Abstract
For many decades, selenium (Se) has been known as a potential anti-cancer agent that can also improve the function of immune cells in a variety of solid tumors. However, there is no report on the role of Se on CD4+ T cell subsets like CD4+CD25+FOXP3+ regulatory T cells (Tregs) in lymphoma patients. In this randomized clinical trial, we investigated the effect of 3-month Se consumption on the frequency of CD4+CD25+FOXP3+ Tregs and the expression of immune checkpoint receptors in thirty-two non-Hodgkin lymphoma (NHL) patients (16 patients with Se (Se+) and 16 without Se (Se−) consumption) with diffuse large B-cell lymphoma (DLBCL) subtype at stable remission. The change in the frequency of Tregs and expression of immune checkpoint receptors including CTLA-4, LAG-3, TIM-3, and PD-L1 genes were evaluated after 3 months in both groups using flow cytometry and SYBR Green Real-time PCR method, respectively. The results showed that the frequency of CD4+CD25+FOXP3+ Tregs and expression of immune checkpoint receptors did not significantly change after 3-month Se consumption in DLBCL patients. However, alteration in the frequency of CD4+CD25−FOXP3+ Treg subsets was positively correlated with change in CTLA-4, LAG-3, and TIM-3 expression in the Se+ group. Three-month Se supplementation did not prevent relapse in Se+ group. Taken together, Se supplementation alone did not affect the frequency of CD4+CD25+FOXP3+ Tregs, expression of checkpoint receptors, and prevention of relapse in DLBCL patients at stable remission phase but might influence the functional properties of other Treg subsets like CD4+CD25−FOXP3+ Tregs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02889-5.
Keywords: Selenium (Se), Diffuse large B-cell lymphoma (DLBCL), Regulatory T cells (Tregs), Immune checkpoint receptors
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), affecting B lymphocytes. The immune system especially T cells, has an indispensable role in the development and progression of the disease [1, 2]. CD4+CD25+FOXP3+ regulatory T cells (Tregs) are a specialized subgroup of CD4 + T cells that are responsible for controlling the immune response especially in human malignancies [3]. The high number of CD4+CD25+FOXP3+ Tregs has been proven to be associated with poor prognosis in various solid tumors [4–6]. However, recent studies are showing that the increased number of peripheral blood Tregs might be associated with a favorable outcome of lymphoma patients like DLBCL [7–9]. One of the key regulatory molecules that modulate the effector function of Tregs, as well as conventional T cells, are immune checkpoint receptors; a group of cell surface receptors affecting the magnitude of the immune responses [10]. The most common immune checkpoint receptors are cytotoxic T-lymphocyte-associated protein 4 (CTLA-4; CD152), lymphocyte activation gene-3 (LAG-3; CD223), T cell immunoglobulin and mucin domain-containing protein-3 (TIM-3), programmed cell death protein 1 (PD-1), and PD-1 ligand 1/2 (PD-L1/2) [10]. Aberrant expression of these receptors in most of the human malignancies has led to the introduction of a new group of anti-cancer drugs, also known as “checkpoint inhibitors” to combat the immunosuppressive microenvironment created by tumor cells itself [11, 12].
Selenium (Se) is an essential trace element implicated in many biological processes including regulation of the redox reactions, thyroid metabolism, detoxification, and antioxidant system [13, 14]. These functions are mediated through its incorporation into selenoproteins in the form of selenocysteine (SeCys) and selenomethionine (SeMet) which at least, 25 of them have been identified in humans [14, 15]. Increasing evidences are showing that Se can be used as a potential anti-cancer agent in a variety of solid tumors such as prostate, lung, or colorectal cancers [13, 15–17]. The anti-cancer properties of selenium have been proven to be mediated by the production of two metabolites; hydrogen selenide (HSe−) and methyl selenol (CH3Se−) [15, 17]. These metabolites have a key role in the generation of reactive oxygen species (ROS) which are detrimental for cancer cells [15, 17]. Also, they can directly make DNA damage and induce cell apoptosis in cancer cells [15, 17].
Recent studies have demonstrated that high dose sodium selenite consumption has a synergistic effect with chemotherapy to improve the clinical outcome of intermediate and high-grade NHL patients [18–20]. On the other hand, the beneficial role of Se on the immune system has gained substantial interests especially for boosting the anti-cancer cellular immune responses in human malignancies [21, 22]. Although the underlying mechanism is not clearly defined, the positive impact of the Se on cellular immunity has been attributed to the fact that Se can stimulate Ca+2 mobilization in T cells leading to increasing TCR signaling associated with enhanced T cell proliferation and effector functions [21–23]. Moreover, it has been shown that Se supplementation can skew the balance of Th1/Th2 cells, depending on its dose of administration [23]. Despite this, there is no evidence of the role of Se on other CD4+ T-helper cell subsets like CD4+CD25+FOXP3+ Tregs in lymphoma patients, especially NHL ones.
Therefore, in this study, we investigated whether high dose Se consumption alone can affect the frequency of peripheral blood CD4+CD25+FOXP3+ Tregs, the expression of immune checkpoint receptors, and also prevent relapse of NHL patients with DLBCL subtype at stable remission.
