi S, Liu W, Jia C, Wang G, Hua X, Tai Y, Zhang Q, Chen G. Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med Oncol. 2013;30:663. doi: 10.1007/s12032-013-0663-z. [DOI] [PubMed] [Google Scholar]
- 77.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2, 3-dioxygenase and prostaglandin E2. Blood J Am Soc Hematol. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 78.Oyer JL, Gitto SB, Altomare DA, Copik AJ. PD-L1 blockade enhances anti-tumor efficacy of NK cells. Oncoimmunology. 2018;7:e1509819. doi: 10.1080/2162402X.2018.1509819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Juliá EP, Amante A, Pampena MB, Mordoh J, Levy EM. Avelumab, an IgG1 anti-PD-L1 immune checkpoint inhibitor, triggers NK cell-mediated cytotoxicity and cytokine production against triple negative breast cancer cells. Front Immunol. 2018;9:2140. doi: 10.3389/fimmu.2018.02140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sarvaria A, Jawdat D, Madrigal JA, Saudemont A. Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front Immunol. 2017;8:329. doi: 10.3389/fimmu.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liang S, Niu L, Xu K, Wang X, Liang Y, Zhang M, Chen J, Lin M. Tumor cryoablation in combination with natural killer cells therapy and herceptin in patients with HER2-overexpressing recurrent breast cancer. Mol Immunol. 2017;92:45–53. doi: 10.1016/j.molimm.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 82.Lin M, Liang S, Wang X, Liang Y, Zhang M, Chen J, Niu L, Xu K. Percutaneous irreversible electroporation combined with allogeneic natural killer cell immunotherapy for patients with unresectable (stage III/IV) pancreatic cancer: a promising treatment. J Cancer Res Clin Oncol. 2017;143:2607–2618. doi: 10.1007/s00432-017-2513-4. [DOI] [PubMed] [Google Scholar]
- 83.Sakamoto N, Ishikawa T, Kokura S, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13:1–13. doi: 10.1186/s12967-015-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li R, Wang C, Liu L, et al. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother. 2012;61:2125–2133. doi: 10.1007/s00262-012-1260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pérez-Martínez A, Fernández L, Valentín J, et al. A phase I/II trial of interleukin-15–stimulated natural killer cell infusion after haplo-identical stem cell transplantation for pediatric refractory solid tumors. Cytotherapy. 2015;17:1594–1603. doi: 10.1016/j.jcyt.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 86.Ardolino M, Raulet DH. Cytokine therapy restores antitumor responses of NK cells rendered anergic in MHC I-deficient tumors. Oncoimmunology. 2016;5:e1002725. doi: 10.1080/2162402X.2014.1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fiedler W, Zeller W, Peimann C-J, Weh H-J, Hossfeld D. A phase II combination trial with recombinant human tumor necrosis factor and gamma interferon in patients with colorectal cancer. Klin Wochenschr. 1991;69:261–268. doi: 10.1007/BF01666852. [DOI] [PubMed] [Google Scholar]
- 88.Hayakawa M, Hatano T, Ogawa Y, Gakiya M, Ogura H, Osawa A. Treatment of advanced renal cell carcinoma using regional arterial administration of lymphokine-activated killer cells in combination with low doses of rlL-2. Urol Int. 1994;53:117–124. doi: 10.1159/000282651. [DOI] [PubMed] [Google Scholar]
- 89.Ueda Y, Yamagishi H, Tanioka Y, Fujiwara H, Fuji N, Itoh T, Fujiki H, Yoshimura T, Oka T. Clinical application of adoptive immunotherapy and IL-2 for the treatment of advanced digestive tract cancer. Hepatogastroenterology. 1999;46:1274–1279. [PubMed] [Google Scholar]
- 90.Law TM, Motzer RJ, Mazumdar M, et al. Phase iii randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer. 1995;76:824–832. doi: 10.1002/1097-0142(19950901)76:5<824::aid-cncr2820760517>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 91.Hashimoto W, Tanaka F, Robbins PD, Taniguchi M, Okamura H, Lotze MT, Tahara H. Natural killer, but not natural killer T, cells play a necessary role in the promotion of an innate antitumor response induced by IL-18. Int J Cancer. 2003;103:508–513. doi: 10.1002/ijc.10844. [DOI] [PubMed] [Google Scholar]
