Abstract
A case report detailing, for the first time, a case of laboratory-confirmed zoster in an astronaut on board the International Space Station is presented. The findings of reduced T-cell function, cytokine imbalance, and increased stress hormones which preceded the event are detailed. Relevance for deep space countermeasures is discussed.
Key words: Immune system dysregulation, zoster, spaceflight, countermeasures
Dysregulation of the human immune system during spaceflight, associated with latent herpesviruses reactivation, is a well-documented phenomenon.1,2 In general, the phenomenon consists of reduced T-cell and natural killer cell function, inflammation, and reactivation of latent herpesviruses. Latent virus reactivation is controlled primarily by T cells, and elevated reactivation or clinical zoster is observed in terrestrial patients associated with diseases of T-cell compromise such as HIV, autoimmunity, stress, or use of immunosuppressive medications.3 Contributing factors that lead to latent virus reactivation during spaceflight include stress, isolation, microgravity, and circadian misalignment. In select crew members, symptomology may occur.4,5 In a previous case report, we detailed persistent atopic dermatitis associated with HSV-1 reactivation that occurred during a space mission.6 In that case, rash flares coincided with mission stressors, and the case was controlled with topical and oral steroids. Evidence suggests a mechanistic link between diminished immune function, particularly reduced T-cell function, and latent herpesvirus reactivation in astronauts.
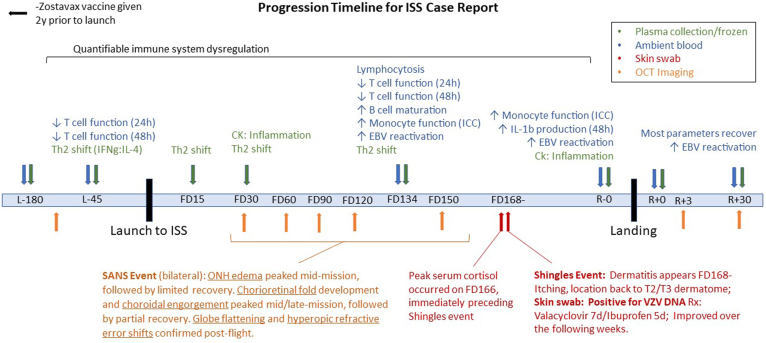
We now report a case of laboratory-confirmed herpes zoster (shingles) in an astronaut during an approximately 6-month spaceflight on board the International Space Station (ISS). The crew member had never previously experienced zoster, autoimmunity, or any other condition associated with immune compromise, nor had the crew member ever been treated with any immunosuppressive medications. During the mission, the crew member participated in spacewalks and other typical mission events that constitute acute stressors, overlaying the chronic stress associated with spaceflight. Fig 1 shows the complete time line of both the immune system dysregulation, biosample analysis, clinical symptomology, and treatments. The array of immune-monitoring assays is summarized by peripheral leukocyte distribution, T-cell function, plasma cytokines, stress hormones, and latent herpesvirus reactivation. Details regarding specific assays and methodology may be found in Crucian et al1 and Mehta et al.2 During flight, ambient blood was collected and returned to Earth twice, at approximately the middle of the mission and immediately before landing. Collections are timed to occur near undocking of some vehicle (eg, Soyuz, SpaceX) that can provide the immediate return of the ambient sample to Earth. Plasma was collected, centrifuged, and stored frozen on board the ISS at 4 time points across the mission’s duration. Relevant clinical information was provided by the crew member and the National Aeronautics and Space Administration (NASA) crew surgeons.
Fig 1.
Timeline of mission duration, biosample collection, medication use, research, and clinical findings in the case study ISS astronaut.