Patients and methods
Trial design and patients’ criteria
The present study is a randomized clinical trial performed in thirty-two non-Hodgkin’s lymphoma (NHL) patients with Diffuse Large B-cell (DLBCL) subgroup who referred to central Hematology and Oncology Clinic from July 2017 to July 2018. All patients were at a stable remission phase who had undergone chemotherapy previously and were followed for two consecutive years. The remission phase was defined as patients without evidence of recurrence by taking the history of the disease and physical examination and also normal tests including complete blood count, blood urea and creatinine, liver function test, serum hemoglobin (Hb), erythrocyte sedimentation rate (ESR), and lactate dehydrogenase (LDH) level. Exclusion criteria were as follows: Patients who were unable to continue selenium for any reason, patients with a history of comorbid disease including renal, heart, pulmonary, and/or liver failure as well as HIV, HBV, HCV positive patients and those who were treated with corticosteroids for some reasons.
The study protocol was approved by the Shiraz University of Medical Sciences (SUMS) Review Board as well as the Local Ethics Committee and also was registered at the Iranian Clinical Trial registry website (IRCT). After explaining the objectives of the study, all participants signed their informed written consent.
Intervention
All patients were in the remission phase after chemotherapy and were randomly divided into two groups of 16 individuals based on the patient registration number (according to the order of referral to the clinic) using a computer-based random cultivar generator. Participants in the case group (Se+) were given selenium while the control group (Se−) was given no selenium. Selenium tablets were administered orally at a dose of 200 µg/day (Bronson Companies, LLC, USA) for 3 consecutive months.
Selenium toxicity
The recommended amount of selenium in the dietary of adults is 55 µg per day, and selenium poisoning has been observed with high doses of selenium reported in dietary supplements with the common symptoms of poisoning including nausea, diarrhea, fatigue, hair loss, joint pain, discolored nails, or brittleness [24]. In this study, all patients were followed for possible toxicity associated with Se consumption, and no signs of selenium poisoning were observed in these patients at the end of the study indicating that such dose is well-tolerated by patients.
Mononuclear cell isolation
Five-milliliters of peripheral blood was collected from all patients in Ethylenediaminetetraacetic acid (EDTA)-treated tubes at the beginning of the study and after 3 months. The peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-hypaque (Inno-Train, Germany) density gradient centrifugation at 400 × g for 20 min. Cells were washed twice with phosphate-buffered saline (PBS) buffer prior to flow cytometry staining and gene expression analysis.
Flow cytometry analysis of CD4+CD25+FOXP3+ Tregs
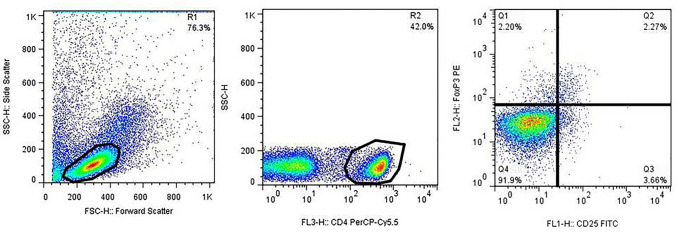
The washed cells were adjusted to 5 × 105 cells per 100 µl staining buffer containing 5% fetal bovine serum (FBS) in PBS 1X. For Tregs enumeration, the following procedure was used. Cells were first stained for surface markers using PerCP-Cy™5.5 mouse anti-human CD4 (Clone SK3) (BD Biosciences, USA) and FITC mouse anti-human CD25 (Clone M-A251) (BD Biosciences, USA) antibodies by incubation with these antibodies at room temperature for 20 min in the dark followed by washing step with staining buffer at 250 × g centrifugation speed for 10 min. Then, cells were fixed and permeabilized according to the manufacturer’s instruction using human FOXP3 buffer set (BD Biosciences, USA) followed by washing with staining buffer at 250 × g centrifugation speed for 10 min. For intracellular staining of FOXP3, cells were stained with PE mouse anti-human FOXP3 antibody (Clone 259D/C7) (BD Biosciences, USA) and incubated at room temperature for 20 min in the dark. After washing step, cell pellets were resuspended in 200–300 µl staining buffer and analyzed by FACSCalibure flow cytometer (BD Biosciences, USA) and FlowJo software. The lymphocyte population was first defined based on the Forward vs. Side scatter plot. The percentage of Tregs was calculated by the selection of CD4+ T cells within the lymphocyte population followed by the gating of the CD25+FOXP3+ cells among the CD4+ cells (Fig. 1). Finally, the frequency of Tregs was compared before and after 3 months in each patient group.
Fig. 1.