- 92.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 93.Multhoff G, Seier S, Stangl S, et al. Targeted natural killer cell-based adoptive immunotherapy for the treatment of patients with NSCLC after radiochemotherapy: a randomized phase II clinical trial. Clin Cancer Res. 2020;26:5368–5379. doi: 10.1158/1078-0432.CCR-20-1141. [DOI] [PubMed] [Google Scholar]
- 94.Knorr DA et al (2014) Clinical utility of natural killer cells in cancer therapy and transplantation. Seminars in immunology. Vol 26, No 2. Academic Press [DOI] [PMC free article] [PubMed]
- 95.Liang S, Lin M, Niu L, Xu K, Wang X, Liang Y, Zhang M, Du D, Chen J. Cetuximab combined with natural killer cells therapy: an alternative to chemoradiotherapy for patients with advanced non-small cell lung cancer (NSCLC) Am J Cancer Res. 2018;8:879. [PMC free article] [PubMed] [Google Scholar]
- 96.Ishikawa T, Okayama T, Sakamoto N, et al. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with I g G 1 antibody in patients with gastric or colorectal cancer. Int J Cancer. 2018;142:2599–2609. doi: 10.1002/ijc.31285. [DOI] [PubMed] [Google Scholar]
- 97.Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, Klingemann H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–632. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 98.Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, Tonn T. NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother. 2016;65:485–492. doi: 10.1007/s00262-015-1761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gong J-H, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- 100.Božič J, Stoka V, Dolenc I. Glucosamine prevents polarization of cytotoxic granules in NK-92 cells by disturbing FOXO1/ERK/paxillin phosphorylation. PLoS ONE. 2018;13:e0200757. doi: 10.1371/journal.pone.0200757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Montagner IM, Penna A, Fracasso G, Carpanese D, Pietà AD, Barbieri V, Zuccolotto G, Rosato A. Anti-PSMA CAR-engineered NK-92 cells: an off-the-shelf cell therapy for prostate cancer. Cells. 2020;9:1382. doi: 10.3390/cells9061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu A, Wiesner S, Xiao J, Ericson K, Chen W, Hall WA, Low WC, Ohlfest JR. Expression of MHC I and NK ligands on human CD133+ glioma cells: possible targets of immunotherapy. J Neurooncol. 2007;83:121–131. doi: 10.1007/s11060-006-9265-3. [DOI] [PubMed] [Google Scholar]
- 103.Bolourian A, Mojtahedi Z. Possible damage to immune-privileged sites in natural killer cell therapy in cancer patients: side effects of natural killer cell therapy. Immunotherapy. 2017;9:281–288. doi: 10.2217/imt-2016-0137. [DOI] [PubMed] [Google Scholar]
- 104.Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, Ohno T. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24:1861–1871. [PubMed] [Google Scholar]
- 105.Mantia CM, Buchbinder EI. Immunotherapy toxicity. hematology/oncology. Clinics. 2019;33:275–290. doi: 10.1016/j.hoc.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 106.Haanen J, Carbonnel F, Robert C, Kerr K, Peters S, Larkin J, Jordan K. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 107.Liu D, Tian S, Zhang K, Xiong W, Lubaki NM, Chen Z, Han W. Chimeric antigen receptor (CAR)-modified natural killer cell-based immunotherapy and immunological synapse formation in cancer and HIV. Prot Cell. 2017;8:861–877. doi: 10.1007/s13238-017-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barkholt L, Alici E, Conrad R, et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy. 2009;1:753–764. doi: 10.2217/imt.09.47. [DOI] [PubMed] [Google Scholar]