All study-related immune parameters (leukocyte distribution, T-cell function, plasma cytokine concentrations, and latent virus reactivation) were essentially within the laboratory-established “normal” ranges at baseline samplings 180 days before launch. By 45 days before launch, some dysregulation of immune parameters was already evident, including reductions in T-cell function, and a “TH2 cell shift” in plasma IFN-γ and IL-4 concentrations (see Fig E1 in the Online Repository at www.jaci-global.org). The crew member did experience very minimal, yet detectable, EBV reactivation before the mission, at the baseline sampling 180 days before launch. As of the mid-mission ambient blood collections, these dysregulations continued, but an increase in innate cell and monocyte function (detected via intracellular cytokine production) also appeared, as well as a substantial increase in reactivation of EBV (see Table E1 in the Online Repository at www.jaci-global.org). Following this sampling, but before landing, the zoster outbreak occurred. Precisely 2 days before the zoster outbreak, at flight day 166, the highest cortisol levels of all other in-flight measured levels were observed (see Fig E1). This patten of immune system alterations is somewhat typical of those observed in astronauts who experience the subclinical reactivation of latent herpesviruses, and it is also typical (although lesser in magnitude) for those observed in terrestrial patients with zoster.7
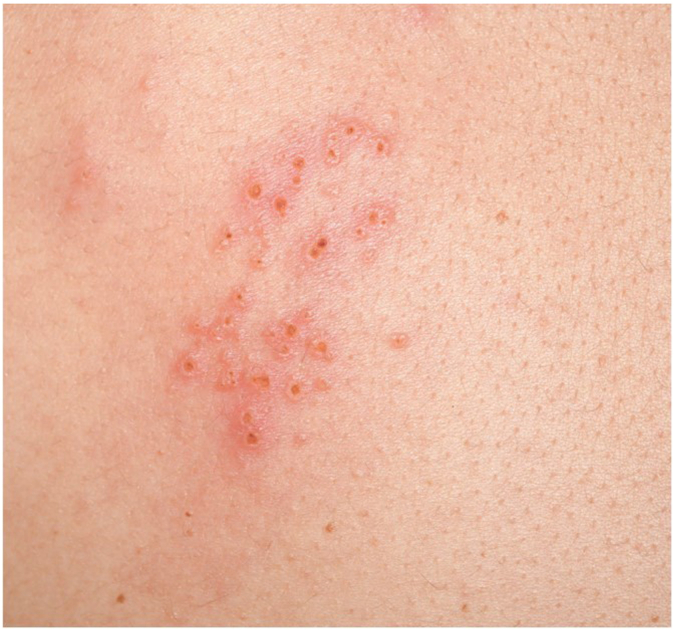
The initial symptoms on flight day 168 included left back discomfort migrating to the front side and an erythematous rash of the back with vesicles under armpit, extending to top of left chest in the T2/T3 dermatome (Fig 2). On the basis of the crew member’s symptoms, rash description, and images of the rash, a clinical diagnosis of shingles was made by the crew surgeons. The crew member was immediately given oral valacyclovir, 1 g 3 times per day for 7 days, and ibuprofen, 400 mg every 6 hours, as needed for pain or discomfort. The crew member reported mild discomfort, sleep disturbance, itching, malaise, and headache coincident with the rash, while remaining afebrile with vital signs within the patient’s baseline range. A rash skin swab was collected and frozen to be later assessed terrestrially for virus DNA. After 7 days, the patient took ibuprofen every 6 hours for 2 more days and then sporadically for another 3 days as needed. Both drugs were available in the on-board medical kit. On the basis of vitals, lack of congestion or symptoms of upper respiratory infection, ability to clear ears, lack of musculoskeletal pain that could confound decompression sickness symptoms, and other indicators, the crew member was deemed medically fit to participate in contingency spacewalks should one be required on an emergency basis. The crew member’s symptoms and physical examination findings improved significantly 2 to 3 weeks after onset; however, mild residual itching was reported through approximately 3 weeks following the outbreak. After analysis after the crew member’s return to Earth, the in-flight skin lesion swab did test positive for varicella zoster virus (1 × 107 copies/mL) by real-time quantitative PCR (cycle threshold = 19), confirming that this was a case of varicella-zoster virus reactivation to development of clinical zoster (see Table E1). The Zostavax (Merck & Co, Rahway, NJ) vaccine had been administered to this crew member approximately 2 years before the flight.
Fig 2.
Image of shingles rash taken by the crew member during the ISS mission.
Following resolution of the zoster symptoms, a final ambient blood sampling was performed before landing. These samples demonstrated that the reductions in T-cell function had essentially resolved to baseline values; however, both detectable inflammation (plasma cytokines) and increased monocyte function (according to intracellular cytokine assay) persisted, as did the shedding of EBV. All monitored parameters had mostly returned to baseline 30 days after return.
Noteworthy is a parallel symptomatic event. By flight day 30, this crew member also developed clinically concerning spaceflight-associated neuroocular syndrome (SANS). Although its etiology remains unclear, SANS is characterized by a constellation of ocular anatomic changes during spaceflight that can be asymptomatic or can induce vision alterations.8 Multiple Spectralis Optical Coherence Tomography images (Heidelberg Engineering GmbH, Heidelberg, Germany) were collected during the mission and processed terrestrially to monitor ocular integrity. This crew member developed bilateral optic nerve head edema that peaked mid-mission, followed by limited recovery during flight (Fig 1). Bilateral chorioretinal fold formation and choroidal engorgement peaked mid-to-late mission, followed by partial recovery during flight. These parameters recovered after flight. In addition, postflight magnetic resonance imaging (Siemens) and eye examinations detected globe flattening and refractive error shifts (greater than +0.75 D) in both eyes. Although microgravity-associated fluid shifts and genetic variants in 1-carbon metabolism have been suggested as causes for SANS, whether persistent inflammation or virus reactivation could also play a role in its pathogenesis is unknown.