Flow cytometry analysis of Tregs. For Treg quantification, the lymphocytes (R1) were defined based on the forward vs. side scatter data acquisition plotting. Then, the CD4+ cells (R2) were first selected in the lymphocyte population (R1) followed by the gating of CD25+FOXP3+ cells (Q2 quadrant) within CD4+ lymphocytes
RNA isolation and cDNA synthesis
Total RNA was extracted from PBMCs using Trizol solution (Invitrogen, USA) according to the manufacturer’s instructions. The quality of extracted RNA was measured by calculating the absorption ratio of 260/280 by Nanodrop (ThermoFisher, USA). Then, cDNA was synthesized using the Primescript™ RT reagent kit (Takara, Japan) according to its protocol in the T100 thermocycler (Bio-Rad Laboratories, USA).
SYBR green real-time PCR
Real-time PCR amplification was performed using SYBR® Premix Ex Taq™ II (Tli RNase H Plus) (Takara, Japan) and designed primers specific for each mRNA in an iQ5 thermocycler (BioRad Laboratories, USA). The specific forward and reverse primers for CTLA-4, LAG-3, TIM-3, and PD-L1 genes were designed using AlleleID version 6, Oligo software version 7, and Molecular Beacon software and rechecked with BLAST program (Table 1). The expression of the CTLA-4, PD-L1, LAG-3, and TIM-3 genes was normalized to the GAPDH as the housekeeping gene. Finally, the relative expression of targeted genes was calculated using 2−ΔΔCT method.
Table 1.
The primer sequence and PCR products for immune checkpoint receptors
| Gene | Primer sequences (5′ → 3′) | PCR product length (bp) |
|---|---|---|
| CTLA-4 forward primer | 5′-TTCTTCTCTTCATCCCTGTCTTCT-3′ | 130 |
| CTLA-4 reverse primer | 5′-CGGACCTCAGTGGCTTTG-3′ | |
| PD-L1 forward primer | 5′-AGGGCATTCCAGAAAGAT-3′ | 118 |
| PD-L1 reverse primer | 5′-GCTACCATACTCTACCACATA-3′ | |
| LAG-3 forward primer | 5′-CTTCTTGGAGCAGCAGTG-3′ | 133 |
| LAG-3 reverse primer | 5′-AAAGGAGCAGAGAAAGGAC-3′ | |
| TIM-3 forward primer | 5′-GTCATCAAACCAGCCAAGG-3′ | 113 |
| TIM-3 reverse primer | 5′-AGTGTCTGTGTCTCTGCT-3′ |
Statistical analysis
All statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) version 22.0. Quantitative variables were described as mean ± standard deviation (SD) and median. The quantitative data were compared before and after treatment by the non-parametric Wilcoxon test. To evaluate the correlation between quantitative variables, Spearman correlation test was performed. P value < 0.05 was considered as statistically significant.
Results
This study included thirty-two DLBCL patients at remission phase post-chemotherapy treatment that were categorized into two groups; the Se+ group (those who consumed Se) and the Se− group (those who did not consume Se) for 3 months, post-chemotherapy treatment. The mean age of patients was 46.25 ± 16.13 years and 52.64 ± 16.15 years in Se+ and Se– groups, respectively. Also, the male/female ratio was 5/11 and 8/8 in Se+ and Se– groups, respectively. The clinical and laboratory characteristics of patients at the beginning of the study are presented in Table 2. Patients were followed for recurrence of the disease for 2 years. Among four relapsed patients (three in Se+ and one in Se− groups), one individual died in each group during a 2-year follow-up. Two patients in Se− group failed to continue the trial and so, were excluded from the study. Therefore, we continued our trial with 30 DLBCL patients (16 Se+ and 14 Se−).
Table 2.
The demographic and laboratory characteristics of Se+ and Se− groups
| Variable | Se+ (n = 16) | Sel− (n = 14) |
|---|---|---|
| Age (years) | ||
| Mean ± SD | 46.25 ± 16.13 | 52.64 ± 16.15 |
| Range (years) | 23–83 | 23–82 |
| Sex | ||
| Male (%) | 5 (26.7) | 7 (46.7) |
| Female (%) | 11 (73.3) | 7 (53.3) |
| Laboratory characteristics | Mean ± SD | Mean ± SD |
|---|---|---|
| WBC count (× 103/ml) | 6.16 ± 2.18 | 4.72 ± 1.75 |
| Platelet count (× 103/ml) | 245.46 ± 59.49 | 186.73 ± 59.78 |
| Hb level (g/dl) | 13.6 ± 2.15 | 12.61 ± 2.53 |
| ESR (mm/h) | 12 ± 6.52 | 12.5 ± 8.82 |
| LDH (U/l) | 244.12 ± 61.86 | 362 ± 94.77 |
WBC white blood cells, Hb hemoglobin, ESR erythrocyte sedimentation rate, LDH lactate dehydrogenase
Change in the frequency of CD4+CD25+FOXP3+ Tregs
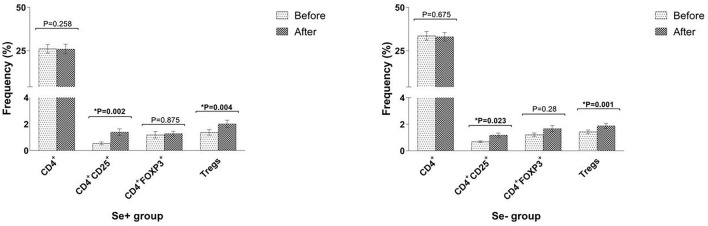