- 109.Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, Rigatos G, Papamichail M, Perez SA. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1781–1789. doi: 10.1007/s00262-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang YJ, Park JC, Kim HK, Kang JH, Park SY. A trial of autologous ex vivo-expanded NK cell-enriched lymphocytes with docetaxel in patients with advanced non-small cell lung cancer as second-or third-line treatment: phase IIa study. Anticancer Res. 2013;33:2115–2122. [PubMed] [Google Scholar]
- 111.Motohashi S, Ishikawa A, Ishikawa E, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non–small cell lung cancer. Clin Cancer Res. 2006;12:6079–6086. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- 112.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17:6287–6297. doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin M, Xu K, Liang S, Wang X, Liang Y, Zhang M, Chen J, Niu L. Prospective study of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced renal cell cancer. Immunol Lett. 2017;184:98–104. doi: 10.1016/j.imlet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 114.Dillman RO, Duma CM, Ellis RA, Cornforth AN, Schiltz PM, Sharp SL, DePriest MC. Intralesional lymphokine-activated killer cells as adjuvant therapy for primary glioblastoma. J Immunother. 2009;32:914–919. doi: 10.1097/CJI.0b013e3181b2910f. [DOI] [PubMed] [Google Scholar]
- 115.Tonn T, Schwabe D, Klingemann HG, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–1570. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 116.Geller MA, Cooley S, Judson PL, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mardanpour P, Nayernia K, Khodayari S, Khodayari H, Molcanyi M, Hescheler J. Application of stem cell technologies to regenerate injured myocardium and improve cardiac function. Cell Physiol Biochem. 2019;53:101–120. doi: 10.33594/000000124. [DOI] [PubMed] [Google Scholar]
- 118.Khodayari S, Khodayari H, Amiri AZ, Eslami M, Farhud D, Hescheler J, Nayernia K. Inflammatory microenvironment of acute myocardial infarction prevents regeneration of heart with stem cells therapy. Cell Physiol Biochem. 2019;53:887–909. doi: 10.33594/000000180. [DOI] [PubMed] [Google Scholar]
- 119.Nayernia K, Nolte J, Michelmann HW, et al. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;11:125–132. doi: 10.1016/j.devcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 120.Guan K, Nayernia K, Maier LS, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 121.Mitalipov S, Wolf D. Totipotency, pluripotency and nuclear reprogramming. Berlin: Engineering of stem cells. Springer; 2009. pp. 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Paepe C, Krivega M, Cauffman G, Geens M, Van de Velde H. Totipotency and lineage segregation in the human embryo. Mol Hum Reprod. 2014;20:599–618. doi: 10.1093/molehr/gau027. [DOI] [PubMed] [Google Scholar]
- 123.Ishiuchi T, Torres-Padilla M-E. Towards an understanding of the regulatory mechanisms of totipotency. Curr Opin Genet Dev. 2013;23:512–518. doi: 10.1016/j.gde.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 124.Zhou L-q, Dean J. Reprogramming the genome to totipotency in mouse embryos. Trends Cell Biol. 2015;25:82–91. doi: 10.1016/j.tcb.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Falco G, Lee S-L, Stanghellini I, Bassey UC, Hamatani T, Ko MS. Zscan4: a novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Dev Biol. 2007;307:539–550. doi: 10.1016/j.ydbio.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De Iaco A, Planet E, Coluccio A, Verp S, Duc J, Trono D. DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat Genet. 2017;49:941–945. doi: 10.1038/ng.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hendrickson PG, Doráis JA, Grow EJ, et al. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat Genet. 2017;49:925–934. doi: 10.1038/ng.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fadloun A, Le Gras S, Jost B, Ziegler-Birling C, Takahashi H, Gorab E, Carninci P, Torres-Padilla M-E. Chromatin signatures and retrotransposon profiling in mouse embryos reveal regulation of LINE-1 by RNA. Nat Struct Mol Biol. 2013;20:332. doi: 10.1038/nsmb.2495. [DOI] [PubMed] [Google Scholar]