A recent report suggests that biomedical countermeasures already deployed to the ISS have positively benefited immunity and reduced virus reactivation in astronauts.9 The current case demonstrates that select astronauts still experience persistent immune system dysregulation that can cross the threshold to clinical symptomology. Unfortunately, the countermeasures deployed to the ISS (large exercise devices, frequent resupply, functional food/nutritional supplementation, and immediate communication with Earth) remain largely incompatible with the Artemis program’s deep space missions owing to operational constraints; examples include far less habitable volume, reduced power availability, and less frequent resupply. Clinical risk and the severity of cases during deep space missions are likely to be increased and will therefore require implementation of new or modified countermeasures. To that end, a group of international scientists recently proposed an “immunity-restorative” countermeasure protocol that is specifically compatible with deep space missions.10 In a separate study, we have already shown that prophylactic administration of an antiviral drug only (valacyclovir) is a safe and successful intervention to reduce EBV and herpes simplex virus 1 shedding in Antarctic expeditioners.11 The full countermeasure protocol for deep space missions (including supplements, specific exercise, and stress relief) is currently being validated at Palmer Station, Antarctica, which is a suitable ground-based spaceflight analog.
Disclosure statement
Supported by the NASA Human Research Program, Human Health and Countermeasures Element.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Consent: The NASA Longitudinal Survey of Astronaut Health Board has reviewed this product and classified it as nonattributable. The specific crew member has reviewed this article. Written informed consent was obtained from the crew member giving permission to access the medical and research data contained herein and to publish this case (including all visual elements) in this nonattributable format.
Acknowledgments
We wish to thank the ISS crew member for participating in the research study, for allowing individual medical and research data to be released in the format presented here, and for assisting in preparation of this article. We also wish to acknowledge the support provided by the Johnson Space Center Clinical Laboratory and the Johnson Space Center Mission Integration Team supporting the relevant flight investigations.
Supplementary data
References
- 1.Crucian B., Stowe R.P., Mehta S., Quiriarte H., Pierson D., Sams C. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity. 2015;1 doi: 10.1038/npjmgrav.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta S.K., Laudenslager M.L., Stowe R.P., Crucian B.E., Feiveson A.H., Sams C.F., et al. Latent virus reactivation in astronauts on the International Space Station. NPJ Microgravity. 2017;3:11. doi: 10.1038/s41526-017-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gershon A.A., Breuer J., Cohen J.I., Cohrs R.J., Gershon M.D., Gilden D., et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crucian B., Babiak-Vazquez A., Mehta S., Pierson D., Ott M., Sams C. Incidence of clinical symptoms during long-duration spaceflight. Int J Gen Med. 2016 Nov 3;9:383–391. doi: 10.2147/IJGM.S114188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crucian B., Johnston S., Mehta S., Stowe R., Uchakin P., Quiriarte H., et al. A case of persistent skin rash and rhinitis with immune system dysregulation onboard the International Space Station. J Allergy Clin Immunol Pract. 2016;4:759. doi: 10.1016/j.jaip.2015.12.021. 62.e8. [DOI] [PubMed] [Google Scholar]
- 6.Mehta S.K., Szpara M.L., Rooney B.V., Diak D.M., Shipley M.M., Renner D.W., et al. Dermatitis during spaceflight associated with HSV-1 reactivation. Viruses. 2022;14:789. doi: 10.3390/v14040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunz H.E., Makedonas G., Mehta S.K., Tyring S.K., Vangipuram R., Quiriarte H., et al. Zoster patients on Earth and astronauts in space share similar immunologic profiles. Life Sci Space Res (Amst) 2020;25:119–128. doi: 10.1016/j.lssr.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Lee A.G., Mader T.H., Gibson C.R., Brunstetter T.J., Tarver W.J. Space flight-associated neuro-ocular syndrome (SANS) Eye (Lond) 2018;32:1164–1167. doi: 10.1038/s41433-018-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crucian B.E., Makedonas G., Sams C.F., Pierson D.L., Simpson R., Stowe R.P., et al. Countermeasures-based improvements in stress, immune system dysregulation and latent herpesvirus reactivation onboard the International Space Station - relevance for deep space missions and terrestrial medicine. Neurosci Biobehav Rev. 2020;115:68–76. doi: 10.1016/j.neubiorev.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Makedonas G., Mehta S., Choukèr A., Simpson R.J., Marshall G., Orange J.S., et al. Specific immunologic countermeasure protocol for deep-space exploration missions. Front Immunol. 2019;10:2407. doi: 10.3389/fimmu.2019.02407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta S.K., Diak D., Rooney B.V., Krieger S.S., Nelman-Gonzalez M., Locke J., et al. Antiviral treatment with valacyclovir reduces virus shedding in saliva of Antarctic expeditioners. Front Virol. 2023;3 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.