The frequency of CD4+, CD4+CD25+, CD4+FOXP3+ lymphocytes, and CD4+CD25+FOXP3+ Tregs was evaluated in both Se+ and Se− groups at the beginning of the study and 3 months later (Fig. 2). The mean fluorescent intensity (MFI) of FOXP3 was measured and considered as the mean expression level. The results showed that in Se+ group, the median of CD4+CD25+ lymphocytes (activated T cells) as well as Tregs was increased following 3-months Se consumption (median 0.39% vs. 1.14%; *P = 0.002 and 1.14% vs. 1.6%; *P = 0.004, respectively). Like Se+ group, the median of CD4+CD25+ lymphocytes, as well as Tregs, was also elevated after 3 months in Se− group (median 0.71% vs. 1.04%; *P = 0.023 and 1.24% vs. 1.6%; *P = 0.001, respectively), indicating that the increase in CD4+CD25+ cells (activated T cells) and Tregs’ frequency in Se+ group cannot be attributed to the Se consumption. Besides, there was no significant difference in CD4+, CD4+FOXP3+ lymphocytes as well as FOXP3 MFI in both Se+ and Se− groups after 3 months (P > 0.05). The change in all T cell subsets and FOXP3 MFI was calculated by subtracting their initial value from their corresponding value at the end of the study (3 months later). Accordingly, no significant difference was observed in CD4+ , CD4+CD25+, CD4+FOXP3+, CD4+CD25+FOXP3+ Tregs as well as FOXP3 MFI change between Se+ and Se− groups (P > 0.05).
Fig. 2.
Change in the frequency of Tregs and related subsets after 3 months in Se+ and Se− groups. Change in the frequency of Tregs and related subsets was calculated after 3 months in both Se+ and Se− groups. Data are presented as mean ± SD and analyzed by Graphpad Prism version 8. P value < 0.05 was considered as statistically significant. Se selenium, Tregs regulatory T cells
A positive correlation was observed between change in the CD4+FOXP3+ lymphocytes and FOXP3 MFI difference (rs = 0.802, *P = 0.001) after 3-month Se administration in the Se+ group. However, this difference was not significant in Se− control group (P > 0.05).
Expression of the immune checkpoint receptors
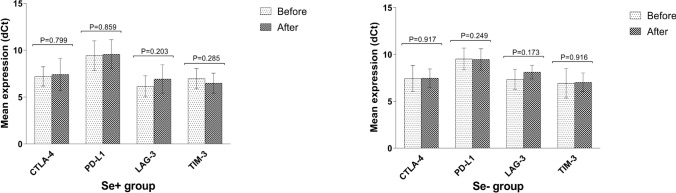
The mean expression level of immune checkpoint receptors including CTLA-4, PD-L1, LAG-3, and TIM-3 was evaluated at the beginning of the study and after 3 months in both Se+ and Se− groups. Accordingly, it was observed that the expression of CTLA-4, PD-L1, LAG-3, and TIM-3 immune checkpoint receptors was not significantly changed in both Se+ and Se− groups after 3 months (P > 0.05).
The change in the expression level of immune checkpoint receptors was evaluated by subtracting their initial values from their corresponding values after 3 months. Accordingly, there was a positive correlation between change in the TIM-3 with CTLA-4 (rs = 0.976, *P < 0.001), PD-L1 (rs = 0.867, *P = 0.001) and LAG-3 (rs = 0.713, *P = 0.009) and also, PD-L1 with CTLA-4 (rs = 0.867, *P = 0.002) and TIM-3 (rs = 0.867, *P = 0.001) genes after 3 months in Se+ group (Fig. 3).
Fig. 3.
Change in the expression of immune checkpoint receptors after 3 months in Se+ and Se− groups. Change in the mean expression level of immune checkpoint receptors (dCt) was defined after 3 months in both Se+ and Se− groups. Data are presented as mean ± SD and analyzed by Graphpad Prism version 8. P value < 0.05 was considered as statistically significant. Se selenium
However, in Se− group, change in TIM-3 expression was positively correlated with CTLA-4 (rs = 0.829, *P = 0.042) and PD-L1 (rs = 0.893, *P = 0.007) variation. Also, there was a positive correlation between change in PD-L1 with CTLA-4 (rs = 0.886, *P = 0.019) after 3 months in Se− group (Fig. 3).
Alteration in FOXP3 MFI was also positively associated with change in CTLA-4 (rs = 0.721, *P = 0.019), PD-L1 (rs = 0.75, *P = 0.02) and TIM-3 (rs = 0.721, *P = 0.019) mean gene expression in Se+ group. However, in Se− group, a positive correlation was observed between change in FOXP3 MFI and CTLA-4 (rs = 0.829, *P = 0.042) mean gene expression.
Correlation between change in CD4+CD25+FOXP3+ Tregs frequency and immune checkpoint expression
The change in the frequency of CD4+CD25+FOXP3+ Tregs and also immune checkpoint receptor expression was calculated after 3 months in both groups. Then, the correlation between the difference in Tregs frequency and immune checkpoint receptors was evaluated.