- 129.Hu Z, Tan DEK, Chia G, et al. Maternal factor NELFA drives a 2C-like state in mouse embryonic stem cells. Nat Cell Biol. 2020;22:175–186. doi: 10.1038/s41556-019-0453-8. [DOI] [PubMed] [Google Scholar]
- 130.King NM, Perrin J. Ethical issues in stem cell research and therapy. St Cell Res Ther. 2014;5:1–6. doi: 10.1186/scrt474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yilmaz A, Benvenisty N. Defining human pluripotency. Cell St Cell. 2019;25:9–22. doi: 10.1016/j.stem.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 132.Boroviak T, Loos R, Bertone P, Smith A, Nichols J. The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nat Cell Biol. 2014;16:513–525. doi: 10.1038/ncb2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sugimura R, Jha DK, Han A, et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature. 2017;545:432–438. doi: 10.1038/nature22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, Kaufman DS. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schmidt R, Plath K. The roles of the reprogramming factors Oct4, Sox2 and Klf4 in resetting the somatic cell epigenome during induced pluripotent stem cell generation. Genome Biol. 2012;13:1–11. doi: 10.1186/gb-2012-13-10-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fiedorowicz K, Rozwadowska N, Zimna A, et al. Tissue-specific promoter-based reporter system for monitoring cell differentiation from iPSCs to cardiomyocytes. Sci Rep. 2020;10:1–13. doi: 10.1038/s41598-020-58050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Y, Mah N, Prigione A, Wolfrum K, Andrade-Navarro MA, Adjaye J. A transcriptional roadmap to the induction of pluripotency in somatic cells. St Cell Rev Rep. 2010;6:282–296. doi: 10.1007/s12015-010-9137-2. [DOI] [PubMed] [Google Scholar]
- 138.Castro-Viñuelas R, Sanjurjo-Rodríguez C, Piñeiro-Ramil M, et al. Generation and characterization of human induced pluripotent stem cells (iPSCs) from hand osteoarthritis patient-derived fibroblasts. Sci Rep. 2020;10:1–13. doi: 10.1038/s41598-020-61071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim Y, Rim YA, Yi H, Park N, Park S-H, Ju JH. The generation of human induced pluripotent stem cells from blood cells: an efficient protocol using serial plating of reprogrammed cells by centrifugation. St Cells Int. 2016;2016:1–9. doi: 10.1155/2016/1329459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Seki T, Yuasa S, Oda M, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell St Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 141.Zhou T, Benda C, Dunzinger S, et al. Generation of human induced pluripotent stem cells from urine samples. Nat Protoc. 2012;7:2080. doi: 10.1038/nprot.2012.115. [DOI] [PubMed] [Google Scholar]
- 142.Zapata-Linares N, Rodriguez S, Mazo M, Abizanda G, Andreu EJ, Barajas M, Prosper F, Rodriguez-Madoz JR. Generation and characterization of human iPSC line generated from mesenchymal stem cells derived from adipose tissue. St Cell Res. 2016;16:20–23. doi: 10.1016/j.scr.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 143.Wu KH, Wang SY, Xiao QR, Yang Y, Huang NP, Mo XM, Sun J. Small-molecule–based generation of functional cardiomyocytes from human umbilical cord–derived induced pluripotent stem cells. J Cell Biochem. 2019;120:1318–1327. doi: 10.1002/jcb.27094. [DOI] [PubMed] [Google Scholar]
- 144.Yulin X, Lizhen L, Lifei Z, Shan F, Ru L, Kaimin H, Huang H. Efficient generation of induced pluripotent stem cells from human bone marrow mesenchymal stem cells. Folia Biol. 2012;58:221. [PubMed] [Google Scholar]
- 145.Teng YD. Functional multipotency of stem cells: biological traits gleaned from neural progeny studies. Seminars in cell & developmental biology. Elsevier; 2019. pp. 74–83. [DOI] [PubMed] [Google Scholar]
- 146.Wu Y, Zeng J, Roscoe BP, et al. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat Med. 2019;25:776–783. doi: 10.1038/s41591-019-0401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zou Y, Zhao Y, Xiao Z, Chen B, Ma D, Shen H, Gu R, Dai J. Comparison of regenerative effects of transplanting three-dimensional longitudinal scaffold loaded-human mesenchymal stem cells and human neural stem cells on spinal cord completely transected rats. ACS Biomater Sci Eng. 2020;6:1671–1680. doi: 10.1021/acsbiomaterials.9b01790. [DOI] [PubMed] [Google Scholar]
- 148.Khodayari S, Khodayari H, Alizadeh AM. A glance into the future cardiac stem cells. Tehran Univ Med J TUMS Publ. 2016;74:223–235. [Google Scholar]
- 149.Goodarzi A, Khanmohammadi M, Ai A, et al. Simultaneous impact of atorvastatin and mesenchymal stem cells for glioblastoma multiform suppression in rat glioblastoma multiform model. Mol Biol Rep. 2020;47:7783–7795. doi: 10.1007/s11033-020-05855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yvernogeau L, Gautier R, Petit L, et al. In vivo generation of haematopoietic stem/progenitor cells from bone marrow-derived haemogenic endothelium. Nat Cell Biol. 2019;21:1334–1345. doi: 10.1038/s41556-019-0410-6. [DOI] [PubMed] [Google Scholar]
- 151.Kao I-T, Yao C-L, Kong Z-L, Wu M-L, Chuang T-L, Hwang S-M. Generation of natural killer cells from serum-free, expanded human umbilical cord blood CD34+ cells. St Cells Dev. 2007;16:1043–1052. doi: 10.1089/scd.2007.0033. [DOI] [PubMed] [Google Scholar]
- 152.Chen Y-R, Yan X, Yuan F-Z, et al. The use of peripheral blood-derived stem cells for cartilage repair and regeneration in vivo: a review. Front Pharmacol. 2020 doi: 10.3389/fphar.2020.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blanchet M-R, Bennett JL, Gold MJ, Levantini E, Tenen DG, Girard M, Cormier Y, McNagny KM. CD34 is required for dendritic cell trafficking and pathology in murine hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2011;184:687–698. doi: 10.1164/rccm.201011-1764OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Knorr DA, Ni Z, Hermanson D, Hexum MK, Bendzick L, Cooper LJ, Lee DA, Kaufman DS. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. St Cells Transl Med. 2013;2:274–283. doi: 10.5966/sctm.2012-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tabatabaei-Zavareh N, Vlasova A, Greenwood CP, Takei F. Characterization of developmental pathway of natural killer cells from embryonic stem cells in vitro. PLoS ONE. 2007;2:e232. doi: 10.1371/journal.pone.0000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+ 7+ NK progenitor. Blood. 1994;83(9):2594–2601. [PubMed] [Google Scholar]
- 157.Woll PS, Martin CH, Miller JS, Kaufman DS. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol. 2005;175:5095–5103. doi: 10.4049/jimmunol.175.8.5095. [DOI] [PubMed] [Google Scholar]
- 158.Ni Z, Knorr DA, Clouser CL, Hexum MK, Southern P, Mansky LM, Park I-H, Kaufman DS. Human pluripotent stem cells produce natural killer cells that mediate anti-HIV-1 activity by utilizing diverse cellular mechanisms. J Virol. 2011;85:43–50. doi: 10.1128/JVI.01774-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bonanno G, Mariotti A, Procoli A, Corallo M, Scambia G, Pierelli L, Rutella S. Interleukin-21 induces the differentiation of human umbilical cord blood CD34-lineage-cells into pseudomature lytic NK cells. BMC Immunol. 2009;10:1–15. doi: 10.1186/1471-2172-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McCullar V, Oostendorp R, Panoskaltsis-Mortari A, Yun G, Lutz CT, Wagner JE, Miller JS. Mouse fetal and embryonic liver cells differentiate human umbilical cord blood progenitors into CD56-negative natural killer cell precursors in the absence of interleukin-15. Exp Hematol. 