The results revealed that alteration in the level of CD4+CD25+FOXP3+ Tregs was not correlated with the difference in mean expression of immune checkpoint receptors after 3 months in both Se+ and Se− groups. However, the change in the frequency of CD4+FOXP3+ cells was positively correlated with CTLA-4 (rs = 0.745, *P = 0.013), LAG-3 (rs = 0.721, *P = 0.019) and TIM-3 (rs = 0.745, *P = 0.013) difference in Se+ group. Although the difference was not significant in Se− group (P > 0.05).
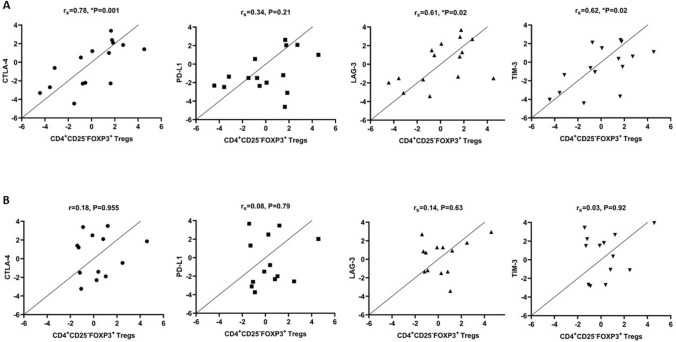
Since the CD4+FOXP3+ lymphocytes are enriched with both CD25+ (CD4+CD25+FOXP3+) and CD25− (CD4+CD25−FOXP3+) Tregs, we next analyzed the frequency of the later population in our patients. The results showed that there was no significant difference between baseline and 3-month CD4+CD25−FOXP3+ Tregs in both Se+ and Se− groups (P > 0.05). Accordingly, a positive correlation was observed between change in CD4+CD25−FOXP3+ Tregs and variation in CD4+FOXP3+ lymphocytes (rs = 0.851, *P < 0.001), FOXP3 MFI (rs = 0.622, *P = 0.018) as well as change in the CTLA-4 (rs = 0.78, *P = 0.001), LAG-3 (rs = 0.61, *P = 0.02), and TIM-3 (rs = 0.62, *P = 0.02) immune checkpoint receptor expression after 3 months in Se+ group (Fig. 4).
Fig. 4.
The relationship between change in the frequency of CD4+CD25−FOXP3+ Tregs and expression of immune checkpoint receptors in Se+ (a) and Se− (b) groups. Data are presented as mean ± SD and analyzed by Graphpad Prism version 8. P value < 0.05 was considered as statistically significant. Se selenium, rs Spearman correlation coefficient
Discussion
Dietary Se has long been considered as a potent antioxidant supplement due to the its fundamental role in regulation of antioxidant systems like glutathione (GSH) peroxidase and thioredoxin/glutaredoxin reductase (Trx/Grx) [14]. In this regard, Se can neutralize the augmented reactive oxygen species (ROS) products and thus, mitigate its associated cellular damage. On the other hand, Se and Se-containing compounds have gained substantial interests as favorable anti-cancer agents for many decades [15, 17]. Generally, the anti-carcinogenic effect of Se is attributed to its selective cytotoxicity against cancer cells by controlling the redox system, which promotes cancer cell apoptosis [15, 17].
In addition to alleviating the cytotoxicity of chemotherapy on normal cells, the immuno-stimulatory property of Se has been considered as a promising avenue to boost the anti-tumor immune response in human malignancies [21, 23]. In this regard, it has been demonstrated that Se deficiency arises from inadequate Se uptake can give rise to the dampening of the immune system, possibly increasing the susceptibility to cancers [21, 23].
Also, recent studies have shown that the low serum Se level at diagnosis has been associated with poor outcome of patients with hematological malignancies like NHL [25, 26]. The immune-stimulatory properties of Se are related to the fact that Se supplementation has been shown to promote T cell proliferation, natural killer (NK) cells activity, and also antibody (Ab) production [21, 23]. Additionally, there are some evidences showing that Se can upregulate CD4+CD25+ Tregs in mice models of autoimmune thyroiditis and chronic colitis [27, 28]. However, little is known about the role of Se on human CD4+ T cell subsets like CD4+CD25+FOXP3+ Tregs number and expression of immune checkpoint receptors in cancers especially lymphoid malignancies.
In this study, the effect of Se consumption on the frequency of CD4+CD25+FOXP3+ Tregs and immune checkpoint receptor expression was evaluated in DLBCL patients at stable remission phase who had taken Se for 3 months post-chemotherapy treatment. The results showed that 3-month Se consumption did not affect the frequency of CD4+CD25+FOXP3+ Tregs and expression of immune checkpoint receptors in these patients.
The beneficial effect of Se supplementation in lymphoid cancers especially NHL has been investigated in various studies. In a study by Asfour et al. in 40 newly diagnosed NHL patients (20 with Se and 20 without Se consumption), it was demonstrated that high-dose orally administrated sodium selenite (0.2 mg/kg/day) for 7 days was so effective to induce apoptosis of lymphoma cells when was used in combination with chemotherapy [19]. In a separate study by this group in fifty newly diagnosed NHL patients (25 with Se and 25 without Se consumption), it was shown that those patients who received standard chemotherapy along with adjuvant sodium selenite 0.2 mg/kg/day for 30 days, had significantly decreased Bcl-2 level in bone marrow (BM) samples, increased CD4/CD8 ratio and also improved clinical outcomes like higher complete remission (CR) and lower relapse rate as well as longer overall survival [20]. This group also showed that newly diagnosed NHL patients who received chemotherapy along with 5-day sodium selenite at dose 0.2 mg/kg/day had a significant reduction in neutrophils apoptosis and consequently, a significant decrease in infection rate following chemotherapy [18]. Supporting these studies, when is used in conjunction with chemotherapy and radiotherapy, Se compounds can substantially ameliorate the toxicity of chemotherapy and radiotherapy on normal cells while preserving cytotoxicity on malignant cells, thereby, the efficacy of chemotherapy and radiotherapy will be improved [29].
The lack of association between Se supplementation and change in CD4+CD25+FOXP3+ Treg frequency and checkpoint receptors in our study might be due to the limited number of patients, duration of Se consumption as well as the dose of Se intake. One of the important issues about our study compared to the above-mentioned investigations was that all of our patients were at a stable remission phase and received Se alone post-chemotherapy treatment (due to the ethical issues). Therefore, considering the relapse of patients in both Se+ and Se− groups, it seems that Se supplementation alone is not sufficient to prevent relapse that occurred in the absence of chemotherapy in DLBCL patients at remission phase.
Besides, the type of Se compound that patients received might be so important. Although there is not enough data about the differential effect of various types of Se compounds on the immune system, it can be helpful to specify which type of Se supplements is more effective to amplify the immune response in cancer patients. This suggestion needs to be tested by more in vitro and in vivo studies.
One of the limitations of our study was that we had no data about the baseline level of Se in our DLBCL patients which might influence the efficiency of Se supplementation on the immune system. Accordingly, the assessment of initial serum Se level in these patients as well as its change during the study should be noticed.
Until now, there is no report on the role of Se supplementation on checkpoint receptors expression in cancer patients. However, in a study by Nair et al., it was revealed that methylseleninic acid (MSA) can sensitize ovarian cancer cell line A2780 to T cell-mediated killing by decreasing PDL1 surface expression on cancer cells [30].
The other finding of our study was the positive correlation between change in FOXP3 MFI with PDL-1 and TIM-3 expression change in Se+ group suggesting that altered FOXP3 expression in Se+ group following Se consumption may affect the functional properties of CD4+CD25+FOXP3+ Tregs by changing PD-L1 and TIM-3 marker expression.
We also observed a positive correlation between alteration in the frequency of CD4+CD25−FOXP3+ Tregs and CTLA-4, LAG-3, and TIM-3 change in the Se+ group. According to these results, since these immune checkpoint receptors are known to be essential for the regulation of Tregs (as well as effector T cells) function, it can be assumed that the function of these cells might be affected by Se consumption.
Previously, we showed that high peripheral blood CD4+CD25+FOXP3+ Tregs might be associated with favorable prognosis of lymphoma patients including lower rate of relapse and better response to chemotherapy [7]. Given the significance of Tregs in the clinical outcome of lymphoma patients, it is tempting that such patients may benefit from positive effect of Se on Treg function. This hypothesis needs to be evaluated in larger DLBCL population. Consequently, in addition to Treg frequency, functional analysis of Tregs during Se supplementation can be highly informative to elucidate the different aspects of the Se effect on Treg lymphocytes. Also, further characterization of Tregs, as well as evaluation of other Treg subsets like Tr1 and Th3 cells, will improve our knowledge about the effect of Se therapy on various Treg subpopulations.
The change in the intratumoral FOXP3+ Tregs in the lymph node (LN) specimen of patients following Se supplementation is another important issue that should be noticed. Since our study population was patients who were at stable remission phase and thereby had no tumor burden, taking tissue specimen from these patients was not reasonable. Despite this, we performed immunohistochemical staining just for detection of FOXP3+ Tregs in the LN tissue of three patients who relapsed after two-year follow-up. Accordingly, different patterns of FOXP3+ cells were observed (Supplementary Fig. 1) showing that FOXP3+ Tregs can be detectable in the tumor microenviroment of the relapsed patients.
Considering this, assessment of the change in the intratumoral FOXP3+ Tregs in newly diagnosed untreated DLBCL patients (which have available LN tissue) who relapsed following Se supplementation and its correlation with the peripheral blood Tregs might be more impressive and is highly recommended in the future study.
Conclusion
Taken together, to the best of our knowledge, it was shown for the first time that the 3-month Se consumption alone, did not affect the frequency of CD4+CD25+FOXP3+ Tregs, expression of immune checkpoint receptors, and prevention of relapse occurrence in DLBCL patients at stable remission phase. However, it might affect the functional properties of CD4+CD25−FOXP3+ Tregs which needs to be evaluated by more studies.