2008;36:598–608. doi: 10.1016/j.exphem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Frias AM, Porada CD, Crapnell KB, Cabral JM, Zanjani ED, Almeida-Porada G. Generation of functional natural killer and dendritic cells in a human stromal-based serum-free culture system designed for cord blood expansion. Exp Hematol. 2008;36:61–68. doi: 10.1016/j.exphem.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Luevano M, Madrigal A, Saudemont A. Generation of natural killer cells from hematopoietic stem cells in vitro for immunotherapy. Cell Mol Immunol. 2012;9:310–320. doi: 10.1038/cmi.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Grzywacz B, Kataria N, Sikora M, Oostendorp RA, Dzierzak EA, Blazar BR, Miller JS, Verneris MR. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood. 2006;108:3824–3833. doi: 10.1182/blood-2006-04-020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Perez SA, Mahaira LG, Sotiropoulou PA, et al. Effect of IL-21 on NK cells derived from different umbilical cord blood populations. Int Immunol. 2006;18:49–58. doi: 10.1093/intimm/dxh348. [DOI] [PubMed] [Google Scholar]
- 165.Ning H, Lei H-E, Xu Y-D, Guan R-L, Venstrom JM, Lin G, Lue TF, Xin Z, Lin C-S. Conversion of adipose-derived stem cells into natural killer-like cells with anti-tumor activities in nude mice. PLoS ONE. 2014;9:e106246. doi: 10.1371/journal.pone.0106246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, De Witte T, Schaap N, Dolstra H. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS ONE. 2011;6:e20740. doi: 10.1371/journal.pone.0020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Davis ZB, Felices M, Verneris MR, Miller JS. Natural killer cell adoptive transfer therapy: exploiting the first line of defense against cancer. Cancer J Sudbury Mass. 2015;21:486. doi: 10.1097/PPO.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 169.Lehmann D, Spanholtz J, Osl M, Tordoir M, Lipnik K, Bilban M, Schlechta B, Dolstra H, Hofer E. Ex vivo generated natural killer cells acquire typical natural killer receptors and display a cytotoxic gene expression profile similar to peripheral blood natural killer cells. St Cells Dev. 2012;21:2926–2938. doi: 10.1089/scd.2011.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sadelain M, Rivière I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang W, Jiang J, Wu C. CAR-NK for tumor immunotherapy: clinical transformation and future prospects. Cancer Lett. 2020;472:175–180. doi: 10.1016/j.canlet.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 172.Siegler EL, Zhu Y, Wang P, Yang L. Off-the-shelf CAR-NK cells for cancer immunotherapy. Cell St Cell. 2018;23:160–161. doi: 10.1016/j.stem.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 173.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell St Cell. 2018;23:181–192. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Veluchamy JP, Kok N, van der Vliet HJ, Verheul HM, de Gruijl TD, Spanholtz J. The rise of allogeneic natural killer cells as a platform for cancer immunotherapy: recent innovations and future developments. Front Immunol. 2017;8:631. doi: 10.3389/fimmu.2017.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Torikai H, Mi T, Gragert L, et al. Genetic editing of HLA expression in hematopoietic stem cells to broaden their human application. Sci Rep. 2016;6:1–11. doi: 10.1038/srep21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Carlsten M, Childs RW. Genetic manipulation of NK cells for cancer immunotherapy: techniques and clinical implications. Front Immunol. 2015;6:266. doi: 10.3389/fimmu.2015.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kooreman NG, Kim Y, de Almeida PE, et al. Autologous iPSC-based vaccines elicit anti-tumor responses in vivo. Cell St Cell. 2018;22:501–513. doi: 10.1016/j.stem.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mrózek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87(7):2632–2640. [PubMed] [Google Scholar]