Study of a larger population, evaluation of other Treg subsets like Tr1 and Th3 cells as well as the assessment of the Se effect on Treg frequency and function in both peripheral blood and also in the tumor microenvironment when is used in combination with chemotherapy in newly diagnosed patients might be more impressive and is highly recommended.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1 Immunohistochemical staining of the FOXP3+ Tregs in the lymph node (LN) specimen. Hematoxylin and eosin (H&E)-stained formalin-fixed and paraffin-embedded (FFPE) 3µm tissue blocks of normal tonsile and LN of DLBCL patients at relapsed phase were stained with anti-FOXP3 antibody (236A/E7) (ab20034, 1:100, Abcam); Sample with no recognizable staining was known as negative (−); slight staining was known as weakly positive (+); moderate staining was known as moderately positive (++), and high staining was known as strongly positive (+++). (A) normal tonsile, (B) negative, (C) weakly and (D) moderately positive staining of the FOXP3+ Tregs in three relapsed DLBCL patients. The pictures were captured in 200X and 400X magnification by OLYMPUS microscope. The arrows show the presence of FOXP3+ Tregs (JPEG 2959 kb)
Acknowledgements
This study was funded by a grant provided by Shiraz University of Medical Sciences (Grant Number 93-01-01-8106), approved by the Clinical Trial Registry of Shiraz University of Medical Sciences (RCT Number: IR.SUMS.REC.1396.S210) and also registered in the Iranian Registry of Clinical Trials (IRCT ID: IRCT20200128046292N1).
Author’s contribution
DM contributed to study design, analysis and interpretation of data. SN and RM contributed to performing the research. KM and GH contributed to interpretation of data and critically revision of the manuscript. AN contributed to study design, analysis and interpretation of data, writing paper and performing the research.
Funding
This study was financially supported by a grant provided by Shiraz University of Medical Sciences (Grant Number 93-01-01-8106).
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest.
Ethical approval
This study was performed according to the ethical standards of the Ethical Committee of Shiraz University of Medical Sciences and in compliance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shankland KR, Armitage JO, Hancock BW. Non-hodgkin lymphoma. Lancet. 2012;380(9844):848–857. doi: 10.1016/S0140-6736(12)60605-9. [DOI] [PubMed] [Google Scholar]
- 2.Ansell SM. Harnessing the power of the immune system in non-Hodgkin lymphoma: immunomodulators, checkpoint inhibitors, and beyond. Hematol 2014 Am Soc Hematol Educ Prog Book. 2017;2017(1):618–21. doi: 10.1182/asheducation-2017.1.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Arena G, Vitale C, Coscia M, Festa A, Di Minno NMD, De Feo V, et al. Regulatory T Cells and their prognostic relevance in hematologic malignancies. J Immunol Res. 2017;2017:1832968. doi: 10.1155/2017/1832968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 5.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13(3):902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 7.Dehghani M, Kalani M, Golmoghaddam H, Ramzi M, Arandi N. Aberrant peripheral blood CD4 (+) CD25 (+) FOXP3 (+) regulatory T cells/T helper-17 number is associated with the outcome of patients with lymphoma. Cancer Immunol Immunother. 2020;69(9):1917–1928. doi: 10.1007/s00262-020-02591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Głowala-Kosińska M, Chwieduk A, Nieckula J, Saduś-Wojciechowska M, Grosicki S, Rusin A, et al. Association of circulating regulatory T cell number with the incidence and prognosis of diffuse large B-cell lymphoma. Eur J Haematol. 2013;91(2):122–128. doi: 10.1111/ejh.12144. [DOI] [PubMed] [Google Scholar]
- 9.Cha Z, Gu H, Zang Y, Wang Z, Li J, Huang W, et al. The prevalence and function of CD4(+)CXCR5(+)Foxp3(+) follicular regulatory T cells in diffuse large B cell lymphoma. Int Immunopharmacol. 2018;61:132–139. doi: 10.1016/j.intimp.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19(18):4917–4924. doi: 10.1158/1078-0432.CCR-12-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 12.La-Beck NM, Jean GW, Huynh C, Alzghari SK, Lowe DB. Immune checkpoint inhibitors: new insights and current place in cancer therapy. Pharmacother J Hum Pharmacol Drug Ther. 2015;35(10):963–76. doi: 10.1002/phar.1643. [DOI] [PubMed] [Google Scholar]
- 13.Evans SO, Khairuddin PF, Jameson MB. Optimising selenium for modulation of cancer treatments. Anticancer Res. 2017;37(12):6497–6509. doi: 10.21873/anticanres.12106. [DOI] [PubMed] [Google Scholar]
- 14.Rayman MP. Selenium and human health. Lancet. 2012;379(9822):1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 15.Wallenberg M, Misra S, Björnstedt M. Selenium cytotoxicity in cancer. Basic Clin Pharmacol Toxicol. 2014;114(5):377–386. doi: 10.1111/bcpt.12207. [DOI] [PubMed] [Google Scholar]
- 16.Davis CD. Selenium supplementation and cancer prevention. Curr Nutr Rep. 2012;1(1):16–23. doi: 10.1007/s13668-011-0003-x. [DOI] [Google Scholar]
- 17.Gandin V, Khalkar P, Braude J, Fernandes AP. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic Biol Med. 2018;127:80–97. doi: 10.1016/j.freeradbiomed.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Asfour IA, El Shazly S, Fayek MH, Hegab HM, Raouf S, Moussa MA. Effect of high-dose sodium selenite therapy on polymorphonuclear leukocyte apoptosis in non-Hodgkin's lymphoma patients. Biol Trace Elem Res. 2006;110(1):19–32. doi: 10.1385/BTER:110:1:19. [DOI] [PubMed] [Google Scholar]
- 19.Asfour IA, El-Tehewi MM, Ahmed MH, Abdel-Sattar MA, Moustafa NN, Hegab HM, et al. High-dose sodium selenite can induce apoptosis of lymphoma cells in adult patients with non-Hodgkin’s lymphoma. Biol Trace Elem Res. 2009;127(3):200. doi: 10.1007/s12011-008-8240-6. [DOI] [PubMed] [Google Scholar]
- 20.Asfour IA, Fayek M, Raouf S, Soliman M, Hegab HM, El-Desoky H, et al. The impact of high-dose sodium selenite therapy on Bcl-2 expression in adult non-Hodgkin’s lymphoma patients: correlation with response and survival. Biol Trace Elem Res. 2007;120(1–3):1–10. doi: 10.1007/s12011-007-0029-5. [DOI] [PubMed] [Google Scholar]
- 21.Avery JC, Hoffmann PR. Selenium, selenoproteins, and immunity. Nutrients. 2018;10(9):1203. doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann PR, Berry MJ. The influence of selenium on immune responses. Mol Nutr Food Res. 2008;52(11):1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16(7):705–743. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacFarquhar JK, Broussard DL, Melstrom P, Hutchinson R, Wolkin A, Martin C, et al. Acute selenium toxicity associated with a dietary supplement. Arch Intern Med. 2010;170(3):256–261. doi: 10.1001/archinternmed.2009.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens J, Waters R, Sieniawska C, Kassam S, Montoto S, Fitzgibbon J, et al. Serum selenium concentration at diagnosis and outcome in patients with haematological malignancies. Br J Haematol. 2011;154(4):448–456. doi: 10.1111/j.1365-2141.2011.08744.x. [DOI] [PubMed] [Google Scholar]
- 26.Last KW, Cornelius V, Delves T, Sieniawska C, Fitzgibbon J, Norton A, et al. Presentation serum selenium predicts for overall survival, dose delivery, and first treatment response in aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2003;21(12):2335–2341. doi: 10.1200/JCO.2003.06.145. [DOI] [PubMed] [Google Scholar]
- 27.Sang L-X, Chang B, Zhu J-F, Yang F-L, Li Y, Jiang X-F, et al. Sodium selenite ameliorates dextran sulfate sodium-induced chronic colitis in mice by decreasing Th1, Th17, and γδT and increasing CD4 (+) CD25 (+) regulatory T-cell responses. World J Gastroenterol. 2017;23(21):3850. doi: 10.3748/wjg.v23.i21.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue H, Wang W, Li Y, Shan Z, Li Y, Teng X, et al. Selenium upregulates CD4+ CD25+ regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD. H-2h4 mice. Endocr J. 2010;57(7):595–601. doi: 10.1507/endocrj.K10E-063. [DOI] [PubMed] [Google Scholar]
- 29.Lobb RJ, Jacobson GM, Cursons RT, Jameson MB. The interaction of selenium with chemotherapy and radiation on normal and malignant human mononuclear blood cells. Int J Mol Sci. 2018;19(10):3167. doi: 10.3390/ijms19103167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair D, Rådestad E, Khalkar P, Diaz-Argelich N, Schröder A, Klynning C, et al. Methylseleninic acid sensitizes ovarian cancer cells to T-cell mediated killing by decreasing PDL1 and VEGF levels. Front Oncol. 2018;8:407. doi: 10.3389/fonc.2018.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Immunohistochemical staining of the FOXP3+ Tregs in the lymph node (LN) specimen. Hematoxylin and eosin (H&E)-stained formalin-fixed and paraffin-embedded (FFPE) 3µm tissue blocks of normal tonsile and LN of DLBCL patients at relapsed phase were stained with anti-FOXP3 antibody (236A/E7) (ab20034, 1:100, Abcam); Sample with no recognizable staining was known as negative (−); slight staining was known as weakly positive (+); moderate staining was known as moderately positive (++), and high staining was known as strongly positive (+++). (A) normal tonsile, (B) negative, (C) weakly and (D) moderately positive staining of the FOXP3+ Tregs in three relapsed DLBCL patients. The pictures were captured in 200X and 400X magnification by OLYMPUS microscope. The arrows show the presence of FOXP3+ Tregs (JPEG 2959 kb)